Abstract
Background.
Existing evidence indicates household income as a predictor of health-related quality of life (HRQoL) following a colorectal cancer (CRC) diagnosis. This association likely varies with neighborhood socioeconomic status (nSES), but evidence is limited.
Methods.
We included data from 1355 CRC survivors participating in the population-based Puget Sound Colorectal Cancer Cohort (PSCCC). Survivors reported current annual household income; we measured HRQoL via the Functional Assessment of Cancer Therapy - Colorectal (FACT-C) tool. Using neighborhood data summarized within a 1-km radial buffer of Census block group centroids, we constructed a multidimensional nSES index measure. We employed survivors’ geocoded residential addresses to append nSES score for Census block group of residence. With linear general estimating equations clustered on survivor location, we evaluated associations of household income with differences in FACT-C mean score, overall and stratified by nSES. We used separate models to explore relationships for wellbeing subscales.
Results.
We found lower household income to be associated with clinically meaningful differences in overall FACT-C scores (<$30K: −13.6, 95% CI: −16.8, −10.4) and subscale wellbeing after a recent CRC diagnosis. Relationships were slightly greater in magnitude for survivors living in lower SES neighborhoods.
Conclusion.
Our findings suggest that recently diagnosed lower income CRC survivors are likely to report lower HRQoL, and modestly more so in lower SES neighborhoods.
Impact.
Findings from this work will aid future investigators’ ability to further consider the contexts in which the income of CRC survivors can be leveraged as a means of improving HRQoL
Keywords: Colorectal, cancer, survivorship, income, neighborhood socioeconomic status
INTRODUCTION
In 2020, approximately 148,000 individuals will be diagnosed with colorectal cancer (CRC) in the United States (US).1 Roughly two-thirds of these new cases will live at least five years following diagnosis, reflecting advancements in early detection and the effectiveness of available treatments.1-3 However, even among those with a favorable prognosis, the physical, psychosocial, and financial effects of a CRC diagnosis and treatment may still lead to substantial long-term strain for CRC survivors and their families.4-6 For survivors, these hardships may result in progression or recurrence of the disease, development of second cancers, and a lower likelihood of survival.6-8 Thus, as CRC detection and treatment modalities improve, better understanding of factors critical to health-related quality of life (HRQoL) after diagnosis in CRC survivors can aid in the development of interventions that enhance both survivorship and survival.1, 9-11
In the US and in Europe, CRC survivors often report poorer overall HRQoL and wellbeing relative to individuals of similar demographics in the general population.8, 12, 13 Prior evidence also suggests that a complex intersection of factors influence HRQoL after a CRC diagnosis – factors including demographics, stage at diagnosis and initial treatment received, time since diagnosis, and socioeconomic characteristics.4, 14 In particular, HRQoL disparities between survivors have been strongly linked to socioeconomic inequities, such as inequities in household income around the time of diagnosis, that result in differential access to supportive medical care and curative treatment.1, 8-10, 15 Prior evidence indicates that CRC survivors with lower household income at diagnosis are more likely than higher-income patients to delay or forgo care, likely leading to less effective treatment, poorer wellbeing, and shorter survival.6, 12, 14, 16 This may suggest that compared with low-income CRC survivors, higher-income survivors are more able to convert preexisting financial resources, as well as power, prestige, and social connections, into timely health care accessing behaviors or other health-related opportunities.6, 8, 17, 18
Neighborhood socioeconomic disadvantage may also be related to HRQoL after a CRC diagnosis, as neighborhood socioeconomic status (nSES) influences the presence and cost of elements in the built environment (e.g., health care facilities, healthy food options, walkability features) and social environment (e.g., social interaction opportunities), which in turn affect CRC survivors’ ability to convert financial resources to behaviors.19-21 Past studies have noted relationships between area-level disadvantage or lower nSES and poorer CRC outcomes, including late-stage diagnosis and poorer survival, even after accounting for individual-level socioeconomic factors and other prognostic characteristics.16, 21, 22
However, while reports consistently indicate a link between higher income and higher HRQoL in recently diagnosed and longer-term CRC survivors,8, 12, 13, 23-25 no previous work has examined whether relationships differ given residential nSES context. If the apparent benefit of higher household income exists only for survivors who live in higher nSES neighborhoods, it may mean that living in lower nSES neighborhoods can lessen or even block the ability to convert income-related resources to behaviors and opportunities.20, 26, 27
Combining data from a population-based cohort of CRC survivors from the Seattle-Puget Sound region with neighborhood socioeconomic environment data from the American Community Survey (ACS), we cross-sectionally assessed the relationship of current annual household income with self-reported HRQoL following a recent diagnosis of CRC, exploring both overall and nSES-stratified associations. Findings from this work will improve future investigators’ ability to better consider the socioeconomic contexts influencing the role of income leveraged as a means of improving HRQoL in CRC survivors.10, 11, 27-29
MATERIALS AND METHODS
Study population
Our study population included CRC survivors who were diagnosed between 2016-2018 and who subsequently participated in the Puget Sound Colorectal Cancer Cohort (PSCCC), a study of CRC risk and survival that serves as an extension of the Seattle Colon Cancer Family Registry (S-CCFR) cohort study. We employ the National Cancer Institute’s definition of survivor, in which a person is considered a survivor from diagnosis through the end of life.30
Details of earlier but similar recruitment phases as part of the S-CCFR (1997-2008) been previously published elsewhere;31 PSCCC cohort recruitment and creation of our analysis’ study population can be found in Figure 1. Briefly, CRC survivors were ascertained via the population-based Cancer Surveillance System (CSS) of western Washington state, a participating cancer registry in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. Survivors were eligible for PSCCC participation given a diagnosis of incident, invasive CRC; an age of 20-74 years at the time of diagnosis; and residence within the CSS’s 13-county catchment area. At the time of our data analysis, there had been 1355 CRC survivors who had been enrolled into the PSCCC, who had completed a standardized risk factor survey in English at the time of study enrollment, and whose residential address at diagnosis met CSS’s geocoding accuracy standards and was mappable to a Census block group.
Figure 1.
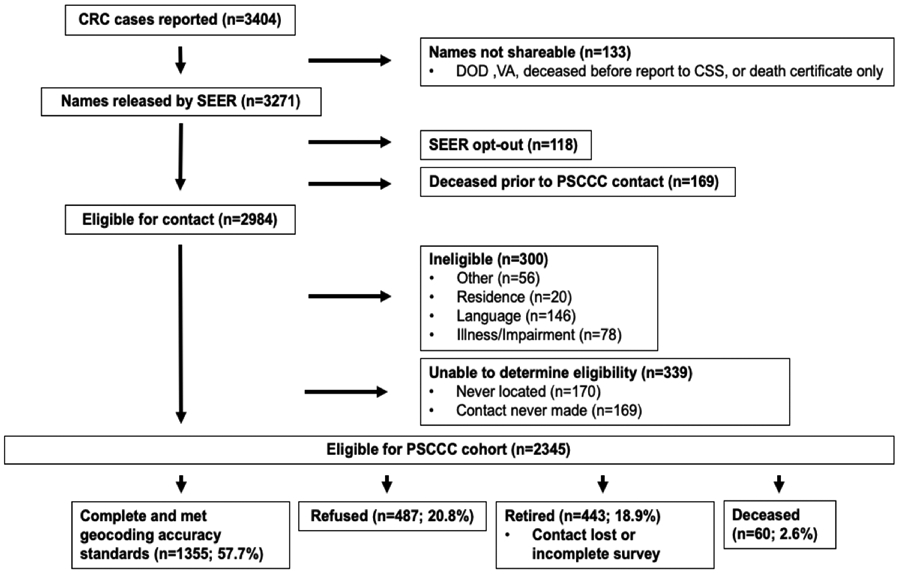
Flowchart of PSCCC cohort recruitment and study population creation for this analysis.
Data collection
According to study protocol, participating survivors completed a risk factor survey at PSCCC enrollment via structured phone interview, self-administered online survey, or paper survey. Surveys were administered a median of 5.4 months after diagnosis (range: 2.8-32.1 months), and collected data on individual-level socioeconomic factors (e.g., household income, educational attainment, race/ethnicity), demographics (e.g., age, gender, marital status), lifestyle behaviors (e.g., cigarette smoking status), health status factors (e.g., height, weight, self-reported physician diagnosis of diabetes or digestive comorbidities), and HRQoL.32, 33 For the present analysis, we only used PSCCC survey measures referring to a time period current to study enrollment.
We also obtained information on tumor characteristics at the time of diagnosis (i.e., primary tumor site) and residential address at cancer diagnosis from the CSS cancer registry.
Household income
We ascertained current annual household income for PSCCC survivors using a single question from the enrollment survey – “Which of the following best describes your current total annual household income from all sources?” – that had seven possible responses, ranging from “<$15K” to “≥$70K”. Based on the range of our available data as well as cutpoints from recent literature, we employed a four-category definition of annual income: <$30K, $30-69K, ≥$70K, and an “unknown/prefer not to answer” category which included survivors who either did not know or declined to report income.8 We included this last group in our main analyses since not knowing or declining to report income may be a function of unmeasured social norms that may also impact HRQoL in CRC survivors.6, 26 During 2016-2018, the lower boundary for the highest household earnings group ($70K) approximately represented the median annual household income for the study region.34 Although scaling our household income measure by household size would likely be relevant to the present analyses, we were unable to do so since the PSCCC survey did not collect information on household membership.
Neighborhood socioeconomic status (nSES)
Using ACS 2014-2018 five-year estimates, we measured six data elements - median household income, median housing unit value, percentage of households earning income from investments, percentage of persons aged ≥ 25 years who have completed high school, percentage of persons aged ≥ 25 years who have completed a college degree, and percentage of persons aged ≥ 16 years in a managerial or professional occupation – in each of the 2010 Census block groups in the CSS’s 13-county catchment area (n=3256 Census block groups in Clallam, Grays Harbor, Island, Jefferson, King, Kitsap, Mason, Pierce, San Juan, Skagit, Snohomish, Thurston, and Whatcom counties, Washington). These six ACS data elements were previously identified by Diez Roux and colleagues via factor analysis. 35, 36 Because Census block group boundaries are defined by population size, typically encompassing 600-3000 people, rather than land area, we decided that it would not work to use these units to represent neighborhoods in a study population including both urban and rural participants. Instead, we summarized ACS data elements within 1-kilometer (1km) radial buffers around Census block group centroids so as to have a consistent neighborhood size. To create the nSES measure, we transformed income-based variables (i.e., median household income and median housing unit value) using the natural logarithm, z-score transformed all six data elements, and then summed z-scored measures together to create the multidimensional index of relative neighborhood deprivation. In the index, lower nSES scores indicated lower neighborhood SES.35, 36
For all participating PSCCC CRC survivors, the CSS cancer registry provided us with the residential address at diagnosis in an already geocoded form. Geocoding protocol for CSS stipulated that all residential addresses be standardized to US postal service format and then geocoded to latitude/longitude coordinates employing, in order of priority, rooftop accuracy or street-level accuracy.37 Employing this geocoded address, we identified each survivor’s Census block group of residence and linked them to their nSES index score. For statistical analyses, we first classified CRC survivors into nSES quartiles and then created a dichotomous measure grouping survivors in two most disadvantaged quartiles as living in “lower nSES” neighborhoods and survivors in the two most advantaged quartiles as residing in “higher nSES” neighborhoods.38
Health-related quality of life (HRQoL)
We assessed HRQoL using the Functional Assessment of Cancer Therapy - Colorectal (FACT-C) tool included on the enrollment survey.32, 33, 39This tool, a version of the FACT-General (FACT-G) measure of self-reported HRQoL specific to individuals undergoing treatment for CRC, has been found to be reproducible and comparable to other measures of HRQoL.32, 40 The FACT-C comprises five subscales of wellbeing: physical (PWB, seven items), social/family (SWB, seven items), emotional (EWB, six items), functional (FWB, seven items), and CRC-specific (CCS, seven items). Subscale items referred to the prior week and were scored on a scale of 0 (“not at all”) to 4 (“very much”) based on predefined scoring guidelines. Survivors were required to have answered at least half of a subscale’s items to have a score for that subscale. We summed subscales together to create our measure of overall HRQoL. Higher overall HRQoL and subscale wellbeing scores indicated better outcomes. The overall HRQoL measure had a possible score range of 0-136 points, while subscale wellbeing scores ranged from 0-28 (PWB, SWB, FWB, CCS) or 0-24 (EWB). For both the FACT-G and FACT-C, prior evidence indicates a five-point difference in raw overall HRQoL scores and a two-point difference in wellbeing subscale scores to be associated with meaningful differences for both clinical and subjective health indicators.33, 41
Statistical analysis
We used generalized estimating equation (GEE) models with Gaussian distributions and clustered on participating survivors’ residential neighborhood to approximate associations of current annual household income with differences in HRQoL scores and 95% confidence intervals (CIs), overall and stratified by nSES. The GEE model type yields population average estimates of relationships with robust standard error estimates to account for potential non-independence of survivors clustered within certain areas of the study region.42, 43 We constructed separate models for overall HRQoL and each wellbeing subscale, for the overall study population and nSES-stratified analyses, and for several sensitivity analyses (i.e., the addition of health behavior and health status covariates, limiting the study population by survivor characteristics, and different methods in accounting for unknown household income).
We selected model covariates using a directed acyclic graph and defined them as shown in Table 1; we removed survivors preferring to not answer survey questions on education and marital status (n=20) from models producing adjusted estimates. We estimated the statistical significance of interactions of income categories with nSES level using a Wald test of the interaction coefficient compared with zero. Two-sided tests were considered statistically significant at the α=0.05 level. We conducted all statistical analyses in STATA/SE 16 (College Station, TX). This research was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center in Seattle, Washington.
Table 1.
Variations of mean overall health-related quality of life (HRQoL) scores by characteristics of PSCCC colorectal cancer survivors (N=1355), in the overall study population and by neighborhood socioeconomic status (nSES), Seattle-Puget Sound Region, 2016-2018
| Neighborhood socioeconomic status (nSES) |
||||||
|---|---|---|---|---|---|---|
| Overall study population |
Lower nSES | Higher nSES | ||||
| N (%) | FACT-C Mean (95%CI) | N (%) | FACT-C Mean (95%CI) | N (%) | FACT-C Mean (95%CI) | |
| Total | 1355 (−) | 103.3 (102.2, 104.3) | 591 (−) | 101.4 (99.8, 103.1) | 764 (−) | 104.5 (103.4, 106.0) |
| Household income, in USD($) | ||||||
| <$30K | 292 (22) | 93.0 (90.6, 95.5) | 186 (32) | 92.3 (89.1, 95.4) | 106 (14) | 94.5 (90.6, 98.5) |
| $30-69K | 320 (24) | 104.5 (102.4, 106.5) | 171 (29) | 102.9 (100.0, 105.8) | 149 (19) | 106.2 (103.4, 109.1) |
| ≥$70K | 641 (47) | 107.0 (105.7, 108.4) | 185 (31) | 108.1 (105.6, 110.6) | 456 (60) | 106.6 (105.0, 108.2) |
| Unknown/Prefer not to answer | 102 (7) | 105.3 (101.6, 109.0) | 49 (8) | 105.2 (99.4, 111.0) | 53 (7) | 105.3 (100.6, 110.0) |
| Educational attainment | ||||||
| ≤High school degree | 277 (20) | 99.7 (97.2, 102.2) | 175 (30) | 98.1 (94.9, 101.3) | 102 (13) | 102.6 (98.7, 106.5) |
| Some college or vocational/technical degree | 455 (34) | 102.9 (101.0, 104.7) | 225 (38) | 100.9 (98.2, 103.6) | 230 (30) | 104.8 (102.2, 107.3) |
| ≥College degree | 609 (45) | 105.1 (103.6, 106.6) | 183 (31) | 104.6 (101.9, 107.4) | 426 (56) | 105.2 (103.5, 106.9) |
| Prefer not to answer | 14 (1) | 110.27 (103.2, 117.3) | 8 (1) | 113.1 (101.4, 124.7) | 6 (1) | 106.5 (101.1, 111.9) |
| Age at diagnosis, in years | ||||||
| <55 | 527 (39) | 100.5 (98.8, 102.2) | 208 (35) | 98.7 (95.8, 101.6) | 319 (42) | 101.6 (99.5, 103.8) |
| 55–64 | 409 (30) | 102.1 (100.2, 104.0) | 196 (33) | 100.0 (97.2, 102.7) | 213 (28) | 103.9 (101.3, 106.5) |
| ≥65 | 419 (31) | 108.0 (106.2, 109.7) | 187 (32) | 106.0 (103.2, 108.8) | 232 (30) | 109.8 (107.6, 111.9) |
| Gender | ||||||
| Male | 740 (55) | 103.6 (102.2–105.0) | 309 (52) | 102.0 (99.6, 104.3) | 431 (56) | 104.8 (103.0–106.6) |
| Female | 615 (45) | 102.9 (101.4–104.5) | 282 (48) | 101.0 (98.5, 103.4) | 333 (44) | 104.7 (102.7–106.6) |
| Marital status | ||||||
| Married or living as married | 895 (66) | 105.3 (104.1, 106.6) | 354 (60) | 103.4 (101.3, 105.4) | 541 (71) | 106.5, (105.0, 108.1) |
| Not living as married | 452 (33) | 99.4 (97.5, 101.2) | 231 (39) | 98.2 (95.6, 100.8) | 221 (29) | 100.6 (98.0, 103.1) |
| Prefer not to answer | 8 (1) | 96.9 (83.0, 110.7) | 6 (1) | 101.0 (84.7, 117,2) | 2 (<1) | 84.6 (58.9, 110.2) |
Notes: HRQoL measured using the Functional Assessment of Cancer Therapy – Colorectal (FACT-C); all measured survivor-level characteristics current to the time of PSCCC enrollment
RESULTS
Our analyses included data from 1355 CRC survivors participating in the PSCCC (Table 1). These survivors had a median age at cancer diagnosis of 59 years (range: 23-74 years) and reported a mean overall HRQoL score of 103.3 points (95% CI: 102.2, 104.3). At study baseline, roughly 47% of survivors reported their current annual household income to be at least the regional median (≥$70K) and 45% reported having attained at least a four-year college degree. The majority of survivors were male (55%) and married or living as married (66%). In the overall study population, mean overall HRQoL scores appeared to vary by income, education, age at diagnosis, and marital status. Mean HRQoL scores also appeared slightly lower for survivors who were at an individual-level socioeconomic disadvantage – a household income under $70K or no education beyond a high school degree or equivalent – and who resided in a lower nSES neighborhood. While we again observed income gradients with mean subscale wellbeing scores in the overall study population, we did not see differences by nSES (Table 2).
Table 2.
Variations of mean overall health-related quality of life (HRQoL) and subscale wellbeing scores by current annual household income of PSCCC colorectal cancer survivors, in the overall study population and by neighborhood socioeconomic status (nSES), Seattle-Puget Sound Region, 2016-2018 (N=1355)
| Neighborhood socioeconomic status (nSES) |
||||
|---|---|---|---|---|
| Overall study population |
Lower nSES | Higher nSES | ||
| Score Mean (95%CI) | Score Mean (95%CI) | Score Mean (95%CI) | ||
| Overall HRQoL | Total | 103.3 (102.2, 104.3) | 101.4 (99.8, 103.1) | 104.5 (103.4, 106.0) |
| Household income, in USD($) | ||||
| <$30K | 93.0 (90.6, 95.5) | 92.3 (89.1, 95.4) | 94.5 (90.6, 98.5) | |
| $30-69K | 104.5 (102.4, 106.5) | 102.9 (100.0, 105.8) | 106.2 (103.4, 109.1) | |
| ≥$70K | 107.0 (105.7, 108.4) | 108.1 (105.6, 110.6) | 106.6 (105.0, 108.2) | |
| Unknown | 105.3 (101.6, 109.0) | 105.2 (99.4, 111.0) | 105.3 (100.6, 110.0) | |
| PWB | Total | 21.0 (20.7, 21.3) | 20.5 (20.0, 21.0) | 21.4 (21.0, 21.7) |
| Household income, in USD($) | ||||
| <$30K | 18.9 (18.2, 19.6) | 18.8 (17.8, 19.7) | 19.1 (18.0, 20.3) | |
| $30-69K | 21.1 (20.5, 21.7) | 21.0 (20.1, 21.8) | 21.4 (20.5, 21.2) | |
| ≥$70K | 21.7 (21.3, 22.1) | 21.6 (20.8, 22.4) | 21.7 (21.3, 22.2) | |
| Unknown | 22.1 (21.1, 23.1) | 21.7 (20.1, 23.4) | 22.5 (21.2, 23.7) | |
| SWB | Total | 22.4 (22.1, 22.6) | 22.1 (21.6, 22.5) | 22.6 (22.3, 23.0) |
| Household income, in USD($) | ||||
| <$30K | 20.2 (19.6, 20.9) | 20.3 (19.4, 21.3) | 20.0 (18.8, 21.2) | |
| $30-69K | 22.3 (21.7, 22.9) | 22.2 (21.3, 23.0) | 22.5 (21.7, 23.4) | |
| ≥$70K | 23.4 (23.1, 23.8) | 23.7 (23.1, 24.3) | 23.3 (22.9, 23.7) | |
| Unknown | 22.1 (21.1, 23.1) | 22.3 (20.7, 23.8) | 22.0 (20.6, 23.3) | |
| EWB | Total | 19.4 (19.2, 19.6) | 19.3 (18.9, 19.6) | 19.6 (19.3, 19.8) |
| Household income, in USD($) | ||||
| <$30K | 18.2 (17.6, 18.8) | 18.1 (17.4, 18.9) | 18.3 (17.4, 19.2) | |
| $30-69K | 20.0 (19.5, 20.4) | 19.6 (19.0, 20.2) | 20.4 (19.8, 21.0) | |
| ≥$70K | 19.7 (19.4, 20.0) | 20.0 (19.5, 20.6) | 19.6 (19.2, 20.0) | |
| Unknown | 19.4 (18.6, 20.2) | 19.1 (17.9, 20.3) | 19.7 (18.5, 20.8) | |
| FWB | Total | 19.4 (19.1, 19.7) | 18.7 (18.2, 19.2) | 19.9 (19.5, 20.3) |
| Household income, in USD($) | ||||
| <$30K | 16.3 (15.5, 17.1) | 16.0 (15.0, 16.9) | 16.9 (15.6, 18.1) | |
| $30-69K | 19.8 (19.2, 20.5) | 19.2 (18.3, 20.2) | 20.5 (19.6, 21.4) | |
| ≥$70K | 20.5 (20.1, 21.0) | 20.7 (19.9, 21.5) | 20.5 (20.0, 21.0) | |
| Unknown | 19.9 (18.8, 21.1) | 20.2 (18.6, 21.9) | 19.7 (18.1, 21.3) | |
| CCS | Total | 21.1 (20.8, 21.3) | 20.8 (20.4, 21.2) | 21.3 (20.9, 21.6) |
| Household income, in USD($) | ||||
| <$30K | 19.4 (18.8, 20.0) | 19.1 (18.3, 19.9) | 20.1 (19.2, 20.9) | |
| $30-69K | 20.8 (20.1, 21.6) | 21.0 (20.3, 21.7) | 21.5 (20.8, 22.2) | |
| ≥$70K | 21.6 (21.3, 22.0) | 22.1 (21.5, 22.8) | 21.5 (21.0, 21.9) | |
| Unknown | 21.7 (20.9, 22.5) | 21.9 (20.8, 23.0) | 21.2 (20.5, 22.7) | |
Notes: HRQoL and wellbeing measured using the Functional Assessment of Cancer Therapy – Colorectal (FACT-C); PWB – Physical wellbeing; SWB – Social and family wellbeing; EWB – Emotional wellbeing; FWB – Functional wellbeing; CCS – Colorectal cancer-specific wellbeing.
After adjusting estimates for survivor-level characteristics, we found evidence in our overall study population that lower household income was related to large and clinically meaningful differences in HRQoL after a recent CRC diagnosis (Table 3). Specifically, compared to survivors with at least the regional median household income (≥$70K), survivors in the lowest income group (<$30K) reported a difference in mean HRQoL score (in points) of 13.6 (95% CI: 16.8, 10.4), and lower wellbeing across all subscales. For overall HRQoL and all wellbeing subscales excepting PWB, these relationships appeared modestly stronger in magnitude with residence in a lower nSES neighborhood; we observed this to be particularly true for associations between income and FWB. However, we detected little evidence of statistically significant interactions between household income and residential nSES in their influence on HRQoL following a CRC diagnosis.
Table 3.
Associations of current household income with differences in overall health-related quality of life (HRQoL) and subscale wellbeing scores in recently diagnosed colorectal cancer (CRC) survivors, overall and by neighborhood socioeconomic status (nSES) (N=1335) a
| Neighborhood socioeconomic status |
|||||
|---|---|---|---|---|---|
| Overall study population N=1335 |
Lower nSES N=579 |
Higher nSES N=756 |
|||
| Household income, in USD($) |
FACT-C Difference (95% CI) | FACT-C Difference (95% CI) | FACT-C Difference (95% CI) |
P for interaction |
|
| Overall HRQoL | <$30K | −13.6 (−16.8, −10.4) | −15.6 (−20.2, −11.0) | −11.8 (−16.5, −7.2) | 0.39 |
| $30-69K | −3.7 (−6.4, −0.9) | −6.2 (−10.3, −2.1) | −1.4 (−5.1, 2.4) | 0.08 | |
| ≥$70K | ref (-) | ref (-) | ref (-) | - | |
| Unknown | −3.1 (−7.3, 1.1) | −4.4 (−11.1, 2.3) | −2.5 (−7.8, 2.9) | 0.65 | |
| PWB | <$30K | −2.9 (−3.9, −2.0) | −3.1 (−4.5, −1.7) | −2.7 (−4.0, −1.4) | 0.90 |
| $30-69K | −1.0 (−1.8, −0.2) | −1.2 (−2.4, 0.1) | −0.8 (−1.9, 0.3) | 0.90 | |
| ≥$70K | ref (-) | ref (-) | ref (-) | - | |
| Unknown | −0.1 (−1.2, 1.1) | −0.6 (−2.6, 1.5) | 0.4 (−1.0 , 1.7) | 0.54 | |
| SWB | <$30K | −3.0 (−3.9, −2.2) | −3.2 (−4.4, −2.1) | −3.0 (−4.3, −1.6) | 0.86 |
| $30-69K | −1.2 (−1.9, −0.4) | −1.5 (−2.5, −0.4) | −0.9 (−1.9, 0.1) | 0.38 | |
| ≥$70K | ref (-) | ref (-) | ref (-) | - | |
| Unknown | −1.5 (−2.6, −0.5) | −1.7 (−3.4, 0.1) | −1.5 (−2.9, −0.1) | 0.82 | |
| EWB | <$30K | 1.6 (−2.4, −0.9) | −2.1 (−3.1, −1.0) | −1.4 (−2.5, −0.3) | 0.69 |
| $30-69K | −0.1 (−0.7, 0.5) | −0.8 (−1.7, 0.1) | 0.5 (−0.3, 1.3) | 0.03 | |
| ≥$70K | ref (-) | ref (-) | ref (-) | - | |
| Unknown | −0.7 (−1.6, 0.2) | −1.4 (−2.8, 0.1) | −0.3 (−1.5, 1.0) | 0.26 | |
| FWB | <$30K | −4.0 (−5.0, −3.1) | −4.7 (−6.1, −3.3) | −3.3 (−4.7, −1.9) | 0.27 |
| $30-69K | −0.9 (−1.8, −0.1) | −1.7 (−3.0, −0.4) | −0.1 (−1.3, 1.1) | 0.06 | |
| ≥$70K | ref (-) | ref (-) | ref (-) | - | |
| Unknown | −0.8 (−2.2, 0.5) | −0.8 (−2.8, 1.2) | −1.0 (−2.8, 0.8) | 0.90 | |
| CCS | <$30K | 2.0 (−2.8, −1.2) | −2.6 (−3.8, −1.5) | −1.5 (−2.6, −0.4) | 0.08 |
| $30-69K | −0.5 (−1.2, 0.2) | −1.2 (−2.2, −0.1) | −0.1 (−1.0, 0.8) | 0.09 | |
| ≥$70K | ref (-) | ref (-) | ref (-) | - | |
| Unknown | 0.1 (−0.9, 1.0) | −0.1 (−1.5, 1.3) | −0.1 (−1.3, 1.3) | 0.85 | |
PWB – Physical wellbeing; SWB – Social and family wellbeing; EWB – Emotional wellbeing; FWB – Functional wellbeing; CCS – Colorectal cancer-specific wellbeing.
All estimates adjusted for educational attainment (≤high school degree, some college or vocational/technical degree, ≥college degree), age at diagnosis (<55, 55–64, ≥65 years), gender (male, female), and marital status (living as married or married, not living as married).
Survivors who preferred not to report educational attainment and marital status (N=20) removed from analysis
Our findings were consistent across sensitivity analyses, including adjusting model estimates for survivor smoking status and health status factors (Supplemental Table S1); limiting the study population to survivors residing in urban areas, survivors diagnosed at a local stage of disease, younger (<55 years at diagnosis) survivors, single survivors, and survivors with less than a year between their CRC diagnosis and PSCCC enrollment (Supplemental Table S2); stratifying results by tumor site (i.e., colon cancer survivors and rectal cancer survivors) (Supplemental Table S3); and using a complete case analysis or multiple imputation by chained equations (MICE) modeling as an alternative method to account for survivors with unknown income (Supplemental Table S4).44 Differences by nSES were greater in magnitude for survivors diagnosed at a local disease stage as well as for younger survivors (Supplemental Table S2).
DISCUSSION
Within this population-based cohort of recently diagnosed CRC survivors in the Seattle-Puget Sound region, we found associations between lower household income and lower HRQoL similar to those observed in prior studies of cancer survivors in the US.6, 8, 15, 24 In addition, we noted that the estimates for all of these relationships were slightly greater in magnitude for survivors residing in lower nSES neighborhoods. These findings may indicate that lower income CRC survivors living in socioeconomically disadvantaged neighborhoods, may not have sufficient cancer health-related resources in their area, thus potentially leading to increased non-medical out-of-pocket costs (e.g., transportation to health and supportive care,) to obtain necessary resources outside of their neighborhood.6, 14, 20, 26, 27 Although these relationships are cross-sectional, and warrant future research to tease out underlying mechanisms and points of intervention, our findings highlight that the association of income with a specific, measurable survivorship outcome (i.e., HRQoL) following a cancer diagnosis may vary with neighborhood socioeconomic context. 8, 12, 24 While these findings are unsurprising, they do impart critical contextual evidence to future investigators wishing to design intervention-supporting research that address income-related CRC survivorship needs.10, 11, 45 In addition, these findings could also support background research for future studies of the relationships between income-related material social needs (e.g., housing instability, food insecurity) and HRQoL in cancer survivors. 15
Our findings of an association between lower income and poorer overall HRQoL after a CRC diagnosis, and our conclusion that income is a critical determinant of survivor wellbeing, are consistent with previous findings from both population- and clinic-based studies.6, 8, 12, 13, 24 Specifically, a recent cohort study in New Mexico found that newly diagnosed CRC survivors reporting a current annual household income of <$30K had a 5.13 point (95% CI:8.56, 1.71) lower physical function PROMIS score compared to survivors reporting an income of ≥$70K;8 similarly, a study of African American cancer survivors, including 231 CRC survivors, in Detroit noted unadjusted mean FACT-G scores that were 18 points lower in the lowest income group (<$20K) compared to the highest income group (>$80K).15 An older population-based study of long-term CRC survivors in the Seattle-Puget Sound region also observed a significant association (p=0.005) between higher levels of annual income and self-evaluated overall HRQoL,24 as did a recent clinic-based study in Germany.13 However, we noted that the estimated associations in our study were stronger in magnitude compared with similar estimates past investigations. These inconsistencies are likely partly due to the type of patient reported outcome metric used; for example, PROMIS items compare cancer survivors to a general disease-free population while FACT items are specific to patient populations and focus on disease-specific concerns.41, 46 In addition, some difference in findings is attributable to the demographics of our study population, the years for which data was collected, and our study region.
To the best of our knowledge, we are the first survivorship study to explore the whether relationships between income and HRQoL in CRC survivors vary with nSES. Prior evidence from a registry-based study in The Netherlands indicated that long-term CRC survivors living in socioeconomically disadvantaged postcode areas were 50% more likely than survivors in advantaged postcode areas to report clinically meaningful anxiety and depression symptoms.12 While this prior study did not look area socioeconomic context as a modifier of income effects, these findings corroborate our conclusions that living in a socioeconomically disadvantaged neighborhood likely influences the HRQoL of CRC survivors. In addition, this previous investigation’s findings align with our observation that lower income CRC survivors living in lower nSES neighborhoods experienced lower FWB compared with lower income survivors living in higher nSES neighborhoods.32, 33 The strength of our FWB subscale estimates may suggest that financial hardship-related strain impedes CRC survivors’ ability to function in activities of daily living, perhaps especially for those living in disadvantaged socioeconomic environments.
Increasing evidence suggests that many cancer survivors in the US are likely to experience some form of cancer- and treatment-related financial hardships.1, 4, 6, 26, 47, 48 These financial burdens – including lost income, increased costs of daily living (e.g., transportation, childcare, non-irritating foods), and increased medical expenditures (e.g., insurance premiums, deductibles, copayments, out-of-pocket costs) – may be substantial both for newly diagnosed and long-term survivors,14, 17, 26, 49 and may lead to delays or forgoing of medical and supportive care.6, 14, 17, 26, 49, 50 The psychological strain of cancer-related financial burdens has also been associated with lower HRQoL6, 51 after diagnosis as well as anxiety and depression. 5, 6, 12, 51 In addition, this strain has also been linked to poorer disease prognosis via stress-related biological processes influencing cancer progression.7, 52 Critically, the impacts of cancer-related financial issues are inequitably exacerbated for low income and working age (<65 years) survivors, whose financial precarity likely predates their diagnosis and whose out-of-pocket cancer-related expenditures constitute a greater proportion of available income.6, 14, 17 Previous evidence indicates that cancer survivors with pre-existing financial or socioeconomic precarity are less likely than socioeconomically stable survivors to adhere to recommended screening and treatment guidelines.1, 6, 14, 49, 53 In the US, CRC is second only to breast cancer in terms of average cancer costs; however, CRC is more likely than breast cancer to be diagnosed at a later stage of disease, leading to higher care costs in the short- and long-term. 9, 17, 54, 55 In addition, growing evidence of differences in insurance needs, care needs, and social needs suggest the importance of disaggregating cancer survivors and exploring CRC survivor-specific associations.9, 56-58
Notably, the ability to leverage existing income to cover cancer- and treatment-related costs and access necessary resources is highly dependent on local resource availability and accessibility, which is heavily influenced by nSES.6, 20, 21, 38Neighborhoods at a socioeconomic disadvantage may have difficulty in attracting quality cancer care specialists and facilities, as well as affordable and culturally sensitive health care services.16, 20 In contrast, neighborhoods with a relative SES advantage may attract high quality medical services and supportive resources, but the relatively higher costs of these assets, in combination with the higher costs of daily living in the neighborhood, may preclude lower income survivors from timely or overall access, which may help explain why we did not find greater differences between lower and higher nSES neighborhoods.6, 14, 15, 17, 20, 27 Our finding that the association of lower income and lower HRQoL was modestly greater for CRC survivors in lower SES neighborhoods may indicate that necessary services and resources are not immediately present or easily accessible to survivors living in these areas and thus survivors may need to convert additional income-related resources to access care and support at a farther distance from home.4, 14, 16, 59, 60
Our findings and interpretations need to be considered in light of key limitations. First, survivors included in our study may systematically differ from the underlying survivor population in the region, including if survivors with severe disease, pre-existing financial precarity, or lower HRQoL were unable to participate; conversely, our participating survivors may have ongoing lower HRQoL that influenced their income level prior to study enrollment. Because both our income and HRQoL measures refer to the current enrollment time period, we likely limited the amount of possible reverse interpretation. We tried to limit the amount of participation bias in our estimates by basing our study nSES distribution on the nSES distribution for the underlying survivor population in the region and also by using categories of current household income found in recent survivorship literature.8 However, almost half of participating survivors reported into our highest income category (≥$70K), the median household income for our region and time period of study.34 Due to the structure of our survey, we were not able to further disaggregate our income measure. We also had additional limitations to our participant recruitment, including being limited to English-language participants. Second, our analysis is cross-sectional, and household income measured at a single time point is an imperfect proxy for how financial precarity, accumulated wealth or debt, and inequities affect HRQoL and wellbeing after a CRC diagnosis.26 A third potential drawback to this work is our use of radial buffers to define participating survivors’ residential neighborhoods. While a theoretical improvement on administrative boundaries, circular buffers may not align with survivors’ perceived communities and daily activity spaces, or their view of available and accessible services and resources.19 For this analysis, we also lacked information on each survivor’s length of residency in their residential neighborhood. Further, our observed associations are relative to our study region and the geographic unit underlying our neighborhood buffer (i.e., summarized Census block group-level data).61 The Seattle-Puget Sound area is currently one of the wealthiest regions in the US, and our results would likely have been different in another region or across different sizes of geographic units (e.g., tracts, counties) within our region of study.15, 34 Finally, our analyses were limited by sample size, which left us unable to further parse lower household income categories and to stratify associations by other socioeconomic characteristics, such as race/ethnicity. Although structural racism – and its impacts on wealth, power, prestige, quality of life, and ability to access quality healthcare - is an obvious confounder of our associations, the majority of our study population was non-Hispanic White (73%) and the minority Black (4%) and we decided that it was not appropriate to proxy adjust estimates for an administrative race measure.20
Despite these limitations, we utilized the largest population-based study population of CRC survivors to-date in assessing the relationships between household income and HRQoL. Further, this is the first study to explore whether these associations vary with neighborhood context. Future research will need to evaluate these relationships in other populations, other geographic locations, with respect to the 2020 COVID-19 pandemic, and with additional measures of socioeconomic position and financial precarity.
Supplementary Material
ACKNOWLEDGMENTS
Financial support: This work was supported by the National Cancer Institute of the National Institutes of Health –T32 CA094061-18 (PI: Neugut,/Terry), T32 CA094880 (PI: Newcomb), K05 CA152715 (PI: Newcomb), and R01 CA196337(PI: Newcomb) – and the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health – T32 ES0115459.
We would like to thank the PSCCC and CSS teams for their help in preparing the PSCCC cohort and cancer registry datasets, respectively, for this study. We would also like to extend our appreciation and gratitude to the CRC survivors participating in the PSCCC.
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest
REFERENCES
- 1.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. March 5 2020;doi: 10.3322/caac.21601 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Jemal A, Thun MJ, Hao Y, Ward EM. Trends in the incidence of colorectal cancer in relation to county-level poverty among blacks and whites. J Natl Med Assoc. December 2008;100(12):1441–4. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. January 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 4.El-Shami K, Oeffinger KC, Erb NL, et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin. November 2015;65(6):427–55. doi: 10.3322/caac.21286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Head B, Harris L, Kayser K, Martin A, Smith L. As if the disease was not enough: coping with the financial consequences of cancer. Support Care Cancer. March 2018;26(3):975–987. doi: 10.1007/s00520-017-3918-y [DOI] [PubMed] [Google Scholar]
- 6.Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. October 15 2013;119(20):3710–7. doi: 10.1002/cncr.28262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma A, Walker LG, Monson JR. Baseline quality of life factors predict long term survival after elective resection for colorectal cancer. Int J Surg Oncol. 2013;2013:269510. doi: 10.1155/2013/269510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDougall JA, Blair CK, Wiggins CL, et al. Socioeconomic disparities in health-related quality of life among colorectal cancer survivors. J Cancer Surviv. June 2019;13(3):459–467. doi: 10.1007/s11764-019-00767-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. September 2019;69(5):363–385. doi: 10.3322/caac.21565 [DOI] [PubMed] [Google Scholar]
- 10.Miller KD, Pandey M, Jain R, Mehta R. Cancer Survivorship and Models of Survivorship Care: A Review. Am J Clin Oncol. December 2015;38(6):627–33. doi: 10.1097/COC.0000000000000153 [DOI] [PubMed] [Google Scholar]
- 11.U.S. Centers for Medicare & Medicaid Services. Accountable Health Communities Model. Accessed Jan, 2019. https://innovation.cms.gov/initiatives/ahcm/
- 12.Andrykowski MA, Aarts MJ, van de Poll-Franse LV, Mols F, Slooter GD, Thong MS. Low socioeconomic status and mental health outcomes in colorectal cancer survivors: disadvantage? advantage?… or both? Psychooncology. November 2013;22(11):2462–9. doi: 10.1002/pon.3309 [DOI] [PubMed] [Google Scholar]
- 13.Han CJ, Gigic B, Schneider M, et al. Risk factors for cancer-related distress in colorectal cancer survivors: one year post surgery. J Cancer Surviv. March 12 2020;doi: 10.1007/s11764-019-00845-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akinyemiju T, Waterbor JW, Pisu M, Moore JX, Altekruse SF. Availability of Healthcare Resources and Colorectal Cancer Outcomes Among Non-Hispanic White and Non-Hispanic Black Adults. J Community Health. April 2016;41(2):296–304. doi: 10.1007/s10900-015-0096-z [DOI] [PubMed] [Google Scholar]
- 15.Hastert TA, McDougall JA, Strayhorn SM, Nair M, Beebe-Dimmer JL, Schwartz AG. Social needs and health-related quality of life among African American cancer survivors: Results from the Detroit Research on Cancer Survivors study. Cancer. November 23 2020;doi: 10.1002/cncr.33286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y, Wimberly MC. Geographic Variations of Colorectal and Breast Cancer Late-Stage Diagnosis and the Effects of Neighborhood-Level Factors. J Rural Health. April 2017;33(2):146–157. doi: 10.1111/jrh.12179 [DOI] [PubMed] [Google Scholar]
- 17.Pisu M, Henrikson NB, Banegas MP, Yabroff KR. Costs of cancer along the care continuum: What we can expect based on recent literature. Cancer. November 1 2018;124(21):4181–4191. doi: 10.1002/cncr.31643 [DOI] [PubMed] [Google Scholar]
- 18.Phelan JC, Link BG, Tehranifar P. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav. 2010;51 Suppl:S28–40. doi: 10.1177/0022146510383498 [DOI] [PubMed] [Google Scholar]
- 19.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. February 2010;1186:125–45. doi: 10.1111/j.1749-6632.2009.05333.x [DOI] [PubMed] [Google Scholar]
- 20.White K, Haas JS, Williams DR. Elucidating the role of place in health care disparities: the example of racial/ethnic residential segregation. Health Serv Res. June 2012;47(3 Pt 2):1278–99. doi: 10.1111/j.1475-6773.2012.01410.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez SL, Shariff-Marco S, DeRouen M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer. July 15 2015;121(14):2314–30. doi: 10.1002/cncr.29345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagedoorn P, Vandenheede H, Vanthomme K, Gadeyne S. Socioeconomic position, population density and site-specific cancer mortality: A multilevel analysis of Belgian adults, 2001–2011. Int J Cancer. January 1 2018;142(1):23–35. doi: 10.1002/ijc.31031 [DOI] [PubMed] [Google Scholar]
- 23.Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer. March 15 2000;88(6):1294–303. [PubMed] [Google Scholar]
- 24.Ramsey SD, Berry K, Moinpour C, Giedzinska A, Andersen MR. Quality of life in long term survivors of colorectal cancer. Am J Gastroenterol. May 2002;97(5):1228–34. doi: 10.1111/j.1572-0241.2002.05694.x [DOI] [PubMed] [Google Scholar]
- 25.Short PF, Mallonee EL. Income disparities in the quality of life of cancer survivors. Med Care. January 2006;44(1):16–23. doi: 10.1097/01.mlr.0000188986.84819.3a [DOI] [PubMed] [Google Scholar]
- 26.Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial Hardships Experienced by Cancer Survivors: A Systematic Review. J Natl Cancer Inst. February 2017;109(2)doi: 10.1093/jnci/djw205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovasi GS, Hutson MA, Guerra M, Neckerman KM. Built environments and obesity in disadvantaged populations. Epidemiol Rev. 2009;31:7–20. doi: 10.1093/epirev/mxp005 [DOI] [PubMed] [Google Scholar]
- 28.Shariff-Marco S, Yang J, John EM, et al. Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the Neighborhood and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. May 2014;23(5):793–811. doi: 10.1158/1055-9965.EPI-13-0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eagle A. Health care villages and districts create caring communities. Healh Facilities Management Magazine: American Hospital Association; 2017. [Google Scholar]
- 30.(NCI) NCI. Accessed 22 Dec 2020, https://www.cancer.gov/publications/dictionaries/cancer-terms/search/survivor/?searchMode=Begins
- 31.Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. November 2007;16(11):2331–43. [DOI] [PubMed] [Google Scholar]
- 32.Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. May 1999;8(3):181–95. [DOI] [PubMed] [Google Scholar]
- 33.Yost KJ, Cella D, Chawla A, et al. Minimally important differences were estimated for the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) instrument using a combination of distribution- and anchor-based approaches. J Clin Epidemiol. December 2005;58(12):1241–1251. doi: 10.1016/j.jclinepi.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 34.Seattle Office of Planning & Community Development. Population & Demographics. Accessed 15 October 2019, https://www.seattle.gov/opcd/population-and-demographics
- 35.Diez-Roux AV, Kiefe CI, Jacobs DR Jr., et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. August 2001;11(6):395–405. [DOI] [PubMed] [Google Scholar]
- 36.Hastert TA, Beresford SA, Sheppard L, White E. Disparities in cancer incidence and mortality by area-level socioeconomic status: a multilevel analysis. J Epidemiol Community Health. February 2015;69(2):168–76. doi: 10.1136/jech-2014-204417 [DOI] [PubMed] [Google Scholar]
- 37.National Cancer Institute (NCI). Surveillance, Epidemiology, and End Results Program (SEER) Summary Stage 2018. Accessed 15 October 2019, https://seer.cancer.gov/tools/ssm/
- 38.Lovasi GS, Neckerman KM, Quinn JW, Weiss CC, Rundle A. Effect of individual or neighborhood disadvantage on the association between neighborhood walkability and body mass index. Am J Public Health. February 2009;99(2):279–84. doi:AJPH.2008.138230 [pii] 10.2105/AJPH.2008.138230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis CM, Wolf WA, Xun P, Sandler RS, He K. Racial differences in dietary changes and quality of life after a colorectal cancer diagnosis: a follow-up of the Study of Outcomes in Colorectal Cancer Survivors cohort. Am J Clin Nutr. June 2016;103(6):1523–30. doi: 10.3945/ajcn.115.126276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ganesh V, Agarwal A, Popovic M, et al. Comparison of the FACT-C, EORTC QLQ-CR38, and QLQ-CR29 quality of life questionnaires for patients with colorectal cancer: a literature review. Support Care Cancer. August 2016;24(8):3661–8. doi: 10.1007/s00520-016-3270-7 [DOI] [PubMed] [Google Scholar]
- 41.Brucker PS, Yost K, Cashy J, Webster K, Cella D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof. June 2005;28(2):192–211. doi: 10.1177/0163278705275341 [DOI] [PubMed] [Google Scholar]
- 42.Lovasi GS, Jacobson JS, Quinn JW, Neckerman KM, Ashby-Thompson MN, Rundle A. Is the environment near home and school associated with physical activity and adiposity of urban preschool children? J Urban Health. December 2011;88(6):1143–57. doi: 10.1007/s11524-011-9604-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rundle A, Neckerman KM, Freeman L, et al. Neighborhood food environment and walkability predict obesity in New York City. Environ Health Perspect. March 2009;117(3):442–7. doi: 10.1289/ehp.11590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin D. Multiple imputtion for nonresponse in surveys. Wiley; 1987. [Google Scholar]
- 45.Demark-Wahnefried W, Schmitz KH, Alfano CM, et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J Clin. January 2018;68(1):64–89. doi: 10.3322/caac.21441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen RE, Potosky AL, Reeve BB, et al. Validation of the PROMIS physical function measures in a diverse US population-based cohort of cancer patients. Qual Life Res. October 2015;24(10):2333–44. doi: 10.1007/s11136-015-0992-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regenbogen SE, Veenstra CM, Hawley ST, et al. The personal financial burden of complications after colorectal cancer surgery. Cancer. October 1 2014;120(19):3074–81. doi: 10.1002/cncr.28812 [DOI] [PubMed] [Google Scholar]
- 48.Pearce A, Tomalin B, Kaambwa B, et al. Financial toxicity is more than costs of care: the relationship between employment and financial toxicity in long-term cancer survivors. J Cancer Surviv. February 2019;13(1):10–20. doi: 10.1007/s11764-018-0723-7 [DOI] [PubMed] [Google Scholar]
- 49.Pisu M, Azuero A, Benz R, McNees P, Meneses K. Out-of-pocket costs and burden among rural breast cancer survivors. Cancer Med. March 2017;6(3):572–581. doi: 10.1002/cam4.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegel RL, Jakubowski CD, Fedewa SA, Davis A, Azad NS. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am Soc Clin Oncol Educ Book. March 2020;40:1–14. doi: 10.1200/EDBK_279901 [DOI] [PubMed] [Google Scholar]
- 51.Gupta D, Lis CG, Grutsch JF. Perceived cancer-related financial difficulty: implications for patient satisfaction with quality of life in advanced cancer. Support Care Cancer. September 2007;15(9):1051–6. doi: 10.1007/s00520-007-0214-2 [DOI] [PubMed] [Google Scholar]
- 52.Bortolato B, Hyphantis TN, Valpione S, et al. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer treatment reviews. January 2017;52:58–70. doi: 10.1016/j.ctrv.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 53.Weaver KE, Rowland JH, Bellizzi KM, Aziz NM. Forgoing medical care because of cost: assessing disparities in healthcare access among cancer survivors living in the United States. Cancer. July 15 2010;116(14):3493–504. doi: 10.1002/cncr.25209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. January 19 2011;103(2):117–28. doi: 10.1093/jnci/djq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ekwueme DU, Yabroff KR, Guy GP Jr., et al. Medical costs and productivity losses of cancer survivors--United States, 2008–2011. MMWR Morb Mortal Wkly Rep. June 13 2014;63(23):505–10. [PMC free article] [PubMed] [Google Scholar]
- 56.Hastert TA, Kyko JM, Reed AR, et al. Financial Hardship and Quality of Life among African American and White Cancer Survivors: The Role of Limiting Care Due to Cost. Cancer Epidemiol Biomarkers Prev. July 2019;28(7):1202–1211. doi: 10.1158/1055-9965.EPI-18-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hastert TA, Ruterbusch JJ, Nair M, et al. Employment Outcomes, Financial Burden, Anxiety, and Depression Among Caregivers of African American Cancer Survivors. JCO Oncol Pract. March 2020;16(3):e221–e233. doi: 10.1200/JOP.19.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shariff-Marco S, Von Behren J, Reynolds P, et al. Impact of Social and Built Environment Factors on Body Size among Breast Cancer Survivors: The Pathways Study. Cancer Epidemiol Biomarkers Prev. April 2017;26(4):505–515. doi: 10.1158/1055-9965.EPI-16-0932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fitzgerald TL, Lea CS, Brinkley J, Zervos EE. Colorectal cancer outcome inequalities: association between population density, race, and socioeconomic status. Rural Remote Health. 2014;14(3):2668. [PubMed] [Google Scholar]
- 60.McDougall JA, Banegas MP, Wiggins CL, Chiu VK, Rajput A, Kinney AY. Rural Disparities in Treatment-Related Financial Hardship and Adherence to Surveillance Colonoscopy in Diverse Colorectal Cancer Survivors. Cancer Epidemiol Biomarkers Prev. November 2018;27(11):1275–1282. doi: 10.1158/1055-9965.EPI-17-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaix B, Rosvall M, Lynch J, Merlo J. Disentangling contextual effects on cause-specific mortality in a longitudinal 23-year follow-up study: impact of population density or socioeconomic environment? Int J Epidemiol. June 2006;35(3):633–43. doi: 10.1093/ije/dyl009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


