Abstract
Neurodegenerative disorders impact more than one billion individuals worldwide and are intimately tied to metabolic disease that can affect another nine hundred individuals throughput the globe. Nicotinamide is a critical agent that may offer fruitful prospects for neurodegenerative diseases and metabolic disorders, such as diabetes mellitus. Nicotinamide protects against multiple toxic environments that include reactive oxygen species exposure, anoxia, excitotoxicity, ethanol-induced neuronal injury, amyloid (Aß) toxicity, age-related vascular disease, mitochondrial dysfunction, insulin resistance, excess lactate production, and loss of glucose homeostasis with pancreatic β-cell dysfunction. However, nicotinamide offers cellular protection in a specific concentration range with dosing outside of this range leading to detrimental effects. The underlying biological pathways of nicotinamide that involve the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), the mechanistic target of rapamycin (mTOR), AMP activated protein kinase (AMPK), and mammalian forkhead transcription factors (FoxOs) may offer insight for the clinical translation of nicotinamide into a safe and efficacious therapy through the modulation of oxidative stress, apoptosis, and autophagy. Nicotinamide is a highly promising target for the development of innovative strategies for neurodegenerative disorders and metabolic disease, but the fruits of this foundation depend greatly on gaining further understanding of nicotinamide’s complex biology.
Keywords: Alzheimer’s disease, AMP activated protein kinase (AMPK), autophagy, apoptosis, dementia, diabetes mellitus, forkhead transcription factors, FoxO, mechanistic target of rapamycin (mTOR), oxidative stress, silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), sirtuin, stem cells
1. Nicotinamide
Nicotinamide is the amide form of the vitamin B3 (niacin). Nicotinamide is obtained in the body either as a dietary source and supplement, such as from animal sources or plants, or through synthesis in the body [1]. Nicotinic acid is the alternative form of the water-soluble vitamin B3 [2]. The primary form of niacin in dietary plant sources is nicotinic acid that is rapidly absorbed through the gastrointestinal epithelium [3]. Nicotinamide is then obtained through the conversion of nicotinic acid in the liver or through the hydrolysis of the coenzyme ß-nicotinamide adenine dinucleotide (NAD+). Once present in the body, nicotinamide is a precursor for the coenzyme NAD+ [4, 5]. Nicotinamide also is required for the synthesis of nicotinamide adenine dinucleotide phosphate (NADP+) [6]. Nicotinamide is changed to its mononucleotide form (NMN) with the enzyme nicotinic acid/nicotinamide adenylyltransferase. NMN is converted to the dinucleotides NAD+ and NAAD+. NAAD+ yields NAD+ through NAD+ synthase [7]. NAD+ also can be synthesized through nicotinamide riboside kinase that phosphorylates nicotinamide riboside to NMN [8, 9].
Nicotinamide through NAD+ can be directly utilized by cells to synthesize NAD+ [1, 5, 10–12]. Nicotinamide participates in energy metabolism through the tricarboxylic acid cycle by utilizing NAD+ in the mitochondrial respiratory electron transport chain for the production of ATP, DNA synthesis, and DNA repair [13–15]. These cellular pathways are critical for energy metabolism and can impact normal physiology as well as disease processes [12, 16–19]. For example, lack of nicotinamide can lead to fatigue, loss of appetite, pigmented rashes of the skin, and oral ulcerations. Severe states of deficiency lead to pellagra that is characterized by cutaneous rashes, oral ulcerations, gastrointestinal difficulties, and cognitive disability [16, 20, 21]. Pellagra can occur as a result of conditions that lead to depressed nicotinamide levels or during the inability to absorb nicotinamide. The inability to absorb tryptophan that causes Hartnup’s disease, isoniazid treatment, or carcinoid syndrome also can be associated with pellagra. Excessive alcohol consumption with poor dietary intake can result in severe nicotinamide loss and insufficient gastrointestinal absorption [22, 23].
2. Nicotinamide and Oxidative Stress
Nicotinamide can affect cellular survival and longevity through different pathways that involve oxidative stress, apoptosis, and autophagy [24]. Reactive oxygen species (ROS) are generated during oxidative stress [18, 25]. These include nitrogen based free radical species, such as nitric oxide and peroxynitrite, and oxygen derivatives involving superoxide free radicals, hydrogen peroxide, and singlet oxygen [26–28]. One source of ROS are mitochondria. Mitochondria yield adenosine triphosphate (ATP) through the oxidation of glucose, pyruvate, and NAD+ that exist in the cytosol. In the tricarboxylic acid cycle, NAD+ and flavin adenine dinucleotide (FAD) are reduced to NADH and FADH2. The redox energy from NADH and FADH2 is transferred to oxygen through the electron transport chain. This process facilitates protons to be transferred from respiratory complexes I, III, and IV in the inner membrane to the intermembrane space with a subsequent proton gradient that is formed across the inner membrane. Complex V (ATP synthase) then accumulates the energy from this gradient to produce ATP from adenosine diphosphate (ADP) and inorganic phosphate (Pi). With the aerobic production of ATP, the generation of ROS occurs [29–36].
Some studies suggest that ROS may be necessary for the promotion of extended lifespan [37]. This may require a careful balance in ROS generation that appears necessary for the generation of ROS to limit cell injury and extend lifespan. Moderate levels of ROS may be required for the tolerance against metabolic, mechanical, and oxidative stressors [38]. The generation of brief periods of ROS during ischemia-reperfusion models may limit cellular injury [39, 40] through several different pathways such as those that involve the mechanistic target of rapamycin (mTOR) [29, 41–46] or Wnt signaling [47–49]. However, at increased levels, ROS through oxidative stress can result in mitochondrial and other organelle injury, DNA damage, protein misfolding, cell demise, and the promotion of aging [50–54].
Depletion of NAD+ has been associated with aging. The maintenance of adequate NAD+ stores has been linked to a reduction in the aging process and increased resistance to oxidative stress [19]. As a result, nicotinamide through NAD+ generation may reduce ROS and prevent cellular senescence [1]. Pathways associated with nicotinamide can limit oxidative stress to increase life span [55], limit vascular disease [11, 20], alleviate mitochondrial stress [56, 57], ischemic injury [58], drug toxicity [59], and neurodegenerative disorders [60–62].
3. Nicotinamide, Apoptosis, and Autophagy
Apoptosis can ensue at elevated levels of ROS generation and involve mitochondrial dysfunction during oxidative stress [63–67]. Apoptosis has both an early and late phase [68, 69]. The early phase consists of phosphatidylserine (PS) asymmetry loss on the plasma membrane [70–72]. The later phase results in genomic DNA degradation [72, 73]. Apoptosis begins through a cascade of nuclease and protease activation that leads to caspase activation [35, 68, 74]. Mitochondrial dysfunction leads to the opening of the mitochondrial membrane permeability transition pore, release of cytochrome c, and apoptotic caspase activation [75–77]. Loss of cellular membrane PS asymmetry activates inflammatory cells to seek out cells with membrane asymmetry and remove them through engulfment [71, 78]. If this process can be prevented, then cells remain functional despite externalization of membrane PS residues [68, 72]. However, the destruction of cellular DNA is usually not considered to be a reversible process [68].
Apoptosis leads to cell death in multiple disease processes. Suppression of cellular apoptosis can increase cell survival in Alzheimer’s disease (AD) [79–82], epilepsy [79, 83, 84], retinal disease [85, 86], Parkinson’s disease (PD) [68, 81, 87], trauma [88], spinal cord injury [89, 90], and neuronal, renal, lung, and vascular cells [45, 91–93]. Apoptotic injury also can lead to long-term disability through progressive neuronal loss such as during subarachnoid hemorrhage [94, 95].
Nicotinamide can influence both phases of apoptotic cell death. Nicotinamide can prevent exposure of plasma membrane PS residues [70–72, 96–99] to prevent inflammatory cell activation [4, 24, 100–102]. Nicotinamide can limit cardiovascular injury by blocking membrane PS exposure in vascular cells [5, 101], since membrane PS residue externalization in vascular cells can lead to hypercoagulation states [103] and cellular inflammation [104, 105]. Nicotinamide can reverse a previously sustained insult. Post-treatments studies with nicotinamide that can follow apoptotic injury in “real-time” show that early cellular apoptotic injury can be reversed [5, 61, 100–102, 106].
Interestingly, it appears that a reduction in nicotinamide levels during nicotinamidase expression can sometimes lead to increased cellular survival and longevity [55, 62]. Nicotinamide can inhibit silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) by intercepting an ADP-ribosyl-enzyme-acetyl peptide intermediate with the regeneration of NAD+ (transglycosidation) [107]. Nicotinamidase expression prevents both apoptotic early PS membrane exposure and late DNA degradation. In addition, inhibition of SIRT1 activity either by pharmacological methods or siRNA gene silencing is detrimental to cell survival during oxidative stress and blocks nicotinamidase protection, further supporting that SIRT1 activity may be necessary for nicotinamidase protection during oxidative stress. It has been hypothesized that sirtuins also may prevent nicotinamide from assisting with DNA repair by altering the accessibility of DNA damaged sites for repair enzymes [108].
Other pathways of programmed cell death, such as autophagy, also may be involved during oxidative stress [12, 44, 109–114]. Autophagy can impair endothelial progenitor cells, and lead to mitochondrial oxidative and endoplasmic reticulum stress [63, 115]. However, autophagy also may be necessary for the removal of misfolded proteins and to eliminate non-functioning mitochondria [112] that has been shown to maintain β-cell function and prevent the onset of diabetes mellitus [116]. Autophagy recycles cytoplasmic organelles and components for tissue remodeling [68, 117] and can remove non-functional organelles [41, 111, 118]. Macroautophagy recycles organelles and sequesters cytoplasmic proteins into autophagosomes within cells. Autophagosomes subsequently combine with lysosomes to become degraded and begin a course for recycling [68]. Microautophagy is a process for lysosomal membrane invagination. Components of the cell cytoplasm are sequestered and digested. Chaperone-mediated autophagy is a process that depends upon cytosolic chaperones to move components of the cytoplasm across lysosomal membranes.
Autophagy also plays a significant role with several disease processes. Autophagy activation that can eliminate or sequester intracellular accumulations that lead to cell death may influence disease progression, such as in PD [79, 119–123], cognitive impairment and AD [68, 122, 124, 125], amyotrophic lateral sclerosis [126–128], Huntington’s disease (HD) [68, 129], and traumatic brain injury [79, 130, 131].
Nicotinamide is linked to SIRT1 to oversee cellular function and autophagy [30, 132–138]. SIRT1 through the transfer of the acetyl residue from the acetyllysine residue of histones to the ADP-ribose moiety of NAD+ can lead to the production of nicotinamide. SIRT1 is a histone deacetylase that can transfer acetyl groups from ε-N-acetyl lysine amino acids to the histones of deoxyribonucleic acid (DNA) to control transcription [68, 127, 135, 136, 138–148]. Physiological concentrations of nicotinamide noncompetitively inhibit SIRT1, suggesting that nicotinamide is a physiologically relevant regulator of SIRT1 enzymes [149]. As a result, in relation to cell longevity, it is the lower concentrations of nicotinamide that can function as an inhibitor of sirtuins that are necessary for the promotion of increased lifespan and cellular survival [55, 61, 62, 100, 101, 106, 150], at least in yeast and metazoans [10, 151, 152].
Nicotinamide and SIRT1 function through autophagic pathways that necessitate a tight oversight of SIRT1 activity [69, 76, 79, 111, 112, 153]. Nicotinamide can promote the delayed induction of autophagy and subsequently decreased survival in cancer cells [154]. During nicotinamide administration, mitochondrial autophagy (mitophagy) can lead to an increased NAD+/NADH ratio [18, 155, 156]. Chronic administration of nicotinamide can lead to skeletal muscle lipotoxicity and glucose intolerance during autophagy activation [156]. As an inhibitor of SIRT1, nicotinamide through autophagy can limit cancer cell growth and in combination with chemotherapeutic agents lead to apoptotic cell death [154, 157–160]. Through SIRT1 inhibition, nicotinamide may exert anti-inflammatory properties, promote SIRT activity as a result of the cellular conversion of nicotinamide to NAD+, and affect the transcriptional regulation of inflammatory genes [161]. As a result, nicotinamide has been shown to be cytoprotective through SIRT1 to prevent palmitate-induced hepatotoxicity through SIRT1-dependent induction of autophagy [162].
Nicotinamide maintains a significant relationship with the mechanistic target of rapamycin (mTOR) pathways and autophagy to influence cellular survival [30, 43, 45, 46, 52, 93, 117, 163–166]. mTOR, a 289-kDa serine/threonine protein kinase, is a vital pathway for nicotinamide to control cellular metabolism [12, 30, 41, 46, 52, 165, 167–169]. mTOR is the principal component of the protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) [170–172]. mTORC1 and mTORC2 are further divided into subcomponents. mTORC1 consists of Raptor, the proline rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR interacting protein), and mammalian lethal with Sec13 protein 8, termed mLST8 (mLST8) [173]. mTORC1 activity can be controlled through multiple pathways, such as through PRAS40, by preventing the association of p70 ribosomal S6 kinase (p70S6K) and the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) with Raptor [174, 175]. mTORC2 consists of Rictor, mLST8, Deptor, the mammalian stress-activated protein kinase interacting protein (mSIN1), and the protein observed with Rictor-1 (Protor-1) [174, 176]. mTORC2 controls cytoskeleton remodeling through PKCα and cell migration through the Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2 and through Rho signaling [177].
Nicotinamide can lead to the activation of autophagy and inhibit mTOR. During the enhanced activation of autophagy, nicotinamide can limit ß-amyloid (Aß) toxicity and improve cognition [2, 178], reduce metabolic dysfunction through the maintenance of mitochondria [12, 16, 56, 179], maintain metabolic homeostasis [31, 180, 181], block neuronal ischemic injury [182] and endothelial injury [92], and increase survival of hypoxic myocardial cells [183]. Yet, limits in autophagy activation may be necessary. Interneuron progenitor growth in the brain requires mTOR activity with the inhibition of autophagy [184]. Autophagy activation also can lead to injury of endothelial progenitor cells, result in mitochondrial oxidative stress, and block new blood vessel formation during elevated glucose exposure [185]. Inhibition of autophagy can limit infarct size and rescue cerebral neurons during stroke and oxidative stress [186].
4. Nicotinamide and Neurodegenerative Disease
Acute and chronic neurodegenerative diseases affect a large number of individuals throughout the globe [119, 187–190]. Neurodegenerative disorders can impact more than one billion individuals. This number represents approximately fifteen percent of the world’s population. Approximately seven million die each year from neurodegenerative disorders [41]. Nervous system diseases comprise over six hundred disorders that can progressively lead to death and disability [119, 190, 191]. Furthermore, neurodegenerative disorders are expected to increase in prevalence throughout the globe. For example, sporadic cases of AD are increasing in the world with dementia now ranked as the 7th leading cause of death [190]. Dementia occurs in all countries throughout the world at a significant financial burden [192]. Greater than five million people suffer with cognitive disorders in the US and most of these cases, at least sixty percent, are from AD [41]. Currently, fifty million people in the world, or five percent of the global population, have dementia. By 2030, dementia will affect eighty-two million individuals, and by 2050, one hundred fifty-two million individuals will suffer with dementia. In addition, caring for dementia is considered a significant cost factor with more than $800 billion USD a year required for dementia care at the present time [192].
In addition, metabolic disorders also can lead to neurodegeneration and affect all cellular systems [168]. In the peripheral nervous system, at least seventy percent of individuals with diabetes mellitus (DM) can develop diabetic peripheral neuropathy. DM can lead to autonomic neuropathy [193] and peripheral nerve disease [42, 194–197]. In the central nervous system, DM can result in insulin resistance and loss of cognition [41, 124, 147, 168, 190, 198–200]. DM can affect several cellular pathways that lead to cognitive loss and dementia [138, 201–206]. DM also has been linked to mental illness [207, 208], cerebral vascular injury [63, 138, 209–212], impairment of microglial activity [124, 190, 198, 199], and impairment of stem cell development [30, 138, 201–205, 213]. DM leads to vascular endothelial dysfunction [138, 147, 214–216], cardiovascular disease [49, 215, 217–224], retinal disease [48, 225, 226], and immune and infectious disorders [52, 137, 227–231].
Nicotinamide provides cellular protection for both neuronal [61, 232, 233] and vascular cells [4, 5, 10, 11]. In neuronal cells, nicotinamide protects against free radical injury [102], anoxia [106], excitotoxicity [234], homocysteine toxicity [235], ethanol-induced neuronal injury [23], oxygen-glucose deprivation [61, 236], and Aß toxicity [138, 237]. Nicotinamide can protect against ultraviolet light in endothelial corneal cells [92], age-related vascular dysfunction [20], endothelial mitochondrial dysfunction [101], and vascular mimicry during cancer [238].
Nicotinamide prevents oxidant-induced apoptotic neuronal injury in a specific concentration range [4]. As a result, limited concentrations of nicotinamide and NAD+ are critical for neuronal survival [11, 156, 239]. Administration of nicotinamide in a range of 5.0 – 25.0 mmol/L can significantly protect neurons during oxidative stress injuries and apoptosis. This concentration range is similar to other injury paradigms in both animal models [240] and in cell culture models [5, 101, 102]. Nicotinamide improves cognitive function and neuronal cell survival following cortical trauma [241], limits axonal degeneration [242], prevents spinal cord injury [243, 244], blocks neuronal death during toxic agent exposure [245] and lessens disability in models of PD [246–248].
Nicotinamide has been shown to also utilize pathways of mammalian forkead transcription factors to block apoptotic neuronal cell death [1, 10, 166, 249, 250]. Mammalian forkhead transcription factors (FoxOs) can affect multiple neurodegenerative disorders [69, 127, 251, 252]. Since the discovery of the Drosophila melanogaster gene forkhead, over one hundred forkhead genes and nineteen human subgroups have been identified that range from FOXA to FOXS [127, 201, 253]. The mammalian FOXO proteins of the O class have important relevance to neurodegenerative disorders and include the members FOXO1, FOXO3, FOXO4, and FOXO6 [109, 127, 143, 146, 201, 252, 254–261]. Forkhead proteins are also known as forkhead in rhabdomyosarcoma (FKHR) (FOXO1), FKHRL1 (forkhead in rhabdomyosarcoma like protein 1) (FOXO3a), the Drosophila gene forkhead (fkh), Forkhead RElated ACtivator (FREAC)-1 and -2, and the acute leukemia fusion gene located in chromosome X (AFX) (FOXO4) [127]. FoxO proteins are transcription factors that bind to deoxyribonucleic acid (DNA) through the FoxO-recognized element in the C-terminal basic region of the forkhead DNA binding domain [69]. Post-translational changes include FoxO protein phosphorylation or acetylation change the binding of the C-terminal basic region to DNA to prevent transcriptional activity and block FoxO activity [262]. Additional factors may affect forkhead binding to DNA. These include N-terminal region of the recognition helix variations, electrostatic distribution changes, and sequestering FoxO proteins in the nucleus of cells [263].
Nicotinamide has been shown to inhibit FoxO protein activity [61] and is protective through two separate mechanisms of post-translational modification of FoxO3a [201, 253, 257]. Nicotinamide not only can maintain phosphorylation of FoxO3a and inhibit its activity to potentially block caspase 3 activity [61], but also can reduce caspase activity and preserve the integrity of the FoxO3a protein to block FoxO3a proteolysis. During oxidative stress, an initial inhibitory phosphorylation of FoxO3a at the regulatory phosphorylation sites (Thr32 and Ser253) occurs [61, 264]. Loss of phosphorylated FoxO3a integrity can subsequently ensue by caspase activity that can increase the vulnerability of neurons to apoptotic injury [61] since FoxO3a proteolysis results in pro-apoptotic amino-terminal (Nt) fragments that can become biologically active and lead to cellular injury [265]. Nicotinamide, through the phosphorylation of FoxO3a at regulatory sites that possess high affinity for protein kinase B (Akt) can prevent apoptotic cell injury [61]. In addition, decrease of caspase 3 activity by nicotinamide appears to be tied to a unique regulatory mechanism that blocks the proteolytic degradation of phosphorylated FoxO3a by caspase 3. Since FoxO3a has been shown to be a substrate for caspase 3-like proteases at the consensus sequence DELD304A [265], inhibition of caspase 3 activity prevents the destruction of phosphorylated FoxO3a during oxidative stress [61], suggesting that nicotinamide maintains a regulatory loop through the modulation of caspase 3 and the preservation of phosphorylated FoxO3a integrity.
5. Nicotinamide and Metabolic Disease
Metabolic disease that includes DM affects a broad spectrum of the world’s population [41, 113, 168, 190, 259, 266–270]. Approximately five hundred million individuals have DM [26, 138, 271–274]. An additional four hundred million individuals either suffer from metabolic disease or are at risk for developing DM [63, 221, 274, 275]. The number of individuals with DM is expected to rise to seven hundred million individuals by the year 2045 [274]. At least thirty-five million individuals are diagnosed with DM [268]. Seven million individuals over the age of eighteen remain undiagnosed with DM and almost thirty-five percent of adults in the US had prediabetes based on their fasting glucose and hemoglobin A1c (HbA1c) levels in the year 2018 [276]. Obesity and excess body fat can increase the risk for developing DM in young individuals [57] and can affect stem cell proliferation, aging, inflammation, oxidative stress injury, and mitochondrial function [249, 277–283]. The care for patients with DM equals approximately $760 billion USD and consumes more than seventeen percent of the Gross Domestic Product in the US [274, 284].
Nicotinamide has a vital role during metabolic dysfunction and DM [1, 5, 10, 12, 57, 179, 285]. Nicotinamide reduces insulin resistance and glucose release with additional pathways to prevent the development and progression of DM [286–288]. Nicotinamide (niacin) blocks skeletal muscle atrophy during DM [289] and reduces brain inflammation during DM [290]. In animal models, nicotinamide can maintain normal fasting blood glucose with streptozotocin-induced DM [291, 292] and prevent oxidative stress pathways that lead to cell death and apoptosis [22, 100, 101, 293, 294]. Nicotinamide also can improve glucose utilization, block excess lactate production, and enhance electrophysiologic capacity in ischemic animal models [295]. Oral nicotinamide administration at a dose of 1200mg/m2/day has been shown to protect pancreatic β-cell function and limits clinical disease in islet-cell antibody-positive first-degree relatives of type-1 DM [296]. Patients with recent onset type-1 DM receiving nicotinamide (25mg/kg) in combination with intensive insulin therapy for up to two years demonstrate significantly reduce HbA1c levels [297]. Yet, prolonged exposure of nicotinamide has been reported to result in impaired pancreatic β-cell function and cell growth [298, 299]. Nicotinamide also may block cytochromes P450 and hepatic metabolism [300]. As a result, the duration of nicotinamide administration may influence efficacy of treatment since long-term administration also has been reported to support glucose intolerance in some animal models [156].
Nicotinamide is reliant upon mTOR pathways to offer cellular protection during metabolic disease and DM. Nicotinamide has a fine control over cellular metabolism through mTOR pathways such as p70S6K, 4EBP1, and AMP activated protein kinase (AMPK). Both p70S6K and 4EBP1 in the mTOR pathway are required by nicotinamide to protect against radiation-induced apoptosis [301]. p70S6K and 4EBP1 activation also can enhance insulin secretion in pancreatic β-cells and increase resistance to β-cell streptozotocin toxicity and obesity in mice [302]. With nicotinamide, mTOR activity plays a significant role to maintain metabolic homeostasis. During the loss of mTOR activity, reduced β-cell function, insulin resistance, and decreased insulin secretion can result and lead to DM progression [303]. Decreased activity of mTOR has been shown to increase mortality in a mouse model of DM [304]. Translocation of glucose transporters to the plasma membrane in skeletal muscle can be blocked in the absence of mTOR activity [305].
Yet as previously described, nicotinamide can lead to the activation of autophagy with mTOR inhibition to reduce metabolic dysfunction through the maintenance of mitochondria [12, 16, 56, 179] and also maintain metabolic homeostasis [31, 180, 181]. These observations suggest that a careful balance of mTOR activity is required for the efficacy of nicotinamide. As an example, if mTOR activity becomes elevated, mTOR and p70S6K can lead to glucose intolerance by inhibiting the insulin receptor substrate 1 (IRS-1) [306]. At times, mTOR inhibition may be required to reduce stroke infarct size during models of DM [307], block cardiac hypertrophy [308], protect vascular cells from oxidative stress [45], prevent retinal degeneration [133], and maintain a balance between pancreatic β-cell proliferation and cell size [309].
Nicotinamide employs AMPK to oversee cellular metabolism [190, 219, 310, 311]. AMPK prevents mTORC1 activity through the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex that blocks mTORC1 [145, 312]. Nicotinamide can reduce intracellular mitochondrial stress in hypoxic cardiomyocytes through the activation of AMPK [56]. Pathways of nicotinamide also may be necessary with AMPK to allow skeletal muscle cells to sense and react to nutrient availability [313]. Nicotinamide in conjunction with AMPK recently has been shown to decrease metabolic abnormalities in polycystic ovary syndrome [314]. AMPK activation during metabolic disease can promote insulin sensitivity, fatty acid oxidation, and mitochondrial biogenesis that results in the generation of ATP and serves to limit oxidative stress [12, 41]. In line with the endothelial protective properties of nicotinamide that can rely potentially upon AMPK [61, 101, 315], AMPK can limit insulin resistance [316] and protect endothelial progenitor cells during periods of hyperglycemia [219]. AMPK activation also can strengthen memory retention in models of AD and DM [310], may assist with Aß elimination in the brain [317], foster tau clearance [318], and reduce chronic inflammation in the nervous system [79, 144, 312].
With AMPK signaling, nicotinamide may not always yield cellular protection during metabolic disorders. AMPK and autophagy pathways may require oversight during metabolic disease and DM [52, 113, 124, 168, 198, 229, 319]. For example, increased activity of autophagy has been shown to result in the loss of cardiac and liver tissue in diabetic rats [320]. Toxic advanced glycation end products (AGEs) during metabolic disorders can yield autophagy activation and vascular smooth muscle proliferation that may lead to in atherosclerosis [321] as well as cardiomyopathy [322]. During high glucose exposure, autophagy can impair endothelial progenitor cells, lead to mitochondrial oxidative stress [115], and prevent angiogenesis [185].
6. Conclusion and Future Perspectives
Neurodegenerative disorders impact more than one billion individuals in the world and at least seven million individuals die each year from neurodegenerative disorders. In addition, metabolic disorders lead to neurodegenerative diseases and impact over nine hundred individuals throughput the globe when cone considers those with active disease and individuals presently at risk for developing metabolic disease. Nicotinamide plays a critical role for the treatment of both neurodegenerative diseases and metabolic disorders, such as DM. In neuronal and vascular systems, nicotinamide protects against oxidative stress, anoxia, excitotoxicity, ethanol-induced neuronal injury, Aß toxicity, ultraviolet light, age-related vascular disease, mitochondrial dysfunction, and vascular mimicry during cancer. In regard to metabolic disorders, nicotinamide reduces insulin resistance, blocks skeletal muscle atrophy during DM, maintains normal fasting blood glucose, improves glucose utilization, blocks excess lactate production, and protects pancreatic β-cell function.
Nicotinamide intimately oversees pathways tied to oxidative stress, apoptosis, and autophagy (Fig. 1). Through nicotinamide, the maintenance of adequate NAD+ stores has been linked to reduction in the aging process and increased resistance to oxidative stress. Nicotinamide can block apoptotic cell death during the early phase with membrane PS asymmetry and the later phase with genomic DNA degradation. Nicotinamide also relies upon pathways of autophagy such as to reduce metabolic dysfunction through the maintenance of mitochondrial function and to maintain metabolic homeostasis. Yet, nicotinamide has been shown to offer cellular protection in a specific concentration range with dosing outside of this range or prolonged administration leading to detrimental effects. The underlying pathways of nicotinamide that involve SIRT1, mTOR, FoxOs, and AMPK may offer insight into these observations for the efficacy and safety of nicotinamide. These pathways require a fine balance in control since each has the potential to foster cellular demise, mitochondrial oxidative stress, and loss of metabolic homeostasis. Nicotinamide presents significant promise for the development of innovative treatments for neurodegenerative disorders and metabolic disease, but the success of such programs rests on gaining further understanding of the complex relationship nicotinamide holds with the pathways of oxidative stress, apoptosis, autophagy, SIRT1, mTOR, FoxOs, and AMPK.
Figure 1: Nicotinamide Pathways for Neurodegenerative Disorders and Metabolic Disease.
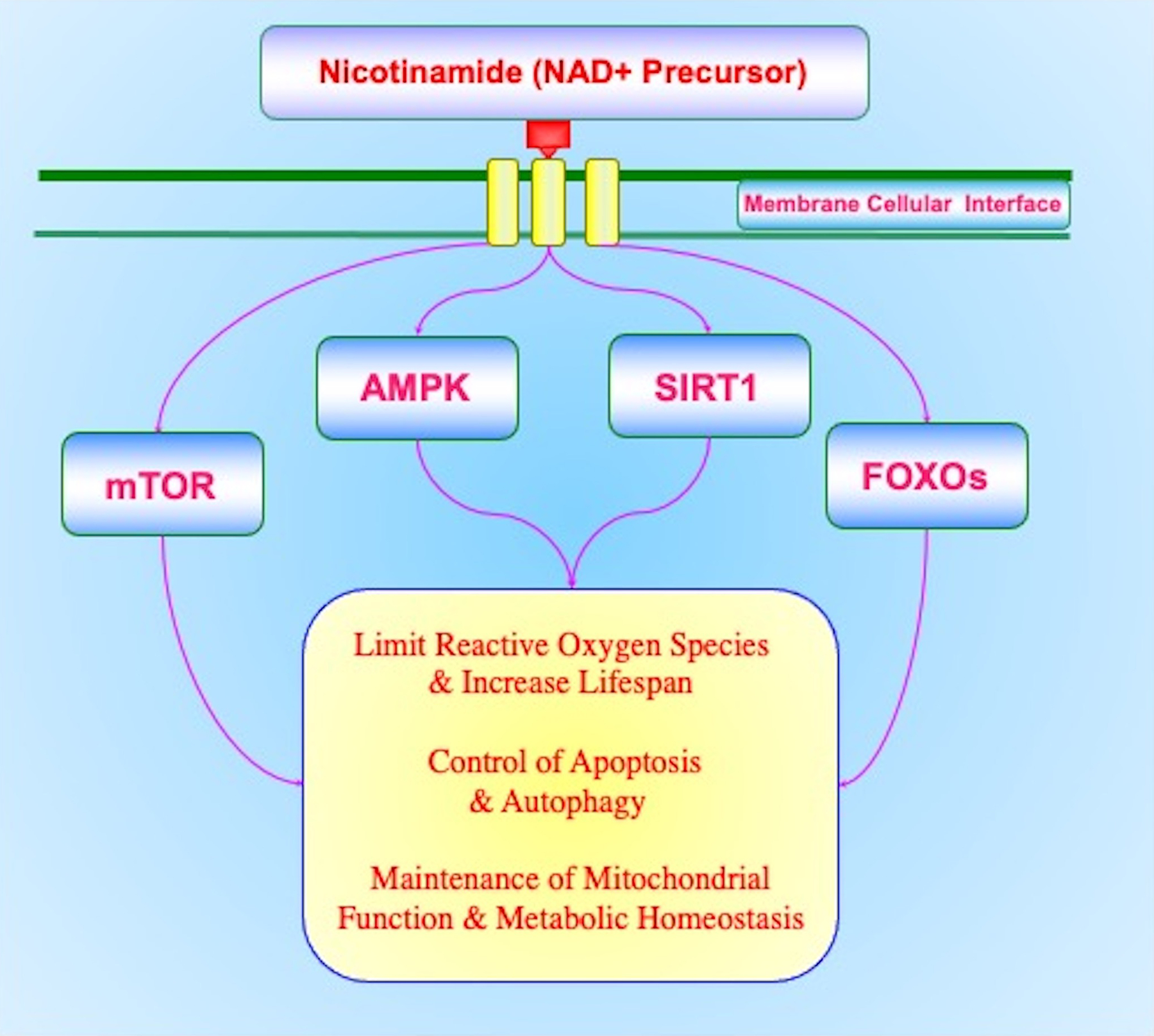
Nicotinamide is vital for the development of treatment strategies for neurodegenerative diseases and metabolic disorders. Nicotinamide relies upon a complex relationship with the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), the mechanistic target of rapamycin (mTOR), AMP activated protein kinase (AMPK), mammalian forkead transcription factors (FoxOs), oxidative stress (reactive oxygen species), apoptosis, and autophagy. Each of these pathways for nicotinamide requires a fine balance in control to maximize clinical efficacy and limit unwanted effects such as cellular demise, mitochondrial oxidative stress, and loss of metabolic homeostasis.
Acknowledgments:
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Footnotes
Competing Interests: There are no conflicts of interest to declare.
References
- 1.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14(9):3446–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braidy N, Liu Y. NAD+ therapy in age-related degenerative disorders: A benefit/risk analysis. Exp Gerontol 2020:110831. [DOI] [PubMed] [Google Scholar]
- 3.Rex A, Fink H. Pharmacokinetic aspects of reduced nicotinamide adenine dinucleotide (NADH) in rats. Front Biosci 2008;13:3735–41. [DOI] [PubMed] [Google Scholar]
- 4.Li F, Chong ZZ, Maiese K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front Biosci 2004;9:2500–20. [DOI] [PubMed] [Google Scholar]
- 5.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci 2003;24(5):228–32. [DOI] [PubMed] [Google Scholar]
- 6.Jackson TM, Rawling JM, Roebuck BD, Kirkland JB. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J Nutr 1995;125(6):1455–61. [DOI] [PubMed] [Google Scholar]
- 7.Wojcik M, Seidle HF, Bieganowski P, Brenner C. Glutamine-dependent NAD+ synthetase. How a two-domain, three-substrate enzyme avoids waste. J Biol Chem 2006;281(44):33395–402. [DOI] [PubMed] [Google Scholar]
- 8.Khan JA, Forouhar F, Tao X, Tong L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert opinion on therapeutic targets. 2007;11(5):695–705. [DOI] [PubMed] [Google Scholar]
- 9.Khan JA, Xiang S, Tong L. Crystal structure of human nicotinamide riboside kinase. Structure. 2007;15(8):1005–13. [DOI] [PubMed] [Google Scholar]
- 10.Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem 2006;13(8):883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiese K Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother 2008;62(4):218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiese K New Insights for nicotinamide: Metabolic disease, autophagy, and mTOR. Frontiers in bioscience (Landmark edition). 2020;25:1925–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell Mol Life Sci 2004;61(1):19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin SJ, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol 2003;15(2):241–6. [DOI] [PubMed] [Google Scholar]
- 15.Hageman GJ, Stierum RH. Niacin, poly(ADP-ribose) polymerase-1 and genomic stability. Mutat Res 2001;475(1–2):45–56. [DOI] [PubMed] [Google Scholar]
- 16.Castro-Portuguez R, Sutphin GL. Kynurenine pathway, NAD(+) synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan. Exp Gerontol 2020;132:110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng Y, Wang Y, Jiang C, Fang Z, Zhang Z, Lin X, et al. Nicotinamide induces mitochondrial-mediated apoptosis through oxidative stress in human cervical cancer HeLa cells. Life Sci 2017;181:62–9. [DOI] [PubMed] [Google Scholar]
- 18.Maiese K The bright side of reactive oxygen species: lifespan extension without cellular demise. J Transl Sci 2016;2(3):185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poljsak B, Milisav I. NAD(+) as the link between oxidative stress, inflammation, caloric restriction, exercise, DNA repair, longevity and health span. Rejuvenation Res 2016;19(5):406–15. [DOI] [PubMed] [Google Scholar]
- 20.Csicsar A, Tarantini S, Yabluchanskiy A, Balasubramanian P, Kiss T, Farkas E, et al. Role of endothelial NAD+ deficiency in age-related vascular dysfunction. Am J Physiol Heart Circ Physiol 2019;316(6):H1253–H66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams AC, Hill LJ, Ramsden DB. Nicotinamide, NAD(P)(H), and Methyl-Group Homeostasis Evolved and Became a Determinant of Ageing Diseases: Hypotheses and Lessons from Pellagra. Current gerontology and geriatrics research. 2012;2012:302875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ieraci A, Herrera DG. Nicotinamide Protects against Ethanol-Induced Apoptotic Neurodegeneration in the Developing Mouse Brain. PLoS Med 2006;3(4):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ieraci A, Herrera DG. Nicotinamide Inhibits Ethanol-Induced Caspase-3 and PARP-1 Over-activation and Subsequent Neurodegeneration in the Developing Mouse Cerebellum. Cerebellum (London, England). 2018. [DOI] [PubMed] [Google Scholar]
- 24.Maiese K Nicotinamide: Oversight of Metabolic Dysfunction through SIRT1, mTOR, and Clock Genes. Curr Neurovasc Res 2020;17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiese K, Chong ZZ, Hou J, Shang YC. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol 2010;45(3):217–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maiese K New Insights for Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev 2015;2015(2015:875961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefano GB, Kream RM. Dysregulated mitochondrial and chloroplast bioenergetics from a translational medical perspective (Review). Int J Mol Med 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tafani M, Sansone L, Limana F, Arcangeli T, De Santis E, Polese M, et al. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid Med Cell Longev 2016;2016:3907147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiese K Molecules to Medicine with mTOR: Translating Critical Pathways into Novel Therapeutic Strategies. Academic Press, Elsevier. 2016;ISBN 9780128027332. [Google Scholar]
- 30.Maiese K Prospects and Perspectives for WISP1 (CCN4) in Diabetes Mellitus. Curr Neurovasc Res 2020;17(3):327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doroftei B, Ilie OD, Cojocariu RO, Ciobica A, Maftei R, Grab D, et al. Minireview Exploring the Biological Cycle of Vitamin B3 and Its Influence on Oxidative Stress: Further Molecular and Clinical Aspects. Molecules. 2020;25(15):3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Feng Y, Wang XX, Truong D, Wu YC. The Critical Role of SIRT1 in Parkinson’s Disease: Mechanism and Therapeutic Considerations. Aging Dis 2020;11(6):1608–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mladenovic Djordjevic A, Loncarevic-Vasiljkovic N, Gonos ES. Dietary restriction and oxidative stress: friends or enemies? Antioxid Redox Signal 2020. [DOI] [PubMed] [Google Scholar]
- 34.Mocayar Marón FJ, Ferder L, Reiter RJ, Manucha W. Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J Steroid Biochem Mol Biol 2020;199:105595. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z, et al. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front Mol Neurosci 2020;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao HY, Li HY, Jin J, Jin JZ, Zhang LY, Xuan MY, et al. L-carnitine treatment attenuates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. Korean J Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scialo F, Sriram A, Fernandez-Ayala D, Gubina N, Lohmus M, Nelson G, et al. Mitochondrial ROS Produced via Reverse Electron Transport Extend Animal Lifespan. Cell Metab 2016;23(4):725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawler JM, Rodriguez DA, Hord JM. Mitochondria in the middle: Exercise preconditioning protection of striated muscle. J Physiol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You H, Li T, Zhang J, Lei Q, Tao X, Xie P, et al. Reduction in Ischemic Cerebral Infarction is Mediated through Golgi Phosphoprotein 3 and Akt/mTOR Signaling following Salvianolate Administration. Curr Neurovasc Res 2014;11(2):107–13. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Y, Fang H, Lin S, Shen S, Tao L, Xiao J, et al. Qiliqiangxin Protects Against Cardiac Ischemia-Reperfusion Injury via Activation of the mTOR Pathway. Cell Physiol Biochem 2015;37(2):454–64. [DOI] [PubMed] [Google Scholar]
- 41.Maiese K Cognitive impairment with diabetes mellitus and metabolic disease: innovative insights with the mechanistic target of rapamycin and circadian clock gene pathways. Expert Rev Clin Pharmacol 2020;13(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai C, Xiao X, Zhang Y, Xiang B, Hoyer D, Shen J, et al. Curcumin attenuates colistin-induced peripheral neurotoxicity in mice. ACS Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- 43.Deng D, Yan J, Wu Y, Wu K, Li W. Morroniside suppresses hydrogen peroxide-stimulated autophagy and apoptosis in rat ovarian granulosa cells through the PI3K/AKT/mTOR pathway. Human & experimental toxicology. 2020:960327120960768. [DOI] [PubMed] [Google Scholar]
- 44.Jayaraj RL, Beiram R, Azimullah S, Mf NM, Ojha SK, Adem A, et al. Valeric Acid Protects Dopaminergic Neurons by Suppressing Oxidative Stress, Neuroinflammation and Modulating Autophagy Pathways. International journal of molecular sciences. 2020;21(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng J, Chen Y, Wang J, Qiu J, Chang C, Bi F, et al. EGCG protects vascular endothelial cells from oxidative stress-induced damage by targeting the autophagy-dependent PI3K-AKT-mTOR pathway. Ann Transl Med 2020;8(5):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang J, Suo H, Song J. Protective role of mitoquinone against impaired mitochondrial homeostasis in metabolic syndrome. Critical reviews in food science and nutrition. 2020;20:1–19. [DOI] [PubMed] [Google Scholar]
- 47.Liu JD, Deng Q, Tian HH, Pang YT, Deng GL. Wnt/Glycogen Synthase Kinase 3beta/beta-catenin Signaling Activation Mediated Sevoflurane Preconditioning-induced Cardioprotection. Chin Med J (Engl) 2015;128(17):2346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maiese K Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural regeneration research. 2015;10(4):518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther 2008;118(1):58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jarero-Basulto J, Rivera-Cervantes M, Gasca-Martínez D, García-Sierra F, Gasca-Martínez Y, Beas-Zárate C. Current Evidence on the Protective Effects of Recombinant Human Erythropoietin and Its Molecular Variants against Pathological Hallmarks of Alzheimer’s Disease. Pharmaceuticals (Basel, Switzerland). 2020;13(424):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li N, Yue L, Wang J, Wan Z, Bu W. MicroRNA-24 alleviates isoflurane-induced neurotoxicity in rat hippocampus via attenuation of oxidative stress. Biochem Cell Biol 2020;98(2):208–18. [DOI] [PubMed] [Google Scholar]
- 52.Maiese K The Mechanistic Target of Rapamycin (mTOR): Novel Considerations as an Antiviral Treatment. Curr Neurovasc Res 2020;17(3):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Speer H, D’Cunha NM, Alexopoulos NI, McKune AJ, Naumovski N. Anthocyanins and Human Health-A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants (Basel, Switzerland). 2020;9(5):366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie T, Ye W, Liu J, Zhou L, Song Y. The Emerging Key Role of Klotho in the Hypothalamus-Pituitary-Ovarian Axis. Reprod Sci 2020. [DOI] [PubMed] [Google Scholar]
- 55.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem 2008;283(41):27810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai YF, Wang L, Liu WY. Nicotinamide pretreatment alleviates mitochondrial stress and protects hypoxic myocardial cells via AMPK pathway. European review for medical and pharmacological sciences. 2019;23(4):1797–806. [DOI] [PubMed] [Google Scholar]
- 57.Maiese K, Chong ZZ, Shang YC, Hou J. Novel Avenues of Drug Discovery and Biomarkers for Diabetes Mellitus. Journal of clinical pharmacology. 2011;51(2):128–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perez-Lobos R, Lespay-Rebolledo C, Tapia-Bustos A, Palacios E, Vio V, Bustamante D, et al. Vulnerability to a Metabolic Challenge Following Perinatal Asphyxia Evaluated by Organotypic Cultures: Neonatal Nicotinamide Treatment. Neurotox Res 2017. [DOI] [PubMed] [Google Scholar]
- 59.Mahmoud YI, Mahmoud AA. Role of nicotinamide (vitamin B3) in acetaminophen-induced changes in rat liver: Nicotinamide effect in acetaminophen-damaged liver. Exp Toxicol Pathol 2016;68(6):345–54. [DOI] [PubMed] [Google Scholar]
- 60.Marshall CA, Borbon IA, Erickson RP. Relative efficacy of nicotinamide treatment of a mouse model of infantile Niemann-Pick C1 disease. Journal of applied genetics. 2016. [DOI] [PubMed] [Google Scholar]
- 61.Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab 2004;24(7):728–43. [DOI] [PubMed] [Google Scholar]
- 62.Chong ZZ, Maiese K. Enhanced Tolerance against Early and Late Apoptotic Oxidative Stress in Mammalian Neurons through Nicotinamidase and Sirtuin Mediated Pathways. Curr Neurovasc Res 2008;5(3):159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maiese K mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes. 2015;6(2):217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mikhed Y, Daiber A, Steven S. Mitochondrial Oxidative Stress, Mitochondrial DNA Damage and Their Role in Age-Related Vascular Dysfunction. International journal of molecular sciences. 2015;16(7):15918–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parmar MS, Syed I, Gray JP, Ray SD. Curcumin, Hesperidin, and Rutin Selectively Interfere with Apoptosis Signaling and Attenuate Streptozotocin-Induced Oxidative Stress-Mediated Hyperglycemia. Curr Neurovasc Res 2015. [DOI] [PubMed] [Google Scholar]
- 66.Perez-Gallardo RV, Noriega-Cisneros R, Esquivel-Gutierrez E, Calderon-Cortes E, Cortes-Rojo C, Manzo-Avalos S, et al. Effects of diabetes on oxidative and nitrosative stress in kidney mitochondria from aged rats. Journal of bioenergetics and biomembranes. 2014. [DOI] [PubMed] [Google Scholar]
- 67.Wang P, Xing Y, Chen C, Chen Z, Qian Z. Advanced glycation end-product (AGE) induces apoptosis in human retinal ARPE-19 cells via promoting mitochondrial dysfunction and activating the Fas-FasL signaling. Biosci Biotechnol Biochem 2015;80(2):1–7. [DOI] [PubMed] [Google Scholar]
- 68.Maiese K The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): oversight for neurodegenerative disorders. Biochem Soc Trans 2018;46(2):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maiese K Targeting the core of neurodegeneration: FoxO, mTOR, and SIRT1. Neural regeneration research. 2021;16(3):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res 2010;7(2):95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal 2010;22(9):1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taveira GB, Mello EO, Souza SB, Monteiro RM, Ramos AC, Carvalho AO, et al. Programmed cell death in yeast by thionin-like peptide from Capsicum annuum fruits involving activation of capases and extracelullar H(+) flux. Bioscience reports. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hou J, Wang S, Shang YC, Chong ZZ, Maiese K. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res 2011;8(3):220–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhowmick S, D’Mello V, Caruso D, Abdul-Muneer PM. Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. Journal of molecular medicine (Berlin, Germany). 2019. [DOI] [PubMed] [Google Scholar]
- 75.Finelli MJ, Liu KX, Wu Y, Oliver PL, Davies KE. Oxr1 improves pathogenic cellular features of ALS-associated FUS and TDP-43 mutations. Hum Mol Genet 2015;24(12):3529–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maiese K Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Curr Neurovasc Res 2015;12(2):173–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Millet A, Bouzat P, Trouve-Buisson T, Batandier C, Pernet-Gallay K, Gaide-Chevronnay L, et al. Erythropoietin and Its Derivates Modulate Mitochondrial Dysfunction after Diffuse Traumatic Brain Injury. J Neurotrauma 2016. [DOI] [PubMed] [Google Scholar]
- 78.Hou J, Chong ZZ, Shang YC, Maiese K. FoxO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol Cell Endocrinol 2010;321(2):194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maiese K Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol 2016;82(5):1245–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saleem S, Biswas SC. Tribbles Pseudokinase 3 Induces Both Apoptosis and Autophagy in Amyloid-beta-induced Neuronal Death. J Biol Chem 2017;292(7):2571–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ullah R, Khan M, Shah SA, Saeed K, Kim MO. Natural Antioxidant Anthocyanins-A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients. 2019;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang CJ, Li JH, Zhang Z, Zhang JY, Liu SQ, Yang J. Suppression of MIF-induced neuronal apoptosis may underlie the therapeutic effects of effective components of Fufang Danshen in the treatment of Alzheimer’s disease. Acta Pharmacol Sin 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.El-Missiry MA, Othman AI, Amer MA, Sedki M, Ali SM, El-Sherbiny IM. Nanoformulated ellagic acid ameliorates pentylenetetrazol-induced experimental epileptic seizures by modulating oxidative stress, inflammatory cytokines and apoptosis in the brains of male mice. Metab Brain Dis 2019. [DOI] [PubMed] [Google Scholar]
- 84.Yue J, Liang C, Wu K, Hou Z, Wang L, Zhang C, et al. Upregulated SHP-2 expression in the epileptogenic zone of temporal lobe epilepsy and various effects of SHP099 treatment on a pilocarpine model. Brain Pathol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Almasieh M, Catrinescu MM, Binan L, Costantino S, Levin LA. Axonal Degeneration in Retinal Ganglion Cells is Associated with a Membrane Polarity-Sensitive Redox Process. J Neurosci 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tao Y, Li C, Yao A, Qu Y, Qin L, Xiong Z, et al. Intranasal administration of erythropoietin rescues the photoreceptors in degenerative retina: a noninvasive method to deliver drugs to the eye. Drug delivery. 2019;26(1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Y, Wang Q, Wang Y, Li J, Lu G, Liu Z. Glutamine protects against oxidative stress injury through inhibiting the activation of PI3K/Akt signaling pathway in parkinsonian cell model. Environmental health and preventive medicine. 2019;24(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dehghanian F, Soltani Z, Khaksari M. Can Mesenchymal Stem Cells Act Multipotential in Traumatic Brain Injury? J Mol Neurosci 2020. [DOI] [PubMed] [Google Scholar]
- 89.Sun F, Li SG, Zhang HW, Hua FW, Sun GZ, Huang Z. MiRNA-411 attenuates inflammatory damage and apoptosis following spinal cord injury. European review for medical and pharmacological sciences. 2020;24(2):491–8. [DOI] [PubMed] [Google Scholar]
- 90.Wang Z, Qiu Z, Gao C, Sun Y, Dong W, Zhang Y, et al. 2,5-hexanedione downregulates nerve growth factor and induces neuron apoptosis in the spinal cord of rats via inhibition of the PI3K/Akt signaling pathway. PLoS One. 2017;12(6):e0179388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu D, Li F, Hou K, Gou X, Fang W, Li Y. XQ-1H attenuates ischemic injury in PC12 cells via Wnt/β-catenin signaling via inhibition of apoptosis and promotion of proliferation. Cell Biol Int 2020. [DOI] [PubMed] [Google Scholar]
- 92.Zhao C, Li W, Duan H, Li Z, Jia Y, Zhang S, et al. NAD(+) precursors protect corneal endothelial cells from UVB-induced apoptosis. Am J Physiol Cell Physiol 2020. [DOI] [PubMed] [Google Scholar]
- 93.Zhou Q, Zhou S, Wang H, Li Y, Xiao X, Yang J. Stable silencing of ROR1 regulates cell cycle, apoptosis, and autophagy in a lung adenocarcinoma cell line. Int J Clin Exp Pathol 2020;13(5):1108–20. [PMC free article] [PubMed] [Google Scholar]
- 94.Simon F, Floros N, Ibing W, Schelzig H, Knapsis A. Neurotherapeutic potential of erythropoietin after ischemic injury of the central nervous system. Neural regeneration research. 2019;14(8):1309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang W, Han P, Xie R, Yang M, Zhang C, Mi Q, et al. TAT-mGluR1 Attenuates Neuronal Apoptosis Through Preventing MGluR1alpha Truncation after Experimental Subarachnoid Hemorrhage. ACS chemical neuroscience. 2018. [DOI] [PubMed] [Google Scholar]
- 96.Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res 2000;59(4):568–80. [DOI] [PubMed] [Google Scholar]
- 97.Schutters K, Reutelingsperger C. Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis. 2010;15(9):1072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wei L, Sun C, Lei M, Li G, Yi L, Luo F, et al. Activation of Wnt/beta-catenin Pathway by Exogenous Wnt1 Protects SH-SY5Y Cells Against 6-Hydroxydopamine Toxicity. J Mol Neurosci 2013;49(1):105–15. [DOI] [PubMed] [Google Scholar]
- 99.Williams CJ, Dexter DT. Neuroprotective and symptomatic effects of targeting group III mGlu receptors in neurodegenerative disease. J Neurochem 2014;129(1):4–20. [DOI] [PubMed] [Google Scholar]
- 100.Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res 2005;2(4):271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chong ZZ, Lin SH, Maiese K. Nicotinamide Modulates Mitochondrial Membrane Potential and Cysteine Protease Activity during Cerebral Vascular Endothelial Cell Injury. J Vasc Res 2002;39(2):131–47. [DOI] [PubMed] [Google Scholar]
- 102.Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J Cereb Blood Flow Metab 2000;20(9):1380–91. [DOI] [PubMed] [Google Scholar]
- 103.Bombeli T, Karsan A, Tait JF, Harlan JM. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89(7):2429–42. [PubMed] [Google Scholar]
- 104.Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res 2002;11(6):863–71. [DOI] [PubMed] [Google Scholar]
- 105.Maiese K, Chong ZZ, Shang YC. Raves and risks for erythropoietin. Cytokine Growth Factor Rev 2008;19(2):145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin SH, Chong ZZ, Maiese K. Nicotinamide: A Nutritional Supplement that Provides Protection Against Neuronal and Vascular Injury. J Med Food 2001;4(1):27–38. [DOI] [PubMed] [Google Scholar]
- 107.Jackson MD, Schmidt MT, Oppenheimer NJ, Denu JM. Mechanism of nicotinamide inhibition and transglycosidation by Sir2 histone/protein deacetylases. J Biol Chem 2003;278(51):50985–98. [DOI] [PubMed] [Google Scholar]
- 108.Kruszewski M, Szumiel I. Sirtuins (histone deacetylases III) in the cellular response to DNA damage--facts and hypotheses. DNA Repair (Amst) 2005;4(11):1306–13. [DOI] [PubMed] [Google Scholar]
- 109.Ali T, Rahman SU, Hao Q, Li W, Liu Z, Ali Shah F, et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J Pineal Res 2020;69(2). [DOI] [PubMed] [Google Scholar]
- 110.Boga JA, Coto-Montes A. ER stress and autophagy induced by SARS-CoV-2: The targets for melatonin treatment. Melatonin Research. 2020;3(3):346–61. [Google Scholar]
- 111.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016;12(1):1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert opinion on therapeutic targets. 2012;16(12):1203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Qi X, Mitter SK, Yan Y, Busik JV, Grant MB, Boulton ME. Diurnal Rhythmicity of Autophagy Is Impaired in the Diabetic Retina. Cells. 2020;9(4):905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wong SQ, Kumar AV, Mills J, Lapierre LR. C. elegans to model autophagy-related human disorders. Prog Mol Biol Transl Sci 2020;172:325–73. [DOI] [PubMed] [Google Scholar]
- 115.Martino L, Masini M, Novelli M, Beffy P, Bugliani M, Marselli L, et al. Palmitate activates autophagy in INS-1E beta-cells and in isolated rat and human pancreatic islets. PLoS ONE. 2012;7(5):e36188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Z, Stanojevic V, Brindamour LJ, Habener JF. GLP1-derived nonapeptide GLP1(28–36)amide protects pancreatic beta-cells from glucolipotoxicity. J Endocrinol 2012;213(2):143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dorvash M, Farahmandnia M, Tavassoly I. A Systems Biology Roadmap to Decode mTOR Control System in Cancer. Interdiscip Sci 2020;12(1):1–11. [DOI] [PubMed] [Google Scholar]
- 118.Preau S, Ambler M, Sigurta A, Kleyman A, Dyson A, Hill NE, et al. Protein recycling and limb muscle recovery after critical illness in slow- and fast-twitch limb muscle. Am J Physiol Regul Integr Comp Physiol 2019. [DOI] [PubMed] [Google Scholar]
- 119.Corti O, Blomgren K, Poletti A, Beart PM. Autophagy in neurodegeneration: New insights underpinning therapy for neurological diseases. J Neurochem 2020;154(4):e15002. [DOI] [PubMed] [Google Scholar]
- 120.Fields CR, Bengoa-Vergniory N, Wade-Martins R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front Mol Neurosci 2019;12:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tatullo M, Marrelli B, Zullo MJ, Codispoti B, Paduano F, Benincasa C, et al. Exosomes from Human Periapical Cyst-MSCs: Theranostic Application in Parkinson’s Disease. Int J Med Sci 2020;17(5):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y, Wu Q, Zhang L, Wang Q, Yang Z, Liu J, et al. Caffeic acid reduces A53T alpha-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol Res 2019;150:104538. [DOI] [PubMed] [Google Scholar]
- 123.Zhou ZD, Selvaratnam T, Lee JCT, Chao YX, Tan EK. Molecular targets for modulating the protein translation vital to proteostasis and neuron degeneration in Parkinson’s disease. Translational neurodegeneration. 2019;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hsieh CF, Liu CK, Lee CT, Yu LE, Wang JY. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Scientific reports. 2019;9(1):840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhou T, Zhuang J, Wang Z, Zhou Y, Li W, Wang Z, et al. Glaucocalyxin A as a natural product increases amyloid beta clearance and decreases tau phosphorylation involving the mammalian target of rapamycin signaling pathway. Neuroreport 2019;30(4):310–6. [DOI] [PubMed] [Google Scholar]
- 126.Francois A, Terro F, Quellard N, Fernandez B, Chassaing D, Janet T, et al. Impairment of autophagy in the central nervous system during lipopolysaccharide-induced inflammatory stress in mice. Molecular brain. 2014;7(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maiese K FoxO Proteins in the Nervous System. Anal Cell Pathol (Amst) 2015;2015:569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sullivan PM, Zhou X, Robins AM, Paushter DH, Kim D, Smolka MB, et al. The ALS/FTLD associated protein C9orf72 associates with SMCR8 and WDR41 to regulate the autophagy-lysosome pathway. Acta neuropathologica communications. 2016;4(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee JH, Tecedor L, Chen YH, Monteys AM, Sowada MJ, Thompson LM, et al. Reinstating aberrant mTORC1 activity in Huntington’s disease mice improves disease phenotypes. Neuron 2015;85(2):303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ye Y, Zhang P, Qian Y, Yin B, Yan M. The Effect of Pyrroloquinoline Quinone on the Expression of WISP1 in Traumatic Brain Injury. Stem cells international. 2017;2017:4782820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang P, Ye Y, Qian Y, Yin B, Zhao J, Zhu S, et al. The effect of pyrroloquinoline quinone on apoptosis and autophagy in traumatic brain injury. CNS Neurol Disord Drug Targets. 2017. [DOI] [PubMed] [Google Scholar]
- 132.Maiese K Healing the Heart with Sirtuins and Mammalian Forkhead Transcription Factors. Curr Neurovasc Res 2020;17(1):1–2. [DOI] [PubMed] [Google Scholar]
- 133.Pan YR, Song JY, Fan B, Wang Y, Che L, Zhang SM, et al. mTOR may interact with PARP-1 to regulate visible light-induced parthanatos in photoreceptors. Cell Commun Signal. 2020;18(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Potthast AB, Nebl J, Wasserfurth P, Haufe S, Eigendorf J, Hahn A, et al. Impact of Nutrition on Short-Term Exercise-Induced Sirtuin Regulation: Vegans Differ from Omnivores and Lacto-Ovo Vegetarians. Nutrients. 2020;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang YL, Zhang CG, Liu H, Zhou Y, Wang YP, Li Y, et al. Ginsenoside Rg1 Inhibits Cell Proliferation and Induces Markers of Cell Senescence in CD34+CD38- Leukemia Stem Cells Derived from KG1α Acute Myeloid Leukemia Cells by Activating the Sirtuin 1 (SIRT1)/Tuberous Sclerosis Complex 2 (TSC2) Signaling Pathway. Med Sci Monit 2020;26:e918207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang GZ, Deng YJ, Xie QQ, Ren EH, Ma ZJ, He XG, et al. Sirtuins and intervertebral disc degeneration: Roles in inflammation, oxidative stress, and mitochondrial function. Clin Chim Acta 2020;508:33–42. [DOI] [PubMed] [Google Scholar]
- 137.Maiese K, Chong ZZ, Shang YC, Wang S. Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol 2011;52(4):1173–85. [PMC free article] [PubMed] [Google Scholar]
- 138.Maiese K SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells. 2015;7(2):235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Charles S, Raj V, Arokiaraj J, Mala K. Caveolin1/protein arginine methyltransferase1/sirtuin1 axis as a potential target against endothelial dysfunction. Pharmacol Res 2017;119:1–11. [DOI] [PubMed] [Google Scholar]
- 140.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert opinion on therapeutic targets. 2012;16(2):167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cui L, Guo J, Zhang Q, Yin J, Li J, Zhou W, et al. Erythropoietin activates SIRT1 to protect human cardiomyocytes against doxorubicin-induced mitochondrial dysfunction and toxicity. Toxicol Lett 2017;275:28–38. [DOI] [PubMed] [Google Scholar]
- 142.Geng C, Xu H, Zhang Y, Gao Y, Li M, Liu X, et al. Retinoic acid ameliorates high-fat diet-induced liver steatosis through sirt1. Science China Life sciences. 2017;60(11):1234–41. [DOI] [PubMed] [Google Scholar]
- 143.Maiese K Forkhead transcription factors: new considerations for alzheimer’s disease and dementia. J Transl Sci 2016;2(4):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maiese K Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer. Curr Neurovasc Res 2017;14(3):299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maiese K Harnessing the Power of SIRT1 and Non-coding RNAs in Vascular Disease. Curr Neurovasc Res 2017;14(1):82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maiese K Novel Treatment Strategies for the Nervous System: Circadian Clock Genes, Non-coding RNAs, and Forkhead Transcription Factors. Curr Neurovasc Res 2018;15(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Maiese K Sirtuins: Developing Innovative Treatments for Aged-Related Memory Loss and Alzheimer’s Disease. Curr Neurovasc Res 2018;15(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Joe Y, Chen Y, Park J, Kim HJ, Rah SY, Ryu J, et al. Cross-talk between CD38 and TTP Is Essential for Resolution of Inflammation during Microbial Sepsis. Cell reports. 2020;30(4):1063–76.e5. [DOI] [PubMed] [Google Scholar]
- 149.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem 2002;277(47):45099–107. [DOI] [PubMed] [Google Scholar]
- 150.Cai AL, Zipfel GJ, Sheline CT. Zinc neurotoxicity is dependent on intracellular NAD levels and the sirtuin pathway. Eur J Neurosci 2006;24(8):2169–76. [DOI] [PubMed] [Google Scholar]
- 151.Porcu M, Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci 2005;26(2):94–103. [DOI] [PubMed] [Google Scholar]
- 152.Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 2007;26(37):5489–504. [DOI] [PubMed] [Google Scholar]
- 153.Maiese K Sirtuin Biology in Medicine: Targeting New Avenues of Care in Development, Aging, and Disease. Elsevier and Academic Press. 2021;(in press). [Google Scholar]
- 154.Han J, Shi S, Min L, Wu T, Xia W, Ying W. NAD(+) Treatment Induces Delayed Autophagy in Neuro2a Cells Partially by Increasing Oxidative Stress. Neurochem Res 2011;36(12):2270–7. [DOI] [PubMed] [Google Scholar]
- 155.Kim SW, Lee JH, Moon JH, Nazim UM, Lee YJ, Seol JW, et al. Niacin alleviates TRAIL-mediated colon cancer cell death via autophagy flux activation. Oncotarget. 2016;7(4):4356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Qi Z, Xia J, Xue X, He Q, Ji L, Ding S. Long-term treatment with nicotinamide induces glucose intolerance and skeletal muscle lipotoxicity in normal chow-fed mice: compared to diet-induced obesity. The Journal of nutritional biochemistry. 2016;36:31–41. [DOI] [PubMed] [Google Scholar]
- 157.Audrito V, Vaisitti T, Rossi D, Gottardi D, D’Arena G, Laurenti L, et al. Nicotinamide Blocks Proliferation and Induces Apoptosis of Chronic Lymphocytic Leukemia Cells through Activation of the p53/miR-34a/SIRT1 Tumor Suppressor Network. Cancer Res 2011;71(13):4473–83. [DOI] [PubMed] [Google Scholar]
- 158.Wang T, Cui H, Ma N, Jiang Y. Nicotinamide-mediated inhibition of SIRT1 deacetylase is associated with the viability of cancer cells exposed to antitumor agents and apoptosis. Oncology letters. 2013;6(2):600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang JG, Zhao G, Qin Q, Wang B, Liu L, Liu Y, et al. Nicotinamide prohibits proliferation and enhances chemosensitivity of pancreatic cancer cells through deregulating SIRT1 and Ras/Akt pathways. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al] 2013;13(2):140–6. [DOI] [PubMed] [Google Scholar]
- 160.Maiese K Sirtuin Biology in Cancer and Metabolic Disease: Cellular Pathways for Clinical Discovery. Elsevier and Academic Press. 2021;(in press). [Google Scholar]
- 161.Zhang XM, Jing YP, Jia MY, Zhang L. Negative transcriptional regulation of inflammatory genes by group B3 vitamin nicotinamide. Mol Biol Rep 2012;39(12):10367–71. [DOI] [PubMed] [Google Scholar]
- 162.Shen C, Dou X, Ma Y, Ma W, Li S, Song Z. Nicotinamide protects hepatocytes against palmitate-induced lipotoxicity via SIRT1-dependent autophagy induction. Nutr Res 2017;40:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Johri MK, Lashkari HV, Gupta D, Vedagiri D, Harshan KH. mTORC1 restricts hepatitis C virus RNA replication through ULK1-mediated suppression of miR-122 and facilitates post-replication events. J Gen Virol 2020;101(1):86–95. [DOI] [PubMed] [Google Scholar]
- 164.Maiese K New Challenges and Strategies for Cardiac Disease: Autophagy, mTOR, and AMP-Activated Protein Kinase. Curr Neurovasc Res 2020. [DOI] [PubMed] [Google Scholar]
- 165.Tian Y, Xiao YH, Geng T, Sun C, Gu J, Tang KF, et al. Clusterin suppresses spermatogenic cell apoptosis to alleviate diabetes-induced testicular damage by inhibiting autophagy via the PI3K/AKT/mTOR axis. Biol Cell. 2020. [DOI] [PubMed] [Google Scholar]
- 166.Tabibzadeh S Signaling pathways and effectors of aging. Frontiers in bioscience (Landmark edition). 2021;26:50–96. [DOI] [PubMed] [Google Scholar]
- 167.Blagosklonny MV. From causes of aging to death from COVID-19. Aging (Albany NY). 2020;12(11):10004–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Maiese K Dysregulation of metabolic flexibility: The impact of mTOR on autophagy in neurodegenerative disease. Int Rev Neurobiol 2020;155:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Saenwongsa W, Nithichanon A, Chittaganpitch M, Buayai K, Kewcharoenwong C, Thumrongwilainet B, et al. Metformin-induced suppression of IFN-alpha via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes. Scientific reports. 2020;10(1):3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hwang SK, Kim HH. The functions of mTOR in ischemic diseases. BMB Rep 2011;44(8):506–11. [DOI] [PubMed] [Google Scholar]
- 171.Maiese K Erythropoietin and mTOR: A “One-Two Punch” for Aging-Related Disorders Accompanied by Enhanced Life Expectancy. Curr Neurovasc Res 2016;13(4):329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Martinez de Morentin PB, Martinez-Sanchez N, Roa J, Ferno J, Nogueiras R, Tena-Sempere M, et al. Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med 2014;14(1):3–21. [DOI] [PubMed] [Google Scholar]
- 173.Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med 2013;19(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Maiese K Driving neural regeneration through the mammalian target of rapamycin. Neural regeneration research. 2014;9(15):1413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Malla R, Ashby CR Jr., Narayanan NK, Narayanan B, Faridi JS, Tiwari AK. Proline-rich AKT substrate of 40-kDa (PRAS40) in the pathophysiology of cancer. Biochem Biophys Res Commun 2015;463(3):161–6. [DOI] [PubMed] [Google Scholar]
- 176.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol 2012;99(2):128–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 2004;6(11):1122–8. [DOI] [PubMed] [Google Scholar]
- 178.Fu L, Liu C, Chen L, Lv Y, Meng G, Hu M, et al. Protective Effects of 1-Methylnicotinamide on Abeta1–42-Induced Cognitive Deficits, Neuroinflammation and Apoptosis in Mice. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2019. [DOI] [PubMed] [Google Scholar]
- 179.Klimova N, Kristian T. Multi-targeted Effect of Nicotinamide Mononucleotide on Brain Bioenergetic Metabolism. Neurochem Res 2019;44(10):2280–7. [DOI] [PubMed] [Google Scholar]
- 180.Li J, Lu Y, Li N, Li P, Su J, Wang Z, et al. Muscle metabolomics analysis reveals potential biomarkers of exercisedependent improvement of the diaphragm function in chronic obstructive pulmonary disease. Int J Mol Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Osorio Alves J, Matta Pereira L, Cabral Coutinho do Rego Monteiro I, Pontes Dos Santos LH, Soares Marreiros Ferraz A, Carneiro Loureiro AC, et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants (Basel, Switzerland). 2020;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tong Y, Elkin KB, Peng C, Shen J, Li F, Guan L, et al. Reduced Apoptotic Injury by Phenothiazine in Ischemic Stroke through the NOX-Akt/PKC Pathway. Brain sciences. 2019;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li W, Zhu L, Ruan ZB, Wang MX, Ren Y, Lu W. Nicotinamide protects chronic hypoxic myocardial cells through regulating mTOR pathway and inducing autophagy. European review for medical and pharmacological sciences. 2019;23(12):5503–11. [DOI] [PubMed] [Google Scholar]
- 184.Ka M, Smith AL, Kim WY. MTOR controls genesis and autophagy of GABAergic interneurons during brain development. Autophagy. 2017:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim KA, Shin YJ, Akram M, Kim ES, Choi KW, Suh H, et al. High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Biol Pharm Bull 2014;37(7):1248–52. [DOI] [PubMed] [Google Scholar]
- 186.Yamada D, Kawabe K, Tosa I, Tsukamoto S, Nakazato R, Kou M, et al. Inhibition of the glutamine transporter SNAT1 confers neuroprotection in mice by modulating the mTOR-autophagy system. Commun Biol 2019;2:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Borowicz-Reutt KK, Czuczwar SJ. Role of oxidative stress in epileptogenesis and potential implications for therapy. Pharmacol Rep 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rey F, Balsari A, Giallongo T, Ottolenghi S, Di Giulio AM, Samaja M, et al. Erythropoietin as a Neuroprotective Molecule: An Overview of Its Therapeutic Potential in Neurodegenerative Diseases. ASN Neuro 2019;11:1759091419871420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Maiese K MicroRNAs for the Treatment of Dementia and Alzheimer’s Disease. Curr Neurovasc Res 2019;16(1):1–2. [DOI] [PubMed] [Google Scholar]
- 190.Maiese K Impacting dementia and cognitive loss with innovative strategies: mechanistic target of rapamycin, clock genes, circular non-coding ribonucleic acids, and Rho/Rock. Neural regeneration research. 2019;14(5):773–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell & bioscience. 2020;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 192.World Health Organization. Global action plan on the public health response to dementia 2017–2025. 2017:1–44. [Google Scholar]
- 193.Albiero M, Poncina N, Tjwa M, Ciciliot S, Menegazzo L, Ceolotto G, et al. Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes. 2014;63(4):1353–65. [DOI] [PubMed] [Google Scholar]
- 194.Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetology & metabolic syndrome. 2014;6(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gomez-Brouchet A, Blaes N, Mouledous L, Fourcade O, Tack I, Frances B, et al. Beneficial effects of levobupivacaine regional anaesthesia on postoperative opioid induced hyperalgesia in diabetic mice. Journal of translational medicine. 2015;13(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Atef MM, El-Sayed NM, Ahmed AAM, Mostafa YM. Donepezil improves neuropathy through activation of AMPK signalling pathway in streptozotocin-induced diabetic mice. Biochem Pharmacol 2019;159:1–10. [DOI] [PubMed] [Google Scholar]
- 197.Dong J, Li H, Bai Y, Wu C. Muscone ameliorates diabetic peripheral neuropathy through activating AKT/mTOR signalling pathway. J Pharm Pharmacol 2019;71(11):1706–13. [DOI] [PubMed] [Google Scholar]
- 198.Caberlotto L, Nguyen TP, Lauria M, Priami C, Rimondini R, Maioli S, et al. Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Scientific reports. 2019;9(1):3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Su M, Naderi K, Samson N, Youssef I, Fulop L, Bozso Z, et al. Mechanisms Associated with Type 2 Diabetes as a Risk Factor for Alzheimer-Related Pathology. Mol Neurobiol 2019;56(8):5815–34. [DOI] [PubMed] [Google Scholar]
- 200.Hu Z, Jiao R, Wang P, Zhu Y, Zhao J, De Jager P, et al. Shared Causal Paths underlying Alzheimer’s dementia and Type 2 Diabetes. Scientific reports. 2020;10(1):4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Maiese K, Chong ZZ, Shang YC, Hou J. FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond). 2009;116(3):191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tang PC, Ng YF, Ho S, Gyda M, Chan SW. Resveratrol and cardiovascular health--promising therapeutic or hopeless illusion? Pharmacol Res 2014;90:88–115. [DOI] [PubMed] [Google Scholar]
- 203.Xiang L, Mittwede PN, Clemmer JS. Glucose Homeostasis and Cardiovascular Alterations in Diabetes. Comprehensive Physiology. 2015;5(4):1815–39. [DOI] [PubMed] [Google Scholar]
- 204.Xu YJ, Tappia PS, Neki NS, Dhalla NS. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart failure reviews. 2014;19(1):113–21. [DOI] [PubMed] [Google Scholar]
- 205.Yao T, Fujimura T, Murayama K, Okumura K, Seko Y. Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in High Glucose-Induced Apoptosis in Rat Cardiac Myocytes and Murine Pancreatic beta-Cells. Cells. 2017;6(4):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fan X, Zhao Z, Wang D, Xiao J. Glycogen synthase kinase-3 as a key regulator of cognitive function. Acta biochimica et biophysica Sinica 2020;52(3). [DOI] [PubMed] [Google Scholar]
- 207.Hadamitzky M, Herring A, Kirchhof J, Bendix I, Haight MJ, Keyvani K, et al. Repeated systemic treatment with rapamycin affects behavior and amygdala protein expression in rats. The international journal of neuropsychopharmacology. 2018;21(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ignacio ZM, Reus GZ, Arent CO, Abelaira HM, Pitcher MR, Quevedo J. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol 2016;82(5):1280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Di Rosa M, Malaguarnera L. Chitotriosidase: A New Inflammatory Marker in Diabetic Complications. Pathobiology. 2016;83(4):211–9. [DOI] [PubMed] [Google Scholar]
- 210.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr Neurovasc Res 2008;5(2):125–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tulsulkar J, Nada SE, Slotterbeck BD, McInerney MF, Shah ZA. Obesity and hyperglycemia lead to impaired post-ischemic recovery after permanent ischemia in mice. Obesity (Silver Spring, Md 2015;24(2):417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xiao FH, He YH, Li QG, Wu H, Luo LH, Kong QP. A genome-wide scan reveals important roles of DNA methylation in human longevity by regulating age-related disease genes. PLoS One. 2015;10(3):e0120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yuan X, Liu Y, Bijonowski BM, Tsai AC, Fu Q, Logan TM, et al. NAD(+)/NADH redox alterations reconfigure metabolism and rejuvenate senescent human mesenchymal stem cells in vitro. Commun Biol 2020;3(1):774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Arildsen L, Andersen JV, Waagepetersen HS, Nissen JBD, Sheykhzade M. Hypermetabolism and impaired endothelium-dependent vasodilation in mesenteric arteries of type 2 diabetes mellitus db/db mice. Diabetes & vascular disease research : official journal of the International Society of Diabetes and Vascular Disease. 2019;16(6):1479164119865885. [DOI] [PubMed] [Google Scholar]
- 215.Ding S, Zhu Y, Liang Y, Huang H, Xu Y, Zhong C. Circular RNAs in Vascular Functions and Diseases. Adv Exp Med Biol 2018;1087:287–97. [DOI] [PubMed] [Google Scholar]
- 216.Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-alpha1/mTOR pathway. Journal of molecular endocrinology. 2019;63(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Alexandru N, Popov D, Georgescu A. Platelet dysfunction in vascular pathologies and how can it be treated. Thromb Res 2012;129(2):116–26. [DOI] [PubMed] [Google Scholar]
- 218.Barchetta I, Cimini FA, Ciccarelli G, Baroni MG, Cavallo MG. Sick fat: the good and the bad of old and new circulating markers of adipose tissue inflammation. Journal of endocrinological investigation. 2019;42(11):1257–72. [DOI] [PubMed] [Google Scholar]
- 219.Chiu SC, Chao CY, Chiang EI, Syu JN, Rodriguez RL, Tang FY. N-3 polyunsaturated fatty acids alleviate high glucose-mediated dysfunction of endothelial progenitor cells and prevent ischemic injuries both in vitro and in vivo. The Journal of nutritional biochemistry. 2017;42:172–81. [DOI] [PubMed] [Google Scholar]
- 220.Maiese K Disease onset and aging in the world of circular RNAs. J Transl Sci 2016;2(6):327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Maiese K, Chong ZZ, Shang YC. Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem 2007;14(16):1729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Maiese K, Chong ZZ, Shang YC, Hou J. Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert opinion on therapeutic targets. 2008;12(7):905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Perez-Hernandez N, Vargas-Alarcon G, Posadas-Sanchez R, Martinez-Rodriguez N, Tovilla-Zarate CA, Rodriguez-Cortes AA, et al. PHACTR1 Gene Polymorphism Is Associated with Increased Risk of Developing Premature Coronary Artery Disease in Mexican Population. International journal of environmental research and public health. 2016;13(8):803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Thackeray JT, Radziuk J, Harper ME, Suuronen EJ, Ascah KJ, Beanlands RS, et al. Sympathetic nervous dysregulation in the absence of systolic left ventricular dysfunction in a rat model of insulin resistance with hyperglycemia. Cardiovasc Diabetol 2011;10:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mishra M, Duraisamy AJ, Kowluru RA. Sirt1- A Guardian of the Development of Diabetic Retinopathy. Diabetes. 2018;67(4):745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ponnalagu M, Subramani M, Jayadev C, Shetty R, Das D. Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine. 2017;95:126–35. [DOI] [PubMed] [Google Scholar]
- 227.Kell DB, Pretorius E. No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biological reviews of the Cambridge Philosophical Society. 2018;93(3):1518–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lin X, Zhang N. Berberine: Pathways to protect neurons. Phytotherapy research : PTR 2018. [DOI] [PubMed] [Google Scholar]
- 229.Maiese K FoxO Transcription Factors and Regenerative Pathways in Diabetes Mellitus. Curr Neurovasc Res 2015;12(4):404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Woodhams L, Al-Salami H. The roles of bile acids and applications of microencapsulation technology in treating Type 1 diabetes mellitus. Therapeutic delivery. 2017;8(6):401–9. [DOI] [PubMed] [Google Scholar]
- 231.Zhao Y, Scott NA, Fynch S, Elkerbout L, Wong WW, Mason KD, et al. Autoreactive T cells induce necrosis and not BCL-2-regulated or death receptor-mediated apoptosis or RIPK3-dependent necroptosis of transplanted islets in a mouse model of type 1 diabetes. Diabetologia 2015;58(1):140–8. [DOI] [PubMed] [Google Scholar]
- 232.Anderson DW, Bradbury KA, Schneider JS. Neuroprotection in Parkinson models varies with toxin administration protocol. Eur J Neurosci 2006;24(11):3174–82. [DOI] [PubMed] [Google Scholar]
- 233.Feng Y, Paul IA, LeBlanc MH. Nicotinamide reduces hypoxic ischemic brain injury in the newborn rat. Brain Res Bull 2006;69(2):117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Slomka M, Zieminska E, Salinska E, Lazarewicz JW. Neuroprotective effects of nicotinamide and 1-methylnicotinamide in acute excitotoxicity in vitro. Folia Neuropathol 2008;46(1):69–80. [PubMed] [Google Scholar]
- 235.Slomka M, Zieminska E, Lazarewicz J. Nicotinamide and 1-methylnicotinamide reduce homocysteine neurotoxicity in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp (Wars) 2008;68(1):1–9. [DOI] [PubMed] [Google Scholar]
- 236.Shen CC, Huang HM, Ou HC, Chen HL, Chen WC, Jeng KC. Protective effect of nicotinamide on neuronal cells under oxygen and glucose deprivation and hypoxia/reoxygenation. J Biomed Sci 2004;11(4):472–81. [DOI] [PubMed] [Google Scholar]
- 237.Turunc Bayrakdar E, Uyanikgil Y, Kanit L, Koylu E, Yalcin A. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Abeta(1–42)-induced rat model of Alzheimer’s disease. Free Radic Res 2014;48(2):146–58. [DOI] [PubMed] [Google Scholar]
- 238.Itzhaki O, Greenberg E, Shalmon B, Kubi A, Treves AJ, Shapira-Frommer R, et al. Nicotinamide inhibits vasculogenic mimicry, an alternative vascularization pathway observed in highly aggressive melanoma. PLoS One. 2013;8(2):e57160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Shear DA, Dixon CE, Bramlett HM, Mondello S, Dietrich WD, Deng-Bryant Y, et al. Nicotinamide Treatment in Traumatic Brain Injury: Operation Brain Trauma Therapy. J Neurotrauma 2016;33(6):523–37. [DOI] [PubMed] [Google Scholar]
- 240.Kiuchi K, Yoshizawa K, Shikata N, Matsumura M, Tsubura A. Nicotinamide prevents N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis in Sprague-Dawley rats and C57BL mice. Exp Eye Res 2002;74(3):383–92. [DOI] [PubMed] [Google Scholar]
- 241.Peterson TC, Hoane MR, McConomy K, Farin F, Bammler T, MacDonald J, et al. A Combination Therapy of Nicotinamide and Progesterone Improves Functional Recovery Following Traumatic Brain Injury. J Neurotrauma 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, et al. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol 2005;170(3):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Brewer KL, Hardin JS. Neuroprotective effects of nicotinamide after experimental spinal cord injury. Acad Emerg Med 2004;11(2):125–30. [DOI] [PubMed] [Google Scholar]
- 244.Isbir CS, Ak K, Kurtkaya O, Zeybek U, Akgun S, Scheitauer BW, et al. Ischemic preconditioning and nicotinamide in spinal cord protection in an experimental model of transient aortic occlusion. Eur J Cardiothorac Surg 2003;23(6):1028–33. [DOI] [PubMed] [Google Scholar]
- 245.Ullah N, Lee HY, Naseer MI, Ullah I, Suh JW, Kim MO. Nicotinamide inhibits alkylating agent-induced apoptotic neurodegeneration in the developing rat brain. PLoS ONE. 2011;6(12):e27093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 246.Anderson DW, Bradbury KA, Schneider JS. Broad neuroprotective profile of nicotinamide in different mouse models of MPTP-induced parkinsonism. Eur J Neurosci 2008;28(3):610–7. [DOI] [PubMed] [Google Scholar]
- 247.Williams A, Ramsden D. Nicotinamide: a double edged sword. Parkinsonism Relat Disord 2005;11(7):413–20. [DOI] [PubMed] [Google Scholar]
- 248.Williams AC, Cartwright LS, Ramsden DB. Parkinson’s disease: the first common neurological disease due to auto-intoxication? Qjm 2005;98(3):215–26. [DOI] [PubMed] [Google Scholar]
- 249.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol 2005;75(3):207–46. [DOI] [PubMed] [Google Scholar]
- 250.Zhang F, Hu Y, Xu X, Zhai X, Wang G, Ning S, et al. Icariin protects against intestinal ischemia-reperfusion injury. J Surg Res 2015;194(1):127–38. [DOI] [PubMed] [Google Scholar]
- 251.Czubowicz K, Jesko H, Wencel P, Lukiw WJ, Strosznajder RP. The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer’s Disease and Other Neurodegenerative Disorders. Mol Neurobiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sanphui P, Das AK, Biswas SC. FoxO3a requires BAF57, a subunit of chromatin remodeler SWI/SNF complex for induction of PUMA in a model of Parkinson’s disease. J Neurochem 2020;154(5):e14969. [DOI] [PubMed] [Google Scholar]
- 253.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med 2008;14(5):219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Maiese K, Chong ZZ, Shang YC. “Sly as a FOXO”: New paths with Forkhead signaling in the brain. Curr Neurovasc Res 2007;4(4):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sooknual P, Pingaew R, Phopin K, Ruankham W, Prachayasittikul S, Ruchirawat S, et al. Synthesis and neuroprotective effects of novel chalcone-triazole hybrids. Bioorg Chem 2020;105:104384. [DOI] [PubMed] [Google Scholar]
- 256.Wang Y, Lin Y, Wang L, Zhan H, Luo X, Zeng Y, et al. TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/FoxO3a signaling pathway in Alzheimer’s disease mice. Aging (Albany NY) 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Maiese K, Chong ZZ, Shang YC, Hou J. A “FOXO” in sight: targeting Foxo proteins from conception to cancer. Med Res Rev 2009;29(3):395–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liu W, Li Y, Luo B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cell Mol Life Sci 2020;77(4):651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Peng S, Li W, Hou N, Huang N. A Review of FoxO1-Regulated Metabolic Diseases and Related Drug Discoveries. Cells. 2020;9(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yaman D, Takmaz T, Yüksel N, Dinçer SA, Şahin F. Evaluation of silent information regulator T (SIRT) 1 and Forkhead Box O (FOXO) transcription factor 1 and 3a genes in glaucoma. Mol Biol Rep 2020. [DOI] [PubMed] [Google Scholar]
- 261.Zhang W, Bai S, Yang J, Zhang Y, Liu Y, Nie J, et al. FoxO1 overexpression reduces Aβ production and tau phosphorylation in vitro. Neurosci Lett 2020;738:135322. [DOI] [PubMed] [Google Scholar]
- 262.Tsai KL, Sun YJ, Huang CY, Yang JY, Hung MC, Hsiao CD. Crystal structure of the human FOXO3a-DBD/DNA complex suggests the effects of post-translational modification. Nucleic Acids Res 2007;35(20):6984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Scodelaro Bilbao P, Boland R. Extracellular ATP regulates FoxO family of transcription factors and cell cycle progression through PI3K/Akt in MCF-7 cells. Biochim Biophys Acta 2013;1830(10):4456–69. [DOI] [PubMed] [Google Scholar]
- 264.Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci 2004;25(11):577–83. [DOI] [PubMed] [Google Scholar]
- 265.Charvet C, Alberti I, Luciano F, Jacquel A, Bernard A, Auberger P, et al. Proteolytic regulation of Forkhead transcription factor FOXO3a by caspase-3-like proteases. Oncogene 2003;22(29):4557–68. [DOI] [PubMed] [Google Scholar]
- 266.Klimontov VV, Bulumbaeva DM, Fazullina ON, Lykov AP, Bgatova NP, Orlov NB, et al. Circulating Wnt1-inducible signaling pathway protein-1 (WISP-1/CCN4) is a novel biomarker of adiposity in subjects with type 2 diabetes. J Cell Commun Signal 2020;14(1):101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Maiese K Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural regeneration research. 2016;11(3):372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. 2020;CS 314227-A:1–30. [Google Scholar]
- 269.Liu L, Hu J, Yang L, Wang N, Liu Y, Wei X, et al. Association of WISP1/CCN4 with Risk of Overweight and Gestational Diabetes Mellitus in Chinese Pregnant Women. Dis Markers 2020;2020:4934206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zaiou M circRNAs Signature as Potential Diagnostic and Prognostic Biomarker for Diabetes Mellitus and Related Cardiovascular Complications. Cells. 2020;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Haldar SR, Chakrabarty A, Chowdhury S, Haldar A, Sengupta S, Bhattacharyya M. Oxidative stress-related genes in type 2 diabetes: association analysis and their clinical impact. Biochemical genetics. 2015;53(4–6):93–119. [DOI] [PubMed] [Google Scholar]
- 272.Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Invited Review: Over-nutrition, mTOR Signaling and Cardiovascular Diseases. Am J Physiol Regul Integr Comp Physiol 2014;307(10):R1198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ye Q, Fu JF. Paediatric type 2 diabetes in China-Pandemic, progression, and potential solutions. Pediatric diabetes. 2018;19(1):27–35. [DOI] [PubMed] [Google Scholar]
- 274.International Diabetes Federation. Diabetes. IDF Diabetes Atlas. 2019(9th Edition). [PubMed] [Google Scholar]
- 275.Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev 2000;16(4):230–6. [DOI] [PubMed] [Google Scholar]
- 276.Maiese K Heightened Attention for Wnt Signaling in Diabetes Mellitus. Curr Neurovasc Res 2020;17(3):215–7. [DOI] [PubMed] [Google Scholar]
- 277.Cernea M, Tang W, Guan H, Yang K. Wisp1 mediates Bmp3-stimulated mesenchymal stem cell proliferation. Journal of molecular endocrinology. 2016;56(1):39–46. [DOI] [PubMed] [Google Scholar]
- 278.Curjuric I, Imboden M, Bridevaux PO, Gerbase MW, Haun M, Keidel D, et al. Common SIRT1 variants modify the effect of abdominal adipose tissue on aging-related lung function decline. Age (Dordr) 2016;38(3):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hill JH, Solt C, Foster MT. Obesity associated disease risk: the role of inherent differences and location of adipose depots. Hormone molecular biology and clinical investigation. 2018;33(2). [DOI] [PubMed] [Google Scholar]
- 280.Liu Z, Gan L, Zhang T, Ren Q, Sun C. Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res 2018;64(1):12455. [DOI] [PubMed] [Google Scholar]
- 281.Maiese K Picking a bone with WISP1 (CCN4): new strategies against degenerative joint disease. J Transl Sci 2016;1(3):83–5. [PMC free article] [PubMed] [Google Scholar]
- 282.Mehta J, Rayalam S, Wang X. Cytoprotective Effects of Natural Compounds against Oxidative Stress. Antioxidants (Basel, Switzerland). 2018;7(10):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Wang AR, Yan XQ, Zhang C, Du CQ, Long WJ, Zhan D, et al. Characterization of Wnt1-inducible Signaling Pathway Protein-1 in Obese Children and Adolescents. Current medical science. 2018;38(5):868–74. [DOI] [PubMed] [Google Scholar]
- 284.Centers for Medicare and Medicaid Services. National Health Expenditure Projections 2018–2027. www.cms.gov.2019.
- 285.Maiese K, Chong ZZ, Shang YC, Wang S. Novel directions for diabetes mellitus drug discovery. Expert opinion on drug discovery. 2013;8(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ahangarpour A, Ramezani Ali Akbari F, Fathi Moghadam H. Effect of C-peptide Alone or in Combination with Nicotinamide on Glucose and Insulin Levels in Streptozotocin-Nicotinamide-Induced Type 2 Diabetic Mice. The Malaysian journal of medical sciences : MJMS 2014;21(4):12–7. [PMC free article] [PubMed] [Google Scholar]
- 287.Folwarczna J, Janas A, Cegiela U, Pytlik M, Sliwinski L, Matejczyk M, et al. Caffeine at a Moderate Dose Did Not Affect the Skeletal System of Rats with Streptozotocin-Induced Diabetes. Nutrients. 2017;9(11):1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Ghasemi A, Khalifi S, Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review). Acta physiologica Hungarica. 2014;101(4):408–20. [DOI] [PubMed] [Google Scholar]
- 289.Guo S, Chen Q, Sun Y, Chen J. Nicotinamide protects against skeletal muscle atrophy in streptozotocin-induced diabetic mice. Archives of physiology and biochemistry. 2019;125(5):470–7. [DOI] [PubMed] [Google Scholar]
- 290.Lee HJ, Yang SJ. Supplementation with Nicotinamide Riboside Reduces Brain Inflammation and Improves Cognitive Function in Diabetic Mice. International journal of molecular sciences. 2019;20(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Reddy S, Bibby NJ, Wu D, Swinney C, Barrow G, Elliott RB. A combined casein-free-nicotinamide diet prevents diabetes in the NOD mouse with minimum insulitis. Diabetes Res Clin Pract 1995;29(2):83–92. [DOI] [PubMed] [Google Scholar]
- 292.Hu Y, Wang Y, Wang L, Zhang H, Zhao B, Zhang A, et al. Effects of nicotinamide on prevention and treatment of streptozotocin-induced diabetes mellitus in rats. Chin Med J (Engl) 1996;109(11):819–22. [PubMed] [Google Scholar]
- 293.Chlopicki S, Swies J, Mogielnicki A, Buczko W, Bartus M, Lomnicka M, et al. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br J Pharmacol 2007;152(2):230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem 2007;282(34):24574–82. [DOI] [PubMed] [Google Scholar]
- 295.Tam D, Tam M, Maynard KI. Nicotinamide modulates energy utilization and improves functional recovery from ischemia in the in vitro rabbit retina. Ann N Y Acad Sci 2005;1053:258–68. [DOI] [PubMed] [Google Scholar]
- 296.Olmos PR, Hodgson MI, Maiz A, Manrique M, De Valdes MD, Foncea R, et al. Nicotinamide protected first-phase insulin response (FPIR) and prevented clinical disease in first-degree relatives of type-1 diabetics. Diabetes Res Clin Pract 2006;71(3):320–33. [DOI] [PubMed] [Google Scholar]
- 297.Crino A, Schiaffini R, Ciampalini P, Suraci MC, Manfrini S, Visalli N, et al. A two year observational study of nicotinamide and intensive insulin therapy in patients with recent onset type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2005;18(8):749–54. [DOI] [PubMed] [Google Scholar]
- 298.Liu HK, Green BD, Flatt PR, McClenaghan NH, McCluskey JT. Effects of long-term exposure to nicotinamide and sodium butyrate on growth, viability, and the function of clonal insulin secreting cells. Endocr Res 2004;30(1):61–8. [DOI] [PubMed] [Google Scholar]
- 299.Reddy S, Salari-Lak N, Sandler S. Long-term effects of nicotinamide-induced inhibition of poly(adenosine diphosphate-ribose) polymerase activity in rat pancreatic islets exposed to interleukin-1 beta. Endocrinology. 1995;136(5):1907–12. [DOI] [PubMed] [Google Scholar]
- 300.Gaudineau C, Auclair K. Inhibition of human P450 enzymes by nicotinic acid and nicotinamide. Biochem Biophys Res Commun 2004;317(3):950–6. [DOI] [PubMed] [Google Scholar]
- 301.Lin F, Xu W, Guan C, Zhou M, Hong W, Fu L, et al. Niacin protects against UVB radiation-induced apoptosis in cultured human skin keratinocytes. Int J Mol Med 2012;29(4):593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hamada S, Hara K, Hamada T, Yasuda H, Moriyama H, Nakayama R, et al. Upregulation of the mammalian target of rapamycin complex 1 pathway by Ras homolog enriched in brain in pancreatic beta-cells leads to increased beta-cell mass and prevention of hyperglycemia. Diabetes. 2009;58(6):1321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57(4):945–57. [DOI] [PubMed] [Google Scholar]
- 304.Sataranatarajan K, Ikeno Y, Bokov A, Feliers D, Yalamanchili H, Lee HJ, et al. Rapamycin Increases Mortality in db/db Mice, a Mouse Model of Type 2 Diabetes. J Gerontol A Biol Sci Med Sci 2016;71(7):850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol 2012;165(7):2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem 2011;286(21):18465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Liu P, Yang X, Hei C, Meli Y, Niu J, Sun T, et al. Rapamycin Reduced Ischemic Brain Damage in Diabetic Animals Is Associated with Suppressions of mTOR and ERK1/2 Signaling. Int J Biol Sci 2016;12(8):1032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zhao Z, Liu H, Guo D. Aliskiren attenuates cardiac dysfunction by modulation of the mTOR and apoptosis pathways. Braz J Med Biol Res 2020;53(2):e8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes. 2011;60(3):827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, et al. AMPK Activation Ameliorates Alzheimer’s Disease-Like Pathology and Spatial Memory Impairment in a Streptozotocin-Induced Alzheimer’s Disease Model in Rats. J Alzheimers Dis 2015;43(3):775–84. [DOI] [PubMed] [Google Scholar]
- 311.Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 2014;171(13):3146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Peixoto CA, de Oliveira WH, da Rocha Araujo SM, Nunes AKS. AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol 2017;298(PtA):31–41. [DOI] [PubMed] [Google Scholar]
- 313.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14(5):661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Nejabati HR, Samadi N, Shahnazi V, Mihanfar A, Fattahi A, Latifi Z, et al. Nicotinamide and its metabolite N1-Methylnicotinamide alleviate endocrine and metabolic abnormalities in adipose and ovarian tissues in rat model of Polycystic Ovary Syndrome. Chem Biol Interact 2020;324:109093. [DOI] [PubMed] [Google Scholar]
- 315.Stevens MJ, Li F, Drel VR, Abatan OI, Kim H, Burnett D, et al. Nicotinamide reverses neurological and neurovascular deficits in streptozotocin diabetic rats. J Pharmacol Exp Ther 2007;320(1):458–64. [DOI] [PubMed] [Google Scholar]
- 316.Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high fat diet feeding in mice. Diabetes. 2014;64(1):36–48. [DOI] [PubMed] [Google Scholar]
- 317.Zhao H, Wang ZC, Wang KF, Chen XY. Abeta peptide secretion is reduced by Radix Polygalaeinduced autophagy via activation of the AMPK/mTOR pathway. Molecular medicine reports. 2015;12(2):2771–6. [DOI] [PubMed] [Google Scholar]
- 318.Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, et al. Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s disease mouse model. J Neurosci 2017;37(9):2449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yamashima T, Ota T, Mizukoshi E, Nakamura H, Yamamoto Y, Kikuchi M, et al. Intake of ω−6 Polyunsaturated Fatty Acid-Rich Vegetable Oils and Risk of Lifestyle Diseases. Adv Nutr 2020;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lee JH, Lee JH, Jin M, Han SD, Chon GR, Kim IH, et al. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med 2014;46:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Hu P, Lai D, Lu P, Gao J, He H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med 2012;29(4):613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Lee Y, Hong Y, Lee SR, Chang KT. Autophagy contributes to retardation of cardiac growth in diabetic rats. Lab Anim Res 2012;28(2):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]


