Abstract
Introduction:
Sickle cell disease (SCD) is the most common abnormal genetic blood disease that affects ~100,000 Americans. Approximately 20% to 37% of children with sickle cell anemia have silent cerebral infarcts by the age of 14 years old. Neurocognitive deficits are identified in infants and preschool children with SCD. The purpose of this systematic literature review is to provide a comprehensive understanding of the prevalence, severity, and the associated risk factors for neurodevelopmental delays (NDDs) in children with SCD 5 years of age and younger.
Methods:
Systematic search of 6 databases identified 2467 potentially relevant publications and 8 were identified through a manual search. Only 24 articles met the inclusion criteria.
Results:
We identified an increased prevalence of NDDs (cognitive, motor, or both). Children experienced deficits with language, attention and behavior, executive functioning, school readiness and/or academic performance, and motor skills (fine and gross motor functioning). Risk factors include silent cerebral infarcts and strokes, SCD genotype (HbSS > HbSC), other biologic, and social factors.
Conclusion:
NDDs are common in children ages 0 to 5 years old with SCD. There is an opportunity to improve adherence to national guideline recommendations and early detection practices by pediatricians, hematologists, and other health care providers.
Keywords: sickle cell anemia, sickle cell disease, neurocognitive or neurodevelopmental delay, silent cerebral infarct, stroke, pediatrics
Sickle cell disease (SCD) is the most common abnormal genetic blood disease that affects ~100,000 Americans.1,2 The prevalence of stroke in children with SCD is 5% to 10% in the absence of primary stroke prevention with transcranial Doppler (TCD) screening and transfusion.3-7 Up to 37% of children with sickle cell anemia have been reported to have silent cerebral infarcts (SCIs) by the age of 14 years, while 27% have been reported before the age of 6 years old.8 Across many scientific studies, the most frequent reported prevalence of SCI is ~20% to 35%.7,8 The polymerization of hemoglobin S with low oxygen tension causes an anatomical change in the red blood cells from round donut like shape to a sickle shape. This causes membrane injury, hemolysis, and increased levels of free hemoglobin in the plasma.9 Some of the disease related complications include vaso-occlusive episodes, moderate to severe anemia, as well as stroke and SCI.3,4,7
Many children with SCD experience neurodevelopmental delays (NDDs), which result in poor school readiness skills, academic performance, grade retention, failure to graduate from high school, limited higher education attainment, and reduced employment.10,11 Strokes and SCI contribute to NDD with lifelong implications. In the United States, most people with SCD are African American and have lower socioeconomic status (SES). Both economic status and parent education levels are associated with NDD.12,13 While there is some information about the prevalence of NDD in children with SCD, there remain many gaps in our understanding.
There is limited understanding surrounding when NDD begin. There is evidence that NDD can be present as early as infancy and are highly prevalent in adulthood.14,15 In school age children, we know that NDD and academic problems are more frequently noticed in elementary school by the child’s academic instructor(s) and/or parents. However, we are uncertain of the prevalence and the magnitude of developmental delays in children ages 0 to 5 years old with SCD.
There are numerous exploratory studies that have investigated neurocognitive functioning in SCD.14,16,17 However, much is still unknown about the overall prevalence of NDD, severity, and associated risk factors of children living with SCD. Some of those gaps include understanding the prevalence of NDD in children with SCD ages 0 to 5 years old.3,18,19 Many risk factors have been identified, but there remain environmental, biologic, and socioeconomic risk factors for NDD that have not been evaluated in children with SCD.14,16,17 Early detection of NDD in young children with SCD is important to promote optimal development. The purpose of this systematic literature review is to provide a comprehensive understanding of the prevalence, severity, and associated risk factors in children with SCD ≤5 years of age. To our knowledge, this is the first systematic literature review to focus on young children with SCD and NDD.
METHODS
A systematic literature review was conducted with the guidance of a medical librarian using the following research databases: CINHAL, ClinicalTrials.gov, Embase, PsycINFO, PubMed, and Scopus. The databases were searched using the following search terms: sickle cell and child and either cognitive dysfunction or neuropsychologic test. For instance, in PubMed sickle cell was searched using the following mesh term: “Anemia, Sickle Cell”[Mesh] and child was searched using: “Child”[Mesh] OR “Infant”[Mesh] OR “Pediatrics”[Mesh]. The PubMed search terms for cognitive dysfunction included: “Cognitive Dysfunction”[Mesh] OR “Child Development”[Mesh] OR “Cognition”[Mesh] OR “Neurodevelopmental Disorders”[Mesh] OR “Executive Function”[Mesh] OR “Cognition Disorders”[Mesh] and the neuropsychologic test was searched using: “Neuropsychological Tests”[Mesh]. Please refer to Figure 1 for the search strategy example using PubMed database.
FIGURE 1.

Search Strategy for Medline.
Literature Search
Studies published in peer reviewed journals that evaluated NDD in young children with SCD were the focus of this review. Article eligibility were studies that included SCD children between the ages of 0 and 5 years and written and/or translated into the English language. Studies that were included for review were observational, experimental, and qualitative research projects. Studies were excluded if they were dissertations, case reports, editorials, letters, commentaries, meta-analyses, systematic reviews, conference papers or reports, books, and clinical trials without posted results. Additional exclusion criteria were studies with SCD children older than 5 years old as well as studies with sickle cell trait versus disease. Research studies were also excluded if the results were not represented or corresponded to the specific age category of children between ages 0 months to 5 years and 11 months. For example, if the authors reported the results for a broader age category of children (such as children between 2 and 10 y old), and if it was not possible to identify results for children ages 0 to 5 years old, the study was not included.
Screening
Two independent investigators (L.M.J.K. and P.T.) screened the citations selected as a result of the search strategy. The review process was completed in 3 screening tiers. The first screening tier was title review, the second tier was abstract review, and the final tier was full text review. The first independent investigator (L.M.J.K.) reviewed all titles and abstracts for project relevance. The second independent investigator (P. T.) randomly reviewed 10% of the abstracts. Of these abstracts, the 2 independent investigators agreed on 96% of the abstracts selected for full text evaluation. The abstracts with disagreement, L.K. and P.T. discussed and obtained final agreement. The second investigator randomly reviewed 10% of the full text articles including 13 of the 130 articles. Of these articles, the 2 investigators agreed on 77% of the full text articles. If the independent investigators were unable to come to an agreement at any screening tier level than they collectively discussed and resolved any issues and/or concerns. Please refer to the PRISMA Diagram in Figure 2.20
FIGURE 2.
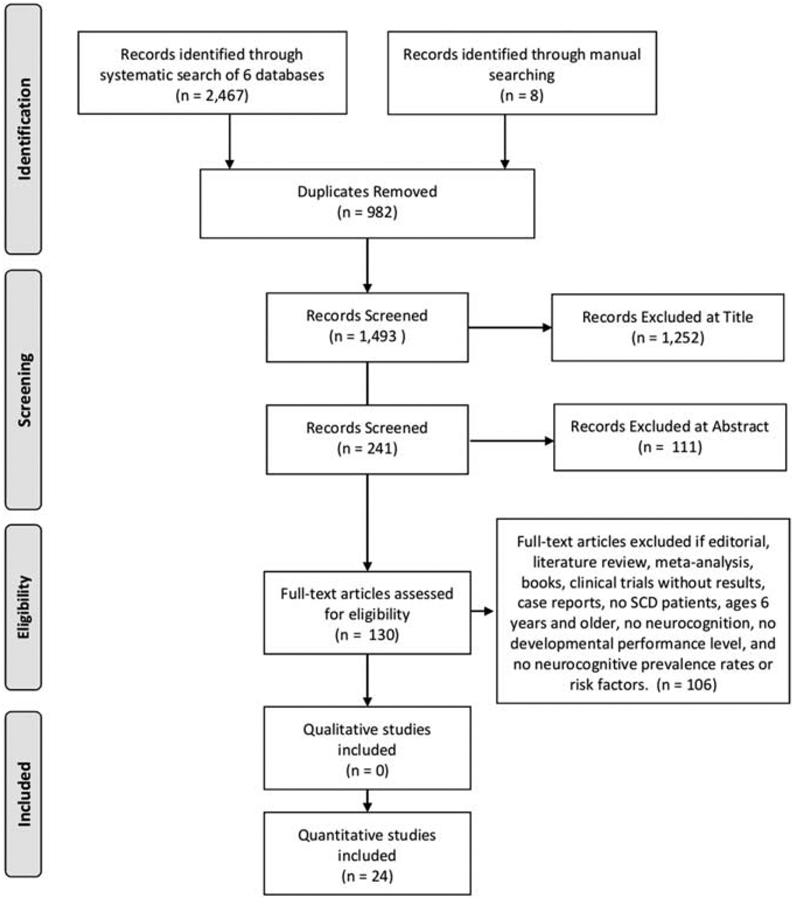
PRISMA 2009 Flow Diagram. SCD indicates sickle cell disease.
Data Extraction and/or Analysis
The Matrix Method was used to synthesize findings into 1 table (Table, Supplemental Digital Content 1, http://links.lww.com/JPHO/A432).21 Each of the research journal articles were evaluated and organized in chronologic order based on the primary author’s last name and year. Each study was abstracted into the following categories: reference number based on the author’s last name and year, sample size and related characteristics, prevalence, severity, and risk factors. A NDD is a decline in the growth and development of a child which is because of a decline in either their cognitive, motor, or both cognitive and motor functions.
RESULTS
The search strategy resulted in 2467 research publications identified through 6 databases and 8 publications that were identified during review of other papers (Fig. 2).20 On the basis of the inclusion and exclusion criteria, removal of duplicates, as well as excluded titles and abstracts, there were 130 full text articles that were eligible for review. Of those publications, only 24 research articles met criteria for inclusion in this systematic literature review (Table, Supplemental Digital Content 1, http://links.lww.com/JPHO/A432 includes the final report of the retained publications for this systematic literature review).
Overview
Supplemental Table (Supplemental Digital Content 1, http://links.lww.com/JPHO/A432) includes 24 research articles that reported neurodevelopmental function and/or dysfunction as a primary outcome. Study samples included participants from birth to younger than 6 years old. Sample sizes ranged from 16 to 344, with studies occurring in the United States (n= 18), United Kingdom (n= 4), Italy (n= 1), and Nigeria (n= 1). The sample size of participants with SCD varied from <50 children (54.2%, n= 13/24), 50 to 100 children (33.3%, n= 8/24), or >100 children (12.5%, n= 3/24). Please refer to supplemental Table (Supplemental Digital Content 1, http://links.lww.com/JPHO/A432) for further details. The most frequent design was the case control study and it was reported in 11 studies. Of the 19 studies that specified SCD genotypes, only 4 studies reported 100% HbSS genotype. The remaining 15 studies differentiated 2 or more SCD genotypes. Genotype HbSS was the most frequent at 49% to 97% and the sample size ranged from n= 21 to n= 201 participants. HbSC was the second most frequent genotype reported in 14 studies at 8% (n= 1) to 43% (n= 38). HbSβ+ thalassemia was reported in 7 studies and the frequency ranged from 2% (n= 1) to 18% (n= 9). HbSβo thalassemia was reported in 6 studies and the frequency ranged from 2% (n= 1) to 4.1% (n= 14). HbSS with hereditary persistence of fetal hemoglobin (SS with HPFH) was reported in 1 study and the same sample size was 5% (n= 2). Of the 17 studies that reported sex, 12 included more males while 5 reported more females. Of the 10 studies that reported ethnicity, African American ethnicity was most frequently reported at 86% to 100% of its participants (n= 8). In 2 of the 10 studies, 100% of the participants were Black British. Of the 3 studies that used 2 or more categories to describe ethnicity, only 1 study included Hispanics, which represented 27% of the sample. Similarly, only 1 study reported findings for Jamaican and/ or Caribbean participants and they represented 14% of the sample. Of the 9 studies that reported primary caregiver education level, the majority of the studies reported high school as the highest education level obtained (n= 7), while some college or college graduate was the highest education level reported (n= 3). Of the 9 studies that reported annual family income, the lowest annual income ranged from 0 to $10,000 to <$39,999 and the highest annual income ranged from $40,000 to > $150,000 (n= 6). One study reported a mean annual family income as $8,600 and 2 studies reported the Middle British annual income as the highest income.
After reviewing all 24 articles, children with SCD had an increased risk or prevalence of NDD (cognitive, motor, or both). Of all the articles, 45.8% (n= 11) included both cognitive and motor skills of the children with SCD, 41.7% (n= 10) either cognitive or motor skills, while 12.5% (n= 3) did not specify the type of NDD.22-24
Neurocognitive function assessments included language, attention and behavior, executive function, as well as working memory. A variety of assessment tools were used to measure neurocognitive function and included age-appropriate measures. Fifty-eight percent (n= 14) of the studies reported the degree of neurocognitive impairment. In these studies, findings ranged from children with SCD with no impairment25-28 or below average functioning.23,24,29-36 Ten percent to 70% of the children with SCD had neurocognitive scores that were below average.
Of the 10 studies that measured language ability and function, 7 found deficits to be common. Language in children with SCD ranged from normal and appropriate language development (through parent survey,37 parent-child survey,38 and neurodevelopmental measure39) to failed screening tests with speech and/or communication deficits (through neurodevelopmental measures).29,32,34,40-43 These language deficits were present in 18% (n=9/50) to 70% (n=21/30) of children with SCD. Children with SCD had lower verbal intelligence quotient (IQ),34,40 performance IQ,34 as well as receptive32 and expressive language29,32,42,43 scores. Children with SCD ages 12 to 18 months had higher language scores than other children with SCD ages 32 to 40 months old, suggesting a decline with age.41
Children with SCD often experience attention and/or behavioral problems. Of the 24 research articles, attention (through neurodevelopmental measures30,31,40 or a parent questionnaires31,43) was measured in 4 studies and behavioral abnormalities (through neurodevelopment measure25 or parent rated surveys41) was measured in 2 studies. Only 1 study reported that toddlers did not show any school related differences in attention.43 In 1 longitudinal study, the children with SCD who did not develop a SCI or stroke, 88% (n= 7/8) of those tested displayed attention difficulties.30 In addition, children with SCD experienced more difficulty with selective attention, had below average attention performance scores, and had a higher number of errors during their assessment tasks.31,40 In 1 study, 97% (n= 187/193) to 99% (n= 190/193 to 192/193) of the children with SCD scored higher than the 85th percentile for all areas of the behavioral scales.25 In addition, parents rated the children with HbSS/Sβ° (64%, n= 39/61) as having less activity then the children with HbSC/Sβ+ (36%, n= 22/61) (mean activity scores = 4.4 vs. 4.7, respectively, P < 0.05).41
Children with SCD experienced abnormalities with executive functioning. Of the 5 studies that measured executive functioning, all 5 found deficits to be common. These studies reported related findings that children with SCD had poorer executive functioning as compared with healthy peers (through neurodevelopmental measures30,31,35,36,41 and/or parent report31). For example, 88% (n= 7/8) of the children with no SCI or stroke had executive function deficits.30 Children with SCD could perform the task given to them but they had significant underlying cognitive deficiencies.35 Children with higher neurologic risk (especially those children with genotypes SS and Sβ0) had poorer executive functioning scores41 and lower processing speed scores36 than other children with lower neurologic risk (genotypes SC and Sβ+).36,41
Children with SCD experienced memory deficits. Four studies reported working memory deficits in children with SCD (through neurodevelopmental measures26,31,39,41 and/or parental report31). Two studies reported lower memory scores in children with SCD.26,41 In 1 of the 2 studies, found children with higher neurologic risk had poorer working memory as compared with children with lower neurologic risk.41 Only 1 study reported no difference in the memory scores of children with SCD.39 However, 1 study reported higher working memory in children with SCD, but the findings were not significant.31
Not only do children with SCD experience NDD but they also experience challenges in their school readiness and even in their overall academic performance. School readiness is assessed in preschool children to evaluate how prepared the child is for primary school (through neurodevelopmental measures26,39 and/or school readiness tests/evaluations26,39). Whereas, academic performance evaluates how proficient the child is at completing academic course-work, and it may identify related learning problems and/or failed grade(s) over time (through neurodevelopmental measures,30,35 academic performance evaluations,30,35 and/or parent questionnaires43). Four of 5 studies reported school readiness and/or academic challenges of children with SCD. There were no differences in the school readiness skills of the children with SCD as compared with the healthy children.35,39 The 3 remaining studies reported a decline in school readiness and/or academic performance of the children with SCD.26,30,43 For example, a longitudinal study followed a group of children for an average of 14 years.30 In this cohort 75% (n= 6/8) of the children with SCI had academic challenges and four children failed at least 1 grade level.30 In another study, 50% (n= 5/10) to 70% (n= 7/10) of children with SCD were deficient in school readiness.26 In addition, children (especially toddlers) with language difficulties had early onset learning challenges (n= 11/41, P= 0.045).43
Psychomotor function included assessments of fine motor and gross motor skills. Of the 24 studies, 46% (n= 11) reported findings of children with fine motor and/or gross motor skills with and without deficiencies (through neurodevelopmental measures,24-26,32,33,36,41,42,44 parent surveys,32,37,41,44 parent-child surveys,38 and/or home observation32,41). Half of these studies reported findings for both fine and gross motor skills.24-26,32,38,42 Of the 11 studies, only 18% (n= 2) reported that majority of the children with SCD had an average37 or borderline normal motor skills.33 Of the remaining 82% (n= 9) of studies that reported abnormal motor skill functioning, 6% (n= 11/193) to 35% (n= 18/52) of the children experienced deficiencies in their motor skills.24-26,32,36,38,41,42,44 The range of children with fine motor skill deficiencies ranged from 14% (n=7/50) to to 35% (n=18/52) and the percentage of children with gross motor skill deficiencies was about 8% (n=4/50).36,42 Two studies reported an abnormal motor functioning in the older toddlers versus the younger toddlers and they found that older children had poorer motor functioning than the younger children.41,44 The child’s risk increased from 15% (n=66 with 42 participants with HbSS and 24 with HbSC or other genotype) at 6 months of age to 28% (n=26 with 17 participants with HbSS and 9 participants with HbSC or other genotype) at 36 months of age.44 Another important finding was 35% (n=18/52) of children with higher neurologic risk had lower fine motor scores as compared with 4% (n=1/25) of children with lower neurologic risks.36
Children with SCD who experience SCIs and strokes are at an increased risk for NDD. Of the 7 studies that measured SCIs and stroke, all the studies found deficits to be common (through developmental surveillance program,22 neurodevelopmental measures,23,30,34,36,42 ICD-9 codes,22 and/or parent surveys42,43). Of the 24 studies, 29% (n= 7) reported findings of children with stroke or SCI,22,30,43 TCD,23,36,42,43 and/or MRI/MRA34 results. Of the 7 research studies that discussed stroke, 100% (n= 7) reported an abnormal performance in the children with SCD which was directly related to the presence of a SCI and/or stroke.22,23,30,34,36,42,43 The children with SCD who were at risk for a stroke or those who had a SCI or stroke were more likely to have cognitive abnormalities,23,30,34,36,42,43 developmental delays,22,23 academic challenges,30 and/or multiple disabilities.22 The children who experienced cognitive delays had deficiencies in language and communication,42,43 fine motor skills,36,42 gross motor skills,42 and syntactic processing speeds.36 Children with higher TCD velocities were at greater risk for NDD and their risk increased from low risk at 3 months of age to moderate high-risk at 12 months of age.23
SCI and/or stroke are common complications in children with SCD and it is an important risk factor for NDD.22,23,30,34,36,42,43 Of all the studies that reported findings related to SCI and/or stroke, 1 study obtained the child’s stroke diagnosis from the International Classification of Disease, 9th edition (ICD-9) code which was located in the child’s medical record,22 TCD23,36,42,43 results were used to assess the child’s stroke risk while MRI/MRA30,34,36 results were used for a definitive stroke diagnosis. Some children with SCD experienced SCI or stroke before the age of 5 years old.22,30 A longitudinal study reported 27% (n= 10/37) of the children had SCI before the age of 5 years old while 32% (n= 12/37) had SCI after the age of 5 years old.30 In another study, the association with SCD and a developmental disability was attributed to the presence of a stroke [observed/expected ratio was 130, n= 38, 95% confidence interval (CI): 69-222, P < 0.0001].22 They also reported that the association with stroke increased in children with SCD who had a developmental disability and were 5 years old or less (observed/expected ratio was 143, n= 28, 95% CI: 69-263, P < 0.0001).22 In addition, the association with stroke increased in children with a developmental disability with stroke at age 5 to 10 years old (observed/expected ratio was 300, n= 4, 95% CI: 62-877, P < 0.0001).22 TCD screenings were used to examine for stroke risk and MRA was used to evaluate for overt stroke.43 Researchers reported 17% (n= 5/30) of the children with normal language screenings had abnormal TCD results or overt stroke while 55% (n= 6/11) of children with abnormal language screenings had abnormal TCD results or overt stroke (P < 0.05).43 Other researchers reported 36% (n= 23/64) of the children had abnormal MRI results while 73% (n= 47/64) had abnormal MRA results.34 In that same study, 66.7% (n= 12/18) of the children ages 4 to 6.6 years old did not have a brain lesion (stroke or SCI), while 16.7% (n= 3/18) had a lesion that was <500 mm3 and 16.7% (n= 3/18) had a lesion that was >500 mm3.34 However, there was no correlation between SCI and neurocognitive functioning.34 The remaining 6 studies reported stroke and/or the risk of stroke as a predictor for neurocognitive decline in children with SCD.22,23,30,36,42,43
Many study teams investigated biologic demographics and related social determinants of health as associated risk factors for neurocognitive decline in children with SCD. Sixty-seven percent (n= 16) of the studies reported risk factors that were related to the biologic demographics and/or social determinants of health of children with SCD (through neurodevelopmental measures,23-25,28,29,31,33-36,39,41,42,44 developmental surveillance program,22 ICD-9 code,22 parent surveys,28,31,32,41,42,44 and/or home observations28,32,41,42). Of these 16 studies, 50% (n= 8) reported no risk for either 1 or more biologic demographics or social determinants of health.22,24,28,29,31,32,41,42 Of the 16 studies, 75% (n= 12) reported associations related to the child’s biologic demographics and/or social determinants of health.23,25,28,29,32-36,39,41,44
The biologic demographics or risk factors that were associated with neurodevelopmental decline in children with SCD include SCD genotype, age, and sex. Biologic demographics were assessed and reported in 63% (n= 15) of all 24 research studies, while only 38% (n= 9) of all the 24 studies reported abnormal findings for the biologic demographics for children with SCD. For instance, the high-risk genotypes were associated with working memory deficits,41 higher TCD velocities and lower processing speeds,36 lower auditory discrimination skill development,39 as well as poorer cognitive and psychomotor development.44 Older age was associated with poor cognitive functioning,25,34,36,41,44 academic challenges,35 and developmental delays.23 Several studies reported as the child grew older in age many of the children experienced difficulties with communication, daily living, socialization, school readiness skills, and had lower IQ scores and longer processing speeds.25,34-36,41 Of these 5 studies, the age of the children did not exceed 5 years old,25,36,41 while the age exceed 5 years in 2 studies.34,35 Developmental delays occurred as early as 3 to 12 months of age and increased in prevalence over time.23 Cognitive abnormalities increased in age from 12 to 24 months.44 Male sex was associated with delays in neurocognitive function and neurodevelopment.33,34
The social determinants of health-related risk factors that were associated with neurodevelopmental decline in children with SCD include annual family income or SES, as well as parental education level. Social determinants of health were assessed and reported in 33% (n= 8) of the 24 research studies, while only 21% (n= 5) of all the 24 research studies reported abnormal findings. Lower annual family income was negatively associated with poorer cognitive and psychomotor scores.25 While, higher annual family income was positively associated with the HOME environment32 (HOME, Home Observation for Measurement of the Environment), which is a measurement tool that assessed the parent-child interaction as well as various stimuli within the environment. Higher annual family income was also positively associated with higher cognitive performance scores28,34 such as language, memory, attention, and visual spatial. Lower parental education was associated with lower cognitive scores25 and screening tool failure.29 Higher parental education was associated with higher language development, memory, and attention in the children with SCD.28
Screening tool performance level as well as other factors were predictors of neurodevelopmental decline in children with SCD. Many tools were used to assess neurocognitive functioning in children with SCD (through neurodevelopmental measures,23,29,31,34,40,44 auditory attention tasks,40 parent surveys,31,32,43,44 and/or home observations32). Of the many tools, 33.3% (n= 8) reported a correlation between the child’s performance on the screening tools and their neurocognitive functioning.23,29,31,32,34,40,43,44 Lower cognitive screening results were associated with language or speech problems,29 higher TCD velocities,23 repeated grades as they aged,43 a learned helplessness attributional style of coping,44 and reduced attention or focus.40 Home exposure to learning materials and parental involvement was a factor that was positively associated with the child’s cognitive scores.32 One study reported no association between the child’s IQ scores and their neurodevelopment functioning.31 These researchers reported that the younger children did not display deficits in their early development which were more commonly displayed in the older children.31 In this study, the children had normal IQ levels but had domain specific deficits.31 However, in another study there was a trend for full IQ and performance IQ scores which showed a decreased in these scores as the child aged.34
There were additional risk factors that did not meet the above specific categories. Of these miscellaneous risk factors, 29% (n=7) of all the 24 research studies assessed and reported findings (through neurodevelopmental measures,23,28,33,35,41,42,45 parental surveys,28,41,42 and/or home observations28,41,42). The additional factors that were associated with neurocognitive decline in children with SCD included co-morbidities such as pneumonia,33 low arterial oxygen saturation,23,45 school absenteeism >7 days35 a decline in physical activity levels,41 and premature births.42 However, preschool or primary school attendance was positively associated with cognitive improvements such as language development.28
DISCUSSION
NDD were common in children with SCD, beyond what is observed in normative populations and demographically similar controls. Children showed deficits in many cognitive domains (example language, memory, attention, and even executive functioning). Developmental delays begin early in infancy and neurocognitive decline continues as the child ages. In this systematic literature review, the 2 neurodevelopmental domains with the highest confidence across studies were motor and language. In addition, the domains with moderate confidence were attention/behavior, executive function, as well as poor school readiness and/or academic performance. Deficits in these neurodevelopmental domains support the need for early educational interventions, age-appropriate learning tools both at home and at school, as well as immediate, frequent and ongoing surveillance of developmental skills by health care providers.
One of the most important findings was the motor deficit of children with SCD. There was a high prevalence of children with fine and/or gross motor deficits, which was reported in 38% (n=9/24) of the articles. If motor skills are not evaluated in children with SCD, deficits can remain undetected and/or unnoticed by health care providers and educators. Ongoing screening practices of both fine and gross motor skills are essential to children with SCD. Early interventions for physical therapy and occupational therapy can be accessed and implemented through state early intervention programs. However, for these programs to be utilized by children with motor deficits, there must be an initial evaluation and/or screening.
Children with SCD were deficient in their motor as well as their language skills. Twenty-nine percent (n= 7/24) of the research articles reported children with SCD as having lower verbal IQ, receptive, expressive, and/or language scores. Children with language deficits should be properly screened and assessed to evaluate the specific type of deficiency present. Once a diagnosis is identified then tailored interventions should be used to promote optimum growth and development of each individual child. Parental education is also significant in early recognition of warning signs and/or problems that may be present in children with SCD.
Implications from this systematic review are supported by the American Academy of Pediatrics (AAP). In January of 2020, AAP published a clinical report which outlines a universal screening model as well as a developmental surveillance for pediatricians and other pediatric providers to use while rendering care to young children.46 These new guidelines encourage health care providers to continue routine well-child visits with an emphasis on recognizing children at risk for developmental delays during annual well-child visits of children ages 4- and 5-year old.46 It is important that pediatricians, hematologist, and advanced practice providers adopt and follow the guidelines promoting early screening of developmental delays. It is also important that children with SCD be screened frequently and regularly during every developmental stage and/or milestone.
This systematic literature review also highlights many risk factors that are associated with NDD in children with SCD. The risk factors with the highest confidence across studies were a diagnosis of either a stroke or SCI, elevated TCD screening results, and high-risk genotypes (SS and Sβ°, which is hemoglobin S beta-O thalassemia). The risk factors with moderate confidence were age (older children vs. younger children) as well as SES and/or low family income. Early identification of these risk factors is important in children with SCD because prompt intervention may help to improve their health, academic, and social outcomes over time. With early interventions, children with SCD will have an enhanced opportunity to excel scholastically, socially, and developmentally.
Stroke was a high confidence predictor of NDD in children with SCD. Therefore, interventions should focus on early screening and identification strategies. The NHLBI guidelines indicate that children with sickle cell anemia (with high-risk genotypes, SS and Sβo) should receive TCD screenings beginning at age 2 years.7 TCD screenings should be completed annually until the child is 16 years old. Transfusion therapy is the standard in care for children with SCD who have experienced overt stroke to prevent additional strokes. Transfusion reduces the risk of stroke by 92% in those with abnormal TCD; primary stroke prevention is essential to prevent strokes in children with sickle cell anemia.7
Future research should focus on early screening practices for all children with SCD beginning as early as infancy and should be performed regularly with every developmental stage and/or milestone. Other researcher opportunities are needed to determine if children with SCD and NDD received supplemental treatment (such as chronic blood transfusions, hydroxyurea, and other interventions) as well as to determine if there are variations in the NDD of children who received additional treatment as compared with those children who did not. Large prospective cohort and/or longitudinal studies should be conducted. The use of local and national registries and public health SCD surveillance can help monitor progress with adherence to screening guidelines and assess for long-term health outcomes. Studies should include uniform screening practices and/or interventions for children with SCD per the NHLBI and AAP guidelines.7 Dissemination and implementation projects should also be conducted to determine the best strategies to disseminate these guidelines to pediatricians and SCD providers nationally.
In addition to improving screening, there is an opportunity to improve referrals for definitive follow up after deficits are detected. Specific therapies include neurocognitive, educational, physical, occupational, behavior, and/or psychologic. In addition, other therapies (such as mind and memory games, motor skills training, academic tutoring, and speech-language training) may even be beneficial in children with deficits to help improve their overall functioning at home, school, and within various social environments.
There were several limitations including the inability to extract individual level data to assess more refined weighting of risk factors. Another limitation across multiple studies were the small sample sizes. Approximately, 54.2% of the research studies had <50 children with SCD. The small number of participants can limit the interpretation of findings as compared with studies with a larger representation of children with SCD. Small sample sizes can limit the researchers’ ability to adjust for variations in the outcome(s). In addition, meta-analysis was not performed for this systematic literature review because of the large percentage of small sample sizes, inconsistent variables and measurements used, the lack of an overarching neurodevelopmental domain, and lack of a common research design for all 24 studies.
Across all 24 studies, there were a variety of neurodevelopmental measures used to examine NDD in children with SCD. For instance, the Bayley Scales of Infant Development (BSID-II) was used to measure mental and motor functioning and average scores ranged from 85 to 115, while impairment was detected with scores <70.25,33 BSID-II scores <85 placed the child in a high neurodevelopmental risk category.44 In another study, BSID-II score was converted to a z-score (mean= 0 and SD= 1) and NDDs were present if the score was ≥ 1 SD below the mean.30 BSID-III had an average score between 8 and 12, but scores <7 were considered abnormal.32 Variations in the measurement of NDD were inconsistent from 1 study to the next. There were differences in the measurement tools as well as the definitions of NDD which created variability in the findings. PhenX recommends using uniform measures so we can compare similar outcomes within our population of interest.47 By using the same or comparable measurement tools across various settings, researchers can easily compare similarities and contrast differences among studies. This will help to inform a standard for clinical practice and research methodology.
CONCLUSION
In this systematic review of children with SCD ages 0 to 5 years old, NDD were common and included a wide range of cognitive and motor deficits. A variety of biologic and social factors were important risk factors as well. There is an opportunity to improve adherence to NHLBI recommended screening criteria for NDD in all children with SCD beginning at a very early age. Early detection practices for NDD in children with SCD age 0 to 5 years old is important for each child’s individual growth and development for every moment of their childhood into adulthood.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention. Facts about sickle cell disease. 2019. Available at: https://www.cdc.gov/ncbddd/sicklecell/facts.html. Accessed January 25, 2020.
- 2.Hassell KL Population estimates of sickle cell disease in the US. Am J Prev Med. 2010;38:S512–S521. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong FD, Thompson RJ Jr, Wang W, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle Cell disease. Am Acad Pediatr. 1996;97:864–870. [PubMed] [Google Scholar]
- 4.Balkaran B, Char G, Morris JS, et al. Stroke in a cohort of patients with homozygous sickle cell disease. J Pediatr. 1992;120:360–366. [DOI] [PubMed] [Google Scholar]
- 5.Berg C, Edwards DF, King A Executive function performance on the children’s kitchen task assessment with children with sickle cell disease and matched controls. Child Neuropsychol. 2012;18:432–448. [DOI] [PubMed] [Google Scholar]
- 6.Oluwole OB, Noll RB, Winger DG, et al. Cognitive functioning in children from Nigeria with sickle cell anemia. Pediatr Blood Cancer. 2016;63:1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. Evidence-based management of sickle cell disease: expert panel report, 2014. Available at: https://www.nhlbi.nih.gov/sites/www.nhlbi.nih.gov/files/sickle-cell-disease-report.pdf. Accessed January 24, 2020.
- 8.DeBaun MR, Armstrong FD, McKinstry RC, et al. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. 2012;119:4587–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vekilov PG. Sickle-cell haemoglobin polymerization: is it the primary pathogenic event of sickle-cell anaemia? Br J Haematol. 2007;139:173–184. [DOI] [PubMed] [Google Scholar]
- 10.Day S, Chismark E The cognitive and academic impact of sickle cell disease. J Sch Nurs. 2006;22:330–335. [DOI] [PubMed] [Google Scholar]
- 11.King AA, DeBaun MR, White DA Need for cognitive rehabilitation for children with sickle cell disease and strokes. Expert Rev Neurother. 2008;8:291–296. [DOI] [PubMed] [Google Scholar]
- 12.Yarboi J, Compas BE, Brody GH, et al. Association of social-environmental factors with cognitive function in children with sickle cell disease. Child Neuropsychol. 2017;23:343–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarboi J, Prussien KV, Bemis H, et al. Responsive parenting behaviors and cognitive function in children with sickle cell disease. J Pediatr Psychol. 2019;44:1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King AA, Strouse JJ, Rodeghier MJ, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am J Hematol. 2014;89:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackin RS, Insel P, Truran D, et al. Neuroimaging abnormalities in adults with sickle cell anemia: associations with cognition. Neurology. 2014;82:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams GT, Snieder H, McKie VC, et al. Genetic risk factors for cerebrovascular disease in children with sickle cell disease: design of a case-control association study and genomewide screen. BMC Med Genet. 2003;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epping AS, Myrvik MP, Newby RF, et al. Academic attainment findings in children with sickle cell disease. J Sch Health. 2013;83:548–553. [DOI] [PubMed] [Google Scholar]
- 18.Kral MC, Brown RT, Connelly M, et al. Radiographic predictors of neurocognitive functioning in pediatric sickle cell disease. J Child Neurol. 2006;21:37–44. [DOI] [PubMed] [Google Scholar]
- 19.Ruffieux N, Njamnshi AK, Wonkam A, et al. Association between biological markers of sickle cell disease and cognitive functioning amongst Cameroonian children. Child Neuropsychol. 2013;19:143–160. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrard J Health Sciences Literature Review Made Easy: The Matrix Method, 4th ed. Burlington: Jones & Bartlett Publishers; 2014. [Google Scholar]
- 22.Ashley-Koch A, Murphy CC, Khoury MJ, et al. Contribution of sickle cell disease to the occurrence of developmental disabilities: a population-based study. Genet Med. 2001;3:181–186. [DOI] [PubMed] [Google Scholar]
- 23.Hogan AM, Kirkham FJ, Prengler M, et al. An exploratory study of physiological correlates of neurodevelopmental delay in infants with sickle cell anaemia. Br J Haematol. 2005;132:99–107. [DOI] [PubMed] [Google Scholar]
- 24.Wang WC, Grover R, Gallagher D, et al. Developmental screening in young children with sickle cell disease: results of a cooperative study. Am J Pediatr Hematol Oncol. 1993;15:87–91. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong FD, Elkin TD, Brown RC, et al. Developmental function in toddlers with sickle cell anemia. Pediatrics. 2013;131:e406–e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chua-Lim C, Moore RB, McCleary G, et al. Deficiencies in school readiness skills of children with sickle cell anemia: a preliminary report. South Med J. 1993;86:397–402. [DOI] [PubMed] [Google Scholar]
- 27.Kramer MS, Rooks Y, Washington LA, et al. Pre-and postnatal growth and development in sickle cell anemia. J Pediatr. 1980;96:857–860. [DOI] [PubMed] [Google Scholar]
- 28.Tarazi RA, Grant ML, Ely E, et al. Neuropsychological functioning in preschool-age children with sickle cell disease: the role of illness-related and psychosocial factors. Child Neuropsychol. 2007;13:155–172. [DOI] [PubMed] [Google Scholar]
- 29.Aygun B, Parker J, Freeman MB, et al. Neurocognitive screening with the Brigance preschool screen-II in 3-year-old children with sickle cell disease. Pediatr Blood Cancer. 2011;56:620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cancio MI, Helton KJ, Schreiber JE, et al. Silent cerebral infarcts in very young children with sickle cell anaemia are associated with a higher risk of stroke. Br J Haematol. 2015;171:120–129. [DOI] [PubMed] [Google Scholar]
- 31.Downes M, Kirkham FJ, Telfer PT, et al. Assessment of executive functions in preschool children with sickle cell anemia. J Int Neuropsychol Soc. 2018b;24:949–954. [DOI] [PubMed] [Google Scholar]
- 32.Drazen CH, Abel R, Gabir M, et al. Prevalence of developmental delay and contributing factors among children with sickle cell disease. Pediatr Blood Cancer. 2016;63:504–510. [DOI] [PubMed] [Google Scholar]
- 33.Glass P, Brennan T, Wang J, et al. Neurodevelopmental deficits among infants and toddlers with sickle cell disease. J Develop Behavl Pediatrics. 2013;34:399–405. [DOI] [PubMed] [Google Scholar]
- 34.Montanaro M, Colombatti R, Pugliese M, et al. Intellectual function evaluation of first generation immigrant children with sickle cell disease: the role of language and sociodemographic factors. Ital J Pediatr. 2013;39:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olatunya OS, Oke OJ, Kuti BP, et al. Factors influencing the academic performance of children with sickle cell anaemia in Ekiti, South West Nigeria. J Trop Pediatr. 2018;64:67–74. [DOI] [PubMed] [Google Scholar]
- 36.Schatz J, Schlenz A, Reinman L, et al. Developmental screening in pediatric sickle cell disease: disease-related risk and screening outcomes in 4 year-olds. J Develop Behavl Pediatrics. 2017;38:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gentry B, Hall L, Dancer J A parental survey of speech, language, and physical development of infants and toddlers with sickle cell disease. Percept Mot Skills. 1997;85:1105–1106. [DOI] [PubMed] [Google Scholar]
- 38.Gentry B, Hayes BT, Dancer J, et al. Language and motor skills of preschool children with sickle cell disease. Percept Mot Skills. 1997;84:486. [DOI] [PubMed] [Google Scholar]
- 39.Steen RG, Hu XJ, Elliott VE, et al. Kindergarten readiness skills in children with sickle cell disease: evidence of early neurocognitive damage? J Child Neurol. 2002;17:111–116. [DOI] [PubMed] [Google Scholar]
- 40.Downes M, Kirkham FJ, Telfer PT, et al. Altered neurophysiological processing of auditory attention in preschool children with sickle cell disease. J Pediatr Psychol. 2018;43:856–869. [DOI] [PubMed] [Google Scholar]
- 41.Schatz J, Roberts CW Neurobehavioral impact of sickle cell disease in early childhood. J Int Neuropsychol Soc. 2007;13:933–943. [DOI] [PubMed] [Google Scholar]
- 42.Schatz J, McClellan CB, Puffer ES, et al. Neurodevelopmental screening in toddlers and early preschoolers with sickle cell disease. J Child Neurol. 2008;23:44–50. [DOI] [PubMed] [Google Scholar]
- 43.Schatz J, Schlenz AM, Smith KE, et al. Predictive validity of developmental screening in young children with sickle cell disease: a longitudinal follow-up study. Develop Med Child Neurol. 2018;60:520–526. [DOI] [PubMed] [Google Scholar]
- 44.Thompson RJ Jr, Gustafson KE, Bonner MJ, et al. Neurocognitive development of young children with sickle cell disease through three years of age. J Pediatr Psychol. 2002;27:235–244. [DOI] [PubMed] [Google Scholar]
- 45.Hogan AM, Telfer PT, Kirkham FJ, et al. Precursors of executive function in infants with sickle cell anemia. J Child Neurol. 2012;28:1197–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipkin PH, Macias MM Promoting optimal development: identifying infants and young children with developmental disorders through developmental surveillance and screening. Pediatrics. 2020;145:e20193449. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton CM, Strader LC, Pratt JG, et al. The PhenX Toolkit: get the most from your measures. Am J Epidemiol. 2011;174:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


