Abstract
Patient: Male, 35-year-old
Final Diagnosis: Esophageal perforation
Symptoms: Emesis
Medication:—
Clinical Procedure: —
Specialty: Critical Care Medicine
Objective:
Rare disease
Background:
Esophageal necrosis is a rare entity characterized by the presence of extensive circumferential necrosis of the esophagus. It generally affects older adults who have associated chronic pathologies and has a reported mortality rate of approximately 32%. Most patients with esophageal necrosis have a complex clinical course.
Case Report:
We present the case of a 37-year-old man with idiopathic chronic renal failure who presented to the Emergency Department with sudden esophageal necrosis and mediastinitis, associated with invasive candidiasis. Diagnosis was challenging owing to the rarity of the condition. The patient required intensive care management and multiple surgical procedures.
Conclusions:
Esophageal necrosis is an uncommon pathology that can be fatal because of associated complications. Its pathophysiology is unclear, and its treatment is based on the control of local injury and signs and symptoms. Acute esophageal necrosis associated with invasive Candida sp. infection is even more infrequent, with only a few cases reported in the literature.
Keywords: Candida albicans; Emergency Service, Hospital; Esophageal Diseases; Necrosis
Background
Esophageal necrosis is a rare entity characterized by the presence of extensive circumferential necrosis of the esophagus. It was initially described by Brennan in 1967 [1]. In 1990, it was characterized endoscopically by Goldenberg et al [2] who gave it the name “black esophagus”, based on the anatomical presentation they observed [3]. Esophageal necrosis generally affects older adults with associated chronic pathologies, mal-nourished patients, immunocompromised patients, or those with multiple comorbidities. According to the main hypothesis, the condition of a low-flow state plus weakened base tissue favors ischemia. Most patients with esophageal necrosis have a complex clinical course, with a reported mortality rate of approximately 32% [3], and a large proportion of patients (< 25%) have sequelae due to related complications [3,4]. Esophageal necrosis has also been associated with invasive infection by Candida sp., which worsens patient outcomes.
Case Report
A 37-year-old man with a history of idiopathic chronic renal failure underwent renal replacement therapy. He had consulted his primary care clinician for multiple episodes of brown-colored emesis, which was associated with dizziness, asthenia, and adynamia. A relative indicated that the patient presented with diarrhea with a 4-day evolution that had already resolved. Subsequently, there was deterioration of the patient’s consciousness and respiratory pattern; therefore, his airway was ensured, and he was referred to a tertiary hospital.
The patient was admitted to our institution in poor general condition, with a heart rate of 132 beats per min, blood pressure of 55/39 mmHg, mean blood pressure of 44.3 mmHg, oxygen saturation of 97%, with a tracheal tube, FIO2 of 70%, and a temperature of 36.4°C. His Glasgow score was 8/15, and he had normally reactive, isochoric pupils, no obvious traumatic lesions, distal coldness of the extremities, and a capillary refill time of 4 s.
The patient’s arterial gases at admission showed metabolic acidosis, with hyperlactatemia (pH, 7.31; pCO2, 27 mmHg; pO2, 226 mmHg; HCO3, 13.6 mmol/L; Base excess, −12.6 mmol/L; lactate, 9.1; glucose, 178 mg/dL; sodium, 131 mmol/L; and potassium, 5.1 mmol/L). Paraclinical examinations showed leukocytosis, neutrophilia, moderate anemia, high nitrogen concentrations, and metabolic acidosis, with lactic acidemia (leukocytes, 14 890×103/uL; neutrophils, 77.3%; lymphocytes, 14.4%; hemoglobin, 8.1 g/dL; hematocrit, 25.4%; platelets, 395 000×103/uL; prothrombin time, 14.3 s; partial thromboplastin time, 29.7 s; blood urea nitrogen, 141mg/dL; creatinine, 9.48 mg/dL; and total bilirubin, 0.14 mg/dL, indirect bilirubin, 0.05 mg/dL, and direct bilirubin, 0.09 mg/dL).
An emergency ultrasound was performed with a 2 to 4 MHz transducer, which showed a small, hyperdynamic left ventricle, a right ventricle smaller than the left ventricle, no obvious signs of right ventricular overload, and no pericardial effusion. There was no free fluid in the abdomen and no hemothorax or pneumothorax in the pulmonary evaluation. A plain chest X-ray showed mixed opacities, which were predominantly alveolar, forming patches of bilateral consolidations and nodular images (Figure 1).
Figure 1.
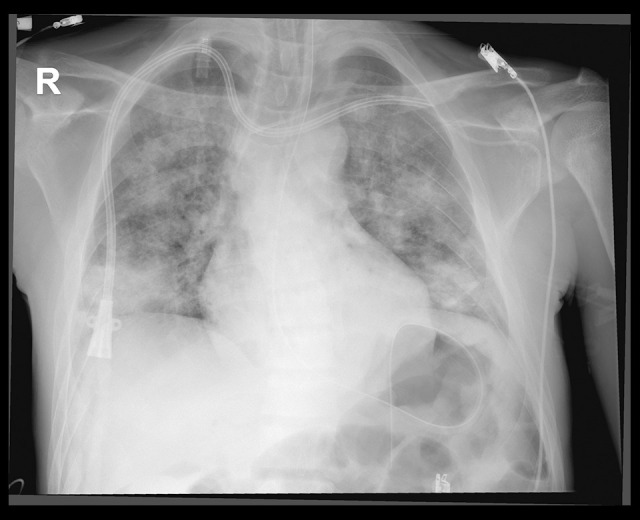
Mixed opacities predominantly alveolar, forming patches of bilateral consolidations and nodular images.
A total computed tomography (CT) scan of the abdomen was conducted, and fluid resuscitation, vasopressor support, and empirical antibiotic therapy were started for suspicion of an intestinal obstruction or other intra-abdominal etiology.
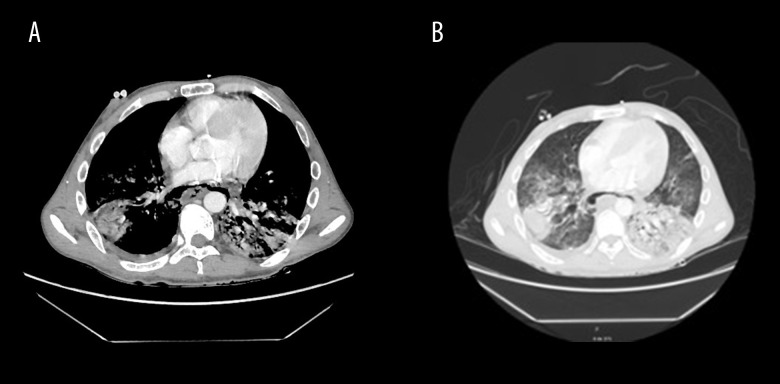
The CT scan showed no evidence of intestinal perforation, bleeding, or an intra-abdominal inflammatory/infectious process; however, there were signs of posterior pneumomediastinum, with thickening of the walls of the distal third of the esophagus and consolidations of an infectious appearance in both lung bases, associated with scarce bilateral pleural effusion (Figure 2A, 2B). An endoscopic examination of the upper digestive tract was performed and showed extensive esophageal necrosis from the cricopharyngeal muscle to the esophageal-gastric junction transmurally at 30 cm, with a perforation of approximately 15 mm at 35 cm, as well as a normal stomach and diffuse severe ulceration and duodenitis of the bulb (Figure 3).
Figure 2.
(A, B) Signs of posterior pneumomediastinum, with thickening of the walls of the distal third of the esophagus and consolidations of infectious appearance in both lung bases, associated with scarce bilateral pleural effusion.
Figure 3.
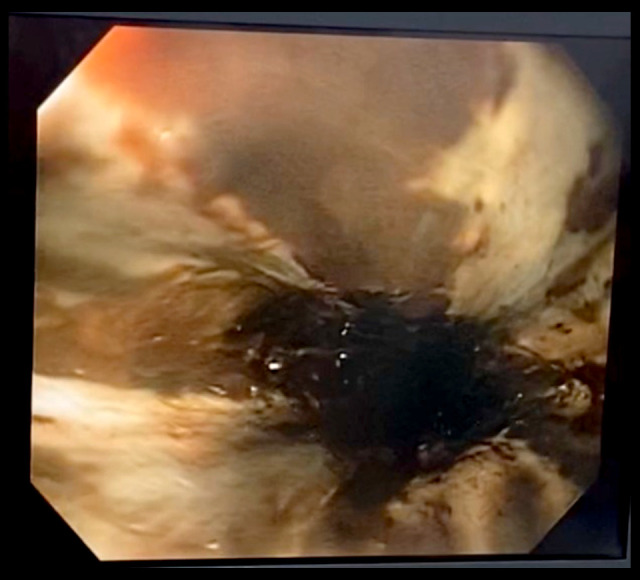
Extensive esophageal necrosis from the cricopharyngeal muscle to the esophagealgastric junction transmurally at 30 cm, with a perforation of approximately 15 mm at 35 cm, as well as a normal stomach and diffuse severe ulceration and duodenitis of the bulb.
The patient’s relative was asked about the possible intake of caustics or nonsteroidal anti-inflammatory drugs, but the family member denied that the patient had contact with corrosive substances or consumed other medications.
We performed a left cervicotomy and right thoracotomy for mediastinal drainage; the procedures showed an air-dissected superior mediastinum with scarce purulent fluid, necrotic esophagus, and perforation, with periesophageal collections, pus, and purulent detritus in addition to 300 cc of purulent pleural collection. The patient was transferred to the Intensive Care Unit, where he required management with high-dose vasopressors, inotropic support, transfusion of blood products, and broad-spectrum empirical antibiotic treatment with vancomycin, meropenem, and caspofungin. The patient presented an unfavorable course, and the high vasoactive requirement persisted the following day, so methylene blue was initiated. On hospitalization day 3, owing to the patient’s gradual improvement, he underwent transhiatal esophagectomy, with gastrostomy and bilateral thoracostomy. After the surgical procedure, a satisfactory evolution and improvement of blood pressure was achieved, allowing progressive weaning from vasopressor support, inotropic agents, and mechanical ventilation, with successful extubation on day 4 of hospitalization.
On day 5, the patient was taken to have the cavity checked and the abdomen closed. The peritoneal fluid culture indicated the presence of hyphae, and esophageal pathology reports described ischemic necrosis, secondary to severe acute trans-mural inflammation, which was compatible with invasive infection by Candida sp. After the surgical procedure, the patient again presented clinical deterioration that required intubation, and vasopressor support was restarted. Faced with refractory respiratory and cardiac failure, CT angiography of the thorax and upper hemiabdomen was performed; a subsegmental pulmonary embolism and suspicion of the presence of a foreign-body embolization in the right brachiocephalic trunk and superior cava were reported. Given the patient’s clinical deterioration, a new cavity review was performed and no evidence of ischemia or intestinal obstruction was found. The patient continued to deteriorate. He did not respond to medical management for hypotension and refractory respiratory failure and died.
Discussion
Esophageal necrosis is a rare entity [1], which generally affects the distal region of the esophagus (97%) and ends abruptly at the gastroesophageal junction, although some cases report damage to the proximal and diffuse tissue of this organ [4,5]. It has an incidence of 0.01% to 0.28% [6–8] and a prevalence of 6% [9]; however, there can be significant underreporting owing to the critical state of the patients, the subclinical presentation, and the delay in performing endoscopic examination of the upper digestive tract [10]. As was the case with the present patient, esophageal necrosis is not an entity that is encountered regularly, given its low incidence.
Esophageal necrosis presents more often in men than in women, with a 3: 1 ratio [11], and the average age of onset is 75 years in published cases [3,7,9].
Its etiology is multifactorial and has been primarily associated with the “double-hit” hypothesis [10], which describes an initial event in which a state of low flow occurs, which is associated with a second event that is related to a poor barrier system, alteration of the repairing capacity of the mucosa, and a weakened compensation system. This is why esophageal necrosis is most frequently described in patients who are malnourished, immunocompromised, or have multiple comorbidities [12].
The main associated comorbidities are diabetes mellitus, chronic alcohol consumption, arterial hypertension, chronic kidney disease, hyperlipidemia, and malignancy [11–13].
Other specific risk factors have been described [14], such as diabetic ketoacidosis [15], the use of drugs, such as cocaine [16], hypothermia [1], gastrointestinal pathology, such as acute gastric outlet obstruction [17], malignancy [18], surgical interventions, and different infections by bacteria, viruses and fungi, such as Candida sp. [19].
The typical clinical picture includes gastrointestinal bleeding in 75% of cases, with hematemesis in 66% of cases and melena in 33% of cases [3–13], which is associated with shock in 36% of cases and abdominal or substernal pain in 28% of cases [13]. Patients can also present with other symptoms, such as nausea, vomiting, dysphagia, fever, and syncope [9]. In the majority of reported cases, anemia has been observed as a predominant sign, although it may not be found, owing to the hyperacute clinical picture [20,21]. Hyperlactatemia and leukocytosis [21] are other findings, which reflect the patient’s state of hypoperfusion and inflammation.
The criterion standard for diagnosis is endoscopic examination of the upper digestive tract, and the characteristic circumferential black coloration that does not go beyond the gastroesophageal junction [3–9] is sufficient to determine this diagnosis. There are other possible endoscopic findings [9], such as different signs of bleeding and the presence of gastric ulcerations [22] or duodenal ulcers [23]. A biopsy analysis is not required [9] to confirm the diagnosis, and performing one can increase the risk of complications in the patient. A biopsy sample can be considered an additional tool to clarify the cause in cases of extensive mucosal necrosis, which can extend to deeper layers and be associated with vascular thrombosis and severe inflammatory changes [5]. When obtaining a tissue sample, it is imperative to conduct studies that search for infections and malignancy, since these are possible etiologies.
The main complications are esophageal perforation (5.7%), which leads to the occurrence of mediastinitis or the formation of abscesses, and esophageal stenosis (10.2%), which is probably related to impaired mucosal healing [13]. There are few cases reported in the literature with esophageal perforation associated with necrosis, and only 6 of the 130 cases were evaluated [14]. All of these patients presented with mediastinitis [3].
The overall mortality rate associated with acute esophageal necrosis is estimated to be approximately 32% to 50% [3,24]. Mortality is usually associated with underlying diseases and, specifically, is secondary to necrosis in approximately 6% of cases [3,13].
On some occasions, spontaneous resolution has been described with no additional complications and is associated with healing changes visualized on endoscopic examination and usually occurs within the first 2 weeks after the first image is taken [10,19,25]. On the other hand, cases related to invasive infection by Candida sp. have been described, and although there are few reports in the literature [27,28], it has been observed mainly in patients with a history of diabetes mellitus [29], immunosuppression due to renal transplantation [30], and the use of corticosteroids to treat rheumatic diseases [28–31]. Mortality in these cases has been more than 65%, with only 3 reports indicating a good outcome, in young patients [27–33].
The treatment of acute esophageal necrosis and its pathophysiology are not completely clear. Therefore, there is no specific related treatment at present. Management is mainly supportive and is associated with the treatment of precipitating conditions or etiology.
The placement of a nasogastric tube should be avoided owing to the risk of perforation [26]. Then, first, the rapid replacement of fluid and of blood products should be initiated to improve perfusion. Second, a proton pump inhibitor should be used to protect the esophageal mucosa from acid reflux. Finally, surgical treatment of the associated pathologies or complications may or may not be required [3,4,8].
Surgical treatment, which is indicated in patients with complications, is initially based on control of the infection site [21]. Damage control surgery is generally used and is based on extrapolated data from patients with hemodynamic instability of a traumatic origin, since there are few related studies in patients without trauma [34,35]. Damage control surgery is performed to restore physiological stability and is followed by a definitive intervention to improve the survival of these patients, who have a high risk of mortality inherent in their hemodynamic status [35].
Acute esophageal necrosis with perforation is not a common etiology of shock in patients who present to the Emergency Department. Despite early diagnosis and management with digestive endoscopy and damage control surgery, the mortality of patients with esophageal perforation is high, especially in the presence of multiorgan failure, as was the case with our patient.
Conclusions
Esophageal necrosis is an uncommon pathology that can be fatal due to complications. Its pathophysiology is unclear, and treatment is based on control of local injury and signs and symptoms. Acute esophageal necrosis associated with invasive infection of Candida sp. is even more infrequent, with only a few cases reported in the literature.
References:
- 1.Brennan JL. Case of extensive necrosis of the oesophageal mucosa following hypothermia. J Clin Pathol. 1967;20(4):581–84. doi: 10.1136/jcp.20.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg SP, Wain SL, Marignani P. Acute necrotizing esophagitis. Gastroenterology. 1990;98(2):493–96. doi: 10.1016/0016-5085(90)90844-q. [DOI] [PubMed] [Google Scholar]
- 3.Gurvits E, Shapsis A, Lau N, et al. Acute esophageal necrosis: A rare syndrome. J Gastroenterol. 2007;42(1):29–38. doi: 10.1007/s00535-006-1974-z. [DOI] [PubMed] [Google Scholar]
- 4.Gurvits GE, Cherian K, Shami MN, et al. Black esophagus: New insights and multicenter international experience in 2014. Dig Dis Sci. 2015;60(2):444–53. doi: 10.1007/s10620-014-3382-1. [DOI] [PubMed] [Google Scholar]
- 5.Jessurun J, Cui I, Aristi-Urista G. Acute (gangrenous) esophageal necrosis (black esophagus). A rare form of injury with specific histologic features and diverse clinical associations with a common pathogenesis. Hum Pathol. 2019;87:44–50. doi: 10.1016/j.humpath.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Moretó M, Ojembarrena E, Zaballa M, et al. Idiopathic acute esophageal necrosis: Not necessarily a terminal event. Endoscopy. 1993;25:534–38. doi: 10.1055/s-2007-1009121. [DOI] [PubMed] [Google Scholar]
- 7.Augusto F, Fernandes V, Cremers M, et al. Acute necrotizing esophagitis: A large retrospective case series. Endoscopy. 2004;36:411–15. doi: 10.1055/s-2004-814318. [DOI] [PubMed] [Google Scholar]
- 8.Soussan EB, Savoye G, Hochain P, et al. Acute esophageal necrosis: A 1-year prospective study. Gastrointest Endosc. 2002;56(2):213–17. doi: 10.1016/s0016-5107(02)70180-6. [DOI] [PubMed] [Google Scholar]
- 9.Dias E, Santos-Antunes J, Macedo G. Diagnosis and management of acute esophageal necrosis. Ann Gastroenterol. 2019;32(6):529. doi: 10.20524/aog.2019.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan H, Ahmed M, Daoud M, et al. Acute esophageal necrosis: A view in the dark. Case Rep Gastroenterol. 2019;13(1):25–31. doi: 10.1159/000496385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullah W, Mehmood A, Micaily I, Khan MS. Comprehensive review of acute esophageal necrosis. BMJ Case Rep. 2019;12(2):e227967. doi: 10.1136/bcr-2018-227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sha J, Savlania A, Bush N, et al. Three cases of an unusual cause of haematemesis: Black oesophagus. Trop Doct. 2020;50(2):152–54. doi: 10.1177/0049475519900756. [DOI] [PubMed] [Google Scholar]
- 13.Abdullah HM, Ullah W, Abdallah M, et al. Clinical presentations, management, and outcomes of acute esophageal necrosis: A systemic review. Expert Rev Gastroenterol Hepatol. 2019;13:507–14. doi: 10.1080/17474124.2019.1601555. [DOI] [PubMed] [Google Scholar]
- 14.Correia J, Castelo LF, Fischer WG, Felipe-Silva A. Black esophagus. Autops Case Rep. 2019;9(1):e2018077. doi: 10.4322/acr.2018.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Usmani A, Samarany S, Nardino R, Shaib W. Black esophagus in a patient with diabetic ketoacidosis. Conn Med. 2011;75(8):467–68. [PubMed] [Google Scholar]
- 16.Ullah W, Abdullah HMA, Rauf A, Saleem K. Acute oesophageal necrosis: A rare but potentially fatal association of cocaine use. BMJ Case Rep. 2018;2018:bcr-2018-225197. doi: 10.1136/bcr-2018-225197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kram M, Gorenstein L, Eisen D, Cohen D. Acute esophageal necrosis associated with gastric volvulus. Gastrointest Endosc. 2000;51:610–12. doi: 10.1016/s0016-5107(00)70304-x. [DOI] [PubMed] [Google Scholar]
- 18.Vohra I, Bashar A, Almoghrabi A. Black esophagus: Acute esophageal necrosis. Clin Gastroenterol Hepatol. 2020;19(2):e16. doi: 10.1016/j.cgh.2019.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Gaissert HA, Breuer CK, Weissburg A, Mermel L. Surgical management of necrotizing Candida esophagitis. Ann Thorac Surg. 1999;67(1):231–33. doi: 10.1016/s0003-4975(98)01144-8. [DOI] [PubMed] [Google Scholar]
- 20.Maubert A, Frey S, Rahili A, et al. Acute esophageal necrosis: Case report of an unknown entity. Int J Surg Case Rep. 2019;61:188–90. doi: 10.1016/j.ijscr.2019.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grigoriy E. Black esophagus: Acute esophageal necrosis syndrome. World J Gastroenterol. 2010;16(26):3219. doi: 10.3748/wjg.v16.i26.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumann DA, 2nd, Francis DL, Baron TH. Proximal black esophagus: A case report and review of the literature. Gastrointest Endosc. 2009;70:180–81. doi: 10.1016/j.gie.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 23.Lacy BE, Toor A, Bensen SP, et al. Acute esophageal necrosis: Report of two cases and a review of the literature. Gastrointest Endosc. 1999;49:527–32. doi: 10.1016/s0016-5107(99)70058-1. [DOI] [PubMed] [Google Scholar]
- 24.Grudell AB, Mueller PS, Viggiano TR. Black esophagus: Report of six cases and review of the literature, 1963–2003. Dis Esophagus. 2006;19(2):105–10. doi: 10.1111/j.1442-2050.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- 25.Haddad I, Alomari M, El Kurdi B, et al. A case of black esophagus. Cureus. 2019;11(9):e5577. doi: 10.7759/cureus.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafa S, Sharma N, Keshishian J, Dellon ES. The black esophagus: A rare but deadly disease. ACG Case Rep J. 2016;3(2):88–91. doi: 10.14309/crj.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gock M, Schäfer M, Perren A, et al. Fatal esophageal perforation caused by invasive candidiasis. Ann Thorac Surg. 2005;80(3):1120–22. doi: 10.1016/j.athoracsur.2004.02.147. [DOI] [PubMed] [Google Scholar]
- 28.Piubelli MLM, Felipe-Silva A, Kanegae MY, Ferraz de Campos FP. Fatal necrotizing Candida esophagitis in a patient with leukocytoclastic cutaneous vasculitis and ankylosing spondylitis. Autops Case Rep. 2019;9(2):e2018070. doi: 10.4322/acr.2018.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yong K, Young S. Black esophagus with concomitant candidiasis developed after diabetic ketoacidosis. World J Gastroenterol. 2007;13(42):5662–63. doi: 10.3748/wjg.v13.i42.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones JM, Glass NR, Belzer FO. Fatal Candida esophagitis in two diabetics after renal transplantation. Arch Surg. 1982;117(4):499–501. doi: 10.1001/archsurg.1982.01380280079016. [DOI] [PubMed] [Google Scholar]
- 31.Aghdam F, Sund S. Invasive esophageal candidiasis with chronic mediastinal abscess and fatal pneumomediastinum. Am J Case Rep. 2016;17:466. doi: 10.12659/AJCR.898053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones JM. Necrotizing Candida esophagitis: Failure of symptoms and roentgenographic findings to reflect severity. JAMA. 1980;244(19):2190–91. doi: 10.1001/jama.244.19.2190. [DOI] [PubMed] [Google Scholar]
- 33.Jones JM, Glass NR, Belzer FO. Fatal Candida esophagitis in two diabetics after renal transplantation. Arch Surg. 1982;117(4):499–501. doi: 10.1001/archsurg.1982.01380280079016. [DOI] [PubMed] [Google Scholar]
- 34.Lamb CM, MacGoey P, Navarro AP, Brooks AJ. Damage control surgery in the era of damage control resuscitation. Br J Anaesth. 2014;113(2):242–49. doi: 10.1093/bja/aeu233. [DOI] [PubMed] [Google Scholar]
- 35.Benz D, Zsolt B. Damage control surgery: Current state and future directions. Curr Opin Crit Care. 2017;23(6):491–97. doi: 10.1097/MCC.0000000000000465. [DOI] [PubMed] [Google Scholar]