Abstract
Background
Helicobacter pylori is estimated to affect about half the world's population and is considered as the main cause of chronic gastritis and peptic ulcer disease. Eradication of H. pylori infection accelerates ulcer healing and prevents relapse, reducing incidence of H. pylori‐related gastric diseases. Numerous studies have provided evidence that the oral cavity could be a potential reservoir for H. pylori. The presence of oralH. pylori might affect the efficiency of eradication therapy and act as a causal force for its recurrence. Conversely, other investigators have indicated that the colonization and growth of H. pylori differs between the oral cavity and the stomach. Considering the open debate on the topic, it's necessary to clarify whether periodontal therapy is an effective adjunctive treatment for gastric H. pylori infection.
Objectives
To assess the effects of periodontal therapy plus eradication therapy versus eradication therapy alone for gastric H. pylori infection. The secondary objective is to compare the non‐recurrence rate at long‐term follow up in different treatment groups.
Search methods
We identified randomized controlled trials (RCTs) by searching the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 8), MEDLINE (1946 to August 2015), EMBASE (1980 to August 2015), and the Chinese Biomedical Database (1978 to August 2015). We also searched both ClinicalTrials.gov and the WHO ICTRP portal in October 2015. We handsearched the reference lists of included studies to identify relevant trials.
Selection criteria
RCTs comparing periodontal therapy plus eradication treatment with eradication treatment alone, regardless of language of publication.
Data collection and analysis
Two reviewers selected the trials that met the inclusion criteria and extracted the details of each study independently. The data were pooled using both fixed‐effect and random‐effects models and results calculated as odds ratios (OR) with their 95% confidence intervals (CIs) based on an intention‐to‐treat analysis. However, because there was little difference in the results from these two models, we only reported the results from the fixed‐effect model.
Main results
We included seven small RCTs involving 691 participants aged 17 to 78 years in our meta analyses. The primary result showed that periodontal therapy combined with H. pylori eradication treatment increased the eradication rate of gastric H. pylori compared with eradication treatment alone (OR 2.15; 95% CI 1.47 to 3.14; P < 0.0001) in people with H. pylori infection. In addition, periodontal therapy also had benefits on long‐term gastric H. pylori eradication. After eradication of H. pylori, the non‐recurrence rate of gastric H. pylori infection increased in participants treated with periodontal therapy compared with those who received eradication therapy alone (OR 3.60; 95% CI 2.11 to 6.15; P < 0.00001). According to the GRADE approach, the overall quality of the evidence was 'moderate' for eradication rate of gastric H.pylori and 'low' for non‐recurrence rate of gastric H. pylori.
Authors' conclusions
Overall, periodontal therapy could increase the efficiency of H. pylori eradication and the non‐recurrence rate of gastricH. pylori. In view of the limited number and quality of included studies, it will be necessary to conduct more well‐designed, multicenter, and large‐scale RCTs to determine the effects of periodontal therapy in eradicating gastric H. pylori and suppressing the recurrence of this bacterium in the stomach.
Keywords: Adolescent, Adult, Aged, Humans, Middle Aged, Helicobacter pylori, Helicobacter Infections, Helicobacter Infections/prevention & control, Helicobacter Infections/therapy, Periodontics, Periodontitis, Periodontitis/therapy, Randomized Controlled Trials as Topic, Recurrence, Secondary Prevention, Secondary Prevention/methods
Plain language summary
Dental hygiene in addition to drug treatment for Helicobacter pylori eradication
Background
Helicobacter pylori is a bacteria that is considered to be the main cause of long‐term inflammation and ulcers of the stomach, and research has also linked it to diseases such as cancer of the stomach and lymph nodes. Globally, the bacteria affects about half the world's population. To eliminate H. pylori infection and prevent its recurrence, physicians can use various combinations of medications, including antibiotics and proton pump inhibitors (which reduce stomach acid production). This is known as eradication therapy. However, H. pylori may also reside inside the mouth, and researchers do not know whether or not its presence there changes the effectiveness of eradication therapy aimed at the stomach. Given this open debate, it is necessary to clarify whether periodontal therapy is an effective added treatment forH. pylori infection of the stomach and whether its use combined with eradication therapy can prevent recurrence better than eradication therapy alone. Periodontal therapy consists of procedures carried out to support the health of the structures in the mouth that support teeth, such as the jaw bone and gums. It includes oral hygiene instruction, toothbrushing, the use of mouthwash, and the professional removal of dental plaque and tartar from the teeth and gum line. This summary of a Cochrane review represents what we know about the benefits and harms of periodontal therapy as an accompanying treatment for H. pylori infection of the stomach in adults.
Study characteristics
After searching for all relevant studies to August 2015, we found seven small randomized controlled trials (considered the highest quality study design) involving 691 participants aged 17 to 78 years.
Key results
The results indicated that periodontal therapy as an added treatment had some benefits on eradication of gastricH. pylori for short‐term and long‐term follow‐up. The eradication (the reduction of the prevalence of H. pylori in stomach to normal range) and non‐recurrence (the proportion of participants that remained free of gastric H. pylori after successful eradication therapy) rates increased in people who received periodontal therapy plus eradication treatment, when compared with those who received eradication treatment alone.
Quality of the evidence
Because there were not very many participants or trials included in our analyses, we cannot draw a firm conclusion about the use of periodontal therapy for all patients with gastric H. pylori infection in clinical practice.
Based on the results in this review, large‐scale randomized controlled trials comparing periodontal therapy plus eradication treatment with eradication treatment alone would be useful to generate stronger evidence on the use of periodontal therapy as an additional treatment for patients with gastric diseases caused by H. pylori.
Summary of findings
Summary of findings for the main comparison. Eradication therapy combined with periodontal therapy versus eradication therapy alone for gastric Helicobacter pylori infection.
| Eradication therapy combined with periodontal therapy versus eradication therapy alone for gastric Helicobacter pylori infection | |||||
| Patient or population: patients with gastric Helicobacter pylori infection Settings: outpatient clinic Intervention: eradication therapy combined with periodontal therapy versus eradication therapy alone | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Eradication therapy alone | Eradication therapy combined with periodontal therapy | ||||
| Eradication rate of gastric H. pylori | 60 per 100 | 76 per 100 (69 to 82) | OR 2.15 (1.47 to 3.14) | 543 (6 studies) | ⊕⊕⊕⊝ moderatea |
| Non‐recurrence rate of gastric H. pylori | 29 per 100 | 60 per 100 (47 to 72) | OR 3.60 (2.11 to 6.15) | 299 (3 studies) | ⊕⊕⊝⊝ lowb,c,d |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded one level due to risk of bias (only Zaric 2009 described methods of randomization). bDowngraded one level due to risk of bias (all patients in Jia 2009a were successfully treated for gastric H. pylori after triple eradication therapy). cAlthough OR value was 3.60, we decided not to upgrade the quality of evidence because all patients in Jia 2009a were successfully treated for gastric H. pylori after triple eradication therapy. dDowngraded one level due to imprecision (less than 400 total participants).
Background
Description of the condition
The prevalence of Helicobacter pylori (H. pylori) is estimated to affect about half the world's population, possibly reaching between 70% to 90% in developing countries and hovering between 25% to 50% in developed countries (Go 2002). Of all individuals infected with H. pylori, only a small percentage develops related diseases. Diseases associated with H. pylori infection commonly happen at earlier ages in developing countries (Balaban 1997), which is probably due to various host‐related factors, the difference in strains of H. pylori, and the duration of infection and environmental factors, including socioeconomic status (Yamaoka 2010). H. pylori infection is considered to be the main cause of chronic gastritis and peptic ulcer disease (Suerbaum 2002). Most individuals infected with H. pylori have also have gastritis. Many of them are never diagnosed because they have so few or no symptoms, but about 10% to 20% of them will develop gastric or duodenal ulcer, with the latter being much more prevalent (Schlemper 1996). A paper from Serbia showed that 15% to 25% of patients had duodenal ulcer, while 13% had gastric ulcer (Sokić‐Milutinović 2004). H. pylori is also linked with gastric adenocarcinoma (cancerous tumour) and mucosa‐associated lymphoid tissue (MALT) lymphoma. An H. pylori‐positive individual has less than 3% lifetime risk of developing adenocarcinoma and a 1% risk of developing MALT lymphoma (Balaban 1997; Kusters 2006; Peek 2002). Eradication of H. pylori infection accelerates ulcer healing and prevents relapse, reducing the incidence of H. pylori‐related gastric diseases (Ford 2006; Graham 1991; Hopkins 1996; Kim 1998; Tytgat 1994).
Description of the intervention
Triple therapy, including a proton pump inhibitor (PPI, a drug that reduces gastric acid secretion in the stomach and accelerates the healing of peptic ulcer) plus two antibiotics, such as clarithromycin and amoxicillin or metronidazole, is the most common first‐line clinical intervention regimen (Asaka 2001; Hunt 1999; Shirin 2004; Wolle 2007). Taking into account the high prevalence of clarithromycin resistance, quadruple therapy (PPI + bismuth + metronidazole + tetracycline) is used as an alternate strategy. In recent years, there have also been reports proposing sequential therapy (agents administered in sequence) and concomitant therapy (non‐bismuth quadruple therapy) as alternatives that produce eradication rates similar to PPI‐triple therapy. In fact, however, the results of recent studies have shown that documented eradication rates are at their lowest level in history (Vakil 2009).This could be due to incomplete elimination of H. pylori, leading to recrudescence of the same strain, or to reinfection with a new strain, with recrudescence being the more common cause of recurrence (Gisbert 2006; Xia 1997). Generally, the recurrence rate of H. pylori in developing countries is about 13%, although one report from India indicated that in that setting it may be as high as 40% (Rimbara 2011); on the other hand, in developed countries estimates of recurrence are under 3% (Niv 2008). The causes of this imbalance seem to be the limitations related to hygiene and socioeconomic constraints.
How the intervention might work
It is critical to identify factors contributing to the recrudescence of gastricH. pylori. Apart from the stomach, H. pylori also has been found in the distal esophagus, the proximal duodenum, the colonic contents, and the oral cavity, including tonsil and adenoid tissue (Cover 2009; Eyigor 2009). Considerable research has been published on the relationship between the oral cavity and gastric H. pylori infection, with numerous studies providing evidence that the oral cavity may be a potential reservoir for H. pylori. The bacteria has been detected in saliva, dental plaques, and gingival pockets (Burgers 2008; Dowsett 2003; Gebara 2006; Liu 2009; Pytko‐Polonczyk 1996; Song 2000b). Conversely, other investigators have indicated that the oral cavity may not be a reservoir of H. pylori (Olivier 2006; Silva Rossi‐Aguiar 2009). This contention is largely based upon different detection methods for oral H. pylori, compounded by the fact that there are several types of urease‐producing bacteria in the mouth including Streptococcus, Haemophilus sp., and Actinomyces sp. The test based on urease activity is not applicable for diagnosis; culture is a more sensitive method. However, culture is difficult to gather successfully due to the potential for the formation of a viable coccus (Chaudhry 2008). Currently, polymerase chain reaction (PCR) is the most effective method for testing oral H. pylori in clinical settings with high sensitivity and specificity. The specificity depends on the different sets of primers (Song 1999; Song 2000b). A meta‐analysis published in 2011 reported a strong connection between the presence ofH. pylori in the oral cavity and in the stomach (Zou 2011): between 39.5% and 64.0% of patients with gastric H. pylori also have oral H. pylori. In contrast, other studies have found that 83.3% of people with oral H. pylori also test positive for the bacteria in gastric samples (Medina 2010; Song 2000a). However, triple eradication therapy has no or little effect on elimination of oral H. pylori; studies have recorded eradication rates for oral infection below 40% (Gebara 2006; Czesnikiewicz‐Guzik 2007; Miyabayashi 2000). The presence of oral H. pylori might diminish the effect of eradication therapy and act as a causal element in the recurrence of H. pylori infection. Furthermore, Sheu 2007 have proposed that the presence of dental disease could predispose patients to recurrent H. pylori infection even after successful eradication.
Considering the important role of the oral cavity in gastric H. pylori infection, periodontal therapy, including oral hygiene procedures and dental plaque and dental calculus control measures, may be a potentially effective adjunctive treatment for gastric H. pylori infection (Namiot 2007; Jia 2009b; Zaric 2009).
Why it is important to do this review
It is important to clarify whether periodontal therapy is an effective adjunctive treatment for gastric H. pylori infection. Therefore, it is necessary to perform a meta‐analysis on the basis of current randomized controlled trials (RCTs) to understand the available data more comprehensively and to evaluate the evidence for the effectiveness of periodontal therapy in eradication of gastric H. pylori infection.
Objectives
To assess the effects of periodontal therapy plus eradication therapy versus eradication therapy alone for gastric H. pylori infection. The secondary objective is to compare the non‐recurrence rate at long‐term follow up in different treatment groups.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) comparing periodontal therapy plus eradication therapy versus eradication therapy alone.
Types of participants
Adults (≥ 17 years) objectively diagnosed as positive for gastric H. pylori, with or without oral H. pylori infection, were eligible for inclusion. Investigators confirmed presence of gastric H. pylori by serology, rapid urease test (RUT), histology or culture of endoscopic antral/body biopsy specimens, or urea breath test (UBT). The sensitivity and specificity of these methods were similar (Ricci 2007). For our meta‐analyses, at least one positive result of above tests confirmed the positive gastric H. pylori diagnosis. For oral H. pylori, investigators used the PCR analysis to obtain an accurate result.
We excluded studies that involved participants who had received any eradication treatment of H. pylori infection within four weeks prior to the study.
Types of interventions
We included all RCTs that used periodontal therapy combined with eradication therapy for the treatment of gastric H. pylori infection. Eligible H. pylori eradication regimens included triple therapy, quadruple therapy, sequential therapy, and concomitant therapy. We excluded trials or trial arms that treated participants with dual therapy because of the low eradication rate. Periodontal therapy considered for this review comprises oral hygiene training, removal of supragingival and accessible subgingival bacterial plaque and calculus by periodontal scaling, periodontal root planing and chemotherapeutic agents (e.g. irrigation of periodontal pockets). However, we excluded trials with oral surgical treatment. Periodontal therapy began with eradication therapy.
Types of outcome measures
Primary outcomes
Eradication rate of gastric H. pylori.
We assessed eradication at four weeks after therapy. We considered eradication successful in case of a negative result for that UBT, serology, RUT, or histology or culture of an endoscopic antral/body biopsy specimen.
Secondary outcomes
Non‐recurrence rate of gastric H. pylori (at least six months after eradication therapy).
Search methods for identification of studies
We conducted searches to identify all published and unpublished trials for this review, applying no restriction with regard to language of publication.
Electronic searches
We identified trials by searching the following electric databases.
The Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 8); see Appendix 1.
MEDLINE (1946 to August 2015); see Appendix 2.
EMBASE (1980 to August 2015); see Appendix 3.
Chinese Biomedical Database (1978 to August 2015).
ClinicalTrials.gov (last searched October 2015).
WHO ICTRP portal (last searched October 2015).
The Cochrane UGPD Group ran the searches in CENTRAL, MEDLINE, and EMBASE, while QR searched the Chinese Biomedical Database (CBM) in parallel.
Searching other resources
We handsearched the reference list of each included study to identify relevant trials. We also searched reference lists of abstracts from all relevant conference proceedings.
Data collection and analysis
Selection of studies
In order to assess studies for inclusion, two review authors independently reviewed abstracts from relevant trials using pre‐specified inclusion criteria. When we could not include or exclude a record solely on the basis of the abstract, we reviewed the article in full. In case of disagreement, a third author decided whether to include the study in the review.
Data extraction and management
Two authors independently extracted details of each study using a pre‐defined data collection form. We recorded the study design, participants, setting, timing, interventions, participant characteristics, and outcomes, in as much detail as possible. We resolved disagreements by consultation with the senior author. We also entered outcome data into an Excel database to check for potential input errors.
Assessment of risk of bias in included studies
Two review authors independently assessed the methodological quality of each study in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The senior author participated by resolving discrepancies in order to reach a consensus.
We assessed trials according to the following criteria.
Did the trial use adequate random sequence generation?
We assessed the allocation sequence as truly random, unclear, or not stated. We defined 'truly random' as: computer‐generated random numbers, published random number table, coin toss, shuffled cards.
Was there adequate allocation concealment?
We assessed allocation concealment as adequate, inadequate, or unclear.
We defined adequate concealment as follows: trialists were ignorant of the allocation of each recruited participant when they were entered in the study. Appropriate methods included central randomization schemes, pharmacy‐based schemes, opaque sealed envelopes.
Did trials adequately prevent knowledge of the allocated interventions during the study?
We assessed allocation concealment as triple blind, double blind, single blind, not blinded, or unclear.
When the participants, investigator and outcome assessors were not aware of the treatments, we defined the trial as 'triple blind'.
If two of them were not aware of the treatments, we defined the trial as 'double blind'.
If only one was not aware of the treatments, we defined the trial as 'single blind'.
We judged trials as blinded, not blinded, or unclear on the basis of the available information.
Did investigators adequately address incomplete outcome data?
We noted the completeness of follow‐up and whether investigators reported the intention‐to‐treat (ITT) analysis.
Measures of treatment effect
We calculated the odds ratio to analyze data on the eradication rate of H. pylori and the non‐recurrence rate of gastricH. pylori infection.
Dealing with missing data
We tried to contact the authors of original RCTs for missing information. We calculated the outcome with the intention‐to‐treat analytical approach. We considered drop‐outs as individuals for whom treatment failed.
Assessment of heterogeneity
We assessed statistical heterogeneity with the ChI2 test with significance set at P value 0.10. We quantified heterogeneity using the I2 statistic (Higgins 2002). We judged I2 greater than 25% as heterogeneous. We considered clinical heterogeneity and the heterogeneity of study quality.
Assessment of reporting biases
According to the number of included trials, we determined whether it was necessary to prepare a funnel plot for assessing publication bias (Egger 1997); as there were relatively few trials, we decided that this step was not necessary.
Data synthesis
We used Review Manager software (RevMan 2011) to pool and analyze the trial data and used forest plots to present the results of this meta‐analysis. All data were dichotomous outcomes. We expressed the results as the odds ratios (ORs) with their corresponding 95% confidence intervals (CIs). We pooled data using both fixed‐effect and random‐effects models. We reported only the results of the fixed‐effect model when both models led to the same conclusion. Otherwise we reported the results from fixed‐effect and random‐effects models.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analysis based on the participants with or without oralH. pylori infection, regardless of the type and the duration of eradication therapy and antibiotics.
We also carried out subgroup analysis based on the different durations of periodontal therapy.
During eradication therapy only.
Continued until testing the result of eradication therapy was complete.
Continued for longer.
If data had been sufficient, we would also have performed subgroup analyses on the basis of different levels of economic development (developed countries or developing countries) and whether or not the participants were diagnosed with periodontitis and prescribed periodontal therapy.
Sensitivity analysis
We conducted a sensitivity analysis by removing trials where investigators did not perform ITT analysis to check how robust the results were. We also performed sensitivity analysis according to relevant clinical features and to determine whether the pooled results changed when we excluded low quality studies from this meta‐analysis. We assessed the quality of included studies by modified Jadad scale and defined the studies with less than four points as low quality.
Results
Description of studies
Results of the search
Examining the results of the electronic searches from CENTRAL, MEDLINE, EMBASE, and the CBM in November 2010 and the updated search in August 2015, we identified a total of 82 records after excluding duplicates. We did not find any additional relevant trials by handsearching the reference lists of included trials. The search flow chart is shown in Figure 1. After reading titles and abstracts, we excluded 54 irrelevant references. We evaluated the full text of 28 other records, excluding 20 records for the reasons described in the Characteristics of excluded studies table. Finally, we included seven small RCTs (described in eight reports) that met all of the eligibility criteria for this review.
1.
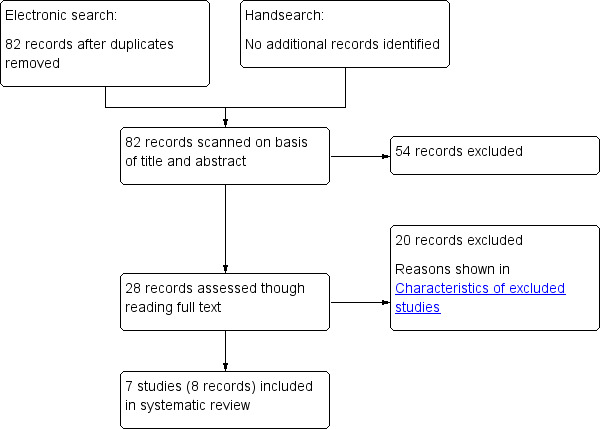
Search flow diagram
Included studies
Overall, we included seven small RCTs from eight reports involving 691 participants. One included study took place in the Clinical Center Zveadara in Belgrade, Serbia (Zaric 2009), while the others were Chinese (Jia 2009a; Jin 2003; Jin 2007; Liu 2012; Lv 2006; Wang 2014). Six RCTs assessed the effects of periodontal therapy plus eradication treatment for gastric H. pylori. Three RCTs observed the role of periodontal therapy on recurrence rate of gastric H. pylori. The periodontal therapy in these studies included oral hygiene education, toothbrushing, mouthwash with or without special agents, dental plaque control (ultrasonic scaling and subgingival scaling). The aim of these meta‐analyses is to assess the effect of periodontal treatment on gastric H. pylori, regardless of which professional interventions the trials used. We regrouped the participants of Jia 2009a into two groups: eradication therapy alone and eradication treatment plus oral dental plaque control, with or without professional care. In Wang 2014, we only extracted data from the two groups that met the inclusion criteria.
The criteria for entering the study were similar in all seven studies. Three RCTs recruited 194 participants positive for both gastric H. pylori and periodontitis, including chronic gastritis and peptic ulcer participants (Jin 2003; Jin 2007; Zaric 2009). In the four other studies, participants had gastric diseases associated with H. pylori but did not have a diagnosis for periodontitis (Jia 2009a; Liu 2012; Lv 2006; Wang 2014). The infection of H. pylori was confirmed by 13C or 14C urea breath test, RUT, histology, or PCR, and some studies also detected the presence of oral H. pylori using PCR, urease test, or H. pylori flagellin test (Lv 2006; Zaric 2009; Wang 2014). After completion of treatment, all trials except Zaric 2009 used the urea breath test to assess whether participants were infected with primary H. pylori. The age of the individuals in six of the included studies varied between 17 and 78 years, but Lv 2006 did not report the details of baseline data, such as mean age and sex ratio. In all studies, baseline data appeared similar across the intervention and control groups. For more details about included studies see Characteristics of included studies.
Excluded studies
We excluded 20 studies after reading the full text. Please see Characteristics of excluded studies.
Four of these trials met all the inclusion criteria except randomization. The most remarkable of these is Jia 2009b, a controlled clinical trial that elucidated the relationship between dental plaque control and H. pylori infection of gastric mucosa. That study divided a total of 110 participants with gastritis or peptic ulcer into two groups according to participant preferences and adherence instead of randomization. All participants received systemic gastric H. pylori eradication treatment, then 59 participants in the intervention group received periodontal therapy, including toothbrushing, mouthrinse, scaling, root planing and polishing, whereas the individuals in the control group used only their routine daily oral care procedures without any special dental plaque control. Six months after eradication treatment, investigators used the 13C urea breath test to detect the prevalence of gastric H. pylori. The result showed that the eradication rate was 76.3% in the intervention group according to an intention‐to‐treat analysis, compared with 15.7% in the control group; investigators concluded that long‐term professional dental plaque control is beneficial to gastric H. pylori eradication and prevention of H. pylori reinfection.
In Hou 2002, the authors divided participants into three groups based on the depth of the periodontal pocket instead of randomization. Participants with periodontal pockets less than 4 mm received eradication therapy only. Further, all participants with periodontal pockets deeper than 4 mm received eradication therapy, with or without periodontal therapy; however, the trial reports failed to explicitly mention random allocation. Likewise, Ye 2012 did not report a random grouping design. We therefore classified these trials as controlled clinical trials, and the results indicated that periodontal therapy including scaling and mouthwash could increase the eradication rate of gastric H. pylori infection when combined with triple eradication treatment. The other noteworthy trial is Tarullo 2001, which recruited participants with periodontitis and concomitant gastric disturbances who tested positive for H. pylori after two cycles of antibiotic polytherapy. The study randomized participants into placebo (10 participants) and treatment groups (14 participants), with treated participants receiving ablation of dental plaques, toilette of periodontal pockets with special agents for prevention against bacteria, and mouthwash. The placebo group received the same procedure but without the special agents. All participants were followed up for 24 months to detect gastric symptoms and the presence of H. pylori. However, this trial failed to describe many details, such as the use of eradication treatment during the process of oral therapy, the presence of H. pylori in the control group after two years, and so on. We tried to contact the authors to get more details, but the trial paper did not provide any email or mailing address. Therefore, there were not enough details to decide whether or not the trial used triple therapy in both groups.
Risk of bias in included studies
Two authors independently undertook the quality evaluation of every eligible study, assessing methods of randomization, allocation concealment, blinding, strategy for addressing incomplete outcome data, selective reporting and other possible sources of bias. We present the summary of the 'Risk of bias' assessment in Figure 2 and Figure 3.
2.
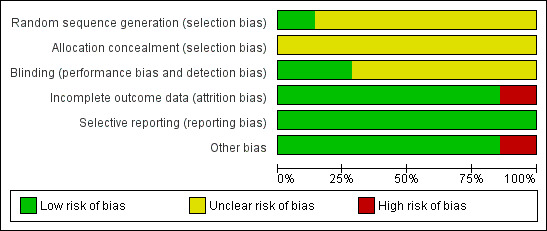
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
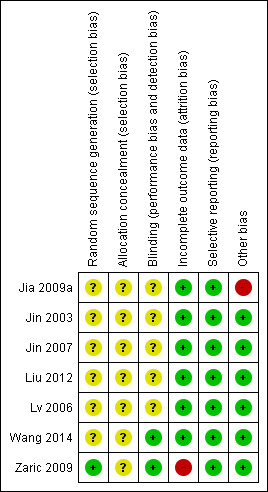
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
None of the trials reported on the generation of random sequence or allocation concealment. After contacting authors of each trial, we obtained further information on Zaric 2009, which used a block randomization method for group allocations. We deemed the risk of allocation bias in the other studies to be unclear.
Blinding
The aim of this meta‐analysis was to assess the effect of periodontal therapy on eradication of gastric H. pylori, so it is difficult to blind the operators, such as dentists carrying out treatments, and the participants who received oral hygiene education and professional oral care in clinical practice. However, the outcome of interest was the presence of H. pylori, which is an objective result. For this reason, we judged that blinding of the outcome assessor was sufficient to prevent knowledge of group allocation in terms of H. pylori detection. The examiners of Zaric 2009 who detected the presence of gastric H. pylori were blinded, whereas Wang 2014 was double blind (participants and outcome assessors). We judged both of these trials to be at low risk in this domain. The remaining five studies did not describe whether blinding was used (Jia 2009a; Jin 2003; Jin 2007; Liu 2012; Lv 2006), so we considered them to carry an unclear risk.
Incomplete outcome data
Jia 2009a excluded five cases in intervention group 1 because the participants discontinued use of mouthwash, along with eight cases in intervention group 2 because participants did not adhere to strict dental plaque control. Investigators used the intention‐to‐treat analysis to minimize bias in this analysis, so we considered it to be at low risk. We also judged Liu 2012 to be at low risk, because even though it lost 16 cases to follow‐up, authors undertook both intention‐to‐treat and per‐protocol analyses. Authors of Zaric 2009 confirmed through correspondence that some participants from both groups did not complete the study. However, their paper did not report the exact number or the reasons, and so we judged the trial to be at high risk in this domain. In the remaining four trials, there were no drop‐outs after randomization (Jin 2003; Jin 2007; Lv 2006; Wang 2014), and we assigned the trials a low risk for this domain.
Selective reporting
All trials were free from bias due to selective reporting (Jia 2009a; Jin 2003; Jin 2007; Liu 2012; Lv 2006; Zaric 2009; Wang 2014).
Other potential sources of bias
Jia 2009a reported that all participants were negative for gastric H. pylori infection by 13C urea breath test after H. pylori eradication treatment, considering the efficacy rate of eradication therapy to be 100%. Since studies have suggested that the eradication rate of anti‐H. pylori treatment is historically low (Vakil 2009), we considered that there could be some participants with treatment failure who were not reported. The remaining six trials were free from bias due to other potential sources (Jin 2003; Jin 2007; Liu 2012; Lv 2006; Zaric 2009; Wang 2014).
Effects of interventions
See: Table 1
Eradication rate of gastric H. pylori
Six RCTs reported eradication rates after evaluating 543 participants from China and Serbia at one to three months (Jin 2003; Jin 2007; Liu 2012; Lv 2006; Wang 2014; Zaric 2009). Combining the results of all of the studies, eradication rates of gastric H. pylori were 75.5% (206/273) in participants receiving eradication therapy plus periodontal treatment, and 60.0% (162/270) in participants receiving eradication therapy alone. Compared with only eradication treatment, periodontal therapy given along with eradication treatment significantly increased the eradication rate of gastric H. pylori (OR 2.15; 95% CI 1.47 to 3.14; P < 0.0001; test of heterogeneity P = 0.78, I2 = 0%), see Analysis 1.1. We downgraded the outcome from high to moderate quality due to risk of bias.
Non‐recurrence rate of gastric H. pylori
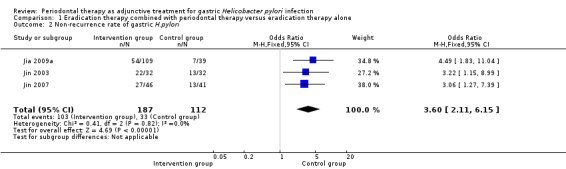
Three trials investigated the recurrence rate after successful gastric H. pylori eradication (Jia 2009a; Jin 2003; Jin 2007). A total of 299 participants with gastric H. pylori received treatment with eradication therapy; after one month, 13C or 14C urea breath testing confirmed the successful eradication of H. pylori in 272 participants. At 6‐ to 12‐month follow‐up, the non‐recurrence rate of gastric H. pylori infection was significantly higher in participants who received adjunctive periodontal therapy compared with those receiving eradication therapy alone (OR 3.60; 95% CI 2.11 to 6.15; P < 0.00001), see Analysis 1.2. The quality of evidence was low due to risk of bias and imprecision.
Subgroup analysis
We conducted the planned subgroup analyses according to oral H. pylori status and duration of periodontal therapy. There was no significant difference between any of the subgroups.
OralH. pylori status
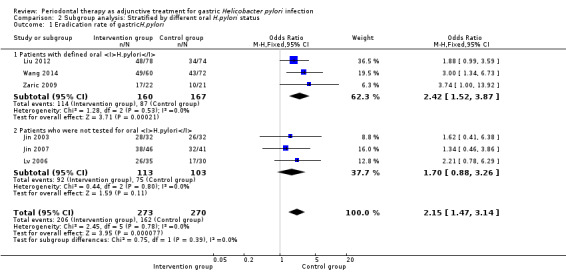
Three trials included participants with both gastric and oral H. pylori infection to assess the gastric H. pylori eradication rate (Liu 2012; Zaric 2009; Wang 2014). Periodontal therapy increased the eradication rate in the meta‐analysis (OR 2.42; 95% CI 1.52 to 3.87; P = 0.0002). The other three trials recruited participants with gastric H. pylori regardless of oral H. pylori status, and there was no significant difference between the intervention and control group (OR 1.70; 95% CI 0.88 to 3.26; P = 0.11); see Analysis 2.1.
Duration of periodontal therapy
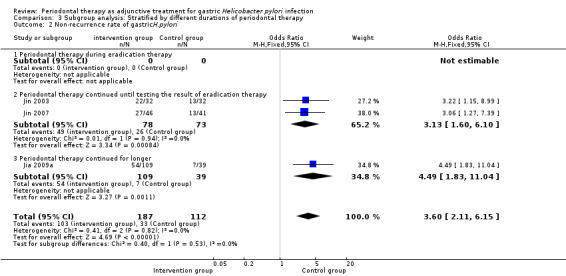
Two trials treated participants simultaneously with periodontal therapy and eradication treatment, stopping both treatments at the same time (Lv 2006; Zaric 2009). The eradication rate in the intervention group was higher (OR 2.72; 95% CI 1.20 to 6.14; P = 0.02) than in the control group. In the other four studies, periodontal therapy began with eradication therapy and continued until the time of testing gastric H. pylori (Jin 2003; Jin 2007; Liu 2012; Wang 2014). There was a significant decrease in the eradication rate between intervention and control groups (OR 2.02; 95% CI 1.31 to 3.10; P = 0.001).
Two of the trials assessed recurrence after treatment, with data showing that in the intervention group, the non‐recurrence rate was significantly higher compared with the control group (OR 3.13; 95% CI 1.60 to 6.10; P = 0.0008). In one trial, participants received periodontal therapy for half a year, that is, five months after testing the eradication rate (Jia 2009a). Compared with eradication treatment alone, periodontal therapy significantly increased the gastric H. pylori non‐recurrence rate (OR 4.49; 95% CI 1.83 to 11.04; P = 0.001); see Analysis 3.1 and Analysis 3.2.
Sensitivity analysis
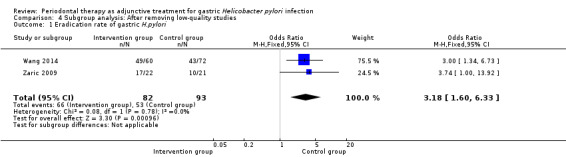
Using the modified Jadad scale, we classified five trials as low quality studies, as the score was three points (Jia 2009a; Jin 2003; Jin 2007; Liu 2012; Lv 2006). We excluded these five trials and analyzed the results of Wang 2014 and Zaric 2009. The outcome showed that periodontal therapy increased the efficiency rate of eradication treatment significantly (OR 3.18; 95% CI 1.60 to 6.33; P = 0.001), see Analysis 4.1.
'Summary of Findings' table
We created a 'Summary of findings' table using all the outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and used GRADEpro software. We justified all decisions to down‐ or up‐grade the quality of studies using footnotes and made comments to aid the reader's understanding of the review where necessary. We considered whether there was any additional outcome information that was not able to be incorporated into meta‐analyses and planned to note this in the comments and state if it supports or contradicts the information from the meta‐analyses.
Discussion
Summary of main results
We included seven RCTs in this meta‐analysis, which involved 382 participants in the intervention groups and 309 participants in the control groups. Results for the review's primary outcome showed that the use of periodontal therapy could increase the eradication rate of H. pylori treatment. Subgroup analysis based on status of oral H. pylori found that the result was consistent with the total study meta‐analysis for participants with oral H. pylori. However, in participants who were not tested for oral H. pylori, there was no significant difference in the eradication rate compared with the control group.
Researchers have considered the oral cavity to be a potential reservoir for H. pylori (Song 2000b). Becauseeradication therapies have not usually been effective in clearing H. pylori from the oral cavity (Gebara 2006), the colonization and growth of oral H. pylori could spread to the stomach during deglutition and affect the eradication of gastric H. pylori. For this reason, the use of periodontal therapy should have different effects on gastric H. pylori eradication depending on participants' oral status. In the subgroup analysis examining this aspect, studies collected data from participants with or without oralH. pylori as the same group, which could explain why we found no difference between periodontal therapy plus eradication treatment versus eradication treatment alone. Another subgroup analysis based on the duration of periodontal therapy showed the same results as the overall findings, with no significant difference between any of the subgroups, regardless of the duration of periodontal therapy.
We also conducted a sensitivity analysis, excluding the low quality studies and analyzing data from the two remaining trials (175 participants) to evaluate the eradication rate. There still was a significant increase in the eradication rate in those receiving adjunctive periodontal therapy compared with the control group. However, more high quality studies are needed to draw a reliable conclusion.
The results of our analyses on the secondary outcome showed that the use of periodontal therapy could increase the non‐recurrence rate after eradication therapy. The results of the subgroup analysis stratified by different durations of periodontal therapy was consistent with the overall result on non‐recurrence rate.
Overall completeness and applicability of evidence
This meta‐analysis included participants with gastric H. pylori, regardless of oralH. pylori infection. However, only a few published RCTs have assessed periodontal therapy as adjunctive treatment for gastric H. pylori. More high quality randomized control trials comparing eradication treatment plus periodontal therapy versus eradication treatment alone are required. In addition, RCTs should be conducted based on oralH. pylori infection and duration of periodontal therapy. In summary, this review is applicable to people with gastricH. pylori infection.
Quality of the evidence
Considering the lack of description regarding methods of the random sequence generation, allocation concealment and blinding, we only judged Wang 2014 and Zaric 2009 to be of high quality. In the other studies, authors just mentioned 'randomization' in text, but did not describe their methods. We considered that there were two possible explanations: one was that authors used the adequate methods for randomization, allocation concealment, and blinding, but did not report these processes accurately or completely; the other possibility was that the trials did not use standard methods during the performance of the trials. If the latter was the case, the trials were not truly randomized controlled trials.
With the exception of Zaric 2009, all of the studies adequately reported follow‐up. Investigators followed up all participants in four studies from one month to one year (Jin 2003; Jin 2007; Lv 2006; Wang 2014). The other two trials lost some participants to follow‐up or excluded them due to poor adherence (Jia 2009a; Lv 2006). All trials were free from selective reporting. In addition, Jia 2009a reported that all participants were free of gastricH. pylori after triple therapy. We considered that potential biases probably exist. Overall, the quality of included trials was not good enough to draw a firm conclusion. Moreover, all seven included trials took place in two developing countries—China and Serbia—where overall health indicators are not as good as they are in developed countries. For this reason, different daily oral hygiene routines may have a differential impact on oral hygiene status in developed versus developing countries, so the significant effect of periodontal therapy for gastric H. pylori in developing countries may not be generalizable to developed countries.
Potential biases in the review process
We searched CENTRAL, MEDLINE, EMBASE, the CBM, ClinicalTrials.gov and the WHO ICTRP portal for published, unpublished and ongoing studies. We could not obtain the details of all identified studies from reading the publications or contacting the trial authors. The limited search and the incompleteness of study details could affect the accuracy and objectivity of the results of this systematic review.
Agreements and disagreements with other studies or reviews
There is only one meta‐analysis assessing the effects of dental plaque control and periodontal therapy on prevention of gastric H. pylori recurrence (Bouziane 2012), and we did not identify any meta‐analysis review evaluating the use of periodontal therapy for the gastric H. pylori eradication rate. Bouziane 2012 included three trials with 298 participants. One was a controlled clinical trial, and the other two were RCTs that we also included in our review. Reviewers used the absence of recurrence of gastric H. pylori as an outcome, calculating the risk ratio (RR) but not the odds ratio (OR) during data analysis. The results showed that in patients with gastric diseases, periodontal therapy as adjunctive treatment significantly decreased the RRs of persistence of gastric H. pylori. Another review by Li 2012 found that periodontal therapy could not only kill oral H. pylori but also increase the eradication rate of gastricH. pylori and reduce recurrence of this bacterial infection, and the conclusions suggested the use of periodontal therapy to improve the efficiency of gastric H. pylori.
Authors' conclusions
Implications for practice.
In this meta‐analysis, periodontal therapy had benefits on the treatment for gastric H. pylori. The eradication rate increased in patients who received periodontal therapy plus eradication, particularly in those with both gastric and oral H. pylori. After long‐term follow‐up, patients treated with periodontal therapy had a higher non‐recurrence rate compared to those without basic oral therapy. The low quality of the included RCTs and incomplete reporting about methodology introduce some uncertainty with regard to these findings, and there is still a need for more high quality, large‐scale, multicenter randomized controlled trials to obtain clear and reliable evidence to recommend using periodontal therapy for patients with gastric diseases associated with H. pylori.
Implications for research.
In this review, we considered the vast majority of included trials to be of low quality due to the lack of adequate description of randomization method, allocation concealment, and blinding. There is a need for more high quality trials to investigate the effects of adjunctive periodontal therapy on the eradication and recurrence of gastric H. pylori. Well‐designed, large‐scale randomized controlled trials with long‐term follow‐up should be performed in both developing and developed countries to gain reliable evidence and to understand the applicability of these therapies across different settings and socioeconomic contexts.
Acknowledgements
We thank Professor KeHu Yang and Bin Ma for their guidance and help. We thank the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group (UGPD) for their support.
Appendices
Appendix 1. CENTRAL search strategy
h‐pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
h‐pylori infection.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
Helicobacter pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
Helicobacter pylori infection.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
PPI.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
proton pump inhibitor.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
or/1‐6
(clarithromycin or 6‐o‐methylerythromycin or abbott‐56268 or biaxin or clarith or klaricid or "te 031").mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(amoxicillin or actimoxi or almodan or amix or amopen or amoram or amoxicot or amoxidin or amoxil or amoxymed or amrit or biomox or brl 2333 or clamoxyl or dispermox or flemoxin solutab or galenamox or larotid or moxatag or moxilin or p‐hydroxyampicillin or penamox or polymox or respillin or rimoxallin or senox or sumox or trimox or utimox or wymox or zoxycil).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(2 methyl 5 nitroimidazole 1 ethanol or anabact or bayer 5360 or clont or danizol or edg dentalgel or elyzol or flagyl or gineflavir or metric or metro iv or metrocream or metrodzhil or metrogel or metrolotion or metrolyl or metronizole or metrotop or metrovex or metrozol or metryl or nidazol or noritate or norzol or nydamax or obagi or protostat or rozex or satric or trichopol or tricom or trivazol or vagilen or vaginyl or vandazole or vitazol or zadstat or zidoval).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(chlorhexidine or acclean or acriflex or avagard or betasept or calgon vesta or chloraprep or chlorhexamed or chlorhexigard or chlorohex 2000 or chlorostat or corsodyl or curasept ads 220 or cx antiseptic dusting or denticare or dyna‐hex or eludril or excel or gibitan or habistat or hexidine or hibiclens or hibident or hibiscrub or hibisol or hibitane or mk 412a or novalsan or oris or oro clense or peridex or periochip or periogard or periosep or perisol or phiso‐med or pre‐scrub ii or rotersept or sebidin a or spectrum‐4 or sterexidine or steripod pink or tubulicid or unisept or uriflex c).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(omeprazole or h 16868 losec or nexium or omesec or prilosec or rapinex or ulcergard or zegerid).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(ranitidine or ah 19065 or azanplus or biotidin or gr 122311x or pylorid or raciran or raniberl or ranisen or rantec or sostril or taladine or tritec or wal‐zan or zaedoc or zantac).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(tinidazole or bioshik or fasigin or fasigyne or tindamax or tricolam).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
or/8‐14
(periodontal and (disease$ or therap$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(oral hygiene or buccal cavity or dental hygiene).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
periodontal scaling.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
periodontal root planing.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
subgingival bacterial plaque.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(gingival and (pocket$ or plaque$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
or/16‐21
7 and 15 and 22
Appendix 2. MEDLINE search strategy
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/1‐8
exp animals/ not humans.sh.
9 not 10
helicobacter pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
helicobacter.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
H‐pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
or/12‐15
PPI.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
proton pump inhibitor.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(amoxicillin or actimoxi or almodan or amix or amopen or amoram or amoxicot or amoxidin or amoxil or amoxymed or amrit or biomox or brl 2333 or clamoxyl or dispermox or flemoxin solutab or galenamox or larotid or moxatag or moxilin or p‐hydroxyampicillin or penamox or polymox or respillin or rimoxallin or senox or sumox or trimox or utimox or wymox or zoxycil).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(clarithromycin or 6‐o‐methylerythromycin or abbott‐56268 or biaxin or clarith or klaricid or "te 031").mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(2 methyl 5 nitroimidazole 1 ethanol or anabact or bayer 5360 or clont or danizol or edg dentalgel or elyzol or flagyl or gineflavir or metric or metro iv or metrocream or metrodzhil or metrogel or metrolotion or metrolyl or metronizole or metrotop or metrovex or metrozol or metryl or nidazol or noritate or norzol or nydamax or obagi or protostat or rozex or satric or trichopol or tricom or trivazol or vagilen or vaginyl or vandazole or vitazol or zadstat or zidoval).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(chlorhexidine or acclean or acriflex or avagard or betasept or calgon vesta or chloraprep or chlorhexamed or chlorhexigard or chlorohex 2000 or chlorostat or corsodyl or curasept ads 220 or cx antiseptic dusting or denticare or dyna‐hex or eludril or excel or gibitan or habistat or hexidine or hibiclens or hibident or hibiscrub or hibisol or hibitane or mk 412a or novalsan or oris or oro clense or peridex or periochip or periogard or periosep or perisol or phiso‐med or pre‐scrub ii or rotersept or sebidin a or spectrum‐4 or sterexidine or steripod pink or tubulicid or unisept or uriflex c).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(omeprazole or h 16868 losec or nexium or omesec or prilosec or rapinex or ulcergard or zegerid).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(ranitidine or ah 19065 or azanplus or biotidin or gr 122311x or pylorid or raciran or raniberl or ranisen or rantec or sostril or taladine or tritec or wal‐zan or zaedoc or zantac).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(tinidazole or bioshik or fasigin or fasigyne or tindamax or tricolam).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
or/17‐25
periodontal therap$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
oral hygiene.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(buccal cavity or cavitas oris or mouth or oral cavity or dental hygiene).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(periodontal and (disease$ or therap$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
periodontal scaling.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
periodontal root planing.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
((subgingival or supragingival) adj5 bacterial plaque).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(periodontal adj10 chemotherapeutic agent$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(irrigation adj10 periodontal pocket$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
gingival pocket.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
or/27‐36
11 and 16 and 26 and 37
Appendix 3. EMBASE search strategy
Clinical trial.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
Randomized controlled trial.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
Randomization.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
Single‐blind method.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
Double‐blind method.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
Cross‐over studies.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
random allocation.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
placebo.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
randomi?ed controlled trial$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
Rct.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
Random allocation.tw.
randomly allocated.tw.
allocated randomly.tw.
(allocated adj2 random).tw.
single blind$.tw.
double blind$.tw.
((treble or triple) adj blind$).tw.
placebo$.tw.
prospective study.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
or/1‐19
case study.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
case report.tw.
abstract report/ or letter.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
or/21‐23
20 not 24
H‐pylori.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
H‐pylori infection.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
Helicobacter pylori.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
helicobacter pylori infection.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
or/26‐29
PPI.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
proton pump inhibitor$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
(clarithromycin or 6‐o‐methylerythromycin or abbott‐56268 or biaxin or clarith or klaricid or "te 031").mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
(amoxicillin or actimoxi or almodan or amix or amopen or amoram or amoxicot or amoxidin or amoxil or amoxymed or amrit or biomox or brl 2333 or clamoxyl or dispermox or flemoxin solutab or galenamox or larotid or moxatag or moxilin or p‐hydroxyampicillin or penamox or polymox or respillin or rimoxallin or senox or sumox or trimox or utimox or wymox or zoxycil).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
(chlorhexidine or acclean or acriflex or avagard or betasept or calgon vesta or chloraprep or chlorhexamed or chlorhexigard or chlorohex 2000 or chlorostat or corsodyl or curasept ads 220 or cx antiseptic dusting or denticare or dyna‐hex or eludril or excel or gibitan or habistat or hexidine or hibiclens or hibident or hibiscrub or hibisol or hibitane or mk 412a or novalsan or oris or oro clense or peridex or periochip or periogard or periosep or perisol or phiso‐med or pre‐scrub ii or rotersept or sebidin a or spectrum‐4 or sterexidine or steripod pink or tubulicid or unisept or uriflex c).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
(omeprazole or h 16868 losec or nexium or omesec or prilosec or rapinex or ulcergard or zegerid).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
(ranitidine or ah 19065 or azanplus or biotidin or gr 122311x or pylorid or raciran or raniberl or ranisen or rantec or sostril or taladine or tritec or wal‐zan or zaedoc or zantac).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
(tinidazole or bioshik or fasigin or fasigyne or tindamax or tricolam).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
or/31‐38
(periodontal and (therap$ or disease$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
(oral hygiene or buccal cavity or dental hygiene).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
periodontal scaling.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
oral cavity.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
dental plaque$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
gingival pocket$.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
periodontal root planing.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
periodontitis.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
preventive dentistry.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
mouth hygiene.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
oral hygiene.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
subgingival bacterial plaque.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
(tooth adj4 (calculus or plaque)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
or/40‐52
25 and 30 and 39 and 53
Data and analyses
Comparison 1. Eradication therapy combined with periodontal therapy versus eradication therapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eradication rate of gastricH.pylori | 6 | 543 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.47, 3.14] |
| 2 Non‐recurrence rate of gastric H.pylori | 3 | 299 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.60 [2.11, 6.15] |
1.1. Analysis.
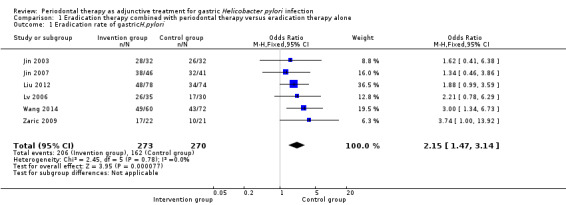
Comparison 1 Eradication therapy combined with periodontal therapy versus eradication therapy alone, Outcome 1 Eradication rate of gastricH.pylori.
1.2. Analysis.
Comparison 1 Eradication therapy combined with periodontal therapy versus eradication therapy alone, Outcome 2 Non‐recurrence rate of gastric H.pylori.
Comparison 2. Subgroup analysis: Stratified by different oral H.pylori status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eradication rate of gastricH.pylori | 6 | 543 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.47, 3.14] |
| 1.1 Patients with defined oral H.pylori | 3 | 327 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.42 [1.52, 3.87] |
| 1.2 Patients who were not tested for oral H.pylori | 3 | 216 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.88, 3.26] |
2.1. Analysis.
Comparison 2 Subgroup analysis: Stratified by different oral H.pylori status, Outcome 1 Eradication rate of gastricH.pylori.
Comparison 3. Subgroup analysis: Stratified by different durations of periodontal therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eradication rate of gastricH.pylori | 6 | 543 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.47, 3.14] |
| 1.1 Periodontal therapy during eradication therapy | 2 | 108 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.20, 6.14] |
| 1.2 Periodontal therapy continued until testing the result of eradication therapy | 4 | 435 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.31, 3.10] |
| 1.3 Periodontal therapy continued for longer | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Non‐recurrence rate of gastricH.pylori | 3 | 299 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.60 [2.11, 6.15] |
| 2.1 Periodontal therapy during eradication therapy | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Periodontal therapy continued until testing the result of eradication therapy | 2 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [1.60, 6.10] |
| 2.3 Periodontal therapy continued for longer | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.49 [1.83, 11.04] |
3.1. Analysis.

Comparison 3 Subgroup analysis: Stratified by different durations of periodontal therapy, Outcome 1 Eradication rate of gastricH.pylori.
3.2. Analysis.
Comparison 3 Subgroup analysis: Stratified by different durations of periodontal therapy, Outcome 2 Non‐recurrence rate of gastricH.pylori.
Comparison 4. Subgroup analysis: After removing low‐quality studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eradication rate of gastric H.pylori | 2 | 175 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.18 [1.60, 6.33] |
4.1. Analysis.
Comparison 4 Subgroup analysis: After removing low‐quality studies, Outcome 1 Eradication rate of gastric H.pylori.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jia 2009a.
| Methods | Randomized clinical trial | |
| Participants | Country: China Sample size: 148 patients with gastritis and peptic ulcer (group 1, 54; group 2, 55; control 39) Sex(M/F): Group 1 (31/23), Group 2 (30/25), Control group (21/18) Age: Group 1 (25 to 72 years), Group 2 (26 to 70 years), Control group (23 to 69 years) H. pylori testing: 13C urea breath test Oral H. pylori status: not tested |
|
| Interventions | Intervention group 1: H. pylori eradication for 6 weeks, followed by daily plaque control for 6 months Intervention group 2: H. pylori eradication for 6 weeks, followed by daily plaque control and oral professional intervention for 6 months Control group: H. pylori eradication for 6 weeks only H. pylori eradication: omeprazole 20 mg, amoxicillin 500 mg and metronidazole 400 mg, twice daily for 2 weeks, followed by ranitidine 300 mg every night and bismuth citrate 110 mg 4 times a day for 4 weeks. Daily plaque control: toothbrushing performed with Bass method half an hour after each meal for at least 3 min. The toothbrush changed every month. 10 mL mouthwash containing chlorhexidine was used for 1 min twice a day. Dental floss or interdental brush was used if needed. Oral professional intervention: oral professional examination and hygiene education were performed every other week. Ultrasonic scaling and subgingival scaling were performed to remove dental plaque (until testing the result of eradication therapy). |
|
| Outcomes | Recurrence rate of gastric H. pylori (6 months after eradication) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in article |
| Allocation concealment (selection bias) | Unclear risk | Not described in article |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data were reported, including the excluded participants with nonadherence. The intention‐to‐treat analytical approach was used. |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported. |
| Other bias | High risk | After H. pylori eradication treatment, all patients were confirmed without gastric H. pylori infection by 13C urea breath test. We considered whether the efficacy rate of eradication therapy could reach 100%. |
Jin 2003.
| Methods | Randomized clinical trial | |
| Participants | Country: China Sample size: 64 patients with peptic ulcer and periodontitis (intervention, 32: control, 32) Sex (M/F): 41/23 Age: 19 to 66 years H. pylori testing: 14C urea breath test Oral H. pylori status: not tested |
|
| Interventions | Intervention group: H. pylori eradication for 2 weeks plus periodontal treatment for 4 weeks Control group: H. pylori eradication for 2 weeks only H. pylori eradication: omeprazole 20 mg, once a day, amoxicillin 1.0 g and metronidazole 400 mg, twice a day, for 2 weeks Periodontal treatment: oral hygiene education, ultrasonic scaling and subgingival scaling, and mouthwash for 1 month with Compound Borax Solution (until testing the result of eradication therapy) |
|
| Outcomes | Eradication rate of gastric H. pylori (1 month after eradication) Recurrence rate of gastric H. pylori (12 months after eradication) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in article |
| Allocation concealment (selection bias) | Unclear risk | Not described in article |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up until the end of trial. The intention‐to‐treat analytical approach was used. |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported. |
| Other bias | Low risk | — |
Jin 2007.
| Methods | Randomized clinical trial | |
| Participants | Country: China Sample size: 87 patients with peptic ulcer and periodontitis (intervention, 46; control, 41) Sex (M/F): 53/34 Age: 17 to 65 years H. pylori testing: 14C urea breath test Oral H. pylori status: not tested |
|
| Interventions | Intervention group: H. pylori eradication for 1 week plus periodontal treatment for 4 weeks Control group: H. pylori eradication for 1 week only H. pylori eradication: omeprazole 20 mg, clarithromycin 0.5 g and amoxicillin 1.0 g, twice a day for 1 week Periodontal treatment: oral hygiene education, ultrasonic scaling and subgingival scaling, and mouthwash for 1 month with Compound Borax Solution (until testing the result of eradication therapy) |
|
| Outcomes | Eradication rate of gastric H. pylori (1 month after eradication) Recurrence rate of gastric H. pylori (12 months after eradication) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in article |
| Allocation concealment (selection bias) | Unclear risk | Not described in article |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up until the end of trial. The intention‐to‐treat analytical approach was used. |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported. |
| Other bias | Low risk | — |
Liu 2012.
| Methods | Randomized clinical trial | |
| Participants | Country: China Sample size: 152 patients with peptic ulcer (intervention, 78; control, 74) Sex (M/F): not reported Age: Intervention group (22 to 68 years), Control group (23 to 70 years) H. pylori testing: rapid urease test and 14C urea breath test Oral H. pylori status: positive |
|
| Interventions | Intervention group: H. pylori eradication for 2 weeks plus periodontal treatment for 4 weeks Control group: H. pylori eradication for 2 weeks only H. pylori eradication: omeprazole 20 mg, metronidazole 400 mg and amoxicillin 1.0 g, twice a day for 2 weeks Periodontal treatment: oral hygiene education for teaching the Bass brushing method, ultrasonic scaling and subgingival scaling, and mouthwash. Toothbrushing performed half an hour after each meal for at least 3 min. 10 mL mouthwash containing chlorhexidine for 1 min twice a day (until testing the result of eradication therapy) |
|
| Outcomes | Eradication rate of gastric H. pylori (1 month after eradication) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in article |
| Allocation concealment (selection bias) | Unclear risk | Not described in article |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 patients lost to follow‐up, but intention‐to‐treat analytical approach used |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported. |
| Other bias | Low risk | — |
Lv 2006.
| Methods | Randomized clinical trial. | |
| Participants | Country: China Sample size: 65 patients with peptic ulcer and chronic gastritis (intervention, 35; control, 30) Sex (M/F): not reported Age: not reported H. pylori testing: rapid urease test or histology Oral H. pylori status: tested, but the results were not used for assessing effect of oral H. pylori on eradication of gastric H. pylori |
|
| Interventions | Intervention group: H. pylori eradication and periodontal treatment for 4 weeks Control group: H. pylori eradication for 2 weeks only H. pylori eradication: bismuth potassium citrate capsules 220 mg and amoxicillin 0.5 g, twice a day, plus metronidazole 0.3 g 3 times per day Periodontal treatment: ultrasonic scaling and subgingival scaling were performed by dentist. Rhizoma Coptidis aerosol used after each meal, 3 times a day for 4 weeks (during triple therapy) |
|
| Outcomes | Eradication rate of gastric H. pylori (1 month after eradication) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in article |
| Allocation concealment (selection bias) | Unclear risk | Not described in article |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described in article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up until the end of trial. The intention‐to‐treat analytical approach was used. |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported. |
| Other bias | Low risk | — |
Wang 2014.
| Methods | Randomized clinical trial | |
| Participants | Country: China Sample size: 277 individuals were recruited in this study, but only 132 adult patients were included for this review. 132 adult patients suffered from both stomach and oral H. pylori infections (intervention, 60; control, 72) Sex (M/F): 121/156 Age: 3 to 77 years H. pylori testing: 13C urea breath test Oral H. pylori status: positive |
|
| Interventions | Intervention group: H. pylori eradication for 2 weeks plus periodontal treatment for 1 month Control group: H. pylori eradication for 2 weeks only H. pylori eradication: standard dose PPIs were used twice a day (PPIs include: lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40 mg, rabeprazole 20 mg, and esomperazole 40 mg), and tetracycline 500 mg, twice a day, plus amoxicillin 1000 mg twice a day (or bismuth subsalicylate 525 mg, once daily, plus metronidazole 250 mg 4 times per day) Periodontal treatment: mouthwash with 20 mL solution was performed twice a day (in the morning and at the night before bed) for 1 month (during triple therapy). The mouthwash solution contained lysine (0.4%) and glycerol monolaurate (0.2%). |
|
| Outcomes | Eradication rate of gastric H. pylori | |
| Notes | Only participants infected with oral Hp, but without gastric Hp were collected in this study. Mouthwash solution provided a 72.58% effectiveness rate on the oral Hp infection, but traditional drug gastric eradication and teeth cleaning had less than a 10% effectiveness rate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in article |
| Allocation concealment (selection bias) | Unclear risk | Not described in article |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind (participants and outcome assessors) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up until the end of test. |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported. |
| Other bias | Low risk | — |
Zaric 2009.
| Methods | Randomized clinical trial | |
| Participants | Country: Serbia Sample size: 66 individuals were selected in this study, but only 43 patients were included for this review. 43 patients with both gastric and oral H. pylori infection, all patients with periodontitis (intervention, 21; control, 22). Sex (M/F): 29/37 Age: 19 to 78 years H. pylori testing: PCR Oral H. pylori status: H. pylori positive |
|
| Interventions | Intervention group: H. pylori eradication and periodontal treatment for 7 days Control group: H. pylori eradication for 7 days only H. pylori eradication: amoxicillin 2.0 g, clarithromycin 1.0 g, pantoprazole 80 mg, once a day for 7 days Periodontal treatment: oral hygiene orientation, ultrasonic scaling and root planing for removing plaque and calculus, irrigation of periodontal pockets with 0.12% chlorhexidine‐gluconate (during triple therapy) |
|
| Outcomes | Eradication rate of gastric H. pylori eradication rates (3 months after eradication) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization method was performed for treatment allocations (author correspondence) |
| Allocation concealment (selection bias) | Unclear risk | Not described in article |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single blind (blinding of outcome assessment): the examiner who was responsible for H. pylori detection was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | From both groups, a number of patients did not complete the study due to different reasons, but the authors did not report the exact number or the reasons (author correspondence). |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported |
| Other bias | Low risk | — |
ITT: intention‐to‐treat; PCR: polymerase chain reaction; PPI: proton pump inhibitor.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avcu 2001 | Not treated with any periodontal therapy Conclusion: H pylori positivity in dental plaque was correlated with oral hygiene index (OHI) scores. The patients with poor OHI scores had the most frequent gastric recurrence of H. pylori, compared with those with fair OHI scores and good OHI scores. |
| Bago 2011 | Not treated with any periodontal therapy Conclusion: Nearly half of the patients with gastricH. pylori harboured H. pylori in the oral cavity. The eradication therapy was very effective in the treatment of oral H. pylori. |
| Bai 2014 | Failed to extract the details of the trial. There are not enough details to know how long the periodontal therapy lasted. Conclusion: Periodontal therapy combined with triple therapy could increase the eradication rate of gastric H. pylori, when compared with triple therapy only. |
| Hou 2002 | Not a randomized clinical trial, though it met other inclusion criteria. The patients were assigned into 3 groups based on the depth of periodontal pocket. Conclusion: Periodontal therapy may affect the eradication of gastric H. pylori. Periodontal therapy increased eradication rate in patients with both gastric H. pylori and periodontitis non‐significantly, when combined with triple eradication treatment. For long‐term follow‐up, the eradication rate was reduced significantly in the intervention group compared with control group after 1 year. |
| Hou 2003 | Not treated with any periodontal therapy Conclusion: the eradication rate of gastric H. pylori was correlated with the presence of oral H. pylori. The patients with oral H. pylori had a low eradication rate compared with those without oral H. pylori. |
| Hou 2004 | Gastic H. pylori eradication rate was not observed. The aim of this study is to evaluate the role of periodontal therapy on oral H. pylori infection. |
| Ierardi 1998 | Not compliant with aim of this meta‐analysis. The aim was to investigate the possible relationship between 'objective' halitosis and H. pylori. Conclusion: Halitosis was correlated with H. pylori, since eradication with triple therapy may resolve the symptom. |
| Jia 2009b | Not a randomized clinical trial, though complied with other inclusion criteria Conclusion: Long‐term (6 months) professional dental plaque control was correlated with less gastric H. pylori reinfection. The prevalence of gastric H. pylori infection in patients with professional dental plaque control was significantly lower than those in the control group. |
| Li 2012 | A review |
| Miyabayashi 2000 | Not treated with any periodontal therapy Conclusion: The outcome of eradication therapy and the recurrence of gastricH. pylori infection were affected by H. pylori in oral cavity. The eradication rate in patients with oral H. pylori was significantly lower compared with those without oral H. pylori. Inversely, recurrence of gastric H. pylori was significantly higher in patients with oral H. pylori 2 years after successful treatment. |
| Namiot 2001 | Not treated with any periodontal therapy. Retrospective review of the relationship between natural teeth status and the efficacy of H. pylori eradication. Conclusion: Natural teeth status may influence the outcome of H. pylori eradication. The number of teeth was higher and caries index lower in successfully eradicated patients with triple regimen (omeprazole, amoxicillin, tinidazole), compared with those with eradication failure. |
| Namiot 2004 | Not treated with any periodontal therapy. The aim was to assess the effect of salivary secretion on the efficacy of gastric H. pylori eradication. Conclusion: Reduced salivary secretion was associated with eradication failure. |
| Namiot 2007 | Not treated with any periodontal therapy. Retrospective review of the relationship between oral health, oral hygiene practices and the efficacy of H. pylori eradication. Conclusion: Oral health and oral hygiene practices seem unlikely to increase the efficacy of H. pylori eradication from the stomach. |
| Qian 2014 | Patients in intervention group treated with triple eradication and periodontal pocket rinse with sodium chloride solution. Patients in control group treated with triple eradication and periodontal therapy, including supragingival scaling, subgingival scaling, mouthwash with tinidazole, periodontal pocket rinse with sodium chloride solution and hydrogen peroxide. Study did not meet other inclusion criteria. Conclusion: Periodontal therapy combined with triple therapy could improve the eradication rate non‐significantly in patients with both gastric H. pylori and chronic periodontitis. For long‐term follow‐up, the eradication rate was increased significantly in the intervention group compared with control group, after 3, 6 and 12 months. |
| Tarullo 2001 | Patients in both groups were not treated with triple eradication therapy. Patients in the treatment group received ablation of dental plaques, toilette of periodontal pockets with special agents for prevention against bacteria, and mouthwash. The placebo group received the same procedure except for the presence of the special agents. Conclusion: Based on the 2‐year follow‐up results, periodontal pockets treatment for patients with gastric H. pylori infection led to long‐term H. pylori eradication. |
| Xu 2001 | Patients treated with dual therapy for eradication of H. pylori. The abstract was not consistent with full text. Conclusion: The use of mouthrinse solution containing amoxicillin, metronidazole and chlorhexidine did not increase the eradication rate of gastric H. pylori when combined with eradication treatment. |
| Yang 2013 | Failed to extract the details of the trial. There are not enough details to know how long the periodontal therapy lasted. Conclusion: In patients with periodontitis and peptic ulcer, the periodontal treatment in combination with triple therapy could increase the eradication of H. pylori. |
| Ye 2012 | Not a randomized clinical trial, though complied with other inclusion criteria. Conclusion: Mouthwash with Chinese herbal medicine plus triple therapy significantly increased eradication of gastric H. pylori, when compared with triple therapy alone. |
| Zhao 2000 | Oral surgical treatment was included in periodontal therapy. Conclusion: No significant differences in H. pylori eradication rate could be found between the patients with periodontal treatment plus bismuth triple therapy and those with bismuth triple therapy alone. The recurrence rate in the former were significantly lower than in the latter group. |
| Özdemir 2001 | Not treated with any periodontal therapy. The aim was to detect the presence of H. pylori in dental plaque and tongue scrapings of patients with chronic gastritis and to investigate the effect of eradication treatment on H. pylori in dental plaque, tongue scrapings and gastric mucosa. Conclusion: patients with gastric H. pylori infection had higher rates of H. pylori colonization in dental plaque and tongue scrapings. Triple eradication therapy failed to respond to H. pylori in dental plaque and tongue scrapings. |
Differences between protocol and review
In the protocol stage, we planned to include randomized controlled trials comparing periodontal therapy plus triple therapy with triple therapy alone. However, in the review, we actually included patients treated with eradication therapy comprising triple therapy, quadruple therapy, sequential therapy and concomitant therapy, only excluding dual therapy because of the low eradication rate. According to the comments of statistical editor and considering the balance of randomisation, we used 'non‐recurrence rate' instead of 'recurrence rate' to evaluate periodontal therapy for gastric H. pylori at long‐term follow up. Additionally, we searched both ClinicalTrials.gov and the WHO ICTRP portal to reduce potential bias as much as possible.
Contributions of authors
Qian Ren drafted the review protocol and modified it according to the opinions of the editors and peer reviewers. Qian Ren undertook the literature search, extracted and analyzed data, and wrote the review. WeiXin Li performed the literature search, original data extraction, and analysis. Xiang Yan and YongNing Zhou participated in analysing data and interpreting the results. All authors contributed to the completed systematic review.
Sources of support
Internal sources
The First Hospital of Lanzhou University, China.
External sources
The Evidence‐Based Medicine Center of Lanzhou University, China.
Declarations of interest
QR: none known.
XY: none known.
YNZ: none known.
WXL: none known.
New
References
References to studies included in this review
Jia 2009a {published data only}
- Jia CL, Jiang GS, Yang XX, Dou HQ, Li CR. Effects onHelicobacter pylori reinfection in gastric mucosa by two oral plaque control methods. Huaxi Kongqiang Yixue Zazhi [West China Journal of Stomatology] 2009;27(2):172‐4. (Chinese). [PubMed] [Google Scholar]
Jin 2003 {published data only}
- Jin F, Yuan F, Jiang HL, Huang XQ, Ma HM, Le QL, et al. Effect of triple therapy associated with initial periodontal therapy on the eradication of gastric Helicobacter pylori. Zhongguo Wuzhenxue Zazhi [Chinese Journal of Misdiagnostics] 2003;3(10):1485‐6. (Chinese). [Google Scholar]
- Yuan F, Jin F, Ma HM, Le QL, Zhu JZ. Effect of initial periodontal therapy on the eradication rate of gastric Helicobacter pylori in patients with peptic ulcer. Xiandai Shiyong Yixue [Modern Practical Medicine] 2003;15(9):549‐50. [Google Scholar]
Jin 2007 {published data only}
- Jin F, Yuan F, Zhang ZJ, Le QL, Zhu JZ. Effect of eradication treatment associated with initial periodontal therapy on the eradication rate of gastric Helicobacter pylori. Shiyong Yixue Zazhi [The Journal of Practical Medicine] 2007;23(5):677‐8. (Chinese). [Google Scholar]
Liu 2012 {published data only}
- Liu Z, Meng WD, Wang ZK. Effect of dental plaque on eradication rate of gastric Helicobacter pylori in patients with peptic ulcer. Zhongguo Wuzhenxue Zazhi [Chinese Journal of Misdiagnostics] 2012;12(5):1038. (Chinese). [Google Scholar]
Lv 2006 {published data only}
- Lv X, Yao BT. The effect on Hp relative gastrosia with Rhizoma Coptidis aerosol clearing away the Helicobacter pylori in the dental plaque. Zhonghua Linchuangyixue Zazhi [Chinese Journal of Clinical Practical Medicine] 2006;7(2):28‐30. (Chinese). [Google Scholar]
Wang 2014 {published data only}
- Wang XM, Yee KC, Hazeki‐Taylor N, Li J, Fu HY, Huang ML, et al. Oral Helicobacter pylori, its relationship to successful eradication of gastric H. pylori and saliva culture confirmation. Journal of Physiology and Pharmacology 2014;65(4):559‐66. [PubMed] [Google Scholar]
Zaric 2009 {published data only}
- Zaric S, Bojic B, Jankovic Lj, Dapcevic B, Popovic B, Cakic S, et al. Periodontal therapy improves gastric Helicobacter pylori eradication. Journal of Dental Research 2009;88(10):946‐50. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Avcu 2001 {published data only}
- Avcu N, Avcu F, Beyan C, Ural AU, Kaptan K, Ozyurt M, et al. The relationship between gastric‐oral Helicobacter pylori and oral hygiene in patients with vitamin B12‐deficiency anemia. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 2001;92(2):166‐9. [DOI] [PubMed] [Google Scholar]
Bago 2011 {published data only}
- Bago I, Bago J, Plečko V, Aurer A, Majstorović K, Budimir A. The effectiveness of systemic eradication therapy against oralHelicobacter pylori. Journal of Oral Pathology and Medicine 2011;40(5):428‐32. [DOI] [PubMed] [Google Scholar]
Bai 2014 {published data only}
- Bai HH, Wu F, Zhao B. Influence of initial periodontal therapy on the eradication of gastric Helicobacter pylori. Zhonghua Shiyan He Linchuang Ganranbing Zazhi [Chinese Journal of Experimental and Clinical Infectious Diseases] 2014;8(5):642‐4. (Chinese). [Google Scholar]
Hou 2002 {published data only}
- Hou HL, Meng HX, Hu WJ, Yang ZP, Wang JW. [Effect of initial periodontal therapy on the eradication of gastric Helicobacter pylori]. Shiyong Kouqiang Yixue Zazhi [Journal of Practical Stomatology] 2002;18(3):198‐200. (Chinese). [Google Scholar]
Hou 2003 {published data only}
- Hou HL, Meng HX, Hu WJ, Wang JW. [The relationship between Helicobacter pylori in oral cavity and the Hp infection in stomach]. Zhonghua Kou Qiang Yi Xue Za Zhi [Chinese Journal of Stomatology] 2003;38(5):327‐9. (Chinese). [PubMed] [Google Scholar]
Hou 2004 {published data only}
- Hou HL, Meng HX, Hu FL, Cheng H, Wang JW. [Influence of initial periodontal therapy on the presence of oral Helicobacter pylori and the relation of genotype of Helicobacter pylori in oral cavity and with that in stomach]. Shiyong Kouqiang Yixue Zazhi [Journal of Practical Stomatology] 2004;20(3):353‐7. (Chinese). [Google Scholar]
Ierardi 1998 {published data only}
- Ierardi E, Amoruso A, Notte T, Francavilla R, Castellaneta S, Marrazza E, et al. Halitosis and Helicobacter pylori: a possible relationship. Digestive Diseases and Sciences 1998;43(12):2733‐7. [DOI] [PubMed] [Google Scholar]
Jia 2009b {published data only}
- Jia CL, Jiang GS, Li CH, Li CR. Effect of dental plaque control on infection of Helicobacter pylori in gastric mucosa. Journal of Periodontology 2009;80(10):1606‐9. [DOI] [PubMed] [Google Scholar]
Li 2012 {published data only}
- Li Y, Zhang WD. [Periodontal treatment improve the H. pylori infection eradication rate]. Yixue Yu Zhexue [Medicine and Philosophy] 2012;33(453):20‐1. [Chinese]. [Google Scholar]
Miyabayashi 2000 {published data only}
- Miyabayashi H, Furihata K, Shimizu T, Ueno I, Akamatsu T. Influence of oral Helicobacter pylori on the success of eradication therapy against gastric Helicobacter pylori. Helicobacter. 2000;5(1):30‐7. [DOI] [PubMed] [Google Scholar]
Namiot 2001 {published data only}
- Namiot DB, Namiot Z, Kemona A, Gołebiewska M. Dental status and efficacy of Helicobacter pylori eradication [Uzȩbienie naturalne a skuteczność leczenia eradykacyjnego bakterii Helicobacter pylori]. Polskie Archiwum Medycyny Wewnetrznej 2001;105(4):291‐5. (Polish). [PubMed] [Google Scholar]
Namiot 2004 {published data only}
- Namiot Z, Namiot DB, Kemona A, Stasiewicz J. Effect of antibacterial therapy and salivary secretion on the efficacy of Helicobacter pylori eradication in duodenal ulcer patients. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2004;97(6):714‐7. [DOI] [PubMed] [Google Scholar]
Namiot 2007 {published data only}
- Namiot DB, Namiot Z, Kemona A, Bucki R, Gotebiewska M. Oral health status and oral hygiene practices of patients with peptic ulcer and how these affect Helicobacter pylori eradication from the stomach. Helicobacter. 2007;12(1):63‐7. [DOI] [PubMed] [Google Scholar]
Özdemir 2001 {published data only}
- Özdemir A, Mas MR, Şahin S, Sağlamkaya U, Ateşkan U. Detection of Helicobacter pylori colonization in dental plaques and tongue scrapings of patients with chronic gastritis. Quintessence International 2001;32(2):131‐4. [PubMed] [Google Scholar]
Qian 2014 {published data only}
- Qian J, Zhou G, Zhong LJ. [Effect of initial periodontal therapy on eradication rate of gastric Helicobacter pylori in patients with gastric ulcer]. Zhongguo Yaofang [China Pharmacy] 2014;12:2091‐2. Chinese. [Google Scholar]
Tarullo 2001 {published data only}
- Tarullo A, Tattoli M, Cagiano R. Persistent eradication of Helicobacter pylori after systemic politherapy associated with periodontal pockets treatment with metronidazole and calcium sulphate. European Review for Medical and Pharmacological Sciences 2001;5(4):127‐9. [PubMed] [Google Scholar]
Xu 2001 {published data only}
- Xu LH, Gu HY, Wu Z, Cui CF. [The effect of mouthrinse solution on the eradication of Helicobacter pylori]. Kouqiang Yixue [Stomatology] 2001;21(3):142‐3. Chinese. [Google Scholar]
Yang 2013 {published data only}
- Yang H, Li XY, Liu C, Lu ZY. [Effect of different treatment on eradication of Helicobacter pylori]. Beijing Kouqiang Yixue [Beijing Journal of Stomatology] 2013;21(5):268‐70. Chinese. [Google Scholar]
Ye 2012 {published data only}
- Ye DG. [The curative effect and nursing guidance of the Chinese herbal medicine mouth wash for treatment of H. pylori infection]. Zhongguo Weisheng Chanye [China Health Industry] 2012;9(7):26, 29. (Chinese). [Google Scholar]
Zhao 2000 {published data only}
- Zhao R, Xu YZ, Duan YQ, Yao SK, Zhang LW, Huang J. [Effect of periodontics treatment on eradication of Helicobacter pylori infection in gastric mucosa]. Hebei Yike Daxue Xuebao [Journal of Hebei Medical University] 2000;21(2):90‐2. (Chinese). [Google Scholar]
Additional references
Asaka 2001
- Asaka M, Sugiyama T, Kato M, Satoh K, Kuwayama H, Fukuda Y, et al. A multicenter, double‐blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter 2001;6(3):254‐61. [DOI] [PubMed] [Google Scholar]
Balaban 1997
- Balaban DH, Peura DA. Helicobacter pylori associated with peptic ulcer and gastritis. In: Lamont JT editor(s). Gastrointestinal Infections. Diagnosis and Management. New York: Marcel Dekker Inc., 1997:29‐69. [Google Scholar]
Bouziane 2012
- Bouziane A, Ahid S, Abouqal R, Ennibi O. Effect of periodontal therapy on prevention of gastric Helicobacter pylori recurrence: a systematic review and meta‐analysis. Journal of Clinical Periodontology 2012;39(12):1166‐73. [DOI] [PubMed] [Google Scholar]
Burgers 2008
- Burgers R, Schneider‐Brachert W, Reischl U, Behr KA, Lehn N, Schalz G, et al. Helicobacter pylori in human oral cavity and stomach. European Journal of Oral Sciences 2008;116(4):297‐304. [DOI] [PubMed] [Google Scholar]
Chaudhry 2008
- Chaudhry S, Iqbal HA, Khan AA, Izhar M, Butt AK, Akhter MW, et al. Helicobacter pylori in dental plaque and gastric mucosa: correlation revisited. Journal of the Pakistan Medical Association 2008;58(6):331‐4. [PubMed] [Google Scholar]
Cover 2009
- Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology 2009;136(6):1863‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czesnikiewicz‐Guzik 2007
- Czesnikiewicz‐Guzik M, Loster B, Bielanski W, Guzik TJ, Konturek PC, Zapala J, et al. Implications of oral Helicobacter pylori for the outcome of its gastric eradication therapy. Journal of Clinical Gastroenterology 2007;41(2):145–51. [DOI] [PubMed] [Google Scholar]
Dowsett 2003
- Dowsett SA, Kowolik MJ. Oral Helicobacter pylori: can we stomach it?. Critical Reviews in Oral Biology and Medicine 2003;14(3):226‐33. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eyigor 2009
- Eyigor M, Eyigor H, Gultekin B, Aydin N. Detection of Helicobacter pylori in adenotonsiller tissue specimens by rapid urease test and polymerase chain reaction. European Archives of Oto‐Rhino‐Laryngology 2009;266(10):1611‐3. [DOI] [PubMed] [Google Scholar]
Ford 2006
- Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003840.pub4] [DOI] [PubMed] [Google Scholar]
Gebara 2006
- Gebara EC, Faria CM, Pannuti C, Chehter L, Mayer MP, Lima LA. Persistence of Helicobacter pylori in the oral cavity after systemic eradication therapy. Journal of Clinical Periodontology 2006;33(5):329‐33. [DOI] [PubMed] [Google Scholar]
Gisbert 2006
- Gisbert JP, Luna M, Gomez B, Herrerias JM, Mones J, Castro‐Fernandez M, et al. Recurrence of Helicobacter pylori infection after several eradication therapies: long‐term follow‐up of 1000 patients. Alimentary Pharmacology and Therapeutics 2006;23(6):713‐9. [DOI] [PubMed] [Google Scholar]
Go 2002
- Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Alimentary Pharmacology and Therapeutics 2002;16(Suppl 1):3‐15. [DOI] [PubMed] [Google Scholar]
Graham 1991
- Graham DY, Lew GM, Evans DG, Evans DJ Jr, Klein PD. Effect of triple therapy (antibiotics plus bismuth) on duodenal ulcer healing. A randomized controlled trial. Annals of Internal Medicine 1991;115(4):266‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hopkins 1996
- Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology 1996;110(4):1244‐52. [DOI] [PubMed] [Google Scholar]
Hunt 1999
- Hunt RH, Fallone CA, Thomson AB. Canadian Helicobacter pylori Consensus Conference update: infections in adults. Canadian Helicobacter Study Group. Canadian Journal of Gastroenterology 1999;13(3):213‐7. [DOI] [PubMed] [Google Scholar]
Kim 1998
- Kim N, Lim SH, Lee KH, Jung HC, Song IS, Kim CY. Helicobacter pylori reinfection rate and duodenal ulcer recurrence in Korea. Journal of Clinical Gastroenterology 1998;27(4):321‐6. [DOI] [PubMed] [Google Scholar]
Kusters 2006
- Kusters JG, Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clinical Microbiology Reviews 2006;19(3):449‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2009
- Liu Y, Yue H, Li A, Wang J, Jiang B, Zhang Y, et al. An epidemiologic study on the correlation between oral Helicobacter pylori and gastric H. pylori. Current Microbiology 2009;58(5):449‐53. [DOI] [PubMed] [Google Scholar]
Medina 2010
- Medina ML, Medina MG, Martín GT, Picón SO, Bancalari A, Merino LA. Molecular detection of Helicobacter pylori in oral samples from patients suffering digestive pathologies. Medicina Oral, Patologia Oral, y Cirugia Bucal 2010;15(1):e38‐42. [PubMed] [Google Scholar]
Niv 2008
- Niv Y. H. pylori recurrence after successful eradication. World Journal of Gastroenterology 2008;14(10):1477‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olivier 2006
- Olivier BJ, Bond RP, Zyl WB, Delport M, Slavik T, Ziady C, et al. Absence of Helicobacter pylori within the oral cavities of members of a healthy South African community. Journal of Clinical Microbiology 2006;44(2):635‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peek 2002
- Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature Reviews Cancer 2002;2(1):28‐37. [DOI] [PubMed] [Google Scholar]
Pytko‐Polonczyk 1996
- Pytko‐Polonczyk J, Konturek SJ, Karczewska E, Bielanski W, Kaczmarczyk‐Stachowska A. Oral cavity as permanent reservoir of Helicobacter pylori and potential source of reinfection. Journal of Physiology and Pharmacology 1996;47(1):121‐9. [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ricci 2007
- Ricci C, Holton J, Vaira D. Diagnosis of Helicobacter pylori: invasive and non‐invasive tests. Best Practice & Research. Clinical Gastroenterology 2007;21(2):299‐313. [DOI] [PubMed] [Google Scholar]
Rimbara 2011
- Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nature Reviews Gastroenterology & Hepatology 2011;8(2):79‐88. [DOI] [PubMed] [Google Scholar]
Schlemper 1996
- Schlemper RJ, Werf SD, Biemond I, Lamers CB. Seroepidemiology of gastritis in Japanese and Dutch male employees with and without ulcer disease. European Journal of Gastroenterology and Hepatology 1996;8(1):33‐9. [DOI] [PubMed] [Google Scholar]
Sheu 2007
- Sheu BS, Cheng HC, Yang YJ, Wu JJ. The presence of dental disease can be a risk factor for recurrent Helicobacter pylori infection after eradication therapy: a 3‐year follow‐up. Endoscopy 2007;39(11):942‐7. [DOI] [PubMed] [Google Scholar]
Shirin 2004
- Shirin H, Birkenfeld S, Shevah O, Levine A, Epstein J, Boaz M, et al. Application of Maastricht 2‐2000 guidelines for the management of Helicobacter pylori among specialists and primary care physicians in Israel: are we missing the malignant potential of Helicobacter pylori?. Journal of Clinical Gastroenterology 2004;38(4):322‐5. [DOI] [PubMed] [Google Scholar]
Silva Rossi‐Aguiar 2009
- Silva Rossi‐Aguiar VP, Navarro‐Rodriguez T, Mattar R, Siqueira de Melo Peres MP, Correa Barbuti R, Silva FM, et al. Oral cavity is not a reservoir for Helicobacter pylori in infected patients with functional dyspepsia. Oral Microbiology and Immunology 2009;24(3):255‐9. [DOI] [PubMed] [Google Scholar]
Sokić‐Milutinović 2004
- Sokić‐Milutinović A, Todorović V, Milosavljević T. Pathogenesis of Helicobacter pylori infection‐‐bacterium and host relationship [Patogeneza infekcije sa Helicobacter pylori‐‐odnos bakterije i domaćina]. Srpski Arhiv za Celokupno Lekarstvo 2004;132(9‐10):340‐4. [DOI] [PubMed] [Google Scholar]
Song 1999
- Song Q, Haller B, Schmid RM, Adler G, Bode G. Helicobacter pylori in dental plaque. A comparison of different PCR primer sets. Digestive Diseases and Sciences 1999;44(3):479‐84. [DOI] [PubMed] [Google Scholar]
Song 2000a
- Song Q, Lange T, Spahr A, Adler G, Bode G. Characteristic distribution pattern of Helicobacter pylori in dental plaque and saliva detected with nested PCR. Journal of Medical Microbiology 2000;49(4):349‐53. [DOI] [PubMed] [Google Scholar]
Song 2000b
- Song Q, Spahr A, Schmid RM, Adler G, Bode G. Helicobacter pylori in the oral cavity: high prevalence and great DNA diversity. Digestive Diseases and Sciences 2000;45(11):2162‐7. [DOI] [PubMed] [Google Scholar]
Suerbaum 2002
- Suerbaum S, Michetti P. Helicobacter pylori infection. New England Journal of Medicine 2002;347(15):1175‐86. [DOI] [PubMed] [Google Scholar]
Tytgat 1994
- Tytgat GN. Review article: treatments that impact favourably upon the eradication of Helicobacter pylori and ulcer recurrence. Alimentary Pharmacology and Therapeutics 1994;8(4):359‐68. [DOI] [PubMed] [Google Scholar]
Vakil 2009
- Vakil N. H. pylori treatment: new wine in old bottles?. American Journal of Gastroenterology 2009;104(1):26‐30. [DOI] [PubMed] [Google Scholar]
Wolle 2007
- Wolle K, Malfertheiner P. Treatment of Helicobacter pylori. Best Practice & Research. Clinical Gastroenterology 2007;21(2):315‐24. [DOI] [PubMed] [Google Scholar]
Xia 1997
- Xia HX, Talley NJ, Keane CT, O'Morain CA. Recurrence of Helicobacter pylori infection after successful eradication: nature and possible causes. Digestive Diseases and Sciences 1997;42(9):1821‐34. [DOI] [PubMed] [Google Scholar]
Yamaoka 2010
- Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nature Reviews. Gatroenterology & Hepatology 2010;7(11):629‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zou 2011
- Zou QH, Li RQ. Helicobacter pylori in the oral cavity and gastric mucosa: a meta‐analysis. Journal of Oral Pathology & Medicine 2011;40(4):317‐24. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ren 2011
- Ren Q, Yan X, Zhou Y, Li WX. Periodontal therapy as adjunctive treatment for gastric Helicobacter pylori infection. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD009477] [DOI] [PMC free article] [PubMed] [Google Scholar]


