Abstract
Monitoring therapeutic response in patients with metastatic castration-resistant prostate cancer (mCRPC) can be challenging. We set out to determine if 18F-fluciclovine PET/CT could be a useful imaging biomarker for response to docetaxel chemotherapy in patients with mCRPC. Seven patients with mCRPC had 18F-fluciclovine PET/CT scheduled at baseline and after 1 and 6 cycles of chemotherapy. The sum of SUVmax from the prostate/bed and up to 5 metastatic bone and soft tissue/visceral lesions were recorded. The SUVpeak of the hottest lesion (PERCIST-like) was also recorded. In comparison to the baseline scan, a decrease of ≥30% was considered response; new lesions or >30% increase was progressive disease; change of <30% was stable disease. Bone scintigraphy and CT were acquired at baseline and after the 6th cycle. Response assessment was based on the Prostate Cancer Clinical Trial Working Group 3 recommendations. All (7/7) enrolled patients completed the 1st and 2nd scans, while 4/7 patients completed all 3 scans. PET response correlated with PSA response in 3/7 (42.9%) patients after 1 cycle of docetaxel, and 3/4 (75%) patients after 6 cycles of docetaxel, respectively. Bone scan and CT correlated with PSA response in 1/4 (25%) patients. There was no significant correlation between baseline 18F-fluciclovine PET parameters or changes in PET parameters and time to PSA progression. In conclusion, this exploratory study showed that 18F-fluciclovine PET/CT has better correlation with PSA response than CT or bone scan in patients with mCRPC treated with docetaxel. 18F-fluciclovine PET/CT however did not predict time to PSA progression.
Keywords: Prostate cancer, 18F-fluciclovine, docetaxel, metastatic castration-resistant prostate cancer, therapy response
Introduction
Androgen deprivation therapy is widely used in the treatment of patients with prostate cancer. While most patients with prostate cancer will respond to androgen deprivation, many will eventually progress to metastatic castrate-resistant prostate cancer (mCRPC) with poor prognosis. Chemotherapy regimens for mCRPC improve overall survival in patients with mCRPC [1-3]. Docetaxel is a first-line chemotherapy regimen for mCRPC with good overall response rates [4] and is more recently employed in the management of newly diagnosed metastatic hormone-sensitive prostate cancer. With advances in options for the treatment of prostate cancer, including chemotherapy and novel hormonal agents in newly diagnosed patients, it has become increasingly important to monitor treatment response. Yet, therapy response assessment remains a challenge [5].
Monitoring therapeutic response has traditionally been accomplished using serum biomarkers such as prostate-specific antigen (PSA), and imaging biomarkers such as bone scanning for skeletal disease, and computed tomography (CT) for nodal and soft tissue disease [6]. Bone scans are limited by high false positivity due to the flare effect [7]. Anatomic imaging such as CT is also limited by the inability of Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 to reliably assess response in soft tissue and bone metastasis in which metabolic response may be decoupled from morphologic characteristics. Molecular imaging has become important in the evaluation of response, with several studies demonstrating that molecular imaging provides an independent assessment of response to therapy and prognostic information [8-11].
Amino acid metabolism is upregulated in many tumors including prostate carcinoma. Anti-1-amino-3-F-18-fluorocyclobutane-1-carboxylic acid (18F-fluciclovine) is a synthetic amino acid analog that has demonstrated utility for the staging of prostate carcinoma compared to conventional imaging [12-15] and has been approved by the United States Food and Drug Administration for imaging of suspected recurrent prostate cancer [16]. In-vitro studies with 18F-fluciclovine have shown uptake correlates with amino acid transporter expression in castration-resistant prostate cancer cells [17]. 18F-fluciclovine PET activity has been shown to reflect cancer cell metabolism, therefore, 18F-fluciclovine PET activity in metastatic castrate-resistant prostate cancer may correlate with tumor burden as well as the response of the cancer cells to cytotoxic chemotherapy. In this exploratory study, we set out to understand if 18F-fluciclovine PET/CT would better reflect the response to docetaxel chemotherapy in patients with mCRPC compared with conventional imaging biomarkers and to also assess the correlation between 18F-fluciclovine uptake and time to PSA progression.
Materials and methods
Patients
This study was a prospective Institutional Review Board-approved trial requiring written informed consent. Inclusion criteria were castration-resistant metastatic prostate carcinoma (castrate serum testosterone <50 ng/dl or 1.7 nmol/l and three consecutive rises in PSA one week apart, resulting in two 50% increases over the nadir, and PSA >2 ng/ml) with radiologic evidence of skeletal metastases and/or nodal involvement eligible to commence chemotherapy utilizing standard regimen of docetaxel 75 mg/m2 administered intravenously every 21 days with appropriate pre-medications (steroids and anti-emetics) given over 4-6 cycles.
Conventional imaging and PSA biomarkers
Each patient had the standard of care conventional staging per institutional protocol including Technetium 99m ([99m Tc]-methylene diphosphonate (MDP)) bone scanning and CT or MR within 60 days of the PET/CT at baseline, after the 6th cycle and at one year if able. Each patient also had serum PSA assays at baseline and before administration of each cycle of chemotherapy.
18F-fluciclovine PET/CT
Each patient had a baseline 18F-fluciclovine PET/CT before the commencement of chemotherapy and after the 1st and 6th cycle of chemotherapy. 18F-fluciclovine was administered under FDA Investigational New Drug (IND) 72,437 and was synthesized using the FastLab Cassette System (GE Healthcare). Safety monitoring during the drug infusion was performed and no adverse events were recorded. All subjects were required to fast for four hours to normalize their neutral amino acid levels.
PET/CT was acquired on a GE Discovery-690 16 slice integrated PET/CT scanner (GE Healthcare, Waukesha, WI) with oral contrast, without intravenous contrast. 18F-fluciclovine (367.0±21.1 MBq) was administered as an intravenous bolus injection. Subsequently, a low-dose CT scan (120 kV, automA, maximum 160 mA) was completed from skull base to thighs for anatomic correlation and attenuation correction of emission data. At 4 minutes after radiotracer infusion, PET image acquisition of 7 consecutive 2 minutes per frame beds was completed starting from the thighs and extending superiorly to the skull base. This was immediately repeated to obtain dual time point (initial and delayed) data.
Images were reconstructed with iterative technique and interpreted on a MimVista workstation (MIM Software, Cleveland, OH). Reconstruction parameters utilized VUE point FX with 3 iterations/24 subsets and 6.4 mm filter cutoff, and reconstructed slice thickness was 3.75 mm.
Image analysis
Conventional imaging
All conventional imaging were interpreted per the usual standard of care but a targeted research interpretation was utilized for this study.
On CT or MR, bi-dimensional measurements of up to five soft-tissue lesions were recorded. These were used to determine Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria [18]. There were no bone lesions with a soft tissue component.
Bone scans findings were interpreted based on recommendations from Prostate Cancer Clinical Trial Working Group 3 (PCCTWG3) and a specialized Bone Scan Assessment Tool include in the appendix of the following reference was utilized [19].
18F-fluciclovine PET/CT
Three-dimensional regions of interest (ROI) were drawn at a MimVista workstation (MIM Software, Cleveland, OH) using the PET-Edge tool when possible, or otherwise conformational ROIs were utilized to record uptake in regions of physiologic and abnormal 18F-fluciclovine uptake.
18F-fluciclovine uptake parameters [SUV (mean, maximum (max), and peak)] were recorded in the prostate/bed and up to 5 metastatic bone and soft tissue lesions each.
Therapy response assessment
Imaging response to treatment was evaluated independently for 18F-fluciclovine PET/CT and conventional imaging. Each was compared to the PSA response.
Conventional imaging and PSA biomarkers
Assessment of response on CT or MR followed RECIST 1.1 criteria [18]. Response on PSA and bone scan were based on recommendations from the PCCTWG3 [19]. For additional analysis, patients were also dichotomized as either having progressive disease (≥ 25% increased PSA) or non-progressive disease (decreasing or stable PSA).
18F-fluciclovine PET/CT
The summation of 18F-fluciclovine uptake parameters (SUVmax, SUVmean) from all indexed lesions per patient was recorded from each PET scan, and the same lesions were evaluated on subsequent scans. The presence of new lesions was also recorded. A decrease in summed uptake parameters of ≥30% was considered response (R), while the appearance of new lesions or >30% increase in summed uptake parameters was considered progressive disease (PD). Stable disease was defined as a change of <30% summed uptake parameters between scans.
To also achieve a modified PET response criteria for solid tumors (PERCIST) 1.0 analysis, the SUVpeak of the single hottest lesion from each PET scan was also assessed for response using the above criteria.
Statistical analysis
Differences in 18F-fluciclovine uptake between the baseline PET and the measurements at each time point during the two follow-up PET scans (after one cycle and after 4-6 cycles of chemotherapy) were compared using two-sided paired t-test. Correlation between the response on 18F-fluciclovine PET scan after 1 and 6 cycles of chemotherapy (or sooner at end of chemotherapy per patient condition) and the clinical response after 6 cycles of chemotherapy (or sooner at end of chemotherapy per patient condition) as measured by standard parameters including PSA and routine radiologic objective measurements (bone scan and RECIST 1.1) was done. Association was determined using Spearman’s correlation coefficient and Chi-square test. Significance level was set at P<0.05 for all tests. All analyses were done using SPSS version 23 (IBM, Armonk, NY).
Results
Patients
Seven patients with metastatic castration-resistant prostate cancer were recruited. The average age was 79.0±5.5 years. Median PSA was 63.43 ng/ml (range 6.67-1300.00 ng/ml) and the median Gleason score (Grade group) was 4+4 (4), with a range of 7-8 Gleason score (Table 1). All patients in the study completed the 1st and 2nd 18F-fluciclovine PET/CT, while 4/7 patients completed all 3 PET/CT scans. One of the patients died during the study period from causes related to disease progression, one patient did not tolerate docetaxel after the second dose, while one patient was switched after cycle 3 from docetaxel to cabazitaxel and carboplatin due to disease progression. Baseline bone scans and conventional imaging (CT and/or magnetic resonance imaging (MRI)) were done within 2 weeks of PET scans, which was well within the specified 60 days (Figure 1).
Table 1.
Patients’ characteristics
| ID | Age | PSA 1 | Gleason score | SUVmax of hottest lesion (baseline) | PSA 2 | SUVmax of hottest lesion (after cycle 1) | PSA 3 | SUVmax of hottest lesion (after cycle 6) |
|---|---|---|---|---|---|---|---|---|
| 1 | 74 | >1300 | 4+4=8 | 7.6 | >1300 | 14.5 | - | - |
| 2 | 86 | 63.43 | 4+4=8 | 8.7 | 53.08 | 4.8 | 10.01 | 5.0 |
| 3 | 70 | 70.18 | 4+4=8 | 5.1 | 50.94 | 5.1 | 50.23 | 6.1 |
| 4 | 84 | 270.29 | 4+4=8 | 10.5 | 253.69 | 8.9 | 64.54 | 6.1 |
| 5 | 78 | 16.89 | 4+4=8 | 6.5 | 26.09 | 6.4 | - | - |
| 6 | 79 | 20.79 | 4+3=7 | 11.5 | 35.02 | 12.6 | 33.76 | 12.2 |
| 7 | 78 | 6.67 | 4+3=7 | 6.5 | 5.87 | 9.6 | - | - |
Figure 1.
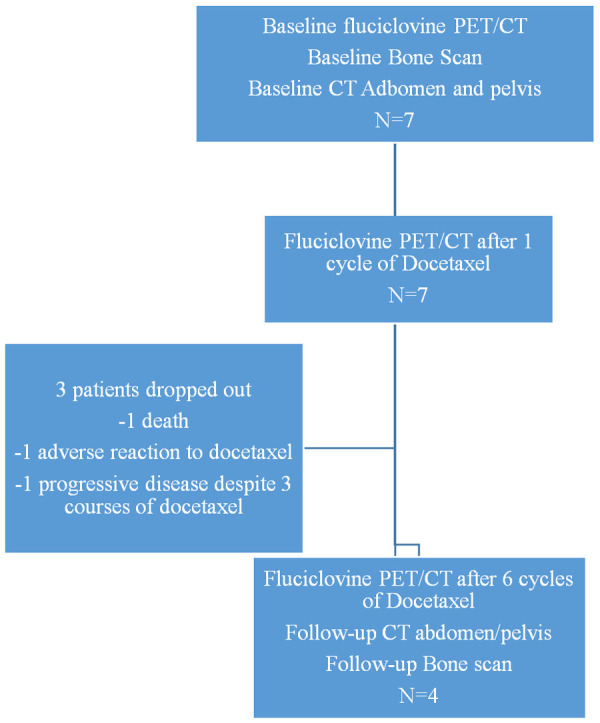
Study flowchart.
Evaluation of therapy response
Biochemical (PSA) response
Based upon PCCTWG 3 criteria, 1/7 (14.3%) patients had PSA response, 4/7 (57.1%) patients had stable PSA, while 2/7 (28.6%) had progression after the first cycle of docetaxel. Of the four patients that completed 6 cycles of docetaxel, 2/4 (50%) had PSA response, 1/4 (25%) had stable PSA, while 1/4 (25%) had PSA progression (Table 3).
Table 3.
Changes in summed SUVmax and PSA in individual patients with mCRPC after 1 and 6 cycles of Docetaxel
| Patient | % ∆ after the 1st cycle | % ∆ after the 6th cycle | % ∆ PSA after 1st cycle | % ∆ PSA after the 6th cycle |
|---|---|---|---|---|
| 1 | 26.3 | - | 0.0 | - |
| 2 | -28.6 | -47.2 | -51.7 | -84.2 |
| 3 | -11.9 | -13.2 | -20.7 | -28.5 |
| 4 | -20.6 | -34.4 | -6.1 | -75.8 |
| 5 | -6.2 | - | 100 | - |
| 6 | -2.2 | 0.8 | 68.3 | 62.5 |
| 7 | 65.8 | - | 22.4 | - |
Δ= change.
CT abdomen and pelvis
In the 4/7 patients who completed cycle 6, stable disease was present in all patients per RECIST 1.1 criteria.
Bone scan
In the 4/7 patients who completed cycle 6, 2/4 (50%) patients were adjudged to have progressive disease, while 2/4 (50%) had stable disease per PCCTWG 3 criteria.
18F-fluciclovine PET/CT
All PET results given are based on the initial time point. Delayed time point data did not improve on initial time point data.
Table 2 presents a summary of the mean of summed PET parameters at baseline, after the first and sixth cycles of chemotherapy. All patients recruited in this study had bone metastasis detected on baseline PET/CT; 6/7 (85.7%) patients had soft tissue disease including 4/7 (57.1%) with nodal disease.
Table 2.
18F-fluciclovine uptake parameters in patients with mCRPC
| Sum of lesions (mean ± SD) | |||||
|---|---|---|---|---|---|
|
| |||||
| PET parameters | Baseline PET (n=7 patients/49 lesions) | After 1st cycle (n=7 patient/49 lesions) | After 6th cycle (n=4 patients/23 lesions) | ∆ after 1st cycle (%) | ∆ after 6th cycle (%) |
| Summed SUVmax | 47.6±16.0 | 48.1±20.4 | 36.3±17.3 | 0.4±11.5 (0.8) | -9.8±11.5 (-20.6) |
| Summed SUVmean | 30.7±10.3 | 31.8±13.2 | 23.3±9.7 | 1.1±8.3 (3.6) | -6.4±7.1 (-20.8) |
| SUVpeak hottest lesion PERCIST-like (mean ± SD) | |||||
| SUVpeak* | 6.2±2.1 | 6.9±3.2 | 5.8±2.8 | 0.7±2.7 (11.3) | -1.0±2.0 (16.1) |
n=1, single hottest lesion;
Δ= change.
After one cycle of Docetaxel (n=7), based on the differences in the summed SUVmax, stable disease was found in 6/7 (85.7%) patients, while progression was noted in 1/7 patients. Using summed SUVmean, 5/7 (71.4%) patients had stable disease and progressive disease in 2/7 patients.
Based on SUVpeak in the hottest lesion (PERCIST-like analysis), 1/7 patients had response, 4/7 patients had stable disease and 2/7 patients had disease progression.
After the sixth cycle of chemotherapy (n=4), based on the differences in the summed SUVmax, and SUVmean, 3/4 (75%) patients had response to therapy, while one of four (25%) patients had disease progression.
Based on SUVpeak in the hottest lesion (PERCIST-like analysis), response was present in 2/4 (50%) patients and stable disease in 2/4 (50%) patients.
Correlation of imaging with biochemical (PSA) response
Conventional imaging
After completion of 6 cycles of docetaxel, CT response based on RECIST 1.1 revealed stable disease in all 4 patients irrespective of increasing or decreasing PSA, thus correlating with PSA response in 1/4 (25%) patients.
Therapy response based on bone scan and PCCTWG3 criteria suggested progressive disease in 2/4 (50%) patients despite reducing PSA, while despite rising PSA in one patient, bone scan suggested stable disease. Thus, bone scan correlated with biochemical response in only 1/4 patients.
18F-fluciclovine PET
There was no significant correlation of summed SUVmax or SUVmean, with the baseline PSA at the time of the baseline scan.
After the first cycle (n=7), PET response using the difference in sums of either SUVmax, compared to parameters at baseline PET correlated with PSA response after the first cycle in 3/7 (42.9%) patients, while PET response using summed SUVmean correlated with PSA response in 2/7 (28.6%) patients.
Using SUVpeak of the hottest lesion (PERCIST-like), there was correlation with PSA response in 3/7 (42.9%) patients after the first cycle.
After the sixth cycle (n=4), PET response using summed differences of SUVmax, SUVmean compared to baseline PET was concordant with PSA response after the sixth cycle in 3/4 (75%) patients.
SUVpeak of the hottest lesion had a similar correlation with PSA response after the sixth cycle.
Progressive versus non-progressive disease
After 6 cycles of chemotherapy, the mean SUVmax and SUVmean were significantly higher in patients with progressive disease (≥25% increased PSA) versus non-progressive (P<0.05). This difference was also seen with SUVpeak of the hottest lesion (Table 4).
Table 4.
18F-fluciclovine uptake in patients with progressive versus non-progressive mCRPC
| Parameters | Progressive Disease | Non-Progressive Disease | p-value |
|---|---|---|---|
| Mean SUVmax (summed) | |||
| Baseline | 59.5±1.4 | 43.0±16.9 | 0.09 |
| Post-cycle 1 | 57.1±3.0 | 44.5±23.8 | 0.31 |
| Post-cycle 6 | 61.0 | 28.1±6.4 | 0.04 |
| Mean SUVmean (summed) | |||
| Baseline | 37.3±1.7 | 28.1±11.3 | 0.15 |
| Post-cycle 1 | 36.0±0.4 | 30.1±15.8 | 0.46 |
| Post-cycle 6 | 37.1 | 18.7±3.7 | 0.04 |
| SUVpeak hottest lesion | |||
| Baseline | 7.0±2.1 | 5.6±2.2 | 0.14 |
| Post-cycle 1 | 9.0±3.8 | 5.3±1.9 | 0.42 |
| Post-cycle 6 | 9.8 | 4.5±1.0 | <0.04 |
Correlation with PSA
The changes in 18F-fluciclovine uptake correlated with changes in PSA after 1 and 6 cycles of docetaxel. This however was a non-significant trend.
Correlation between 18F-fluciclovine PET and conventional imaging response
18F-fluciclovine PET response criteria using the summed SUVmax, SUVmean, and SUVpeak of the hottest lesion correlated with RECIST 1.1 and bone scan in 2/4 (50%) patients.
An overview of the correlation between biochemical response and imaging is provided in Table 5.
Table 5.
Correlation of imaging with PSA for assessment of therapy response in patients with mCRPC on Docetaxel
| A. After one cycle | ||||
|
| ||||
| Patient | 18F-fluciclovine PET | PSA | ||
|
| ||||
| SUVmax | SUVmean | SUVpeak hottest lesion | ||
|
| ||||
| 1 | SD | PD | PD | S |
| 2 | SD | SD | R | R |
| 3 | SD | SD | SD | S |
| 4 | SD | SD | SD | S |
| 5 | SD | SD | SD | P |
| 6 | SD | SD | SD | P |
| 7 | PD | PD | PD | S |
|
| ||||
| B. After six cycles | ||||
|
| ||||
| Patient | 18F-fluciclovine PET (Summed SUVmax, SUVmean, SUVpeak hottest lesion) | RECIST | Bone scan | PSA |
|
| ||||
| 1 | - | - | - | - |
| 2 | R | SD | PD | R |
| 3 | SD | SD | SD | S |
| 4 | R | SD | PD | R |
| 5 | - | - | - | - |
| 6 | SD | SD | SD | P |
| 7 | - | - | - | - |
Key: R: response; S: stable; P: progression; SD: stable disease; PD: progressive disease.
Figures 2 and 3 are representative images from patients with non-response. Figures 4 and 5 are representative images from a patient with response to docetaxel.
Figure 2.
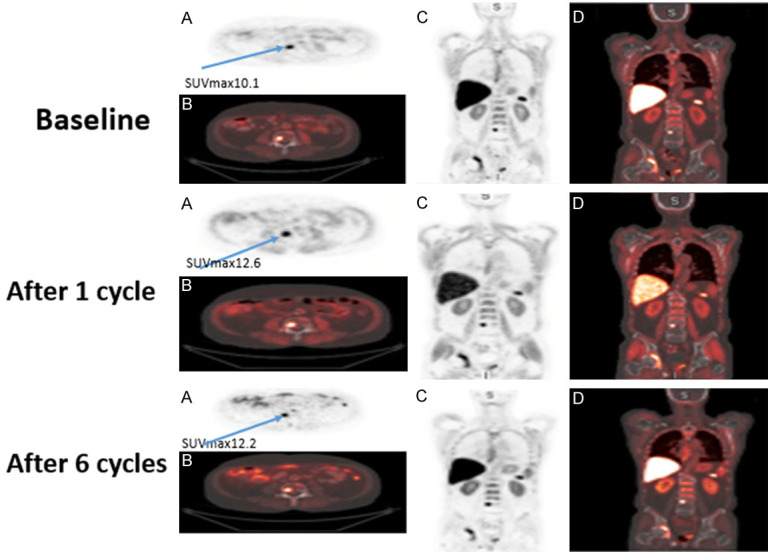
Representative case of non-response. A 79-year-old male with metastatic castration-resistant prostate cancer, Gleason score 4+3, baseline PSA 20.79 ng/ml which increased to 33.83 ng/ml after 6 cycles of chemotherapy. A. Axial image of fluciclovine PET with abnormal uptake in the L4 vertebra. B. Axial image of fused fluciclovine PET/CT with abnormal uptake in the L4 vertebra. C. Coronal image of fluciclovine PET. D. Coronal image of fused fluciclovine PET/CT. Images demonstrate increased intensity of fluciclovine uptake in the same lesion on subsequent imaging after chemotherapy.
Figure 3.
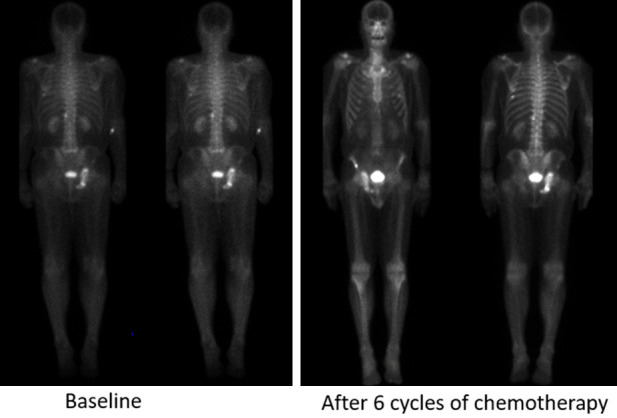
Technituium-99 bone scans in a 79-year-old male with metastatic castration-resistant prostate cancer, Gleason score 4+3, baseline PSA 20.79 ng/ml showing progressive disease after 6 cycles of chemotherapy in comparison to baseline scan.
Figure 4.
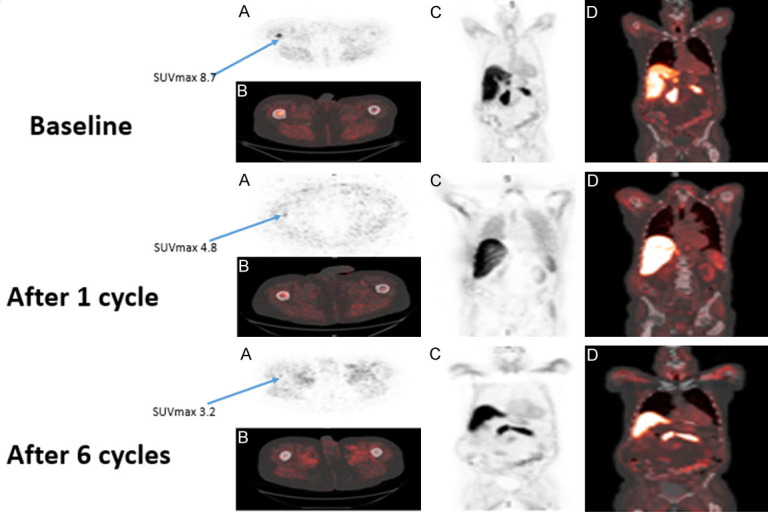
Representative case of response. 86 y/o M with metastatic castration-resistant prostate cancer, Gleason score 4+4, baseline PSA 63.43 ng/ml, which decreased to 10.01 ng/ml after 6 cycles of chemotherapy. A. Axial image of fluciclovine PET with abnormal uptake in the right proximal femur. B. Axial image of fused fluciclovine PET/CT with abnormal uptake in the right proximal femur. C. Coronal image of fluciclovine PET. D. Coronal image of fused fluciclovine PET/CT. Images demonstrate decreased intensity of fluciclovine uptake in the same lesion on subsequent imaging after chemotherapy.
Figure 5.
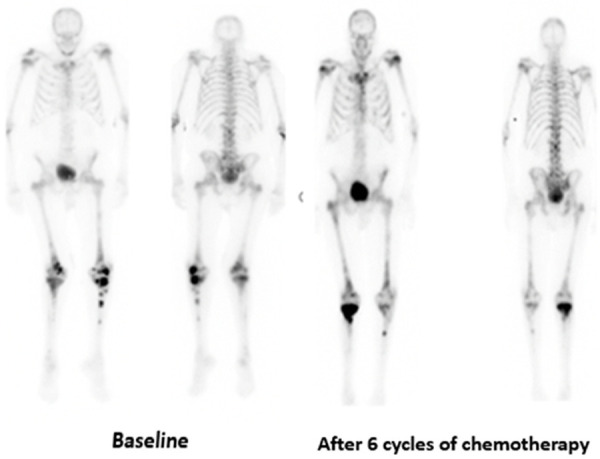
Technituium-99 bone scans in an 86-year-old male with metastatic castration-resistant prostate cancer, Gleason score 4+4, baseline PSA 63.43 ng/ml showing stable disease after 6 cycles of chemotherapy in comparison to baseline bone scans.
Time to PSA progression
The median time to PSA progression in the evaluable patients (n=5) was 199 days (interquartile range 115.5-321.0 days); see Supplementary Table 1. Baseline 18F-fluciclovine uptake parameters did not correlate with time to progression. Also, there was no correlation between time to PSA progression and change in PET parameters after 1 and 6 cycles of chemotherapy (Table 6).
Table 6.
Correlation of time to PSA progression with change in summed PET-parameters
| ∆ PET parameter after the 1st cycle | Pearson Correlation | p-value | ∆ PET parameter after the 6th cycle | Pearson Correlation | p-value |
|---|---|---|---|---|---|
| SUVmax | 0.51 | 0.25 | SUVmax | 0.70 | 0.10 |
| SUVmean | 0.67 | 0.16 | SUVmean | 0.83 | 0.23 |
| SUVpeak hottest lesion | 0.74 | 0.13 | SUVpeak hottest lesion | 0.74 | 0.10 |
Δ= change.
Discussion
Determining response to chemotherapy in patients with metastatic castration-resistant prostate cancer with current conventional imaging modalities such as CT and bone scan remains a challenge [6,7]. In this exploratory study, we set out to determine the value of 18F-fluciclovine PET/CT in the assessment of response to the first-line chemotherapeutic agent docetaxel, and its correlation with time to PSA progression.
We found a 43% correlation of response assessment using 18F-fluciclovine PET parameters (SUVmax, and SUVpeak of the hottest lesion) with PSA response after a single cycle of docetaxel; however, after completion of 6 cycles of docetaxel, 18F-fluciclovine PET parameters correlated with the standard of care biochemical (PSA) response in 75% of patients. In comparison to RECIST 1.1 and bone scan, 18F-fluciclovine PET correlated better with the biochemical response to therapy in patients with mCRPC. The changes in 18F-fluciclovine uptake correlated with changes in PSA after 1 and 6 cycles of docetaxel. This however was a non-significant trend. There was no significant correlation of uptake parameters with time to PSA progression.
Currently, assessment of therapy response in prostate cancer is based on changes in serum PSA, clinical assessment, and imaging using RECIST 1.1 and bone scans. The role of molecular imaging in the assessment of therapy response has been gaining attention. Studies making use of 2-deoxy-2-[18F]fluoro-D-glucose (FDG) [8], carbon-11 choline (11C-choline) [9,20], 18F-fluorocholine (18F-choline) [10] and Gallium-68 prostate-specific membrane antigen (68Ga-PSMA) [11,21] in the evaluation of therapy response in the setting of metastatic prostate cancer have reported varying results.
Our finding that PET parameters correlated better with biochemical response than RECIST 1.1 or bone scan is similar to the findings of previous similar studies making use of molecular imaging in the evaluation of response to chemotherapy in patients with mCRPC. Jadvar reported that FDG uptake decreases concordantly with PSA and contributes independent prognostic information on overall survival in men with mCRPC [8]. Studies done by Ceci et al [9] and Caroli et al [10] making use of 11C-choline and 18F-choline, respectively, reported promising results in the evaluation of therapy response in patients with mCRPC. Yet, Schwarzenbock did not find a correlation between change in choline uptake in 11C-choline PET/CT and response assessment in patients with mCRPC treated with docetaxel chemotherapy [20]. Seitz et al in a study of 16 patients with mCRPC undergoing docetaxel chemotherapy, reported that 68Ga-PSMA-11 PET/CT correlated better with PSA response, with 56% correlation in patients with mCRPC compared to 33% using RECIST 1.1 [11]. Other molecular imaging studies making use of 68Ga-PSMA-11 PET/CT for the assessment of therapy response in patients with metastatic prostate cancer have reported similar findings of significant correlation between response to chemotherapy and PET parameters [21,22].
After a single cycle of docetaxel, there was limited correlation between PSA response and 18F-fluciclovine PET response, however, there was no statistical difference in the PET parameters between patients with progressive disease versus those who had non-progressive disease. This trend was however different after the completion of 6 cycles, with a correlation of 75% using PET parameters and a significant difference in SUVmax, SUVmean, and SUVpeak of the hottest lesion between patients with progressive disease versus non-progressive disease. The finding of a 75% correlation of PET with PSA response in patients with mCRPC treated with docetaxel is higher than 56% reported by Seitz [11] and 64% reported by Ceci [9]. These disparities may be related to differences in sample size and study design. Though speculative, 18F-fluciclovine PET may be a valuable tool in the assessment of early therapy response after a single cycle of docetaxel, and overall response after completing six cycles of docetaxel.
There was no correlation between 18F-fluciclovine uptake parameters at the baseline or after 6 cycles of chemotherapy and the time to PSA progression. The role of PET in the evaluation of time to progression or overall survival in patients with mCRPC has not been widely reported. Jadvar et al in a study of 87 patients with mCRPC demonstrated that the sum of SUVmax derived from FDG PET/CT is a useful imaging biomarker for predicting overall survival in men with mCRPC [8]. De Giorgi et al reported in the study evaluating 18F-choline in a study of 36 patients with mCRPC treated with enzalutamide that PET/CT was an independent predictor of progression-free survival, but not overall survival [23]. The disparity with 18F-fluciclovine PET from the above studies may be related to the small sample size in this exploratory study. Overall survival was not assessed in this small cohort of patients, as the study was not powered to determine this. Further evaluation in larger prospective studies is encouraged.
The small sample size is an obvious limitation of this study. This was an exploratory study designed to assess the possible role of 18F-fluciclovine PET as a marker of therapy response in patients with mCRPC. It is practically difficult to recruit this cohort of patients with extensive metastatic disease who also served as controls for themselves over a prolonged period. As noted only 4 patients could complete the study. This study may prove useful in the design of future studies. Serum PSA was used as the reference standard for therapy response in this study; however, there have been questions raised about the consistent applicability of PSA levels with treatment response [9,24]. PSA levels however remain a primary reference method of objective assessment of patients with prostate cancer. Finally, while PERCIST 1.0 criteria has been established for evaluation of response to therapy in FDG PET [25], it has not been evaluated for 18F-fluciclovine; therefore, we employed a modified PERCIST with SUVpeak in the assessment of response to therapy in this study.
Conclusion
This exploratory study suggests that 18F-fluciclovine PET/CT seems to better correlate with PSA response than CT or bone scan for assessment of treatment response in patients with metastatic castration-resistant prostate cancer on docetaxel. The changes in 18F-fluciclovine uptake correlated with changes in PSA after 1 and 6 cycles of docetaxel. After 6 cycles of chemotherapy, the mean SUVmax, SUVmean, and SUVpeak of the hottest lesion were significantly higher in patients with progressive disease versus non-progressive disease. 18F-fluciclovine PET/CT may however be limited in the prediction of time to PSA progression in this population. Larger studies are required to confirm the value of 18F-fluciclovine PET as an imaging biomarker for response assessment. Future studies evaluating therapy response in a different cohort of patients, on androgen deprivation therapy, are encouraged.
Acknowledgements
We acknowledge Bridget Fielder, RN, Kathy Vaughn, Fenton G. Ingram, RT(R), CNMT, PET, Seraphinah Lawal, RT(R), CNMT, PET, Ronald J. Crowe, RPh, BCNP, and the cyclotron/synthesis team from Emory University Center for Systems Imaging.
Disclosure of conflict of interest
Funding was received from Blue Earth Diagnostics Ltd, Nihon Medi-Physics Co., Ltd, and Emory University School of Medicine Catalyst and Seed funding program. Emory University: Blue Earth Diagnostics Ltd. provided 18F-fluciclovine synthesis cassettes to Emory University. Entitled to royalties derived from the sale of products related to the research described in this manuscript. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies. AAA, OAA: Funding is or has been received from Blue Earth Diagnostics Ltd. and Nihon Medi-Physics Co., Ltd. through the Emory University Office of Sponsored Projects for other clinical trials using 18F-fluciclovine. DMS: Funding is or has been received from Blue Earth Diagnostics Ltd. and Nihon Medi-Physics Co., Ltd. through the Emory University Office of Sponsored Projects for other clinical trials using 18F-fluciclovine. Participates through the Emory Office of Sponsored Projects in sponsored grants including those funded or partially funded by Telix Pharmaceuticals (US) Inc; Advanced Accelerator Applications; FUJIFILM Pharmaceuticals U.S.A., Inc; Amgen Inc. Consultant: Syncona; AIM Specialty Health; Global Medical Solutions Taiwan; Progenics Pharmaceuticals, Inc.
Supporting Information
References
- 1.Delanoy N, Hardy-Bessard AC, Efstathiou E, Le Moulec S, Basso U, Birtle A, Thomson A, Krainer M, Guillot A, De Giorgi U, Hasbini A, Daugaard G, Bahl A, Chowdhury S, Caffo O, Beuzeboc P, Spaeth D, Eymard JC, Flechon A, Alexandre J, Helissey C, Butt M, Priou F, Lechevallier E, Deville JL, Goupil MG, Morales R, Thiery-Vuillemin A, Gavrikova T, Barthelemy P, Sella A, Fizazi K, Baciarello G, Fererro JM, Laguerre B, Verret B, Hans S, Oudard S. Sequencing of taxanes and new androgen-targeted therapies in metastatic castration-resistant prostate cancer: results of the international multicentre retrospective CATS database. Eur Urol Oncol. 2018;1:467–475. doi: 10.1016/j.euo.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J. Clin. Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 3.Oudard S, Fizazi K, Sengelov L, Daugaard G, Saad F, Hansen S, Hjalm-Eriksson M, Jassem J, Thiery-Vuillemin A, Caffo O, Castellano D, Mainwaring PN, Bernard J, Shen L, Chadjaa M, Sartor O. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J. Clin. Oncol. 2017;35:3189–3197. doi: 10.1200/JCO.2016.72.1068. [DOI] [PubMed] [Google Scholar]
- 4.Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Ippolito JE, Kane CJ, Kuettel MR, Lang JM, McKenney J, Netto G, Penson DF, Plimack ER, Pow-Sang JM, Pugh TJ, Richey S, Roach M, Rosenfeld S, Schaeffer E, Shabsigh A, Small EJ, Spratt DE, Srinivas S, Tward J, Shead DA, Freedman-Cass DA. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 5.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, van der Poel HG, van der Kwast TH, Rouviere O, Wiegel T, Mottet N. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Cook GJ, Azad G, Padhani AR. Bone imaging in prostate cancer: the evolving roles of nuclear medicine and radiology. Clin Transl Imaging. 2016;4:439–447. doi: 10.1007/s40336-016-0196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jadvar H, Desai B, Ji L, Conti PS, Dorff TB, Groshen SG, Pinski JK, Quinn DI. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J Nucl Med. 2013;54:1195–1201. doi: 10.2967/jnumed.112.114116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceci F, Castellucci P, Graziani T, Schiavina R, Renzi R, Borghesi M, Di Tullio P, Brunocilla E, Ardizzoni A, Fanti S. (11)C-Choline PET/CT in castration-resistant prostate cancer patients treated with docetaxel. Eur J Nucl Med Mol Imaging. 2016;43:84–91. doi: 10.1007/s00259-015-3177-4. [DOI] [PubMed] [Google Scholar]
- 10.Caroli P, De Giorgi U, Scarpi E, Fantini L, Moretti A, Galassi R, Celli M, Conteduca V, Rossi L, Bianchi E, Paganelli G, Matteucci F. Prognostic value of 18F-choline PET/CT metabolic parameters in patients with metastatic castration-resistant prostate cancer treated with abiraterone or enzalutamide. Eur J Nucl Med Mol Imaging. 2018;45:348–354. doi: 10.1007/s00259-017-3866-2. [DOI] [PubMed] [Google Scholar]
- 11.Seitz AK, Rauscher I, Haller B, Kronke M, Luther S, Heck MM, Horn T, Gschwend JE, Schwaiger M, Eiber M, Maurer T. Preliminary results on response assessment using (68)Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2018;45:602–612. doi: 10.1007/s00259-017-3887-x. [DOI] [PubMed] [Google Scholar]
- 12.Schuster DM, Votaw JR, Nieh PT, Yu W, Nye JA, Master V, Bowman FD, Issa MM, Goodman MM. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med. 2007;48:56–63. [PubMed] [Google Scholar]
- 13.Schuster DM, Taleghani PA, Nieh PT, Master VA, Amzat R, Savir-Baruch B, Halkar RK, Fox T, Osunkoya AO, Moreno CS, Nye JA, Yu W, Fei B, Wang Z, Chen Z, Goodman MM. Characterization of primary prostate carcinoma by anti-1-amino-2-[(18)F] -fluorocyclobutane-1-carboxylic acid (anti-3-[(18)F] FACBC) uptake. Am J Nucl Med Mol Imaging. 2013;3:85–96. [PMC free article] [PubMed] [Google Scholar]
- 14.Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, Master VA, Nye JA, Odewole OA, Osunkoya AO, Savir-Baruch B, Alaei-Taleghani P, Goodman MM. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191:1446–1453. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fei B, Abiodun-Ojo OA, Akintayo AA, Akin-Akintayo O, Tade F, Nieh PT, Master VA, Alemozaffar M, Osunkoya AO, Goodman MM, Schuster DM. Feasibility and initial results: fluciclovine positron emission tomography/ultrasound fusion targeted biopsy of recurrent prostate cancer. J Urol. 2019;202:413–421. doi: 10.1097/JU.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bach-Gansmo T, Nanni C, Nieh PT, Zanoni L, Bogsrud TV, Sletten H, Korsan KA, Kieboom J, Tade FI, Odewole O, Chau A, Ward P, Goodman MM, Fanti S, Schuster DM, Willoch F. Multisite experience of the safety, detection rate and diagnostic performance of fluciclovine ((18)F) positron emission tomography/computerized tomography imaging in the staging of biochemically recurrent prostate cancer. J Urol. 2017;197:676–683. doi: 10.1016/j.juro.2016.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ono M, Oka S, Okudaira H, Nakanishi T, Mizokami A, Kobayashi M, Schuster DM, Goodman MM, Shirakami Y, Kawai K. [(14)C]Fluciclovine (alias anti-[(14)C]FACBC) uptake and ASCT2 expression in castration-resistant prostate cancer cells. Nucl Med Biol. 2015;42:887–892. doi: 10.1016/j.nucmedbio.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, Antonarakis ES, Beer TM, Carducci MA, Chi KN, Corn PG, de Bono JS, Dreicer R, George DJ, Heath EI, Hussain M, Kelly WK, Liu G, Logothetis C, Nanus D, Stein MN, Rathkopf DE, Slovin SF, Ryan CJ, Sartor O, Small EJ, Smith MR, Sternberg CN, Taplin ME, Wilding G, Nelson PS, Schwartz LH, Halabi S, Kantoff PW, Armstrong AJ. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J. Clin. Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarzenbock SM, Eiber M, Kundt G, Retz M, Sakretz M, Kurth J, Treiber U, Nawroth R, Rummeny EJ, Gschwend JE, Schwaiger M, Thalgott M, Krause BJ. Prospective evaluation of [(11)C]Choline PET/CT in therapy response assessment of standardized docetaxel first-line chemotherapy in patients with advanced castration refractory prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2105–2113. doi: 10.1007/s00259-016-3439-9. [DOI] [PubMed] [Google Scholar]
- 21.Schmuck S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, Wester HJ, Ross TL, Bengel FM, Derlin T. Initial experience with volumetric (68)Ga-PSMA I&T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J Nucl Med. 2017;58:1962–1968. doi: 10.2967/jnumed.117.193581. [DOI] [PubMed] [Google Scholar]
- 22.Schmidkonz C, Cordes M, Schmidt D, Bauerle T, Goetz TI, Beck M, Prante O, Cavallaro A, Uder M, Wullich B, Goebell P, Kuwert T, Ritt P. (68)Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:1862–1872. doi: 10.1007/s00259-018-4042-z. [DOI] [PubMed] [Google Scholar]
- 23.De Giorgi U, Caroli P, Scarpi E, Conteduca V, Burgio SL, Menna C, Moretti A, Galassi R, Rossi L, Amadori D, Paganelli G, Matteucci F. (18)F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur J Nucl Med Mol Imaging. 2015;42:1276–1283. doi: 10.1007/s00259-015-3042-5. [DOI] [PubMed] [Google Scholar]
- 24.Sandblom G, Ladjevardi S, Garmo H, Varenhorst E. The impact of prostate-specific antigen level at diagnosis on the relative survival of 28,531 men with localized carcinoma of the prostate. Cancer. 2008;112:813–819. doi: 10.1002/cncr.23235. [DOI] [PubMed] [Google Scholar]
- 25.de Langen AJ, Vincent A, Velasquez LM, van Tinteren H, Boellaard R, Shankar LK, Boers M, Smit EF, Stroobants S, Weber WA, Hoekstra OS. Repeatability of 18F-FDG uptake measurements in tumors: a metaanalysis. J Nucl Med. 2012;53:701–708. doi: 10.2967/jnumed.111.095299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


