Abstract
Development of the methods to examine the molecular targets of biologically active compounds is one of the most important subjects in experimental biology/biochemistry. To evaluate the usability of the (7-nitro-2,1,3-benzoxadiazole)-thioether (NBD-S) probe for this purpose, bioactive chemical probe (1) as the cellulose biosynthesis (CB) inhibitor was synthesized and tested. As a result, a variety of fluorescently-labeled particles and organelles were found in the columella root cap cells of radish plants. Of note, well-defined cellular organelles were clearly recognized in the detaching root cap cells (border-like cells). These results imply that the bioactive NBD-S chemical probe could be a valuable direct-labeling reagent. Analysis of these fluorescent substances would be helpful in providing new information on defined molecular targets and events.
Keywords: Chemical probe, Fluorescence, Indaziflam, NBD, Root cap, Triaziflam
Abbreviations: CB, cellulose biosynthesis; Cys, cysteine; CW, cell wall; Lys, lysine; NBD, nitrobenzoxadaizole; NBD-S, (7-nitro-2,1,3-benzoxadiazole)-thioether; NBD-O, (7-nitro-2,1,3-benzoxadiazole)-ether; NBD-N, (7-nitro-2,1,3-benzoxadiazole)-amine; NBD-Cl, 4-chloro-7-nitro-2,1,3-benzoxadiazole; DIEA, N,N-diisopropylethylamine; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; DMSO, N,N-dimethylsulfoxide
Graphical abstract
Highlights
•Nobel S-NBD type chemical probe for cellulose biosynthesis inhibitors was prepared.
•This S-NBD type probe was designed for triaziflam and indaziflam.
•This S-NBD type probe labeled columella and detaching root cap cells fluorescent.
•S-NBD probe would be practical as a target exploring tool compound.
1. Introduction
Bioactive compounds taken up into organisms must interact with target molecules to be bioactive. Thus, identification of the target molecule/s is a vital task in the field of biochemistry/molecular biology. If it were possible to know exactly where the bioactive compounds are translocated and performed their biological activities/function, the identification processes would be fairly accelerated. Considering that bioactive compounds exclusively interact with the target molecules, and if they were labeling-reactive, they could make a mark on the target molecules by themselves.
Nitrobenzoxadaizole (NBD) group is known to be one of such labeling groups, and has been characterized by their advantageous labeling manner. The NBD-thioether (NBD-S) and NBD-ether (NBD-O) themselves are non-fluorescent before labeling, but turns amines fluorescent as NBD-amine (NBD-N) [[1], [2], [3], [4], [5], [6]]. A variety of non-fluorescent NBD-S probes have been developed to turn free cysteine (Cys) fluorescent [1,2,[4], [5], [6]], because NBD-S is highly reactive with the thiol group in Cys (HS-Cys) to yield NBD-S-Cys, which readily changes into fluorescent NBD–NH–Cys by an intra-molecular rearrangement reaction. Therefore, the NBD-S group could be used as the labeling group to create a fluorescent mark on the target molecules with the bioactive chemical probe.
Cellulose is a major component of the plant cell wall (CW), and cellulose biosynthesis (CB) considerably influences their shape and growth. Interestingly, some CB inhibitors are used as herbicides, for example, triaziflam (Idetop™) [7,8] and indaziflam (Alion™) [9] are known as amino-triazine type CB inhibiting herbicides (Fig. 1A). Indaziflam (Alion™) has been proposed to affect the distribution or mobility of cellulose synthases as a possible herbicidal mechanism [9], however, their molecular targets are still unknown. Thus, in this study we approached their target organelles/molecules with which triaziflam or indaziflam are interacting in the plant cells, and adopted a direct labeling method using the NBD-S moiety. We designed chemical probe (1) (Fig. 1A) which has the amino-triazine moiety as the ligand for CB inhibition activity and reactive NBD-S moiety for the labeling performance.
Fig. 1.
Chemical structures of the CB inhibiting amino-triazine type herbicides and synthetic scheme for chemical probe (1). (A) Chemical structures of the amino-triazine type CB inhibitor herbicides and chemical probe (1). (B) Synthetic scheme for chemical probe (1). Reagents and conditions: (a) HCl/n-hexane, ice-cold, 30 min, 71%; (b) dicyandiamide/n-octane, 170 °C, 4 h; (c) S-t-boc-2-mercaptopropionic acid ethyl ester, CH3ONa/CH3OH, rt, overnight. (d) CH3ONa/CH3OH, rt, overnight. Thiol amino-triazine (4) was obtained in 17% yield from (2). e) NBD-Cl, DIEA/CH3OH, rt, 0.5 h, 60%.
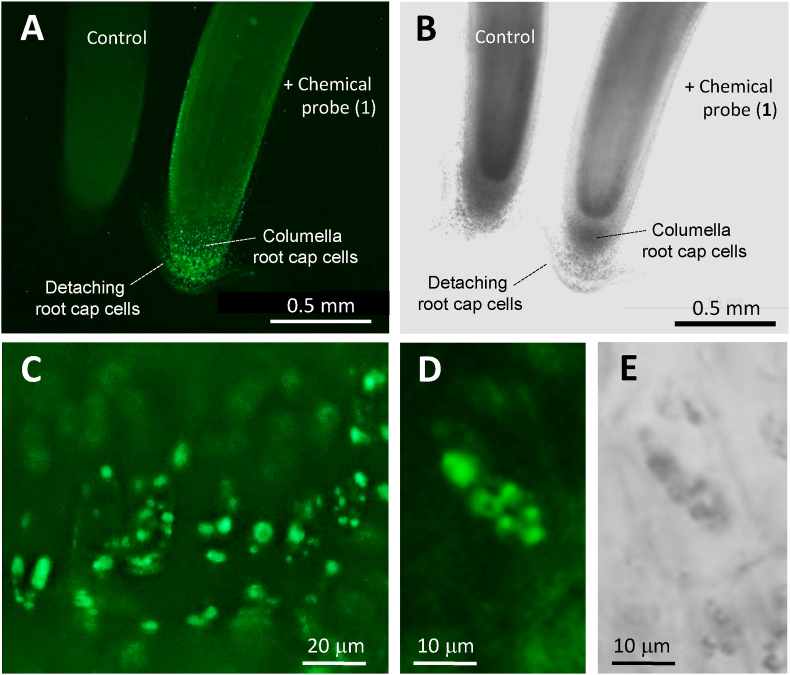
Chemical probe (1) showed herbicidal properties similar to that of triaziflam and indaziflam, and the labeling reactivity of turning Cys and Lys fluorescent in vitro (Fig. 2). Labeling experiments in vivo using radish plants yielded fluorescent substances in the root cap cells (Fig. 3). Further analysis indicated that these clear fluorescent particles were located in the columella cells, which are commonly known to be active in the cell differentiation accompanying the cell wall modification [10,11]. Of note, some small fluorescent organelles were clearly identified in the detaching root cap cells, called border-like cells (Fig. 3D). To the best of our knowledge, these present observations would be the first microscopic observation of the fluorescently-labeled target candidates with the bioactive NBD-S chemical probe. By the application example for triaziflam and indaziflam, present results suggest that the bioactive S-NBD probe might be a useful reagent in target-identification experiments. Herein, synthesis, properties and labeling usability of chemical probe (1) were described.
Fig. 2.
Biological activity and chemical reactivity of chemical probe (1). (A) Phytotoxic activity of chemical probe (1), triaziflam and indaziflam against 5 day-old radish plants. 1: control, 2: chemical probe (1) (10−7 M), 3: chemical probe (1) (10−6 M), 4: triaziflam (10−6 M), 5: indaziflam (10−6 M). (B) Fluorescence intensity from chemical probe (1) (2 × 10−5 M) with Cys or Lys in HEPES buffer (pH 8) containing 10% DMSO. ●; + Cys (1 × 10−3 M), ◯; + Cys (1 × 10−4 M), ■; + Lys (1 × 10−3 M), ◆; chemical probe (1) only. λex = 450 nm. (C) Time course fluorescence intensity from the solution containing chemical probe (1) (2 × 10−5 M) and Cys. ●; + Cys (1 × 10−3 M), ◯; + Cys (1 × 10−4 M). λex/λem = 450/550 nm. (D) Effects of reaction pH on the time course fluorescence intensity from the solution containing chemical probe (1) (2 × 10−5 M). ●; + Cys (1 × 10−3 M, pH 8), ◯; + Cys (1 × 10−3 M, pH 7). ■; + Lys (1 × 10−3 M, pH 8), □; + Lys (1 × 10−3 M, pH 7). λex/λem = 450/550 nm.
Fig. 3.
In vivo labeling experiments with chemical probe (1). (A) A typical fluorescence microscope image of primary roots of radish plant. Left: control, Right: treated with chemical probe (1) (2 × 10−5 M) for 30 min. Fluorescent particles were clearly recognized in the root cap cells. (B) Optical microscope image of (A). (C) A magnified image of the fluorescent particles in the columella root cap cells treated with chemical probe (1) in (A). (D) A magnified image of the fluorescent detaching root cap cells treated with chemical probe (1) in (A). Well-defined various small fluorescent organelles were clearly recognized. (E) Optical microscope image of (D).
2. Materials and methods
2.1. Reagents and apparatus
4-Chloro-7-nitro-2,1,3-benzoxadiazole (NBD-Cl), di-tert-butyl dicarbonate, dicyandiamide, N,N-diisopropylethylamine (DIEA), (±)-ethyl 2-mercaptopropionate, (R)-(−)-1,2,3,4-tetrahydro-1-naphtylamine, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were all purchased from Tokyo Chemical Industry. Triaziflam and indaziflam were from commercial products. Silica-gel 60 F254 plates and silica gel 60 (0.063–0.200 mm) were purchased from Merck and used for thin-layer chromatography and column chromatography, respectively. Other reagents and organic solvents were purchased from FUJIFIUM Wako Pure Chemical Corporation. 1H NMR spectra were recorded on a JOEL JNM-ECS400 (400 MHz) spectrometer with tetramethylsilane (0.00 ppm) as an internal standard. For mass spectrometry analysis, Exactive orbitrap high resolution mass spectrometry (Thermo Fischer) or TSQ Ultra MS (Thermo Fischer) equipped with electrospray ionization probes were used. For fluorescence analysis, SpectraMax M5 multimode microplate reader (Molecular devices) was used.
2.2. Bioassay of the compounds
Biological activity and inhibition property of the compounds were evaluated by phytotoxicity against radish plants (Raphanus sativus L.). As a typical example, the treatment procedure of triaziflam was described as follows. To a filter paper (55 mm in diameter) in a glass Petri dish, 650 μl of 6 mM triaziflam in acetone (10 mg triaziflam was dissolved in 5 ml of acetone) was applied. After volatilization of the solvent, 3.9 ml of water was added to give the test solution of 1 × 10−3 M. Four seeds of radish (R. sativus L.) were placed on the filter paper, and the dish was closed with a lid. After incubation (25 °C, with a light/dark cycle of 12/12 h) for 5 days, phytotoxic effects on the radish plants were investigated. For lower concentration assays, 6 M triaziflam solution was diluted and used. Other compounds were applied in the same manner.
2.3. Fluorescent emission analysis of the compounds
Fluorescent emission from the sample solution was analyzed using a fluorescence spectrometer. As a typical example, the procedure for preparing the analyte solution containing chemical probe (1) and Cys was described as follows. To a 4 ml of HEPES buffer (1 × 10−2 M, pH 8), 0.5 ml of chemical probe (1) (2 × 10−4 M) in DMSO and 0.5 ml of Cys (1 × 10−2 M) in HEPES buffer (1 × 10−2 M, pH 8) was added. After mixing, 3 ml of the solution was transferred into a glass cuvette (4 ml capacity, light path length = 10 mm) and subjected to analysis at 23 °C.
Fluorescence microscope images of radish roots were obtained using an Olympus Fluorescence Microscope BX51 (Filter: U-MGFPHQ). Three-day old radish plants (R. sativus L.) grown up in the dark at 25 °C, were dipped in a solution prepared from 4.5 ml of HEPES buffer (1 × 10−2 M) and 0.5 ml of 2 × 10−2 M chemical probe (1) in DMSO. After incubation for 30 min at 25 °C, the radish plants were rinsed in HEPES buffer (1 × 10−4 M, pH 8) and immediately observed under a fluorescence microscope. Control materials were prepared in the same manner without chemical probe (1).
2.4. Chemical synthesis
2.4.1. Thiol amino triazine (4)
To a solution of n-hexane (100 ml) containing 25 g of (R)-(−)-1,2,3,4-tetrahydro-1-naphtylamine (2) (0.17 mol) in an ice-water bath, 36% hydrochloric acid (20 ml, 0.20 mol) was added dropwise over 30 min with vigorous stirring. Crystalline precipitate was collected by filtration and washed with acetone to give 22.0 g (0.12 mol) of HCl salt of amine (2) in 71% yield. Ten g of the HCl salt of amine (2) (55 mmol) and dicyandiamide (4.6 g, 55 mmol) in n-decane (200 ml) was heated at 170 °C for 4 h in an oil bath. After removal of the solvent by decantation, the precipitated product of biguanide·HCl (3) was dissolved in methanol (200 ml). To this solution, 22 ml of 5 M sodium methoxide (0.11 mol) in methanol was added dropwise with stirring at room temperature. After 30 min, 13.0 g of (±)-ethyl S-(tert-butoxycarbonyl)-2-mercaptopropionate (55 mmol), prepared from ethyl 2-mercaptopropionate and di-tert-butyl dicarbonate, was added and stirred overnight at room temperature. After removal of the solvent under reduced pressure, water was added to the reaction mixture and extracted with ethyl acetate. Removal of the solvent gave an oily crude product, which was successively subjected to deprotection reaction with 13 ml of 5 M sodium methoxide (65 mmol) in methanol (100 ml) at room temperature, overnight. The product obtained by ethyl acetate extraction was purified by silica-gel column chromatography (n-hexane: ethyl acetate = 1 : 1) to give 4.0 g (13 mmol) of thiol amino-triazine (4) as colorless solids in 17% yield from (R)-(−)-1,2,3,4-tetrahydro-1-naphtylamine (2). 1H NMR (400 MHz, CDCl3, TMS) δ (ppm) 1.58 (m, 3H, CH3), 1.84 (m, 3H, (CH2)3), 2.05 (m, 1H, (CH2)3), 2.25 (m, 1H, SH), 2.76 (m, 2H, (CH2)3), 3.70 (m, 1H, S–CH–CH3), 5.30 (m, 1H, CH–NH), 5.60 (m, 3H, NH), 7.10 (m, 1H, PhH), 7.17 (m, 2H, PhH), 7.31 (m, 1H, PhH). HRMS (ESI) m/z: [M+H]+ calcd for C15H20N5S; 302.14394, found 302.14291.
2.4.2. Preparation of chemical probe (1)
To a methanol solution (20 ml) containing thiol amino-triazine (4) (2.7 g, 9 mmol) and DIEA (1.2 g, 9 mmol), NBD-Cl (1.8 g, 9 mmol) was added and stirred for 30 min at room temperature under N2. After removal of the solvent, the crude product was subjected to silica-gel column chromatography (n-hexane: ethyl acetate = 1 : 1) to yield 2.5 g (5.4 mmol) of chemical probe (1) as a mixture of isomers in 60% yield as slightly yellow solids. 1H NMR (400 MHz, CDCl3, TMS) δ (ppm) 1.81 (m, 3H, CH3), 1.88 (m, 3H, (CH2)3), 2.05 (m, 1H, (CH2)3), 2.80 (m, 2H, (CH2)3), 4.52 (q, J = 7.0 Hz, 1/4H, S–CH–CH3), 4.54 (q, J = 7.0 Hz, 1/4H, S–CH–CH3), 4.67 (q, J = 7.0 Hz, 1/4H, S–CH–CH3), 4.70 (q, J = 7.0 Hz, 1/4H, S–CH–CH3), 5.23 (m, 3H, NH), 5.58 (m, 1H, CH–NH), 7.10 (m, 1H, PhH), 7.18 (m, 2H, PhH), 7.23 (m, 1H, PhH), 7.51 (d, J = 8.0 Hz, 1/4H, C CH–CH C), 7.53 (d, J = 8.0 Hz, 1/4H, C CH–CH C), 7.61 and 7.63 (d, J = 8.0 Hz, 2/4H, C CH–CH C), 8.11 (d, J = 8.0 Hz, 1/4H, C CH–CH C), 8.30 (d, J = 8.0 Hz, 1/4H, C CH–CH C), 8.35 (d, J = 8.0 Hz, 1/4H, C CH–CH C), 8.37 (d, J = 8.0 Hz, 1/4H, C CH–CH C). HRMS (ESI) m/z: [M+H]+ calcd for C21H21N8O3S; 465.14573, found 465.14584.
2.4.3. NBD labelling of ethyl 4-amino-butanate by chemical probe (1)
To a DMSO solution (10 ml) containing ethyl 4-aminobutanate·HCl (167 mg, 1 mmol) and DIEA (260 mg, 2 mmol), chemical probe (1) (465 mg, 1 mmol) was added under N2 with stirring for 30 min at room temperature. The reaction mixture was poured into water and extracted with ethyl acetate. After removal of the solvent, the reaction mixture was purified by silica-gel column chromatography (n-hexane: ethyl acetate = 2 : 1) to yield 120 mg of ethyl 4-(N-Nitrobenzoxadaizole) aminobutanate in 41% yield as a yellow powdery product. 1H NMR (400 MHz, CDCl3, TMS) δ (ppm) 1.28 (t, J = 7.2 Hz, 3H, CH3–CH2), 2.15 (quin, J = 6.8 Hz, 2H, CH2–CH2–CH2), 2.55 (t, J = 6.8 Hz, 2H, CH2–CH2–CH2), 3.57 (br q, J = 6.8 Hz, 2H, CH2–CH2–CH2), 4.20 (q, J = 7.2 Hz, 2H, CH3–CH2), 6.22 (d, J = 8.5 Hz, 1H, C CH–CH C), 6.85 (br s, 1H, NH), 8.51 (d, J = 8.5 Hz, 1H, C CH–CH C). MS (ESI) m/z: [M+H]+ calcd for C12H15N4O5; 295.27, found 295.18.
3. Results
3.1. Design and synthesis of chemical probe (1)
Triaziflam and indaziflam are amino-triazine type compounds with substituents of an aryl-alkyl amine and a fluoro alkane (Fig. 1A), and stereochemistry of the aryl-alkyl amine was required to be (R)-configuration for the CB inhibiting activity [8]. Then, to prepare the bioequivalent probe, we used commercially available (R)-(−)-1,2,3,4-tetrahydro-1-naphthylamine (2) as the aryl-alkyl amine moiety (Fig. 1B). It has been reported that the herbicidal activity did not decrease when (1-fluoro-1-methyl) ethyl moiety was changed into substituents with larger molecular size in triaziflam [7]. We speculated that the labeling module could be introduced in this position, and thiol amino-triazine (4) was designed to utilize NBD-S as the labeling module (Fig. 1B). Formation of the triazine ring was achieved by reacting biguanide·HCl intermediate (3, not isolated) with tert-boc-protected ethyl 2-mercaptopropionate using sodium methoxide as a base, according to the previous condition [7]. Since TLC monitoring of the reaction suggested that the t-boc group was partially deprotected, the crude product of the triazine ring formation was successively subjected to further treatment of sodium methoxide to complete the de-protection. As a result, thiol amino-triazine (4) was obtained as colorless solids in 17% yield from (R)-(−)-1,2,3,4-tetrahydro-1-naphthylamine (2) (Fig. 1B). Successive NBD derivatization of thiol amino-triazine (4) was carried out with NBD-Cl in the presence of DIEA in methanol to yield chemical probe (1) as slightly yellow solids in 60% yield. Since diastereomeric isomers of chemical probe (1) were unseparable, biological and labeling properties were evaluated as a mixture of the isomers.
3.2. Herbicidal property of chemical probe (1)
Herbicidal properties of chemical probe (1) were examined with radish plants (Fig. 2A). Roots of the control radish plant grew normally (healthy) with slightly opened cotyledons on top of the red hypocotyl. Treatment with chemical probe (1) at 1 × 10−7 M caused strong inhibition of the root elongation with swelling of the tip, in contrast to no damage against the cotyledon and hypocotyl. With a higher concentration of 1 × 10−6 M, chemical probe (1) completely inhibited root formation, with hypocotyl bleaching and formation of dark pigments on its surface as well as with the application of triaziflam and indaziflam. Chemical probe (1) showed remarkably strong phytotoxicity against the roots, and its inhibition properties were like those of triaziflam and indaziflam. It has been reported that triaziflam and indaziflam show strong phytotoxicity against plant roots. Triaziflam induces typical swelling of the meristematic root tips [8], and indaziflam induces the radial swelling and ectopic lignification in roots [9]. These strong activities accompanied root tip swelling, hypocotyl bleaching and formation of dark pigments (Fig. 2A) indicating that chemical probe (1) retained herbicidal activity and properties of triaziflam and indaziflam as the bioactive probe.
3.3. Reactivity of chemical probe (1) with Cys and Lys in vitro
Labeling reactivity of chemical probe (1) in vivo was evaluated by measuring the fluorescent emission intensity. Chemical probe (1) was mixed with Cys or Lys in HEPES buffer solution (pH 8), and fluorescence emission from the solution was measured after 30 min. It was found that the solution containing chemical probe (1) and Cys was strongly fluorescent, while chemical probe (1) alone in the buffer was non-fluorescent (Fig. 2B). Time course changes of the fluorescence emission showed that the reaction of chemical probe (1) with Cys was very fast reaching a maximum level within 5 min in both concentrations of 1 × 10−4 M and 1 × 10−3 M (Fig. 2C) with pH 8. A higher pH condition (pH 8) was kinetically preferable for the reaction than with pH 7 in both Cys and Lys (Fig. 2D). Higher reactivity of chemical probe (1) with Cys than Lys could be explained by the rapid exchange reaction known as the thiol sulfide exchange between thiol in Cys and sulfide in the probes [1]. Although an increase around 540 nm was small when chemical probe (1) was mixed with Lys (Fig. 2B), this result indicated that chemical probe (1) could react with amines to some extent. To ensure its reactivity, chemical probe (1) was mixed with ethyl 4-amino-butanate in the presence of DIEA in DMSO, and the reaction product was isolated and analyzed by 1H NMR. The structure of the reaction product was elucidated to be the NBD derivative of ethyl 4-amino-butanate. Therefore, although its reactivity was not so high, amino groups, such as in Lys residues were proven to turn fluorescent with chemical probe (1).
3.4. Fluorescent labeling experiments with chemical probe (1) in vivo
Since chemical probe (1) inhibited root development of the radish plant at fairly low concentrations as well as indaziflam and triaziflam (Fig. 2A), cellular activities including CW biosynthesis in these front root cap cells might be possibly the targets. Chemical probe (1) was applied to living radish plants to examine whether fluorescent products were yielded. Three-day old radish plants were treated with chemical probe (1) for 30 min, and their primary roots were imaged by fluorescent microscopy. To clarify the differences of emission magnitude between treated and non-treated plant (R. sativus L.) materials, they were compared side by side (Fig. 3A and B). It was clearly observed that green bright fluorescence was emitted from the root cap treated with chemical probe (1) (Fig. 3A). Only infinite emission was observed from the control root whose existence was identified by the optical microscopic image (Fig. 3B). These results clearly indicated that chemical probe (1) was absorbed into the plant root tissues, and reacted with cellular substances. In the enlarged view, fluorescent particles located in the columella root cap cells were clearly observed (Fig. 3C). Moreover, various small fluorescent organelles were clearly identified in the detaching root cap cells (border-like cells) (Fig. 3D).
4. Discussion
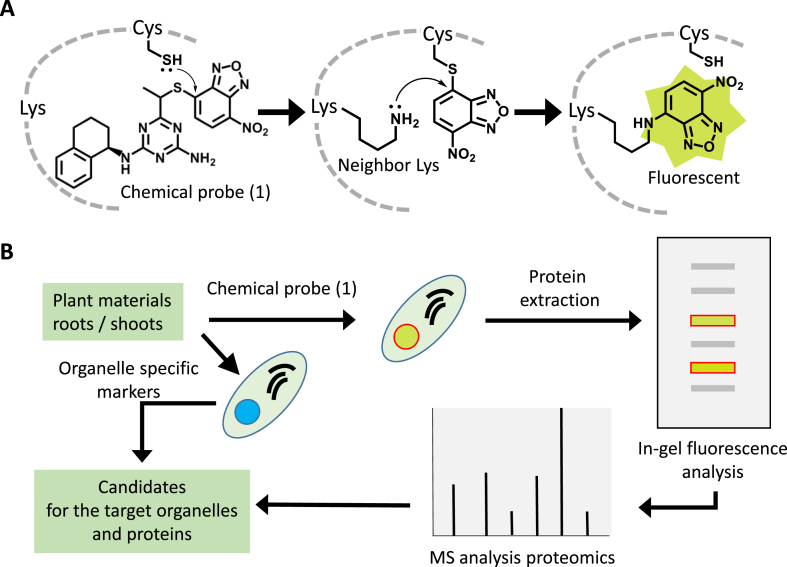
Consistent with previous results on the NBD-S type probes [1,2,[4], [5], [6]], chemical probe (1) itself was non-fluorescent but turned Cys fluorescent as NBD–NH–Cys (Fig. 2B). Chemical probe (1) would be the first bioactive probe with S-NBD as the labeling module. Since chemical probe (1) showed the high labeling reactivity for free Cys (Fig. 2B), we expected chemical probe (1) would efficiently react also with the Cys included in the target molecules and successive reaction turning the neighbor Lys fluorescent (Fig. 4A). In fact, some fluorescent substances were yielded in the root cap cells in vivo labeling experiments (Fig. 3A). Especially, various fluorescent particles were clearly recognized in the columella root cap cells (Fig. 3C), and moreover, characteristic well-defined fluorescent cellular organelles, which appeared to be vesicles or chromosomes, were clearly recognized in the detaching root cap cells (border-like cells) as shown in Fig. 3D. Clear bodies of these cells were advantageous for cytochemical microscopic observation to identify the target organelles (Fig. 3E). It was highly possible that these organelles included the target molecules; because the fluorescence indicated that chemical probe (1) was translocated and reacted at there. It has been understood that the root cap cells have specialized functions by the specific CW modification accompanying subcellular organization [10,11]. Border-like cells are naturally-released top areas as defensive protection for the root in Brassicaceae species [10,11]. It has been reported that these cells are metabolically active expressing unique mRNA transcripts and proteins concerning specific CW modification accompanying subcellular organization [12,13], and it could be speculated that chemical probe (1) might react with these specific proteins functioning in cell division or differentiation. In addition, stem cells could be used for labeling experiments to obtain further information on the action mechanism. Present study showed that the bioactive chemical probe (1) with S-NBD possessed similar biological properties of triaziflam and indaziflam and labeling-reactivity in vitro and vivo. Analysis of these fluorescent particles and organelles using organelle-specific markers (Fig. 4B) might provide useful information on the targets of triaziflam and indaziflam. Finally, the bioactive NBD-S chemical probe could be a valuable direct-labeling reagent with fluorescence.
Fig. 4.
Target labeling strategy with the chemical probe (1) and an application example to obtain the target-candidate proteins using chemical probe (1). (A) Chemical probe (1) would react fast with also non-terminus HS-Cys in biomolecules to yield Cys-S-NBD by efficient thiol sulfide exchange reaction [1,2,[4], [5], [6]], which could turn the neighbor Lys in biomolecules fluorescent. Labeling of the Lys could be possibly accelerated via intramolecular-like reaction. (B) Information on the fluorescent metabolites in the plant materials (roots or shoots) treated with chemical probe (1) could be obtained by protein isolation and successive mass-spectrometry (MS) analysis [14]. Additionally, the organelle specific markers would help to identify the candidates as the target organelles.
Credit author statement
Shigeru Tamogami: Methodology, Conceptualization, Investigation, Data curation, Validation, Writing Original-Draft. Ganesh K. Agrawal: Methodology, Conceptualization, Writing-Review and Editing. Randeep Rakwal: Methodology, Conceptualization, Writing-Review and Editing.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by the funds by Akita Prefectural University.
References
- 1.Li Y., Yang Y., Guan X. Benzofurazan sulfides for thiol imaging and quantification in live cells through fluorescence microscopy. Anal. Chem. 2012;84:6877–6883. doi: 10.1021/ac301306s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammers M.D., Pluth M.D. Ratiometric measurement of hydrogen sulfide and cysteine/homocysteine ratios using a dual-fluorophore fragmentation strategy. Anal. Chem. 2014;86:7135–7140. doi: 10.1021/ac501680d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi T., Asanuma M., Nakanishi S., Saito Y., Okazaki M., Dodo K K., Sodeoka M. Turn-ON fluorescent affinity labeling using a small bifunctional O-nitrobenzoxadiazole unit. Chem. Sci. 2014;5:1021–1029. doi: 10.1039/C3SC52704B. [DOI] [Google Scholar]
- 4.Lee D., Kim G., Yin J., Yoon J. An aryl-thioether substituted nitrobenzothiadiazole chemical probe for the selective detection of cysteine and homocysteine. Chem. Commun. 2015;51:6518–6520. doi: 10.1039/C5CC01071C. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y., Guan X. Rapid and thiol-specific high-throughput assay for simultaneous relative quantification of total thiols, protein thiols, and nonprotein thiols in cells. Anal. Chem. 2015;87:649–655. doi: 10.1021/ac503411p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang C., Wu S., Xi Z., Yi L. Design and synthesis of NBD-S-dye dyads for fluorescently discriminative detection of biothiols and Cys/Hcy. Tetrahedron. 2017;73:6651–6656. doi: 10.1016/j.tet.2017.10.020. [DOI] [Google Scholar]
- 7.Nishii M., Kobayashi I., Uemura M., Takematsu T. Patent; 1990. Preparation of Triazine Derivative as Herbicides. WO 9009378. [Google Scholar]
- 8.Grossmann K., Tresch S., Plath P. Triaziflam and diaminotriazine derivatives affect enantioselectively multiple herbicide target sites. Z. Naturforsch. 2001;56c:559–569. doi: 10.1515/znc-2001-7-814. [DOI] [PubMed] [Google Scholar]
- 9.Brabham C., Lei L., Gu Y., Stork J., Barrett M., DeBolt S. Indaziflam herbicidal action: a potent cellulose biosynthesis inhibitor. Plant Physiol. 2014;166:1177–1185. doi: 10.1104/pp.114.241950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawes M., Smith L., Stephenson M. Root organogenesis from single cells released from the root cap of Medicago sp. Plant Cell Tissue Organ Cult. 1991;27:303–308. doi: 10.1007/BF00157595. [DOI] [Google Scholar]
- 11.Weiller F., Moore1 J., Young P., Driouich A., Vivier M. The Brassicaceae species Heliophila coronopifolia produces root border-like cells that protect the root tip and secrete defensin peptides. Ann. Bot. 2017;119:803–813. doi: 10.1093/aob/mcw141. https://doi:10.1093/aob/mcw141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamiya M., Higashio S., Isomoto A., Kim J., Seki M., Miyashima S., Nakajima K. Control of root cap maturation and cell detachment by BEARSKIN transcription factors in Arabidopsis. Development. 2016;143:4063–4072. doi: 10.1242/dev.142331. https://doi:10.1242/dev.142331 [DOI] [PubMed] [Google Scholar]
- 13.Bennett T., Toorn A., Sanchez-Perez G., Campilho A., Willemsen V., Snel B., Scheresa B. SOMBRERO, BEARSKIN1, and BEARSKIN2 regulate root cap maturation in arabidopsis. Plant Cell. 2010;22:640–654. doi: 10.1105/tpc.109.072272. https://doi/10.1105/tpc.109.072272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright M.H., Sieber S. Chemical proteomics approaches for identifying the cellular targets of natural products. Nat. Prod. Rep. 2016;33:681–708. doi: 10.1039/c6np00001k. https://doi:10.1039/c6np00001k [DOI] [PMC free article] [PubMed] [Google Scholar]