Abstract
The influence of psychological stress on the physiology of the cardiovascular system, and on the etiology and outcomes of cardiovascular disease (CVD) has been the object of intense investigation. As a whole, current knowledge points to a “brain-heart axis” that is especially important in individuals with pre-existing CVD. The use of acute psychological stress provocation in the laboratory has been useful to clarify the effects of psychological stress on cardiovascular physiology, immune function, vascular reactivity, myocardial ischemia, neurobiology and cardiovascular outcomes. An emerging paradigm is that dynamic perturbations of physiological and molecular pathways during stress or negative emotions are important in influencing cardiovascular outcomes, and that some patient subgroups, such as women, patients with an early-onset myocardial infarction, and patients with adverse psychosocial exposures, may be at especially high risk for these effects. This review summarizes recent knowledge on mind-body connections in CVD among cardiac patients and highlights important pathways of risk which could become the object of future intervention efforts. As a whole, this research suggests that an integrated study of mind and body is necessary to fully understand the determinants and consequences of CVD.
1. Introduction
Despite extraordinary advances in our understanding of the risk factors and pathophysiology of cardiovascular disease (CVD), this condition remains a major cause of morbidity and mortality in the United States and throughout the world. Dramatic declines in CVD mortality in the United States over the past 40 years represent some enormous progress but have also uncovered troubling disparities as not all segments of the population have benefited equally from such improvements. Young women, Americans who live in rural communities or who are from racial/ethnic minority groups have experienced fewer gains or even a worsening of CVD incidence and case fatality1–8 and these disparities are growing.7–12
One area where knowledge still lags, and which could help us understand population disparities, is the sphere of psychological influences on CVD. For many decades, data from epidemiological studies, clinical research and animal models have pointed to a connection between emotional stress and the likelihood of developing CVD, as well as its progression and adverse outcomes.13, 14 Chronic mental health conditions closely related to stress, such as depression and posttraumatic stress disorder (PTSD), have also been linked to CVD risk and prognosis in epidemiological research.15 However, causal evidence in humans has remained insufficient and mechanisms unclear because of difficulties in measuring stress. Research has been limited by the reliance on self-reported measures and the inability to capture fluctuations of emotions and mood over time and in real life. Consequently, this sphere remains inadequately recognized in clinical medicine and prevention.16 Using modern imaging modalities and controlled testing in the laboratory with experimental mental stress protocols, research from our group and others in the past decade has overcome some of these limitations and has demonstrated that such influences can be powerful.17–21
The goal of this review is to highlight recent knowledge on mind-body connections in CVD with emphasis on individuals with pre-existing CVD, a high-risk group who can be disproportionally affected by the effects of psychological stress.14 We will summarize empirical studies from our laboratory and others that have used mental stress testing in this population, an approach that has proved helpful in the clarification of pathways related to stress and emotions. As a whole, this research suggests that an integrated study of mind and body is necessary to fully understand the determinants and consequences of CVD.
2. Overview of contributions of laboratory research on mental stress
Over the past decade, we centered efforts on the effects of acute psychological stress as measured experimentally in the laboratory on cardiovascular physiology, immune function, myocardial ischemia, neurobiology and cardiovascular outcomes in men and women with CVD.19–29 Others have also reported that acute mental stress is associated with abnormal coronary reactivity, plaque rupture, as well as cardiac arrhythmias.23, 30–34 We have developed the concept that stress and mental health are especially important in influencing cardiovascular outcomes in patient subgroups such as women, patients with an early-onset myocardial infarction (MI), and patients with adverse psychosocial exposures.20, 24, 25, 27, 29, 35–37 A general conceptual framework is that chronic or cumulative stress causes dysregulation of adaptive stress response systems which, in turn, affect CVD risk though downstream effects on autonomic, vascular, immune, metabolic, injury and repair physiology; these effects are exacerbated by dynamic perturbations in response to everyday stressors or recurrent negative emotions (Figure 1).38
Figure 1. A schematic illustration of the main pathways linking psychological stress with cardiovascular risk.
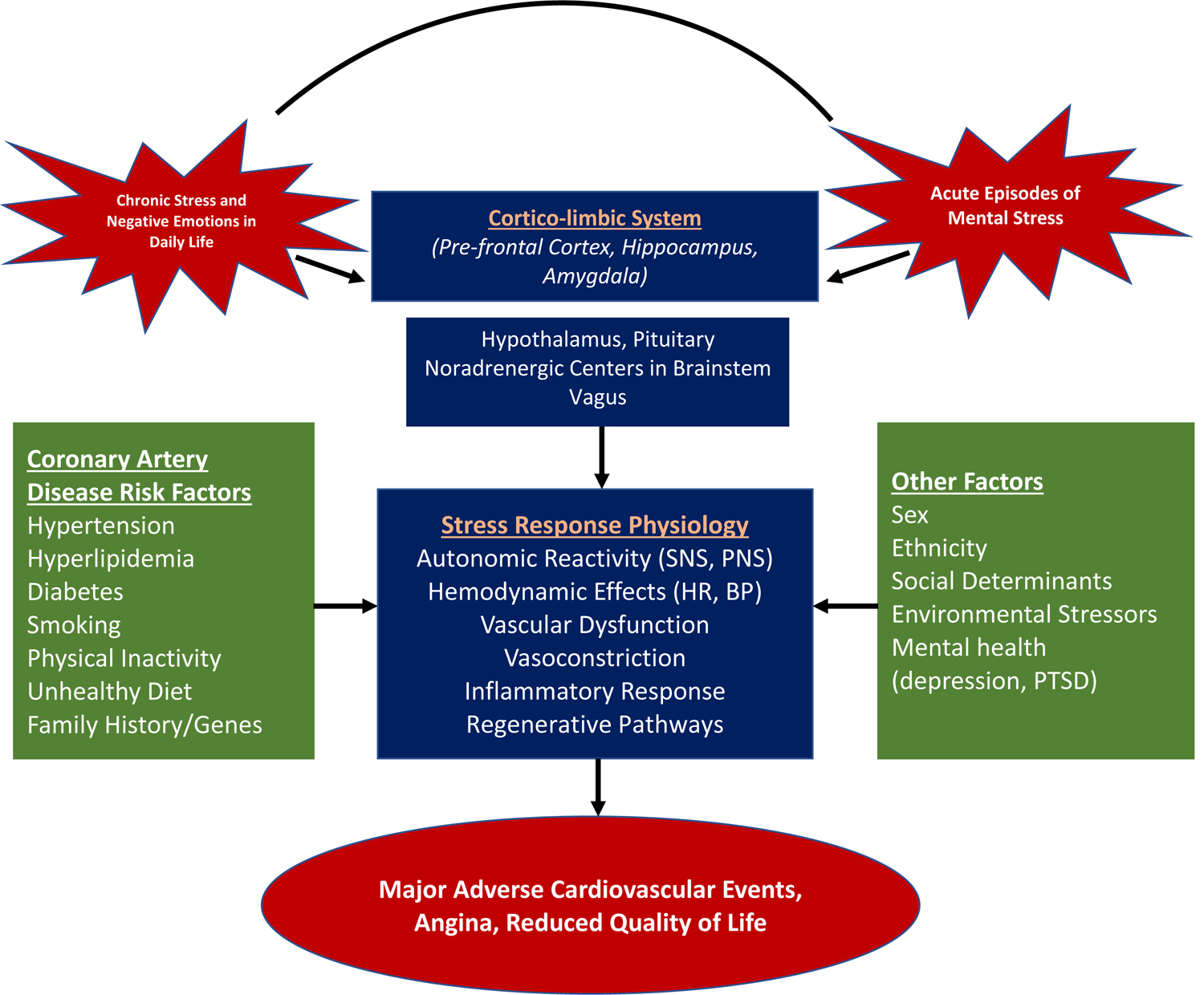
A chronic background of stress acts together and interacts with acute episodes of stress in everyday life to influence regulatory centers in the brain that control emotional responses, neuroendocrine stress systems and the autonomic nervous system. These, in turn, influence a number of risk pathways for cardiovascular disease that are responsive to stress. These processes are modulated by traditional risk factors, behaviors and genetic background, as well as by concurrent social and environmental exposures and mental health conditions.
Abbreviations: SNS: sympathetic nervous system; PNS: parasympathetic nervous system; HR: heart rate; BP: blood pressure; PTSD: posttraumatic stress disorder.
Both enhanced and blunted cardiovascular reactivity to mental stress have previously been implicated in CVD risk,39–41 but until recently almost no objective data existed for a possible link with clinical outcomes. A major recent finding from our laboratory is that dynamic changes of vascular and immune function measures with mental stress are related to adverse patient outcomes independent of respective baseline (resting) levels and are often better predictors of adverse outcomes than resting levels. We have observed this pattern with transient endothelial dysfunction with mental stress measured by flow-mediated vasodilation (FMD),21 with stress-induced peripheral vasoconstriction,26 and with the inflammatory response to mental stress (especially in women).29 Using positron emission tomography (PET) imaging of the brain, we have shown that mental stress-induced myocardial ischemia and peripheral vasoconstrictive responses to mental stress are related to activation in brain areas involved in the stress response, emotion and autonomic regulation of the cardiovascular system,28, 42 and that activation of these areas is associated with cardiovascular outcomes,19 as well as with angina symptoms43 and obesity44 in patients with CVD. These data directly support brain-heart connections and suggest that variations in individuals’ responses to acute stress represent mechanisms through which stress leads to increased risk of CVD-related morbidity and mortality.
3. Neurobiology of stress in relation to CVD
Stressful exposures and symptoms of psychiatric disorders related to stress, like PTSD and depression, and even stressful environmental exposures such as ambient noise,45 are processed in the brain and affect heart function through output pathways to the heart. A network of brain areas involved in memory and fear, including the hippocampus, medial prefrontal cortex, and amygdala, are involved in the brain’s response to stress.46 The hippocampus plays a critical role in memory and is also sensitive to stress. Studies in animals demonstrated that stress results in damage to neurons in the CA3 region of the hippocampus,47 and stress-related disorders like PTSD and depression are associated with a smaller hippocampal volume and deficits in hippocampal-based declarative memory.48–50 The medial prefrontal cortex also plays an important role in the stress response. This brain structure includes the anterior cingulate, the orbitofrontal cortex, and adjacent areas. These regions regulate neuroendocrine responses to stress via complex pathways involving the brainstem, the hypothalamus and the amygdala. The hippocampus and the medial prefrontal cortex have inhibitory pathways to the amygdala, which in turn activates neuroendocrine responses to stress.51, 52 The amygdala also participates in the stress response to noise.45, 53
Given these known pathways, it is conceivable that psychological stress acts through brain areas involved in stress response regulation (amygdala, insula, medial prefrontal cortex, and hippocampus) to influence CVD risk. Using the paradigm of inducing mental stress in the laboratory in conjunction with simultaneous heart and brain imaging,54 we studied heart and brain correlates of stress in CVD patients. Patients with CVD underwent the stress of performing mental arithmetic or giving a speech with negative feedback in conjunction with PET imaging of brain blood flow and myocardial perfusion imaging using single-photon emission computed tomography (SPECT) of the heart. Using these techniques, we found that patients with a history of traumatic stress and depression where more likely to develop myocardial ischemia with mental stress, typically without chest pain.37, 55 We also found that CVD patients with mental stress-induced myocardial ischemia had increased anterior cingulate function with mental stress compared to CVD patients without ischemia,42 and brain activation in these regions correlated with increased stress-induced peripheral vasoconstriction.28 Furthermore, in a series of studies, we observed that CVD patients have differing brain activation patterns to mental stress based on gender,56 obesity,57 symptoms of angina,43, 58 history of early trauma,59 and co-occurring depression.60, 61
Emphasizing the central role of the brain in these mechanisms, mental stress-induced activation of the rostro-medial prefrontal cortex was prospectively associated with adverse outcomes in CVD patients.19 This is an area that is key in the regulation of emotion and is also involved in immune and autonomic function. As opposed to other regulatory areas of the medial prefrontal cortex, this region is specifically activated during social stressors that include embarrassment and social rejection.19 Notably, we found that decreased heart rate variability and increased secretion of inflammatory markers [interleukin 6 (IL-6)] during mental stress mediated the relationship between brain activation and adverse cardiac outcomes, accounting for 33% and 16% of the association, respectively.19
In summary, multiple lines of evidence show that stress acts through key brain areas to activate peripheral sympathetic and neurohormonal responses that may increase the risk for CVD. The pathways involved are complex and encompass brain, inflammatory, and autonomic responses with stress, suggesting multiple potential future targets for treatment.
4. Autonomic nervous system mechanisms and stress regulation
The autonomic nervous system is an integral component of stress physiology. Sympathetic hyperactivity and parasympathetic withdrawal are core aspects of the acute stress response; these lead to increased heart rates and blood pressure in most individuals undergoing laboratory stress challenges.23, 62 These changes, and the cascade of inflammatory and prothrombotic effects that are instigated by autonomic activation during acute mental stress, could increase the likelihood of plaque rupture.14, 63
Chronic stress, on the other hand, can result in dysregulation of the autonomic nervous system, likely stemming from the brain mechanisms highlighted above.35, 41, 64 Dysregulation can manifest as hyper-reactivity or blunted activity.41 Because autonomic activation is essential for activities that include cognitive and physical tasks, a blunting of autonomic regulatory activity can lead to impaired quality of life in domains that require adequate blood circulation during everyday function. This can lead to fatigue and worse cognitive function.65 Both enhanced and blunted cardiovascular reactivity to mental stress have been related to CVD risk factors.39–41
Biomarkers of autonomic activity may help evaluate the physiologic effects of mental stress, risk stratify individuals, and help evaluate certain treatments. One candidate electrocardiographic biomarker is heart rate variability, which describes the variation in heart rate that occurs in response to various physiologic responses, ranging from respiration (high-frequency heart rate variability) to circadian variation (ultra-low frequency heart rate variability). Heart rate variability has been extensively studied as a noninvasive measure of autonomic modulation that predicts adverse CVD outcomes.66, 67 Heart rate variability and other measures of autonomic dysfunction such as pre-ejection period, galvanic skin response, and T-wave amplitude have promise as quantifiable, unbiased measures of stress physiology that can be measured with ambulatory electrocardiography, track treatment progress, and predict future CVD risk as well.67, 68 Studies have consistently shown a robust dose-response relationship between reduced heart rate variability and both PTSD and depression.64 More recently, research has also suggested that low heart rate variability can predict the development of worsening depressive symptoms and future PTSD development in trauma exposed individuals.69, 70 Unlike psychiatric conditions, the lack of stigma for metrics of autonomic dysfunction make them more likely to be useful in engaging patients and motivating them towards behavioral interventions.
5. Vascular function
In the largest and most comprehensive study investigating the effects of mental stress in patients with CVD, the Mental Stress Ischemia Prognosis Study (MIPS),22 we found that laboratory induction of mental stress was associated with autonomic activation together with coronary and peripheral microvascular constriction, protracted increase in arterial stiffness, and endothelial dysfunction.23, 62, 71 These findings are similar to those reported in previous smaller studies of individuals without CVD.72–74
Adverse environmental exposures can also cause psychological stress and affect vascular function. Ambient noise, for example, is a recognized environmental stressor.75 In human experimental models nighttime aircraft noise induced a decrease in endothelial function, a marked increase in sympathetic activation and increase in systolic blood pressure, particularly in patients with established coronary artery disease.76, 77 Traffic noise has also been linked to arterial inflammation.53
Whereas normal coronary arteries dilate in response to mental stress, atherosclerotic epicardial coronary arteries tend to constrict, at least partly due to alpha-adrenergic activation.78 By measuring peripheral vasomotion using pulsatile digital arterial tonometry in patients with CVD, we found that men tended to have greater peripheral vasoconstriction compared to women,24, 79 potentially due to the vasodilatory effect of estrogen and sex-based differences in the sensitivity of adrenergic receptors.80 By testing coronary vascular responses during acute mental stress, we also found a significant correlation between coronary microvascular changes with mental stress and the coronary endothelium-dependent, but not endothelium-independent function.23 Thus, patients with worse coronary endothelial function had reduced microvascular vasodilation in response to mental stress. Moreover, there was a strong correlation between the magnitude of coronary microvascular vasodilation and digital peripheral microvascular constriction during mental stress, demonstrating that vasoreactivity to mental stress is a generalized phenomenon. It is likely that inflammatory and oxidative pathways play a role in the link between psychological stress and vascular function, as discussed in section 8.
To further illustrate the interplay of brain and CVD, we showed that stress-induced vasoreactivity is associated with activation of brain areas involved in emotion and autonomic regulation. Specifically, we found that CVD patients with stress-induced peripheral vasoconstriction have differential patterns of brain activation with mental stress in areas involved in emotional regulation, including the insula, the parietal cortex and the medial prefrontal cortex.28
6. Vascular regenerative pathways
Chronic stress and its associated neurobiological changes also result in activation of the bone marrow.17, 18 Exposure to cardiovascular risk factors and injury promotes progenitor cell mobilization from the bone marrow and ultimately may lead to depletion of progenitor cells.81 Progenitor cell counts are considered to be an index of endogenous regenerative capacity and are independent predictors of adverse cardiovascular outcomes.82, 83 Progenitor cells expressing the CD34 epitope have the potential to differentiate into hematopoietic, endothelial, and other lineages. These progenitor cells are mobilized after acute mental stress, an effect that is mediated through activation of the beta-adrenergic receptor activation in the bone marrow and the production of cytokines.84, 85 Levels of stromal cell-derived factor-1 (SDF1) or CXCL12, a chemokine that plays a central role in the recruitment of progenitor cells in response to ischemia, are also stimulated by acute mental stress and increase more in patients who develop ischemia with mental stress.86
7. Prognostic value of mental stress-induced vascular perturbations
Whether mental stress-induced vascular alterations have a prognostic implication has not been extensively studied. In the MIPS study, we demonstrated that greater digital vasoconstriction during mental stress, measured using digital tonometry, was an independent predictor of adverse events during follow-up.26 In MIPS, we also demonstrated that the transient decline in endothelial function, measured as flow-mediated vasodilation of the brachial artery in response to mental stress, was an independent predictor of adverse CVD events.21 Both the magnitude of PC mobilization with mental stress and the change in SDF1 level induced by mental stress were independent predictors of adverse incident cardiovascular events. Finally, we found that activation of specific brain areas during mental stress involved in executive function, stress activation and the limbic system were associated with greater mobilization of progenitor cells.87
These results are consistent with the model that activation of specific brain areas with mental stress leads to hemodynamic and vascular responses that, in turn, alter regenerative pathways, and these responses have pathogenic implications on the long-term prognosis of patients with CAD (Figure 2).
Figure 2. Pathophysiological pathways responsive to acute stress that are postulated to be determinants of adverse prognosis with mental stress.
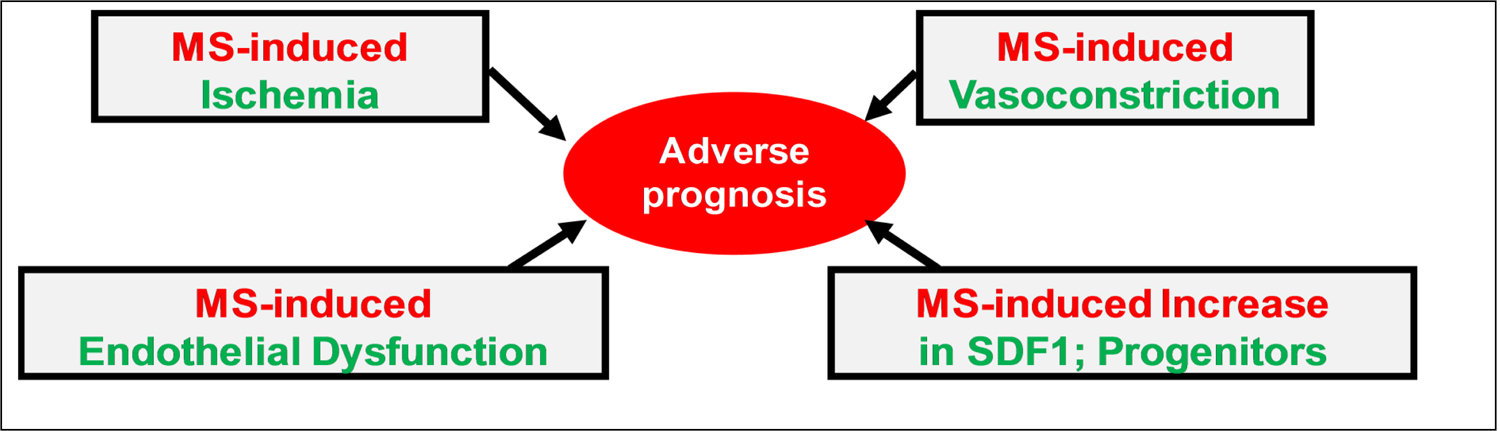
MS: mental stress; SDF1: stromal cell-derived factor-1.
8. Inflammatory/immune and oxidative mechanisms
Inflammatory mechanisms play a well-established role in atherosclerosis and coronary artery disease, and acute psychological stress is associated with an increase in inflammatory markers and certain chemokines.88, 89 Therefore, the immune system is clearly at the intersection between psychological stress and adverse cardiac events. Nevertheless, the mechanistic connections between dysregulated immune responses, psychological stress and CVD are not fully understood.
An important goal of our studies has been to elucidate the pathways connecting psychological stress with CVD through immune mechanisms and interrelated endothelial and microvascular function.25, 29, 35, 37, 89 Using a well-established stress protocol, we examined changes in cytokines and other immune molecules in response to a controlled laboratory stressor. We collected blood sequentially at 45 and 90 minutes after the mental stress and employed a highly-sensitive electrochemiluminescence assay system. The working hypothesis was that individual differences in the sensitivity to daily stressors have pathophysiological correlates in neurocircuitry and autonomic reactivity, as well as in the provocation of inflammatory responses.42, 90 In support of these mechanisms, we recently reported that individuals with higher activation of the rostromedial prefrontal cortex in response to a psychological stress challenge had greater increases in stress-induced IL-6 levels.19
Among young women with CVD, we found higher levels of circulating IL-6 at rest, and an enhanced IL-6 response to stress, compared to men of a similar age and CVD status.25 This suggests that accentuated IL-6 responses to mental stress among women may be associated with a greater risk for major adverse cardiac events. Indeed, among women with stable CVD, there was a significant positive relationship between stress-induced IL-6 and adverse cardiovascular events, while there was no such association among men.29 A similar female-specific relationship was found for the chemokine monocyte chemoattractant protein-1 (MCP-1, also referred to as CCL2). The mechanism connecting this sex specific enhanced inflammatory response to adverse cardiac outcomes has not been elucidated. However, studies in animal models, and to a lesser extent in humans, suggest that sex differences in neuronal circuitry and bidirectional brain-immune interactions may explain some aspects of the over-activation of inflammatory responses to stress in women compared with men.91 Considering the specific role of the rostromedial prefrontal cortex in processing and reacting to social stressors, and our finding that post-mental stress IL-6 levels correlated with activation of this brain region, a future direction of our studies is to examine sex and gender differences in brain immune interactions in relation to adverse cardiac outcomes. Overall, as the mind-body connections in CVD continue to be revealed, individual differences in immune responses to psychological stress will undoubtedly be discovered to play an important role.
It is well established that there is extensive intercommunication between the sympathetic nervous system and the innate immune system.92 Accordingly, transient inflammatory responses to mental stress may result in part from activation of the sympathetic nervous system, perhaps by mobilization of monocytes. Oxidative pathways are likewise interconnected with the immune and sympathetic nervous systems, which presents additional mechanisms by which mental stress can influence cardiovascular disease risk.93, 94 There is a large literature showing that psychological stress can increase reactive oxygen species and cause lipid peroxidation and DNA damage.94 These stress-associated alterations of reactive oxygen species occur in both the periphery and the brain (especially through activation of microglia).94 Oxidative damage, in turn, plays a well-established role in endothelial dysfunction.93 Considering the importance of endothelial dysfunction in the microvascular response to mental stress (described above), reactive oxygen species are likely important intermediates in the connection between psychological stress and cardiovascular disease.
9. Mental stress-induced myocardial ischemia
In approximately 1 in 6 patients with clinically stable CHD, acute mental stress in the laboratory can trigger myocardial ischemia, a phenomenon that can be detected with myocardial perfusion imaging.20, 95 Mental stress-induced myocardial ischemia is analogous to ischemia provoked by exercise (e.g., treadmill testing), except that the stimulus is psychological rather than physical.96, 97
While the prognostic significance of ischemia with mental stress was suspected for many years based on a limited number of small studies that measured mental stress-induced changes in left ventricular dysfunction,97 recent studies show that mental stress ischemia measured with myocardial perfusion imaging (the gold standard for ischemia detection) is also prospectively associated with an elevated risk of adverse cardiovascular events.98 The excess risk associated with mental stress ischemia is approximately two-fold after multivariable adjustment. Cardiovascular risk factors, other clinical characteristics, and even psychological disturbances do not explain the excess risk associated with mental stress ischemia, suggesting that this phenomenon has clinical significance beyond the assessment of other known risk factors.
Myocardial ischemia provoked by mental stress has distinct characteristics compared with exercise stress ischemia. It is usually silent, occurs at a lower hemodynamic workload has not been consistently related with coronary atherosclerosis, and can occur in patients who do not have a positive conventional stress test or have been successfully revascularized.71, 95, 99 These characteristics suggest that ischemia with mental stress is subtended by unique mechanisms. Many factors have been associated with mental stress ischemia, including abnormal peripheral vasomotion, psychological conditions, platelet reactivity, inflammation, and metabolic risk factors,23, 24, 96, 100, 101 but the exact mechanisms remain unclear.
While systemic vascular resistance falls in response to exercise, it rises with mental stress, due to peripheral vasoconstriction and increasing left ventricular afterload.96 Patients who develop mental stress ischemia have a more pronounced peripheral vasoconstriction with mental stress.62, 71, 102 In the MIPS study of patients with stable CVD, the major independent vascular predictors of mental stress ischemia were a greater hemodynamic response during mental stress, measured as the rate-pressure product change, and a greater magnitude of peripheral vasoconstriction, measured as digital vasoconstriction during mental stress 62 (Figure 3), suggesting that a combination of reduced coronary perfusion due to generalized vasoconstriction and higher myocardial demand, increase the likelihood of myocardial ischemia with mental stress, findings suggested also by some previous studies.96
Figure 3. Pathophysiological determinants of mental stress-induced myocardial ischemia and its consequences.
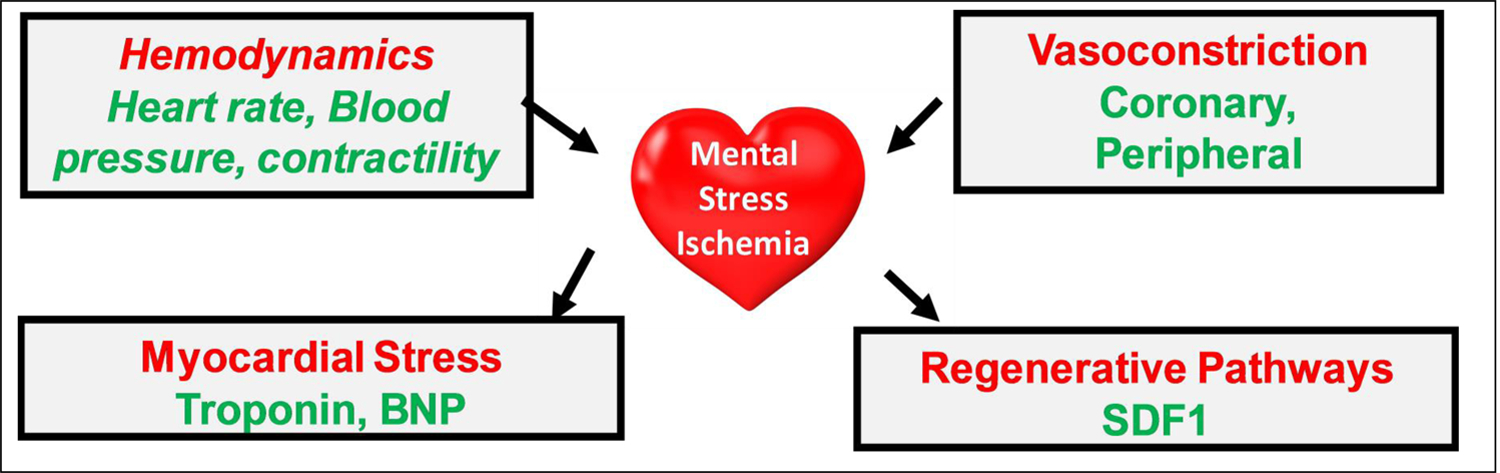
BNP: brain natriuretic peptide; SDF1: stromal cell-derived factor-1.
10. Special at-risk populations
Women seem to be particularly susceptible to psychological stressors, and women are twice as likely to be diagnosed with conditions such as depression and PTSD.103, 104 Studies clearly demonstrate that depression is a major contributor to poor CVD outcomes in women diagnosed with a myocardial infarction.104, 105 Furthermore, women, especially younger women, are more likely to develop mental stress ischemia than men,20, 106 even in absence of obstructive coronary disease.99 In the settings of acute and chronic coronary syndromes, women are more likely to have ischemia with no obstructive coronary arteries (INOCA).20, 24 Comorbid psychological factors and vital exhaustion are highly prevalent in these patients, and psychosocial stress can contribute to and exacerbate angina.107 In addition to angina during exercise, INOCA patients often report rest angina at low hemodynamic workloads, emotional-stress induced angina, as well as symptoms even after cessation of exercise.108 In the absence of definitive treatment, INOCA patients with persistent angina make repeated visits to emergency departments and physician offices for chest pain, have reduced health-related quality of life, and high symptom-related disability.109–111 They undergo repeated cycles of diagnostic testing and hospitalization for general evaluation, but percutaneous coronary interventions or other approaches to revascularization to reduce ischemia are not options for persons with INOCA as there is no culprit lesion(s) to target. Compared to asymptomatic women without INOCA, those with INOCA have more microvascular vasoconstriction in response to mental stress.112 Among women, microvascular reactivity with stress plays a more pronounced role in mental stress ischemia than in men,20, 24 and women with ischemia with mental stress, but not men, are more likely to report angina in everyday life.107 This suggests that, at least among women, mental stress ischemia could be a marker of myocardial ischemia occurring in everyday life.
Myocardial infarction patients with PTSD,37 depression,113 and anger,114 are also more likely to develop mental stress ischemia. Women with CVD and myocardial infarction patients with PTSD, in addition to showing a higher propensity for mental stress ischemia, also exhibit a heightened inflammatory response to acute stress.25, 27 Furthermore, we have observed that stress-induced IL-6 is a predictor of subsequent CVD events in women with CVD, but not in men.29 We have also found that patients with CVD who have a phenotype of chronic psychological distress defined as a combination of symptoms of depression, PTSD, anxiety, anger, hostility, and perceived stress, have a higher risk of subsequent cardiovascular events, especially among women.115 These observations suggest that there are subgroups of CVD patients, including women and patients with psychological distress and mental health conditions, who are disproportionally vulnerable to mental stress ischemia. They also indicate that inflammation and vascular function are key pathways of risk linking psychological stress, mental stress ischemia and adverse outcomes in these at-risk patient populations.
A potentially related condition that is more common in women, where the brain-heart connection is clearly evident, is Takotsubo syndrome. This is a condition where intense emotional or physical stress triggers a catecholamine surge, characteristic ventricular wall motion abnormalities, and acute heart failure.116
Certain racial/ethnic groups, such as African Americans, may also be more susceptible to the adverse cardiovascular consequences of psychological risk factors in part through exposure to adverse social determinants of health.117–121 African American patients report more adverse life events, discrimination, lower income, more chronic stressors, and negative emotions, but whether these psychosocial factors have synergistic effects with traditional risk CVD factors, or directly contribute to pathobiological mechanisms differentially in African Americans vs. other groups is unclear.122
11. Management and secondary prevention
Clinical and translational studies designed to modulate the autonomic nervous system and mitigate adverse physiological responses to stress are the next frontier in biobehavioral medicine. While there is growing evidence that psychological stress contributes to CVD, it has not been systematically targeted as a risk factor in clinical care. In the busy clinical care of cardiology and primary care clinics, a thorough history of psychological risk factors and stressful exposures may not be feasible, but brief screening tools for depression and anxiety can alert the clinician to refer to a biobehavioral expert to address these factors (or a mental health specialist in severe cases). The recent ACC/AHA guidelines for prevention of CVD recommend that clinicians address psychological factors as well as social determinants of health when devising an individualized treatment plan for the cardiac patient.123 Biological responses to stress can be modulated by techniques such as yoga, deep breathing, meditation, biofeedback, and noninvasive vagal nerve stimulation. These modalities may directly modulate autonomic function, have neuroplastic effects on the brain, reduce inflammation, reduce arrhythmia burden, improve quality of life, and reduce the risk of CVD events.124 Stress management training that includes group support, education, and cognitive behavioral therapy, may also improve perceived stress and reduce the risk of CVD events in patients undergoing cardiac rehabilitation.125
Many of the techniques discussed in this review that are performed in the laboratory to study acute mental stress may have future clinical applications for patient monitoring. For example, ambulatory devices for the assessment of heart rate variability, or the assessment of peripheral vasoconstriction, could be useful in tracking everyday stress in individuals undergoing biobehavioral therapies. More work is needed, however, to standardize measurement techniques and algorithms, as well as to identify a clinically relevant magnitude of change achievable with interventions through randomized controlled trials.
12. Conclusions and future directions
Recent research advances, using modern imaging modalities and controlled testing in the laboratory, provide growing evidence that brain-heart influences are powerful contributors of risk in individuals with CVD. These recent discoveries highlight the possibility for objective evaluation of psychological stress reactivity and its cardiovascular effects through laboratory-based and ambulatory metrics, and will hopefully inspire new randomized controlled trials that may eventually improve our ability to risk stratify patients and enhance the well-being of the heart-brain axis. Despite recent calls,16, 126, 127 this sphere remains insufficiently recognized in clinical medicine and prevention, as there is a tendency for health care professionals to focus on the physical aspects of the disease and neglect mental health in their evaluation and treatment of cardiac patients. Increased attention to this area should shift the prevailing paradigm of cardiovascular risk assessment to incorporate mental health and psychological factors. It is also hoped that this growing body of knowledge will stimulate future mechanistic research and the development of innovative interventions that could transform CVD prevention and clinical care. Future studies are needed to assess the mechanisms of stress on pathobiology using powerful approaches such as proteomics and metabolomics, as well as using real-time assessments for capturing stress and negative emotions via wearable technologies. Intervention studies in diverse populations are urgently needed, to shed light into treatment modalities that are effective in all segments of the population.
Highlights.
A “brain-heart axis” exerts influences on the prognosis of individuals with cardiovascular disease.
Acute psychological stress provocation in the lab has helped clarify stress-related pathophysiological pathways.
Dynamic perturbations of physiological and molecular systems during stress affect cardiovascular outcomes.
Some patient subgroups are at especially high risk for these effects.
Acknowledgments
Financial support
This work was supported by NIH grants P01 HL101398, R01 HL109413, R01HL109413-S1, P20HL113451, P01HL086773, R56HL126558, R01 HL125246, R01HL136205, T32 HL130025, TL1TR002382, UL1TR002378, UL1TR000454, UL1 TR000424, KL2TR000455, K23HL127251, K24HL077506, K24 MH076955, and K12HD085850.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Wilmot KA, O’Flaherty M, Capewell S, Ford ES and Vaccarino V. Coronary Heart Disease Mortality Declines in the United States From 1979 Through 2011: Evidence for Stagnation in Young Adults, Especially Women. Circulation. 2015;132:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izadnegahdar M, Singer J, Lee MK, Gao M, Thompson CR, Kopec J and Humphries KH. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. Journal of women’s health. 2014;23:10–7. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D’Onofrio G, Lichtman JH and Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. Journal of the American College of Cardiology. 2014;64:337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabet A, Danchin N, Juilliere Y and Olie V. Acute coronary syndrome in women: rising hospitalizations in middle-aged French women, 2004–14. European heart journal. 2017;38:1060–1065. [DOI] [PubMed] [Google Scholar]
- 5.Lehto HR, Lehto S, Havulinna AS, Ketonen M, Lehtonen A, Kesaniemi YA, Airaksinen KJ and Salomaa V. Sex differences in short- and long-term case-fatality of myocardial infarction. European journal of epidemiology. 2011;26:851–61. [DOI] [PubMed] [Google Scholar]
- 6.Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, Porterfield D, Blankstein R, Rosamond WD, Bhatt DL and Caughey MC. Twenty Year Trends and Sex Differences in Young Adults Hospitalized With Acute Myocardial Infarction. Circulation. 2019;139:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossard M, Latifi Y, Fabbri M, Kurmann R, Brinkert M, Wolfrum M, Berte B, Cuculi F, Toggweiler S, Kobza R, Chamberlain AM and Moccetti F. Increasing Mortality From Premature Coronary Artery Disease in Women in the Rural United States. J Am Heart Assoc. 2020;9:e015334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross SH, Mehra MR, Bhatt DL, Nasir K, O’Donnell CJ, Califf RM and Warraich HJ. Rural-Urban Differences in Cardiovascular Mortality in the US, 1999–2017. JAMA. 2020;323:1852–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casper M, Kramer MR, Quick H, Schieb LJ, Vaughan AS and Greer S. Changes in the Geographic Patterns of Heart Disease Mortality in the United States: 1973 to 2010. Circulation. 2016;133:1171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulshreshtha A, Goyal A, Dabhadkar K, Veledar E and Vaccarino V. Urban-rural differences in coronary heart disease mortality in the United States: 1999–2009. Public health reports. 2014;129:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaughan AS, Ritchey MD, Hannan J, Kramer MR and Casper M. Widespread recent increases in county-level heart disease mortality across age groups. Ann Epidemiol. 2017;27:796–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer MR, Valderrama AL and Casper ML. Decomposing Black-White Disparities in Heart Disease Mortality in the United States, 1973–2010: An Age-Period-Cohort Analysis. Am J Epidemiol. 2015;182:302–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen BS. Brain on stress: how the social environment gets under the skin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109 Suppl 2:17180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kivimaki M and Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol. 2018;15:215–229. [DOI] [PubMed] [Google Scholar]
- 15.Levine GN, Cohen BE, Commodore-Mensah Y, Fleury J, Huffman JC, Khalid U, Labarthe DR, Lavretsky H, Michos ED, Spatz ES and Kubzansky LD. Psychological Health, Well-Being, and the Mind-Heart-Body Connection: A Scientific Statement From the American Heart Association. Circulation. 0:CIR.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 16.Levine GN, Cohen BE, Commodore-Mensah Y, Fleury J, Huffman JC, Khalid U, Labarthe DR, Lavretsky H, Michos ED, Spatz ES, Kubzansky LD, American Heart Association Council on Clinical C, Council on Arteriosclerosis T, Vascular B, Council on C, Stroke N, Council on L and Cardiometabolic H. Psychological Health, Well-Being, and the Mind-Heart-Body Connection: A Scientific Statement From the American Heart Association. Circulation. 2021:CIR0000000000000947. [DOI] [PubMed]
- 17.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA and Pitman RK. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tawakol A, Osborne MT, Wang Y, Hammed B, Tung B, Patrich T, Oberfeld B, Ishai A, Shin LM, Nahrendorf M, Warner ET, Wasfy J, Fayad ZA, Koenen K, Ridker PM, Pitman RK and Armstrong KA. Stress-Associated Neurobiological Pathway Linking Socioeconomic Disparities to Cardiovascular Disease. Journal of the American College of Cardiology. 2019;73:3243–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moazzami K, Wittbrodt MT, Lima BB, Nye JA, Mehta PK, Pearce BD, Almuwaqqat Z, Hammadah M, Levantsevych O, Sun YV, Raggi P, Garcia EV, Goetz M, Quyyumi AA, Bremner JD, Vaccarino V and Shah AJ. Higher Activation of the Rostromedial Prefrontal Cortex During Mental Stress Predicts Major Cardiovascular Disease Events in Individuals With Coronary Artery Disease. Circulation. 2020;142:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, Shah AJ, Alkhoder A, Levantsevych O, Gay H, Obideen M, Huang M, Lewis TT, Bremner JD, Quyyumi AA and Raggi P. Mental Stress-Induced-Myocardial Ischemia in Young Patients With Recent Myocardial Infarction: Sex Differences and Mechanisms. Circulation. 2018;137:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima BB, Hammadah M, Kim JH, Uphoff I, Shah A, Levantsevych O, Almuwaqqat Z, Moazzami K, Sullivan S, Ward L, Kutner M, Ko YA, Sheps DS, Bremner JD, Quyyumi AA and Vaccarino V. Association of Transient Endothelial Dysfunction Induced by Mental Stress With Major Adverse Cardiovascular Events in Men and Women With Coronary Artery Disease. JAMA Cardiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V and Quyyumi AA. The Mental Stress Ischemia Prognosis Study: Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosom Med. 2017;79:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammadah M, Kim JH, Al Mheid I, Samman Tahhan A, Wilmot K, Ramadan R, Alkhoder A, Khayata M, Mekonnen G, Levantsevych O, Bouchi Y, Kaseer B, Choudhary F, Gafeer MM, Corrigan FE 3rd, Shah AJ, Ward L, Kutner M, Bremner JD, Sheps DS, Raggi P, Vaccarino V, Samady H, Mavromatis K and Quyyumi AA. Coronary and Peripheral Vasomotor Responses to Mental Stress. Journal of the American Heart Association. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Isakadze N, Shah A, Levantsevych O, Pimple PM, Kutner M, Ward L, Garcia EV, Nye J, Mehta PK, Lewis TT, Bremner JD, Raggi P, Quyyumi AA and Vaccarino V. Sex Differences in Hemodynamic and Microvascular Mechanisms of Myocardial Ischemia Induced by Mental Stress. Arterioscler Thromb Vasc Biol. 2018;38:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan S, Hammadah M, Wilmot K, Ramadan R, Pearce BD, Shah A, Kaseer B, Gafeer MM, Lima BB, Kim JH, Ward L, Ko YA, Lewis TT, Hankus A, Elon L, Li L, Bremner JD, Raggi P, Quyyumi A and Vaccarino V. Young Women With Coronary Artery Disease Exhibit Higher Concentrations of Interleukin-6 at Baseline and in Response to Mental Stress. J Am Heart Assoc. 2018;7:e010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JH, Almuwaqqat Z, Hammadah M, Liu C, Ko YA, Lima B, Sullivan S, Alkhoder A, Abdulbaki R, Ward L, Bremner JD, Sheps DS, Raggi P, Sun YV, Shah AJ, Vaccarino V and Quyyumi AA. Peripheral Vasoconstriction During Mental Stress and Adverse Cardiovascular Outcomes in Patients With Coronary Artery Disease. Circulation research. 2019;125:874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lima BB, Hammadah M, Wilmot K, Pearce BD, Shah A, Levantsevych O, Kaseer B, Obideen M, Gafeer MM, Kim JH, Sullivan S, Lewis TT, Weng L, Elon L, Li L, Bremner JD, Raggi P, Quyyumi A and Vaccarino V. Posttraumatic stress disorder is associated with enhanced interleukin-6 response to mental stress in subjects with a recent myocardial infarction. Brain Behav Immun. 2019;75:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah A, Chen C, Campanella C, Kasher N, Evans S, Reiff C, Mishra S, Hammadah M, Lima BB, Wilmot K, Al Mheid I, Alkhoder A, Isakadze N, Levantsevych O, Pimple PM, Garcia EV, Wittbrodt M, Nye J, Ward L, Lewis TT, Kutner M, Raggi P, Quyyumi A, Vaccarino V and Bremner JD. Brain correlates of stress-induced peripheral vasoconstriction in patients with cardiovascular disease. Psychophysiology. 2019;56:e13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sullivan S, Young A, Hammadah M, Lima BB, Levantsevych O, Ko YA, Pearce BD, Shah AJ, Kim JH, Moazzami K, Driggers EG, Haffar A, Ward L, Herring I, Hankus A, Lewis TT, Mehta PK, Bremner JD, Raggi P, Quyyumi A and Vaccarino V. Sex differences in the inflammatory response to stress and risk of adverse cardiovascular outcomes among patients with coronary heart disease. Brain Behav Immun. 2020;90:294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strike PC, Magid K, Brydon L, Edwards S, McEwan JR and Steptoe A. Exaggerated platelet and hemodynamic reactivity to mental stress in men with coronary artery disease. Psychosom Med. 2004;66:492–500. [DOI] [PubMed] [Google Scholar]
- 31.Levine SP, Towell BL, Suarez AM, Knieriem LK, Harris MM and George JN. Platelet activation and secretion associated with emotional stress. Circulation. 1985;71:1129–34. [DOI] [PubMed] [Google Scholar]
- 32.Wallen NH, Held C, Rehnqvist N and Hjemdahl P. Effects of mental and physical stress on platelet function in patients with stable angina pectoris and healthy controls. European heart journal. 1997;18:807–15. [DOI] [PubMed] [Google Scholar]
- 33.Peacock J and Whang W. Psychological distress and arrhythmia: risk prediction and potential modifiers. Progress in cardiovascular diseases. 2013;55:582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dakak N, Quyyumi AA, Eisenhofer G, Goldstein DS and Cannon RO, 3rd. Sympathetically mediated effects of mental stress on the cardiac microcirculation of patients with coronary artery disease. The American journal of cardiology. 1995;76:125–30. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan S, Kelli HM, Hammadah M, Topel M, Wilmot K, Ramadan R, Pearce BD, Shah A, Lima BB, Kim JH, Hardy S, Levantsevych O, Obideen M, Kaseer B, Ward L, Kutner M, Hankus A, Ko YA, Kramer MR, Lewis TT, Bremner JD, Quyyumi A and Vaccarino V. Neighborhood poverty and hemodynamic, neuroendocrine, and immune response to acute stress among patients with coronary artery disease. Psychoneuroendocrinology. 2019;100:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaccarino V and Bremner JD. Behavioral, emotional and neurobiological determinants of coronary heart disease risk in women. Neurosci Biobehav Rev. 2017;74:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lima BB, Hammadah M, Pearce BD, Shah A, Moazzami K, Kim JH, Sullivan S, Levantsevych O, Lewis TT, Weng L, Elon L, Li L, Raggi P, Bremner JD, Quyyumi A and Vaccarino V. Association of Posttraumatic Stress Disorder With Mental Stress-Induced Myocardial Ischemia in Adults After Myocardial Infarction. JAMA Netw Open. 2020;3:e202734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaccarino V and Bremner JD. Psychiatric and behavioral aspects of cardiovascular disease. In: Zipes DP, Libby P, Bonow RO, Mann DL and Tomaselli GF, eds. Braunwald’s Heart Disease - A Textbook of Cardiovascular Medicine, 11th ed Philadelphia, PA: Elsevier-Saunders; 2019: 1879–1889. [Google Scholar]
- 39.Chida Y and Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–32. [DOI] [PubMed] [Google Scholar]
- 40.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G and Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. [DOI] [PubMed] [Google Scholar]
- 41.Phillips AC, Ginty AT and Hughes BM. The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2013;90:1–7. [DOI] [PubMed] [Google Scholar]
- 42.Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, Al Mheid I, Lima BB, Garcia EV, Nye J, Ward L, Kutner MH, Raggi P, Pearce BD, Shah AJ, Quyyumi AA and Vaccarino V. Brain Correlates of Mental Stress-Induced Myocardial Ischemia. Psychosom Med. 2018;80:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moazzami K, Wittbrodt MT, Alkhalaf M, Lima BB, Nye JA, Mehta PK, Quyyumi AA, Vaccarino V, Bremner JD and Shah AJ. Association Between Mental Stress-Induced Inferior Frontal Cortex Activation and Angina in Coronary Artery Disease. Circ Cardiovasc Imaging. 2020;13:e010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moazzami K, Wittbrodt MT, Lima BB, Levantsevych O, Kaseer B, Martini A, Haffar A, Nye JA, Quyyumi AA, Shah A, Vaccarino V and Bremner JD. Neural Correlates of Stress and Abdominal Obesity in Patients With Coronary Artery Disease. Psychosom Med. 2020;82:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munzel T, Steven S, Hahad O and Daiber A. The sixth sense is involved in noise-induced stress responses and vascular inflammation: evidence for heightened amygdalar activity in response to transport noise in man. European heart journal. 2020;41:783–785. [DOI] [PubMed] [Google Scholar]
- 46.Bremner JD. Stress and human neuroimaging studies. . In: Conrad CD, ed. The Handbook of Stress: Neuropsychological Effects on the Brain: Wiley-Blackwell; 2011. [Google Scholar]
- 47.Nibuya M, Morinobu S and Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. The Journal of Neuroscience. 1995;15:7539–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bremner JD. Changes in brain volume in major depression. Depression: Mind and Brain. 2005;2:38–46. [Google Scholar]
- 49.Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N and Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–31. [DOI] [PubMed] [Google Scholar]
- 50.Bremner JD, Hoffman M, Afzal N, Cheema FA, Novik O, Ashraf A, Brummer M, Nazeer A, Goldberg J and Vaccarino V. The environment contributes more than genetics to smaller hippocampal volume in posttraumatic stress disorder (PTSD). Journal of Psychiatric Research. 2020. [DOI] [PMC free article] [PubMed]
- 51.Milad MR and Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–73. [DOI] [PubMed] [Google Scholar]
- 52.Davis M The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. [DOI] [PubMed] [Google Scholar]
- 53.Osborne MT, Radfar A, Hassan MZO, Abohashem S, Oberfeld B, Patrich T, Tung B, Wang Y, Ishai A, Scott JA, Shin LM, Fayad ZA, Koenen KC, Rajagopalan S, Pitman RK and Tawakol A. A neurobiological mechanism linking transportation noise to cardiovascular disease in humans. European heart journal. 2020;41:772–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soufer R, Bremner JD, Arrighi JA, Cohen I, Zaret BL, Burg MM and Goldman-Rakic P. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6454–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bremner JD, Cheema FA, Ashraf A, Afzal N, Fani N, Reed J, Musselman DL, Ritchie JC, Faber T, Votaw JR, Nemeroff CB and Vaccarino V. Effects of a cognitive stress challenge on myocardial perfusion and plasma cortisol in coronary heart disease patients with depression. Stress and Health. 2009;25:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasher N, Wittbrodt MT, Alam ZS, Lima BB, Nye JA, Campanella C, Ladd S, Hammadah M, Shah AJ, Raggi P, Quyyumi AA, Vaccarino V and Bremner JD. Sex differences in brain activation patterns with mental stress in patients with coronary artery disease. Biology of Sex Differences. 2019;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moazzami K, Wittbrodt MT, Lima BB, Levantsevych O, Kaseer B, Martini A, Haffar A, Nye JA, Quyyumi AA, Shah AJ, Vaccarino V and Bremner JD. Neural correlates of stress and abdominal obesity in patients with coronary artery disease. Psychosomatic Medicine. 2020;82:272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wittbrodt MT, Moazzami K, Shah AJ, Lima BB, Hammadah M, Mehta PK, Quyyumi AA, Vaccarino V, Nye JA and Bremner JD. Neural responses during acute mental stress are associated with angina pectoris. Journal of Psychosomatic Research. 2020;134:110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wittbrodt MT, Moazzami K, Lima BB, Alama A, Corry D, Hammadah M, Campanella C, Ward L, Quyyumi AA, Shah AJ, Vaccarino V, Nye JA and Bremner JD. Early childhood trauma alters neurological responses to mental stress in patients with coronary artery disease. Journal of Affective Disorders. 2019;254:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bremner JD, Fani N, Cheema FA, Ashraf A and Vaccarino V. Effects of a mental stress challenge on brain function in coronary artery disease patients with and without depression. Health Psychology. 2019;38:910–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bremner JD, Campanella C, Khan Z, Fani N, Kasher N, Evans S, Reiff C, Mishra S, Ladd S, Nye JA, Raggi P and Vaccarino V. Brain mechanisms of stress and depression in coronary artery disease. Journal of Psychiatric Research. 2019;109:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O’Neal WT, Sullivan S, Tahhan AS, Kelli HM, Ramadan R, Pimple P, Sandesara P, Shah AJ, Ward L, Ko YA, Sun Y, Uphoff I, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Sheps DS, Raggi P, Vaccarino V and Quyyumi AA. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. Int J Cardiol. 2017;243:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang DO, Eo JS, Park EJ, Nam HS, Song JW, Park YH, Park SY, Na JO, Choi CU, Kim EJ, Rha SW, Park CG, Seo HS, Kim CK, Yoo H and Kim JW. Stress-associated neurobiological activity is linked with acute plaque instability via enhanced macrophage activity: a prospective serial 18F-FDG-PET/CT imaging assessment. European heart journal. 2021. [DOI] [PubMed]
- 64.Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD and Vaccarino V. Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biological psychiatry. 2013;73:1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah AJ, Su S, Veledar E, Bremner JD, Goldstein FC, Lampert R, Goldberg J and Vaccarino V. Is heart rate variability related to memory performance in middle-aged men? Psychosom Med. 2011;73:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shah AS, Alonso A, Whitsel EA, Soliman EZ, Vaccarino V and Shah AJ. Association of Psychosocial Factors With Short-Term Resting Heart Rate Variability: The Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2021;10:e017172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang SC, Wu YL and Tsai PS. Heart Rate Variability and Risk of All-Cause Death and Cardiovascular Events in Patients With Cardiovascular Disease: A Meta-Analysis of Cohort Studies. Biol Res Nurs. 2020;22:45–56. [DOI] [PubMed] [Google Scholar]
- 68.van Lien R, Neijts M, Willemsen G and de Geus EJ. Ambulatory measurement of the ECG T-wave amplitude. Psychophysiology. 2015;52:225–37. [DOI] [PubMed] [Google Scholar]
- 69.Huang M, Shah A, Su S, Goldberg J, Lampert RJ, Levantsevych OM, Shallenberger L, Pimple P, Bremner JD and Vaccarino V. Association of Depressive Symptoms and Heart Rate Variability in Vietnam War-Era Twins: A Longitudinal Twin Difference Study. JAMA Psychiatry. 2018;75:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah A and Vaccarino V. Heart Rate Variability in the Prediction of Risk for Posttraumatic Stress Disorder. JAMA Psychiatry. 2015;72:964–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V and Quyyumi AA. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J Am Heart Assoc. 2013;2:e000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O’Connor G, Betteridge J, Klein N, Steptoe A and Deanfield JE. Mental Stress Induces Transient Endothelial Dysfunction in Humans. Circulation. 2000;102:2473–2478. [DOI] [PubMed] [Google Scholar]
- 73.Xue Y-T, Tan Q-w, Li P, Mou S-f, Liu S-j, Bao Y, Jiao H-c and Su W-G. Investigating the role of acute mental stress on endothelial dysfunction: a systematic review and meta-analysis. Clinical Research in Cardiology. 2015;104:310–319. [DOI] [PubMed] [Google Scholar]
- 74.Vlachopoulos C, Kosmopoulou F, Alexopoulos N, Ioakeimidis N, Siasos G and Stefanadis C. Acute mental stress has a prolonged unfavorable effect on arterial stiffness and wave reflections. Psychosom Med. 2006;68:231–7. [DOI] [PubMed] [Google Scholar]
- 75.Munzel T, Kroller-Schon S, Oelze M, Gori T, Schmidt FP, Steven S, Hahad O, Roosli M, Wunderli JM, Daiber A and Sorensen M. Adverse Cardiovascular Effects of Traffic Noise with a Focus on Nighttime Noise and the New WHO Noise Guidelines. Annu Rev Public Health. 2020;41:309–328. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt F, Kolle K, Kreuder K, Schnorbus B, Wild P, Hechtner M, Binder H, Gori T and Munzel T. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin Res Cardiol. 2015;104:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt FP, Basner M, Kroger G, Weck S, Schnorbus B, Muttray A, Sariyar M, Binder H, Gori T, Warnholtz A and Munzel T. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. European heart journal. 2013;34:3508–14a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ Jr., Ganz P and Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. The New England journal of medicine. 1991;325:1551–6. [DOI] [PubMed] [Google Scholar]
- 79.Hassan M, Li Q, Brumback B, Lucey DG, Bestland M, Eubanks G, Fillingim RB and Sheps DS. Comparison of peripheral arterial response to mental stress in men versus women with coronary artery disease. The American journal of cardiology. 2008;102:970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller VM and Duckles SP. Vascular Actions of Estrogens: Functional Implications. Pharmacological Reviews. 2008;60:210–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al Mheid I, Hayek SS, Ko YA, Akbik F, Li Q, Ghasemzadeh N, Martin GS, Long Q, Hammadah M, Maziar Zafari A, Vaccarino V, Waller EK and Quyyumi AA. Age and Human Regenerative Capacity Impact of Cardiovascular Risk Factors. Circulation research. 2016;119:801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fadini GP, Mehta A, Dhindsa DS, Bonora BM, Sreejit G, Nagareddy P and Quyyumi AA. Circulating stem cells and cardiovascular outcomes: from basic science to the clinic. European heart journal. 2019. [DOI] [PMC free article] [PubMed]
- 83.Patel RS, Li Q, Ghasemzadeh N, Eapen DJ, Moss LD, Janjua AU, Manocha P, Kassem HA, Veledar E, Samady H, Taylor WR, Zafari AM, Sperling L, Vaccarino V, Waller EK and Quyyumi AA. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circulation research. 2015;116:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL, McCarthy PL, Abrams SI and Repasky EA. beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest. 2019;129:5537–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Elenkov IJ, Wilder RL, Chrousos GP and Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 86.Hammadah M, Samman Tahhan A, Mheid IA, Wilmot K, Ramadan R, Kindya BR, Kelli HM, O’Neal WT, Sandesara P, Sullivan S, Almuwaqqat Z, Obideen M, Abdelhadi N, Alkhoder A, Pimple PM, Levantsevych O, Mohammed KH, Weng L, Sperling LS, Shah AJ, Sun YV, Pearce BD, Kutner M, Ward L, Bremner JD, Kim J, Waller EK, Raggi P, Sheps D, Vaccarino V and Quyyumi AA. Myocardial Ischemia and Mobilization of Circulating Progenitor Cells. Journal of the American Heart Association. 2018;7:e007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Almuwaqqat Z, Wittbrodt M, Nye J, Moazzami K, Lima BB, Shah AJ, Blackburn E, Zhao JY, Lin J, Sun Y, Quyyumi AA, Vaccarino V and Bremner JD. Brain Regions Activation During Stress and Accelerated Biological Aging. Circulation. 2020;141:2. [Google Scholar]
- 88.Marsland AL, Walsh C, Lockwood K and John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun. 2017;64:208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hammadah M, Sullivan S, Pearce B, Al Mheid I, Wilmot K, Ramadan R, Tahhan AS, O’Neal WT, Obideen M, Alkhoder A, Abdelhadi N, Mohamed Kelli H, Ghafeer MM, Pimple P, Sandesara P, Shah AJ, Hosny KM, Ward L, Ko YA, Sun YV, Weng L, Kutner M, Bremner JD, Sheps DS, Esteves F, Raggi P, Vaccarino V and Quyyumi AA. Inflammatory response to mental stress and mental stress induced myocardial ischemia. Brain Behav Immun. 2018;68:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kop WJ, Weissman NJ, Zhu J, Bonsall RW, Doyle M, Stretch MR, Glaes SB, Krantz DS, Gottdiener JS and Tracy RP. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. The American journal of cardiology. 2008;101:767–73. [DOI] [PubMed] [Google Scholar]
- 91.Martinez-Muniz GA and Wood SK. Sex differences in the inflammatory consequences of stress: Implications for pharmacotherapy. Journal of Pharmacology and Experimental Therapeutics. 2020;375:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dunn AJ. Neurochemical and Endocrine Responses to Immune Activation: the Role of Cytokines. The Neuroimmunological Basis of Behavior and Mental Disorders. 2009:19–33.
- 93.Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L and Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. [DOI] [PubMed] [Google Scholar]
- 94.Maes M, Kubera M, Obuchowiczwa E, Goehler L and Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett. 2011;32:7–24. [PubMed] [Google Scholar]
- 95.Burg MM and Soufer R. Psychological Stress and Induced Ischemic Syndromes. Current cardiovascular risk reports. 2014;8:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arri SS, Ryan M, Redwood SR and Marber MS. Mental stress-induced myocardial ischaemia. Heart. 2016;102:472–80. [DOI] [PubMed] [Google Scholar]
- 97.Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, Kutner M and Vaccarino V. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. The American journal of cardiology. 2014;114:187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Almuwaqqat Z, Lima B, Young A, Sullivan S, Shah AJ, Hammadah M, Garcia M, Levantsevych O, Elon L, Garcia E, Bremner D, Raggi P, Lewis T, Quyyumi AA and Vaccarino V. Mental stress-induced myocardial ischemia as a marker for adverse cardiovascular events after MI. Journal of the American College of Cardiology. 2020;75:1.31918815 [Google Scholar]
- 99.Almuwaqqat Z, Sullivan S, Hammadah M, Lima BB, Shah AJ, Abdelhadi N, Fang SY, Wilmot K, Al Mheid I, Bremner JD, Garcia E, Nye JA, Elon L, Li L, O’Neal WT, Raggi P, Quyyumi AA and Vaccarino V. Sex-Specific Association Between Coronary Artery Disease Severity and Myocardial Ischemia Induced by Mental Stress. Psychosomatic Medicine. 2019;81:57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang W, Boyle SH, Ortel TL, Samad Z, Velazquez EJ, Harrison RW, Wilson J, Kuhn C, Williams RB, O’Connor CM and Becker RC. Platelet aggregation and mental stress induced myocardial ischemia: Results from the Responses of Myocardial Ischemia to Escitalopram Treatment (REMIT) study. Am Heart J. 2015;169:496–507 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah R, Burg MM, Vashist A, Collins D, Liu J, Jadbabaie F, Graeber B, Earley C, Lampert R and Soufer R. C-reactive protein and vulnerability to mental stress-induced myocardial ischemia. Molecular medicine (Cambridge, Mass). 2006;12:269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burg MM, Graeber B, Vashist A, Collins D, Earley C, Liu J, Lampert R and Soufer R. Noninvasive detection of risk for emotion-provoked myocardial ischemia. Psychosom Med. 2009;71:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shah AJ, Ghasemzadeh N, Zaragoza-Macias E, Patel R, Eapen DJ, Neeland IJ, Pimple PM, Zafari AM, Quyyumi AA and Vaccarino V. Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J Am Heart Assoc. 2014;3:e000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vaccarino V, Badimon L, Bremner JD, Cenko E, Cubedo J, Dorobantu M, Duncker DJ, Koller A, Manfrini O, Milicic D, Padro T, Pries AR, Quyyumi AA, Tousoulis D, Trifunovic D, Vasiljevic Z, de Wit C, Bugiardini R and Reviewers ESCSDG. Depression and coronary heart disease: 2018 position paper of the ESC working group on coronary pathophysiology and microcirculation. European heart journal. 2020;41:1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, Freedland KE, Jaffe AS, Leifheit-Limson EC, Sheps DS, Vaccarino V, Wulsin L, American Heart Association Statistics Committee of the Council on E, Prevention, the Council on C and Stroke N. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350–69. [DOI] [PubMed] [Google Scholar]
- 106.Vaccarino V, Wilmot K, Al Mheid I, Ramadan R, Pimple P, Shah AJ, Garcia EV, Nye J, Ward L, Hammadah M, Kutner M, Long Q, Bremner JD, Esteves F, Raggi P and Quyyumi AA. Sex Differences in Mental Stress-Induced Myocardial Ischemia in Patients With Coronary Heart Disease. J Am Heart Assoc. 2016;5:DOI: 10.1161/JAHA.116.003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pimple P, Hammadah M, Wilmot K, Ramadan R, Al Mheid I, Levantsevych O, Sullivan S, Garcia EV, Nye J, Shah AJ, Ward L, Mehta P, Raggi P, Bremner JD, Quyyumi AA and Vaccarino V. Chest Pain and Mental Stress-Induced Myocardial Ischemia: Sex Differences. Am J Med. 2018;131:540–547 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN and Coronary Vasomotion Disorders International Study G. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 109.Jespersen L, Abildstrom SZ, Hvelplund A, Galatius S, Madsen JK, Pedersen F, Hojberg S and Prescott E. Symptoms of angina pectoris increase the probability of disability pension and premature exit from the workforce even in the absence of obstructive coronary artery disease. European heart journal. 2013;34:3294–303. [DOI] [PubMed] [Google Scholar]
- 110.Handberg E, Johnson BD, Arant CB, Wessel TR, Kerensky RA, von Mering G, Olson MB, Reis SE, Shaw L, Bairey Merz CN, Sharaf BL, Sopko G and Pepine CJ. Impaired coronary vascular reactivity and functional capacity in women: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE) Study. Journal of the American College of Cardiology. 2006;47:S44–9. [DOI] [PubMed] [Google Scholar]
- 111.Safdar B, Ong P and Camici PG. Identifying Myocardial Ischemia due to Coronary Microvascular Dysfunction in the Emergency Department: Introducing a New Paradigm in Acute Chest Pain Evaluation. Clin Ther. 2018;40:1920–1930. [DOI] [PubMed] [Google Scholar]
- 112.Mehta PK, Hermel M, Nelson MD, Cook-Wiens G, Martin EA, Alkhoder AA, Wei J, Minissian M, Shufelt CL, Marpuri S, Hermel D, Shah A, Irwin MR, Krantz DS, Lerman A and Noel Bairey Merz C. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction: Results from the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) study. Int J Cardiol. 2018;251:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wei J, Pimple P, Shah AJ, Rooks C, Bremner JD, Nye JA, Ibeanu I, Murrah N, Shallenberger L, Raggi P and Vaccarino V. Depressive symptoms are associated with mental stress-induced myocardial ischemia after acute myocardial infarction. PLoS One. 2014;9:e102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pimple P, Shah A, Rooks C, Bremner JD, Nye J, Ibeanu I, Murrah N, Shallenberger L, Kelley M, Raggi P and Vaccarino V. Association between anger and mental stress-induced myocardial ischemia. Am Heart J. 2015;169:115–21 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pimple P, Lima BB, Hammadah M, Wilmot K, Ramadan R, Levantsevych O, Sullivan S, Kim JH, Kaseer B, Shah AJ, Ward L, Raggi P, Bremner JD, Hanfelt J, Lewis T, Quyyumi AA and Vaccarino V. Psychological Distress and Subsequent Cardiovascular Events in Individuals With Coronary Artery Disease. J Am Heart Assoc. 2019;8:e011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghadri JR and Templin C. The InterTAK Registry for Takotsubo Syndrome. European heart journal. 2016;37:2806–2808. [DOI] [PubMed] [Google Scholar]
- 117.Lewis TT, Lampert R, Charles D and Katz S. Expectations of Racism and Carotid Intima-Media Thickness in African American Women. Psychosom Med. 2019;81:759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lewis TT, Everson-Rose SA, Colvin A, Matthews K, Bromberger JT and Sutton-Tyrrell K. Interactive effects of race and depressive symptoms on calcification in African American and white women. Psychosomatic Medicine. 2009;71:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Topel ML, Kim JH, Mujahid MS, Sullivan SM, Ko YA, Vaccarino V, Quyyumi AA and Lewis TT. Neighborhood Socioeconomic Status and Adverse Outcomes in Patients With Cardiovascular Disease. The American journal of cardiology. 2019;123:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Islam SJ, Kim JH, Baltrus P, Topel ML, Liu C, Ko YA, Mujahid MS, Vaccarino V, Sims M, Mubasher M, Khan A, Ejaz K, Searles C, Dunbar S, Pemu P, Taylor HA, Quyyumi AA and Lewis TT. Neighborhood Characteristics and Ideal Cardiovascular Health Among Black Adults: Results From the Morehouse-Emory Cardiovascular (MECA) Center for Health Equity. Ann Epidemiol. 2020. [DOI] [PMC free article] [PubMed]
- 121.Saelee R, Vaccarino V, Sullivan S, Hammadah M, Shah A, Wilmot K, Abdelhadi N, Elon L, Pimple P, Kaseer B, Levantsevych O, Bremner JD and Lewis TT. Longitudinal associations between self-reported experiences of discrimination and depressive symptoms in young women and men post- myocardial infarction. J Psychosom Res. 2019;124:109782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW, American Heart Association Council on Quality of C, Outcomes Research CoE, Prevention CoC, Stroke Nursing CoL, Cardiometabolic H and Stroke C. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132:873–98. [DOI] [PubMed] [Google Scholar]
- 123.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Munoz D, Smith SC Jr., Virani SS, Williams KA Sr., Yeboah J and Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2019;74:e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shah AJ, Wittbrodt MT, Bremner JD and Vaccarino V. Cardiovascular Pathophysiology from the Cardioneural Perspective and its Clinical Applications. Trends in Cardiovascular Medicine. 2021. [DOI] [PMC free article] [PubMed]
- 125.Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, Ingle K, Miller P and Hinderliter A. Enhancing Cardiac Rehabilitation With Stress Management Training. Circulation. 2016;133:1341–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suglia SF, Koenen KC, Boynton-Jarrett R, Chan PS, Clark CJ, Danese A, Faith MS, Goldstein BI, Hayman LL, Isasi CR, Pratt CA, Slopen N, Sumner JA, Turer A, Turer CB, Zachariah JP, American Heart Association Council on E, Prevention, Council on Cardiovascular Disease in the Y, Council on Functional G, Translational B, Council on C, Stroke N, Council on Quality of C and Outcomes R. Childhood and Adolescent Adversity and Cardiometabolic Outcomes: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e15–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, Freedland KE, Jaffe AS, Leifheit-Limson EC, Sheps DS, Vaccarino V, Wulsin L and American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation. 2014;129:1350–69. [DOI] [PubMed] [Google Scholar]


