ABSTRACT
Understanding the mechanisms of embryonic cell cycles is a central goal of developmental biology, as the regulation of the cell cycle must be closely coordinated with other events during early embryogenesis. Quantitative imaging approaches have recently begun to reveal how the cell cycle oscillator is controlled in space and time, and how it is integrated with mechanical signals to drive morphogenesis. Here, we discuss how the Drosophila embryo has served as an excellent model for addressing the molecular and physical mechanisms of embryonic cell cycles, with comparisons to other model systems to highlight conserved and species-specific mechanisms. We describe how the rapid cleavage divisions characteristic of most metazoan embryos require chemical waves and cytoplasmic flows to coordinate morphogenesis across the large expanse of the embryo. We also outline how, in the late cleavage divisions, the cell cycle is inter-regulated with the activation of gene expression to ensure a reliable maternal-to-zygotic transition. Finally, we discuss how precise transcriptional regulation of the timing of mitosis ensures that tissue morphogenesis and cell proliferation are tightly controlled during gastrulation.
KEY WORDS: Cell cycle, Cytoplasmic flows, Signaling waves, Gastrulation, Maternal-to-zygotic transition
Summary: This Review discusses emerging quantitative principles for how embryos coordinate cell cycle timing and morphogenesis.
Introduction
In many species, early development initiates with rapid and almost synchronous mitotic divisions that slow over time as the embryo approaches the key developmental transition to gastrulation (Foe et al., 1993; O'Farrell, 2015). These early divisions are much faster than somatic divisions in adult tissues and do not contain gap phases of the cell cycle (Farrell and O'Farrell, 2014); the cell cycle simply oscillates between DNA synthesis and mitosis. This happens because the supply of maternal mRNAs deposited in the oocyte during gametogenesis allows the cell cycle oscillator to run essentially as an unperturbed biochemical clock (Murray and Kirschner, 1989). In Drosophila, these early pre-gastrulation divisions are nuclear divisions that take place in a syncytium, i.e. a large multinucleated cell, whereas in vertebrates these rapid divisions include separation of the cytoplasm.
The large size of embryos and speed of divisions impose a need for coordination across several hundred micrometers over timescales of a few minutes or less. Thus, the regulation of these early cleavage divisions requires specialized mechanisms. Long-range coordination is achieved through gradients or traveling waves of the cell cycle oscillator. Moreover, the cell cycle-dependent regulation of cytoskeletal dynamics generates physical forces, which further facilitate embryo-wide collective dynamics.
In most metazoans, there is a transition prior to gastrulation from synchronous and rapid cell divisions to patterned divisions, which often corresponds with the establishment of cell fate specification domains (Foe, 1989; Kane et al., 1992; Arora and Nüsslein-Volhard, 1992; Murakami et al., 2004). This transition and remodeling of the cell cycle coincides with the activation of zygotic transcription (Newport and Kirschner, 1982; Lasko, 2013; Farrell and O'Farrell, 2014; Yartseva and Giraldez, 2015; Yuan et al., 2016; Jukam et al., 2017). In many species, the timing of these events is linked to the changing nuclear-to-cytoplasmic (N/C) ratio that naturally occurs when cells divide without growth (Newport and Kirschner, 1982; Edgar et al., 1986). Thus, both before and after gastrulation, the dynamics of cell cycle regulation are key to early transitions in embryonic development.
Here, we will review recent progress that dissects the spatial regulation of the cell cycle oscillator and how biochemical and mechanical signals are integrated to ensure proper coordination of early development. We will also discuss recent progress on how embryos control the number of cleavage divisions and how transcription can ensure the precise timing of the cell cycle during gastrulation. This Review will focus primarily on recent insights from the Drosophila model system, where quantitative live-imaging tools have enabled the measurement of cell cycle dynamics with high spatiotemporal precision. However, we will also discuss how mechanisms similar to those observed in the fly embryo have been uncovered in other models, including frog, fish, worm, mouse and starfish embryos.
Molecular mechanisms of cell cycle control in the pre-gastrulation embryo
The cell cycle machinery is highly conserved and has been extensively reviewed elsewhere (Heim et al., 2017; Morgan, 2006). Here, we briefly introduce the key molecular mechanisms pertinent to this Review. Cyclin-dependent kinases (Cdks) drive progression through the mitotic cell cycle (Heim et al., 2017). Activation of Cdks requires the binding of a cyclin, and different cyclins bind and activate different Cdks at each cell cycle stage (Nigg, 1995; Jeffrey et al., 1995). The oscillating concentration of cyclins throughout the cell cycle is the initial driver of mitotic periodicity (Glotzer et al., 1991; Morgan, 1997; Murray, 2004). A central control mechanism of the oscillations is cyclin degradation at mitotic exit (Holloway et al., 1993; Parry and O'Farrell, 2001). In the vast cytoplasm of developing embryos, this degradation initiates near dividing spindles and chromosomes, and can drive either local or global downregulation of Cdk1 activity (Evans et al., 1983; van der Velden and Lohka, 1994; Edgar et al., 1994a; Su et al., 1998; Huang and Raff, 1999; Ban et al., 2007; Bischof et al., 2017). This dependence primarily on autonomous, oscillatory Cdk1 activity for cell cycle progression is unique to the simple cell cycles in the early embryo, which lack gap phases, whereas in somatic cells the cell cycle can be paused at the G1/S or G2/M transitions (Elledge, 1996; Morgan, 1997).
In addition to changes in Cdk1 activity driven by oscillations in cyclin concentration, feedback mechanisms are also important for the regulation of Cdk1 activity. At mitotic entry, two inhibitory phosphorylation events, mediated by the kinases Wee1 and Myt1 (Campbell et al., 1995; Mueller et al., 1995a,b), shape Cdk1 activity (Fig. 1Ai). Wee1 tyrosine kinase phosphorylates Cdk1 at the Y15 residue (Parker and Piwnica-Worms, 1992), whereas Myt1 can phosphorylate both Y15 and T14 residues (Kornbluth et al., 1994; Mueller et al., 1995a). Cdc25 phosphatases antagonize the action of Wee1 and Myt1 by removing Y15 and T14 inhibitory phosphate groups from Cdk1 (Kumagai and Dunphy, 1992; Morgan, 1997). Cdk1 participates in a positive-feedback loop by inactivating Wee1 and activating Cdc25 (Mueller et al., 1995b; Kumagai and Dunphy, 1992; Hoffmann et al., 1993). This positive-feedback loop is important for the regulation of mitotic entry (Solomon et al., 1990; Sha et al., 2003; Pomerening et al., 2003; Stumpff et al., 2004; Tsai et al., 2014) and integrates inputs from an additional layer of regulation – the DNA replication checkpoint – to ensure that nuclei do not enter mitosis before completion of DNA replication. A major effector of this checkpoint is the protein kinase Chk1, which controls Cdk1 activity by activating Wee1 and inhibiting Cdc25 (Fig. 1A-B) (Tang et al., 1993; Peng et al., 1997; Sanchez et al., 1997; Lee et al., 2001; Shimuta et al., 2002; Uto et al., 2004).
Fig. 1.
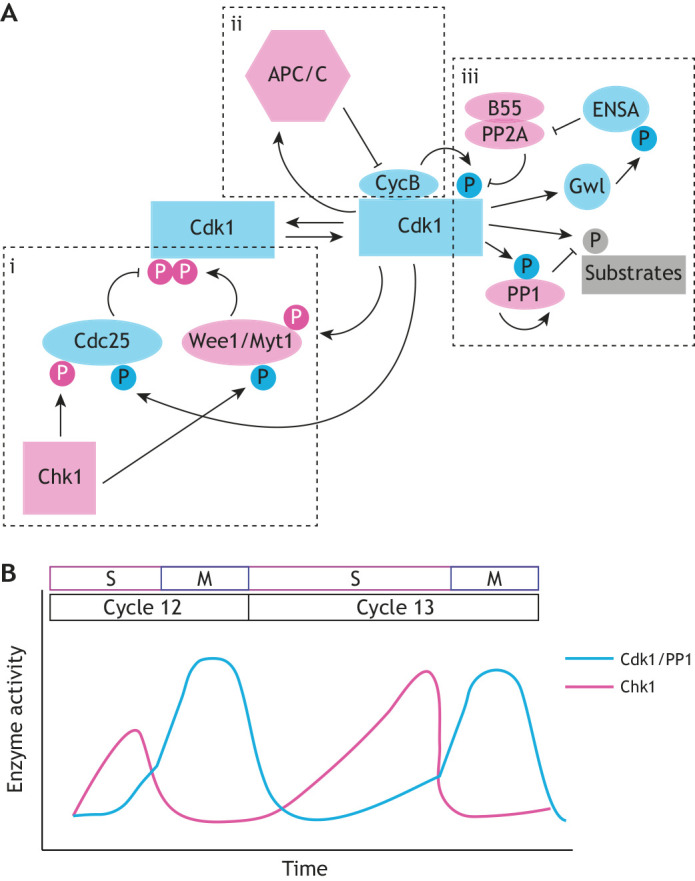
Cdk1 activity oscillates in the cell cycle through a network of positive- and negative-feedback loops. (A) Cdk1 activity is regulated by negative phosphorylation, positive phosphorylation and feedback from APC/C and phosphatases. Pink-labeled enzymes and phosphorylations indicate negative feedback on Cdk1 activity; cyan-labeled enzymes and phosphorylations indicate positive feedback on Cdk1 activity. (i) Inhibitory phosphorylation of Cdk1 is regulated by the kinases Wee1 and Myt1, which phosphorylate Cdk1 at T14 and Y15. The phosphatase Cdc25 counteracts the T14/Y15 phosphorylation and is itself regulated by the kinase Chk1. (ii) Cyclin B binding to Cdk1 results in an activating phosphorylation event at T161. The APC/C protein complex is activated by Cdk1 and provides negative feedback by resetting cyclin B levels. (iii) Phosphatases play an important role in antagonizing Cdk1 activity directly and on Cdk1 substrates. Cdk1 activates the phosphatase PP1, which then removes phosphorylation from other Cdk1 substrates. The phosphatase PP2A, when associated with the B55 regulatory subunit, acts as a direct inhibitor of Cdk1 by removing the T161 activating phosphorylation. Cdk1 promotes its own activity in a positive-feedback loop by phosphorylating Gwl, which inhibits PP2A-B55 through activation of ENSA. (B) Feedback loops lead to changes in the Cdk1 phosphorylation state and thus oscillations of Cdk1 activity within the cell cycle. During the early divisions of the embryo, the cell cycle oscillates between mitosis (M) and DNA synthesis (S), without gap phases. The ratio of active Cdk1 to PP1 is high (cyan line) as nuclei undergo mitosis, and drops as nuclei exit mitosis and enter S phase. The steep slope of the switch from inactive to active Cdk1 is reflective of measures of Cdk1 and Chk1 activity from Deneke et al. (2016), and illustrates the bistability of Cdk1 activity in which Cdk1 quickly switches between two stable states.
Following mitosis, a negative-feedback mechanism resets Cdk1 activity through Cdk1-dependent activation of the anaphase-promoting complex/cyclosome (APC/C) (Pines, 2011) (Fig. 1Aii). The APC/C is a large complex of proteins that has E3-ubiquitin ligase activity and thus promotes ubiquitylation and degradation of cyclins, leading to reduced Cdk1 activity (King et al., 1995; Irniger et al., 1995). Mitotic exit is also facilitated by phosphatases that remove mitotic phosphorylation from Cdk1 targets (Fig. 1Aiii). Protein phosphatase 2A (PP2A) associates with the regulatory subunit B55, forming a holoenzyme that is a major Cdk1 antagonist (Mochida et al., 2009). Protein phosphatase 1 (PP1) directly removes mitotic phosphorylation laid down by Cdk1 and it additionally facilitates the activation of PP2A (Wu et al., 2009; Grallert et al., 2015; Heim et al., 2017). Both PP2A and PP1 undergo cell cycle-dependent oscillations in their activity towards mitotic targets. Specifically, both phosphatases have low activity at the onset of mitosis and their activity rapidly increases at mitotic exit (Mochida et al., 2009; Wu et al., 2009) (Fig. 1B). Several feedback mechanisms contribute to this. For PP1, direct phosphorylation by Cdk1 and self-dephosphorylation, as well as regulation of co-factors (inhibitor 1 and inhibitor 2), have been implicated (Wu et al., 2009). PP2A activity is also regulated by feedback from Cdk1. The kinase greatwall (Gwl) inhibits PP2A-B55 by phosphorylating endosulfine alpha (ENSA), which binds and inhibits PP2A (Mochida et al., 2010). Gwl is in turn phosphorylated and activated by Cdk1, so that phosphatase activity remains low while Cdk1 activity is high (Hégarat et al., 2014). Collectively, the positive- and negative-feedback loops highlighted above provide avenues by which the cell cycle oscillator can be programmed during embryogenesis.
Traveling waves coordinate the rapid cell cycle events in developing embryos
Trigger waves: an early model for propagating mitotic waves in the embryo
Coordinated mitotic events characterize the early stages of embryogenesis of Drosophila, Xenopus and other systems. These events are coordinated across the large distance of the embryo, a phenomenon that cannot be achieved by simple diffusion (see Box 1). The early divisions of the Drosophila embryo occur within minutes across the 500 µm syncytium (Foe and Alberts, 1983). However, simple diffusion of active Cdk1 would take hours to traverse this distance (Box 1). To overcome this problem, it was theorized that the combination of diffusion and bistability of the cell cycle oscillator could generate trigger waves of Cdk1 activity that would synchronize mitosis across the large embryo (Novak and Tyson, 1993, Box 1). Trigger waves have several desirable features for propagating a robust biological signal across the expanse of the embryo. Specifically, they do not lose amplitude as they travel and they can transfer signals much more rapidly than by simple diffusion (Gelens et al., 2014; Deneke and Di Talia, 2018). In Drosophila embryos and Xenopus egg extracts, imaging studies of mitotic waves and their regulation have uncovered how Cdk1 activity and simple diffusion couple to move signals quickly across the embryo.
Box 1. Bistability and diffusion.
Bistability
Bistability describes the property of dynamic systems with two stable (equilibrium) points, e.g. a low activity and high activity state. When the activity of the system lies between the two stable states, the activity will either increase or decrease to reach a stable equilibrium point. Whether an activity value will evolve toward the low or high activity state depends upon a threshold for the system. Values below the threshold will proceed toward the low steady state, whereas values above the threshold will proceed toward the high steady state. Thus, bistability is usually characterized by rapid transitions between the two states. The cell cycle oscillator is characterized by a series of bistable transitions, where the low and high net activity of Cdk1 and opposing phosphatases represent the two states (Morgan, 2006). In biological systems such as the cell cycle control network, bistability usually arises from nonlinear positive feedback (Ferrell and Ha, 2014).
Diffusion
All molecules inside a cell are subject to random thermal motion. Such motion can be described by the diffusion equation, in which the molecule of interest is characterized by a diffusion constant D. In first approximation, this constant is dependent on properties of the medium and the molecule. Typical values for the intracellular diffusion constant of proteins are of the order D≈1-10 μm2 s−1. A major property of diffusion is that the time (t) needed for molecules to spread across a given distance (l) scales as the square of distance, t≈l2 D−1. Although diffusion can easily spread signals in several seconds across distances on the order of 10 μm, the time needed to spread such signal across 100 μm is 100 times as long. Thus, for rapid communication (within a few minutes) across a large expanse such as the 500 μm Drosophila embryo, simple diffusion is insufficient. Alternative mechanisms, such as fluid flows, direct transport, reaction-diffusion-generated gradients and waves must be adopted. These strategies have the additional advantage that the transported signal does not become dampened during spreading, as is typically observed for signals spreading by simple diffusion.
The trigger wave model proposes that mitotic signaling is propagated as a bistable wave (Box 2). Observing nuclear membrane breakdown in Xenopus egg extract loaded into long, thin Teflon tubes, it was found that mitosis traveled down the tube at a constant velocity, which is consistent with a wave (Chang and Ferrell, 2013). Partitioning the tube caused nuclear breakdown to decouple on the two sides, suggesting that coordination relies on physical coupling. Inhibition of Wee1 activity changed the speed of the mitotic wave front, supporting a role for Cdk1 in mitotic waves (Chang and Ferrell, 2013). Thus, these experimental data are consistent with mitotic waves being described as trigger waves.
Box 2. Reaction-diffusion systems.
Long-range order and rapid signal communication can be established in reaction-diffusion systems. Often in cellular systems, the reaction terms assume the form of protein synthesis and degradation, and non-linear feedback (positive and negative). In this Review, we discuss two mechanisms by which reaction-diffusion systems can generate wave-like spreading of biochemical activity: trigger waves and sweep waves.
Trigger waves
Trigger waves arise from the coupling of diffusion and bistability. As nuclei in the embryo approach mitosis, high Cdk1 activity (mitotic state) in a region causes some of the active Cdk1 molecules to diffuse to neighboring regions, which are in the low state (interphase). In this region, positive feedback can cause Cdk1 to further increase and nuclei to enter the mitotic state. This process will continue until the entire embryo enters the mitotic state. In the Drosophila embryo, experimental and modeling arguments suggest that the speed of a Cdk1 trigger wave would be about 0.4 μm s−1, which implies that it would take about 10 min for a wave to travel across the entire embryo. Yet all nuclei divide within 1-2 min, arguing that a more rapid mechanism is at play. This mechanism was shown to be sweep waves.
Sweep waves
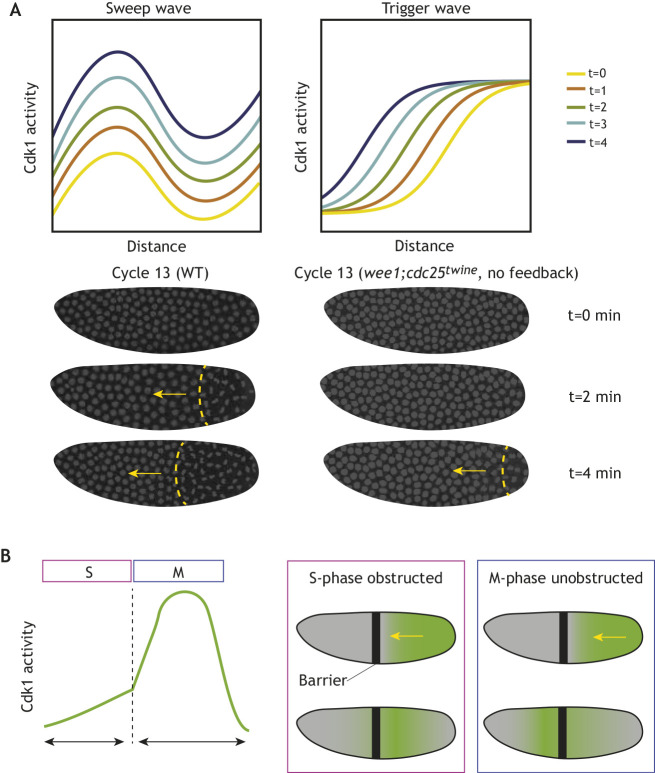
Sweep waves arise when bistability is transient and the transition from a bistable system to a system with only one stable state (e.g. high Cdk1 activity) happens on a much faster timescale than that needed for a wave to propagate. In this case, even if a bistable wave is initiated in a region of the embryo, this wave would not be able to travel far before the activity in the entire system switches towards the high state. To understand how this switch to the high state gives rise to waves, one needs to understand what happens when Cdk1 activity is low during interphase. Using both theoretical and experimental analyses, we showed that the early reaction-diffusion dynamic generates Cdk1 activity gradients that extend for about 100-150 μm. Late in interphase, when bistability is lost, Cdk1 activity rises towards the high stable state at a uniform rate across the entire embryo. The uniform rise of Cdk1 gradients causes wave-like mitotic entry as different regions across the gradient enter mitosis at slightly different times. Thus, the speed of the mitotic wave is inversely proportional to the steepness of the gradient and directly proportional to the rate at which the system moves away from bistability, a theoretical prediction that we confirmed experimentally. Moreover, if the Cdk1 gradients are not very steep and the drive is rapid, very fast mitotic waves will be observed.
Cdk1 activity spreads through Drosophila embryos via sweep waves
Although the above experiments and the bistable nature of mitosis had argued that a bistable wave could explain the dynamics of mitotic waves in early embryos, direct observation of Cdk1 activity waves was crucial to elucidate their biological and physical mechanism (Deneke et al., 2016; Vergassola et al., 2018). Strikingly, observation of Cdk1 activity in vivo demonstrated a novel mechanism of wave-like propagation, a sweep wave (Deneke et al., 2016; Vergassola et al., 2018), that can travel across the embryo even more quickly than a trigger wave (Box 2). This observation was achieved in the Drosophila embryo using a fluorescence resonance energy transfer (FRET) sensor that reports on the phosphorylation state of a Cdk1 target (Gavet and Pines, 2010; Deneke et al., 2016, 2019). Analysis of the spatial profiles of Cdk1 activity demonstrated that they lack the crucial property expected for bistable trigger waves, i.e. a wavefront, in which a high state of Cdk1 activity connects to regions of low Cdk1 activity (Murray, 1989; van Saarloos, 1998). Instead, at the onset of mitosis, gradients of Cdk1 activity that rapidly rise uniformly are observed (Vergassola et al., 2018) (Fig. 2A, Box 2). This phenomenon allows the Drosophila embryonic divisions to progress across the embryo even more rapidly than is theoretically possible by a trigger wave under the same parameters. In fact, weakening the positive-feedback loops that promote Cdk1 activation transforms sweep waves into trigger waves, which leads to slower mitotic waves (Vergassola et al., 2018) (Fig. 2A).
Fig. 2.
Time-dependent sweep waves of Cdk1 activity grow synchronously across the Drosophila embryo to drive faster entry into mitosis. (A) Theoretical Cdk1 activity curves in the Drosophila embryo illustrate the differences between sweep waves and trigger waves. Sweep waves preserve the spatial gradient of Cdk1 activity across the embryo while increasing the overall level of Cdk1 activity. The levels of Cdk1 activity at the left and right ends of each curve sweep upward with time. Unlike sweep waves, trigger waves arise from the invasion of a metastable state by a stable state. The left and right ends of the curves (the metastable and stable points of the bistable system) are roughly unchanged with time. The embryos pictured below right lack any feedback on Cdk1 activity due to mutations in wee1 and in the Drosophila cdc25 homolog, cdc25twine, leading to a slower sharp wave front that propagates across the embryo. Images reproduced, with permission, from Vergassola et al. (2018). (B) In Drosophila embryos, the cell cycle propagates as a wave (green) that relies on diffusion during S phase. A barrier (black) placed during interphase blocks wave propagation. After mitosis is initiated by high Cdk1 activity, the events of mitosis proceed as a wave that is pre-patterned by the early Cdk1 levels and thus propagates through the barrier. Adapted, with permission, from Deneke et al. (2016).
The generation of sweep waves relies on the fact that early reaction-diffusion Cdk1 dynamics result in the establishment of signaling gradients that can span regions of about 100-150 μm (Box 2). Late in interphase, the rapid accumulation of cyclins drives loss of bistability – the only stable state becomes high Cdk1 activity (mitosis) – and thus Cdk1 activity rises at a uniform rate across the embryo. This causes a global uniform increase of the gradients of Cdk1 activity and a wave-like pattern of mitotic entry (Vergassola et al., 2018) (Fig. 2A). After mitotic entry, the other mitotic events follow in a clock-like manner; thus, waves of mitotic exit are also observed (Deneke et al., 2016; Vergassola et al., 2018). Consistently, it was demonstrated that insertion of an impenetrable barrier in early interphase in Drosophila embryos causes mitosis to decouple in the two sides, but insertion in late interphase or early mitosis does not (Deneke et al., 2016) (Fig. 2B).
A qualitative difference between sweep and trigger waves is in the dependency of the wave speed on the rate of cyclin accumulation. For trigger waves, one expects a very small dependency on that rate, whereas the speed of sweep waves shows a strong dependency (Vergassola et al., 2018) (Box 2). This is seen in Drosophila embryos, where there is a decrease in the speed of Cdk1 waves when cyclin A and cyclin B levels are reduced by heterozygous mutations (Vergassola et al., 2018). Moreover, the wave speed is quantitatively captured by the theory of sweep waves in both wild-type and mutant embryos (Vergassola et al., 2018). Recent experimental results in Xenopus extracts might be consistent with a transition from sweep to trigger waves as the cell cycle slows down. Seeding a frog egg extract with fewer nuclei resulted in initially fast cycles that progressively slowed down (Chang and Ferrell, 2013). The speed of the mitotic waves initially demonstrated a strong dependency on cell cycle duration (Nolet et al., 2020), but eventually mitotic waves slowed and achieved speeds independent of cell cycle duration (Nolet et al., 2020; Afanzar et al., 2020). Collectively, these observations suggest that there are two mechanisms for mitotic waves and that the dominating one is determined by how rapidly the system progresses through the cell cycle.
Waves in Xenopus embryos are controlled by cell-autonomous clocks
In Xenopus extracts and Drosophila embryos, simple diffusion can propagate signals across the shared cytoplasm. However, in the intact Xenopus embryo, cytokinesis physically separates the cytoplasm of neighboring cells (Singal and Sanders, 1974; Keller, 1991), yet waves of cell division across multiple cells are still observed (Anderson et al., 2017). Using temperature gradients, it was investigated whether the waves observed from the second cell cycle onwards required diffusion or whether they were regulated by cell-autonomous clocks ticking at different periods (Anderson et al., 2017). Remarkably, these experiments showed that mitotic waves are due to autonomous clocks ticking at different rates, such that cells on the ‘cold’ side of the embryo and cells on the ‘warm’ side of the embryo never caught up with one another through 10 rounds of cell division. Instead, the two sides of the embryo maintained their own periodicity of mitosis (Anderson et al., 2017). Understanding what determines the periodicity of cell-autonomous cycling will reveal interesting insights into the developmental mechanisms of cell cycle control. Intriguingly, embryos that were forced to desynchronize with temperature gradients were viable (Anderson et al., 2017). Such remarkable robustness was previously observed in Drosophila embryos, where temperature gradients can similarly desynchronize the cell cycle, yet patterning seems robustly restored at gastrulation (Lucchetta et al., 2005). How embryos can compensate for significant delays in the timing of mitosis remains an unanswered question. It would be intriguing to find out whether there are developmental checkpoints able to resynchronize developmental pathways or whether the embryo is able to deal with the timing differences gradually by compensatory mechanisms.
Contractility and cytoplasmic flow couple cell cycle oscillations with mechanics in the embryo
In addition to chemical mechanisms that coordinate mitotic divisions across space, the mechanical properties of the early embryo must be tightly controlled to bring about proper morphogenesis. This is achieved by inter-regulation of the cell cycle oscillator and the cytoskeleton, and ensures the positioning of organelles, e.g. nuclei in the Drosophila embryo, and the separation of yolk from cytoplasm in zebrafish.
The cell cycle regulates cortical actomyosin to reposition nuclei in Drosophila
In the early Drosophila embryo, the first six nuclear divisions occur in the center of the embryo (Foe and Alberts, 1983). The first three nuclear divisions are characterized by little nuclear movement. From nuclear cycle 4 to 6, the nuclei gradually spread across the anterior-posterior axis of the embryo. Following this phase, also known as axial expansion, the nuclei progressively migrate to the cortex from nuclear cycle 7 to the end of nuclear cycle 10 (Foe and Alberts, 1983). Early time-lapse imaging studies suggested that axial expansion is controlled by dissolution of an F actin network in the bulk cytoplasm, which initiates cytoplasmic flows that push nuclei along the anterior-posterior axis (von Dassow and Schubiger, 1994). However, it was later proposed that cycles of myosin II activity at the cortex might produce cytoplasmic flows and drive nuclear movements (Royou et al., 2002). Indeed, oscillations of myosin II activity were observed at the cortex of early Drosophila embryos starting at the 4th mitotic division, and these oscillations were shown to depend on Cdk1 activity (Royou et al., 2002). However, as cyclin degradation is restricted to a very small region near the nuclei (Edgar et al., 1994a; Su et al., 1998), this raised the question of how the cell cycle oscillator could control cortical contractions many micrometers away. Such coordination would not be required for the bulk actin model, which could be controlled by cell cycle oscillations restricted around the chromosomes.
Distinguishing whether bulk or cortical actomyosin drives nuclear spreading was made difficult by the fact that most genetic and pharmacological perturbations would affect actomyosin globally across the embryo. How the cell cycle and actomyosin dynamics are integrated to drive nuclear spreading required novel imaging approaches that were introduced by our group (Deneke et al., 2019). Using a FRET biosensor, we measured the ratio of Cdk1 and PP1 activity directly in vivo. Although cyclin degradation is restricted to a region of a few micrometers around the chromosomes, the oscillation of Cdk1/PP1 activity extends to a region of about 50 μm, which is sufficient to couple nuclear and cortical dynamics. PP1 is a major player in the spreading of the oscillation and in the recruitment of myosin II at the cortex (Deneke et al., 2019). Using an optogenetic approach to alter actomyosin contractility specifically at the cortex, we also demonstrated that cortical contractions drive the flow of cytoplasm within the embryo (cytoplasmic streaming), which in turn drives nuclear positioning. More generally, the mechanism by which nuclear spreading is accomplished in the Drosophila embryo is an excellent example of a self-organized system. The cytoplasmic flows responsible for nuclear spreading are driven by the presence of myosin gradients. Myosin gradients are established by the localized oscillations between Cdk1 and phosphatase activity, and are present only when nuclei are unevenly distributed across the embryo (Fig. 3). As soon as nuclei are uniformly positioned across the embryo, myosin oscillations, although still present, are uniform and unable to generate flow. Thus, the embryo generates only the number of cortical contractions required for uniform positioning. This mechanism likely allows the embryo to correct for variability in initial nuclear positioning and to achieve a uniform nuclear distribution across the embryo. This uniformity ensures that the correct number of nuclear divisions precedes gastrulation. Further study of the Drosophila embryo will shed light on the molecular mechanisms that couple the cell cycle with actomyosin contractility, as well as elucidate the physical mechanisms by which cortical contractions generate cytoplasmic streaming. More importantly, investigating whether similar mechanisms work in insect species laying embryos of different sizes and shapes will provide interesting evolutionary insights, alternative mechanisms (Donoughe et al., 2021 preprint), and possibly a framework for how mechanical forces and cytoplasmic flow can scale in embryogenesis.
Fig. 3.
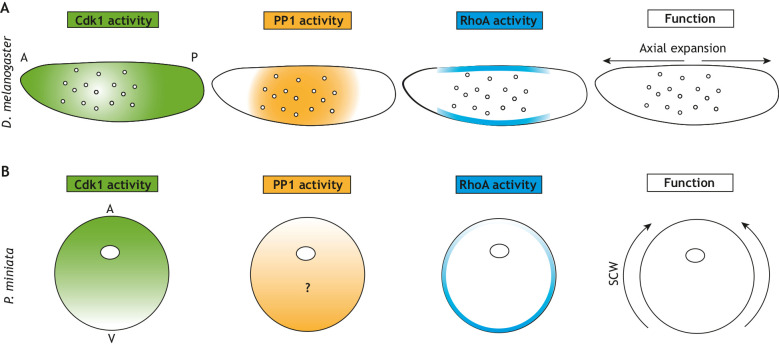
Summary of known activities of Cdk1, PP1 and RhoA in fly and starfish embryos. (A) In the fly embryo, a self-organized system positions nuclei across the embryo syncytia. Cdk1 activity is low around nuclei following mitosis, which leads to local increases in PP1 phosphatase. PP1 can activate RhoA at the cortex, thus coupling nuclear position with activity at the cortex. RhoA then promotes contraction at the cortex and mixing of the cytoplasm to spread nuclei along the anterior-posterior (A-P) axis. (B) A similar self-organizing system might exist in starfish oocytes, which have a gradient of Cdk1 activity that negatively correlates with RhoA activity. The role of PP1 in starfish embryos remains unknown. Cdk1 and RhoA gradients promote contraction at the surface and the propagation of surface contraction waves (SCWs) from the vegetal (V) to animal pole (A).
Cdk1 generates surface contraction waves via RhoA in starfish oocytes
Cell cycle-dependent regulation of cortical contractility has also been studied in starfish oocytes, where it was shown that surface contraction waves (SCWs) are linked to Cdk1 activity (Bement et al., 2015). It was subsequently proposed that SCWs are regulated by a gradient of active Cdk1 that opposes RhoA activity (Fig. 3B) (Bischof et al., 2017). In this model, the gradient of Cdk1 activity results in RhoA activation at the vegetal pole. Feedback mechanisms on RhoA activity then generate a traveling wave. Inhibition of Cdk1 at a specific point in the embryo through local drug application causes RhoA activity to accumulate at that point and change the direction of the SCW. RhoA ultimately activates myosin II, causing waves of cortical contraction (Bischof et al., 2017). In these studies, the levels of cyclin B were taken as a proxy of Cdk1 activity, and moving gradients, similar to those explained by sweep waves (Vergassola et al., 2018), were proposed to position RhoA waves (Bischof et al., 2017; Wigbers et al., 2021). However, the bistable regulation of Cdk1 argues for non-linearity in the relationship between cyclin B and Cdk1 activity. Furthermore, the activities of PP1 and PP2A might contribute to the spatial control of the phosphorylation status of mitotic targets (Mochida et al., 2016; Rata et al., 2018; Kamenz et al., 2020; Deneke et al., 2019). Notably, SCWs have also been observed in Xenopus embryos, originating at the animal pole and moving laterally down the embryo (Rankin and Kirschner, 1997). Propagation of SCWs coincides with mitosis (Rankin and Kirschner, 1997), and it was proposed that they are directly driven by wave-like activation and inactivation of Cdk1, at least during the first cell cycle (Chang and Ferrell, 2013). In the future, the use of cell-cycle biosensors coupled with perturbations of the cell cycle and cytoskeleton will be crucial to reveal how SCWs are controlled by Cdk1 and mitotic phosphatase activities.
Ooplasmic reorganization in zebrafish occurs through cell cycle-dependent regulation of bulk actin polymerization
Coordination between Cdk1 activity and actin polymerization in the cytoplasm also plays important roles in early embryos. Xenopus egg extracts under a coverslip undergo periodic contraction as actin polymerizes and myosin promotes contraction of the filamentous network. This process is mostly active in mitotic extracts, supporting the idea that the cell cycle affects actin dynamics (Field et al., 2011). Recently, this coordination has been shown to play a central role in ooplasmic separation during the first division of zebrafish embryos. Soon after fertilization, there is a directional separation of yolk granules and ooplasm in the embryo, such that yolk granules move ventrally (Beams et al., 1985; Leung et al., 2000; Shamipour et al., 2019). Previous work in zebrafish and other models suggested that ooplasm flow was likely driven by cortical actomyosin contractions (Leung et al., 2000; Munro et al., 2004; Prodon et al., 2008; Klughammer et al., 2018). Indeed, cortical actomyosin disassembly has been observed in the animal pole of zebrafish oocytes, where it was thought to allow streaming of ooplasm into an expanded animal pole (Beams et al., 1985; Fuentes et al., 2018). However, the actomyosin cortex is dispensable for cytoplasmic flow in the fish oocyte (Shamipour et al., 2019). Rather, cell cycle-mediated bursts of bulk actin polymerization drive ooplasm reorganization. Strikingly, actin polymerization proceeds in a wave throughout the time of the first cell division of the zygote. The periodicity of these waves is dependent on Cdk1 activity, with Wee1 inhibition giving rise to faster waves of actin polymerization and Chk1 inhibition slowing the wave. Thus, these experiments led to a model in which bulk actomyosin dynamics controlled by the Cdk1 wave drive ooplasmic segregation (Shamipour et al., 2019). Notably, the positive regulation of bulk actomyosin contractility in mitosis contrasts with regulation at the cortex, where contractility is inhibited at mitotic entry and rises at mitotic exit. This differential regulation is not yet fully elucidated on a mechanistic level. However, it predicts that actomyosin-dependent flows would be observed at different phases of the cell cycle: at interphase for cortical contractions and at mitotic entry for bulk contractions. This prediction is confirmed by observations in Drosophila and zebrafish embryos (Deneke et al., 2019; Shamipour et al., 2019).
Microtubule-dependent regulation of nuclear movements
In addition to actomyosin-dependent cortical dynamics, microtubules also play a role in the spatial distribution of nuclei in Drosophila embryos and Xenopus extracts (Baker et al., 1993; Nguyen et al., 2014; Cheng and Ferrell, 2019; Mitchison, 2020; Pelletier et al., 2020; Deshpande et al., 2019). In fly embryos, microtubules are important in controlling the migration of nuclei to the surface (Baker et al., 1993) following actomyosin-driven axial expansion and in regulating internuclear distance (Telley et al., 2012; Deshpande et al., 2019; de-Carvalho et al., 2020 preprint). Similar processes have been observed and well characterized in Xenopus extracts (Nguyen et al., 2014; Field and Mitchison, 2018; Pelletier et al., 2020). Importantly, these microtubule-driven processes are also linked to the cell cycle. For example, in Drosophila embryos, nuclear migration happens in three cycles (nuclear cycles 7 to 9), in which nuclear movements towards the cortex are restricted to mitotic exit/early interphase (Foe and Alberts, 1983). In the future, it will be important to dissect how the cell cycle oscillator and microtubule dynamics are integrated to ensure that nuclei move towards the cortex at the correct time and in the correct direction.
Cell cycle slowing at the mid-blastula transition promotes embryonic patterning
The mid-blastula transition (MBT) is a distinct stage of embryonic development in most organisms characterized by slowing of the cell cycle and ultimately the inclusion of gap phases. This lengthening of the cell cycle likely gives embryos the time necessary to turn on zygotic transcripts, so the MBT also coincides with a transition from maternal to zygotic control of mRNA products (Fig. 4A). Recent studies have focused on identifying the mechanisms that control this crucial developmental transition by initiating these dramatic changes in transcription and cell cycle control.
Fig. 4.
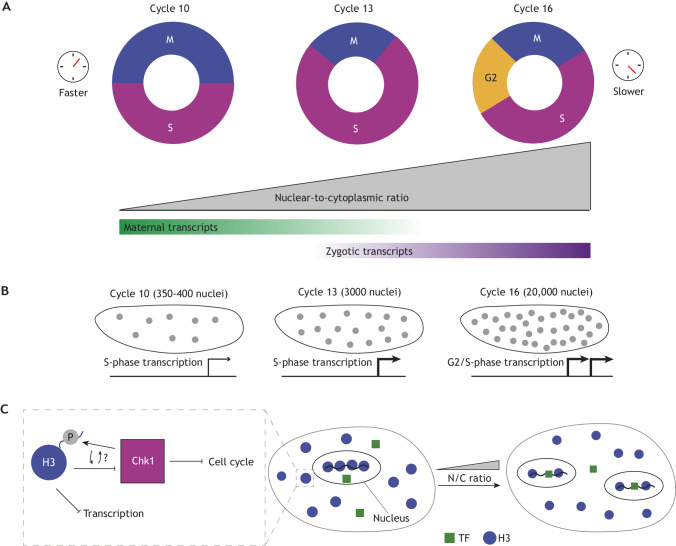
Convergence of multiple events at the Drosophila mid-blastula transition. (A) The cell cycle is remodeled at the mid-blastula transition (MBT) through the lengthening of S phase and then introduction of G2. This slowing of the cell cycle coincides with a transition from maternal to zygotic transcription. (B) Transcriptional output from zygotic nuclei also coincides with the increase in nuclear density. The expression of S-phase and, later, G2-phase genes progressively increases with time due to increased numbers of nuclei and to an increase in the transcription-competent cell cycle length. (C) We illustrate the embryo compartment and the nuclei inside before and after a nuclear division to illustrate titration as the nuclear-to-cytoplasmic (N/C) ratio changes. A titration model has been proposed as a mechanism by which nuclear events, such as DNA content and transcription, might correlate to cell cycle remodeling at the MBT. Early on, the binding of histones (blue) prevents the access of transcription factors to DNA, preventing the expression of zygotic genes. An increase in the N/C ratio leads to less competition between histones and transcription factors (green), promoting the expression of zygotic genes. Moreover, cells spend an increased time in the transcription-competent phases of the cell cycle. Histone proteins themselves may also feedback on Chk1, which inhibits cell cycle progression (left box). Cooperatively, these changes allow the embryo to undergo this crucial moment in development.
Increased Chk1 activity mediates cell cycle slowing at the MBT
Slowing of the cell cycle at the MBT is pronounced and often follows a more gradual slowing of pre-MBT cycles that is observed, e.g. in cycles 10-13 of Drosophila embryogenesis (Sibon et al., 1997; Farrell et al., 2012) and less so in cycles 10-12 in Xenopus (Howe et al., 1995). The timing of cell cycle slowing is tied to DNA content. In Drosophila, one of the clearest illustrations of this concept comes from studies of haploid embryos, which undergo an additional division before cellularization to reach the appropriate DNA content for the MBT (Edgar et al., 1986). The lengthening of the cell cycles preceding the MBT is due to slower DNA replication. The duration of S phase in the last cycle preceding the MBT (cycle 13) is more than three times longer than the duration in cycle 10 (14 versus 4 min), whereas the duration of M phase itself is constant. This cell cycle lengthening is mediated by the DNA replication checkpoint and requires the activity of the checkpoint kinase Chk1. The molecular regulation of Chk1 activity and its impact on Cdk1 have been demonstrated and investigated in both Xenopus (Lee et al., 2001; Shimuta et al., 2002; Petrus et al., 2004) and Drosophila (Sibon et al., 1997; Su et al., 1999; Takada et al., 2007; Royou et al., 2008; Fasulo et al., 2012). Chk1 activity likely increases in response to increased DNA content in the embryo. In Drosophila embryos, it has been shown that increasing Chk1 activity causes Cdk1 activity to rise progressively more slowly during interphase of nuclear cycles 12 and 13 (Fig. 1B), thus delaying the cell cycle to correctly couple DNA replication and mitosis (Deneke et al., 2016). Negative feedback from Cdk1 to Chk1 activity, which has been observed in cell culture (Shiromizu et al., 2006; Enomoto et al., 2009; Xu et al., 2012), might further sharpen and control the transition between S phase and M phase (Yuan et al., 2012; Deneke et al., 2016). This early remodeling of the cell cycle due to S-phase lengthening is followed by a more dramatic slowing at the MBT with the inclusion of gap phases. Cell cycle remodeling at the Drosophila MBT is controlled by downregulating maternal Cdc25 to pause the cell cycle in G2 (Farrell et al., 2012). This downregulation is mainly controlled post-translationally through mechanisms targeting Cdc25 for degradation (Farrell and O'Farrell, 2013; Di Talia et al., 2013). Maternal cdc25 mRNA degradation (Edgar and Datar, 1996) ensures that pulses of zygotic Cdc25 transcription can then drive entry into mitosis in the subsequent cycles (Edgar and O'Farrell, 1990). Regulation of Cdc25 levels is also required for cell cycle slowing in zebrafish and in frogs (Shimuta et al., 2002; Dalle Nogare et al., 2009).
Titration of histone proteins and/or of the DNA replication machinery has been suggested as a mechanism to regulate the cell cycle at the MBT (Collart et al., 2013; Pérez-Montero et al., 2013; Amodeo et al., 2015; Joseph et al., 2017; Liu et al., 2019; Djabrayan et al., 2019). Recently, a molecular mechanism has been proposed by which titration of histones might be linked to activation of Chk1 (Shindo and Amodeo, 2021). The authors show that the tail of histone H3 is a direct competitive inhibitor of Chk1 activity. This model can explain why Chk1 activity is low in earlier cycles, in which maternally deposited H3 is present in excess, and higher in later cycles, when histones have been sufficiently titrated to allow Chk1 activity to rise (Fig. 4C). In zebrafish, recent work has shown that histone proteins directly compete on chromatin with transcription factors necessary for zygotic transcription. Thus, the dilution of histone protein can also time the onset of zygotic transcription (Joseph et al., 2017), a hallmark feature of the MBT together with cell cycle remodeling and degradation of several maternal mRNAs.
Cell cycle control of zygotic gene activation
Titration of factors involved in both DNA replication or zygotic gene activation (Almouzni and Wolffe, 1995; Collart et al., 2013; Amodeo et al., 2015; Joseph et al., 2017) could explain how DNA content impinges on all MBT processes. A complication in this analysis is that the cell cycle and zygotic gene activation are strongly co-regulated. The early cleavage divisions are not conducive to gene expression, as transcription aborts during mitosis and the short interphase only leaves time to transcribe a few short mRNAs (Heyn et al., 2014; Jukam et al., 2017). Thus, lengthening of the cell cycle favors zygotic gene activation. In turn, transcription can promote activation of the DNA replication checkpoint (Sibon et al., 1997; Blythe and Wieschaus, 2015) and zygotic gene products can inhibit the rapid cell cycle (Edgar et al., 1994b), further supporting transcription. These feedback mechanisms are likely to ensure a robust coordination of all the MBT processes, but they have made dissecting the mechanisms driving the control of the MBT difficult. For example, it was recently argued that slowing of the cell cycle is not merely a central feature of the MBT but is the driving process of this developmental transition in Drosophila embryos. Arresting the cell cycle as early as cycle 12 through inhibition of Cdk1 activity triggers the initiation of several MBT processes, namely zygotic gene expression and gastrulation, even when DNA content is below the amount usually needed for the MBT (Strong et al., 2020). Even genes previously identified as dependent upon the embryo reaching a critical N/C ratio were transcribed in Cdk1-inhibited embryos arrested in interphase 12. These and other experiments led the authors to argue that rapid cell cycles are a major inhibitor of the MBT (Strong et al., 2020).
Quantitative arguments support the role of the cell cycle in inhibiting transcription. For example, in cycle 13 embryos there are eight times more nuclei than in cell cycle 10 embryos. Moreover, interphase, i.e. the period compatible with transcription, is at least three times longer (Foe and Alberts, 1983; Farrell and O'Farrell, 2014). Thus, in principle, the embryo would have increased its overall transcriptional capacity by about 25-fold just by nuclear proliferation and cell cycle remodeling (Fig. 4B). Therefore, gradual inhibition of Cdk1 by Chk1 might be the initiating and controlling event of the MBT, ultimately allowing sufficient time for a significant upregulation of zygotic genome activation, and consequent cell cycle remodeling and morphogenetic processes. Live imaging of transcription also revealed a crucial role for the cell cycle in regulating gene expression, while also arguing for effects independent of cell cycle control (Syed et al., 2021). Imaging of zygotic transcription at single-cell resolution in Xenopus embryos revealed significant and systematic differences in the timing of zygotic gene activation. This argued that cell size, which dictates the N/C ratio in individual cells, controls zygotic genome activation (Chen et al., 2019). The importance of the N/C ratio in Xenopus embryos is further supported by observations in embryonic chimeras of Xenopus laevis and Xenopus tropicalis, which result in embryos with different DNA content (Jukam et al., 2021 preprint). The local nature of the sensing of the N/C ratio and its impact on cell cycle control was also suggested in Drosophila, where altering the mechanisms of nuclear positioning can generate embryos with different N/C ratios across the anterior-posterior axis. These different ratios are likely the cause for different cell cycle durations along the embryo (Deneke et al., 2019). Importantly, in zebrafish, cell cycle control and the N/C ratio can be decoupled from zygotic transcription and, instead, genome activation is timed from fertilization through the translation of chromatin modifiers from maternal mRNAs and the deposition of epigenetic marks (Chan et al., 2019).
The MBT is a complex transition point for the early embryo that involves multiple hallmark phenomena, most importantly the slowing of the cell cycle and a significant increase in zygotic transcription (Fig. 4A). Quantitative approaches coupled with classical embryological approaches are likely to reveal the mechanisms by which embryos reproducibly initiate these events and how these processes feedback on one another.
Cell cycle control during gastrulation
The remodeling of the cell cycle during gastrulation is most clearly illustrated in Drosophila when cells first begin to divide asynchronously. After cellularization in the Drosophila embryo, 25 mitotic domains emerge that have local synchrony of cell division but are asynchronous with other neighboring domains in the embryo (Foe, 1989). In these domains, mitosis often begins in few cells and spreads in a wave-like pattern to the boundary of that domain (Foe, 1989). Disruption of the timing of these mitotic patterns can have consequences for embryo viability (Edgar and O'Farrell, 1989). It has been proposed that mitotic domains map to specific fates later in development and may facilitate the establishment of cell populations that show a similar response to differentiation signals (Edgar et al., 1994a; Cambridge et al., 1997). Specifically, mitotic domains must avoid conflicts between cell division and cytoskeletal rearrangements that drive morphogenesis. This is illustrated by the control of mesoderm specification, where a delay is introduced in the cell cycle machinery to ensure that cell divisions always follow the apical constriction process that drives invagination (Grosshans and Wieschaus, 2000).
The spatiotemporal pattern of cell divisions during gastrulation in Drosophila is controlled by the transcriptional activation of string (stg), one of the two Cdc25 phosphatases present in Drosophila (Edgar and O'Farrell, 1989, 1990) (Fig. 5A). Quantitative imaging experiments revealed that almost all cells across the embryo are equally sensitive to Cdc25 levels and that the rapid accumulation of Cdc25 can drive mitotic entry independently of Cdk1-positive feedback (Di Talia and Wieschaus, 2012). At the MBT, both maternal String and Twine (the other Cdc25 phosphatase) are degraded, and thus pulses of Cdc25 transcription become rate-limiting to initiate mitotic divisions 14-16 (Edgar et al., 1994b). The developmental patterning genes that control differentiation and fate specification, rather than input from the cell cycle, promote string transcriptional activation (Edgar et al., 1994a). This was supported by the observation that homozygous mutants in patterning genes lead to spatial changes in mitotic domains or even the loss of entire domains (Edgar et al., 1994a). However, this approach did not provide clear insights into the mechanisms encoding the timing of stg expression. These came from a different genetic approach that used heterozygous deficiencies to perform whole-genome screening for regulators of the timing of division in mitotic domains 1 and 2 (Momen-Roknabadi et al., 2016). The authors found that not only are mitotic domains established by spatially restricted transcriptional regulatory networks, but within a domain, the timing of division is tuned by a subset of those same transcriptional regulators. It was proposed that a balance between inhibitory and activating transcription factors controls mitotic precision in each region of the embryo (Momen-Roknabadi et al., 2016) (Fig. 5B,C). Using a combination of inhibitors and activators to time division might reduce the impact of fluctuations in the levels of the transcriptional regulators. However, both theoretical and experimental work is still needed to address this idea and to elucidate how transcriptional networks can achieve both rapid and precise control of cell cycle timing during gastrulation.
Fig. 5.
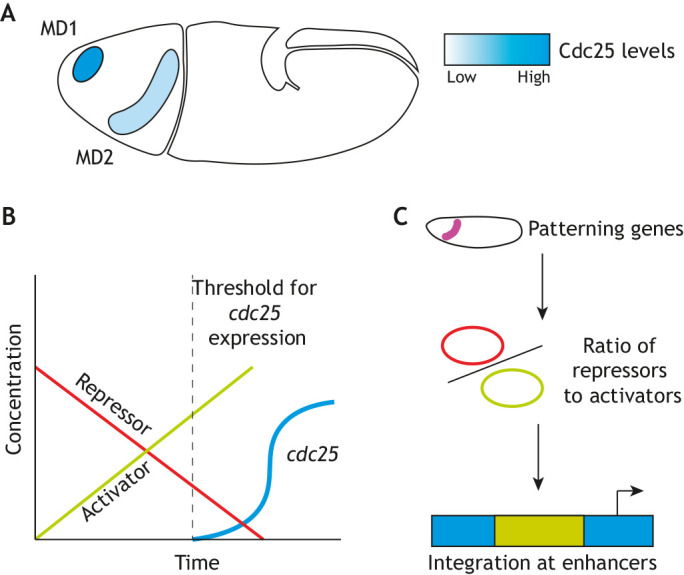
Differential levels in Cdc25 protein result in spatial asynchrony of mitotic divisions in Drosophila. (A) The timing of Cdc25 transcription correlates with the speed of division in mitotic domains (MDs) in Drosophila. MD1 and MD2 are the first two of 25 groups of cells to divide during the asynchronous 14th mitosis in Drosophila embryos. The onset of mitosis correlates to levels of Cdc25 in each mitotic domain. (B) A ratio of activators and repressors to initiate new cdc25 transcription controls the timing of mitosis in gastrulating fly embryos. (C) Using multiple activators and repressors whose expression is responsive to patterning genes allows for robustness and reproducibility of the highly stereotyped divisions that give rise to the proper body plan in Drosophila.
How asynchronous divisions are timed during morphogenesis has also been investigated in the chordate ascidian (sea squirt) Ciona (Ogura and Sasakura, 2016). In Ciona, the 11th mitosis, which occurs after gastrulation, is the first asynchronous mitosis in embryonic development and spreads in a wave across the embryo. Interestingly, asynchrony is already observed in S phase during cell cycle 10. As in cell cycle 11, anterior cells progress more slowly through S phase than posterior cells. However, there is a reciprocal compensation in G2 so that mitosis in cell cycle 10 remains synchronous, whereas in cycle 11 there is no G2 compensation, leading to asynchronous mitosis across the embryo. As in the mitotic domains of the Drosophila gastrula, asynchrony in Ciona also depends on the regulation of Cdc25. Two developmentally regulated transcription factors, ci-AP-2 and ci-GATAb, were shown to promote Cdc25 transcription, and loss of these two proteins in cell cycle 10 can disrupt mitotic synchrony (Ogura and Sasakura, 2016). It will be of great interest to determine whether, similar to Drosophila, the combinatorial action of activators and repressors is responsible for timing divisions. Understanding whether similar strategies are used in these different embryos to obtain precise transcriptional control of mitotic timing has the potential to reveal general insights on how precise timing is achieved during morphogenesis.
Asynchronous cell divisions are not only observed during gastrulation. In the roundworm C. elegans, division is asynchronous as early as the two-cell stage. In this case, the two cells (AB and P1) are of different sizes, with AB being larger and reproducibly dividing earlier than P1 (Sulston et al., 1983; Schierenberg and Wood, 1985; Encalada et al., 2000; Brauchle et al., 2003). Regulation does not occur at the level of transcription, as it takes place before zygotic transcription is active. Rather, the PAR proteins, which regulate anterior-posterior symmetry, partition more Polo kinase PLK-1, another positive regulator of mitosis known to contribute to the control of the mitotic switch (Archambault and Glover, 2009), to the AB cell (Rivers et al., 2008; Budirahardja and Gönczy, 2008). Loss of function in chk-1 and wee-1 in C. elegans diminishes the delay between the AB and P1 cells, suggesting that the feedback on Cdk1 activity through inhibitory phosphorylations is likely important (Brauchle et al., 2003; Michael, 2016). It has been proposed that differences in the nuclear-cytoplasmic ratio might contribute to cell cycle slowing in C. elegans (Brauchle et al., 2003), similar to what is observed in other systems. How polarity cues might play into cell cycle timing in other systems has not been well studied. Comparisons across species will uncover both general principles and species-specific mechanisms of the regulation of cell cycle timing.
Future directions and open questions
The recent use of quantitative imaging tools to dissect the dynamics and molecular mechanisms that couple cell division, morphogenesis, actomyosin dynamics and cytoplasmic flows in developing embryos has given us a better understanding of the coordinated processes that shape early embryogenesis from flies to vertebrates. Here, we have focused on the ways in which molecular mechanisms that control the cell cycle act locally and globally to coordinate development. Developing biochemical sensors of Cdk1 activity in diverse model systems will likely uncover the mechanisms by which embryos coordinate the cell cycle and subsequent physical or morphological events across the expanse of an embryo. These tools have been useful in the Drosophila embryo, and directly measuring Cdk1 activity in vivo in vertebrates may also provide new insights as these embryos have different geometries and perhaps different spatial regulation of the cell cycle. The syncytial nature of the early fly embryo is very different from the cellular nature of fish embryos, for example. Therefore, although the molecular mechanisms across model systems are likely to be conserved, how each species uses these mechanisms for their own unique biology and body plan organization will be an interesting avenue for future work. Moreover, using imaging biosensors and quantitative approaches will uncover how waves and flows contribute to collective decision making in other biological systems (Hubaud et al., 2017; Sonnen et al., 2018; Streichan et al., 2018; Jörg et al., 2019; Saadaoui et al., 2020; De Simone et al., 2021).
In mammals, the series of rapid divisions before gastrulation is slightly delayed in comparison with other vertebrate embryos due the need to establish extra-embryonic tissues soon after fertilization (O'Farrell et al., 2004). However, the rapid divisions of the pre-gastrulating epiblast cells in mammalian embryos share similar cell cycle dynamics with non-mammalian embryos, as gap phases in epiblast cell cycles are shortened or absent (O'Farrell et al., 2004). New technology that enables imaging of mammalian embryos ex vivo or in reconstituted systems (Shahbazi et al., 2016; Deglincerti et al., 2016; McDole et al., 2018; Zheng et al., 2019; Minn et al., 2020; Kohrman et al., 2021) provides developmental biologists with the opportunity to address how cell cycle machinery might coordinate developmental events in mammalian embryos. It will be interesting to see whether mechanisms similar to ones described in this Review are at play in the rapid cell divisions that precede gastrulation and in the transition to asynchronous divisions after gastrulation (Mac Auley et al., 1993). Most importantly, there may be previously unidentified feedback mechanisms involving cell cycle machinery that are crucial for gastrulating mammalian embryos.
Acknowledgements
We thank Brigid Hogan, Daniel Lew, Bernard Mathey-Prevot and members of the Di Talia lab for comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
The authors’ research on cell cycle control in embryogenesis is supported by the National Institutes of Health (R01-GM122936 to S.D.). Deposited in PMC for release after 12 months.
References
- Afanzar, O., Buss, G. K., Stearns, T. and Ferrell, J. E. (2020). The nucleus serves as the pacemaker for the cell cycle. Elife 9, e59989. 10.7554/eLife.59989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almouzni, G. and Wolffe, A. P. (1995). Constraints on transcriptional activator function contribute to transcriptional quiescence during early Xenopus embryogenesis. EMBO J. 14, 1752-1765. 10.1002/j.1460-2075.1995.tb07164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo, A. A., Jukam, D., Straight, A. F. and Skotheim, J. M. (2015). Histone titration against the genome sets the DNA-to-cytoplasm threshold for the Xenopus midblastula transition. Proc. Natl. Acad. Sci. USA 112, E1086-E1095. 10.1073/pnas.1413990112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, G. A., Gelens, L., Baker, J. C. and Ferrell, J. E. (2017). Desynchronizing embryonic cell division waves reveals the robustness of Xenopus laevis development. Cell Rep 21, 37-46. 10.1016/j.celrep.2017.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault, V. and Glover, D. M. (2009). Polo-like kinases: conservation and divergence in their functions and regulation. Nat. Rev. Mol. Cell Biol. 10, 265-275. 10.1038/nrm2653 [DOI] [PubMed] [Google Scholar]
- Arora, K. and Nüsslein-Volhard, C. (1992). Altered mitotic domains reveal fate map changes in Drosophila embryos mutant for zygotic dorsoventral patterning genes. Development 114, 1003-1024. 10.1242/dev.114.4.1003 [DOI] [PubMed] [Google Scholar]
- Baker, J., Theurkauf, W. E. and Schubiger, G. (1993). Dynamic changes in microtubule configuration correlate with nuclear migration in the preblastoderm Drosophila embryo. J. Cell Biol. 122, 113-121. 10.1083/jcb.122.1.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban, K. H., Torres, J. Z., Miller, J. J., Mikhailov, A., Nachury, M. V., Tung, J. J., Rieder, C. L. and Jackson, P. K. (2007). The END network couples spindle pole assembly to inhibition of the anaphase-promoting complex/cyclosome in early mitosis. Dev. Cell 13, 29-42. 10.1016/j.devcel.2007.04.017 [DOI] [PubMed] [Google Scholar]
- Beams, H. W., Kessel, R. G., Shih, C. Y. and Tung, H. N. (1985). Scanning electron microscope studies on blastodisc formation in the zebrafish,Brachydanio rerio. J. Morphol .184, 41-49. 10.1002/jmor.1051840105 [DOI] [Google Scholar]
- Bement, W. M., Leda, M., Moe, A. M., Kita, A. M., Larson, M. E., Golding, A. E., Pfeuti, C., Su, K., Miller, A. L., Goryachev, A. B.et al. (2015). Activator-inhibitor couple between Rho signalling and actin assembly makes the cell cortex an excitable medium. Nat. Cell Biol. 17, 1471-1483. 10.1038/ncb3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof, J., Brand, C. A., Somogyi, K., Májer, I., Thome, S., Mori, M., Schwarz, U. S. and Lénárt, P. (2017). A cdk1 gradient guides surface contraction waves in oocytes. Nat. Commun. 8, 849. 10.1038/s41467-017-00979-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blythe, S. A. and Wieschaus, E. F. (2015). Zygotic genome activation triggers the DNA replication checkpoint at the midblastula transition. Cell 160, 1169-1181. 10.1016/j.cell.2015.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauchle, M., Baumer, K. and Gönczy, P. (2003). Differential activation of the DNA replication checkpoint contributes to asynchrony of cell division in C. elegans embryos. Curr. Biol. 13, 819-827. 10.1016/S0960-9822(03)00295-1 [DOI] [PubMed] [Google Scholar]
- Budirahardja, Y. and Gönczy, P. (2008). PLK-1 asymmetry contributes to asynchronous cell division of C. elegans embryos. Development 135, 1303-1313. 10.1242/dev.019075 [DOI] [PubMed] [Google Scholar]
- Cambridge, S. B., Davis, R. L. and Minden, J. S. (1997). Drosophila mitotic domain boundaries as cell fate boundaries. Science 277, 825-828. 10.1126/science.277.5327.825 [DOI] [PubMed] [Google Scholar]
- Campbell, S. D., Sprenger, F., Edgar, B. A. and O'farrell, P. H. (1995). Drosophila Wee1 kinase rescues fission yeast from mitotic catastrophe and phosphorylates Drosophila Cdc2 in vitro. Mol. Biol. Cell 6, 1333-1347. 10.1091/mbc.6.10.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, S. H., Tang, Y., Miao, L., Darwich-Codore, H., Vejnar, C. E., Beaudoin, J.-D., Musaev, D., Fernandez, J. P., Benitez, M. D. J., Bazzini, A. A.et al. (2019). Brd4 and P300 confer transcriptional competency during zygotic genome activation. Dev. Cell 49, 867-881.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. B. and Ferrell, J. E. (2013). Mitotic trigger waves and the spatial coordination of the Xenopus cell cycle. Nature 500, 603-607. 10.1038/nature12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Einstein, L. C., Little, S. C. and Good, M. C. (2019). Spatiotemporal patterning of zygotic genome activation in a model vertebrate embryo. Dev. Cell 49, 852-866.e7. 10.1016/j.devcel.2019.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X. and Ferrell, J. E. (2019). Spontaneous emergence of cell-like organization in Xenopus egg extracts. Science 366, 631-637. 10.1126/science.aav7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart, C., Allen, G. E., Bradshaw, C. R., Smith, J. C. and Zegerman, P. (2013). Titration of four replication factors is essential for the Xenopus laevis midblastula transition. Science 341, 893-896. 10.1126/science.1241530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Nogare, D. E., Pauerstein, P. T. and Lane, M. E. (2009). G2 acquisition by transcription-independent mechanism at the zebrafish midblastula transition. Dev. Biol. 326, 131-142. 10.1016/j.ydbio.2008.11.002 [DOI] [PubMed] [Google Scholar]
- De-Carvalho, J., Tlili, S., Hufnagel, L., Saunders, T. E. and Telley, I. A. (2020). Aster repulsion drives local ordering in an active system. BioRxiv. 10.1101/2020.06.04.133579 [DOI] [PubMed] [Google Scholar]
- Deshpande, O., De-Carvalho, J., Vieira, D. M. and Telley, I. A. (2019). Astral microtubule crosslinking by feo safeguards uniform nuclear distribution in the drosophila syncytium. SSRN J. 10.1101/859975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone, A., Evanitsky, M. N., Hayden, L., Cox, B. D., Wang, J., Tornini, V. A., Ou, J., Chao, A., Poss, K. D. and Di Talia, S. (2021). Control of osteoblast regeneration by a train of Erk activity waves. Nature 590, 129-133. 10.1038/s41586-020-03085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deglincerti, A., Croft, G. F., Pietila, L. N., Zernicka-Goetz, M., Siggia, E. D. and Brivanlou, A. H. (2016). Self-organization of the in vitro attached human embryo. Nature 533, 251-254. 10.1038/nature17948 [DOI] [PubMed] [Google Scholar]
- Deneke, V. E. and Di Talia, S. (2018). Chemical waves in cell and developmental biology. J. Cell Biol. 217, 1193-1204. 10.1083/jcb.201701158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke, V. E., Melbinger, A., Vergassola, M. and Di Talia, S. (2016). Waves of cdk1 activity in S phase synchronize the cell cycle in drosophila embryos. Dev. Cell 38, 399-412. 10.1016/j.devcel.2016.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke, V. E., Puliafito, A., Krueger, D., Narla, A. V., De Simone, A., Primo, L., Vergassola, M., De Renzis, S. and Di Talia, S. (2019). Self-Organized Nuclear Positioning Synchronizes the Cell Cycle in Drosophila Embryos. Cell 177, 925-941.e17. 10.1016/j.cell.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Talia, S. and Wieschaus, E. F. (2012). Short-term integration of Cdc25 dynamics controls mitotic entry during Drosophila gastrulation. Dev. Cell 22, 763-774. 10.1016/j.devcel.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Talia, S., She, R., Blythe, S. A., Lu, X., Zhang, Q. F. and Wieschaus, E. F. (2013). Posttranslational control of Cdc25 degradation terminates Drosophila's early cell-cycle program. Curr. Biol. 23, 127-132. 10.1016/j.cub.2012.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djabrayan, N. J. V., Smits, C. M., Krajnc, M., Stern, T., Yamada, S., Lemon, W. C., Keller, P. J., Rushlow, C. A., Shvartsman, S. Y. (2019). Metabolic regulation of developmental cell cycles and zygotic transcription. Curr. Biol. 29, 1193-1198. 10.1016/j.cub.2019.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoughe, S., Hoffmann, J., Nakamura, T., Rycroft, C. H. and Extavour, C. G. (2021). Local density determines nuclear movements during syncytial blastoderm formation in a cricket. BioRxiv. 10.1101/2021.04.26.441395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, B. A. and Datar, S. A. (1996). Zygotic degradation of two maternal Cdc25 mRNAs terminates Drosophila's early cell cycle program. Genes Dev. 10, 1966-1977. 10.1101/gad.10.15.1966 [DOI] [PubMed] [Google Scholar]
- Edgar, B. A. and O'farrell, P. H. (1989). Genetic control of cell division patterns in the Drosophila embryo. Cell 57, 177-187. 10.1016/0092-8674(89)90183-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, B. A. and O'farrell, P. H. (1990). The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell 62, 469-480. 10.1016/0092-8674(90)90012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, B. A., Kiehle, C. P. and Schubiger, G. (1986). Cell cycle control by the nucleo-cytoplasmic ratio in early Drosophila development. Cell 44, 365-372. 10.1016/0092-8674(86)90771-3 [DOI] [PubMed] [Google Scholar]
- Edgar, B. A., Lehman, D. A. and O'farrell, P. H. (1994a). Transcriptional regulation of string (cdc25): a link between developmental programming and the cell cycle. Development 120, 3131-3143. 10.1242/dev.120.11.3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar, B. A., Sprenger, F., Duronio, R. J., Leopold, P. and O'farrell, P. H. (1994b). Distinct molecular mechanism regulate cell cycle timing at successive stages of Drosophila embryogenesis. Genes Dev. 8, 440-452. 10.1101/gad.8.4.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge, S. J. (1996). Cell cycle checkpoints: preventing an identity crisis. Science 274, 1664-1672. 10.1126/science.274.5293.1664 [DOI] [PubMed] [Google Scholar]
- Encalada, S. E., Martin, P. R., Phillips, J. B., Lyczak, R., Hamill, D. R., Swan, K. A. and Bowerman, B. (2000). DNA replication defects delay cell division and disrupt cell polarity in early Caenorhabditis elegans embryos. Dev. Biol. 228, 225-238. 10.1006/dbio.2000.9965 [DOI] [PubMed] [Google Scholar]
- Enomoto, M., Goto, H., Tomono, Y., Kasahara, K., Tsujimura, K., Kiyono, T. and Inagaki, M. (2009). Novel positive feedback loop between Cdk1 and Chk1 in the nucleus during G2/M transition. J. Biol. Chem. 284, 34223-34230. 10.1074/jbc.C109.051540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, T., Rosenthal, E. T., Youngblom, J., Distel, D. and Hunt, T. (1983). Cyclin: a protein specified by maternal mRNA in sear urchin eggs that is destroyed at each cleavage division. Cell 33, 389-396. 10.1016/0092-8674(83)90420-8 [DOI] [PubMed] [Google Scholar]
- Farrell, J. A. and O'farrell, P. H. (2013). Mechanism and regulation of Cdc25/Twine protein destruction in embryonic cell-cycle remodeling. Curr. Biol. 23, 118-126. 10.1016/j.cub.2012.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell, J. A. and O'farrell, P. H. (2014). From egg to gastrula: how the cell cycle is remodeled during the Drosophila mid-blastula transition. Annu. Rev. Genet. 48, 269-294. 10.1146/annurev-genet-111212-133531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell, J. A., Shermoen, A. W., Yuan, K. and O'farrell, P. H. (2012). Embryonic onset of late replication requires Cdc25 down-regulation. Genes Dev. 26, 714-725. 10.1101/gad.186429.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasulo, B., Koyama, C., Yu, K. R., Homola, E. M., Hsieh, T. S., Campbell, S. D. and Sullivan, W. (2012). Chk1 and Wee1 kinases coordinate DNA replication, chromosome condensation, and anaphase entry. Mol. Biol. Cell 23, 1047-1057. 10.1091/mbc.e11-10-0832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell, J. E. and Ha, S. H. (2014). Ultrasensitivity part III: cascades, bistable switches, and oscillators. Trends Biochem. Sci. 39, 612-618. 10.1016/j.tibs.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, C. M. and Mitchison, T. J. (2018). Assembly of spindles and asters in xenopus egg extracts. Cold Spring Harb. Protoc. 440-448. 10.1101/pdb.prot099796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field, C. M., Wühr, M., Anderson, G. A., Kueh, H. Y., Strickland, D. and Mitchison, T. J. (2011). Actin behavior in bulk cytoplasm is cell cycle regulated in early vertebrate embryos. J. Cell Sci. 124, 2086-2095. 10.1242/jcs.082263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe, V. E. (1989). Mitotic domains reveal early commitment of cells in Drosophila embryos. Development 107, 1-22. 10.1242/dev.107.1.1 [DOI] [PubMed] [Google Scholar]
- Foe, V. E. and Alberts, B. M. (1983). Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J. Cell Sci. 61, 31-70. 10.1242/jcs.61.1.31 [DOI] [PubMed] [Google Scholar]
- Foe, V. E., Odell, G. M. and Edgar, B. A. (1993). Mitosis and morphogenesis in the Drosophila embryo: Point and counterpoint. In The Development of Drosophila Melanogaster (ed. Bate M. and Martinez Arias A.), pp. 149-300: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Fuentes, R., Mullins, M. C. and Fernández, J. (2018). Formation and dynamics of cytoplasmic domains and their genetic regulation during the zebrafish oocyte-to-embryo transition. Mech. Dev. 154, 259-269. 10.1016/j.mod.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Gavet, O. and Pines, J. (2010). Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev. Cell 18, 533-543. 10.1016/j.devcel.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelens, L., Anderson, G. A. and Ferrell, J. E. (2014). Spatial trigger waves: positive feedback gets you a long way. Mol. Biol. Cell 25, 3486-3493. 10.1091/mbc.e14-08-1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer, M., Murray, A. W. and Kirschner, M. W. (1991). Cyclin is degraded by the ubiquitin pathway. Nature 349, 132-138. 10.1038/349132a0 [DOI] [PubMed] [Google Scholar]
- Grallert, A., Boke, E., Hagting, A., Hodgson, B., Connolly, Y., Griffiths, J. R., Smith, D. L., Pines, J. and Hagan, I. M. (2015). A PP1-PP2A phosphatase relay controls mitotic progression. Nature 517, 94-98. 10.1038/nature14019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans, J. and Wieschaus, E. (2000). A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 101, 523-531. 10.1016/S0092-8674(00)80862-4 [DOI] [PubMed] [Google Scholar]
- Hégarat, N., Vesely, C., Vinod, P. K., Ocasio, C., Peter, N., Gannon, J., Oliver, A. W., Novák, B. and Hochegger, H. (2014). PP2A/B55 and Fcp1 regulate Greatwall and Ensa dephosphorylation during mitotic exit. PLoS Genet. 10, e1004004. 10.1371/journal.pgen.1004004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim, A., Rymarczyk, B. and Mayer, T. U. (2017). Regulation of cell division. Adv. Exp. Med. Biol. 953, 83-116. 10.1007/978-3-319-46095-6_3 [DOI] [PubMed] [Google Scholar]
- Heyn, P., Kircher, M., Dahl, A., Kelso, J., Tomancak, P., Kalinka, A. T. and Neugebauer, K. M. (2014). The earliest transcribed zygotic genes are short, newly evolved, and different across species. Cell Rep 6, 285-292. 10.1016/j.celrep.2013.12.030 [DOI] [PubMed] [Google Scholar]
- Hoffmann, I., Clarke, P. R., Marcote, M. J., Karsenti, E. and Draetta, G. (1993). Phosphorylation and activation of human cdc25-C by cdc2--cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 12, 53-63. 10.1002/j.1460-2075.1993.tb05631.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway, S. L., Glotzer, M., King, R. W. and Murray, A. W. (1993). Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell 73, 1393-1402. 10.1016/0092-8674(93)90364-V [DOI] [PubMed] [Google Scholar]
- Howe, J. A., Howell, M., Hunt, T. and Newport, J. W. (1995). Identification of a developmental timer regulating the stability of embryonic cyclin A and a new somatic A-type cyclin at gastrulation. Genes Dev. 9, 1164-1176. 10.1101/gad.9.10.1164 [DOI] [PubMed] [Google Scholar]
- Huang, J. and Raff, J. W. (1999). The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J. 18, 2184-2195. 10.1093/emboj/18.8.2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubaud, A., Regev, I., Mahadevan, L. and Pourquié, O. (2017). Excitable dynamics and Yap-dependent mechanical cues drive the segmentation clock. Cell 171, 668-682.e11. 10.1016/j.cell.2017.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irniger, S., Piatti, S., Michaelis, C. and Nasmyth, K. (1995). Genes involved in sister chromatid separation are needed for B-type cyclin proteolysis in budding yeast. Cell 81, 269-278. 10.1016/0092-8674(95)90337-2 [DOI] [PubMed] [Google Scholar]
- Jeffrey, P. D., Russo, A. A., Polyak, K., Gibbs, E., Hurwitz, J., Massagué, J. and Pavletich, N. P. (1995). Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature 376, 313-320. 10.1038/376313a0 [DOI] [PubMed] [Google Scholar]
- Jörg, D. J., Caygill, E. E., Hakes, A. E., Contreras, E. G., Brand, A. H. and Simons, B. D. (2019). The proneural wave in the Drosophila optic lobe is driven by an excitable reaction-diffusion mechanism. Elife 8, e40919. 10.7554/eLife.40919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, S. R., Pálfy, M., Hilbert, L., Kumar, M., Karschau, J., Zaburdaev, V., Shevchenko, A. and Vastenhouw, N. L. (2017). Competition between histone and transcription factor binding regulates the onset of transcription in zebrafish embryos. Elife 6, e23326. 10.7554/eLife.23326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam, D., Shariati, S. A. M. and Skotheim, J. M. (2017). Zygotic genome activation in vertebrates. Dev. Cell 42, 316-332. 10.1016/j.devcel.2017.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam, D., Kapoor, R. R., Straight, A. F. and Skotheim, J. M. (2021). The DNA-to-cytoplasm ratio broadly activates zygotic gene expression in Xenopus. BioRxiv. 10.1101/2021.04.18.440334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenz, J., Gelens, L. and Ferrell, J. E. (2020). Bistable, biphasic regulation of PP2A-B55 accounts for the dynamics of mitotic substrate phosphorylation. Curr. Biol. 31, 794-808. 10.1016/j.cub.2020.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane, D. A., Warga, R. M. and Kimmel, C. B. (1992). Mitotic domains in the early embryo of the zebrafish. Nature 360, 735-737. 10.1038/360735a0 [DOI] [PubMed] [Google Scholar]
- Keller, R. (1991). Chapter 5 Early Embryonic Development of Xenopus laevis. In Xenopus laevis: Practical Uses in Cell and Molecular Biology, pp. 61-113: Elsevier. [DOI] [PubMed] [Google Scholar]
- King, R. W., Peters, J. M., Tugendreich, S., Rolfe, M., Hieter, P. and Kirschner, M. W. (1995). A 20S complex containing CDC27 and CDC16 catalyzes the mitosis-specific conjugation of ubiquitin to cyclin B. Cell 81, 279-288. 10.1016/0092-8674(95)90338-0 [DOI] [PubMed] [Google Scholar]
- Klughammer, N., Bischof, J., Schnellbächer, N. D., Callegari, A., Lénárt, P. and Schwarz, U. S. (2018). Cytoplasmic flows in starfish oocytes are fully determined by cortical contractions. PLoS Comput. Biol 14, e1006588. 10.1371/journal.pcbi.1006588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrman, A. Q., Kim-Yip, R. P. and Posfai, E. (2021). Imaging developmental cell cycles. Biophys. J. S0006–3495(21)00374-X. 10.1016/j.bpj.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth, S., Sebastian, B., Hunter, T. and Newport, J. (1994). Membrane localization of the kinase which phosphorylates p34cdc2 on threonine 14. Mol. Biol. Cell 5, 273-282. 10.1091/mbc.5.3.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, A. and Dunphy, W. G. (1992). Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell 70, 139-151. 10.1016/0092-8674(92)90540-S [DOI] [PubMed] [Google Scholar]
- Lasko, P. (2013). Development: new wrinkles on genetic control of the MBT. Curr. Biol. 23, R65-R67. 10.1016/j.cub.2012.12.012 [DOI] [PubMed] [Google Scholar]
- Lee, J., Kumagai, A. and Dunphy, W. G. (2001). Positive regulation of Wee1 by Chk1 and 14-3-3 proteins. Mol. Biol. Cell 12, 551-563. 10.1091/mbc.12.3.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, C. F., Webb, S. E. and Miller, A. L. (2000). On the mechanism of ooplasmic segregation in single-cell zebrafish embryos. Dev. Growth Differ. 42, 29-40. 10.1046/j.1440-169x.2000.00484.x [DOI] [PubMed] [Google Scholar]
- Liu, B., Winkler, F., Herde, M., Witte, C. P. and Großhans, J. (2019). A link between deoxyribonucleotide metabolites and embryonic cell-cycle control. Curr. Biol. 29, 1187-1192. 10.1016/j.cub.2019.02.021 [DOI] [PubMed] [Google Scholar]
- Lucchetta, E. M., Lee, J. H., Fu, L. A., Patel, N. H. and Ismagilov, R. F. (2005). Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature 434, 1134-1138. 10.1038/nature03509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Auley, A., Werb, Z. and Mirkes, P. E. (1993). Characterization of the unusually rapid cell cycles during rat gastrulation. Development 117, 873-883. 10.1242/dev.117.3.873 [DOI] [PubMed] [Google Scholar]
- Mcdole, K., Guignard, L., Amat, F., Berger, A., Malandain, G., Royer, L. A., Turaga, S. C., Branson, K. and Keller, P. J. (2018). In toto imaging and reconstruction of post-implantation mouse development at the single-cell level. Cell 175, 859-876.e33. 10.1016/j.cell.2018.09.031 [DOI] [PubMed] [Google Scholar]
- Michael, W. M. (2016). Cyclin CYB-3 controls both S-phase and mitosis and is asymmetrically distributed in the early C. elegans embryo. Development 143, 3119-3127. 10.1242/dev.141226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn, K. T., Fu, Y. C., He, S., Dietmann, S., George, S. C., Anastasio, M. A., Morris, S. A. and Solnica-Krezel, L. (2020). High-resolution transcriptional and morphogenetic profiling of cells from micropatterned human ESC gastruloid cultures. Elife 9, e59445. 10.7554/eLife.59445.sa2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison, T. J. (2020). Cell biology: size scaling of mitotic spindles. Curr. Biol. 30, R1476-R1478. 10.1016/j.cub.2020.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida, S., Ikeo, S., Gannon, J. and Hunt, T. (2009). Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 28, 2777-2785. 10.1038/emboj.2009.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida, S., Maslen, S. L., Skehel, M. and Hunt, T. (2010). Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330, 1670-1673. 10.1126/science.1195689 [DOI] [PubMed] [Google Scholar]
- Mochida, S., Rata, S., Hino, H., Nagai, T. and Novák, B. (2016). Two bistable switches govern M phase entry. Curr. Biol. 26, 3361-3367. 10.1016/j.cub.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen-Roknabadi, A., Di Talia, S. and Wieschaus, E. (2016). Transcriptional timers regulating mitosis in early drosophila embryos. Cell Rep 16, 2793-2801. 10.1016/j.celrep.2016.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, D. O. (1997). Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13, 261-291. 10.1146/annurev.cellbio.13.1.261 [DOI] [PubMed] [Google Scholar]
- Morgan, D. O. (2006). Cell Cycle: Principles of Control. London: New Science Press. [Google Scholar]
- Mueller, P. R., Coleman, T. R., Kumagai, A. and Dunphy, W. G. (1995a). Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 270, 86-90. 10.1126/science.270.5233.86 [DOI] [PubMed] [Google Scholar]
- Mueller, P. R., Coleman, T. R. and Dunphy, W. G. (1995b). Cell cycle regulation of a Xenopus Wee1-like kinase. Mol. Biol. Cell 6, 119-134. 10.1091/mbc.6.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, E., Nance, J. and Priess, J. R. (2004). Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413-424. 10.1016/j.devcel.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Murakami, M. S., Moody, S. A., Daar, I. O. and Morrison, D. K. (2004). Morphogenesis during Xenopus gastrulation requires Wee1-mediated inhibition of cell proliferation. Development 131, 571-580. 10.1242/dev.00971 [DOI] [PubMed] [Google Scholar]
- Murray, A. W. (2004). Recycling the cell cycle: cyclins revisited. Cell 116, 221-234. 10.1016/S0092-8674(03)01080-8 [DOI] [PubMed] [Google Scholar]
- Murray, A. W. and Kirschner, M. W. (1989). Dominoes and clocks: the union of two views of the cell cycle. Science 246, 614-621. 10.1126/science.2683077 [DOI] [PubMed] [Google Scholar]
- Murray, J. D. (1989). Mathematical Biology. New York: Springer-Verlag. 10.1007/b98868 [DOI] [Google Scholar]
- Newport, J. and Kirschner, M. (1982). A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 30, 675-686. 10.1016/0092-8674(82)90272-0 [DOI] [PubMed] [Google Scholar]
- Nguyen, P. A., Groen, A. C., Loose, M., Ishihara, K., Wuhr, M., Field, C. M. and Mitchison, T. J. (2014). Spatial organization of cytokinesis signaling reconstituted in a cell-free system. Science 346, 244-247. 10.1126/science.1256773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg, E. A. (1995). Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. BioEssays 17, 471-480. 10.1002/bies.950170603 [DOI] [PubMed] [Google Scholar]
- Nolet, F. E., Vandervelde, A., Vanderbeke, A., Piñeros, L., Chang, J. B. and Gelens, L. (2020). Nuclei determine the spatial origin of mitotic waves. Elife 9, e52868. 10.7554/eLife.52868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak, B. and Tyson, J. J. (1993). Numerical analysis of a comprehensive model of M-phase control in Xenopus oocyte extracts and intact embryos. J. Cell Sci. 106, 1153-1168. 10.1242/jcs.106.4.1153 [DOI] [PubMed] [Google Scholar]
- O'Farrell, P. H. (2015). Growing an embryo from a single cell: a hurdle in animal life. Cold Spring Harb. Perspect. Biol. 7, 1-24. 10.1101/cshperspect.a019042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell, P. H., Stumpff, J. and Su, T. T. (2004). Embryonic cleavage cycles: how is a mouse like a fly? Curr. Biol. 14, R35-R45. 10.1016/j.cub.2003.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura, Y. and Sasakura, Y. (2016). Developmental control of cell-cycle compensation provides a switch for patterned mitosis at the onset of chordate neurulation. Dev. Cell 37, 148-161. 10.1016/j.devcel.2016.03.013 [DOI] [PubMed] [Google Scholar]
- Parker, L. L. and Piwnica-Worms, H. (1992). Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 257, 1955-1957. 10.1126/science.1384126 [DOI] [PubMed] [Google Scholar]
- Parry, D. H. and O'farrell, P. H. (2001). The schedule of destruction of three mitotic cyclins can dictate the timing of events during exit from mitosis. Curr. Biol. 11, 671-683. 10.1016/S0960-9822(01)00204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier, J. F., Field, C. M., Furthauer, S., Sonnett, M. and Mitchison, T. J. (2020). Co-movement of astral microtubules, organelles and F-actin by dynein and actomyosin forces in frog egg cytoplasm. Elife 9, e60047. 10.7554/eLife.60047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, C. Y., Graves, P. R., Thoma, R. S., Wu, Z., Shaw, A. S. and Piwinca-Worms, H. (1997). Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277, 1501-1505. 10.1126/science.277.5331.1501 [DOI] [PubMed] [Google Scholar]
- Pérez-Montero, S., Carbonell, A., Morán, T., Vaquero, A. and Azorín, F. (2013). The embryonic linker histone H1 variant of Drosophila, dBigH1, regulates zygotic genome activation. Dev. Cell 26, 578-590. 10.1016/j.devcel.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Petrus, M. J., Wilhelm, D. E., Murakami, M., Kappas, N. C., Carter, A. D., Wroble, B. N. and Sible, J. C. (2004). Altered expression of Chk1 disrupts cell cycle remodeling at the midblastula transition in Xenopus laevis embryos. Cell Cycle 3, 212-217. 10.4161/cc.3.2.647 [DOI] [PubMed] [Google Scholar]
- Pines, J. (2011). Cubism and the cell cycle: the many faces of the APC/C. Nat. Rev. Mol. Cell Biol. 12, 427-438. 10.1038/nrm3132 [DOI] [PubMed] [Google Scholar]
- Pomerening, J. R., Sontag, E. D. and Ferrell, J. E.Jr. (2003). Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nat. Cell Biol. 5, 346-351. 10.1038/ncb954 [DOI] [PubMed] [Google Scholar]
- Prodon, F., Sardet, C. and Nishida, H. (2008). Cortical and cytoplasmic flows driven by actin microfilaments polarize the cortical ER-mRNA domain along the a-v axis in ascidian oocytes. Dev. Biol. 313, 682-699. 10.1016/j.ydbio.2007.11.001 [DOI] [PubMed] [Google Scholar]
- Rankin, S. and Kirschner, M. W. (1997). The surface contraction waves of Xenopus eggs reflect the metachronous cell-cycle state of the cytoplasm. Curr. Biol. 7, 451-454. 10.1016/S0960-9822(06)00192-8 [DOI] [PubMed] [Google Scholar]
- Rata, S., Rodriguez, S. P., Joseph, M. F., Peter, S., Echegaray Iturra, N., Yang, F., Madzvamuse, F., Ruppert, A., Samejima, J. G., Platani, K.et al. (2018). Two interlinked bistable switches govern mitotic control in mammalian cells. Curr. Biol. 28, 3824-3832.e6. 10.1016/j.cub.2018.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers, D. M., Moreno, S., Abraham, M. and Ahringer, J. (2008). PAR proteins direct asymmetry of the cell cycle regulators Polo-like kinase and Cdc25. J. Cell Biol. 180, 877-885. 10.1083/jcb.200710018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royou, A., Sullivan, W. and Karess, R. (2002). Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: its role in nuclear axial expansion and its regulation by Cdc2 activity. J. Cell Biol. 158, 127-137. 10.1083/jcb.200203148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royou, A., McCusker, D., Kellogg, D. R. and Sullivan, W. (2008). Grapes (Chk1) prevents nuclear CDK1 activation by delaying cyclin B nuclear accumulation. J. Cell Biol. 183, 63-75. 1083/jcb.200801153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadaoui, M., Rocancourt, D., Roussel, J., Corson, F. and Gros, J. (2020). A tensile ring drives tissue flows to shape the gastrulating amniote embryo. Science 367, 453-458. 10.1126/science.aaw1965 [DOI] [PubMed] [Google Scholar]
- Sanchez, Y., Wong, C., Thoma, R. S., Richman, R., Wu, Z., Piwinca-Worms, H. and Elledge, S. J. (1997). Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497-1501. 10.1126/science.277.5331.1497 [DOI] [PubMed] [Google Scholar]
- Schierenberg, E. and Wood, W. B. (1985). Control of cell-cycle timing in early embryos of Caenorhabditis elegans. Dev. Biol. 107, 337-354. 10.1016/0012-1606(85)90316-1 [DOI] [PubMed] [Google Scholar]
- Sha, W., Moore, J., Chen, K., Lassaletta, A. D., Yi, C., Tyson, J. J. and Sible, J. C. (2003). Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc. Natl. Acad. Sci. USA 100, 975-980. 10.1073/pnas.0235349100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi, M. N., Jedrusik, A., Vuoristo, S., Recher, G., Hupalowska, A., Bolton, V., Fogarty, N. N. M., Campbell, A., Devito, L., Ilic, D.et al. (2016). Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 18, 700-708. 10.1038/ncb3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamipour, S., Kardos, R., Xue, S.-L., Hof, B., Hannezo, E. and Heisenberg, C.-P. (2019). Bulk actin dynamics drive phase segregation in zebrafish oocytes. Cell 177, 1463-1479.e18. 10.1016/j.cell.2019.04.030 [DOI] [PubMed] [Google Scholar]
- Shimuta, K., Nakajo, N., Uto, K., Hayano, Y., Okazaki, K. and Sagata, N. (2002). Chk1 is activated transiently and targets Cdc25A for degradation at the Xenopus midblastula transition. EMBO J. 21, 3694-3703. 10.1093/emboj/cdf357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo, Y. and Amodeo, A. A. (2021). Excess histone H3 is a competitive Chk1 inhibitor that controls cell-cycle remodeling in the early Drosophila embryo. Curr. Biol. (in press). 10.1016/j.cub.2021.03.035 [DOI] [PubMed] [Google Scholar]
- Shiromizu, T., Goto, H., Tomono, Y., Bartek, J., Totsukawa, G., Inoko, A., Nakanishi, M., Matsumura, F. and Inagaki, M. (2006). Regulation of mitotic function of Chk1 through phosphorylation at novel sites by cyclin-dependent kinase 1 (Cdk1). Genes Cells 11, 477-485. 10.1111/j.1365-2443.2006.00955.x [DOI] [PubMed] [Google Scholar]
- Sibon, O. C., Stevenson, V. A. and Theurkauf, W. E. (1997). DNA-replication checkpoint control at the Drosophila midblastula transition. Nature 388, 93-97. 10.1038/40439 [DOI] [PubMed] [Google Scholar]
- Singal, P. K. and Sanders, E. J. (1974). An ultrastructural study of the first cleavage of Xenopus embryos. J. Ultrastruct Res. 47, 433-451. 10.1016/S0022-5320(74)90019-7 [DOI] [PubMed] [Google Scholar]
- Solomon, M. J., Glotzer, M., Lee, T. H., Philippe, M. and Kirschner, M. W. (1990). Cyclin activation of p34cdc2. Cell 63, 1013-1024. 10.1016/0092-8674(90)90504-8 [DOI] [PubMed] [Google Scholar]
- Sonnen, K. F., Lauschke, V. M., Uraji, J., Falk, H. J., Petersen, Y., Funk, M. C., Beaupeux, M., François, P., Merten, C. A. and Aulehla, A. (2018). Modulation of phase shift between Wnt and Notch signaling oscillations controls mesoderm segmentation. Cell 172, 1079-1090.e12. 10.1016/j.cell.2018.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streichan, S. J., Lefebvre, M. F., Noll, N., Wieschaus, E. F. and Shraiman, B. I. (2018). Global morphogenetic flow is accurately predicted by the spatial distribution of myosin motors. Elife 7, e27454. 10.7554/eLife.27454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong, I. J. T., Lei, X., Chen, F., Yuan, K. and O'farrell, P. H. (2020). Interphase-arrested Drosophila embryos activate zygotic gene expression and initiate mid-blastula transition events at a low nuclear-cytoplasmic ratio. PLoS Biol. 18, e3000891. 10.1371/journal.pbio.3000891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpff, J., Duncan, T., Homola, E., Campbell, S. D. and Su, T. T. (2004). Drosophila Wee1 kinase regulates Cdk1 and mitotic entry during embryogenesis. Curr. Biol. 14, 2143-2148. 10.1016/j.cub.2004.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, T. T., Sprenger, F., Digregorio, P. J., Campbell, S. D. and O'farrell, P. H. (1998). Exit from mitosis in Drosophila syncytial embryos requires proteolysis and cyclin degradation, and is associated with localized dephosphorylation. Genes Dev. 12, 1495-1503. 10.1101/gad.12.10.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, T. T., Campbell, S. D. and O'farrell, P. H. (1999). Drosophila grapes/CHK1 mutants are defective in cyclin proteolysis and coordination of mitotic events. Curr. Biol. 9, 919-922. 10.1016/S0960-9822(99)80399-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, J. E., Schierenberg, E., White, J. G. and Thomson, J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119. 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- Syed, S., Wilky, H., Raimundo, J., Lim, B. and Amodeo, A. A. (2021). The nuclear to cytoplasmic ratio directly regulates zygotic transcription in Drosophila through multiple modalities. Proc. Natl. Acad. Sci. USA 118, e2010210118. 10.1073/pnas.2010210118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada, S., Kwak, S., Koppetsch, B. S. and Theurkauf, W. E. (2007). . grp (chk1) replication-checkpoint mutations and DNA damage trigger a Chk2-dependent block at the Drosophila midblastula transition. Development 134, 1737-1744. 10.1242/dev.02831 [DOI] [PubMed] [Google Scholar]
- Tang, Z., Coleman, T. R. and Dunphy, W. G. (1993). Two distinct mechanisms for negative regulation of the Wee1 protein kinase. EMBO J. 12, 3427-3436. 10.1002/j.1460-2075.1993.tb06017.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telley, I. A., Gáspár, I., Ephrussi, A. and Surrey, T. (2012). Aster migration determines the length scale of nuclear separation in the Drosophila syncytial embryo. J. Cell Biol. 197, 887-895. 10.1083/jcb.201204019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, T. Y.-C., Theriot, J. A. and Ferrell, J. E. (2014). Changes in oscillatory dynamics in the cell cycle of early Xenopus laevis embryos. PLoS Biol. 12, e1001788. 10.1371/journal.pbio.1001788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uto, K., Inoue, D., Shimuta, K., Nakajo, N. and Sagata, N. (2004). Chk1, but not Chk2, inhibits Cdc25 phosphatases by a novel common mechanism. EMBO J. 23, 3386-3396. 10.1038/sj.emboj.7600328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Velden, H. M. W. and Lohkat, M. J. (1994). Cell cycle-regulated degradation of Xenopus Cyclin B2 requires binding to p34cdc2. Mol. Biol. Cell 5, 713-724. 10.1091/mbc.5.7.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Saarloos, W. (1998). Three basic issues concerning interface dynamics in nonequilibrium pattern formation. Phys. Rep. 301, 9-43. 10.1016/S0370-1573(98)00004-0 [DOI] [Google Scholar]
- Vergassola, M., Deneke, V. E. and Di Talia, S. (2018). Mitotic waves in the early embryogenesis of Drosophila: Bistability traded for speed. Proc. Natl. Acad. Sci. USA 115, E2165-E2174. 10.1073/pnas.1714873115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Dassow, G. and Schubiger, G. (1994). How an actin network might cause fountain streaming and nuclear migration in the syncytial Drosophila embryo. J. Cell Biol. 127, 1637-1653. 10.1083/jcb.127.6.1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigbers, M. C., Tan, T. H., Brauns, F., Liu, J., Swartz, S. Z., Frey, E. and Fakhri, N. (2021). A hierarchy of protein patterns robustly decodes cell shape information. Nat. Phys. 17, 578-584. 10.1038/s41567-021-01164-9 [DOI] [Google Scholar]
- Wu, J. Q., Guo, J. Y., Tang, W., Yang, C.-S., Freel, C. D., Chen, C., Nairn, A. C. and Kornbluth, S. (2009). PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat. Cell Biol. 11, 644-651. 10.1038/ncb1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, N., Libertini, S., Black, E. J., Lao, Y., Hegarat, N., Walker, M. and Gillespie, D. A. (2012). Cdk-mediated phosphorylation of Chk1 is required for efficient activation and full checkpoint proficiency in response to DNA damage. Oncogene 31, 1086-1094. 10.1038/onc.2011.310 [DOI] [PubMed] [Google Scholar]
- Yartseva, V. and Giraldez, A. J. (2015). The maternal-to-zygotic transition during vertebrate development: a model for reprogramming. Curr. Top. Dev. Biol. 113, 191-232. 10.1016/bs.ctdb.2015.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, K., Farrell, J. A. and O'farrell, P. H. (2012). Different cyclin types collaborate to reverse the S-phase checkpoint and permit prompt mitosis. J. Cell Biol. 198, 973-980. 10.1083/jcb.201205007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, K., Seller, C. A., Shermoen, A. W. and O'farrell, P. H. (2016). Timing the Drosophila mid-blastula transition: a cell cycle-centered view. Trends Genet. 32, 496-507. 10.1016/j.tig.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y., Xue, X., Shao, Y., Wang, S., Esfahani, S. N., Li, Z., Muncie, J. M., Lakins, J. N., Weaver, V. M., Gumucio, D. L.et al. (2019). Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421-425. 10.1038/s41586-019-1535-2 [DOI] [PMC free article] [PubMed] [Google Scholar]