Abstract
Aims: CD4 T cell count and optimal timing of antiretroviral therapy (ART) during tuberculosis (TB) treatment are challenging. We conducted a meta-analysis to assess the association of CD4 T cell count and timing of ART initiation with immune reconstitution inflammatory syndrome (IRIS) and all-cause mortality of patients co-infected with HIV/TB. Methods: We conducted an electronic search of clinical studies dated from January 1980 to December 2019 in PubMed and EMBASE. Randomized, controlled trials evaluating low-base CD4 T cell count (< 50 cells/μL) versus high-base CD4 T cell count (≥ 50 cells/μL), and/or early ART initiation (1 to 28 days after starting TB treatment) versus delayed ART initiation (≥ 28 days after starting TB treatment) were included. The primary endpoints were all-cause mortality and TB-related immune reconstitution inflammatory syndrome (IRIS-TB). The risk ratio (RR) was calculated as a measure of intervention effect. Mantel-Haenszel method was used to estimate the RR. Results: Ten trials (n = 5226) were conducted in North America, Africa, and Asia. We found that low-baseline CD4 T cell count increased the incidence of TB-associated IRIS (RR, 1.47; 95% CI, 1.24-1.75; I2 = 58%) and all-cause mortality (RR, 2.42; 95% CI, 1.71-3.42; I2 = 41%) compared with high baseline CD4 T cell count, and early ART initiation increased the incidence of TB-associated IRIS compared with delayed ART initiation (RR, 1.80; 95% CI, 1.57-2.07; I2 = 74%). However, early ART initiation did not reduce all-cause mortality (RR, 0.91; 95% CI, 0.74-1.12; I2 = 49%) compared with delayed ART initiation.Conclusions: The present study demonstrates that low-baseline CD4 T cell count (< 50 cells/μL) in patients co-infected with TB-HIV increases the incidence of TB-associated IRIS and all-cause mortality. Early ART initiation (≤ 28 days) in patients co-infected with TB-HIV increases the incidence of TB-associated IRIS. However, evidence is insufficient to refute or support a survival benefit conferred by the comparison between early ART initiation (≤ 28 days) and delayed ART initiation.
Keywords: CD4 T cell count, antiretroviral therapy, immune reconstitution inflammatory syndrome, tuberculosis, human immunodeficiency virus
Introduction
Tuberculosis (TB) is a common cause of deaths in human immunodeficiency virus (HIV)-infected patients. In 2012, about one-third of the one million patients with new TB among those who were HIV-positive died [1]. Among the new cases, more than 70% were in resource-limited settings. Without the use of anti-retroviral therapy (ART), the risk of HIV-infected adults dying during TB treatment ranges from 16% to 37% among those with CD4 T cell counts greater than 350 cells/μL [2-6]. For many reasons, the basic time and optimal timing of CD4+ T cell count to start antiretroviral therapy with anti-TB drugs during the treatment for drug-resistant TB are still challenging [2-6].
The CD4 T-cell counts at baseline and optimal timing of ART initiation in TB-HIV co-infected patients who have begun TB therapy require further definition. The current World Health Organization (WHO) guidelines recommend that for severely immunosuppressed patients (CD4 T-cell counts < 50 cells/μL), TB therapy should be started first, and then retroviral therapy treatment should be initiated “within the first 8 weeks” of starting TB therapy. Further data are shown in various expert reviews [7-10]. To provide an up-to-date summary, we conducted a meta-analysis to assess the association of CD4 T cell count and timing of ART initiation with IRIS and all-cause mortality among TB-HIV co-infected patients.
Materials and methods
Search strategy
The meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses statement. An electronic search of clinical studies dated from January 1980 to December 2019 was independently conducted by two reviewers with English language restrictions in PubMed and EMBASE.
Trial selection
Two reviewers evaluated the eligibility of trials. Disagreements were resolved by negotiation. Randomized, controlled trials that compared low-baseline CD4 T cell count (less than 50 cells/μL) with high-baseline CD4 T cell count (greater or equal to 50 cells/μL), and those that compared TB-HIV co-infected patients who had early ART initiation (≤ 28 days after the initiation of TB therapy) with delayed ART initiation (> 28 days after the initiation of TB therapy) were included in the present study.
Data extraction
Two reviewers independently extracted the data. Trial design, demographic characteristics, and outcome indicators were extracted and the bias was evaluated for each trial. Discrepancies were resolved by reaching consensus through discussion. The primary outcomes considered included IRIS-TB and all-cause mortality.
Data synthesis and analysis
Heterogeneity of trials was assessed by forest plots and tau2 test, and I2 statistic. The results of individual trials were assessed by R (version 3.3.2 “meta” package).
Results
Basic features
Figure 1 shows the process of research identification and selection. The main outcomes of four trials were not related to IRIS-TB [11-14]. The main outcomes of fifteen trials could not be classified by “low CD4/high CD4” or “early/delayed” [15-29]. Four trials were repeated research [30-33]. All of these trials were excluded. A total of 10 studies were included in this meta-analysis [34-43], and their characteristics are listed in Table 1. The studies were conducted in North America, Africa, and Asia and published between 2011 and 2018. The mean length of follow-up of the participants ranged from 8 to 528 days, the mean age ranged between 32 and 38 years, the mean BMI ranged between 17 and 26 kg/m2, the median CD4 T cell count ranged between 25 and 367 cells/μL, and the median log10 HIV-1 RNA viral load ranged from 5.0 to 5.7 copies/mL. Five studies compared low CD4 T cell count with high CD4 T cell count in IRIS-TB. Nine studies compared early ART with delayed ART in IRIS-TB. Three studies compared low CD4 T cell count with high CD4 T cell count regarding all-cause deaths. Six studies compared early ART with delayed ART regarding all-cause deaths. In addition, 827 patients (16%) developed IRIS, and 333 patients (9%) died.
Figure 1.
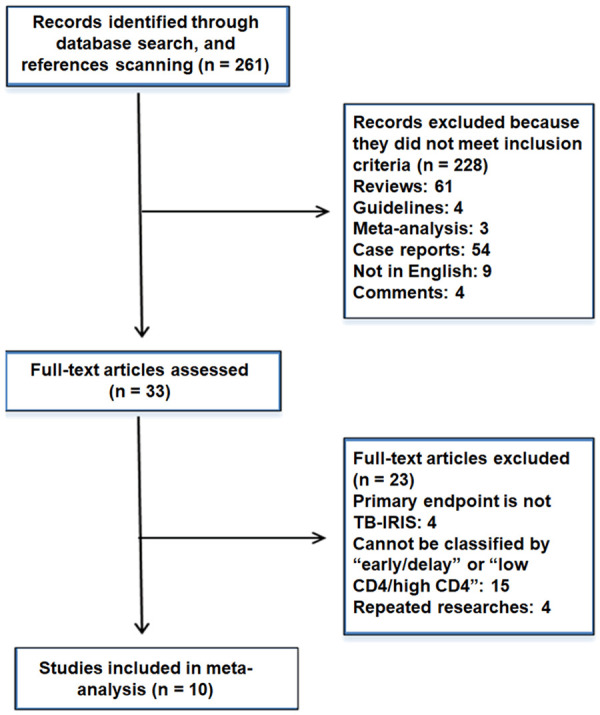
Summary of evidence search and selection. The main outcomes of four trials were not related to IRIS-TB. The main outcomes of fifteen trials cannot be classified by “low CD4/high CD4” or “early/delayed”. Four trials were repeated research. All of these trials were excluded. A total of 10 studies were included in this meta-analysis.
Table 1.
Characteristics of the 10 studies included in the meta-analysis
| Authors | Publication year | Country | Study design | No. of patients | Length of follow-up (wk) | Mean age (y) | No. of male | No. of female | Mean BMI | Median CD4 cell count (cells/μL) | Median HIV-1 RNA viral load-log10 (copies/ml) | Patients with TB at enrolment | Days on tuberculosis therapy at ART start | Incidence of TB-Associated IRIS | Deaths of all-cause |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdool Karim SS et al. | 2011 | South Africa | Randomized clinical trial, RCT | 429 | 18 | 34 | 209 | 220 | 26 | 152 | 5 | 429 | 28 | 61 | 30 |
| Havlir DV et al. | 2011 | USA | Randomized clinical trial, RCT | 806 | 48 | 34 | 501 | 305 | 19 | 76 | 5.4 | 374 | 14 | 61 | |
| Blanc FX et al. | 2011 | Cambodia | Randomized clinical trial, RCT | 661 | 50 | 35 | 425 | 236 | 17 | 25 | 5.7 | 661 | 14/56 | 155 | 149 |
| Manosuthi W et al. | 2012 | Thailand | Randomized clinical trial, RCT | 156 | 54 | 38 | 121 | 35 | 19 | 45 | 5.7 | 156 | 28/84 | 81 | 11 |
| Sinha S et al. | 2012 | India | Randomized clinical trial, RCT | 150 | 54 | 35 | 126 | 24 | 18 | 140 | 5.3 | 150 | 14-28/56-84 | 15 | 16 |
| Laureillard D et al. | 2013 | Cambodia | Randomized clinical trial, RCT | 597 | 48 | 36 | 385 | 212 | 18 | 26 | 5.6 | 530 | 14/56 | 155 | |
| Mfinanga SG et al. | 2014 | South Africa | Randomized clinical trial, RCT | 1675 | 24 | 32 | 922 | 616 | 19 | 367 | 1675 | 14/180 | 171 | 63 | |
| Amogne W et al. | 2015 | Ethiopia | Randomized clinical trial, RCT | 478 | 8 | 36 | 245 | 233 | 19 | 72 | 5.2 | 478 | 7/28/56 | 22 | 64 |
| Haridas V et al. | 2015 | Cambodia | Randomized clinical trial, RCT | 154 | 48 | 154 | 14/56 | 50 | |||||||
| Meintjes G et al. | 2018 | South Africa | Randomized clinical trial, RCT | 120 | 12 | 36 | 73 | 47 | 21 | 49 | 5.6 | 89 | 30 | 56 |
Bias of included studies
A bias evaluation of the included studies is shown in Figure 2. The trials showed a relatively low risk of bias.
Figure 2.
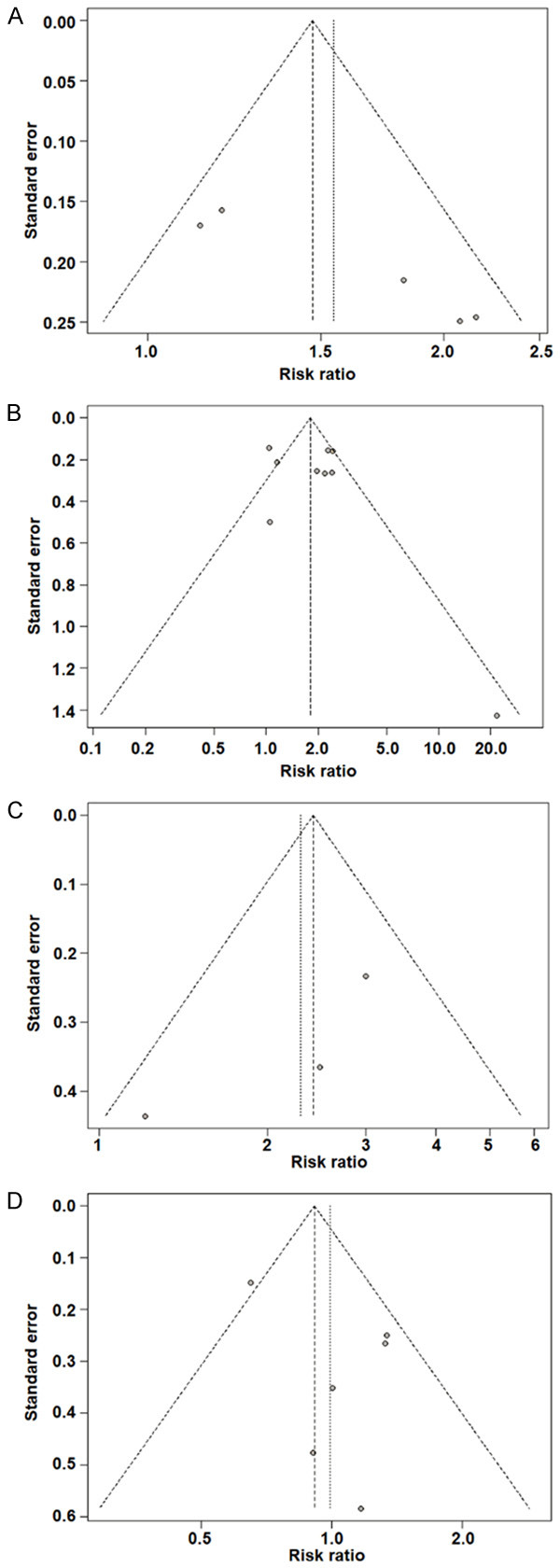
Risk-of-bias assessments of the included studies. A. IRIS-TB comparison between low CD4 T cells and low CD4 T cells at baseline. B. IRIS-TB comparison between early ART initiation and delayed ART initiation. C. All-cause mortality comparison between low CD4 T cells and low CD4 T cells at baseline. D. All-cause mortality comparison between early ART initiation and delayed ART initiation.
Heterogeneity assessment
Figures 3, 4, 5 and 6 show the heterogeneity among the included trials. I2 ranged from 41% to 74%. There was no heterogeneity among the studies.
Figure 3.
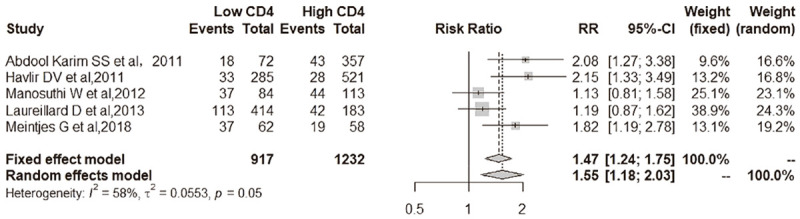
IRIS-TB comparison between low CD4 T cell and low CD4 T cell at baseline. Among 917 participants with low CD4 T cell count at baseline, 238 (26.0%) developed IRIS-TB compared with 176 of 1232 (14.3%) in high CD4 group [5 studies; relative risk, RR, 1.47 (95% CI, 1.24-1.75); I2 = 58%].
Figure 4.
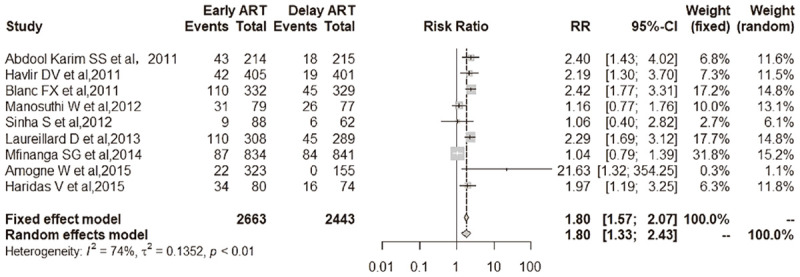
IRIS-TB comparison between early ART initiation and delayed ART initiation. Among 2663 participants with early ART, 488 (18.3%) became IRIS-TB compared with 259 of 2443 (10.6%) in the delayed ART set [9 studies; RR, 1.80 (95% CI, 1.57-2.07); I2 = 74%].
Figure 5.
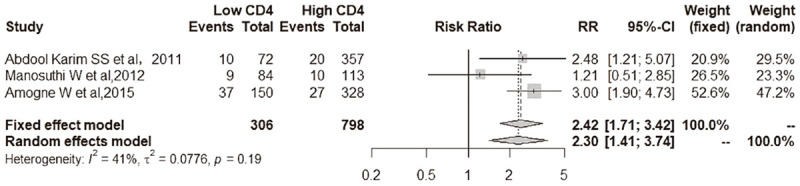
All-cause mortality comparison between low CD4 T cells and low CD4 T cells at baseline. Patients with low CD4 T cell count at baseline [18.3% (56 of 306 patients)] had a higher all-cause mortality than those with high CD4 T cell count at baseline [7.1% (57 of 798 patients)] at the end of follow-up [3 trials; RR, 2.42 (95% CI, 1.71-3.42); I2 = 41%].
Figure 6.
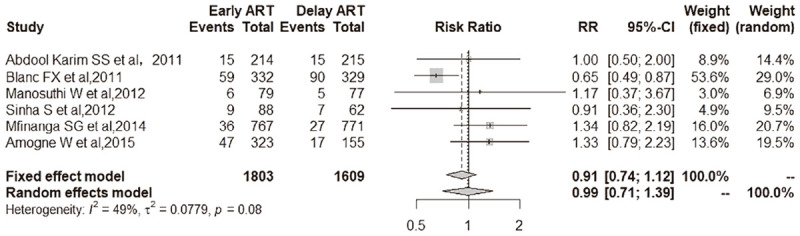
All-cause mortality comparison between early ART initiation and delayed ART initiation. Patients randomly assigned to early ART group [9.5% (172 of 1803 patients)] had insignificantly lower all-cause mortality than those receiving delayed ART [10.0% (161 of 1609 patients)] at the end of follow-up [6 trials; RR, 0.91 (95% CI, 0.74-1.12); I2 = 49%].
IRIS-TB
Low CD4 T cell count versus high CD4 T cell count at base
Among 917 participants with low CD4 T cell count at baseline, 238 (26.0%) developed IRIS-TB compared with 176 of 1232 (14.3%) in high CD4 group [5 studies; relative risk, RR, 1.47 (95% CI, 1.24-1.75); I2 = 58%] (Figure 3). As shown in Figure 3, low CD4 T cell count at baseline was associated with a higher incidence of IRIS-TB than a high CD4 T cell count at baseline for patients.
Early ART initiation versus delayed ART initiation
Among 2663 participants with early ART, 488 (18.3%) became IRIS-TB compared with 259 of 2443 (10.6%) in the delayed ART set [9 studies; RR, 1.80 (95% CI, 1.57-2.07); I2 = 74%] (Figure 4). As shown in Figure 4, early ART was associated with a higher incidence of IRIS-TB than delayed ART for patients.
All-cause mortality
Low CD4 versus high CD4 T cell count at base
Patients with low CD4 T cell count at baseline [18.3% (56 of 306 patients)] had a higher all-cause mortality than those with high CD4 T cell count at baseline [7.1% (57 of 798 patients)] at the end of follow-up [3 trials; RR, 2.42 (95% CI, 1.71-3.42); I2 = 41%] (Figure 5).
Early initiation versus delayed initiation of ART
Patients randomly assigned to the early ART group [9.5% (172 of 1803 patients)] had insignificantly lower all-cause mortality than those receiving delayed ART [10.0% (161 of 1609 patients)] at the end of follow-up [6 trials; RR, 0.91 (95% CI, 0.74-1.12); I2 = 49%] (Figure 6).
Discussion
The present systematic review included 10 trials with 5226 participants to assess the association of timing of ART initiation and CD4 T cell count with IRIS and all-cause mortality among TB-HIV co-infected patients receiving TB therapy.
Overall, patients with baseline CD4 T cell count < 50 cells/μL had a higher incidence of IRIS-TB than those with baseline CD4 T cell count ≥ 50 cells/μL. Patients commencing ART within 28 days after starting TB therapy had a higher incidence of IRIS-TB than those commencing ART more than 28 days after starting TB therapy. Compared to IRIS-TB, mortality benefit is a more important consideration in treating TB-HIV co-infected patients. Overall, patients with baseline CD4 T cell count < 50 cells/μL had higher all-cause mortality than those with baseline CD4 T cell count ≥ 50 cells/μL. By contrast, patients commencing ART within 28 days after starting TB therapy had statistically insignificantly lower all-cause mortality than those commencing ART more than 28 days after starting tuberculosis therapy. Although the present meta-analysis strongly supported timely treatment in patients with CD4 T cell counts less than 50 cells/μL, it had insufficient evidence to support or refute a survival benefit conferred by the comparison between early ART initiation (≤ 28 days) and delayed ART initiation (> 28 days). This indicates that we need more nuanced data to better define the time threshold for early ART.
Except for the incidence of IRIS-TB and mortality, concurrent treatment of HIV and TB still requires consideration of adherence and drug interactions for both infections [44,45]. These considerations highlight the need for more in-depth research to conduct rigorous controlled trials through the use of anti-inflammatory drugs (such as glucocorticoids or non-steroidal anti-inflammatory drugs) in an environment where TB and HIV co-infection are highly prevalent. Such trials are under way in high-burden settings, including South Africa [46].
Our search for reviews on the association of CD4 T cell count and timing of ART initiation with IRIS and mortality among patients co-infected with TB-HIV was updated in December 2019 and identified 2 reviews. Being consistent with our findings, Müller et al. [10] conclude that the risk of IRIS is related to CD4 cell count at the beginning of ART, with a high risk in patients with less than 50 cells/μl. Uthman and colleagues [47] conclude that early ART in HIV-infected adults with newly diagnosed TB improves survival in those with CD4 T cell counts fewer than 50 cells/μL, which is not comparable to our results. This is possibly because that we did not perform a subgroup analysis.
The strengths of our meta-analysis include rigorous methods to control bias during the review process and a exhaustive search for multiple databases to identify eligible randomized, controlled study. However, the present study still had limitations in bias in our analyses. For example, only 3 trials that compared early ART with delayed ART provided enough data to analyze mortality.
In conclusion, the present study demonstrates that low baseline CD4 T cell count (< 50 cells/μL) in TB-HIV co-infected patients increases the incidence of TB-associated IRIS and all-cause mortality. Early ART (≤ 28 days) in TB-HIV co-infected patients increases the incidence of TB-associated IRIS. However, evidence is not enough to refute or support a survival benefit conferred by the comparison between early ART initiation (≤ 28 days) and delayed ART initiation.
Acknowledgements
The authors thank all member of their team for help and dedication. International Science and technology cooperation project of Shanxi Province (2015081033).
The current study was approved by the Ethics Committee of Shanxi Medical University. Informed consent was obtained from all patients.
Disclosure of conflict of interest
None.
References
- 1.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 2.Lucas SB, De Cock KM, Hounnou A, Peacock C, Diomande M, Hondé M, Beaumel A, Kestens L, Kadio A. Contribution of tuberculosis to slim disease in Africa. BMJ. 1994;308:1531–1533. doi: 10.1136/bmj.308.6943.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perriëns JH, St Louis ME, Mukadi YB, Brown C, Prignot J, Pouthier F, Portaels F, Willame JC, Mandala JK, Kaboto M, et al. Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. N Engl J Med. 1995;332:779–784. doi: 10.1056/NEJM199503233321204. [DOI] [PubMed] [Google Scholar]
- 4.Cohen T, Murray M, Wallengren K, Alvarez GG, Samuel EY, Wilson D. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: a postmortem study. PLoS Med. 2010;7:e1000296. doi: 10.1371/journal.pmed.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu R, Mills EJ, Beyene J, Pullenayegum E, Bakanda C, Nachega JB, Devereaux PJ, Thabane L. Impact of tuberculosis on mortality among HIV-infected patients receiving antiretroviral therapy in Uganda: a prospective cohort analysis. AIDS Res Ther. 2013;10:19. doi: 10.1186/1742-6405-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nachega JB, Morroni C, Chaisson RE, Goliath R, Efron A, Ram M, Maartens G. Impact of immune reconstitution inflammatory syndrome on antiretroviral therapy adherence. Patient Prefer Adherence. 2012;6:887–891. doi: 10.2147/PPA.S38897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulle A, Van Cutsem G, Cohen K, Hilderbrand K, Mathee S, Abrahams M, Goemaere E, Coetzee D, Maartens G. Outcomes of nevirapine-and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–539. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 8.Cohen K, Meintjes G. Management of individuals requiring antiretroviral therapy and tuberculosis therapy. Curr Opin HIV AIDS. 2010;5:61–69. doi: 10.1097/COH.0b013e3283339309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uthman OA, Okwundu C, Gbenga K, Volmink J, Dowdy D, Zumla A, Nachega JB. Optimal timing of antiretroviral therapy initiation for HIV-infected adults with newly diagnosed pulmonary tuberculosis: a systematic review and meta-analysis. Ann Intern Med. 2015;163:32–39. doi: 10.7326/M14-2979. [DOI] [PubMed] [Google Scholar]
- 10.Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–261. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva CAM, Graham B, Webb K, Ashton LV, Harton M, Luetkemeyer AF, Bokatzian S, Almubarak R, Mahapatra S, Hovind L, Kendall MA, Havlir D, Belisle JT, De Groote MA. A pilot metabolomics study of tuberculosis immune reconstitution inflammatory syndrome. Int J Infect Dis. 2019;84:30–38. doi: 10.1016/j.ijid.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crump JA, Wu X, Kendall MA, Ive PD, Kumwenda JJ, Grinsztejn B, Jentsch U, Swindells S. Predictors and outcomes of Mycobacterium tuberculosis bacteremia among patients with HIV and tuberculosis co-infection enrolled in the ACTG A5221 STRIDE study. BMC Infect Dis. 2015;15:12. doi: 10.1186/s12879-014-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant PM, Komarow L, Andersen J, Sereti I, Pahwa S, Lederman MM, Eron J, Sanne I, Powderly W, Hogg E, Suckow C, Zolopa A. Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of previously vs. deferred ART during an opportunistic infection. PLoS One. 2010;5:e11416. doi: 10.1371/journal.pone.0011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao HJ, Crump JA, Ramadhani HO, Uiso LO, Ole-Nguyaine S, Moon AM, Kiwera RA, Woods CW, Shao JF, Bartlett JA, Thielman NM. Previously versus postpone fixed dose combination abacavir/lamivudine/zidovudine in patients with HIV and tuberculosis in Tanzania. AIDS Res Hum Retroviruses. 2009;25:1277–1285. doi: 10.1089/aid.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 16.Breton G, Duval X, Estellat C, Poaletti X, Bonnet D, Mvondo Mvondo D, Longuet P, Leport C, Vildé JL. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis. 2004;39:1709–1712. doi: 10.1086/425742. [DOI] [PubMed] [Google Scholar]
- 17.da Silva TP, Giacoia-Gripp CBW, Schmaltz CA, Sant’Anna FM, Saad MH, Matos JA, de Lima ESJCA, Rolla VC, Morgado MG. Risk factors for increased immune reconstitution in response to mycobacterium tuberculosis antigens in tuberculosis HIV-infected, antiretroviral-naïve patients. BMC Infect Dis. 2017;17:606. doi: 10.1186/s12879-017-2700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumarasamy N, Chaguturu S, Mayer KH, Solomon S, Yepthomi HT, Balakrishnan P, Flanigan TP. Incidence of immune reconstitution syndrome in HIV/tuberculosis-coinfected patients after initiation of generic antiretroviral therapy in India. J Acquir Immune Defic Syndr. 2004;37:1574–1576. doi: 10.1097/00126334-200412150-00007. [DOI] [PubMed] [Google Scholar]
- 19.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 20.Manosuthi W, Kiertiburanakul S, Phoorisri T, Sungkanuparph S. Immune reconstitution inflammatory syndrome of tuberculosis among HIV-infected patients receiving antituberculous and antiretroviral therapy. J Infect. 2006;53:357–363. doi: 10.1016/j.jinf.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Michailidis C, Pozniak AL, Mandalia S, Basnayake S, Nelson MR, Gazzard BG. Clinical characteristics of IRIS syndrome in patients with HIV and tuberculosis. Antivir Ther. 2005;10:417–422. doi: 10.1177/135965350501000303. [DOI] [PubMed] [Google Scholar]
- 22.Musselwhite LW, Andrade BB, Ellenberg SS, Tierney A, Belaunzaran-Zamudio PF, Rupert A, Lederman MM, Sanne I, Sierra Madero JG, Sereti I. Vitamin D, D-dimer, interferon γ, and sCD14 levels are independently associated with immune reconstitution inflammatory syndrome: a prospective, international study. EBioMedicine. 2016;4:115–123. doi: 10.1016/j.ebiom.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–161. doi: 10.1164/ajrccm.158.1.9712001. [DOI] [PubMed] [Google Scholar]
- 24.Park WB, Choe PG, Jo JH, Kim SH, Bang JH, Kim HB, Kim NJ, Oh MD, Choe KW. Tuberculosis manifested by immune reconstitution inflammatory syndrome during HAART. AIDS. 2007;21:875–877. doi: 10.1097/QAD.0b013e3280f7751f. [DOI] [PubMed] [Google Scholar]
- 25.Serra FC, Hadad D, Orofino RL, Marinho F, Lourenço C, Morgado M, Rolla V. Immune reconstitution syndrome in patients treated for HIV and tuberculosis in Rio de Janeiro. Braz J Infect Dis. 2007;11:462–465. doi: 10.1590/s1413-86702007000500004. [DOI] [PubMed] [Google Scholar]
- 26.Tadokera R, Meintjes GA, Wilkinson KA, Skolimowska KH, Walker N, Friedland JS, Maartens G, Elkington PT, Wilkinson RJ. Matrix metalloproteinases and tissue damage in HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur J Immunol. 2014;44:127–136. doi: 10.1002/eji.201343593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tieu HV, Ananworanich J, Avihingsanon A, Apateerapong W, Sirivichayakul S, Siangphoe U, Klongugkara S, Boonchokchai B, Hammer SM, Manosuthi W. Immunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in Thailand. AIDS Res Hum Retroviruses. 2009;25:1083–1089. doi: 10.1089/aid.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wendel KA, Alwood KS, Gachuhi R, Chaisson RE, Bishai WR, Sterling TR. Paradoxical worsening of tuberculosis in HIV-infected persons. Chest. 2001;120:193–197. doi: 10.1378/chest.120.1.193. [DOI] [PubMed] [Google Scholar]
- 29.Bonnet M, Baudin E, Jani IV, Nunes E, Verhoustraten F, Calmy A, Bastos R, Bhatt NB, Michon C. Incidence of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome and impact on patient outcome. PLoS One. 2013;8:e84585. doi: 10.1371/journal.pone.0084585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, Khan M, Pienaar J, El-Sadr W, Friedland G, Abdool Karim Q. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidoo K, Yende-Zuma N, Padayatchi N, Naidoo K, Jithoo N, Nair G, Bamber S, Gengiah S, El-Sadr WM, Friedland G, Abdool Karim S. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Ann Intern Med. 2012;157:313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luetkemeyer AF, Kendall MA, Nyirenda M, Wu X, Ive P, Benson CA, Andersen JW, Swindells S, Sanne IM, Havlir DV, Kumwenda J. Tuberculosis immune reconstitution inflammatory syndrome in A5221 STRIDE: timing, severity, and implications for HIV-TB programs. J Acquir Immune Defic Syndr. 2014;65:423–428. doi: 10.1097/QAI.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meintjes G, Wilkinson RJ, Morroni C, Pepper DJ, Rebe K, Rangaka MX, Oni T, Maartens G. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. Aids. 2010;24:2381–2390. doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amogne W, Aderaye G, Habtewold A, Yimer G, Makonnen E, Worku A, Sonnerborg A, Aklillu E, Lindquist L. Efficacy and safety of antiretroviral therapy initiated one week after tuberculosis therapy in patients with CD4 T cell counts < 200 cells/μL: TB-HAART study, a randomized clinical trial. PLoS One. 2015;10:e0122587. doi: 10.1371/journal.pone.0122587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, Gengiah T, Gengiah S, Naidoo A, Jithoo N, Nair G, El-Sadr WM, Friedland G, Abdool Karim Q. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, Madec Y, Marcy O, Chan S, Prak N, Kim C, Lak KK, Hak C, Dim B, Sin CI, Sun S, Guillard B, Sar B, Vong S, Fernandez M, Fox L, Delfraissy JF, Goldfeld AE. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haridas V, Pean P, Jasenosky LD, Madec Y, Laureillard D, Sok T, Sath S, Borand L, Marcy O, Chan S, Tsitsikov E, Delfraissy JF, Blanc FX, Goldfeld AE. IRIS-TB, T-cell activation, and remodeling of the T-cell compartment in highly immunosuppressed HIV-infected patients with TB. Aids. 2015;29:263–273. doi: 10.1097/QAD.0000000000000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, Luetkemeyer AF, Hogg E, Rooney JF, Wu X, Hosseinipour MC, Lalloo U, Veloso VG, Some FF, Kumarasamy N, Padayatchi N, Santos BR, Reid S, Hakim J, Mohapi L, Mugyenyi P, Sanchez J, Lama JR, Pape JW, Sanchez A, Asmelash A, Moko E, Sawe F, Andersen J, Sanne I. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laureillard D, Marcy O, Madec Y, Chea S, Chan S, Borand L, Fernandez M, Prak N, Kim C, Dim B, Nerrienet E, Sok T, Delfraissy JF, Goldfeld AE, Blanc FX. Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome after previously initiation of antiretroviral therapy in a randomized clinical trial. Aids. 2013;27:2577–2586. doi: 10.1097/01.aids.0000432456.14099.c7. [DOI] [PubMed] [Google Scholar]
- 40.Manosuthi W, Mankatitham W, Lueangniyomkul A, Thongyen S, Likanonsakul S, Suwanvattana P, Thawornwan U, Suntisuklappon B, Nilkamhang S, Sungkanuparph S. Time to initiate antiretroviral therapy between 28 days and 12 weeks of tuberculosis treatment in HIV-infected patients: results from the TIME study. J Acquir Immune Defic Syndr. 2012;60:377–383. doi: 10.1097/QAI.0b013e31825b5e06. [DOI] [PubMed] [Google Scholar]
- 41.Meintjes G, Stek C, Blumenthal L, Thienemann F, Schutz C, Buyze J, Ravinetto R, van Loen H, Nair A, Jackson A, Colebunders R, Maartens G, Wilkinson RJ, Lynen L. Prednisone for the prevention of paradoxical tuberculosis-associated IRIS. N Engl J Med. 2018;379:1915–1925. doi: 10.1056/NEJMoa1800762. [DOI] [PubMed] [Google Scholar]
- 42.Mfinanga SG, Kirenga BJ, Chanda DM, Mutayoba B, Mthiyane T, Yimer G, Ezechi O, Connolly C, Kapotwe V, Muwonge C, Massaga J, Sinkala E, Kohi W, Lyantumba L, Nyakoojo G, Luwaga H, Doulla B, Mzyece J, Kapata N, Vahedi M, Mwaba P, Egwaga S, Adatu F, Pym A, Joloba M, Rustomjee R, Zumla A, Onyebujoh P. Previously versus postpone initiation of highly active antiretroviral therapy for HIV-positive adults with newly diagnosed pulmonary tuberculosis (TB-HAART): a prospective, international, randomised, placebo-controlled trial. Lancet Infect Dis. 2014;14:563–571. doi: 10.1016/S1473-3099(14)70733-9. [DOI] [PubMed] [Google Scholar]
- 43.Sinha S, Shekhar RC, Singh G, Shah N, Ahmad H, Kumar N, Sharma SK, Samantaray JC, Ranjan S, Ekka M, Sreenivas V, Mitsuyasu RT. Previously versus postpone initiation of antiretroviral therapy for Indian HIV-Infected individuals with tuberculosis on antituberculosis treatment. BMC Infect Dis. 2012;12:168. doi: 10.1186/1471-2334-12-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nachega JB, Rosenkranz B, Simon G, Chaisson RE, Diacon A, Taljaard J. Management of adult active tuberculosis disease in era of HIV pandemic, current practices and future perspectives. Infect Disord Drug Targets. 2011;11:134–143. doi: 10.2174/187152611795589726. [DOI] [PubMed] [Google Scholar]
- 45.Maartens G, Decloedt E, Cohen K. Effectiveness and safety of antiretrovirals with rifampicin: crucial issues for high-burden countries. Antivir Ther. 2009;14:1039–1043. doi: 10.3851/IMP1455. [DOI] [PubMed] [Google Scholar]
- 46.Lawn SD, Meintjes G. Pathogenesis and prevention of immune reconstitution disease during antiretroviral therapy. Expert Rev Anti Infect Ther. 2011;9:415–430. doi: 10.1586/eri.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]


