Abstract
Objective
To identify risk factors associated with severe COVID-19 infection in a defined Midwestern US population overall and within different age groups.
Patients and Methods
We used the Rochester Epidemiology Project research infrastructure to identify persons residing in a defined 27-county Midwestern region who had positive results on polymerase chain reaction tests for COVID-19 between March 1, 2020, and September 30, 2020 (N=9928). Age, sex, race, ethnicity, body mass index, smoking status, and 44 chronic disease categories were considered as possible risk factors for severe infection. Severe infection was defined as hospitalization or death caused by COVID-19. Associations between risk factors and severe infection were estimated using Cox proportional hazard models overall and within 3 age groups (0 to 44, 45 to 64, and 65+ years).
Results
Overall, 474 (4.8%) persons developed severe COVID-19 infection. Older age, male sex, non-White race, Hispanic ethnicity, obesity, and a higher number of chronic conditions were associated with increased risk of severe infection. After adjustment, 36 chronic disease categories were significantly associated with severe infection. The risk of severe infection varied significantly across age groups. In particular, persons 0 to 44 years of age with cancer, chronic neurologic disorders, hematologic disorders, ischemic heart disease, and other endocrine disorders had a greater than 3-fold increased risk of severe infection compared with persons of the same age without those conditions. Associations were attenuated in older age groups.
Conclusion
Older persons are more likely to experience severe infections; however, severe cases occur in younger persons as well. Our data provide insight regarding younger persons at especially high risk of severe COVID-19 infection.
Abbreviations and Acronyms: BMI, body mass index; CCC, Clinical Classification Codes; COVID-19, coronavirus disease 2019; ICD, International Classification of Diseases; PCR, polymerase chain reaction; REP, Rochester Epidemiology Project; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Since Coronavirus Disease 2019 (COVID-19) was first recognized in December 2019,1 investigators have attempted to identify characteristics associated with higher risk of adverse health outcomes following infection. Several studies have shown that older age, male sex, higher body mass index (BMI) and the presence of diabetes, hypertension, cardiovascular disease, and other comorbidities are associated with more severe outcomes among hospitalized patients.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 Other studies have reported similar characteristics associated with risk of hospitalization and adverse outcomes among persons presenting to emergency departments.14 , 15
However, many persons with COVID-19 develop mild or no symptoms and do not require emergency department visits or hospitalization. Failure to identify and include persons who develop milder cases of COVID-19 may overestimate the risk of severe disease and may result in a failure to recognize risk characteristics for severe COVID-19 infection.16 Capturing less severe COVID-19 cases has been especially difficult in the United States because access to testing has been extremely limited in some populations. In addition, analyses of cases reported to state and territorial health departments may miss potential risk factors that are not routinely included as part of the case report forms. Finally, disease severity has been strongly associated with increasing age. However, severe cases do occur in younger persons, and there are limited data on whether known risk factors may differentially affect different age groups.
To address this gap, we studied persons residing in a 27-county region of southeastern Minnesota and west-central Wisconsin, using the resources of the Rochester Epidemiology Project (REP) medical records-linkage system.17 The REP links together the medical records of persons residing in this region from multiple health care providers for research studies and provides comprehensive health information for this population. One of the health care providers in the REP (Mayo Clinic) rapidly made COVID-19 testing available early in the pandemic and provided testing services to other health care providers in the region. For this reason, COVID-19 testing in this population was more common than in some other areas of the United States, and tests did not need to be strictly reserved for patients who required emergency department or hospital care. Therefore, the REP provides a unique environment to study the epidemiology of COVID-19 in 27 counties of a defined US population. In this study, we report hospitalization and death rates caused by COVID-19 infection, and we provide detailed information on the demographic and clinical characteristics associated with increased risk for hospitalization and death. Finally, we assess whether the impact of potential risk factors differs across 3 age groups.
Methods
Data Source
The REP has been previously described.17 , 18 Briefly, the REP includes linked medical records from local health care providers for 1.7 million persons who have lived in a 27-county Midwest region after January 1, 2010. The REP captures approximately 61% of the entire population residing in this region.17 Health care data from all visits to each health care provider are coded and indexed electronically. Therefore, the REP includes demographic data and comprehensive information about medical diagnoses, hospital admissions, surgical procedures, drug prescriptions, laboratory test results, BMI, and smoking status. This study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Study Population
We searched the REP electronic indexes to identify all persons who resided in the 27-county region and who had a positive nasopharyngeal polymerase chain reaction (PCR) tests for COVID-19 between March 1, 2020, and September 30, 2020 (7 months). Persons who did not provide authorization to use their medical records for research were excluded.
Definition of Potential Risk Factors
We extracted BMI and smoking-status information (never, former, or current) closest to the date of the first positive test result. For persons 0 to 20 years of age, we categorized BMI into 4 categories, using percentiles for height and weight compared with persons of the same age and gender, using criteria defined by the Centers for Disease Control and Prevention (under/normal weight: <85th percentile, overweight: ≥85th percentile to <95th percentile, obese: ≥95th percentile, and unknown).19 For persons 21 years or older, BMI was categorized into 4 categories: under and normal weight (<25 kg/m2), overweight (25 to <30 kg/m2), and obese (≥30 kg/m2). Missing data are shown in Table 1 and described as “unknown.” We then extracted all International Classification of Diseases diagnosis codes (ICD-9 and ICD-10) for the 5 years before the positive test result to capture patient comorbidities. However, we excluded all diagnosis codes that occurred within 14 days before the first positive test to exclude diagnoses that could have been caused by COVID-19 infection. ICD diagnostic codes were then grouped into categories using the Clinical Classifications Software.20 For this study, we focused on only the chronic disease categories and further combined the categories into 48 broader disease groups, as previously defined by Mukherjee and colleagues.21 These categories were used in a previous REP study.22 The final Clinical Classification Codes (CCC), the 48 chronic condition groups, and the number of persons with diagnoses in each group are shown in Supplemental Table 1, available online at http://www.mayoclinicproceedings.org.
Table 1.
Risk of Severe COVID-19 Infection (Hospitalization or Death Caused by COVID-19) by Study Population Characteristics (N=9928)
| Characteristic | N at risk (%) | N severe infections | Unadjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI)b |
|---|---|---|---|---|
| Age group (age at date of first positive test) | ||||
| 0-19 | 1371 (13.8) | 9 | 0.44 (0.22, 0.88) | 0.42 (0.21, 0.84) |
| 20-44 | 5455 (55.0) | 81 | Reference | Reference |
| 45-64 | 2169 (21.9) | 165 | 5.25 (4.03, 6.85) | 2.97 (2.25, 3.93) |
| 65+ | 933 (9.4) | 219 | 17.51 (13.57, 22.60) | 6.38 (4.70, 8.65) |
| Sex | ||||
| Women/girls | 5167 (52.0) | 223 | Reference | Reference |
| Men/boys | 4753 (47.9) | 251 | 1.23 (1.03, 1.47) | 1.43 (1.18, 1.71) |
| Unknown | 8 (0.1%) | 0 | ||
| Race | ||||
| White | 6635 (66.8) | 333 | Reference | Reference |
| Black | 1141 (11.5) | 44 | 0.77 (0.56, 1.05) | 1.49 (1.08, 2.06) |
| Asian | 408 (4.1) | 36 | 1.79 (1.27, 2.53) | 3.61 (2.53, 5.15) |
| Other/unknown | 1744 (17.6) | 61 | 0.69 (0.53, 0.91) | 1.50 (1.07, 2.10) |
| Ethnicity | ||||
| Not Hispanic/Unknown | 8357 (84.2) | 383 | Reference | Reference |
| Hispanic | 1571 (15.8) | 91 | 1.27 (1.01, 1.60) | 1.87 (1.41, 2.49) |
| BMIa category | ||||
| Under/normal weight | 2631 (26.5) | 80 | Reference | Reference |
| Overweight | 2193 (22.1) | 124 | 1.96 (1.48, 2.60) | 1.05 (0.79, 1.40) |
| Obese | 3092 (31.1) | 269 | 3.22 (2.51, 4.12) | 1.60 (1.23, 2.07) |
| Unknown | 2012 (20.3) | 1 | 0.02 (0.00, 0.12) | 0.03 (0.00, 0.23) |
| Smoking status | ||||
| Never | 6,916 (69.7) | 200 | Reference | Reference |
| Former | 2,258 (22.7) | 252 | 4.01 (3.33, 4.83) | 1.20 (0.98, 1.46) |
| Current | 754 (7.6) | 22 | 1.01 (0.65, 1.57) | 0.78 (0.50, 1.21) |
| Number of chronic conditions | ||||
| 0-1 | 4043 (40.7) | 61 | Reference | Reference |
| 2-3 | 1829 (18.4) | 33 | 1.20 (0.78, 1.83) | 0.68 (0.45, 1.05) |
| 4-6 | 1748 (17.6) | 69 | 2.64 (1.87, 3.73) | 0.93 (0.66, 1.33) |
| 7+ | 2308 (23.3) | 311 | 9.41 (7.15, 12.38) | 1.42 (1.04, 1.95) |
CI, confidence interval.
BMI, body mass index.
Adjusted for all other variables in the table.
Definition of Severe COVID-19 Infection
We defined severe COVID-19 infection as either hospitalization or death caused by the infection. We used the REP resources to electronically extract all hospitalizations and deaths that occurred in the 3 months following the first positive test result or through October 31, 2020, whichever came first. The full text of the medical records was then reviewed by J.L.S. and G.S.L. to determine whether the hospitalization or death was caused by the COVID-19 infection.
Analysis
Follow-up began on the date of COVID-19 diagnosis (positive PCR test) and continued for 3 months, until death, or October 31, 2020, whichever came first. Cumulative incidence plots were constructed to visualize the probability of hospitalization and death for 4 age groups (0 to 19, 20 to 44, 45 to 64, 65+), sex (male, female), ethnicity (Hispanic, non-Hispanic), 4 races (White, Black, Asian, and other/unknown), and number of comorbidities (0 to 1, 2 to 3, 4 to 6, 7+).
We next combined hospitalizations and deaths into a single composite end point of “severe COVID-19 infection” and used Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the risk of severe infection among persons with different demographic characteristics, clinical characteristics, and chronic condition categories. We conducted analyses for chronic conditions that were present in at least 50 persons in the study population. All models were adjusted for continuous age, sex, race, ethnicity, BMI, and smoking status. P values were adjusted for multiple comparisons, using the false discovery rate, and an adjusted P value of <0.05 was considered statistically significant.23 Stratified Cox proportional hazards regression models were used to estimate the risk for hospitalization or death among persons with different chronic condition categories by 3 age categories: 0 to 44 years, 45 to 64 years, and 65+ years. All models were adjusted for continuous age within each age group, sex, race, ethnicity, BMI group, and smoking status. Interactions among age groups and each chronic condition were assessed by including an interaction term in overall models. Interaction P values <0.05 were considered statistically significant. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, NC) and R, version 3.2.3.
Results
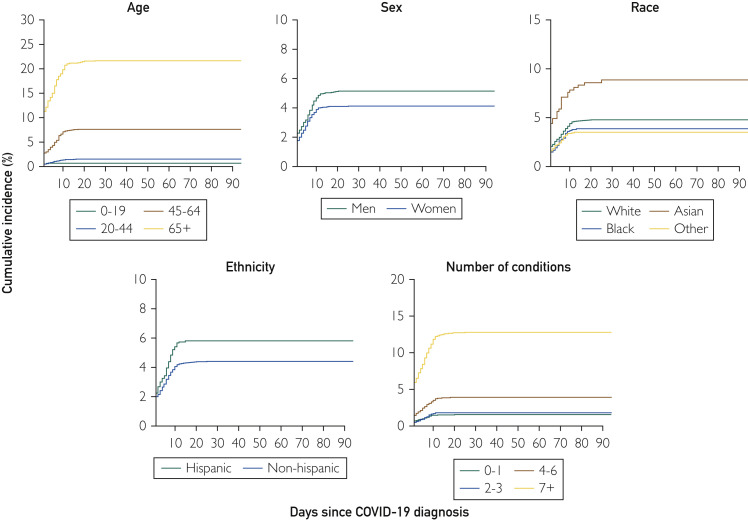
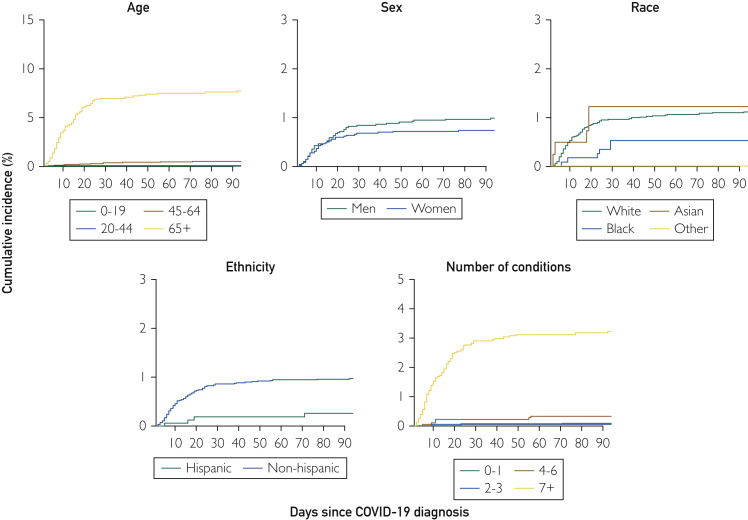
We identified 10,231 persons in the 27-county region with positive COVID-19 PCR test results between March 1, 2020, and September 30, 2020; 9928 (97%) had provided authorization to use their medical records for research and were included in the study. Among these persons, 474 (4.8%) had severe COVID-19 infections. The number and percent of persons with severe infections differed by phase of the pandemic. Between March 1, 2020, and June 30, 2020, 211 (6.6%) had severe COVID-19 infections. Between July 1, 2020, and September 30, 2020, 263 (3.9%) had severe COVID-19 infections. Overall, 402 were hospitalized (4.0%), 54 (0.5%) were hospitalized and later died, and 18 (0.2%) died without hospitalization. The overall cumulative incidence rate of hospitalizations was 4.59 of 100 (95% CI, 4.18 to 5.01) and the overall mortality rate was 0.73 of 100 (95% CI, 0.58 to 0.92). Unadjusted cumulative incidence rates of hospitalization are shown in Figure 1 . Persons 65 years of age or older had the highest rates of hospitalization and death, followed by persons 45 to 64 years of age. Hospitalization rates were also higher in men compared with women and in persons of Hispanic ethnicity. Persons of Asian race had the highest hospitalization rates, and rates increased with an increasing number of chronic conditions (Figure 1). Death rates were highest in persons 65+ years of age, in men, in persons of non-Hispanic ethnicity, in persons of White and Asian races, and in persons with the highest number of chronic conditions (Figure 2 ). Persons with 7 or more chronic conditions showed dramatic increases in the risk of both hospitalization and death compared with the other strata by number of conditions. In general, the curves for hospitalization flattened approximately 10 days after diagnosis, and the curves for mortality flattened after approximately 20 days.
Figure 1.
Cumulative incidence of hospitalization among persons with COVID-19 infections by demographic characteristics and number of chronic conditions. A, Incidence by age; B, incidence by sex; C, incidence by race (White, Black, Asian, and other); D, incidence by ethnicity (Hispanic vs non-Hispanic); E, incidence by number of baseline conditions. Some curves for hospitalization do not start at “0” because the test was performed during hospitalization. Results are not adjusted for patient characteristics.
Figure 2.
Cumulative incidence of death among persons with COVID-19 infections by demographic characteristics and number of chronic conditions. A, Incidence by age; B, incidence by sex; C, incidence by race (White, Black, Asian, and other); D, incidence by ethnicity (Hispanic vs non-Hispanic); E, incidence by number of baseline conditions. Some curves for hospitalization do not start at “0” because the test was performed during hospitalization. Results are not adjusted for patient characteristics.
We next combined hospitalizations and deaths into a single composite end point of “severe COVID-19 infection” (474 severe infections; 4.8% of the study population). Risks of severe infection by age, sex, ethnicity, race, BMI group, smoking status, and number of chronic conditions are shown in Table 1. After adjusting for all of the characteristics in Table 1, older age, male sex, Hispanic ethnicity, Black, Asian, and other or unknown race, obesity, and having 7 or more chronic conditions were associated with an increased risk of severe infection.
We examined the risk of severe COVID-19 infection (hospitalization or death) among persons with different types of chronic conditions, considered one at a time (Table 2 ). In unadjusted analyses, nearly all of the comorbidities were strongly associated with an increased risk of severe infection. After adjustment for demographic characteristics, smoking, and BMI category, associations were attenuated. However, after adjusting for multiple comparisons, most of the chronic condition categories remained significantly associated with risk of severe infection at an adjusted statistical significance threshold of P<0.05 (Table 2). Developmental disorders; personality disorders; and affective disorders, schizophrenia, and other psychoses were most strongly associated with risk of severe infection (Table 2; all HRs >3.0).
Table 2.
Risk of Severe COVID-19 Infection (Hospitalization or Death Caused by COVID-19) by Chronic Condition Categories
| Chronic condition category | N at risk (%) | N of severe infections | Unadjusted hazard ratio (95% CI) | Adjusted hazard ratio (95% CI)a |
|---|---|---|---|---|
| Skin disorders | 3473 (35.0) | 254 | 2.18 (1.82, 2.61) | 1.13 (0.94, 1.37) |
| Osteoarthritis, joint disorders | 3084 (31.1) | 274 | 3.11 (2.60, 3.74) | 1.06 (0.87, 1.29) |
| Other endocrine disorders | 2574 (25.9) | 282 | 4.36 (3.63, 5.23) | 1.67 (1.37, 2.05)b |
| Back problems | 2466 (24.8) | 261 | 3.83 (3.19, 4.59) | 1.53 (1.27, 1.85)b |
| Anxiety, depression, bipolar disorders | 2337 (23.5) | 183 | 2.08 (1.73, 2.50) | 1.49 (1.23, 1.81)b |
| Chronic neurologic disorders | 2090 (21.1) | 268 | 5.10 (4.26, 6.12) | 1.76 (1.45, 2.15)b |
| Other upper respiratory tract disease | 1949 (19.6) | 154 | 2.00 (1.65, 2.43) | 1.29 (1.06, 1.57)b |
| Headache | 1817 (18.3) | 147 | 2.05 (1.68, 2.49) | 1.56 (1.28, 1.91)b |
| Arrhythmias | 1673 (16.9) | 239 | 5.29 (4.42, 6.34) | 1.72 (1.40, 2.10)b |
| Disorders of lipid metabolism | 1662 (16.7) | 281 | 7.73 (6.43, 9.28) | 1.50 (1.20, 1.86)b |
| Hypertension | 1544 (15.6) | 276 | 8.12 (6.76, 9.74) | 1.50 (1.20, 1.88)b |
| Diabetes | 1460 (14.7) | 236 | 6.12 (5.11, 7.33) | 1.38 (1.13, 1.69)b |
| Esophageal disorders | 1334 (13.4) | 169 | 3.72 (3.08, 4.49) | 1.38 (1.13, 1.67)b |
| Conditions associated with dizziness or vertigo | 1060 (10.7) | 117 | 2.83 (2.30, 3.49) | 1.28 (1.04, 1.59)b |
| Substance use disorders | 1022 (10.3) | 78 | 1.75 (1.37, 2.23) | 1.42 (1.09, 1.84)b |
| Deficiency and other anemia | 870 (8.8) | 154 | 5.36 (4.43, 6.50) | 1.76 (1.43, 2.17)b |
| Asthma | 777 (7.8) | 65 | 1.90 (1.46, 2.47) | 1.38 (1.06, 1.80)b |
| Biliary and liver disorders | 767 (7.7) | 143 | 5.54 (4.55, 6.74) | 2.07 (1.70, 2.54)b |
| Thyroid disorders | 761 (7.7) | 121 | 4.38 (3.56, 5.38) | 1.67 (1.34, 2.09)b |
| Bowel disorders | 730 (7.4) | 116 | 4.29 (3.48, 5.29) | 1.28 (1.02, 1.59)b |
| Cancer | 688 (6.9) | 105 | 4.02 (3.24, 5.00) | 1.24 (0.98, 1.56) |
| Peripheral venous disorders | 683 (6.9) | 105 | 4.05 (3.26, 5.03) | 1.32 (1.06, 1.66)b |
| Hematologic disorders | 674 (6.8) | 140 | 6.26 (5.14, 7.63) | 2.24 (1.82, 2.76)b |
| Obstructive pulmonary disorders | 668 (6.7) | 117 | 4.84 (3.93, 5.96) | 1.76 (1.42, 2.19)b |
| Ischemic heart disease | 584 (5.9) | 151 | 8.36 (6.89, 10.14) | 1.86 (1.50, 2.31)b |
| Attention-deficit, conduct, and disruptive behavior disorder | 443 (4.5) | 13 | 0.60 (0.35, 1.04) | 1.35 (0.77, 2.37) |
| Heart-valve disorders | 429 (4.3) | 89 | 5.52 (4.39, 6.96) | 1.46 (1.14, 1.87)b |
| Peripheral and central vascular disorders | 359 (3.6) | 106 | 8.74 (7.04, 10.85) | 1.56 (1.23, 1.99)b |
| Chronic kidney disease | 344 (3.5) | 119 | 10.9 (8.85, 13.42) | 1.89 (1.48, 2.40)b |
| Heart failure | 308 (3.1) | 114 | 11.66 (9.44, 14.4) | 2.44 (1.93, 3.09)b |
| Gastric and duodenal ulcer | 294 (3.0) | 45 | 3.64 (2.68, 4.95) | 1.50 (1.10, 2.05)b |
| Gout and other crystal arthropathies | 188 (1.9) | 43 | 5.57 (4.07, 7.62) | 1.17 (0.85, 1.62) |
| Osteoporosis | 186 (1.9) | 56 | 7.92 (5.99, 10.47) | 1.69 (1.25, 2.30)b |
| Other diseases of bladder and urethra | 172 (1.7) | 39 | 5.58 (4.02, 7.74) | 1.41 (1.00, 1.98) |
| Epilepsy, convulsions | 164 (1.7) | 24 | 3.36 (2.23, 5.07) | 2.05 (1.36, 3.10)b |
| Developmental disorders | 132 (1.3) | 11 | 1.80 (0.99, 3.27) | 3.05 (1.67, 5.57)b |
| Rheumatoid arthritis and related disease | 131 (1.3) | 21 | 3.59 (2.32, 5.56) | 1.11 (0.71, 1.72) |
| Hepatitis | 121 (1.2) | 21 | 3.98 (2.57, 6.16) | 1.69 (1.08, 2.65)b |
| Central nervous system vascular ischemia | 102 (1.0) | 28 | 6.77 (4.62, 9.91) | 1.28 (0.86,1.90) |
| Personality disorders | 82 (0.8) | 11 | 3.01 (1.65, 5.47) | 3.08 (1.68, 5.65)b |
| Tuberculosis | 66 (0.7) | 7 | 2.29 (1.09, 4.83) | 1.81 (0.84, 3.86) |
| Affective disorders, schizophrenia, other psychoses | 64 (0.6) | 19 | 7.53 (4.76, 11.92) | 3.53 (2.21, 5.62)b |
| Paralysis | 64 (0.6) | 17 | 6.48 (3.99, 10.52) | 2.04 (1.25, 3.33)b |
| Systemic lupus erythematosus and connective tissue disorders | 55 (0.6) | 9 | 3.71 (1.92, 7.17) | 1.78 (0.92, 3.45) |
Chronic condition categories are listed in descending order of frequency of occurrence in the population. The reference population for each analysis is all persons without the chronic condition. Conditions that remained significant after adjustment are highlighted in bold type.
BMI, body mass index; CI, confidence interval.
Adjusted for age (continuous variable), sex, race, ethnicity, BMI category, and smoking status.
Significant after adjustment for the false discovery rate.
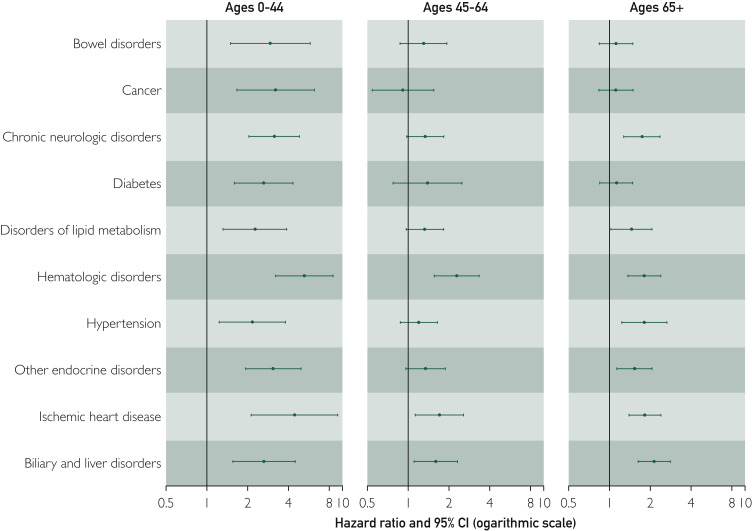
We next stratified our analyses by age and assessed interactions between the chronic condition categories and age groups to determine if associations between the chronic condition categories and risk of severe COVID-19 infection differed by age. Because severe infections were uncommon in persons 0 to 19 years of age (0.7%), and in persons 20 to 44 years of age (1.5%), these 2 age groups were combined in a single group for the stratified analyses. Therefore, we considered 3 age groups: persons 0 to 44 years of age, 45 to 64 years of age, and 65+ years of age. Associations between 10 of the chronic disease categories and risk of severe COVID-19 infection differed significantly across the 3 age groups (interaction P values <0.05; Figure 3 ; Supplemental Table 2, available online at http://www.mayoclinicproceedings.org). The risk of severe COVID-19 for persons with these conditions compared with person without these conditions was highest in persons 0 to 44 years of age compared with the 2 older age groups. In particular, persons 0 to 44 years of age with cancer, chronic neurologic disorders, hematologic disorders, other endocrine disorders, and ischemic heart disease had a 3-fold increased risk of developing severe infection compared with persons of the same age without these conditions (Figure 3; Supplemental Table 2).
Figure 3.
Risk of severe COVID-19 infection among persons with 1 of 10 chronic conditions associated with significant variability by age. Data are presented for 3 age groups: 0 to 44 years, 45 to 64 years, and 65+ years. Hazard ratios are adjusted for age (continuous variable within each age stratum), sex, race, ethnicity, body mass index category, and smoking status.
Finally, most of the persons in our cohort who had developmental disorders, epilepsy or convulsions, and personality disorders were between 0 and 44 years of age (Supplemental Table 2). In this age group, each of these chronic conditions was a strong risk factor for severe COVID-19 infections (developmental disorders HR, 3.79; 95% CI, 1.17 to 12.31; epilepsy or convulsions HR, 7.49; 95% CI, 3.59 to 15.62; personality disorders HR, 3.72; 95% CI, 1.32 to 10.46).
Discussion
In a large Midwestern US population, we found that the risk of severe COVID-19 disease was associated with older age; male sex; Hispanic ethnicity; Asian, Black, and other or mixed race; obesity; and with an increasing number of chronic conditions. Most of the chronic disease categories we studied were significantly associated with an increased risk of severe COVID-19 disease, even after adjusting for patient characteristics and accounting for multiple comparisons. We also found differences in associations among 10 chronic disease categories and severe COVID-19 infection across 3 age groups. Associations were strongest in persons 0 to 44 years of age, suggesting that younger patients with COVID-19 and these conditions may be at especially high risk of severe disease compared with persons 0 to 44 years of age who do not have these conditions compared with the other age groups.
Our study results are in agreement with previous studies that have shown that older age is the primary risk factor for more severe COVID-19 disease.2 , 10 , 15 , 24 , 25 In addition, our findings are consistent with previous studies that have shown that persons with multiple chronic conditions are at higher risk of severe infection.8 , 25, 26, 27, 28, 29, 30 It is not clear why older age is such a strong risk factor for severe disease, but the number of chronic conditions increases with increasing age. However, in our study, older age remained significantly associated with severe infection even after adjustment for the number of comorbidities, suggesting that other factors related to older age (eg, immunosenescence) may also play a role in severity of disease.31
We also found that minority race and ethnicity, obesity, and male sex were associated with an increased risk of severe COVID-19 infection, and these results are consistent with previous studies.13 , 14 , 24 , 25 , 32, 33, 34, 35, 36, 37 By contrast, we did not confirm findings from previous studies that have reported smoking as a risk factor for severe COVID-19 outcomes.37 , 38 Before adjustment, former smokers were at an increased risk of severe infection, but, after adjustment, that association was significantly attenuated. The associations previously reported may have been caused by confounding by older age and by the presence of multiple chronic conditions.
We also found that virtually all the chronic condition categories we examined were significantly associated with increased risk of severe COVID-19 infection, even after adjusting for patient characteristics and multiple comparisons. Our comprehensive assessment of comorbidities demonstrates that an increased risk of severe infection extends well beyond those already established with cardiovascular diseases, their risk factors, chronic lung disease, and cancer. We found, however, that the risk of severe infection in persons with 10 of these conditions differed significantly by age. Overall, persons 0 to 44 years of age were at the lowest risk of severe infection. However, severe cases occurred in 90 persons (1.5%) in this age group. Persons 0 to 44 years of age who also had cancer, chronic neurologic disorders, hematologic disorders, ischemic heart disease, and other endocrine disorders had greater than 3-fold increased risks of severe COVID-19 infection compared with persons of the same age who did not have these conditions. These risks were significantly different across the 3 age groups we studied, suggesting that such conditions are especially concerning in the younger population. Our results were particularly striking for cancer. Cancer was a strong risk factor for severe COVID-19 disease but only in this age group (HR, 3.21; 95% CI, 1.66 to 6.21). Cancer was not a significant risk factor for severe COVID-19 disease in persons 45 to 64 or 65+ years of age.
Finally, we found that some conditions were overrepresented in younger persons in our cohort (0 to 44 years of age) including developmental disorders, epilepsy and convulsions, and personality disorders. There were not enough persons with these conditions to study the association between the conditions and severe COVID-19 infection in the age groups of 45 to 64 and 65+ years. However, in analyses restricted to persons 0 to 44 years of age, each of these chronic condition categories was strongly associated with risk of severe COVID-19 infection.
Strengths and Limitations
Strengths of our study include access to historically collected data on all chronic conditions in a defined Midwestern population across age, sex, and racial and ethnic groups. We note that the REP captures approximately 61% of the population residing in this region and that the characteristics of persons in this region are similar to those of persons residing in the upper Midwest.17 In addition, testing for COVID-19 has been readily available to this population since early in the pandemic for persons with at least 1 symptom of COVID-19, exposure to a known positive case, or for screening before a medical or surgical procedure. These less stringent testing criteria make it more likely that less severe cases were captured. However, we expect to still miss persons who were asymptomatic, sought testing outside of the REP health care partners, or did not seek testing for other reasons. Therefore, hospitalization and death rates may still represent an overestimate of the risk of severe infection in the entire population.
An additional limitation of our study is the inability to verify the ICD codes for this study; ICD codes may be assigned in error (overdiagnosis), and manual review of the medical records is often needed to determine whether a person truly has the disease or condition of interest. We also may have missed people who should have been assigned a code of interest but were not (underdiagnosis). However, because we limited our diagnosis categories to those that are considered chronic conditions, most patients would be seen at least once within the 5-year period we considered for this study, making it unlikely that we missed conditions that require medical treatment. This approach may have caused us to include persons in the chronic disease categories who have recovered from the disease or who have well-controlled chronic conditions. Therefore, we conducted a sensitivity analysis in which we only included chronic conditions that occurred in the 18 months before COVID-19 infection. The number of persons with the chronic conditions declined; however, point estimates were very similar, suggesting that the use of a 5-year window for capturing chronic conditions did not significantly bias our estimates (Supplemental Table 3, available online at http://mayoclinicproceedings.org). Finally, there were 1391 persons with less than 6 months of information between the date of first visit in the REP system and their COVID-19 diagnosis. We may underestimate chronic conditions in this population, and inclusion of these persons in our unexposed group may have biased our study results. We conducted a sensitivity analysis excluding these persons and found that results were again largely unchanged (Supplemental Table 3).
The chronic condition categories that we examined were broad, and some categories included disparate conditions (eg, “Cancer” included all cancers). In addition, the conditions we considered are not all equivalent in severity, and using a simple count of conditions to identify persons with a high disease burden may overestimate disease severity in persons with a high number of less serious conditions. However, our approach is useful as a way to examine a wide range of conditions and to screen for groups of conditions that may increase the risk of severe infection. However, this approach will miss conditions that are important risk factors for severe infection if there were not a sufficient number persons with those conditions within the categories that we studied. Similarly, there were too few severe cases in persons 0 to 19 years of age to study this population as a separate group, and a larger sample size is needed to identify all risk factors in this age group.
Finally, PCR tests for COVID-19 sometimes produce inaccurate results, and we may have missed some infections (false negatives), and we may have incorrectly identified some persons as infected who were not truly infected (false positives). Poor sensitivity will result in an underestimation of all cases, and poor specificity will result in an overestimation of all cases. If demographic and clinical characteristics routinely affect sensitivity and specificity, our results may be biased. A range of PCR tests has been used in this community, and accurate sensitivity and specificity data are not currently available for all tests. Therefore, it is difficult to determine in which direction-testing variability may have biased our study results.
Conclusion
In a large Midwestern US population, we found that older age, male sex, minority ethnicity or race, obesity, and a wide range of chronic conditions were significantly associated with the risk of severe COVID-19 infection. We also found that the risk of severe COVID-19 infection associated with many of the chronic conditions differed by age group, and younger persons in our cohort with these conditions were at especially high risk for severe infection compared with persons of the same age. Older persons are much more likely to experience severe infections; however, severe cases do occur in younger persons as well. Our data provide insight regarding younger persons at especially high risk of severe COVID-19 infection.
Acknowledgments
We thank Kristi Klinger for assistance with preparation of the manuscript.
J.L.S.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. J.L.S.S., G.S.L., W.A.R., D.J.J., and C.M.V. were responsible for the concept and design. J.L.S.S., G.S.L., K.P., M.R.M., D.J.J., C.F., R.M.J., and L.J.R. were responsible for the acquisition, analysis, and interpretation of data. J.L.S.S., G.S.L., W.A.R., D.J.J., and C.M.V. drafted the manuscript. All authors were responsible for the critical revision of the manuscript for important intellectual content. G.S.L., D.J.J., and C.F. were responsible for statistical analysis. J.L.S.S, A.H.L., and C.M.V. obtained funding; and administrative, technical, and material support was provided by K.P., M.R.M., A.H.L., and A.D.N. Supervision was provided by J.L.S.S., D.J.J., A.D.N., and C.M.V.
Footnotes
For editorial comment, see page 2508
Grant Support: This study was supported by the Mayo Clinic COVID-19 Research Fund, the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, the Mayo Clinic Division of Epidemiology, and the Rochester Epidemiology Project (NIA AG 058738). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The contents do not represent the views of the Mayo Clinic or of the National Institute on Aging.
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.World Health Organization Timeline of WHO's response to COVID-19. December, 2020. https://www.who.int/news/item/29-06-2020-covidtimeline
- 2.Chen T., Dai Z., Mo P. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75(9):1788–1795. doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo W., Li M., Dong Y. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24(1):108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang W., Liang H., Ou L. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu L., Chen S., Fu Y. Risk factors associated with clinical outcomes in 323 COVID-19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020;71(16):2089–2098. doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galloway J.B., Norton S., Barker R.D. A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: an observational cohort study. J Infect. 2020;81:282–288. doi: 10.1016/j.jinf.2020.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krittanawong C., Virk H.U.H., Narasimhan B. Coronavirus disease 2019 (COVID-19) and cardiovascular risk: a meta-analysis. Prog Cardiovasc Dis. 2020;63(4):527–528. doi: 10.1016/j.pcad.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R., Liang W., Jiang M. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klang E., Kassim G., Soffer S., Freeman R., Levin M.A., Reich D.L. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020;28(9):1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lighter J., Phillips M., Hochman S. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrilli C.M., Jones S.A., Yang J. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19: studies needed. N Engl J Med. 2020;382(13):1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 17.Rocca W.A., Grossardt B.R., Brue S.M. Data resource profile: expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP) Int J Epidemiol. 2018;47(2) doi: 10.1093/ije/dyx268. 368–368j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rocca W.A., Yawn B.P. St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Clinical Growth Charts. 2017. https://www.cdc.gov/growthcharts/clinical_charts.htm
- 20.Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP). Clinical Classifications Software Refined (CCSR) for ICD-10-CM Diagnoses. November, 2020. https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
- 21.Mukherjee B., Ou H.T., Wang F., Erickson S.R. A new comorbidity index: the health-related quality of life comorbidity index. J Clin Epidemiol. 2011;64(3):309–319. doi: 10.1016/j.jclinepi.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 22.St. Sauver J.L., Warner D.O., Yawn B.P. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88(1):56–67. doi: 10.1016/j.mayocp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 24.Garg S., Kim L., Whitaker M. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019: COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tenforde M.W., Billig Rose E., Lindsell C.J. Characteristics of adult outpatients and inpatients with COVID-19: 11 academic medical centers, United States, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):841–846. doi: 10.15585/mmwr.mm6926e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chudasama Y.V., Gillies C.L., Appiah K. Multimorbidity and SARS-CoV-2 infection in UK Biobank. Diabetes Metab Syndr. 2020;14(5):775–776. doi: 10.1016/j.dsx.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marengoni A., Zucchelli A., Vetrano D.L. Beyond chronological age: frailty and multimorbidity predict in-hospital mortality in patients with coronavirus disease 2019. J Gerontol A Biol Sci Med Sci. 2020;76(3):e38–e45. doi: 10.1093/gerona/glaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maddaloni E., D'Onofrio L., Alessandri F. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II) Cardiovasc Diabetol. 2020;19(1):164. doi: 10.1186/s12933-020-01140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iaccarino G., Grassi G., Borghi C. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian Society of Hypertension. Hypertension. 2020;76(2):366–372. doi: 10.1161/HYPERTENSIONAHA.120.15324. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez-Vasquez A., Azanedo D., Vargas-Fernandez R., Bendezu-Quispe G. Association of comorbidities with pneumonia and death among COVID-19 patients in Mexico: a nationwide cross-sectional study. J Prev Med Public Health. 2020;53(4):211–219. doi: 10.3961/jpmph.20.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crooke S.N., Ovsyannikova I.G., Poland G.A., Kennedy R.B. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu H.E., Ashe E.M., Silverstein M. Race/ethnicity, underlying medical conditions, homelessness, and hospitalization status of adult patients with COVID-19 at an urban safety-net medical center: Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):864–869. doi: 10.15585/mmwr.mm6927a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention COVIDView: A weekly surveillance summary of US COVID-19 activity (week 34) August, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-08-28-2020.pdf
- 34.Hamer M., Gale C.R., Kivimaki M., Batty G.D. Overweight, obesity, and risk of hospitalization for COVID-19: a community-based cohort study of adults in the United Kingdom. Proc Natl Acad Sci USA. 2020;117(35):21011–21013. doi: 10.1073/pnas.2011086117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamer M., Kivimaki M., Gale C.R., Batty G.D. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang F., Xiong Y., Wei Y. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol. 2020;92(11):2536–2542. doi: 10.1002/jmv.26039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Killerby M.E., Link-Gelles R., Haight S.C. Characteristics associated with hospitalization among patients with COVID-19: Metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):790–794. doi: 10.15585/mmwr.mm6925e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Q.W., Meng M., Kumar R. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020;92(10):1915–1921. doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.