Abstract
Patient: Female, 51-year-old
Final Diagnosis: Diabetic ketoacidosis
Symptoms: Abdominal pain • diarrhea
Medication: Pembrolizumab
Clinical Procedure: —
Specialty: Endocrinology and Metabolic
Objective:
Unusual or unexpected effect of treatment
Background:
Immune checkpoint inhibitors (ICIs) are a novel class of antibodies, which have been increasingly utilized in cancer immunotherapies. Pembrolizumab is a humanized IgG4 monoclonal antibody, which acts against programmed cell death (PD)-1 receptors to help restore the body’s T-cell and immune response.
Case Report:
In this case, we present a 51-year-old woman with a past medical history of lung adenocarcinoma and triple-positive breast cancer who was actively receiving therapy with pembrolizumab. Following her second chemo-therapy cycle, she developed a severe case of diabetic ketoacidosis (DKA), with concern for new-onset auto-immune type 1 diabetes mellitus (T1DM), secondary to her recent ICI therapy. The patient was initiated on a high-dose insulin infusion for rapid glycemic control and was successfully transitioned to a subcutaneous regimen approximately 24 h after presentation. She additionally developed other autoimmune-related complications, including hepatoxicity, duodenitis, and a maculopapular rash, which all resolved upon discontinuation of the ICI treatment. Her laboratory test results were consistent with positive anti-glutamic acid decarboxylase (anti-GAD) antibodies and undetectable c-peptides, illustrating the uniqueness of an ICI potentially precipitating an autoimmune T1DM.
Conclusions:
Immune-related adverse events from ICI therapy warrant further investigation to acknowledge the risk of potentially life-threatening adverse reactions, such as the development of DKA. Patients receiving ICI therapy should be educated on signs and symptoms of hyperglycemia, and routine measurements of blood glucose levels should be completed during each chemotherapy cycle. Future research in assessing potential biomarkers of beta cell dysfunction, such as anti-GAD antibodies and c-peptides, is of interest, particularly for patients receiving ICI therapies.
Keywords: Antibodies, C-Peptide, Diabetic Ketoacidosis, Immunotherapy, Pembrolizumab
Background
Immune checkpoint inhibitors (ICIs) offer a novel mechanism in cancer therapies by disinhibiting the immune system and upregulating T-cell activation [1]. Pembrolizumab is a humanized IgG4 monoclonal antibody, which acts against programmed cell death (PD)-1 receptors to potentiate this immune response [2]. Pembrolizumab is indicated for various malignancies including non-small cell lung cancer (NSCLC), as a single agent or in combination with pemetrexed and carboplatin [2,3]. Although highly impactful, ICI therapy has been recognized to induce immune-related adverse events (irAE), including endocrinopathies affecting the thyroid and, less commonly, the pancreas [1].
This case report highlights the degree of severity of developing diabetic ketoacidosis (DKA) following therapy with pembrolizumab. The presence of anti-glutamic acid decarboxylase (anti-GAD) antibodies and an undetectable c-peptide level illustrates the uniqueness of an ICI potentially precipitating an autoimmune type 1 diabetes mellitus (T1DM). Here we present a postulated mechanism of autoantibody formation and the importance of health care professionals educating and recognizing glucose abnormalities during ICI therapy.
Case Report
A 51-year-old woman with a past medical history of lung adenocarcinoma and triple-positive breast cancer presented to the Emergency Department (ED) with concern for new-onset auto-immune T1DM from recent ICI therapy. At the time of admission, the patient weighed 101.6 kg. She was actively receiving chemotherapy every 3 weeks with pembrolizumab 2 mg/kg, pemetrexed 500 mg/m2, and carboplatin 750 mg and remained normoglycemic between treatments. Approximately 2 weeks after her second cycle, she presented to the ED with abdominal pain and progressive diarrhea, with concern for new-onset DKA. Upon admission, her random blood glucose level was 1123 mg/dL, serum ketones were >8.00 mmol/L, anion gap (AG) was 34 mmol/L, and pH was 6.943 on a venous blood gas draw. The following pertinent vital signs were recorded: blood pressure, 107/60 mmHg; heart rate, 120 beats/min; respiratory rate, 20 breaths/min; oxygen saturation, 100% on room air. Her physical examination was positive for abdominal pain, diarrhea, vomiting, fatigue, and dizziness. Based on the patient’s clinical presentation, severe hyperglycemia with detection of ketones, and a wide anion gap metabolic acidosis, the diagnosis of DKA was confirmed. The laboratory abnormalities are shown in Table 1.
Table 1.
Laboratory results on admission.
| Laboratory parameter | Value | Reference |
|---|---|---|
| Serum sodium | 123 | 133–145 mmol/L |
| Serum potassium | 7.1 | 3.5–5.1 mmol/L |
| Serum chloride | 85 | 98–108 mmol/L |
| Serum glucose | 1123 | 70–199 mg/dL |
| Anion gap | 34 | 4–16 mmol/L |
| Hemoglobin A1c | 8.3 | ≤5.6% |
| Beta hydroxybutyrate | >8.00 | ≤0.27 mmol/L |
| Lactate | 4.7 | 0.5–2.2 mmol/L |
| Venous pH | 6.943 | 7.330–7.430 |
| Venous pCO2 | 30 | 36–48 mmHg |
| Venous bicarbonate | 6.1 | 22–26 mmol/L |
| Serum c-peptide | 0.1 | 0.8–5.2 ng/mL |
| Glutamic acid decarboxylase | >250 | 0.0–5.0 IU/mL |
| (GAD65) antibody | ||
| T4 free | 1.05 | 0.89–1.76 ng/dL |
| Thyroid stimulating hormone (TSH) | 0.23 | 0.45–5.33 mIU/L |
This patient was admitted to the Intensive Care Unit (ICU) for management of her hyperglycemic crisis. She had no known history of DM and was treated with standard insulin and rehydration therapy for rapid glycemic control. Of note, her hemoglobin A1c (HbA1c) level upon admission was 8.3%. Her insulin regimen was initiated at 0.1 units/kg/h (10 units/h) for approximately 13 h and then was rapidly titrated down to 2 units/h over an additional 8-h period. The following day, the patient’s anion gap closed and electrolyte abnormalities resolved. She was successfully transitioned to a subcutaneous insulin regimen (30 units of glargine daily) and transferred out of the ICU. A computerized tomography (CT) scan obtained during admission showed no pancreatic abnormalities. Given the presence of an undetectable c-peptide level with remarkably elevated anti-GAD antibodies, the potential differential diagnosis was consistent with autoimmune T1DM.
The patient developed additional autoimmune complications including gastroenteritis, hepatotoxicity, a new skin rash, and concern for progression of pulmonary nodules on CT (Figure 1). One week into her admission, her transaminase levels peaked from a baseline aspartate transaminase (AST) and alanine transaminase (ALT) of 20 and 14 (prior to checkpoint inhibitor therapy) to 90 and 99, respectively. Her chest CT was notable for “increase in size and number of numerous pulmonary nodules, consistent with progressive intrathoracic metastatic disease”. Upon discharge, she was initiated on a weekly prednisone taper starting at 50 mg (0.5 mg/kg) daily for suspected auto-immune-mediated duodenitis due to additional identification of duodenal thickening on CT and erythematous duodenopathy on esophagogastroduodenoscopy. Subsequently, she was prescribed a rigorous subcutaneous insulin regimen (insulin glargine 55 units nightly, NPH 50 units daily, while on steroid therapy, and an insulin regular sliding scale) with close out-patient follow-up with an endocrinologist.
Figure 1.
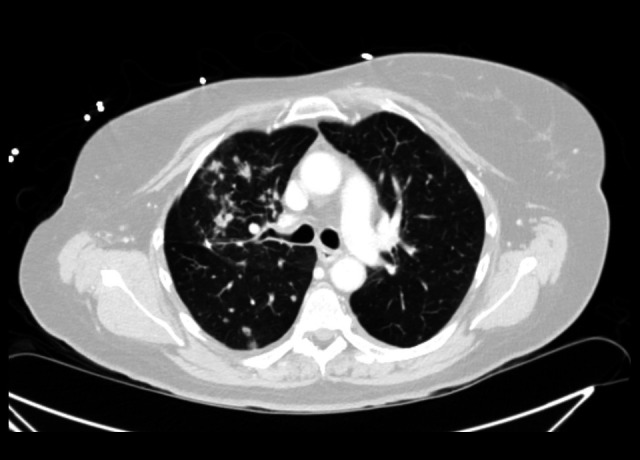
Progression of pulmonary nodules on computed tomography.
Approximately 4 months after her admission and steroid taper completion, her AST and ALT levels had normalized to baseline and her maculopapular rash and duodenitis had resolved. These autoimmune complications may have likely been attributed to the initiation of checkpoint inhibitor therapy, as they quickly resolved with removal of the offending agent. The progression of pulmonary nodules was determined to be consistent with the worsening of her underlying lung adenocarcinoma.
Discussion
This case illustrates the potential for acute development of autoimmune T1DM, secondary to the initiation of ICI therapy. Immune checkpoints, such as PD-1, are small molecules found on the surface of immune cells [1]. Upon binding with its ligands PD-L1 and PD-L2, an inhibitory signaling pathway can be stimulated to suppress an immune response. Similarly, tumor cells can express PD-L1 and bind to the receptor to prevent detection by the host immune system. ICIs are antibodies designed to target these checkpoints, and upon interaction, T cells are activated and help restore an immune response through apoptosis of tumor cells. However, immune checkpoints also play a vital role in maintaining healthy immune homeostasis and preventing autoimmune disorders [1].
By tightly binding to PD-1 receptors on T cells, the immune system is triggered by pembrolizumab to destroy tumor cells, but, due to increased T-cell activation, irAEs can be exhibited. The most common irAEs include hypophysitis and thyroid disorders. Although rare, endocrine irAEs, such as the development of T1DM, have been associated with ICI therapy, owing to the disinhibition of the host immune homeostasis [2,4,5]. The increasing prevalence of reported cases makes this an alarming adverse event, as patients will typically present with life-threatening DKA. The safety of pembrolizumab was assessed in 3 randomized, open-label, active-controlled clinical trials, including 2799 patients with melanoma and/or NSCLC [6–9]. The incidence of developing T1DM, including DKA, was reported in 0.2% of patients. With increasing case reports of endocrine abnormalities, the estimated frequency has been noted to be between 0.2% and 0.8%, but can reach as high as 1.5% in patients on combination immunotherapy regimens [10].
Hypothesized mechanisms of autoantibody production after exposure to PD-1 ligands include enhanced activity of T cells destroying beta islet cells of the pancreas [11,12]. PD-1 is expressed not only on T cells, but also on hematopoietic cells, vascular endothelial cells, and pancreatic islet cells. By blocking the PD-1 pathway with pembrolizumab, autoreactive T cells can be activated and initiate an autoimmune response against pancreatic islet cells. With the breakdown of these cells and dysregulation of insulin production, intracellular proteins, including GAD, are released. These enzymes can then subsequently trigger an immune response and form anti-GAD auto-antibodies, as were detected in our patient. Based on several case reports, the time to onset of a DKA presentation is variable, occurring as early as after a single dose, but a systemic review noted a median time to onset after 3.1 cycles in patients with positive anti-GAD antibodies [5,13].
The clinical manifestations of ICI-related DM will present similarly to typical hyperglycemic crises. The assessment of biochemical and imaging manifestations remains questionable in its sensitivity for detecting DM secondary to ICI therapy. The development of autoimmune antibodies, such as GAD, remains unclear in the pathogenesis of DM from ICI therapy. Some studies report 50% of cases with positive GAD autoantibodies [1]. This is interesting because in patients who develop conventional autoimmune T1DM that is unrelated to ICI use, more than 90% have detectable autoantibodies [14]. The advantages of testing anti-GAD at baseline and utilizing this value as a biomarker during therapy remains unanswered, but warrants further investigation. C-peptide is a measurement of endogenous insulin secretion [1]. Beta islet cells synthesize the pro-hormone proinsulin, which then gets cleaved to insulin and c-peptide. In the setting of impaired insulin secretion, an undetectable level of c-peptide appears consistent with autoimmune DM. Although the utility of c-peptide has not been established in this context, a low level may raise high suspicion for ICI-related DM. HbA1c is a measurement of average blood glucose over the past 8 to 12 weeks. Owing to the rapid development of DM in some patients, the utility of measuring a HbA1c can be limited in truly reflecting the patient’s average blood glucose since initiation of ICI therapy. Pancreatic imaging on CT can be useful in detecting pancreatic atrophy and enlargement or diffuse inflammation. However, our patient had an unremarkable CT scan, which has been similarly reported in other case series, limiting the usage of radiological imaging as a diagnostic factor.
Based on published case reports, ICI-related DM is typically irreversible, requiring long-term insulin therapy [14,15]. Whether to continue ICI treatment once glycemic control has been attained has not yet been established. The American Society of Clinical Oncology and National Comprehensive Cancer Network guidelines recommend withholding therapy until glucose control is achieved [16]. Considering that management of DM can be tailored, patients with advanced malignancies may require continuation of their ICI therapy and require consistent education in recognizing endocrine abnormalities. Although steroidal treatment has been useful in the management of many irAEs, it is reported to be less effective in restoring beta cell function [14]. Currently, the role of steroids in ICI-induced DM is unknown, as they have the potential to worsen blood glucose management, further complicating treatment.
Conclusions
With the increasing usage of these novel anticancer therapies, raising awareness in the medical community is pivotal to not only treating, but preventing these potentially life-threatening irAEs. This case illustrates the severity of complications with recent ICI therapy initiation after presentation to the ED, with severe DKA and development of additional autoimmune-related complications. During ICI therapy, patients should be routinely educated on signs and symptoms of hyperglycemia and when to seek medical attention. Routine measurements of blood glucose levels should be completed during each chemotherapy cycle to facilitate early detection of any glucose abnormalities or life-threatening DKA. The data surrounding the utility of monitoring anti-GAD and c-peptides during therapy remain inconsistent in currently published literature. Future research on potential biomarkers to detect beta cell dysfunction before complete loss of insulin production is of interest and highly warranted.
Abbreviations
- Anti-GAD
anti-glutamic acid decarboxylase;
- CT
computerized tomography;
- DKA
diabetic ketoacidosis;
- DM
diabetes mellitus;
- ED
Emergency Department;
- HbA1c
hemoglobin A1c;
- ICI
immune checkpoint inhibitor;
- ICU
Intensive Care Unit;
- irAE
immune-related adverse events;
- NSCLC
non-small cell lung cancer;
- PD-1
programmed cell death-1;
- T1DM
type 1 diabetes mellitus
References:
- 1.Chang L-S, Barroso-Sousa R, Tolaney SM, et al. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40:17–65. doi: 10.1210/er.2018-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamy TA, Aguasvivas M, Serhan M, et al. Diabetic ketoacidosis triggered by pembrolizumab in a patient with bladder cancer. Diabetes [Internet]. 2018 [cited 2020 Dec 10];67(Suppl. 1):219-LB. https://diabetes.diabetes-journals.org/content/67/Supplement_1/219-LB.
- 3.Keytruda (pembrolizumab) [prescribing information] Whitehouse Station NJ: Merck Sharp & Dohme Corp; 2015. [Google Scholar]
- 4.Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: A systematic review of case reports. PLoS One. 2016;11:e0160221. doi: 10.1371/journal.pone.0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Filette J, Andreescu C, Cools F, et al. A systematic review and meta-analysis of endocrine-related adverse events associated with immune checkpoint inhibitors. Horm Metab Res. 2019;51:145–56. doi: 10.1055/a-0843-3366. [DOI] [PubMed] [Google Scholar]
- 6.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. New Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 7.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–18. doi: 10.1016/S1470-2045(15)00083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 9.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239–51. doi: 10.1016/S1470-2045(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 10.Verlekar P, Price H. Immune-mediated diabetes due to pembrolizumab. Pract Diab. 2017;34:250–51. [Google Scholar]
- 11.Cheema A, Makadia B, Karwadia T, et al. Autoimmune diabetes associated with pembrolizumab: A review of published case reports. World J Oncol. 2018;9:1–4. doi: 10.14740/wjon1085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clotman K, Janssens K, Specenier P, et al. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab. 2018;103:3144–54. doi: 10.1210/jc.2018-00728. [DOI] [PubMed] [Google Scholar]
- 13.Maamari J, Yeung S-CJ, Chaftari PS. Diabetic ketoacidosis induced by a single dose of pembrolizumab. Am J Emerg Med. 2019;37:376. doi: 10.1016/j.ajem.2018.10.040. e1–e2. [DOI] [PubMed] [Google Scholar]
- 14.Quandt Z, Young A, Anderson M. Immune checkpoint inhibitor diabetes mellitus: A novel form of autoimmune diabetes. Clin Exp Immunol. 2020;200:131–40. doi: 10.1111/cei.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuenca JA, Laserna A, Reyes MP, et al. Critical care admission of an HIV patient with diabetic ketoacidosis secondary to pembrolizumab. Case Rep Crit Care. 2020;2020:8671530. doi: 10.1155/2020/8671530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]


