Abstract
The Leadbeater's possum (Gymnobelideus leadbeateri) is a critically endangered marsupial in south-eastern Australia. Among other conservation efforts, free-ranging animals in the two remaining geographically separate populations (highland and lowland) have been extensively studied; however, little is known about their health and mortality. Although some wild populations are frequently monitored, cadavers are rarely recovered for post mortem examination. In June 2019, a recently deceased, wild, adult male lowland Leadbeater's possum was collected from a nest box and a comprehensive post mortem examination was conducted. Microfilariae of a filarioid nematode were observed in testes, liver, lung and skin samples in tissue impression smears and upon histopathological examination. No gross or histological changes were seen associated with the parasites, except for a focal area of tissue damage in the skin, suggesting that the possum is a natural host. Using a PCR-coupled sequencing method the filarioid was identified as a species of Breinlia. Species of Breinlia occur in other Australian marsupials and rodents.
Keywords: Gymnobelideus leadbeateri, Leadbeater's possum, Australia, Breinlia sp. filarioidea, Microfilariae
Graphical abstract
Highlights
-
•
A post mortem examination of a wild endangered Leadbeater's possum was performed.
-
•
Microfilariae were observed in testes, liver, lung and skin.
-
•
The filarioid nematode was identified as Breinlia sp. Using molecular techniques.
-
•
The possum appears to be the natural host of this parasite.
1. Introduction
The Leadbeater's possum (Gymnobelideus leadbeateri) is a critically endangered marsupial with a restricted geographical distribution in south-eastern Australia. Two genetically distinct populations, highland and lowland, are recognised (Hansen et al., 2009). The population of highland possums is found in montane ash forests and subalpine woodlands in the Central Highlands of Victoria (Department of Environment, Land, Water & Planning, 2014). The last extant population of lowland possums inhabits a remnant swamp forest habitat in Yellingbo Nature Conservation Reserve (Smales, 1994; Harley et al., 2005). Listed as critically endangered since 2005 (Harley, 2016), Leadbeater's possums continue to be vulnerable to fire and anthropogenic changes, including the long term legacy of logging (Lindenmayer et al., 1991). Small family colonies of Leadbeater's possums build woven bark-strip nests in multiple tree hollows in mature trees (≥190 years of age) which are a critically scarce resource across much of the species range (Smith and Lindenmayer 1988; Lindenmayer et al., 2012). Conservation measures for this species include habitat protection, revegetation, nest box provision and translocation, in order to maintain breeding habitat and facilitate population genetic diversity and growth (Harley, 2016). The lowland possum population has declined to ≤40 individuals; a genetic rescue programme is presently underway that involves the formation of mixed lowland-highland pairs in captivity to alleviate inbreeding in the lowland population (Zilko et al., 2020).
Morbidity and mortality predilections in free-ranging Leadbeater's possums are not known, and cadavers of adults from wild populations have not previously been studied (Booth, 1995; Lynch, 2012). There is some knowledge of ectoparasites of wild Leadbeater's possums based on a study of live animals examined during conservation efforts (Lindenmayer et al., 1994). The 1994 study and an earlier investigation (Dunnet and Mardon, 1974; Lindenmayer et al., 1994; C. Steventon et al. unpubl data) found mites (including Haemolaelaps anticlea sp. n. and Haemolaelaps cleptusa sp. n.), a tick (Ixodes tasmani) and fleas (including Choristopsylla tristis, Acanthopsylla rothschildii sp., Stephanocircus domrowi and Wurunjerria warnekei).
Previous records of endoparasites are few. In 2015, a lowland Leadbeater's possum died from a respiratory illness soon after collection from lowland habitat and introduction to the captive breeding program; histopathological examination of lung tissue by electron microscopy suggested that a protozoan parasite was involved (proposed was a species of Plasmodium) (Scheelings et al., 2016). In a necropsy report from 1977, a filarioid nematode was reported in granulomatous lesions in the liver of a captive highland possum, but no further investigation was undertaken. In this study, we examined tissues from a recently deceased, adult male lowland Leadbeater's possum found in a nestbox. Molecular approaches were used to characterise and identify a filarioid nematode of the genus Breinlia in a lung sample from this individual.
2. Materials and methods
2.1. Signalment and history
In June 2019, a male lowland Leadbeater's possum was found deceased in a nest box during annual population monitoring in Yellingbo Nature Conservation Reserve (37° 51′ S, 145° 29′ E; c. 110 m asl), located ~50 km east of Melbourne, Victoria, Australia. The reserve includes ~640 ha of riparian forest and swamp forest, extending along four watercourses – the Woori Yallock, Sheep Station, Macclesfield and Cockatoo Creeks. This location receives ~1100 mm of rainfall annually. Daily mean temperatures fluctuate from 13.6 °C in winter to 25.6 °C in summer.
This population has been monitored intensively since 1996. The male individual in this study was first captured in May of 2010 as a young adult of 18–24 months of age. During 2017–2018, when he was >9 years of age, he had been observed to have alopecia and a dermatitis-like lesion along his dorsum and tail. The possum was estimated to be 11 years of age at the time of death, the oldest longevity reported for a wild Leadbeater's possum.
2.2. Post mortem examination
Upon discovery, the cadaver was immediately transported at ambient temperature from the field to the Australian Wildlife Health Center, Healesville Sanctuary, Zoos Victoria, Australia, for post mortem examination. Impression smears of lung, spleen and testicle were performed and stained routinely with Diff Quik (Australian Biostain, Victoria, Australia). Representative tissue samples of all viscera and skin were stored in 10% neutral-buffered formalin and submitted for histopathological examination (Gribbles Veterinary Pathology). These tissues were embedded in paraffin, sectioned at 5 μm and stained with haematoxylin and eosin. Additional sections of lung, brain, liver and spleen were collected and stored individually at −20 °C. This stored lung tissue was subsequently subjected to molecular investigation.
2.3. Molecular analyses
PCR-coupled sequencing was used for the molecular identification of nematode larvae observed in lung tissue. First, total genomic DNA was isolated from frozen lung tissue containing such larvae; ~0.2 g of tissue was placed in 400 μl of extraction buffer (20 mM Tris–HCl [pH 8.0], 100 mM EDTA, and 1% SDS) and 20 μl of proteinase K 20 mg/ml (Promega, WI, USA) overnight and mini-column purification (Wizard DNA Clean-Up Promega, WI, USA), and eluted into 50 μl of H20. Subsequently, 2 μl of this DNA sample were subjected to PCR, targeting 830 bp of the small subunit of the nuclear ribosomal RNA gene (SSU) (cf. Lefoulon et al., 2015) employing primers F18ScF1 (forward: 5′- ACC GCC CTA GTT CTG ACC GTA AA -′3) and F18ScR1 (reverse: 5′- GGT TCA AGC CAC TGC GAT TAA AGC -′3) using the following cycling protocol: 94 °C for 5 min (initial denaturation), followed by 35 cycles of 94 °C for 30 s (denaturation), 58 °C for 45 s (annealing) and 72 °C for 1 min (extension), with a final extension of 72 °C for 5 min. A second nested-PCR assay targeting 650 bp of the mitochondrial cytochrome c oxidase 1 gene (cox1) employing primers FCo1extdF1 (forward: 5′- TAT AAT TCT GTT YTD ACT A -′3) and FCo1extdR1 (reverse: 5′- ATG AAA ATG AGC YAC WAC ATA A -′3) in the primary PCR (Lefoulon et al., 2015) and primers COIintF (forward: 5′- TGA TTG GTG GTT TTG GTA A -′3) and COIintR (reverse: 5′- ATA AGT ACG AGT ATC AAT ATC -′3) in the second PCR (Casiraghi et al., 2001). Both PCRs employed the following cycling protocol: 94 °C for 5 min (initial denaturation), followed by 35 cycles of 94 °C for 30 s (denaturation), 52 °C for 45 s (annealing) and 72 °C for 1 min (extension), with a final extension of 72 °C for 5 min. All PCRs were conducted in a volume of 50 μl containing GoTaq Flexi buffer (Promega, WI, USA), 3.0 mM of MgCl2, 200 μM of each deoxynucleotide triphosphate, 25 pmol of each primer and 1 U of GoTaq DNA polymerase (Promega, WI, USA).
Known test-positive (Onchocerca volvulus DNA), test-negative and no-template controls were included in each PCR run. The intensity and size of all amplicons were assessed by agarose electrophoresis. PCR products were sequenced bi-directionally using a standard protocol, and the SSU (712 bp) and cox1 (662 bp) sequences obtained were separately aligned with reference sequences representing distinct filarioid species and Mastophorus muris as the outgroup obtained from the National Center for Biotechnology Information (NCBI) database. Sequences were aligned using the program MUSCLE (Edgar, 2004), and alignments adjusted manually using the program Mesquite v.3.61 (Maddison and Maddison, 2015). Phylogenetic analyses of sequence data were conducted by Bayesian inference (BI) using Monte Carlo Markov Chain analysis in the program MrBayes v.3.2.6 (Ronquist et al., 2012). The likelihood parameters set for BI analyses of sequence data were based on the Akaike Information Criteria test in IQ-TREE v.2 (Minh et al., 2020), with the number of substitutions (Nst) set at 2 with no rate variation across sites for SSU and (Nst) set at 6 and an invariant gamma-distribution for cox1. Posterior probability (pp) values were calculated by running 2,000,000 generations with four simultaneous tree-building chains. Trees were saved every 100th generation. At the end of each run, the standard deviation of split frequencies was <0.01, and the potential scale reduction factor approached one. For each analysis, a 50% majority rule consensus tree was constructed based on the final 75% of trees generated by BI. Analyses were run three times to ensure convergence and insensitivity to priors. The sequences are available from GenBank (NCBI) under accession nos. MT731343 (SSU) and MT724666 (cox1).
3. Results
3.1. Pathological findings
At gross necropsy, the carcass was emaciated, weighed 84 g and had moderate to marked autolysis. The teeth had age-related attrition. Superficial dermatitis, with alopecia and crusting, was evident, particularly along the dorsum. Dry scabs were present on the feet and left dorsal scapular regions and were consistent with conspecific aggression.
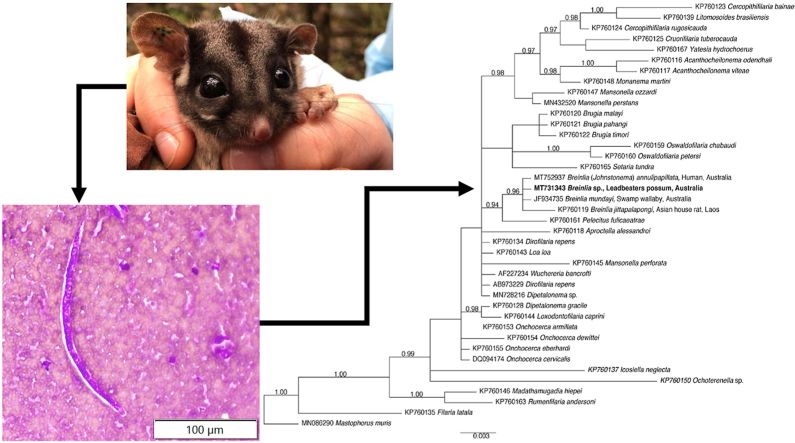
Cytology of the impression smears from the lung (Fig. 1A and B) revealed limited to moderate nucleated cellularity with poor cellular preservation, and an abundant background of blood cells and bacteria (the latter being interpreted as post mortem overgrowth). Numerous microfilariae of up to 180 μm in length and 15 μm in width, with a rounded anterior end and a tapering tail, were identified in the smears. Similarly, testicular smears were moderately cellular with poor cellular preservation and occasional microfilariae (Fig. 1C).
Fig. 1.
Photomicrographs of microfiliariae (arrows) in longitudinal orientation in impression smears of tissues of an aged, adult male Leadbeater's possum. (A) Lung. D-Q stain ( × 40 magnification). (B) Lung. D-Q stain ( × 40 magnification). (C) Testis. D-Q stain ( × 40 magnification). Scale-bar indicates 100 μm.
The interpretation of histological findings was hindered by tissue autolysis. Intravascular microfilaria were present in the lung and liver, and were not associated with inflammation or tissue damage. One section of skin had a focal area of subcutaneous tissue damage with an embedded portion of a microfilaria. Within the lumen of scattered hair follicles were occasional cross-sections of Demodex parasites. There was no discernible, associated follicular inflammation. A small focus of bile duct hyperplasia was detected in the liver.
3.2. Molecular identification of a filarioid of the genus breinlia
The 712 bp SSU sequence obtained from the lung DNA sample matched (over 700 bp) with reference sequences for the filarioid nematodes Breinlia mundayi and had 99% (711/712) identity with sequences representing Breinlia (Johnstonema) annulipapillata (GenBank accession nos. JF934735 and MT752937). The 662 bp of the cox1 sequence obtained had a 92% (552/603 bp) match to Breinlia jittapalapongi and 91% (601/659 bp) match to B. (J.) annulipapillata (accession nos. KP760170 and MT754705; no cox1 gene sequence of B. mundayi was available in the GenBank database).
The phylogenetic tree constructed using SSU sequence data for representative filarioid nematodes and the spiruroid Mastophorus muris as an outgroup (Fig. 2) showed a well-supported branch with the filarioid from the Leadbeater's possum B. mundayi and B. (J.) annulipapillata grouping with B. jittapalapongi. Similarly, for the cox1 tree (Fig. 3), the filarioid sequence from this possum grouped on a strongly-supported branch with B. jittapalapongi and B. (J.) annulipapillata.
Fig. 2.
Relationship of the novel Breinlia sp. Taxon (bold-type) from the lung of the Leadbeater's possum with members of the Onchocercidae, established by phylogenetic analysis of small subunit of nuclear ribosomal RNA gene (SSU) sequence data employing the Bayesian method. Posterior probabilities are indicated at nodes. Mastophorus muris was used as an outgroup.
Fig. 3.
Relationship of the novel Breinlia sp. Taxon (in bold-type) from lung tissue from the Leadbeater's possum with members of the Onchocercidae, established by phylogenetic analysis of cytochrome c oxidase subunit 1 (cox1) sequence data employing the Bayesian method. Posterior probabilities are indicated at nodes. Mastophorus muris was used as an outgroup.
4. Discussion
Using molecular approaches, this study has confirmed the presence of Breinlia species in a Leadbeater's possum. Microfilariae were detected in multiple tissues, but could not be identified conclusively to the genus or species level using histological and cytological methods. Identification to the genus level was achieved using a combined PCR-sequencing and phylogenetic technique. Specific identification was not possible due to scant sequence data for Breinlia species in the GenBank database. Comparisons could only be made to three species of Breinlia, specifically B. jittapalapongi from an Asian house rat (Rattus tanezumi) from Laos (Lefoulon et al., 2015), B. (J.) annulipapillata from the eye of a human in Queensland, Australia (thought to relate to the zoonotic transmission from a macropodid marsupial) (Koehler et al., 2021) and B. mundayi from a swamp wallaby (Wallabia bicolor) from New South Wales, Australia (Laetsch et al., 2012). Based on the results from the phylogenetic analysis of SSU and cox1 sequence data, we could confidently assign this parasite to the genus Breinlia (Fig. 3 and 4).
Twenty-two species of the genus Breinlia, subgenus Breinlia, are known from monotreme, marsupial and rodents hosts in Australia and Papua New Guinea, five species from a sciurid and rodent hosts in South East Asia, and two species from a sciurid and a lorisid host in India (Spratt, 2011; Veciana et al., 2015). Host specificity is highly variable as exemplified by species common to possums, kangaroos and wallabies in Australia, and rodents and other hosts in South East Asia. Filarioid nematodes occurring in Australasian hosts include a number of species of Breinlia, as well as other genera, occurring in the possum families Petauridae, Pseudocheiridae and Phalangeridae (Spratt, 2011).
Minimal pathology was associated with the presence of microfilariae in this possum specimen, despite his geriatric age, concurrent demodicosis and a history of chronic dermatitis, which could otherwise indicate a compromised immune status (Mueller et al., 2020). The cause of death of this individual is unknown, but was likely age-related. Historical necropsy records from 1977 from the Melbourne Veterinary School (Presidente, 1977) describe a granulomatous reaction surrounding small filarioids in the liver and a sectioned filarioid in diseased cardiac muscle from a highland Leadbeater's possum kept in captivity with sugar gliders in Blackburn, Victoria, Australia. Whether this was the same species of filarioid as detected here is unknown.
Previously reported pathological changes associated with species of Breinlia in marsupials are variable. A report of B. oweni in a koala reported only that the parasite was located in the peritoneal cavity (Spratt, 2011). Breinlia pseudocheiri has been identified within the peritoneal and pleural cavities in a diverse range of host species belonging to the possum families Petauridae, Pseudocheiridae and Phalangeridae (Spratt, 2011). Sullivan (in Spratt, 2011) reported that this species in P. peregrinus was associated with host inflammation, including endothelial hypertrophy, perivascular lymphocytic cuffing, meningoencephalitis, and suggested that neurotropism may be a feature of this parasite. This was not apparent in the present case. A single subcutaneous lesion associated with microfilariae of this filarioid concurs with existing knowledge that members of this nematode group elicit cutaneous lesions to attract potential arthopod intermediate hosts to eggs or larvae (Anderson, 1992). Other pathological changes were not observed here, although the degree of autolysis in the tissues from this possum may have masked subtle histological changes.
Four species of Breinlia are known to use species of Aedes mosquitoes as intermediate hosts (Spratt, 2011). The swamp habitat of the lowland Leadbeater's possum may facilitate mosquito-borne parasitism, as was suggested previously for a protozoan infection in a lowland individual (Scheelings et al., 2016). The limited pathological changes seen at necropsy suggests that infection with Breinlia sp. May not cause significant disease in this possum species. The conservation strategy for the lowland Leadbeater's possum relies on translocation of the species to new localities, coupled with gene-pool mixing with highland individuals to allieveate inbreeding. Where possible, disease-screening of translocated animals should include microscopic examination and, ideally, the molecular testing of blood samples. As only three species of Breinlia have been genetically characterised to date (Laetsch et al., 2012; Lefoulon et al., 2015; Koehler et al., 2021), future molecular studies are needed to characterise more congeners by their SSU and cox1 sequences. This would assist in understanding the taxonomy and phylogentic relationships of the genus Breinlia.
Declaration of competing interest
None.
Acknowledgements
C.S. is supported by a Zoos Victoria Scholarship and the Ernest Stewart Memorial Research Grant from the University of Melbourne. The authors thank the Histopathology Department at the Australian Clinical Laboratories for cytology and for histology slide preparation, Dr David Spratt for constructive comments and suggestions on the manuscript and Dr Andrew Stent for assistance with access to histopathology records at the Melbourne Veterinary School. This study was partially supported by a grant from the Australian Research Council (to RBG and AVK). The authors wish to acknowledge Zoos Victoria and the field team, including Arabella Eyre and Vivianna Miritis.
References
- Anderson R. CAB International, University Press; Cambridge: 1992. Nematode Parasites of Vertebrates Their Development and Transmission; p. 437. [Google Scholar]
- Booth R. Veterinary management of Leadbeater's possum (Gymnobelideus leadbeateri) In: Myronuik P.O., editor. International Studbook. Zoological Board of Victoria; Victoria: 1995. pp. 55–68. [Google Scholar]
- Casiraghi M., Anderson T., Bandi C., Bazzocchi C., Genchi C. A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology. 2001;122:93. doi: 10.1017/s0031182000007149. [DOI] [PubMed] [Google Scholar]
- Department of Environment, Land, Water & Planning . 2014. Action Statement No. 62. Leadbeater's Possum Gymnobelideus Leadbeateri. Flora and Fauna Guarantee Act 1988. [Google Scholar]
- Dunnet G.M., Mardon D.K. A monograph of Australian fleas (Siphonaptera) Aust. J. Zool. Suppl. Ser., No. 1974;30:273. [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B.D., Harley D.P.K., Lindenmayer D.B., Taylor A.C. Population genetic analysis reveals a long-term decline of a threatened endemic Australian marsupial. Mol. Ecol. 2009;18:3346–3362. doi: 10.1111/j.1365-294X.2009.04269.x. [DOI] [PubMed] [Google Scholar]
- Harley D.K.P. An overview of actions to conserve Leadbeater's possum Gymnobelideus leadbeateri. Victorian Nat. 2016;133:85–97. [Google Scholar]
- Harley D.K.P., Worley M.A., Harley T.K. The distribution and abundance of Leadbeater's possum Gymnobelideus leadbeateri in lowland swamp forest at Yellingbo Nature Conservation Reserve. Aust. Mammal. 2005;27:7–15. [Google Scholar]
- Koehler A.V., Robson J.M.B., Spratt D.M., Hann J., Beveridge I., Walsh M., McDougall M., Bromely M., Hume A., Sheorey H., Gasser R.B. Ocular filariasis in human caused by Breinlia (Johnstonema) annulipapillata, Australia. Emerg. Infect. Dis. 2021;27:297–300. doi: 10.3201/eid2701.203585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laetsch D.R., Heitlinger E.G., Taraschewski H., Nadler S.A., Blaxter M.L. The phylogenetics of Anguillicolidae (Nematoda: anguillicoloidea), swimbladder parasites of eels. BMC Evol. Biol. 2012;12:60. doi: 10.1186/1471-2148-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefoulon E., Bain O., Bourret J., Junker K., Guerrero R., Cañizales I., Kuzmin Y., Satoto T.B.T., Cardenas-Callirgos J.M., de Souza Lima S. Shaking the tree: multi-locus sequence typing usurps current onchocercid (filarial nematode) phylogeny. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer D.B., Nix H.A., McMahon J.P., Hutchinson M.F., Tanton M.T. The conservation of the Leadbeater's possum, Gymnobelideus leadbeateri (McCoy): a case study of the use of bioclimatic modelling. J. Biogeogr. 1991;18:371–383. [Google Scholar]
- Lindenmayer D.B., Tanton M.T., Viggers K.L. The Fur-inhabiting ectoparasites of Leadbeater's possum Gymnobelideus leadbeateri (Marsupialia: Petauridae) Aust. Mammal. 1994;17:109–111. [Google Scholar]
- Lindenmayer D.B., Blanchard W., McBurney L., Blair D., Banks S., Likens G.E., Franklin J.F., Laurance W.F., Stein J., Gibbons P. Interacting factors driving a major loss of large trees with cavities in a forest ecosystem. PloS One. 2012;7 doi: 10.1371/journal.pone.0041864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. An investigation of the diet of captive Leadbeater's possum Gymnobeledius leadbeateri) In: Myronuik P.O., editor. International Studbook. Zoological Board of Victoria; Victoria: 2012. pp. 55–68. [Google Scholar]
- Maddison W.P., Maddison D.R. Vol. 3.04. 2015. (Mesquite; a Modular System for Evolutionary Analysis). [Google Scholar]
- Minh B.Q., Schmidt H.A., Chernomor O., Schrempf D., Woodhams M.D., von Haeseler A., Lanfear R. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller R.S., Rosenkrantz W., Bensignor E., Karaś-Tęcza J., Paterson T., Shipstone M.A. Diagnosis and treatment of demodicosis in dogs and cats. Vet. Dermatol. 2020;31:4. doi: 10.1111/vde.12806. e2. [DOI] [PubMed] [Google Scholar]
- Presidente P. The University of Melbourne, Veterinary Paraclinical Sciences; 1977. Histopathology Report W557/77. [Google Scholar]
- Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheelings T.S., McLaren P.J., Tatarczuch L., Slocombe R.F. Plasmodium infection in a Leadbeater's possum (Gymnobelideus leadbeateri) Aust. Vet. J. 2016;948:299–304. doi: 10.1111/avj.12466. [DOI] [PubMed] [Google Scholar]
- Smales I. The discovery of Leadbeater's possum, Gymnobelideus leadbeateri McCoy, resident in a lowland swamp woodland. Victorian Nat. 1994;111:178–182. [Google Scholar]
- Smith A.P., Lindenmayer D.B. Tree hollow requirements of Leadbeater's possum and other possums and gliders in timber production ash forests of the Victorian central highlands. Aust. Wildl. Res. 1988;15:347–362. [Google Scholar]
- Spratt D.M. New records of filarioid nematodes (Nematoda: filarioidea) parasitic in Australasian monotremes, marsupials and murids, with descriptions of nine new species. Zootaxa. 2011;2860:1–61. [Google Scholar]
- Veciana M., Bain O., Morand S., Chaisiri K., Douangboupha B., Miquel J., Ribas A. Breinlia (Breinlia) jittapalapongi n. sp. (nematoda: filarioidea) from the asian house rat Rattus tanezumi temminck in Lao PDR. Syst. Parasitol. 2015;90:237–245. doi: 10.1007/s11230-014-9544-x. [DOI] [PubMed] [Google Scholar]
- Zilko J.P., Harley D., Hansen B., Pavlova A., Sunnucks P. Accounting for cryptic population substructure enhances detection of inbreeding depression with genomic inbreeding coefficients: an example from a critically endangered marsupial. Mol. Ecol. 2020;29:2978–2993. doi: 10.1111/mec.15540. [DOI] [PubMed] [Google Scholar]