Abstract
Dysregulation of the Wnt pathway causes various diseases including cancer, Parkinson’s disease, Alzheimer’s disease, schizophrenia, osteoporosis, obesity and chronic kidney diseases. The modulation of dysregulated Wnt pathway is absolutely necessary. In the present study, we evaluated the anti-inflammatory effect and the mechanism of action of Wnt-C59, a Wnt signaling inhibitor, in lipopolysaccharide (LPS)-stimulated epithelial cells and macrophage cells. Wnt-C59 showed a dose-dependent anti-inflammatory effect by suppressing the expression of proinflammatory cytokines including IL6, CCL2, IL1A, IL1B, and TNF in LPS-stimulated cells. The dysregulation of the Wnt/β-catenin pathway in LPS stimulated cells was suppressed by Wnt-C59 treatment. The level of β-catenin, the executor protein of Wnt/β-catenin pathway, was elevated by LPS and suppressed by Wnt-C59. Overexpression of β-catenin rescued the suppressive effect of Wnt-C59 on proinflammatory cytokine expression and nuclear factor-kappa B (NF-κB) activity. We found that the interaction between β-catenin and NF-κB, measured by co-immunoprecipitation assay, was elevated by LPS and suppressed by Wnt-C59 treatment. Both NF-κB activity for its target DNA binding and the reporter activity of NF-κB-responsive promoter showed identical patterns with the interaction between β-catenin and NF-κB. Altogether, our findings suggest that the anti-inflammatory effect of Wnt-C59 is mediated by the reduction of the cellular level of β-catenin and the interaction between β-catenin and NF-κB, which results in the suppressions of the NF-κB activity and proinflammatory cytokine expression.
Keywords: Catenins, Cytokines, Inflammation, NF-kappa B
INTRODUCTION
The Wnt pathway plays essential roles in the regulation of cellular proliferation, differentiation and tissue hemostasis [1]. Dysregulation of the Wnt pathway, however, causes various diseases, including cancer [2], Parkinson’s disease [3], Alzheimer’s disease [4], schizophrenia [5], osteoporosis [6], obesity [7] and chronic kidney diseases [8]. Among these, most studies on the Wnt pathway have focused on its role in cancer [9]: The overactivation of the Wnt pathway constitutes the hallmarks of many types of cancers [10], and Wnt signaling is responsible for the generation of cancer stem cells in many human tumors [11]. Accordingly, numerous compounds targeting the Wnt pathway have been developed as candidate anticancer drugs [9].
Wnt-C59, a Wnt signaling inhibitor developed as an anti-cancer drug candidate, was reported to inhibit the acylation of Wnt, which is required for its secretion [12]. Wnt-C59 showed anti-cancer activity by suppressing the proliferation of various tumor cells [11,13,14]. It also suppressed the growth of nasopharyngeal carcinoma in mice by inhibiting Wnt signaling in the tumor microenvironment [11], and reduces tumor growth in a xenograft mouse model of glioma [13]. The modulation of dysregulated Wnt pathway in other diseases is also necessary, and Wnt-C59 may be a candidate. Kidney-protective effect of Wnt-C59 has been reported: Wnt-C59 was found to attenuate kidney fibrosis in a murine ureteral obstruction model [15], and suppress kidney injury caused by ischemia-reperfusion [16]. Until now, however, the anti-inflammatory effect of Wnt-C59 has not been evaluated.
Recently, it has been reported that Wnt pathway is involved in inflammation: β-Catenin, the executor protein of Wnt pathway was found to interact with nuclear factor-kappa B (NF-κB), the major proinflammatory transcription factor [17-19]. β-Catenin may activate and repress NF-κB depending on the context such as cell and tissue types and the kinds of inflammatory stimulators [20]. The interaction between β-catenin and NF-κB has not fully elucidated during lipopolysaccharide (LPS)-induced inflammation in various cells.
In the present study, we evaluated the anti-inflammatory effect of Wnt-C59 along with its effect on the interaction between β-catenin and NF-κB in LPS-stimulated epithelial cells and macrophage cells.
METHODS
Cell culture and reagents
BEAS-2B human bronchial epithelial cells (Cat. No. CRL-9609) and RAW264.7 murine macrophage cells (Cat. No. TIB-71) were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 μg/ml streptomycin and 100 units/ml penicillin at 37°C in a 5% CO2 atmosphere. Human umbilical vein endothelial cells (Cat. No. CRL-1730) were purchased from ATCC and cultured in Vascular Cell Basal Medium (ATCC; Cat. No. PCS-100-300) supplemented with the Endothelial Cell Growth Kit (ATCC; Cat. No. PCS 100-041).
Anti-LRP6 (Cat. No. #3395, 1:1,000), anti-phospho LRP6 (p-LRP6) (Ser 1490) (Cat. No. #2568, 1:500), anti-Axin (Cat. No. #2087, 1:1,000), anti-GSK-3β (Cat. No. #9315, 1:1,000), anti-phospho GSK-3β (p-GSK-3β) (Ser 9) (Cat. No. #9336, 1:1,000), anti-phospho β-catenin (p-β-catenin) (Ser 33/37) (Cat. No. #2009, 1:500), and Anti-NF-κB p65 (Cat. No. #13681, 1:1,000) primary antibodies and secondary antibodies (anti-mouse; Cat. No. #7076 , 1:1,000 and anti-rabbit; Cat. No. #7074, 1:1,000) were obtained from Cell Signaling Technologies Inc. (Beverly, MA, USA). Anti-β-catenin (Cat. No. 610153, 1:1,000) antibody was purchased from BD Transduction Laboratories Inc. (Lexington, KY, USA). Anti-β-actin (Cat. No. sc-47778, 1:1,000) antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). LPS from Klebsiella pneumoniae (Cat. No. L4268) was purchased from Sigma (St. Louis, MO, USA). Wnt-C59 (Cat. No. hy-15659) was from Med Chem Express (Monmouth Junction, NJ, USA).
Quantitative reverse-transcription (RT) qPCR
Total RNA was isolated using a RNeasy Kit (Cat. No. 74106; Qiagen, Hilden, Germany). One microgram of RNA was reverse transcribed using a cDNA Reverse Transcription kit (Cat. No. 43-688-13; Applied Biosystems Inc., Foster City, CA, USA). RT-qPCR reactions were conducted as described previously [21]. Assay-on-Demand Gene Expression Products (Applied Biosystems) were used for RT-qPCR reactions of IL6 (Cat. No. Hs00174131_m1), CCL2 (Cat. No. Hs00234140_m1), IL1A (Cat. No. Hs00174092_m1), IL1B (Cat. No. Hs01555410_m1), TNF (Cat. No. Hs01113624_g1) and 18S rRNA (Cat. No. Hs99999901_s1) in BEAS-2B human bronchial epithelial cells and human umbilical vein endothelial cells; IL6 (Cat. No. Mm00446190_m1), CCL2 (Cat. No. Mm00441242_m1), IL1A (Cat. No. Mm00439620_m1), IL1B (Cat. No. Mm00434228_m1), TNF (Cat. No. Mm00443258_m1), and 18S ribosomal RNA (Cat. No. Hs99999901_s1) were used in RAW 264.7 murine macrophage cells. For each experimental groups, the mRNA levels were normalized against the 18S ribosomal RNA level and the ratios of normalized mRNA in each groups were compared to that of an untreated group using the comparative Ct method [22].
Cell viability test
The viability of cell was tested by a Cell CountEZ Cell Survival Assay Kit (Cat. No. RKKLD-001, Rockland Immunochemicals, Limerick, PA, USA), which measure the capability of viable mammalian cells to convert hydroxyethyl disulfide into β-mercaptoethanol [23].
Cell lysate preparation and Western blotting
Cells were lysed using RIPA buffer containing 25 mM Tris–HCl (pH 7.6), 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and protease inhibitor cocktail (Cat. No. P8340, Sigma) for 30 min at 4°C. Total cell lysates were obtained after removing the insoluble materials by centrifugation at 20,000 × g for 20 min at 4°C. Western blotting experiment was conducted as described previously [21]. The protein band intensities of the western blots were quantified by ImageJ (NIH, Bethesda, MD, USA).
β-Catenin overexpression
Cells were seeded in 12 well plates at 5 × 105 cells/well, and, on the following day, were transfected with control vector plasmid or β-catenin overexpressing plasmid [24] using lipofectamine 2000 reagent (Cat. No. 11668027; Invitrogen, Carlsbad, CA, USA). After 24 h, cells were stimulated with LPS with or without Wnt-C59 for more analyses.
Measurement of NF-κB activity
Nuclear lysates were collected from cells using a nuclear extraction kit (Cat. No. 40010; Active Motif, Carlsbad, CA, USA). The binding activity of NF-κB to its target DNA sequence (5'-GGGACTTTCC-3’) was measured using a TransAM NF-κB ELISA kit (Cat. No. 40096, Active Motif). Briefly, 10 μg of nuclear protein extract was added to a 96 well plate coated with oligonucleotides containing the target DNA sequence. After incubation and washing, an anti-NF-κB antibody was added to the wells, followed by sequential additions of horseradish peroxidase-conjugated secondary antibody, peroxidase substrate solution and stop solution provided by the kit.
Co-immunoprecipitation of β-catenin and NF-κB
Co-immunoprecipitation assay was performed using an immunoprecipitation kit (Cat. No. K286; BioVision, Mountain View, CA, USA) according to the manufacturer’s instruction. Briefly, cells were lysed with the non-denaturing lysis buffer provided in the kit; the cell lysate was supplemented with 2 μg/ml anti-β-catenin antibody or anti-NF-κB antibody, and incubated overnight at 4°C. After the addition of protein-A/G bead slurry and incubation, beads were collected by centrifugation at 2,000 × g for 2 min. The collected beads were washed thrice with the wash buffer provided in the kit and mixed with 2× SDS PAGE loading buffer. The amount of NF-κB and β-catenin bound to the beads was measured using Western blotting. Protein band intensities were quantified using ImageJ.
Reporter assay of NF-κB-responsive promoter
Cells were co-transfected with a pGL 4.32 vector (Cat. No. E8491; Promega, Madison, WI, USA) containing the NF-κB response element linked to a firefly luciferase reporter gene and a 1:50 ratio of pGL 4.17 vector (Cat. No. E6721, Promega) containing the Renilla luciferase reporter gene. In the following day, cells were harvested after Wnt-C59 treatment and LPS stimulation, and luciferase activities were measured using a Dual-Luciferase Reporter Assay System (Cat. No. E1910, Promega). For each assay, the activity of firefly luciferase was normalized to that of Renilla luciferase to control for variations in transfection efficiency.
Statistical analysis
All data are expressed as the means ± standard deviation of three replicate experiments. Statistically significant differences between experimental groups were detected using unpaired t-tests, and p-values less than 0.05 were considered to be significant. All analyses were performed using SPSS ver. 14 (SPSS, Chicago, IL, USA).
RESULTS
Wnt-C59 suppressed LPS-induced proinflammatory cytokine expression in epithelial cells, endothelial cells and macrophage cells
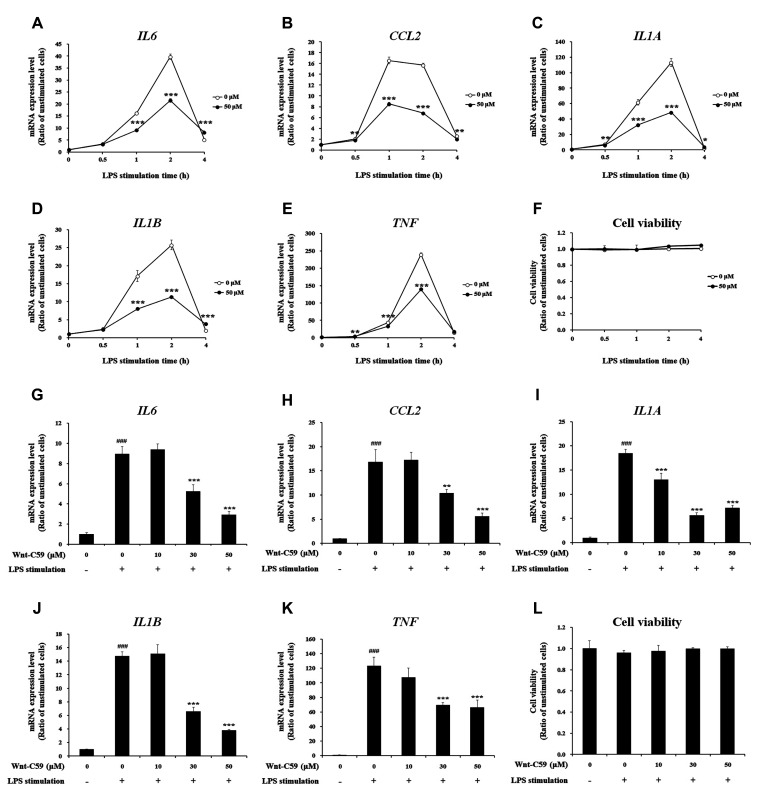
When BEAS-2B human bronchial epithelial cells were stimulated with LPS for 0.5 to 4 h, the mRNA levels of proinflammatory cytokines, such as IL6, CCL2, IL1A, IL1B, and TNF, were markedly elevated. Maximum mRNA expression of proinflammatory cytokines were observed after 2 h of LPS stimulation (Fig. 1A–E). We found that LPS-induced proinflammatory cytokine expression was significantly suppressed by Wnt-C59 treatment. However, the cell viability was not affected by Wnt-C59 (Fig. 1F), showing that the reduction of the proinflammatory cytokine mRNA levels by Wnt-C59 was not mediated by the decreased cell viability. When BEAS-2B cells were stimulated with LPS for 2 h with various concentrations of Wnt-C59, the proinflammatory cytokine mRNA level was suppressed in a dose dependent manner (Fig. 1G–K). Wnt-C59 showed no cytotoxicity (Fig. 1L). We found that Wnt-C59 also significantly suppressed proinflammatory cytokine mRNA expressions with no effect on cell viability in LPS-stimulated human umbilical vein endothelial cells (Supplementary Data 1).
Fig. 1. Suppressive effect of Wnt-C59 on lipopolysaccharide (LPS)-stimulated proinflammatory cytokine expressions in BEAS-2B human bronchial epithelial cells.
(A–F) Cells were treated with 0 or 50 μM of Wnt-C59, followed by LPS stimulation at 0.1 μg/ml for various time periods of 0.5 to 4 h. (A–E) Messenger RNA levels of proinflammatory cytokines were measured by RT-qPCR. (F) Cell viability was measured. Cells treated with 0 or 50 μM of Wnt-C59 with the same time period of LPS stimulation were compared. *p < 0.05, **p < 0.01, ***p < 0.001. (G–L) Cells were treated with 0 to 50 μM of Wnt-C59, followed by LPS stimulation at 0.1 μg/ml for 2 h. (G–K) Messenger RNA levels of proinflammatory cytokines were measured by RT-qPCR. (L) Cell viability was measured. **p < 0.01, ***p < 0.001 compared with cells stimulated with LPS with 0 μM of Wnt-C59. ###p < 0.001 compared with unstimulated cells. Experiments were conducted in triplicate. Data are shown as mean ± standard deviation, and statistical significance was measured by unpaired t-test.
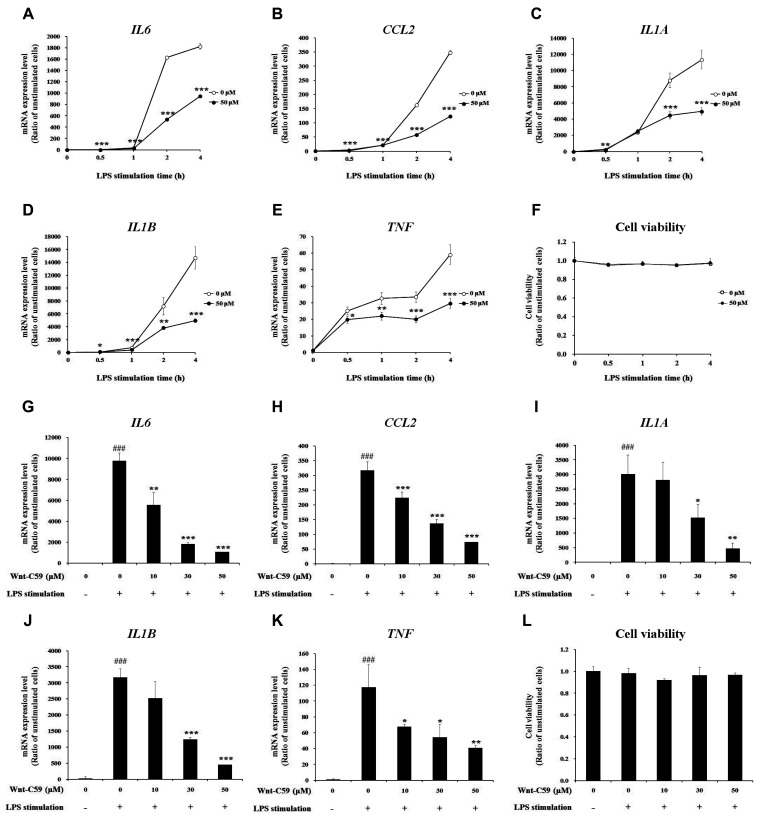
The anti-inflammatory effect of Wnt-C59 was confirmed in RAW264.7 murine macrophage cells. Stimulation of RAW264.7 murine macrophage cells with LPS for 0.5 to 4 h resulted in elevations of the proinflammatory cytokine mRNA levels which were significantly suppressed by treatment of 50 μM Wnt-C59 with no effect on cell viability (Fig. 2A–F). In turn, treatment with 0 to 50 μM of Wnt-C59 suppressed the LPS-induced proinflammatory cytokine expression in a dose-dependent manner with no effect on cell viability (Fig. 2G–L). Our data showed that Wnt-C59 has anti-inflammatory effects in various cell types such as epithelial, endothelial and macrophage cells.
Fig. 2. Suppressive effect of Wnt-C59 on lipopolysaccharide (LPS)-induced proinflammatory cytokine expression in RAW264.7 murine macrophage cells.
(A–F) Cells were treated with 0 or 50 μM of Wnt-C59, followed by LPS stimulation at 0.1 μg/ml for various time periods of 0.5 to 4 h. (A–E) Messenger RNA levels of proinflammatory cytokines were measured by RT-qPCR. (F) Cell viability was measured. Cells treated with 0 or 50 μM of Wnt-C59 with the same time period of LPS stimulation were compared. *p < 0.05, **p < 0.01, ***p < 0.001. (G–L) Cells were treated with 0 to 50 μM of Wnt-C59, followed by LPS stimulation at 0.1 μg/ml for 4 h. (G–K) Messenger RNA levels of proinflammatory cytokines were measured by RT-qPCR. (L) Cell viability was measured. *p < 0.05, **p < 0.01, ***p < 0.001 compared with cells stimulated with LPS with 0 μM of Wnt-C59. ###p < 0.001 compared with unstimulated cells. Experiments were conducted in triplicate. Data are shown as mean ± standard deviation, and statistical significance was measured by unpaired t-test.
Wnt-C59 suppressed Wnt/β-catenin signaling activated by LPS stimulation
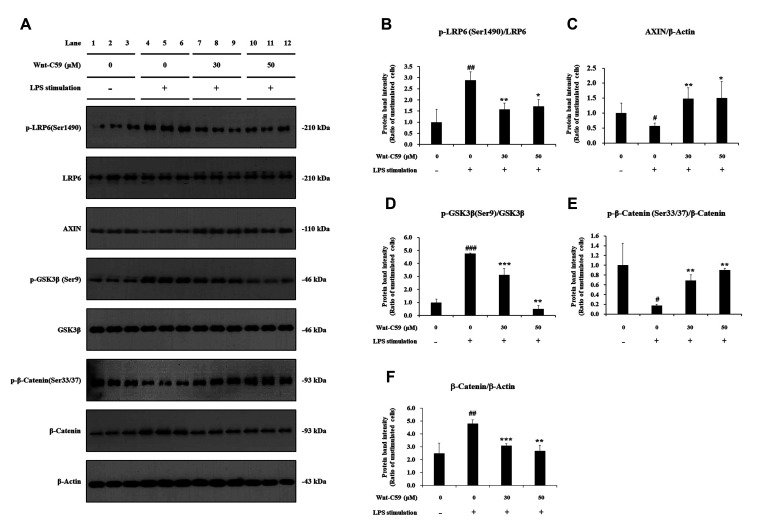
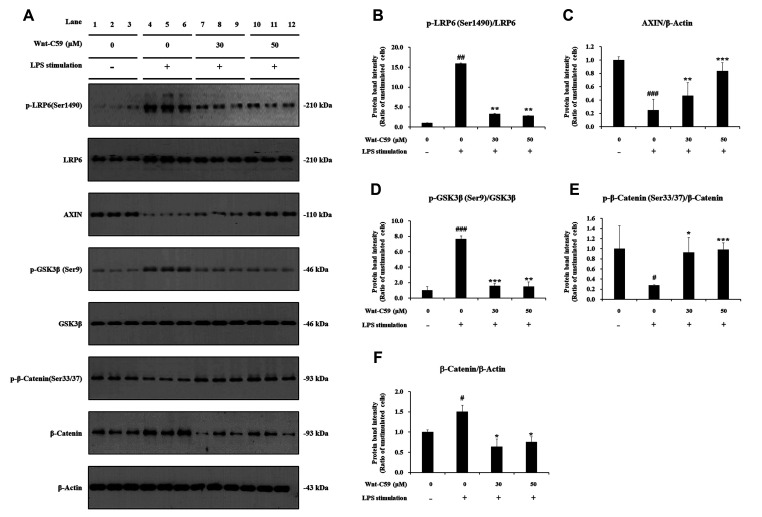
LPS-stimulated BEAS-2B human bronchial epithelial cells were treated with 0, 30, or 50 μM of Wnt-C59, then major proteins of the Wnt/β-catenin pathway were analyzed by Western blotting (Fig. 3A). Original uncut western blot images are shown in Supplementary Data 2. Our data showed that LPS stimulation activated Wnt/β-catenin signaling: LPS significantly increased the level of phospho(p)-LRP6 and p-GSK3β, which are positive regulators of Wnt signaling (Fig. 3B, D), whereas that of AXIN (Axis Inhibition Protein 1), a negative regulator of Wnt signaling, was significantly reduced by LPS (Fig. 3C) compared with untreated control cells. LPS induced significant reduction of phosphorylated β-catenin (p-β-catenin) (Fig. 3E), and elevation of the protein level of β-catenin, the executor protein of Wnt/β-catenin pathway (Fig. 3F). The activation of Wnt/β-catenin pathway by LPS was also found in RAW264.7 murine macrophage cells (Fig. 4A): LPS stimulation increased the levels of p-LRP6, p-GSK3β and β-catenin, but decreased those of AXIN and p-β-catenin (Fig. 4B–F).
Fig. 3. Suppressive effect of Wnt-C59 on lipopolysaccharide (LPS)-induced activation of the Wnt/β-catenin pathway in BEAS-2B human bronchial epithelial cells.
Cells were treated with 0, 30, or 50 μM of Wnt-C59, followed by LPS stimulation at 0.1 μg/ml for 2 h. (A) Cellular protein levels were measured by Western blotting. Original uncut Western blot images were shown in Supplementary Data 2. β-Actin was used an equal loading control. (B–F) Protein band intensities were quantified using ImageJ. *p < 0.05, **p < 0.01, ***p < 0.001 compared with cells stimulated with LPS with 0 μM of Wnt-C59. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with unstimulated cells. Experiments were conducted in triplicate. Data are shown as mean ± standard deviation, and statistical significance was measured by unpaired t-test. p-LRP6, anti-phospho LRP6; p-GSK-3β, anti-phospho GSK-3β; p-β-catenin, anti-phospho β-catenin.
Fig. 4. Suppressive effect of Wnt-C59 on lipopolysaccharide (LPS)-induced activation of the Wnt/β-catenin pathway in RAW264.7 murine macrophage cells.
Cells were treated with 0, 30, or 50 μM of Wnt-C59, followed by LPS stimulation at 0.1 μg/ml for 4 h. (A) Cellular protein levels were measured by Western blotting. Original uncut Western blot images were shown in Supplementary Data 2. β-Actin was used an equal loading control. (B–F) Protein band intensities were quantified using ImageJ. *p < 0.05, **p < 0.01, ***p < 0.001 compared with cells stimulated with LPS with 0 μM of Wnt-C59. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with unstimulated cells. Experiments were conducted in triplicate. Data are shown as mean ± standard deviation, and statistical significance was measured by unpaired t-test. p-LRP6, anti-phospho LRP6; p-GSK-3β, anti-phospho GSK-3β; p-β-catenin, anti-phospho β-catenin.
Our data showed that the Wnt/β-catenin pathway activated by LPS was suppressed by Wnt-C59 in both human bronchial epithelial cells and murine macrophage cells: Wnt-C59 reduced p-LRP6 (Figs. 3B and 4B) and increased AXIN (Figs. 3C and 4C). GSK3β, a kinase which phosphorylates Ser 33/37 residues of β-catenin, is known to be inactivated by its Ser 9 phosphorylation [25,26]. Our data showed that Wnt-C59 reduced the Ser 9 phosphorylation of GSK3β (Figs. 3D and 4D), leading to the increase of Ser 33/37 phosphorylation of β-catenin (Figs. 3E and 4E). β-Catenin, phosphorylated at Ser 33/37 residues is degraded via a destruction complex containing AXIN [25,26]. Wnt-C59 increased both Ser 33/37 phosphorylation of β-catenin (Figs. 3E and 4E) and level of AXIN (Figs. 3C and 4C), which resulted in the reduction in the level of β-catenin (Figs. 3F and 4F). These data demonstrated that LPS-activated Wnt/β-catenin pathway was suppressed by Wnt-C59, resulting in the reduction of β-catenin, the executor protein of Wnt/β-catenin pathway.
Anti-inflammatory effects of Wnt-C59 were rescued by β-catenin overexpression
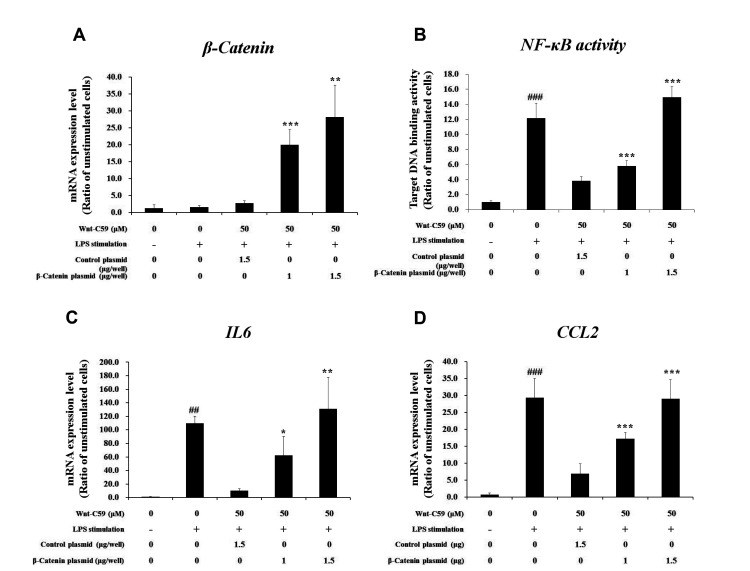
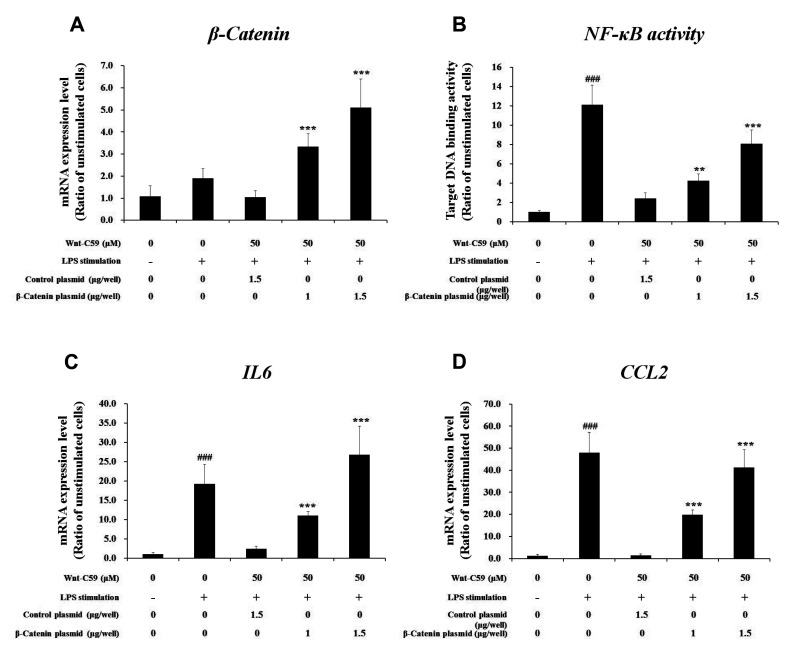
To confirm that anti-inflammatory effects of Wnt-C59 (shown in Figs. 1 and 2) were mediated by the inhibition of Wnt/β-catenin pathway (shown in Figs. 3 and 4), we conducted β-catenin overexpression experiments. We transfected human epithelial or murine macrophage cells with control vector plasmid or β-catenin overexpressing plasmid. Measurement of β-catenin mRNA level showed that transfection was well conducted (Fig. 5A). In the same experimental condition, we measured the target DNA binding activity of NF-κB, a major proinflammatory transcription factor responsible for the expression of proinflammatory cytokines. Our results showed that the NF-κB activity for its target DNA binding , which was suppressed by Wnt-C59, was rescued by β-catenin overexpression (Fig. 5B) in human bronchial epithelial cells, which suggested us that β-catenin might play a role in the regulation of NF-κB activity. LPS-stimulated proinflammatory cytokine expressions, which were suppressed by Wnt-C59, were also rescued by β-catenin overexpression in human bronchial epithelial cells (Fig. 5C, D). Same results were observed in β-catenin overexpression experiments conducted in murine macrophage cells (Fig. 6). These results confirmed that anti-inflammatory effects of Wnt-C59 are dependent on its modulations of the cellular β-catenin level.
Fig. 5. Rescuing effects of β-catenin overexpression on anti-inflammatory effects of Wnt-C59 in BEAS-2B human bronchial epithelial cells.
Cells grown in 12 well plate were transfected with control vector plasmid (1.5 μg/well) or β-catenin overexpressing plasmid (1.0 or 1.5 μg/well). After one day, cells were treated with 50 μM of Wnt-C59, followed by lipopolysaccharide (LPS) stimulation at 0.1 μg/ml for 2 h. (A) Messenger RNA level of β-catenin were measured by RT-qPCR. (B) Nuclear factor-kappa B (NF-κB) activity for target DNA binding was measured by ELISA. (C, D) Messenger RNA levels of proinflammatory cytokines were measured by RT-qPCR. *p < 0.05, **p < 0.01, ***p < 0.001 compared with cells transfected with control plasmid. ##p < 0.01, ###p < 0.001 compared with unstimulated cells. Experiments were conducted in triplicate. Data are shown as mean ± standard deviation, and statistical significance was measured by unpaired t-test.
Fig. 6. Rescuing effects of β-catenin overexpression on anti-inflammatory effects of Wnt-C59 in RAW264.7 murine macrophage cells.
Cells grown in 12 well plate were transfected with control vector plasmid (1.5 μg/well) or β-catenin overexpressing plasmid (1.0 or 1.5 μg/well). After one day, cells were treated with 50 μM of Wnt-C59, followed by lipopolysaccharide (LPS) stimulation at 0.1 μg/ml for 4 h. (A) Messenger RNA level of β-catenin were measured by RT-qPCR. (B) Nuclear factor-kappa B (NF-κB) activity for target DNA binding was measured by ELISA. (C, D) Messenger RNA levels of proinflammatory cytokines were measured by RT-qPCR. **p < 0.01, ***p < 0.001 compared with cells transfected with control plasmid. ###p < 0.001 compared with unstimulated cells. Experiments were conducted in triplicate. Data are shown as mean ± standard deviation, and statistical significance was measured by unpaired t-test.
Wnt-C59 reduced the protein–protein interaction between β-catenin and NF-κB along with the activities of NF-κB and its responsive promoter
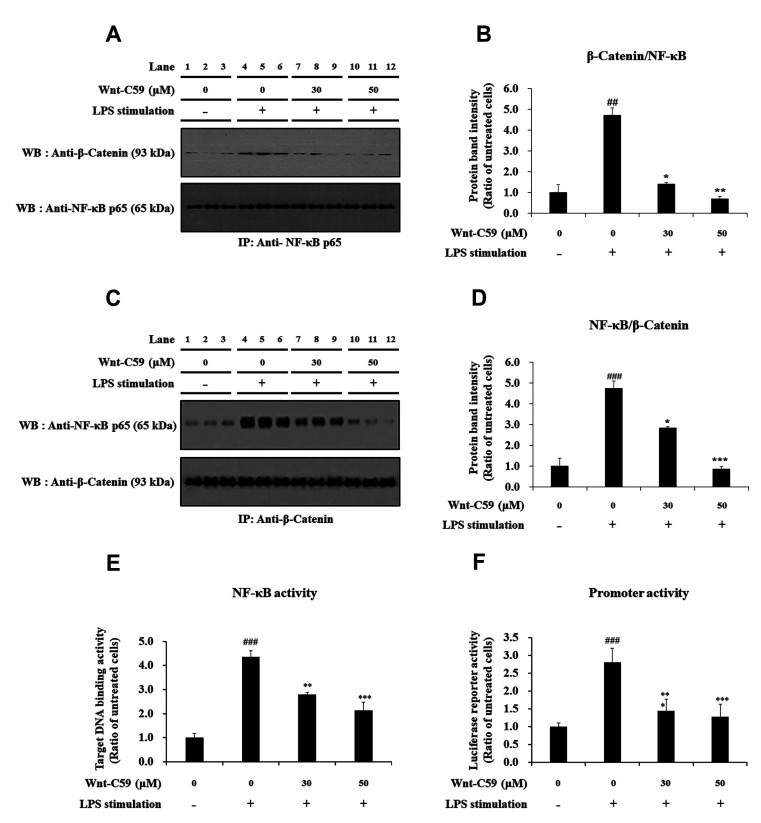
To find the regulatory role of β-catenin on NF-κB, we analyzed the protein–protein interaction between β-catenin and NF-κB via co-immunoprecipitation assays in human bronchial epithelial cells (Fig. 7A–D). The amount of β-catenin co-immunoprecipitated with NF-κB was increased by LPS-stimulation and reduced by Wnt-C59 treatment (Fig. 7A, B). Input data of the co-immunoprecipitation experiments were shown in Supplementary Data 3. The comparison of Fig. 7A and Supplementary Data 3 suggested that the amount of β-catenin bound to NF-κB is proportional to the β-catenin level in the cells. The amount of NF-κB co-immunoprecipitated with β-catenin showed same pattern with that of β-catenin co-immunoprecipitated with NF-κB (Fig. 7C, D). These results showed that protein-protein interaction between β-catenin and NF-κB was elevated by LPS stimulation and reduced by Wnt-C59 treatment. In the same experimental condition, NF-κB activity for its target DNA binding (Fig. 7E) and reporter activity of NF-κB-responsive promoter (Fig. 7F) were also elevated by LPS and reduced by Wnt-C59.
Fig. 7. Suppressive effect of Wnt-C59 on the interaction between β-catenin and NF-κB along with the activities of Nuclear factor-kappa B (NF-κB) and its responsive promoter in BEAS-2B human bronchial epithelial cells.
Cells were treated with 0, 30, or 50 μM of Wnt-C59, followed by lipopolysaccharide (LPS) stimulation at 0.1 μg/ml for 2 h. (A, C) The protein-protein interaction between β-catenin and NF-κB p65 was analyzed by co-immunoprecipitation assays followed by Western blotting. Original uncut Western blot images were shown in Supplementary Data 2. Input samples of co-immunoprecipitation assays are shown in Supplementary Data 3. (B, D) The amount of co-immunoprecipitated β-catenin or NF-κB p65 was measured by ImageJ. (E) NF-κB activity for its target DNA binding was measured by ELISA. (F) Transcriptional activity of NF-κB-responsive promoter was measured by luciferase reporter assay. *p < 0.05, **p < 0.01, ***p < 0.001 compared with cells stimulated with LPS with 0 μM of Wnt-C59. ##p < 0.01, ###p < 0.001 compared with unstimulated cells. Experiments were conducted in triplicate. Data are shown as mean ± standard deviation, and statistical significance was measured by unpaired t-test. IP, immunoprecipitation; WB, Western blot.
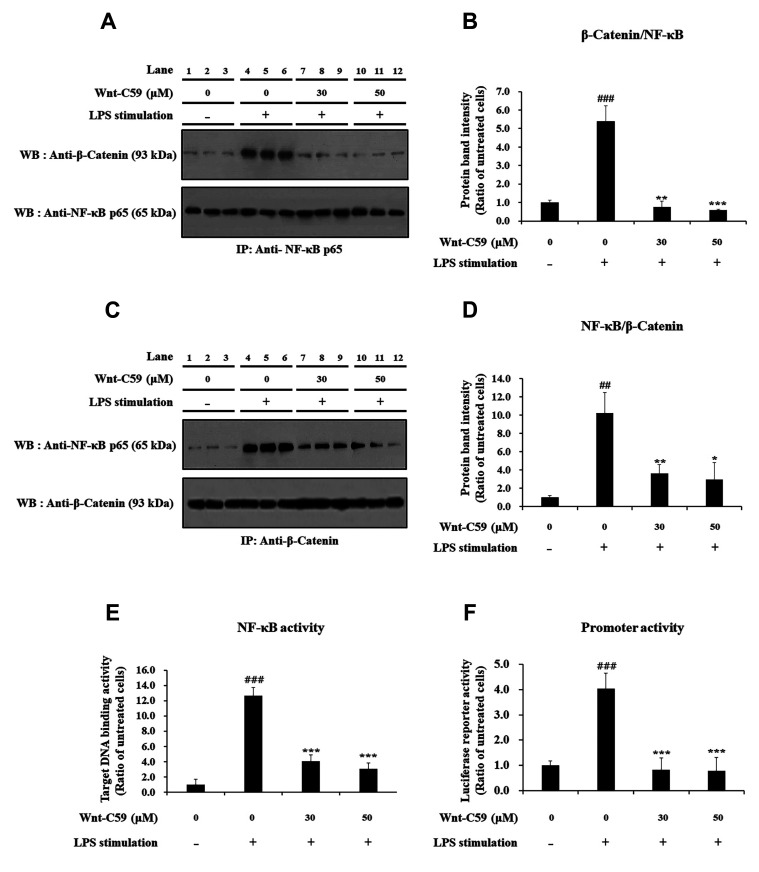
The protein–protein interaction between β-catenin and NF-κB was confirmed in LPS-stimulated murine macrophage cells: The protein-protein interaction between β-catenin and NF-κB (Fig. 8A–D) showed identical patterns with NF-κB activity for target DNA binding (Fig. 8E) and reporter activity of NF-κB-responsive promoter (Fig. 8F). These results suggest that the protein-protein interaction between β-catenin and NF-κB may modulate the target DNA binding of NF-κB and transcriptional activity of a NF-κB-responsive promoter.
Fig. 8. Suppressive effect of Wnt-C59 on the interaction between β-catenin and Nuclear factor-kappa B (NF-κB) along with the activities of NF-κB and its responsive promoter in RAW264.7 murine macrophage cells.
Cells were treated with 0, 30, or 50 μM of Wnt-C59, followed by lipopolysaccharide (LPS) stimulation at 0.1 μg/ml for 4 h. (A, C) The protein-protein interaction between β-catenin and NF-κB p65 was analyzed by co-immunoprecipitation assays followed by Western blotting. Original uncut western blot images were shown in Supplementary Data 2. Input samples of co-immunoprecipitation assays are shown in Supplementary Data 3. (B, D) The amount of co-immunoprecipitated β-catenin or NF-κB p65 was measured by ImageJ. (E) NF-κB activity for its target DNA binding was measured by ELISA. (F) Transcriptional activity of NF-κB-responsive promoter was measured by luciferase reporter assay. *p < 0.05, **p < 0.01, ***p < 0.001 compared with cells stimulated with LPS with 0 μM of Wnt-C59. ##p < 0.01, ###p < 0.001 compared with unstimulated cells. Experiments were conducted in triplicate. Data are shown as mean ± standard deviation, and statistical significance was measured by unpaired t-test. IP, immunoprecipitation; WB, Western blot.
DISCUSSION
In this study, we found the anti-inflammatory effects of Wnt-C59 in epithelial, endothelial and macrophage cells stimulated by LPS: Wnt-C59 suppressed LPS-induced proinflammatory cytokine mRNA expression significantly and dose-dependently (Fig. 1, Supplementary Data 1, and Fig. 2). The expression of proinflammatory cytokine mRNA are mediated by NF-κB binding to target DNAs located near the promoters of their genes followed by activation of transcriptional activities of their promoters [27]. Our data suggest a mechanism of action of the anti-inflammatory effect of Wnt-C59: As a Wnt signaling inhibitor, Wnt-C59 reduced the cellular levels of β-catenin (Figs. 3F and 4F), resulting in the decreased protein-protein interaction between β-catenin and NF-κB (Figs. 7A–D and 8A–D) followed by the concomitant reductions of both target DNA binding activity of NF-κB (Figs. 7E and 8E) and the transcriptional activity of a NF-κB responsive promoter (Figs. 7F and 8F), which may explain the mechanism of decreased proinflammatory cytokine mRNA expression by Wnt-C59 (Figs. 1 and 2). Our data also showed that the anti-inflammatory effects of Wnt-C59 were rescued by β-catenin overexpression (Figs. 5 and 6), which showed that anti-inflammatory effects of Wnt-C59 were caused by the reduction of β-catenin, the main executor protein of Wnt/β-catenin pathway; These data suggested that anti-inflammatory activity of Wnt-C59 is not an off-target effect unrelated with the inhibition of the Wnt/β-catenin pathway.
The findings of this study are supported by recent reports indicating that β-catenin physically interacts with NF-κB [17-19] and this interaction is strengthened following Wnt-induced stabilization of β-catenin [28]. It was also reported that direct protein-protein interaction between NF-κB and β-catenin is involved in TNF-α-induced expression of C-reactive protein, a clinical maker of acute inflammation [29,30]. β-Catenin was also found to elevate the transcriptional activity of NF-κB by interacting with CREB binding protein [31]. The depletion of β-catenin by siRNA reduces NF-κB activation [32]. It should be noted, however, that the interaction between β-catenin and NF-κB may have diverse effects : β-Catenin may activate or repress NF-κB depending on the context such as cell and tissue types and the kinds of inflammatory stimulators [20]. The regulation of NF-κB by Wnt/β-catenin pathway modulators may have different effects on diverse context.
In our previous study, we found that LPS-induced elevation of NF-κB activity and proinflammatory cytokine expression were suppressed by siRNA-mediated knockdown of β-catenin [32], which suggests that β-catenin siRNA can be developed as an anti-inflammatory agents. However, siRNA has difficulty in delivery without proper carriers to be developed into a therapeutic agents [33]. In this study, we used Wnt-C59, a small molecule inhibitor of Wnt signaling, to reduce the cellular level of β-catenin in LPS-stimulated cells (Figs. 3F and 4F). The effects of WNT-C59 on NF-κB activity and proinflammatory cytokine expression were similar to those of the siRNA-mediated β-catenin knockdown which can be rescued by β-catenin overexpression (Figs. 5 and 6).
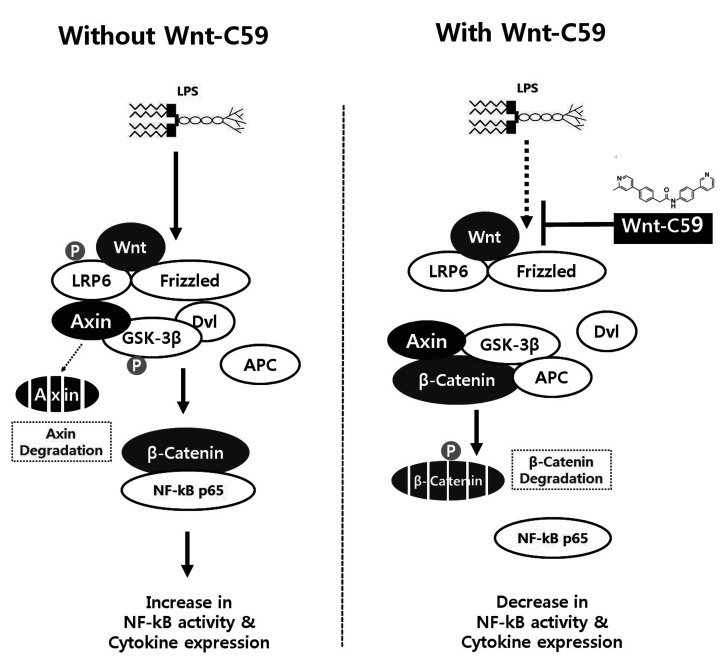
The finding of this study was summarized in Fig. 9. LPS induced Wnt signaling in both epithelial and macrophage cells including the phosphorylation of LRP6 and GSK-3β, degradation of AXIN, and dephosphorylation and increase of β-catenin. β-Catenin interacts with NF-κB to increase its activity and cytokine expression. We found that Wnt-C59 inhibits LPS-induced Wnt signaling resulting in the dephosphorylations of LRP6 and GSK-3β, stabilization of AXIN, and phosphorylation and degradation of β-catenin (Figs. 3 and 4). The protein-protein interaction of β-catenin with NF-κB was reduced resulting in the decrease of NF-κB activity (Figs. 7 and 8) and cytokine expression (Figs. 1 and 2).
Fig. 9. Summary of the findings of this study.
The lipopolysaccharide (LPS)-induced activations of Wnt signaling and β-catenin-NF-κB interaction were shown in left panel. Wnt-C59 was reported to inhibit Wnt signaling by suppressing the secretion of Wnt. The inhibitory effects of Wnt-C59 on Wnt signaling and β-catenin-NF-κB interaction were shown in right panel. NF-κB, nuclear factor-kappa B.
The results of this study showed in vitro anti-inflammatory effect of Wnt-C59. Further study is needed to confirm the anti-inflammatory activity in vivo. In this study, we focused on the cellular level of β-catenin and its interaction with NF-κB. Further studies are necessary on the molecular characterization of the protein-protein interaction between NF-κB and β-catenin as well as the effects of Wnt-C59 on the movement of β-catenin into nucleus, the activity of β-catenin, and the shape of the inactivated β-catenin.
In conclusion, Wnt-C59 showed an anti-inflammatory effects in LPS-stimulated epithelial, endothelial and macrophage cells. Anti-inflammatory effects of Wnt-C59 were mediated by the downregulations of β-catenin and its interaction with NF-κB followed by the reductions of target DNA binding of NF-κB and transcriptional activity of NF-κB responsive promoter. LPS, also called as endotoxin, the major virulence factor of gram negative bacteria, is involved in diverse inflammatory disorders including septic shock, atherosclerosis, metabolic syndrome, chronic fatigue, and so on [34]. Further study on the anti-inflammatory effect of Wnt-C59 may provide a possible treatment for the various diseases caused by gram negative bacterial endotoxin.
SUPPLEMENTARY MATERIALS
Supplementary data including three data can be found with this article online at https://doi.org/10.4196/kjpp.2021.25.4.307.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program (2020R1F1A1064537) of National Research Foundation (NRF) of Korea, and also by Chung-Ang University Research Grants in 2020.
Footnotes
Author contributions: J.J., J.S., and I.S. conducted the experiments. Y.Y. designed the research and analyzed the results. All authors reviewed the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerman ZF, Moon RT, Chien AJ. Targeting Wnt pathways in disease. Cold Spring Harb Perspect Biol. 2012;4:a008086. doi: 10.1101/cshperspect.a008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Hao S, Yang B, Fan Y, Qin X, Chen Y, Hu J. Wnt/β-catenin signaling plays an essential role in α7 nicotinic receptor-mediated neuroprotection of dopaminergic neurons in a mouse Parkinson's disease model. Biochem Pharmacol. 2017;140:115–123. doi: 10.1016/j.bcp.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Vallée A, Lecarpentier Y. Alzheimer disease: crosstalk between the canonical Wnt/Beta-catenin pathway and PPARs alpha and gamma. Front Neurosci. 2016;10:459. doi: 10.3389/fnins.2016.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoseth EZ, Krull F, Dieset I, Mørch RH, Hope S, Gardsjord ES, Steen NE, Melle I, Brattbakk HR, Steen VM, Aukrust P, Djurovic S, Andreassen OA, Ueland T. Exploring the Wnt signaling pathway in schizophrenia and bipolar disorder. Transl Psychiatry. 2018;8:55. doi: 10.1038/s41398-018-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baron R, Gori F. Targeting WNT signaling in the treatment of osteoporosis. Curr Opin Pharmacol. 2018;40:134–141. doi: 10.1016/j.coph.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Chen N, Wang J. Wnt/β-catenin signaling and obesity. Front Physiol. 2018;9:792. doi: 10.3389/fphys.2018.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Zhou CJ, Liu Y. Wnt signaling in kidney development and disease. Prog Mol Biol Transl Sci. 2018;153:181–207. doi: 10.1016/bs.pmbts.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141–149. doi: 10.1016/j.critrevonc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W, He TC. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, Phoon YP, Jin X, Chong SY, Ip JC, Wong BW, Lung ML. Wnt-C59 arrests stemness and suppresses growth of nasopharyngeal carcinoma in mice by inhibiting the Wnt pathway in the tumor microenvironment. Oncotarget. 2015;6:14428–14439. doi: 10.18632/oncotarget.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proffitt KD, Madan B, Ke Z, Pendharkar V, Ding L, Lee MA, Hannoush RN, Virshup DM. Pharmacological inhibition of the Wnt acyltransferase PORCN prevents growth of WNT-driven mammary cancer. Cancer Res. 2013;73:502–507. doi: 10.1158/0008-5472.CAN-12-2258. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Cai H, Sun L, Zhan P, Chen M, Zhang F, Ran Y, Wan J. LGR5, a novel functional glioma stem cell marker, promotes EMT by activating the Wnt/β-catenin pathway and predicts poor survival of glioma patients. J Exp Clin Cancer Res. 2018;37:225. doi: 10.1186/s13046-018-0864-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo BK, van Es JH, van den Born M, Clevers H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc Natl Acad Sci U S A. 2015;112:7548–7550. doi: 10.1073/pnas.1508113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madan B, Patel MB, Zhang J, Bunte RM, Rudemiller NP, Griffiths R, Virshup DM, Crowley SD. Experimental inhibition of porcupine-mediated Wnt O-acylation attenuates kidney fibrosis. Kidney Int. 2016;89:1062–1074. doi: 10.1016/j.kint.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong Q, Jie Y, Ma J, Li C, Xin T, Yang D. Wnt/β-catenin signaling pathway promotes renal ischemia-reperfusion injury through inducing oxidative stress and inflammation response. J Recept Signal Transduct Res. 2021;41:15–18. doi: 10.1080/10799893.2020.1783555. [DOI] [PubMed] [Google Scholar]
- 17.Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, Lin SY, Hung MC. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002;2:323–334. doi: 10.1016/S1535-6108(02)00154-X. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Kim B, Cai L, Choi HJ, Ohgi KA, Tran C, Chen C, Chung CH, Huber O, Rose DW, Sawyers CL, Rosenfeld MG, Baek SH. Transcriptional regulation of a metastasis suppressor gene by Tip60 and beta-catenin complexes. Nature. 2005;434:921–926. doi: 10.1038/nature03452. [DOI] [PubMed] [Google Scholar]
- 19.Sun J, Hobert ME, Duan Y, Rao AS, He TC, Chang EB, Madara JL. Crosstalk between NF-kappaB and beta-catenin pathways in bacterial-colonized intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G129–G137. doi: 10.1152/ajpgi.00515.2004. [DOI] [PubMed] [Google Scholar]
- 20.Ma B, Hottiger MO. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front Immunol. 2016;7:378. doi: 10.3389/fimmu.2016.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang J, Jung Y, Kim Y, Jho EH, Yoon Y. LPS-induced inflammatory response is suppressed by Wnt inhibitors, Dickkopf-1 and LGK974. Sci Rep. 2017;7:41612. doi: 10.1038/srep41612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Zhang D, Ward KM, Prendergast GC, Ayene IS. Hydroxyethyl disulfide as an efficient metabolic assay for cell viability in vitro. Toxicol In Vitro. 2012;26:603–612. doi: 10.1016/j.tiv.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo E, Jho EH. Axin-independent phosphorylation of APC controls beta-catenin signaling via cytoplasmic retention of beta-catenin. Biochem Biophys Res Commun. 2007;357:81–86. doi: 10.1016/j.bbrc.2007.03.117. [DOI] [PubMed] [Google Scholar]
- 25.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119(Pt 3):395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 26.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473. doi: 10.1038/onc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanenkov YA, Balakin KV, Lavrovsky Y. Small molecule inhibitors of NF-kB and JAK/STAT signal transduction pathways as promising anti-inflammatory therapeutics. Mini Rev Med Chem. 2011;11:55–78. doi: 10.2174/138955711793564079. [DOI] [PubMed] [Google Scholar]
- 28.Ma B, van Blitterswijk CA, Karperien M. A Wnt/β-catenin negative feedback loop inhibits interleukin-1-induced matrix metalloproteinase expression in human articular chondrocytes. Arthritis Rheum. 2012;64:2589–2600. doi: 10.1002/art.34425. [DOI] [PubMed] [Google Scholar]
- 29.Choi YS, Hur J, Jeong S. Beta-catenin binds to the downstream region and regulates the expression C-reactive protein gene. Nucleic Acids Res. 2007;35:5511–5519. doi: 10.1093/nar/gkm547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi YS, Hur J, Lee HK, Jeong S. The RNA aptamer disrupts protein-protein interaction between beta-catenin and nuclear factor-kappaB p50 and regulates the expression of C-reactive protein. FEBS Lett. 2009;583:1415–1421. doi: 10.1016/j.febslet.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Koopmans T, Eilers R, Menzen M, Halayko A, Gosens R. β-catenin directs nuclear factor-κB p65 output via CREB-binding protein/p300 in human airway smooth muscle. Front Immunol. 2017;8:1086. doi: 10.3389/fimmu.2017.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang J, Ha JH, Chung SI, Yoon Y. β-catenin regulates NF-κB activity and inflammatory cytokine expression in bronchial epithelial cells treated with lipopolysaccharide. Int J Mol Med. 2014;34:632–638. doi: 10.3892/ijmm.2014.1807. [DOI] [PubMed] [Google Scholar]
- 33.Subhan MA, Torchilin VP. siRNA based drug design, quality, delivery and clinical translation. Nanomedicine. 2020;29:102239. doi: 10.1016/j.nano.2020.102239. [DOI] [PubMed] [Google Scholar]
- 34.Munford RS. Endotoxemia-menace, marker, or mistake? J Leukoc Biol. 2016;100:687–698. doi: 10.1189/jlb.3RU0316-151R. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.