Abstract
Background:
The consequences of an infertility diagnosis extend beyond the pursuit of family building, as infertile women also face increased risks of severe maternal morbidity, cancer, and chronic disease.
Objective:
To examine the association between female infertility and all-cause mortality.
Study Design:
Retrospective analysis of 72,786 infertile women identified in the Optum Clinformatics Datamart from 2003-2019 by infertility diagnosis, testing and treatment codes compared with 3,845,790 non-infertile women seeking routine gynecologic care. Baseline comorbidities were assessed using the presence of ≥1 metabolic syndrome (MetS) diagnoses and the Charlson Comorbidity Index (CCI). The primary outcome of all-cause mortality was identified by linkage to Social Security Administration Death Master File outcomes and medical claims. The association of infertility with mortality was examined using Cox proportional hazard regression while adjusting for age, hypertension, hyperlipidemia, type II diabetes, year of evaluation, smoking, number of visits per year, nulliparity, obesity, region of country, and race.
Results:
Among 16,473,458 person-years of follow up, 13,934 women died. Infertile women had a 32% higher relative risk of death from any cause (0.42% versus 0.35%, aHR 1.32, 95% CI 1.18-1.48) compared to non-infertile women. Mean follow up time per patient was 4.0±3.7 years versus 4.2±3.8 years for infertile and non-infertile women, respectively. When stratified by age < 35 or ≥35 years or baseline medical comorbidity, the association between infertility and mortality remained. Infertile women who delivered a child during the follow up period faced similar increased risk of mortality compared to the overall infertile group. Finally, receipt of fertility treatment was not associated with a higher risk of death compared to receiving an infertility diagnosis or testing alone.
Conclusions:
While absolute risk of death was low in both groups, infertile women faced a higher relative risk of mortality compared to non-infertile women. The association remained across all age, race/ethnicity, morbidity, and delivery strata. Importantly, infertility treatment was not associated with an increased risk of death. These findings reinforce the disease burden associated with infertility and its potential for longer-term sequelae.
Introduction
Infertility, defined by the American Society of Reproductive Medicine as the inability to achieve pregnancy after 12 months of regular unprotected intercourse, has short and long-term health implications[1]. Since the birth of the first IVF baby in 1978, the field of assisted reproductive technology (ART) has developed at a remarkable pace, with rapid advances in ovarian stimulation, IVF techniques and the embryology laboratory. ART has created family building options for patients that were previously unimaginable [2]. While our ability to address the immediate (periconceptional) aspects of infertility have improved, the long-term health effects implications of infertility have received less recognition.
Both male and female infertility have been associated with greater risks of cancer and chronic disease, which are the leading causes of morbidity and mortality [3-10]. These associations suggest that infertility provides a window into future health and has effects beyond the reproductive years [11, 12]. Although nulliparity and infertility are distinct, the former has been associated with increased risk of mortality in large population-based linkage studies[13, 14]. An independent association between infertility and mortality was reported from a secondary analysis of a multicenter randomized clinical trial from 1992-2001[15]. We sought to build upon this prior work by presenting results from a contemporary cohort of infertile women and examining differential effects of infertility and fertility treatment on the subsequent risk of mortality.
Materials and Methods
Patients
We analyzed subjects insured by a plan included in Optum’s de-identifed Clinformatics® Data Mart (CDM), a nationwide commercial and Medicare Advantage claims database, from 2003-2019. Patients are enrolled in Optum’s CDM when their insurance coverage begins and administrative claims submitted for payment by providers and pharmacies are verified, adjudicated, adjusted, and de-identified prior to inclusion. 23% of the US population is covered by a plan included in CDM, and the distribution of ethnicity and region is similar to the US population overall. This study was approved by the Stanford Institutional Review Board.
Women were identified within Optum’s CDM for inclusion in the infertile and control groups based on the following criteria: 1) Age 20-45 years, 2) Enrolled in the insurance claims database for at least 6 months before and after inclusion in the study and 3) presence of specific diagnostic or procedural codes indicating fertility status. Characterization of the infertile and non-infertile groups by ICD 9/10 diagnosis and procedural (CPT) codes has been described in detail previously by our group [3, 4]. In brief, the group of infertile women was comprised of women receiving any of the following during enrollment: 1) an infertility diagnosis, 2) fertility testing, or 3) fertility treatment. Women with an infertility diagnosis were identified by ICD 9/10 diagnosis codes (Supplementary Table 1). Fertility testing was identified through ICD 9/10 diagnosis codes or the presence of a CPT code for hysterosalpingogram (HSG). Patients receiving fertility treatment were identified by the presence of a CPT code for intra-uterine artificial insemination (IUI), follicular puncture for oocyte retrieval, or intrauterine embryo transfer. Pharmacy claims for a prescription for clomiphene citrate or gonadotropins (Follicle Stimulating Hormone, Human Menopausal Gonadotropin, or Human Chorionic Gonadotropin) were also used to identify patients receiving fertility treatment.
The comparison group was composed of women receiving routine gynecologic care who did not have an infertility diagnosis or any procedure codes for fertility testing or treatment during enrollment. These non-infertile patients were identified through: 1) the presence of a claim for a well woman visit, or an encounter for 2) contraceptive management, 3) placement or removal of an IUD, 4) placement of a contraceptive implant, 5) encounter for contraceptive surveillance, or 6) pap smear.
Assessment of Baseline Comorbidities
Baseline comorbidities were assessed using the presence of ≥ 1 metabolic syndrome (MetS) diagnoses and the Charlson Comorbidity Index (CCI). MetS diagnoses included hypertension, hyperlipidemia, type II diabetes, and obesity and were identified using diagnosis codes from inpatient and outpatient records (hypertension: ICD 9: 401–405; ICD 10: I10–I16), hyperlipidemia (ICD 9: 270.2–270.4; ICD 10: E784, E785, E781, E782, E7800), diabetes mellitus (ICD 9: 250; ICD 10: E08–E13), obesity (ICD 9: 278.0; ICD 10: E66.9, E66.01, E66.3, E66.2). While the CCI has traditionally been used in an inpatient setting to evaluate mortality, its use has been validated in both ambulatory and reproductive settings[16, 17]. In addition, an updated CCI was calculated for all patients that is appropriate for use with administrative data and has shown good to excellent discrimination in predicting mortality[18].
Outcome Ascertainment
All-cause mortality was determined by Optum using any of the following strategies: 1) Linkage to Social Security Administration Death Master File, which contains information about individuals who had Social Security numbers and whose deaths were reported to the Social Security Administration from 1962 to the present. If multiple dates were present, verification was performed with death certificates. 2) Claims from Centers for Medicare and Medicaid Services (CMS) data 3) Facility claims where discharge status was designed as “expired” and 4) Member coverage discontinuation due to death. Death records were removed if insurance coverage of claims was found more than 60 days after the death date.
Subgroup of Patients with a Delivery
In both groups, women who became pregnant and had a delivery during the follow up period were identified by diagnosis and procedure codes obtained from literature indicating the conclusion of a pregnancy [19, 20].
Statistical Analysis
The association of infertility with mortality was examined using Cox proportional hazard regression. Upon inclusion in either infertile or non-infertile groups, patients were followed for the duration of their enrollment in the insurance database or until their death, whichever occurred first. The end of enrollment was defined as when insured coverage ended or the last period of available data, and women were censored in the survival analysis at this time point. Analyses were adjusted for age, hypertension, hyperlipidemia, type II diabetes, year of evaluation, smoking, number of visits per year, nulliparity, obesity, region of country and race. We also stratified the data by age (< 35 or ≥35 year), baseline medical comorbidity quantified as number of MetS diagnoses (0 or ≥1) and CCI (score of 0 or ≥1), follow up time (<4 or ≥4 years), race, delivery, receipt of fertility treatment during the follow up period. Identical Cox proportional hazard models were applied for the sub-analyses. All p values were 2-sided with p<0.05 considered statistically significant. Analyses were performed using SAS (version 9.4, SAS Institute, Inc., Cary, NC, USA).
Results
Patient Demographics
The study population included 72,786 infertile women and 3,845,790 non-infertile women. Infertile and non-infertile women were similar in age at the time of enrollment (mean age 33.7±5.8 years versus 32.6±7.5 years, respectively) and followed for comparable time periods (mean follow up time 4.0±3.7 years versus 4.2±3.8 years). Infertile women were more likely to be nulliparous (16.6% versus 8.1%), obese (20.2% versus 14.9%) and smokers (7.3% versus 5.4%) compared to non-infertile women. Epidemiologic studies vary in their findings but some have found a higher prevalence of smoking among infertile women compared to fertile women [21]. Ethnicity, year of evaluation and region of the country was distributed similarly among infertile and non-infertile women (Table 1). Infertile women had a median of 4.0 (range 1.9-7.2) medical visits per year (all types) compared to 2.7 (range 1.3-4.8) visits per year (all types) among non-infertile women. 37% of the women in the cohort had infertility testing, 90% of women in the cohort had an infertility diagnosis and 23% of the infertile cohort underwent fertility treatment. We included patients undergoing fertility testing with the understanding that they would already meet criteria for an infertility diagnosis. However, providers may use testing instead of diagnosis codes for reimbursement purposes thus we sought to capture these women for inclusion in the infertile group.
Table 1.
Patient demographics of infertile and non-infertile patients.
| Infertile | Non-Infertile | ||
|---|---|---|---|
| Number of Patients | 72,786 | 3,845,790 | |
| Age (years) | Mean (SD) | 33.7 (5.8) | 32.6 (7.5) |
| 20-24, (n,%) | 4,331 (6.0) | 698,131 (18.2) | |
| 25-29, (n,%) | 14,053 (19.3) | 780,473 (20.3) | |
| 30-34, (n,%) | 21,532 (29.6) | 743,454 (19.3) | |
| 35-39, (n,%) | 19,714 (27.1) | 719,187 (18.7) | |
| 40-45, (n,%) | 13,156 (18.1) | 904,545 (23.5) | |
| Follow up time (years) | Mean (SD) | 4.0 (3.7) | 4.2 (3.8) |
| 0-1, (n,%) | 12,593 (17.3) | 632,019 (16.4) | |
| 1-2, (n,%) | 17,552 (24.1) | 878,338 (22.8) | |
| 2-3, (n,%) | 10,319 (14.2) | 539,445 (14.0) | |
| 3-4, (n,%) | 6,920 (9.5) | 366,186 (9.5) | |
| 4+, (n,%) | 25,402 (34.9) | 1,429,802 (37.2) | |
| Total (n) | 292,659.0 | 16,180,799.6 | |
| Nulliparity, (n,%) | 12,073 (16.6) | 309,968 (8.1) | |
| Obesity, (n,%) | 14,689 (20.2) | 572,426 (14.9) | |
| Smoking, (n,%) | 5,313 (7.3) | 206,726 (5.4) | |
| Index date, (n,%) | 2003 | 5,827 (8.0) | 334,573 (8.7) |
| 2004 | 6,109 (8.4) | 339,374 (8.8) | |
| 2005 | 4,938 (6.8) | 290,230 (7.6) | |
| 2006 | 5,867 (8.1) | 319,446 (8.3) | |
| 2007 | 5,114 (7.0) | 276,433 (7.2) | |
| 2008 | 4,865 (6.7) | 270,675 (7.0) | |
| 2009 | 3,991 (5.5) | 242,446 (6.3) | |
| 2010 | 3,350 (4.6) | 202,660 (5.3) | |
| 2011 | 3,431 (4.7) | 198,358 (5.2) | |
| 2012 | 3,380 (4.6) | 189,021 (4.9) | |
| 2013 | 3,549 (4.9) | 180,817 (4.7) | |
| 2014 | 3,729 (5.1) | 176,551 (4.6) | |
| 2015 | 4,262 (5.9) | 189,951 (4.9) | |
| 2016 | 4,646 (6.4) | 206,514 (5.4) | |
| 2017 | 4,691 (6.4) | 216,822 (5.6) | |
| 2018 | 5,037 (6.9) | 211,919 (5.5) | |
| Visits per person year | Median (range) | 4.0 (1.9 - 7.2) | 2.7 (1.3 - 4.8) |
| <1, (n,%) | 9,013 (12.4) | 723,672 (18.8) | |
| 1-2, (n,%) | 9,766 (13.4) | 752,178 (19.6) | |
| 2+, (n,%) | 54,007 (74.2) | 2,369,940 (61.6) | |
| Region of the country, (n,%) | Midwest | 16,479 (22.6) | 954,824 (24.8) |
| Northeast | 8,201 (11.3) | 385,803 (10.0) | |
| South | 29,962 (41.2) | 1,732,143 (45.0) | |
| West | 17,919 (24.6) | 766,041 (19.9) | |
| Unknown | 225 (0.3) | 6,979 (0.2) | |
| Unknown | 16,479 (22.6) | 954,824 (24.8) | |
| Race, (n,%) | Asian | 7,129 (9.8) | 197,821 (5.1) |
| Black | 6,972 (9.6) | 364,996 (9.5) | |
| Hispanic | 9,046 (12.4) | 445,851 (11.6) | |
| White | 40,029 (55.0) | 2,365,380 (61.5) | |
| Unknown | 9,610 (13.2) | 471,742 (12.3) |
Risk of Mortality between Infertile and Non-infertile Women
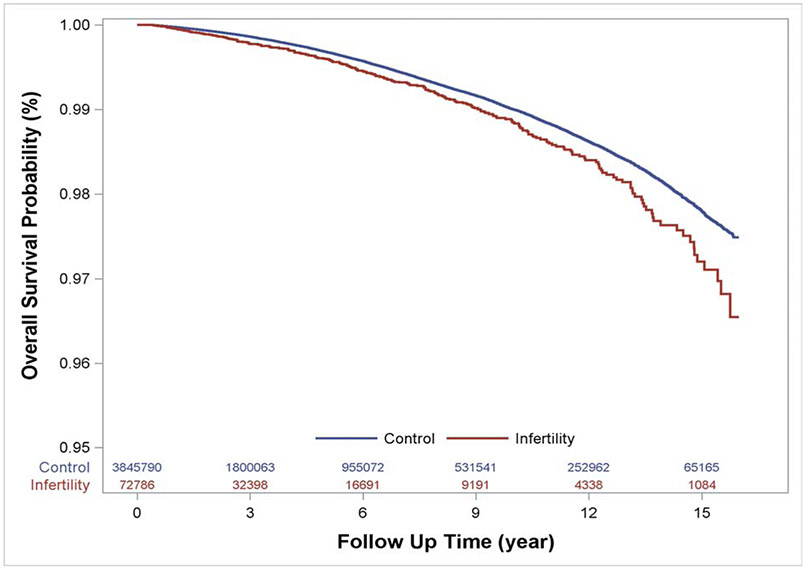
Among 16,473,458 person-years of follow up, 13,934 women died. Among infertile women, there were 307 deaths (0.42%) during the follow up period. Among non-infertile women, there were 13,627 deaths (0.35%). Women were on average 42.2±7.6 years of age at time of death in the infertile and 43.3±8.2 years of age at time of death in the non-infertile group. While absolute rates of death from any cause were low, infertile women had a 32% higher relative risk of death (aHR 1.32, 95% CI 1.18-1.48) compared to non-infertile women during the follow up period. The risk of all-cause mortality for infertile women compared with non-infertile women during the follow up period is depicted in Figure 1. Using the Schoenfeld Residuals method, we concluded the assumption of proportional hazards was valid. A subgroup of the non-infertile and infertile cohorts were matched based on age, follow up time, nulliparity, obesity, smoking, year of visit, region and race. In this analysis, power was limited due to the smaller sample size and number of outcomes but point estimates and interpretations were similar (Supplementary Table 2).
Figure 1.
Adjusted Cox-proportional hazard regression models compare the risk of death from any cause over the study period between infertile and non-infertile women. The survival table represents the number of women available for analysis at each time point.
Stratification by Age and Comorbidities
Analyses were stratified by age and medical comorbidities to further investigate the association between infertility, mortality, and potential confounders (Table 2). When stratified by age < 35 or ≥35 years or baseline medical comorbidity, the association between infertility and mortality remained (aHR 1.39, 95% CI 1.15 - 1.69 and aHR 1.32, 95% CI 1.15-1.52, respectively). When stratified by MetS diagnoses, there was a similar increase in risk of death among infertile patients compared to non-infertile patients across strata (aHR 1.24, 95% CI 1.07 - 1.44 among patients with no prior MetS diagnosis, aHR 1.24, 95% CI 1.05 - 1.47 among patients with at least 1 prior MetS diagnosis. Baseline comorbidities were also quantified using the CCI with similar results as shown in Table 2.
Table 2.
Absolute incidence, events per person years, hazard ratios and 95% confidence intervals (CI) for the association between infertility and mortality.
| Infertile | Non-Infertile | Crude HR (95% CI) | aHR* (95% CI) | |||
|---|---|---|---|---|---|---|
| All | N | 72,786 | 3,845,790 | |||
| Deaths (N, %) | 307 (0.42) | 13,627 (0.35) | 1.27 (1.14 - 1.42) | 1.32 (1.18 -1.48) | ||
| Age (years) | <35 | N | 39,916 | 2,222,058 | ||
| Deaths | 110 (0.28) | 4,181 (0.19) | 1.44 (1.20 - 1.74) | 1.39 (1.15 - 1.69) | ||
| ≥35 | N | 32,870 | 1,623,732 | |||
| Deaths | 197 (0.60) | 9,446 (0.58) | 1.21 (1.05 - 1.40) | 1.32 (1.15 - 1.52) | ||
| MetS Diagnoses** | 0 | N | 54,649 | 3,059,843 | ||
| Deaths | 168 (0.31) | 7,914 (0.26) | 1.24 (1.06 - 1.44) | 1.24 (1.07 - 1.44) | ||
| ≥1 | N | 18,137 | 785,947 | |||
| Deaths | 139 (0.77) | 5,713 (0.73) | 1.22 (1.03 - 1.45) | 1.24 (1.05 - 1.47) | ||
| CCI** | 0 | N | 55,980 | 3,104,100 | ||
| Deaths | 133 (0.24) | 6,966 (0.22) | 1.13 (0.95 - 1.34) | 1.15 (0.97 - 1.37) | ||
| ≥1 | N | 16,806 | 741,690 | |||
| Deaths | 174 (1.04) | 6,661 (0.90) | 1.24 (1.07 - 1.44) | 1.20 (1.03 - 1.39) | ||
| Follow-up time (years) | <4 | N | 47,384 | 2,415,988 | ||
| Deaths | 211 (0.45) | 9,110 (0.38) | 1.13 (0.99 - 1.30) | 1.09 (0.95 - 1.25) | ||
| ≥4 | N | 25,402 | 1,429,802 | |||
| Deaths | 96 (0.38) | 4,517 (0.32) | 1.22 (1.00 - 1.50) | 1.25 (1.02 - 1.53) | ||
| Race | Asian | N | 7,129 | 197,821 | ||
| Deaths | 12 (0.17) | 326 (0.16) | 1.11 (0.62 - 1.97) | 1.33 (0.75 - 2.37) | ||
| Black | N | 6,972 | 364,996 | |||
| Deaths | 47 (0.67) | 1,803 (0.49) | 1.44 (1.08 - 1.92) | 1.40 (1.05 - 1.87) | ||
| Hispanic | N | 9,046 | 445,851 | |||
| Deaths | 18 (0.20) | 1,010 (0.23) | 0.92 (0.58 - 1.46) | 0.90 (0.56 - 1.43) | ||
| White | N | 40,029 | 2,365,380 | |||
| Deaths | 199 (0.50) | 9,037 (0.38) | 1.38 (1.20 - 1.58) | 1.41 (1.23 - 1.63) |
Adjusted for age, hypertension, hyperlipidemia, type II diabetes, year of evaluation, smoking, number of visits per year, nulliparity, obesity, region of country and race.
Adjusted for age, year of evaluation, smoking, number of visits per year, nulliparity, obesity, region of country and race (adjustment for hypertension, hyperlipidemia, type II diabetes not performed because these variables are included in MetS or CCI definition).
Stratification by Race
Risk of death among infertile and non-infertile women was examined by racial subgroups. The majority of patients in both patient groups were White (55% and 61.5%). Infertile White women faced a higher risk of death compared to non-infertile White women with a point estimate similar to the overall analysis (aHR 1.41, 95% CI 1.23-1.63). The association was similar among Black patients (aHR 1.40, 95% CI 1.05-1.87). Among Asian and Hispanic patients, the risk of death was not statistically different between infertile and non-infertile groups (aHR 1.33, 95% CI 0.75-2.37, and aHR 0.90, 95% CI 0.56-1.43, respectively).
Subgroup of All Patients with a Delivery
Risk of mortality was stratified by delivery among patients in both infertile and non-infertile groups (Table 3). Among patients who had a delivery during follow up, there were 14 deaths (0.19%) in the infertile group and 328 deaths (0.16%) in the non-infertile group. Among patients who did not have a delivery during follow up, there were 293 deaths (0.45%) in the infertile group and 13,299 deaths (0.37%) in the non-infertile group. The association between infertility and increased risk of mortality remained among women who had a delivery during follow up (aHR 1.75, 95% CI 1.14-2.70) and among women who did not have a delivery during follow up (aHR 1.30, 95% CI 1.16-1.46).
Table 3.
Absolute incidence, hazard ratios and 95% confidence intervals (CI) for the association between infertility and mortality stratified by delivery during the enrollment period.
| Infertile | Non-Infertile | Crude HR (95% CI) | aHR (95% CI) | |||
|---|---|---|---|---|---|---|
| Delivery during enrollment | Yes | N | 7,538 | 205,385 | ||
| Deaths (N, %) | 14 (0.19) | 328 (0.16) | 1.25 (0.73 - 2.13) | 1.75 (1.14 - 2.70) | ||
| No | N | 65,248 | 3,640,405 | |||
| Deaths (N, %) | 293 (0.45) | 13,299 (0.37) | 1.31 (1.17 - 1.47) | 1.30 (1.16 - 1.46) |
Subgroup of Infertile Patients
Finally, the risk of mortality was examined among infertile patients only, subdivided among infertile patients who received a fertility diagnosis or underwent fertility testing, and infertile patients who underwent fertility treatment. Among 55,858 patient receiving an infertility diagnosis or undergoing fertility testing, there 269 deaths (0.48%). Among 16,928 infertile patients undergoing fertility treatment, there were 38 deaths (0.22%). The risk of death was increased among infertile patients receiving an infertility diagnosis or fertility testing compared to those undergoing fertility treatment (aHR 1.92, 95% CI 1.35-2.72).
Comment
Principal Findings of the Study
From a contemporary cohort of 3,918,576 women age 20-45, we report a small absolute risk of death but a 32% relative increase in risk of death among infertile women compared to non-infertile women. These findings are consistent with prior reports from our group that the consequences of an infertility diagnosis extend beyond the pursuit of family building, as infertile women also face increased risks of severe maternal morbidity, cancer, and chronic disease [3, 4, 22]. Mortality patterns among U.S. residents are tracked by the National Center for Health Statistics. To place our results in context, the death rate in 2017 among all U.S. women ages 25-44 years was 446.7/100,000 (0.45%), which is overall similar to the death rates we report.
Results and Clinical Implications
Counseling an infertile patient who is planning to undertake fertility treatment typically includes a discussion regarding attendant risks. Current counseling largely focuses on peri-procedural and pregnancy-related risks[23]. The growing body of work demonstrating an association between both female and male infertility and increased risk of morbidity and mortality[24] suggests that continued evaluation of infertile patients may be warranted after reproductive pursuits conclude. The fertility evaluation offers an opportunity to screen for additional factors that may affect future health, implement additional periodic health screening measures, and provide counseling regarding the implications of an infertility diagnosis on a woman’s short and long-term health.
Research Implications
Our findings are complementary to several studies reporting an association between male and female infertility, increased risk of morbidity, and increased risk of mortality[6, 7, 11, 12, 25, 26]. Our findings also reinforce the concept of infertility as a distinct class of diagnoses. Female infertility has been associated with increased risk of metabolic syndrome and cardiovascular disease[9, 27]. An increased risk of death among infertile women was first reported from a secondary analysis of a multicenter randomized clinical trial (aHR1.10, 95% CI, 1.02-1.18) [15]. . Our findings reinforce this initial report, with the additional advantage that the data in this analysis were prospectively obtained and not subject to patients’ ability to recall a history of infertility. Furthermore, the ages of patients enrolled in the study was such that during their reproductive years, contemporary fertility treatments were just emerging; the awareness of infertility and treatment options have changed dramatically since the early 1980s[2].
We performed a series of stratified analyses to evaluate if confounding factors (e.g. age, comorbidity, race/ethnicity, maternity) may explain the association between infertility and mortality. We found that the risk of mortality among infertile patients persisted across differences in age (<35 or ≥ 35 years) and comorbidities (Number of MetS diagnoses or CCI). In a subanalysis with propensity matching, the association of infertility and mortality remained among healthy women. These findings suggest that a biologic difference may exist among infertile women leading to an increased risk of mortality that is not solely attributed to increasing age and medical comorbidities.
Childbearing has a protective effect on mortality and is influenced by a myriad of factors that are biologic, social, and economic [13, 14, 28]. Having children prior to ART treatment has been associated with reduced risk of mortality[29]. This association can be attributed to better health among previously fertile women, or a less severe form of infertility among parous women that is associated with a lower risk of death. In a subgroup analysis, we found similar risk of mortality among women who had a delivery during follow up and those who did not.. Although this analysis is limited by small sample size, it suggests that a biologic difference exists between infertile and non-infertile women independent of powerful influencers of mortality including childbirth. Disparate birth outcomes including stillbirth, multiple birth, and birth of a child with significant abnormality can all increase physical and emotional stress, which in turn can influence health and mortality risk among both infertile and non-infertile women; this merits examination in a future study.
Finally, we stratified the risk of mortality among infertile patients who received an infertility diagnosis or fertility testing versus those patients who underwent fertility treatment. While our sub-analyses are limited by small numbers, we report that receipt of fertility treatment (e.g. IUI, in vitro fertilization, or controlled ovarian stimulation) is not independently associated with increased risk of death. These findings are concurrent with large population studies reporting lower risk of death among women treated with IVF compared to unexposed women[30, 31]. Importantly, women who undergo fertility treatment generally have higher education, income and access to care; this “healthy user effect” may explain the association with lower mortality [32]. In addition, physical, psychological, and social resources play a role in fertility treatment initiation, and patients can face multiple barriers to obtaining treatment after an infertility diagnosis [33].
Strengths and Limitations
To our knowledge, this is the first report of an association between infertility and mortality in a contemporary cohort of women diagnosed with infertility and exposed to fertility treatment as it is practiced in the current day. Study strengths include the large size of the cohort, detailed claims-based characterization of infertility, adjustment for multiple covariates and sub-analyses demonstrating that the increased risk of mortality among infertile women persists across all age, race/ethnicity, morbidity, and delivery strata. Limitations to our study design include the relatively young age of the cohort and the modest individual follow-up of 4 years on average, a time when death represents a rare event, and the relatively young age at time of death. Furthermore, since cause of death was not available, we could not determine if mortality events were precipitated by biological disease, accidents, suicide or other causes. In addition, details regarding infertility diagnosis and treatment are not fully captured in insurance claims data. The prevalence of infertility in our patient cohort is lower than reported in national data[34]. This reflects prior reports that only a minority of infertile women seek evaluation and/or treatment[32], and is also due to stringent inclusion criteria to avoid inadvertently including a fertile woman in the infertile cohort. While the prevalence of fertility treatment is in line with the most recent National Health Statistics Report[32], patients could have sought fertility treatment outside of insurance coverage and this would have not been captured in our database. It is also possible that women in the unexposed group had diagnoses of infertility before or after enrollment the database. This misclassification, however, would lead to a regression to a null finding. Despite any association we found likely being an under-estimate, we still found a significant difference between the cohorts. Given the rare nature of the outcome of interest, we were unable to further stratify the infertile cohort by infertility diagnosis. Within this heterogeneous patient population, there may be subsets that are higher risk for later mortality that warrant study in a larger cohort over a longer period of time. For example, the association between infertility and risk of mortality may be more pronounced in female infertility and diluted out by a population of women in IVF-treated couples with only male factor infertility. Overall, however, we anticipate this would have resulted in an underestimate of associations. The limited duration of follow up and lack of access to granular information on why an individual patient changes or loses insurance coverage is an additional limitation of the analysis. However, follow up time was similar between the groups (4.0 years vs 4.2 years for infertile vs non-infertile, respectively). The reported differences between the groups may change as these young patients age beyond the reproductive years and longer term follow up is warranted. We are also limited by the accuracy of coding for infertility diagnoses and procedures, and standard fertility tests such as the HSG could have been performed to evaluate other gynecologic concerns. Additionally, information on parity is not readily available using claims data and births were captured only if they occurred during the follow up period. Finally, an ideal statistical model would incorporate time-dependent covariates. Claims data is not well-suited to this design because not every enrollee is seen at regular intervals, the time of a particular diagnosis may be unreliable, and once a diagnosis is recorded, it is not possible to know if or when it resolves. Validation of these findings in a contemporary, prospective cohort of women evaluated longitudinally at multiple time points past the reproductive years is warranted.
Conclusions
While absolute risk of death was low in both groups, infertile women face a higher relative risk of mortality compared to fertile women, reinforcing the disease burden associated with this diagnosis and its potential for long-term health sequelae. Moreover, the association remained across all age, race/ethnicity, morbidity, and delivery strata. Importantly, fertility treatment was not associated with an increased risk of death. Longer-term studies are needed to address the time course of this association.
Supplementary Material
Condensation: While absolute risk of death was low in both groups, infertile women faced a higher relative risk of mortality compared to non-infertile women.
AJOG at a Glance:
The objective of this study was to examine the association between female infertility and mortality using an insurance claims database.
While absolute risk of death was low in both groups, we report that infertile women have a 32% higher relative risk of death from any cause (0.42% versus 0.35%, aHR 1.32, 95% CI 1.18-1.48) compared to non-infertile women. When stratified by age or baseline medical comorbidity, the association between infertility and mortality remains.
Novel findings include that receipt of fertility treatment was not associated with a higher risk of death compared to receiving an infertility diagnosis or testing alone. Overall, these findings suggest that infertility is not only a distinct disease process, but also a window to future health.
Acknowledgments
Funding: Support for this work was provided by the Eunice Kennedy Shriver NICHD of the National Institutes of Health under award number 1K12HD103084.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Barbara Luke is a research consultant to the Society for Assisted Reproductive Technology (SART). Dr. Ruben Alvero is Scientific Advisor to Hannah Life Technologies and Orchid Bioscience.
Paper presentation information: These findings were presented at the 76th ASRM Virtual Scientific Congress, October 17-22nd, 2020.
References
- 1.Practice Committee of the American Society for Reproductive Medicine. Electronic address, a.a.o., Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril, 2020. 113(3): p. 533–535. [DOI] [PubMed] [Google Scholar]
- 2.Niederberger C, et al. , Forty years of IVF. Fertil Steril, 2018. 110(2): p. 185–324 e5. [DOI] [PubMed] [Google Scholar]
- 3.Murugappan G, et al. , Risk of cancer in infertile women: analysis of US claims data. Hum Reprod, 2019. 34(5): p. 894–902. [DOI] [PubMed] [Google Scholar]
- 4.Murugappan G, et al. , Increased risk of incident chronic medical conditions in infertile women: analysis of US claims data. Am J Obstet Gynecol, 2019. 220(5): p. 473.e1–473.e14. [DOI] [PubMed] [Google Scholar]
- 5.Glazer CH, et al. , Male Infertility and Risk of Nonmalignant Chronic Diseases: A Systematic Review of the Epidemiological Evidence. Semin Reprod Med, 2017. 35(3): p. 282–290. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg ML, et al. , Increased risk of incident chronic medical conditions in infertile men: analysis of United States claims data. Fertility and Sterility, 2016. 105(3): p. 629–636. [DOI] [PubMed] [Google Scholar]
- 7.Eisenberg ML, et al. , Increased risk of cancer in infertile men: analysis of U.S. claims data. J Urol, 2015. 193(5): p. 1596–601. [DOI] [PubMed] [Google Scholar]
- 8.Kurabayashi T, et al. , Ovarian infertility is associated with cardiovascular disease risk factors in later life: A Japanese cross-sectional study. Maturitas, 2016. 83: p. 33–9. [DOI] [PubMed] [Google Scholar]
- 9.Parikh NI, et al. , Subfertility and risk of later life maternal cardiovascular disease. Human Reproduction, 2011. 27(2): p. 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Udell JA, Lu H, and Redelmeier DA, Long-Term Cardiovascular Risk in Women Prescribed Fertility Therapy. Journal of the American College of Cardiology, 2013. 62(18): p. 1704–1712. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg ML, Risks beyond reproduction for infertile men. Fertil Steril, 2016. 105(2): p. 300–1. [DOI] [PubMed] [Google Scholar]
- 12.Choy JT and Eisenberg ML, Male infertility as a window to health. Fertil Steril, 2018. 110(5): p. 810–814. [DOI] [PubMed] [Google Scholar]
- 13.Coleman PK, Reardon DC, and Calhoun BC, Reproductive history patterns and long-term mortality rates: a Danish, population-based record linkage study. Eur J Public Health, 2013. 23(4): p. 569–74. [DOI] [PubMed] [Google Scholar]
- 14.Barclay K, et al. , Reproductive history and post-reproductive mortality: A sibling comparison analysis using Swedish register data. Soc Sci Med, 2016. 155: p. 82–92. [DOI] [PubMed] [Google Scholar]
- 15.Stentz NC, et al. , Infertility and mortality. Am J Obstet Gynecol, 2020. 222(3): p. 251.e1–251.e10. [DOI] [PubMed] [Google Scholar]
- 16.Salonia A, et al. , Are Infertile Men Less Healthy than Fertile Men? Results of a Prospective Case-Control Survey. European Urology, 2009. 56(6): p. 1025–1032. [DOI] [PubMed] [Google Scholar]
- 17.Sundararajan V, et al. , New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. Journal of Clinical Epidemiology, 2004. 57(12): p. 1288–1294. [DOI] [PubMed] [Google Scholar]
- 18.Quan H, et al. , Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. American Journal of Epidemiology, 2011. 173(6): p. 676–682. [DOI] [PubMed] [Google Scholar]
- 19.Ailes EC, et al. , Using insurance claims data to identify and estimate critical periods in pregnancy: An application to antidepressants. Birth Defects Research Part A: Clinical and Molecular Teratology, 2016. 106(11): p. 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett WL, et al. , Utilization of Primary and Obstetric Care After Medically Complicated Pregnancies: An Analysis of Medical Claims Data. Journal of General Internal Medicine, 2014. 29(4): p. 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurent SL, et al. , An epidemiologic study of smoking and primary infertility in women. Fertility and sterility, 1992. 57(3): p. 565–572. [PubMed] [Google Scholar]
- 22.Murugappan G, et al. , Increased risk of severe maternal morbidity among infertile women: analysis of US claims data. Am J Obstet Gynecol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medicine P.C.o.t.A.S.f.R., Evidence-based treatments for couples with unexplained infertility: a guideline. Fertil Steril, 2019. 113: p. 305–322. [DOI] [PubMed] [Google Scholar]
- 24.Luke B, et al. , Is the wrong question being asked in infertility research? Journal of assisted reproduction and genetics, 2016. 33(1): p. 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glazer CH, et al. , Male factor infertility and risk of death: a nationwide record-linkage study. Hum Reprod, 2019. 34(11): p. 2266–2273. [DOI] [PubMed] [Google Scholar]
- 26.Murshidi MM, Choy JT, and Eisenberg ML, Male Infertility and Somatic Health. Urol Clin North Am, 2020. 47(2): p. 211–217. [DOI] [PubMed] [Google Scholar]
- 27.Gleason JL, Shenassa ED, and Thoma ME, Self-reported infertility, metabolic dysfunction, and cardiovascular events: a cross-sectional analysis among U.S. women. Fertil Steril, 2019. 111(1): p. 138–146. [DOI] [PubMed] [Google Scholar]
- 28.Lund E, Arnesen E, and Borgan JK, Pattern of childbearing and mortality in married women--a national prospective study from Norway. Journal of epidemiology and community health, 1990. 44(3): p. 237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vassard D, et al. , Mortality in Women Treated With Assisted Reproductive Technology—Addressing the Healthy Patient Effect. American Journal of Epidemiology, 2018. 187(9): p. 1889–1895. [DOI] [PubMed] [Google Scholar]
- 30.Venn A, et al. , Mortality in a cohort of IVF patients. Human Reproduction, 2001. 16(12): p. 2691–2696. [DOI] [PubMed] [Google Scholar]
- 31.Braat DD, et al. , Maternal death related to IVF in the Netherlands 1984-2008. Hum Reprod, 2010. 25(7): p. 1782–6. [DOI] [PubMed] [Google Scholar]
- 32.Chandra A, Copen CE, and Stephen EH, Infertility service use in the United States: data from the National Survey of Family Growth, 1982-2010. Natl Health Stat Report, 2014(73): p. 1–21. [PubMed] [Google Scholar]
- 33.Domar A, et al. , Understanding the perceptions of and emotional barriers to infertility treatment: a survey in four European countries. Hum Reprod, 2012. 27(4): p. 1073–9. [DOI] [PubMed] [Google Scholar]
- 34.Lepkowski JM, M.W. Davis KE, Groves RM, Van Hoewyk J , The 2006–2010 National Survey of Family Growth: Sample design and analysis of a continuous survey. National Center for Health Statistics. Vital Health Stat, 2010. 150. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.