Abstract
Background
The VENUS trial demonstrated that adding vein of Marshall (VOM) ethanol infusion to catheter ablation (CA) improves ablation outcomes in persistent atrial fibrillation (AF). There was significant heterogeneity in the impact of VOM ethanol infusion on rhythm control.
Objective
To assess the association between outcomes and: 1) achievement of bidirectional perimitral conduction block, and 2) procedural volume.
Methods
VENUS randomized patients (n=343) with persistent AF to CA combined with VOM ethanol, or CA alone. Primary outcome (freedom from AF or tachycardia -AT- longer than 30s after a single procedure) was analyzed by 2 categories: 1) Successful vs no perimitral block. 2) High- (>20 patients enrolled) vs low-volume centers.
Results
In patients with perimitral block, the primary outcome was reached 54.3% after VOM-CA and 37% after CA alone (P=0.01). Among patients without perimitral block, freedom from AF/AT after VOM-CA was 34.0% and 37.0% after CA (P=0.583). In high volume centers, the primary outcome was reached in 56.4% after VOM-CA and 40.2% after CA (P=0.01). In low volume centers, freedom from AF/AT was 30.77% after VOM-CA and 32.61% after CA (P=0.84). In patients with successful perimitral block from high volume centers, the primary outcome was reached in 59% after VOM-CA and 39.1% after CA in (P=0.01). Tests for interaction were significant (P=0.002 for perimitral block and P=0.04 for center volume).
Conclusion
Adding VOM ethanol infusion to CA has greater impact on outcomes when associated with perimitral block and performed in high-volume centers. Perimitral block should be part of VOM procedure.
Keywords: Catheter ablation, Ethanol, Mitral isthmus, Vein of Marshall, Persistent atrial fibrillation
Introduction
Catheter ablation (CA) of persistent atrial fibrillation (AF) has suboptimal results.1 Ablation lesions beyond pulmonary vein isolation -the procedural strategy for paroxysmal AF,2 did not lead to improved outcomes in two clinical trials.3, 4
Arrhythmogenesis originating from the Ligament and Vein of Marshall (VOM) has been associated with AF generation and maintenance. Retrograde balloon cannulation and ethanol infusion in the VOM creates a local ablation,5 eliminates VOM innervation,6 and AF triggers.7 VOM ethanol is uniquely suited to facilitate mitral isthmus ablation,8 and eliminate or prevent perimitral reentry, a common form of ablation failure.
The VENUS trial enrolled patients with persistent AF undergoing their first ablation and demonstrated that VOM ethanol infusion as an adjunct to CA, compared to CA alone, significantly increased the likelihood of remaining free of AF or atrial tachycardia at 6 and 12 months.9
Understanding the factors that may influence the clinical impact of adding VOM ethanol infusion to CA is important. Given the VOM anatomical location in the mitral isthmus, we hypothesized that successful mitral isthmus ablation -as proven by bidirectional conduction block- could have impacted the ablation results.
Ethanol injection into the VOM requires a blend of procedural skills belonging to both interventional cardiology and electrophysiology, and the operator’s familiarity with the procedure’s workflow may impact the outcome of VOM ethanol ablation. In VENUS, there was significant variation in the enrollment volume between centers that could have impacted overall ablation success.
Here we performed a secondary analysis focusing on the impact of achievement of bidirectional perimitral block and of procedural volume on the outcome benefits of adding VOM ethanol infusion to CA.
Methods
Study design
This is a post-hoc analysis from the VENUS trial. Its design and results have been published.9, 10 Briefly, VENUS was a multicenter, randomized clinical trial comparing the rhythm-control effectiveness of two ablation strategies: CA alone or combined with VOM ethanol infusion (VOM-CA) in de novo ablation of persistent AF. The trial was sponsored by the NIH/NHLBI and approved by the Institutional Review Boards of each center, was overseen by the FDA (IND#115,060). Patients were randomly assigned in a 1:1.15 ratio to either CA alone, or VOM-CA. Patients were blinded to randomization outcome, as were the committees evaluating adverse events and electrocardiographic data.
Patients randomized to VOM-CA underwent the VOM procedure before CA. CA followed in the same procedure. Procedural details have been previously described.10
Participants
Patients were recruited from 12 centers in the United States. Patients were eligible if they were between ages 18 and 85 years of age, had symptomatic persistent AF (sustained AF lasting more than 7 days) refractory to at least one antiarrhythmic agent. Participants were excluded if they had previous AF ablation attempts, left atrial diameter or volume exceeding 65 mm or 200 mL, respectively.
Assessments
Clinical assessments, and 12-lead electrocardiograms were obtained at baseline and at 1, 3, 6, 9, and 12 months after the initial ablation. Patients underwent continuous 1-month monitoring (MediLynx, Dallas, TX) at 6 and 12 months after ablation. When present, data from implanted rhythm-monitoring devices (at least 30 days of data from pacemakers, defibrillators, or implanted loop recorders) replaced external monitor data.
During the first 3 months after the randomization procedure (blanking period), recurrent AF/atrial tachycardia was treated with antiarrhythmic drugs or cardioversion as needed, and not considered treatment failure.1
Outcomes
The primary outcome was freedom from AF/atrial tachycardia (AT) lasting longer than 30 seconds after the performance of a single procedure (VOM-CA or CA alone), without the use of antiarrhythmic medications and occurring after the blanking period, including 1-month monitoring at 6 and 12 months. The main secondary endpoint reported here is AF burden, which includes the percentage of time in AF or AT recorded during monitoring.
For the present analysis, we analyzed the study population by the following categories:
Bidirectional perimitral block at the end of the procedure, as reported by the operator, based on differential pacing across the mitral isthmus ablation lesion.
Center’s enrollment volume. We compared centers that performed high vs. low volumes of procedures during the trial. We defined low volume centers as those whose individual enrollment contributed <5% of the study population. Figure S1 shows the distribution of enrolling centers.
Statistical analysis
The primary analysis for efficacy was based on a two-tailed hypothesis test for equality of two independent success proportions. Primary outcome analyses were performed on the as-randomized population. Additionally, a pre-specified analysis excluding patients with failed VOM procedures (as-treated analysis) was also performed. Patient deaths were classified as procedure failures; cases missing primary outcomes were also assumed to be failures. Crude odds ratios for primary outcome failures were determined using Woolf-based 95% confidence intervals (CI). Significance threshold was ≤ 0.05 using two-sided testing.
Data are reported as mean (standard deviation). Two-tailed t-tests were employed for equality of means hypothesis testing. Bartlett’s test was used to test for heteroscedasticity among both treatment arms. If significant, a Welch t-test was performed, for which the degrees of freedom were modified. Categorical data were compared using Chi-squared contingency table analysis. Logistic regression was used to assess interactions between sub-group membership and treatment arm. Confidence intervals (95%) were based on the exponent of the univariate regression coefficient ± 1.96 representing membership (0,1) in the specific sub-group vs all remaining sub-groups. All analyses were performed with the software IBM SPSS Statistics for Macintosh, Version 25.0 (IBM Corp. Released 2017. Armonk, NY: IBM Corp).
Kaplan-Meier analysis.
Kaplan-Meier (KM) analysis was performed for time-to-event estimates of the primary outcome. The log-rank test was used to determine significance between the VOM-CA and CA treatment groups, performed by subgroups as described. Log-rank chi-squared values >3.84 (1 d.f.) were assumed to be significant at the α=0.05 level for all KM tests. Cox proportional hazards regression analysis was used to calculate crude hazard ratios. The proportional hazards (PH) assumption based on Schoenfeld residuals was not significant.
Results
Baseline clinical characteristics in different subgroups
Th study population included 343 patients, of which 185 were randomly assigned to VOM-CA, and 158 to CA.
Figure 1 shows the clinical trial conduct. Of 343 patients enrolled, 245 (71.4%) were recruited in high volume centers, and 98 (28.6%) were recruited in low volume centers.
Figure 1.
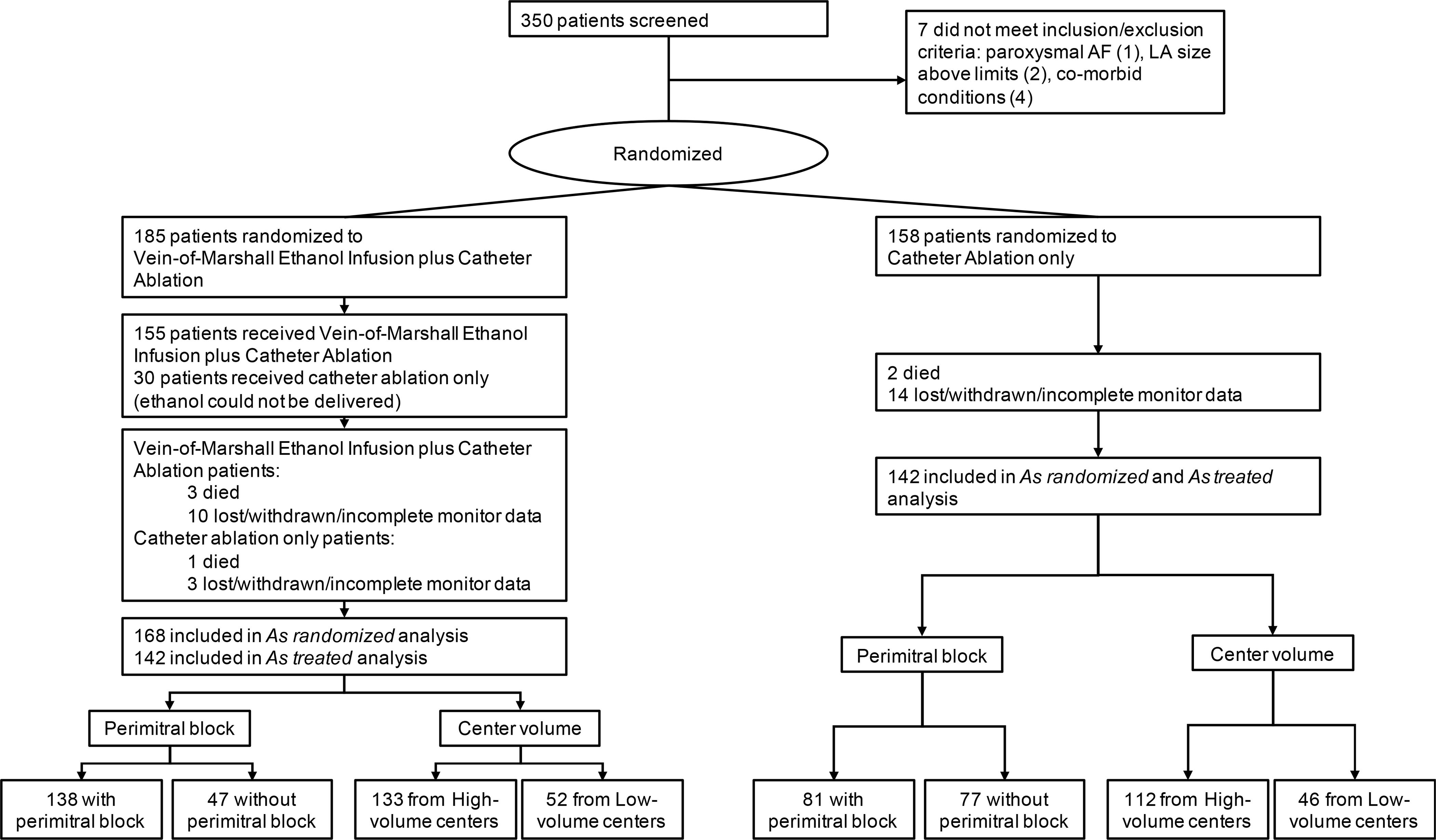
Clinical trial conduct.
Enrollment, randomization of patients and follow up per different groups. AF: atrial fibrillation; LA: left atrium
Successful perimitral block was obtained in 219 (63.8%) trial patients, and 124 (36.2%) had no perimitral block at the end of the ablation. Of the 219 patients with perimitral block, 63% were in the VOM-CA group, and 37% in the CA group.
Baseline characteristics in different groups are shown in Table 1. No differences were found between the groups with and without perimitral block, with the exception of a higher prevalence of prior heart failure in the patients with perimitral block. Compared to low volume centers, high volume centers had more women, higher CHADS-VASC scores and more patients had heart failure.
Table 1.
Patient’s demographic characteristics by group.
| Perimitral block | No perimitral block | P Value | High volume centers | Low volume centers | P Value | |
|---|---|---|---|---|---|---|
| Characteristic | (n=219) | (n=124) | (n=244) | (n=99) | ||
| Age, mean (SD), y | 66.6 (10) | 66.4 (9.3) | 0.89 | 66.6 (10.2) | 66.4(8.7) | 0.85 |
| Male sex - no. (%) | 157 (71.7) | 99 (79.8) | 0.1 | 173 (70.6) | 83 (84.7) | 0.006 |
| Race- no. (%) | 0.44 | 0.29 | ||||
| White | 198 (90.4) | 118 (95.2) | 224 (91%) | 92 (94%) | ||
| Black | 6 (2.7) | 1 (0.8) | 5 (2%) | 2 (2%) | ||
| Hispanic | 14 (6.5) | 2 (1.6) | 13 (5.4%) | 3 (3%) | ||
| Asian | 3 (1.4) | 0 (0) | 1 (0.4%) | 2 (2%) | ||
| Not stated | 9 (4.2) | 5 (4) | 12 (5%) | 2 (2%) | ||
| Medical history and risk factors- no. (%) | ||||||
| Hypertension | 149 (69.6) | 97 (80.2) | 0.04 | 184 (75.4) | 62 (68.1) | 0.21 |
| Diabetes | 50 (23.4) | 32 (26.4) | 0.53 | 64 (26.2) | 18 (19.8) | 0.25 |
| Coronary disease | 57 (26.9) | 35 (28.9) | 0.69 | 71 (29.3) | 21 (23.1) | 0.25 |
| Stroke-TIA | 29 (13.6) | 11 (9.3) | 0.26 | 33 (13.5) | 7 (8.0) | 0.19 |
| Heart failure | 67 (31.3) | 24 (19.8) | 0.03 | 79 (32.4) | 12 (13.2) | <0.001 |
| Body mass Index (kg/m2) a | 30.8 (6.5) | 32.3 (7.3) | 0.06 | 31.4 (6.8) | 31.2 (6.8) | 0.73 |
| CHADS-VASC Score b | 2.8 (1.7) | 2.61 (1.6) | 0.24 | 2.9 (1.7) | 2.3 (1.4) | 0.003 |
| Cardiac parameters | ||||||
| Ejection fraction (%) | 52.37 (9.6) | 53.4 (10) | 0.38 | 52.3 (10) | 53.9 (9.1) | 0.2 |
| Left atrial diameter (mm) | 40.5 (15.3) | 41.8 (14.2) | 0.47 | 39.3 (16.2) | 45.8 (8.6) | 0.001 |
| Left atrial volume (ml) | 115 (47.1) | 119.4 (55.5) | 0.5 | 119.3 (48.8) | 109.2 (53.3) | 0.12 |
| Time from first AF diagnosis | 1 | |||||
| <6 months- no. (%) | 16 (7.3) | 9 (7.3) | 18 (7.3) | 7 (7.1) | ||
| 6 months to 2 years - no. (%) | 90 (41.1) | 51 (41.1) | 92 (37.6) | 49 (50.0) | ||
| >2 years- no. (%) | 113 (51.6) | 64 (51.6) | 135 (55.1) | 42 (42.9) | ||
| Longstanding Persistent AF- no. (%) c | 117 (53.2) | 64 (51.6) | 0.75 | 107 (43.7) | 55 (56.1) | 0.04 |
| Arm | 0.001 | 0.84 | ||||
| CA | 81 (37) | 77 (62.1) | 112 (45.7) | 46 (46.9) | ||
| VOM-CA | 138 (63) | 47 (37.9) | 133 (54.3) | 52 (53.10) | ||
Abbreviations: AF, atrial fibrillation; CHA2DS2-VASc, congestive heart failure, hypertension, age>75 years (doubled), diabetes, stroke/TIA/thromboembolism (doubled), vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque), age 65 to 75 years, sex category (female); TIA, transient ischemic attack.
Calculated as weight in kilograms divided by height in meters squared.
CHA2DS2-VASc score is a clinical estimation of the risk of stroke in patients with AF. Scores range from 0 to 9, and higher scores indicate a greater risk.
Longstanding persistent AF: continuous AF lasting for 1 year or more.
Overall outcomes
In the overall population, freedom from any clinical AF/AT, or >30 seconds of AF/AT on monitoring at 6 and 12 months, or repeat procedures, without the use of antiarrhythmic drugs was reached in 38% (60/158) of patients randomized to CA and in 49.2% (91/185) of patients randomized to VOM-CA (P=0.04), with an odds ratio (OR) of 0.63 (CI: 0.4–0.97). Excluding patients in whom VOM ethanol infusion was attempted but not completed (as treated analysis), ablation success was achieved in 80/155 of the VOM-CA group (51.6%, P=0.02), with an OR of 0.57 (CI: 0.4–0.9).
Table 2 shows all results by different categories.
Table 2:
Primary outcome by both center volume and presence of perimitral block.
| Number | Vein of Marshall-Catheter Ablation No (%) | Catheter Ablation No (%) | Odds Ratio (95% CI) | P-value | |
|---|---|---|---|---|---|
| As randomized | |||||
| Perimitral block all centers | 219 | 75/138 (54.3) | 30/81 (37) | 0.49 (0.28 – 0.87) | 0.01 |
| No perimitral block all centers | 124 | 16/47 (34.0) | 30/77 (37) | 1.24 (0.58–2.64) | 0.58 |
| High volume centers | 245 | 75/133 (56.39) | 45/112 (40.18) | 0.52 (0.32 – 0.86) | 0.01 |
| Low Volume centers | 98 | 16/52 (30.77) | 15/46 (32.61) | 1.09 (0.46 – 2.55) | 0.85 |
| High volume center – Perimitral block | 181 | 69/117 (59) | 25/64 (39.1) | 0.45 (0.24 – 0.83) | 0.01 |
| High volume center – No perimitral block | 64 | 6/16 (37.5) | 20/48 (41.7) | 1.19 (0.37 – 3.81) | 1.19 |
| Low volume center – Perimitral block | 38 | 6/15 (28.6) | 5/17 (29.4) | 1.04 (0.25 – 4.26) | 0.95 |
| Low volume center – No perimitral block | 60 | 10/31 (32.3) | 10/29 (34.5) | 1.1 (0.38 – 3.23) | 0.85 |
| As treated | |||||
| Perimitral block all centers | 207 | 71/126 (56.3) | 30/81 (37) | 0.46 (0.26 – 0.81) | 0.007 |
| No perimitral block all centers | 106 | 9/29 (31) | 30/77 (39 | 1.42 (0.57 – 3.52) | 0.45 |
| High volume centers | 232 | 70/120 (58.3) | 45/112(40.18) | 0.48 (0.28 – 0.81) | 0.006 |
| Low Volume centers | 81 | 10/35 (28.6) | 15/46 (32.61) | 1.2 (0.46 – 3.15) | 0.70 |
| High volume center – Perimitral block | 171 | 66/107 (61.7) | 25/64 (39.1) | 0.4 (0.21 – 0.75) | 0.004 |
| High volume center – No perimitral block | 61 | 4/13 (30.8) | 20/48 (41.7) | 1.6 (0.43 – 5.9) | 0.48 |
| Low volume center – Perimitral block | 36 | 5/19 (26.3) | 5/17 (29.4) | 1.17 (0.27 – 5.02) | 0.84 |
| Low volume center – No perimitral block | 45 | 5/16 (31.3) | 10/29 (34.5) | 1.16 (0.31 – 4.27) | 0.83 |
Primary outcome according to perimitral block
Successful perimitral block was achieved in 74% (137/185) of patients randomized to VOM-CA and in 51.4% (81/158) of patients in the CA group (p<0.001, OR 2.7, 95% CI 1.7–4.2). Perimitral block was obtained in 80.6% (125/155) in VOM-CA patients successfully completing VOM ethanol infusion procedure (as treated, P<0.001, OR 3.9, 95% CI 2.4–6.5). Of note, coronary sinus (CS) ablation was performed in 29.7% of patients in the VOM-CA group vs 46.8% of the patients in the CA group (p<0.001).9
Independent of the randomization group, the primary end point occurred in 105 of 219 patients who had successful perimitral block (47.9%) and in 46 of 124 patients with no perimitral block (37.1%) (P=0.05, OR 0.64, 95% 0.41–1.0).
In patients randomized to VOM-CA who had successful perimitral block, the primary endpoint was achieved in 75 of 138 (54.3%), compared to 30 of 81 (37%) of patients randomized to CA with successful perimitral block (P=0.01, OR 0.49, 95% CI 0.28–0.87).
In patients randomized to VOM-CA in whom perimitral block was not achieved, the primary endpoint occurred in 16 of 47 (34.0%), compared to 30 of 77 (37.0%) of those randomized to CA (P=0.583, OR 1.24, 95% CI 0.58 – 2.64).
The effect of VOM-CA vs CA on the primary end point was significantly different depending on whether perimitral block was achieved or not (P for interaction 0.002). Outcomes differences were more pronounced excluding patients with failed VOM ethanol procedure (as treated analysis, Table 2 and Figure 2).
Figure 2.
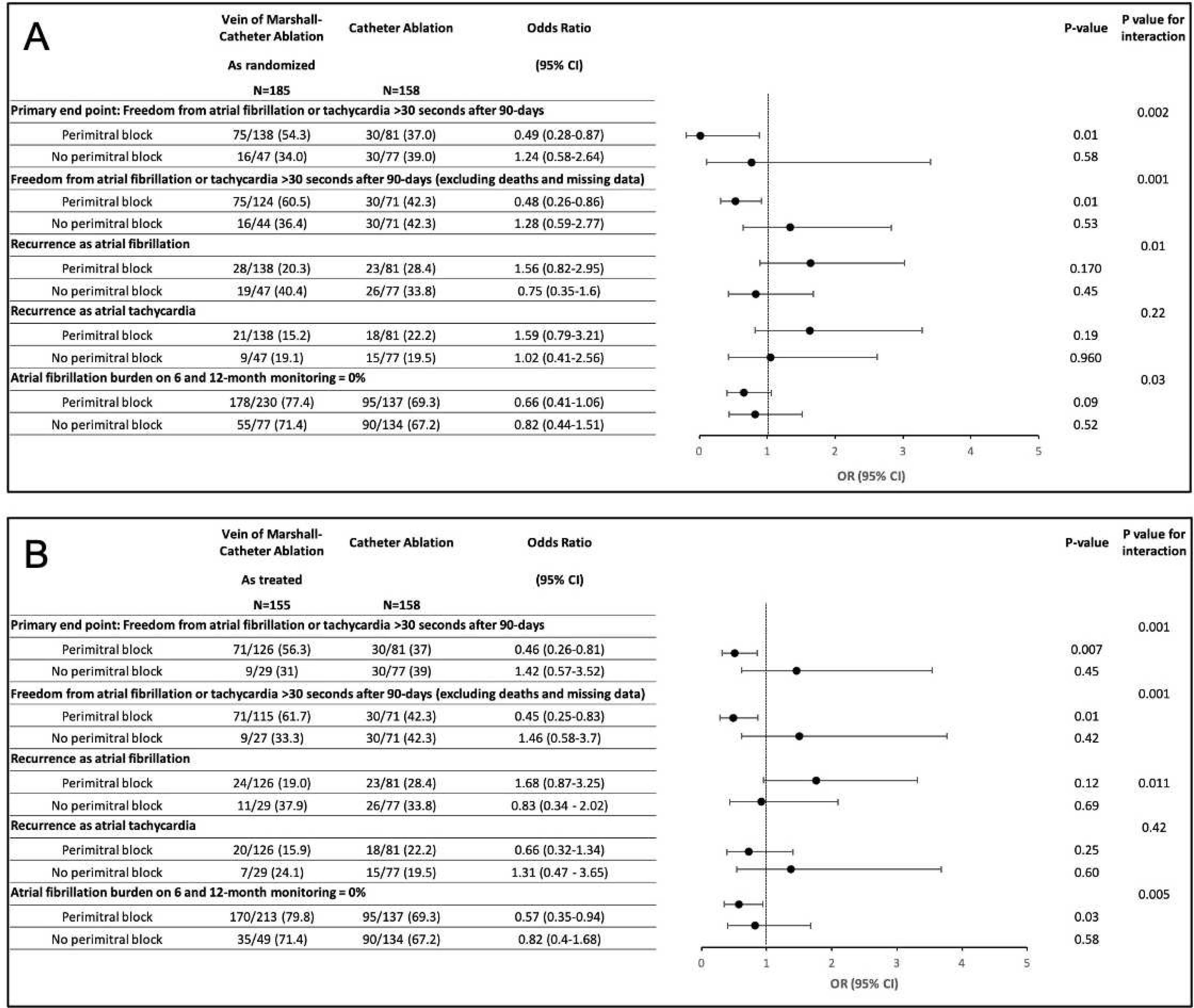
Association of perimitral block with clinical outcomes in as randomized (A) and as treated (B) analysis.
Primary outcome by enrolling center volume
In the high volume center group, we included two centers that enrolled a total of 245 patients (71% of the total). Each of the 9 other centers preformed 5% or less of the cases and were included in the low volume center group (Figure s1). Overall, in 30 patients randomized to VOM-CA, the VOM ethanol injection procedure was not completed due to failure to cannulate the VOM (success in 83.7%). Of those, 17 were from low volume centers and 13 were from high volume centers. The failure rates were 32.7% in low volume centers and 9.8% in high volume centers, (P<0.001; Figure s2).
Independent of the randomization group, the primary endpoint occurred in 31 of 98 patients from low volume centers (31.6%) and in 120 of 245 patients in high volume centers (49%) (P=0.04, OR 0.45, 95% CI 0.26–0.7). In patients randomized to VOM-CA at high volume centers, the primary endpoint occurred in 75 of 133 (56.4%), compared to 45 of 112 (40.2%) patients randomized to CA (P=0.01, OR 0.52, 95% CI 0.31–0.86). In low volume centers, the primary end point occurred after VOM-CA in 16 of 52 (30.77%) and after CA in 15 of 46 (32.61 %) (P=0.84, OR 1.09, 95% CI 0.46–2.55). The effect of VOM-CA vs CA on the primary end point was significantly different between the high vs low volume centers (P for interaction 0.04). Outcomes differences were more pronounced excluding patients with failed VOM ethanol procedure (as treated analysis, Table 2, Figure 3).
Figure 3.
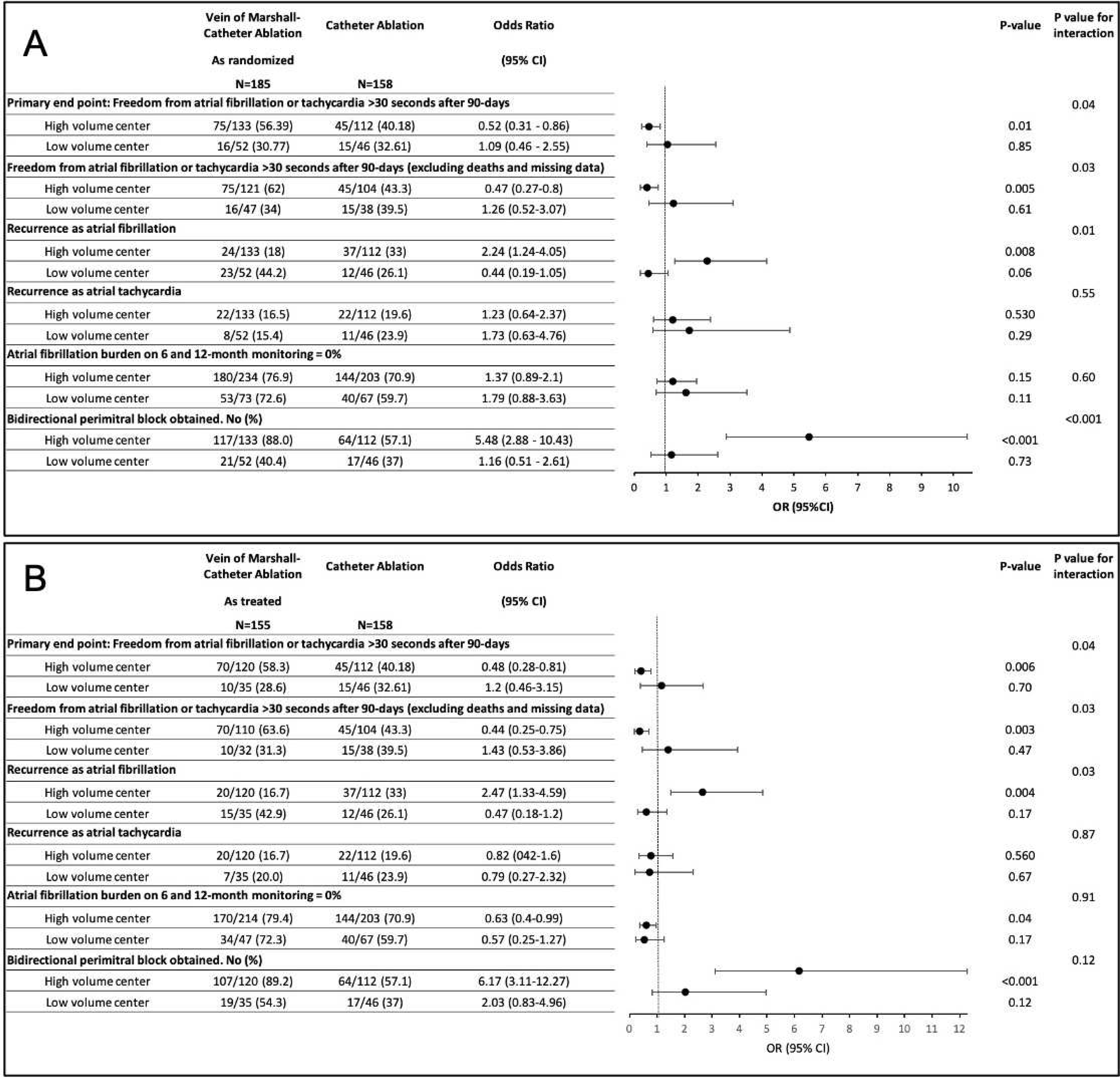
Association of center’s volume with clinical outcome in as randomized (A) and as treated (B).
Combined high-volume center and perimitral block
Independent of the randomization group, 52.8% (181/343) patients were treated in high volume centers and had successful perimitral block. The primary endpoint was reached in 51.9% in those patients (94/181). That was significantly higher than patients with no perimitral block or from low volume center (See Table 2).
In patients randomized to VOM-CA who were from high volume centers and had successful perimitral block, the primary end point occurred in 69 of 117 (59%), compared to in 25 of 64 (39.1%) of those randomized to CA (P=0.01, OR 0.45, 95% CI 0.24–0.83). Excluding patients with unsuccessful VOM infusion (as treated), the primary endpoint was reached in 66 of 107 (61.7%) of the VOM-CA patients, (P=0.005, OR 0.4, 95% CI 0.21–0.75). See Table 2.
Primary endpoint components were consistent with a reduction in recurrence of AF in the VOM-CA group, more pronounced than the reduction in atrial tachycardia recurrences. Subgroups from high-volume centers as well with bidirectional perimitral block had greater reductions in AF than atrial tachycardia (Figure 2 and 3).
Secondary outcomes
AF burden was variable in both groups. Among patients with successful perimitral block, zero burden of AF or tachycardia was achieved in 77.4% (178/230) of monitoring sessions in the VOM-CA group and in 69.3% (95/137) in the CA group (P=0.09). In patients with successful VOM ethanol infusion (as treated), 79.8% (170/213) monitoring sessions had zero AF burden (P=0.03). Among patients without perimitral block, zero burden was achieved in 71.4% (55/77) of monitoring sessions in the VOM-CA group, and in 67.2% (90/134) in the CA group, (P=0.52) and in 71.4% (35/49) of those undergoing successful VOM infusion procedures (as treated, P=0.58).
In the high volume centers, freedom from any AF/AT on 1-month monitoring (zero burden, including after repeat procedures or antiarrhythmic drugs), was achieved in 76.9% (180/234) monitoring sessions of patients randomized to VOM-CA, and in 70.9% (144/203) of monitoring sessions in the CA group (P=0.15). In patients who underwent successful VOM ethanol infusion (as treated), 79.44% (170/214) of monitoring sessions had zero burden (P=0.04).
In the low volume centers, zero burden was achieved in 72.6% (53/73) in the VOM-CA group compared to 59.7% (40/67) of monitoring sessions in the CA group (P=0.11). In patients who underwent successful VOM ethanol infusion procedures (as treated), 72.3% (34/47) of monitoring sessions had zero burden (P=0.17) (figure 3s).
Time-to-event analyses are shown in Figure 4 and 5. Among patients with successful perimitral block, KM analyses showed significant reduction in AF/atrial tachycardia recurrence in VOM-CA group, as randomized (P=0.03, HR 0.63, CI=0.42 − 0.96), as well as in as treated analysis (P=0.02, HR 0.58, CI=0.39 − 0.92). For patients without perimitral block no significant reduction was noted.
Figure 4.
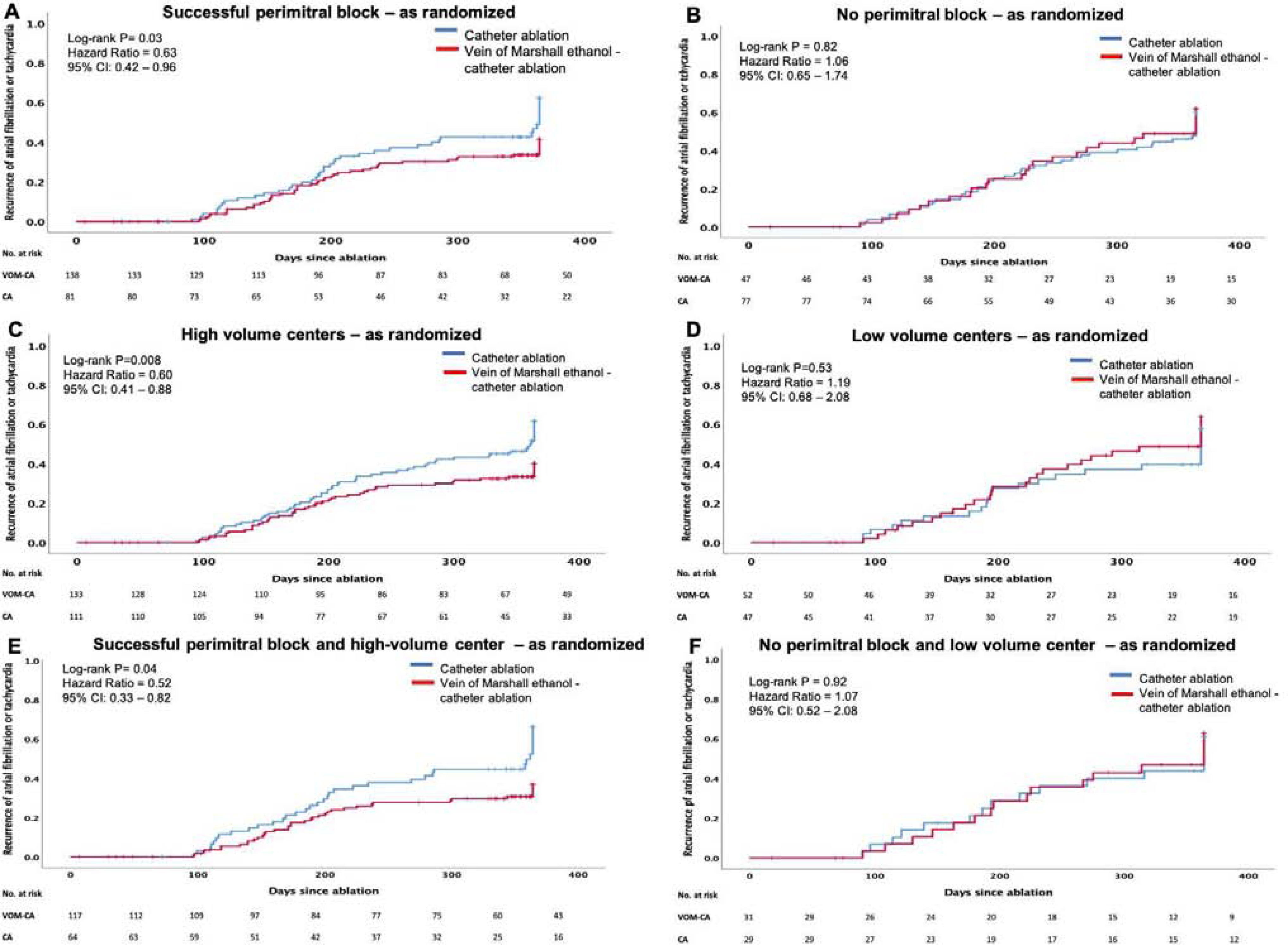
Time to recurrence of atrial fibrillation or tachycardia - as randomized.
A. Successful perimitral block, B. No perimitral block, C. High volume centers, D. Low volume centers, E. Combination of successful perimitral block and high volume center and F. Combination of no perimitral block and low volume center.
Figure 5.
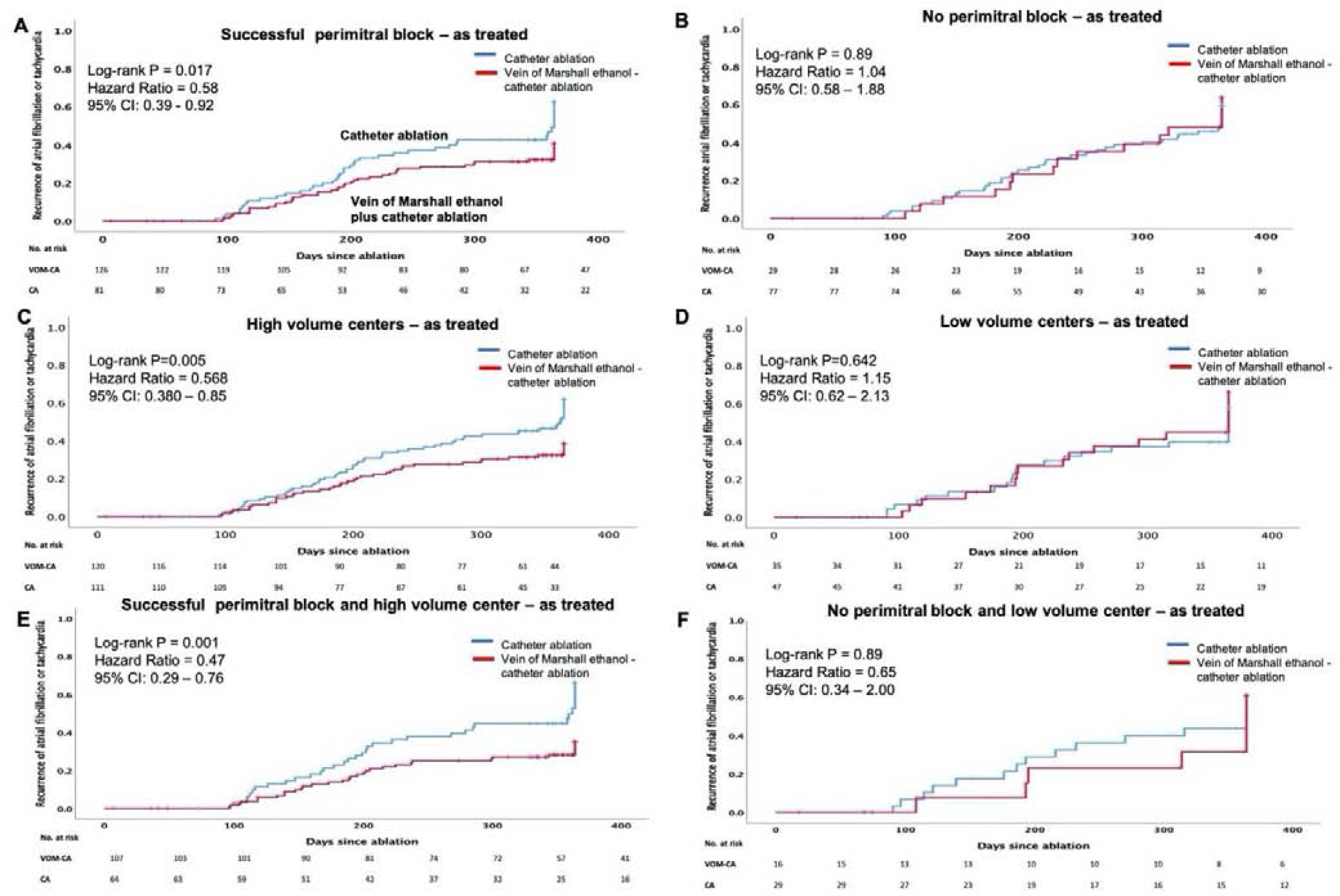
Time to recurrence of atrial fibrillation or tachycardia - as treated.
A. Successful perimitral block, B. No perimitral block, C. High volume centers, D. Low volume centers, E. Combination of successful perimitral block and high volume center and F. Combination of no perimitral block and low volume center.
In high volume centers, there was significant reduction in AF/atrial tachycardia recurrence in the VOM-CA group, in as randomized (P=0.008, HR 0.602, CI=0.41 − 0.88), as well as in as treated analysis (P=0.005, HR 0.568, CI=0.38 − 0.85). For low volume centers no significant reduction was noted.
In patients deriving from high-volume centers in whom perimitral block was achieved, KM plots showed the greatest reduction in AF/atrial tachycardia recurrence in VOM-CA group compared to CA alone (P=0.04, HR 0.52, CI=0.33−0.82), in as randomized well as in as treated analysis (P=0.001, HR 0.47, CI=0.29−0.76).
Discussion
The results of the VENUS trial indicated a favorable impact of VOM ethanol infusion when added to CA of persistent AF; this impact was greater when the VOM ethanol procedure was successfully completed (as treated). In this secondary analysis, we found that VOM ethanol infusion was associated with greater benefits in rhythm control when associated with successful achievement of perimitral block, and when performed at a high-volume center.
Potential therapeutic mechanisms of VOM ethanol are multiple, related to regional myocardial5 and/or neural6 ablation. For these to occur, adequate ethanol delivery to the VOM is necessary. The VOM has substantial variability in size, location, and extent of branching patterns11 that can impact the extent of myocardial and neural ethanol effects. In most cases, the location of ethanol-ablated tissue extends from the most proximal balloon infusion (usually in the VOM take-off from the CS) to variable lengths of the left atrial ridge, anterior to the left pulmonary veins, and area the coincides with the posterior mitral isthmus, commonly ablated to treat or prevent perimitral reentrant atrial tachycardia.12
Mechanistically, achieving perimitral block can be conceived as a marker of a complete ablation in the mitral isthmus. Achieving perimitral block was associated with improved rhythm control regardless of the randomization group. However, the differences in outcomes were greater in the VOM group than in the controls, suggesting that, although perimitral block may be important in determining the impact of VOM ethanol in rhythm control, it may not be the only factor, since perimitral block obtained by VOM ethanol led to better rhythm control than when it was obtained by conventional radiofrequency. Other studies3, 4 have failed to show benefit of ablating the mitral isthmus with radiofrequency. Incomplete mitral isthmus ablation is common, as is conduction recovery in the mitral isthmus.13 It is possible that VOM ethanol leads to a more reliable mitral isthmus ablation, as recently reported,14 less likely to recover conduction. Alternatively, perimitral block may simply be a marker of a more robust ablation of other neural or myocardial AF substrates in the region and not related to local block. The fact that the impact on outcomes was primarily due to reductions in AF recurrence -and not atrial tachycardia, which would have included perimitral flutter- suggests this scenario.
Achieving perimitral block by isolated VOM ethanol infusion is rare.8 Although VOM ethanol infusion leads to a substantial epicardium-to-endocardium ablation in the mitral isthmus, it is not expected to be complete. Given the VOM’s take-off from the posterior wall of the CS and the balloon length, the isthmus’ most annular aspect (including the CS) is unaffected by ethanol and requires conventional radiofrequency ablation.8 Our data support the practical conclusion that, in order to achieve best results of the VOM procedure, a complete ablation of the mitral isthmus -as indicated by bidirectional perimitral block- is necessary.
Rates of unsuccessful completion of the VOM procedure in high-volume centers were less then 10%, more than 3 times lower than in low-volume centers. Since operator training was similar in all centers, it is likely that a critical threshold of experience is needed to complete the procedure reliably. This limits the generalizability of the overall favorable impact of the technique. An important consideration is that in the CA group, success rates where comparable between high- and low-volume centers, which indicates that only the VOM procedure impact was affected by procedural volume.
Overall, the best outcomes were obtained in patients from high-volume centers in whom the VOM procedure was completed (as treated), and perimitral block was obtained. In this subgroup of patients, the benefits of VOM ethanol infusion were most marked (as treated, an absolute difference of 20% success, with a reduction in failure by 60%) suggesting that when technical aspects of VOM are not compromised and the full extent of ethanol effect is achieved in the mitral isthmus, the favorable impact is substantial.
Limitations
The analyses reported here are post-hoc, multiple comparisons. Therefore, results should be considered hypothesis-generating. Given the non-universal feasibility of the VOM procedure, the results are only applicable to ~83% of the patients in whom it is attempted.
The lower success rates in low center volume subgroups, or in patients randomized to VOM-CA without perimitral block have to be interpreted with caution, given the small numbers in these categories. Additionally, there were differences in baseline characteristics -most notably the number of patients with longstanding persistent AF- that could have influenced the results. Perimitral block is a post-randomization characteristic, therefore, it cannot be considered a proper sub-group analysis. However, there is no reason to suspect that randomization to VOM-CA would predispose to perimitral block success for any reason other than the therapeutic effect of ethanol. Achieving perimitral block can be difficult. In the CA group, perimitral block was achieved in only 51%, despite CS ablation in 46.8%. A more determined approach to achieve perimitral block in the control group could have impacted the results.
Conclusions
Among patients with persistent AF, the addition of VOM ethanol infusion to CA led to overall increased chances of remaining free of AF/AT, and the outcome benefits were most marked in patients in whom bidirectional perimitral block was achieved and in high-volume centers. The technical and clinical implications are substantial, since benefit from the VOM procedure seems to be obtained predominantly if perimitral block is achieved. Operators performing VOM procedures should consider efforts to achieve perimitral block to optimize outcome benefits.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health [NIH/NHLBI R01HL115003]; the sponsor had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Conflict of interest: All authors have none to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calkins H, Hindricks G, Cappato R, et al. 2017 hrs/ehra/ecas/aphrs/solaece expert consensus statement on catheter and surgical ablation of atrial fibrillation: Executive summary. J Arrhythm 2017;33:369–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 3.Dixit S, Marchlinski FE, Lin D, et al. Randomized ablation strategies for the treatment of persistent atrial fibrillation: Rasta study. Circ Arrhythm Electrophysiol 2012;5:287–294. [DOI] [PubMed] [Google Scholar]
- 4.Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–1822. [DOI] [PubMed] [Google Scholar]
- 5.Valderrabano M, Chen HR, Sidhu J, Rao L, Ling Y, Khoury DS. Retrograde ethanol infusion in the vein of marshall: Regional left atrial ablation, vagal denervation and feasibility in humans. Circ Arrhythm Electrophysiol 2009;2:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baez-Escudero JL, Keida T, Dave AS, Okishige K, Valderrabano M. Ethanol infusion in the vein of marshall leads to parasympathetic denervation of the human left atrium: Implications for atrial fibrillation. J Am Coll Cardiol 2014;63:1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dave AS, Baez-Escudero JL, Sasaridis C, Hong TE, Rami T, Valderrabano M. Role of the vein of marshall in atrial fibrillation recurrences after catheter ablation: Therapeutic effect of ethanol infusion. J Cardiovasc Electrophysiol 2012;23:583–591. [DOI] [PubMed] [Google Scholar]
- 8.Baez-Escudero JL, Morales PF, Dave AS, et al. Ethanol infusion in the vein of marshall facilitates mitral isthmus ablation. Heart Rhythm 2012;9:1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valderrabano M, Peterson LE, Swarup V, et al. Effect of catheter ablation with vein of marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: The venus randomized clinical trial. JAMA 2020;324:1620–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valderrabano M, Peterson LE, Bunge R, et al. Vein of marshall ethanol infusion for persistent atrial fibrillation: Venus and mars clinical trial design. Am Heart J 2019;215:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valderrabano M, Morales PF, Rodriguez-Manero M, Lloves C, Schurmann PA, Dave AS. The human left atrial venous circulation as a vascular route for atrial pharmacological therapies: Effects of ethanol infusion. JACC Clin Electrophysiol 2017;3:1020–1032. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo S, Wright M, Knecht S, et al. Peri-mitral atrial flutter in patients with atrial fibrillation ablation. Heart Rhythm 2010;7:2–8. [DOI] [PubMed] [Google Scholar]
- 13.Rostock T, O’Neill MD, Sanders P, et al. Characterization of conduction recovery across left atrial linear lesions in patients with paroxysmal and persistent atrial fibrillation. J Cardiovasc Electrophysiol 2006;17:1106–1111. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima T, Pambrun T, Vlachos K, et al. Impact of vein of marshall ethanol infusion on mitral isthmus block: Efficacy and durability. Circ Arrhythm Electrophysiol 2020;13:e008884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


