Abstract
Congenital hyperinsulinism (CHI) occurs most commonly in infants but may also be discovered in older children. It presents with recurrent episodes of hypoglycemia due to high endogenous insulin levels. There is a focal and diffuse form of the disease depending on the extent of pancreatic involvement. Hyperplasia of the islet cells results in hyperfunctioning pancreatic β cells and the ensuing clinical disease. Medical treatment fails in several patients and surgery has been shown to be very effective in improving prognosis and even resolution of disease in the focal form. Several genetic mutations have been uncovered and these may also be predictive of prognosis. Anatomical imaging alone including ultrasound, CT and MRI are rarely able to detect any abnormality in the pancreas. PET plays a major role in the distinction between the focal and diffuse forms of the disease. It also guides surgical intervention by providing information on the location of the focal hyperfunctioning islet cells. Imaging children and infants in this disease is quite challenging. We propose to show the benefit of using two PET tracers in this disease. 18F-FDOPA has been used quite successfully in the evaluation of CHI. 68Ga-DOTATATE has also been described to be helpful although inferior to 18F-FDOPA. We illustrate imaging of CHI patients in 3 different scans and briefly review the literature. 18F-FDOPA as described in the literature is superior but when unavailable 68Ga-DOTATATE may be a reasonable alternative.
Keywords: CHI, congenital hyperinsulinism, DOTATATE, FDOPA, hyperinsulinism, nesidioblastosis, PHI, PHH, persistent hyperinsulinemic hypoglycemia
Introduction
Congenital hyperinsulinism (CHI) previously known as nesidioblastosis or persistent hyperinsulinemic hypoglycemia of infancy occurs most commonly in infants but may also be discovered in older children. It is a congenital disorder due to over-secretion of insulin and does not include acquired conditions of persistent hypoglycemia [1]. Hyperplasia of the islet cells results in hyperfunctioning pancreatic β cells and the ensuing clinical disease. The goal is early diagnosis and treatment in order to prevent any neurological sequelae (i.e developmental delay, seizures) and try to prevent the development of postsurgical diabetes. In some cases, CHI can be transient and will self-resolve by 3-4 months of age. However, in the persistent form of CHI optimal control of blood sugar levels is important in order to avoid any sequelae. A variety of nutritional and medication-based interventions are used to maintain euglycemia and avoid any neurological sequelae. When the latter fails surgical interventions are performed. Long term development in the diffuse form of CHI of glucose intolerance and insulin-dependent diabetes mellitus has been described to occur by 14 years of age in 100% and 91% respectively of 58 patients who underwent subtotal pancreatectomy [2]. Arya et al also reported similar findings [3]. Hence medical treatment fails in several patients and surgery has been shown to be very effective in improving prognosis and even resolution of disease more so in the focal form. Prognosis is therefore excellent in the focal form of CHI when successful surgical resection of the focal lesion is performed. Several genetic mutations have been uncovered and these may also be predictive of prognosis [4-7]. PET plays a major role in the distinction between the focal and diffuse forms of the disease. It can also guide surgical intervention by providing information on the location of the focal hyper-functioning islet cells. This can be further ascertained by complementing genetic testing with molecular imaging. Usually anatomical imaging with ultrasound, computed tomography or magnetic resonance imaging has a low yield in identifying the focal lesion [1,8-15]. Imaging children and infants in this disease is quite challenging. We propose to discuss two cases of focal CHI and review the literature regarding molecular imaging in CHI.
Case presentation
Our first case involved a 2-month-old baby girl found to have homozygous KCNJ11 mutation whom underwent near total pancreatectomy after having an 18F-FDOPA PET scan which showed uptake in the head of the pancreas slightly more prominent than the remainder of the pancreas. 10 months post op her condition improved but she remained on gastrostomy tube feeds, octreotide injections 22 mcg/kg/day divided every 6 hours and pancreatic enzyme administration 5000 units three times a day. This patient’s sister also has a history of CHI and ended up developing diabetes mellitus. Post operatively blood sugars were monitored at home and rarely reported to be under 60 mg/dl. Preoperatively the baby was on 35 mcg/kg/day divided every 6 hours of octreotide injections and diazoxide 22.5 mg every 8 hours and hydrochlorothiazide 7 mg every 12 hours. She suffered preoperatively from multiple episodes of hypoglycemia requiring dextrose infusion and dextrose intravenous pushes as well as glucagon injections. The baby’s 18F-FDOPA scan preoperatively showed focal uptake in the head of the pancreas Figure 1. The scan also showed some uptake in the remainder of the pancreas. - Relative focality noted within the head of the pancreas SUVmax 3.4 compared to the tail SUVmax 2.8. SUVmax ratio (head of pancreas/tail) was 1.21. - This was below the SUVmax ratio threshold of 1.44 identified by Christiansen et al suggesting a diffuse form even-though visual/qualitative evaluation may show some focality as noted in Figure 1 [16]. In the immediate postoperative period a 68Ga-DOTATATE scan was performed due to persistent hypoglycemic episodes that were difficult to control and showed sizeable residual pancreatic tissue in the head of the pancreas Figure 2.
Figure 1.
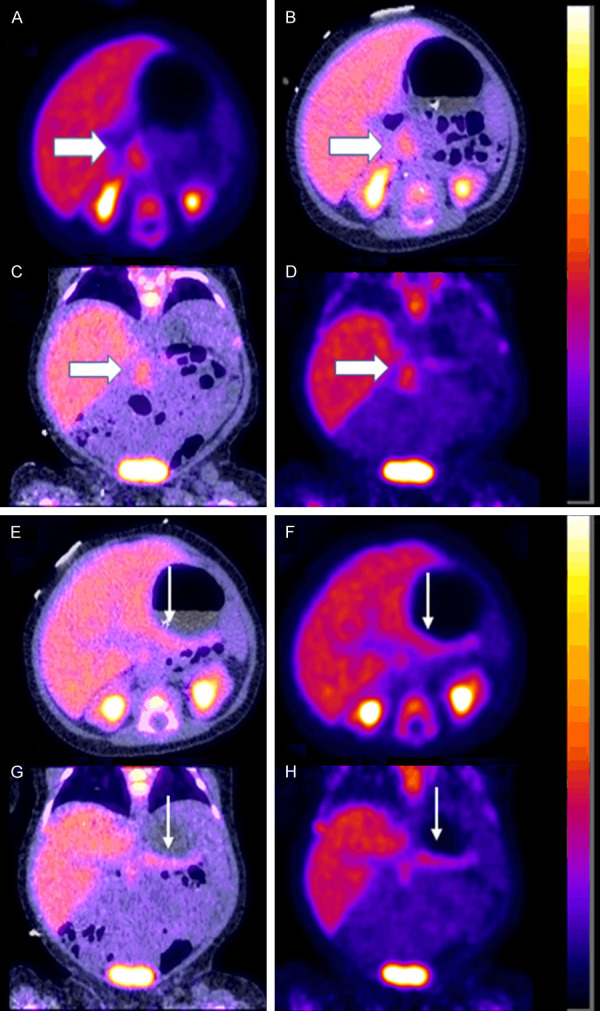
Preoperative 18F-FDOPA PET scan in a 2-month-old baby girl with CHI demonstrating the diffuse form of CHI. A. Axial PET. B. Axial fused PET-CT. C. Coronal fused PET-CT. D. Coronal PET showing moderate uptake in the head of the pancreas (thick white arrow) not meeting criteria for focal disease [16]. E. Axial fused PET-CT. F. Axial PET. G. Coronal fused PET-CT. H. Coronal PET showing mild uptake in the tail of the pancreas (thin white arrow).
Figure 2.
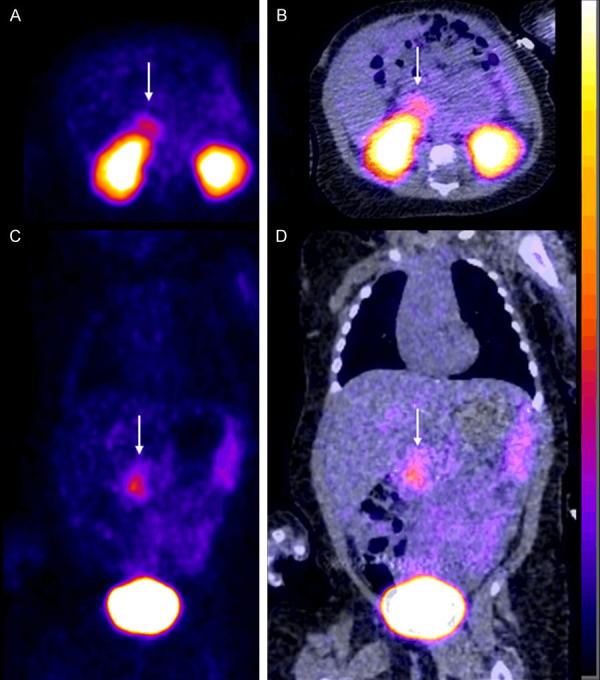
Post-operative 68Ga DOTATATE scan in the 2-month-old baby girl from Figure 1 shows some residual uptake in the head of the pancreas (thin white arrow). A. Axial PET. B. Axial fused PET-CT. C. Coronal PET. D. Coronal fused PET-CT.
Our second case was a 6 months old baby boy found to have heterozygous mutation in ABCC8 gene resulting in focal hyperinsulinism confirmed by histopathology. He was also found to be heterozygous for HNF1A gene. He underwent an 18F-FDOPA PET scan that showed unequivocal focal uptake in the head of the pancreas. - Focal uptake within the head of the pancreas with an SUVmax of 12 compared to the tail SUVmax 2.7. SUVmax ratio of 4.44 - Figure 3. He had a partial pancreatectomy, head uncinate, body, and roux-en-y pancreatico-jejunostomy. This baby was preoperatively on 35 mcg/kg/day divided every 6 hours of octreotide injections and frequent glucose administrations as needed for low blood sugars and gradually switched to octreotide 15 mcg/kg/day divided every 6 hours and with no hypoglycemic episodes prior to discharge.
Figure 3.
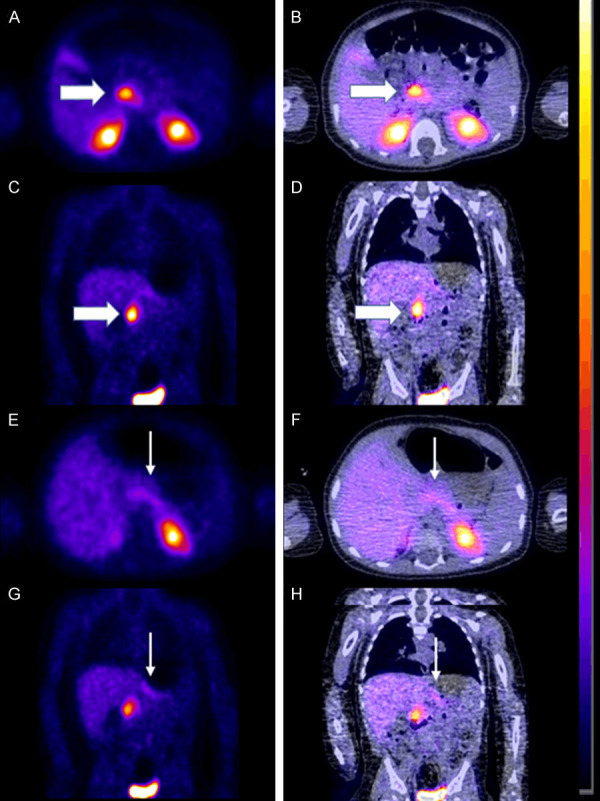
Preoperative 18F-FDOPA PET scan in a 6-month-old baby boy demonstrating the focal form of CHI. A. Axial PET. B. Axial fused PET-CT. C. Coronal PET. D. Coronal fused PET-CT showing intense uptake in the head of the pancreas (thick white arrow). E. Axial PET. F. Axial fused PET-CT. G. Coronal PET. H. Coronal fused PET-CT showing only very mild uptake in the tail of the pancreas (thin white arrow).
Discussion
The management of CHI patients is quite complex. Nuclear medicine imaging with PET plays a pivotal role and guides clinical decision making. Several genetic mutations have been uncovered and may be predictive of prognosis [4-7]. Autosomal dominant syndromes have been reported to have better outcomes and better response to treatment compared to autosomal recessive sydromes [17]. Sulfonylurea gene mutations (SUR1) when coupled with surgical focal adenomatous resection have been associated with better long-term outcomes [18]. Glaser et al reported better outcomes for children with only paternal mutations who entered clinical remission within 16 months, compared to 48 months for those with 2 SUR1 mutations [19]. Keeping in mind that focal forms are associated with better prognosis than diffuse forms, Belleanne-Chantelot et al noted that all focal disease patients in their cohort of 109 diazoxide unresponsive patients had heterozygous mutations, whereas 47% of patients known or suspected to have diffuse disease had homozygous or compound heterozygous mutations [20]. Flanagan et al later on confirmed these findings by using next generation sequencing [21]. Owing to the underlying disease severity and genetic heterogeneity certain mutations have been associated with remission or on the contrary with the late development of complications such as diabetes [22]. PET plays a major role in the distinction between the focal and diffuse forms of the disease and hence impacts prognosis. It can guide surgical intervention by providing information on the location of the focal hyper-functioning islet cells. This can be further ascertained by complementing genetic testing with molecular imaging. However, considering there is still a large number of patients with no associated genetic mutation; PET is of even greater value in these patients [4-7,17-22].
18F-FDOPA PET has been shown to be the reference imaging tool in CHI and is used nowadays quite successfully jointly with genetic testing in defining the focal form and the lesion that needs to be resected. Ultimately affecting the outcome of these patients [23]. FDOPA PET plays a major role in guided treatment and prognosis in patients with and without detectable genetic mutations and is therefore an essential piece of management [4-7,17-22]. 18F-FDOPA (6-[14]-L-fluoro-L-3, 4-dihydroxyphenylalanine) is taken up into the cells via the neutral amino acid transporter (LAT1/4F2hc). 18F-FDOPA PET has widespread utility in the diagnosis of different neuroendocrine tumors including in the evaluation of paragangliomas, pheochromocytomas, carcinoid tumors as well as medullary thyroid cancer. 18F-FDOPA PET can also distinguish between focal vs diffuse CHI [24]. Although insulinoma, a digestive NET generally of a benign nature also induces hyperinsulinism, in congenital CHI a clustered beta-cell hyperplasia is found on histopathology, rather than a definite tumor. In infants with CHI, life-threatening hypoglycemia, seizures and neurological sequelae may occur if the hypoglycemic episodes are not controlled and interrupted. Imaging can localize focal hyperplasia of beta cells in the pancreas and therefore guide curative selective surgical resection, in contrast to the diffuse form, which requires subtotal pancreatectomy when resistant to medical treatment. In an initial study by Ribeiro et al on 15 CHI children (aged 1-14 months) 18F-FDOPA PET showed an abnormal focal pancreatic uptake in 5 patients who then underwent a limited pancreatic resection that was followed by complete clinical remission [13]. Diffuse pancreatic uptake was observed in ten patients, four of whom underwent surgical resection that confirmed 18F-FDOPA PET results where the abnormal beta cells were gathered in small clusters, scattered in the whole pancreas. In contrast, MRI performed in six babies in this study detected no abnormality. These very promising results were confirmed in larger series by the same team and by many others [9,11,12,25-27]. In a recent German study [27], 18F-FDOPA PET/CT with multiphase contrast protocols was performed in 135 CHI patients. All the foci were excised on the basis of 18F-FDOPA PET/CT images and 87-91% of the operated patients could be completely healed. Two meta-analyses have also been recently published. An Italian team concluded that the pooled sensitivity and specificity of 18F-FDOPA PET scans in differentiating between focal and diffuse CHI were 89 and 98%, respectively [8]. In another study an American team aimed to compare the diagnostic performance of 18F-FDOPA PET, pancreatic venous sampling (PVS) and selective pancreatic arterial calcium stimulation with hepatic venous sampling (ASVS) in diagnosing and localizing focal CHI [15]. 18F-FDOPA PET was superior in distinguishing focal from diffuse CHI [summary diagnostic odds ratio (SDOR) = 73.2], compared to PVS (SDOR = 23.5) and ASVS (SDOR = 4.3). Furthermore, it localized focal CHI in the pancreas more accurately than PVS and ASVS (pooled accuracy 82 vs 76 and 64%, respectively) [8,15].
In the adult population 18F-FDOPA is slightly less beneficial because of the increased physiologic baseline pancreatic uptake. Low intralesional AADC activity and the resultant lack of accumulation of the decarboxylated tracer has been postulated as a potential cause [28,29]. Carbidopa premedication as a means to improve scan sensitivity for detecting insulinomas has been explored by Imperiale and his group who looked at 16 hyperinsulinaemic hypoglycaemia patients [30]. Other authors have also looked at the value of FDOPA PET in insulinomas as well [31-33]. In summary, 18F-FDOPA is of major utility to select those infants for surgery and shortens the intervention by guiding the surgical exploration of the pancreas. Premedication has been attempted in order to improve the diagnostic accuracy in adults but there is no patient safety or efficacy data in children.
On the other hand somatostatin receptors (SSTRs) are G-protein coupled membrane receptors that were first described in rat pituitary tumor cells by Schonbrunn and Tashjian in 1978 [34]. Five different human subtypes have been identified, named SSTR1 to 5 [35]. SSTR analogues (SSA) have been used to image a variety of neuroendocrine tumors. Previously SPECT imaging agents radiolabeled with SSA had low sensitivity and were of limited use. However, nowadays PET agents such as 68Ga radiolabeled SSA: 68Ga-DOTA-Phe1-Tyr3-Octreotide (TOC), 68Ga-DOTA-NaI3-Octreotide (NOC), and 68Ga-DOTA-Tyr3-Octreotate (TATE) are being used with great success to image neuroendocrine tumors [36]. They have demonstrated high sensitivity and high specificity [37]. Within the realm of hypoglycemic syndromes in adults 68Ga-DOTATATE PET has been used effectively especially in the evaluation of metastatic insulinomas [38]. Prasad et al evaluated 13 patients with 68Ga-DOTATATE/DOTANOC for clinical hyperinsulinemia. Two patients had malignant insulinoma, eight had non-metastasized insulinoma, and three had CHI [39]. Additionally, Christiansen et al showed that 68Ga-DOTATATE was less sensitive compared to 18F-FDOPA PET in the evaluation of CHI patients, however they did demonstrate high specificity in defining focal lesions [16]. This would suggest that 68Ga-DOTATATE can also be used to define the focal form of CHI although data is scarcer, and it would not be recommended if 18F-FDOPA is available [29]. Considering 68Ga-DOTATATE has been approved by the FDA and EMEA to be used for the evaluation and staging of neuroendocrine tumors and is currently produced and may be purchased commercially it becomes an attractive alternative tool for centers that do not have access to 18F-FDOPA PET scans. In our case 68Ga-DOTATATE was also used to evaluate the extent of residual pancreatic tissue status post subtotal pancreatectomy. One may also note that delayed imaging may offer better characterization of the disease although further studies in a larger group of patients is required to ascertain the benefit of 68Ga-DOTATATE evaluation and of the delayed imaging paradigm.
Conclusions
18F-FDOPA and 68Ga-DOTATATE are critical in the evaluation of CHI patients. 18F-FDOPA as described in the literature is superior and of great value but only when available otherwise 68Ga DOTATATE may be a reasonable alternative as it is more accessible. Regardless of the agent used imaging and interpretation can be challenging.
Disclosure of conflict of interest
None.
References
- 1.Yorifuji T, Horikawa R, Hasegawa T, Adachi M, Soneda S, Minagawa M, Ida S, Yonekura T, Kinoshita Y, Kanamori Y, Kitagawa H, Shinkai M, Sasaki H, Nio M (on behalf of The Japanese Society for Pediatric Endocrinology and The Japanese Society of Pediatric Surgeons) Clinical practice guidelines for congenital hyperinsulinism. Clin Pediatr Endocrinol. 2017;26:127–152. doi: 10.1297/cpe.26.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltrand J, Caquard M, Arnoux JB, Laborde K, Velho G, Verkarre V, Rahier J, Brunelle F, Nihoul-Fékété C, Saudubray JM, Robert JJ, de Lonlay P. Glucose metabolism in 105 children and adolescents after pancreatectomy for congenital hyperinsulinism. Diabetes Care. 2012;35:198–203. doi: 10.2337/dc11-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arya VB, Senniappan S, Demirbilek H, Alam S, Flanagan SE, Ellard S, Hussain K. Pancreatic endocrine and exocrine function in children following near-total pancreatectomy for diffuse congenital hyperinsulinism. PLoS One. 2014;9:e98054. doi: 10.1371/journal.pone.0098054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley CA. Perspective on the genetics and diagnosis of congenital hyperinsulinism disorders. J Clin Endocrinol Metab. 2016;101:815–26. doi: 10.1210/jc.2015-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demirbilek H, Hussain K. Congenital hyperinsulinism: diagnosis and treatment update. J Clin Res Pediatr Endocrinol. 2017;9(Suppl 2):69–87. doi: 10.4274/jcrpe.2017.S007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenfeld E, Ganguly A, De Leon DD. Congenital hyperinsulinism disorders: genetic and clinical characteristics. Am J Med Genet C Semin Med Genet. 2019;181:682–692. doi: 10.1002/ajmg.c.31737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee I, Salomon-Estebanez M, Shah P, Nicholson J, Cosgrove KE, Dunne MJ. Therapies and outcomes of congenital hyperinsulinism-induced hypoglycaemia. Diabet Med. 2019;36:9–21. doi: 10.1111/dme.13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treglia G, Mirk P, Giordano A, Rufini V. Diagnostic performance of fluorine-18-dihydroxyphenylalanine positron emission tomography in diagnosing and localizing the focal form of congenital hyperinsulinism: a meta-analysis. Pediatr Radiol. 2012;42:1372–9. doi: 10.1007/s00247-012-2459-2. [DOI] [PubMed] [Google Scholar]
- 9.Zani A, Nah SA, Ron O, Totonelli G, Ismail D, Smith VV, Ashworth M, Blankenstein O, Mohnike W, De Coppi P, Eaton S, Hussain K, Pierro A. The predictive value of preoperative fluorine-18-L-3,4-dihydroxyphenylalanine positron emission tomography-computed tomography scans in children with congenital hyperinsulinism of infancy. J Pediatr Surg. 2011;46:204–8. doi: 10.1016/j.jpedsurg.2010.09.093. [DOI] [PubMed] [Google Scholar]
- 10.Gopal-Kothandapani JS, Hussain K. Congenital hyperinsulinism: role of fluorine-18L-3, 4 hydroxyphenylalanine positron emission tomography scanning. World J Radiol. 2014;6:252–60. doi: 10.4329/wjr.v6.i6.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otonkoski T, Näntö-Salonen K, Seppänen M, Veijola R, Huopio H, Hussain K, Tapanainen P, Eskola O, Parkkola R, Ekström K, Guiot Y, Rahier J, Laakso M, Rintala R, Nuutila P, Minn H. Noninvasive diagnosis of focal hyperinsulinism of infancy with [18F]-DOPA positron emission tomography. Diabetes. 2006;55:13–8. [PubMed] [Google Scholar]
- 12.Ribeiro MJ, Boddaert N, Bellanné-Chantelot C, Bourgeois S, Valayannopoulos V, Delzescaux T, Jaubert F, Nihoul-Fékété C, Brunelle F, De Lonlay P. The added value of [18F]fluoro-L-DOPA PET in the diagnosis of hyperinsulinism of infancy: a retrospective study involving 49 children. Eur J Nucl Med Mol Imaging. 2007;34:2120–8. doi: 10.1007/s00259-007-0498-y. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro MJ, De Lonlay P, Delzescaux T, Boddaert N, Jaubert F, Bourgeois S, Dollé F, Nihoul-Fékété C, Syrota A, Brunelle F. Characterization of hyperinsulinism in infancy assessed with PET and 18F-fluoro-L-DOPA. J Nucl Med. 2005;46:560–6. [PubMed] [Google Scholar]
- 14.Yang J, Hao R, Zhu X. Diagnostic role of 18F-dihydroxyphenylalanine positron emission tomography in patients with congenital hyperinsulinism: a meta-analysis. Nucl Med Commun. 2013;34:347–53. doi: 10.1097/MNM.0b013e32835e6ac6. [DOI] [PubMed] [Google Scholar]
- 15.Blomberg BA, Moghbel MC, Saboury B, Stanley CA, Alavi A. The value of radiologic interventions and (18)F-DOPA PET in diagnosing and localizing focal congenital hyperinsulinism: systematic review and meta-analysis. Mol Imaging Biol. 2013;15:97–105. doi: 10.1007/s11307-012-0572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christiansen CD, Petersen H, Nielsen AL, Detlefsen S, Brusgaard K, Rasmussen L, Melikyan M, Ekström K, Globa E, Rasmussen AH, Hovendal C, Christesen HT. 18F-DOPA PET/CT and 68Ga-DOTANOC PET/CT scans as diagnostic tools in focal congenital hyperinsulinism: a blinded evaluation. Eur J Nucl Med Mol Imaging. 2018;45:250–261. doi: 10.1007/s00259-017-3867-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton PS, Satin-Smith MS, Herold K, Glaser B, Chiu KC, Nestorowicz A, Permutt MA, Baker L, Stanley CA. Familial hyperinsulinism with apparent autosomal dominant inheritance: clinical and genetic differences from the autosomal recessive variant. J Pediatr. 1998;132:9–14. doi: 10.1016/s0022-3476(98)70477-9. [DOI] [PubMed] [Google Scholar]
- 18.Taguchi T, Suita S, Ohkubo K, Ono J. Mutations in the sulfonylurea receptor gene in relation to the long-term outcome of persistent hyperinsulinemic hypoglycemia of infancy. J Pediatr Surg. 2002;37:593–8. doi: 10.1053/jpsu.2002.31616. [DOI] [PubMed] [Google Scholar]
- 19.Glaser B, Ryan F, Donath M, Landau H, Stanley CA, Baker L, Barton DE, Thornton PS. Hyperinsulinism caused by paternal-specific inheritance of a recessive mutation in the sulfonylurea-receptor gene. Diabetes. 1999;48:1652–7. doi: 10.2337/diabetes.48.8.1652. [DOI] [PubMed] [Google Scholar]
- 20.Bellanné-Chantelot C, Saint-Martin C, Ribeiro MJ, Vaury C, Verkarre V, Arnoux JB, Valayannopoulos V, Gobrecht S, Sempoux C, Rahier J, Fournet JC, Jaubert F, Aigrain Y, Nihoul-Fékété C, de Lonlay P. ABCC8 and KCNJ11 molecular spectrum of 109 patients with diazoxide-unresponsive congenital hyperinsulinism. J Med Genet. 2010;47:752–9. doi: 10.1136/jmg.2009.075416. [DOI] [PubMed] [Google Scholar]
- 21.Flanagan SE, Xie W, Caswell R, Damhuis A, Vianey-Saban C, Akcay T, Darendeliler F, Bas F, Guven A, Siklar Z, Ocal G, Berberoglu M, Murphy N, O’Sullivan M, Green A, Clayton PE, Banerjee I, Clayton PT, Hussain K, Weedon MN, Ellard S. Next-generation sequencing reveals deep intronic cryptic ABCC8 and HADH splicing founder mutations causing hyperinsulinism by pseudoexon activation. Am J Hum Genet. 2013;92:131–6. doi: 10.1016/j.ajhg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palladino AA, Stanley CA. Nesidioblastosis no longer! It’s all about genetics. J Clin Endocrinol Metab. 2011;96:617–9. doi: 10.1210/jc.2011-0164. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee I, Avatapalle B, Padidela R, Stevens A, Cosgrove KE, Clayton PE, Dunne MJ. Integrating genetic and imaging investigations into the clinical management of congenital hyperinsulinism. Clin Endocrinol (Oxf) 2013;78:803–13. doi: 10.1111/cen.12153. [DOI] [PubMed] [Google Scholar]
- 24.Santhanam P, Taieb D. Role of (18) F-FDOPA PET/CT imaging in endocrinology. Clin Endocrinol (Oxf) 2014;81:789–98. doi: 10.1111/cen.12566. [DOI] [PubMed] [Google Scholar]
- 25.Hardy OT, Hernandez-Pampaloni M, Saffer JR, Scheuermann JS, Ernst LM, Freifelder R, Zhuang H, MacMullen C, Becker S, Adzick NS, Divgi C, Alavi A, Stanley CA. Accuracy of [18F]fluorodopa positron emission tomography for diagnosing and localizing focal congenital hyperinsulinism. J Clin Endocrinol Metab. 2007;92:4706–11. doi: 10.1210/jc.2007-1637. [DOI] [PubMed] [Google Scholar]
- 26.Capito C, Khen-Dunlop N, Ribeiro MJ, Brunelle F, Aigrain Y, Crétolle C, Jaubert F, De Lonlay P, Nihoul-Fékété C. Value of 18F-fluoro-L-dopa PET in the preoperative localization of focal lesions in congenital hyperinsulinism. Radiology. 2009;253:216–22. doi: 10.1148/radiol.2532081445. [DOI] [PubMed] [Google Scholar]
- 27.Mohnike W, Barthlen W, Mohnike K, Blankenstein O. Positron emission tomography/computed tomography diagnostics by means of fluorine-18-L-dihydroxyphenylalanine in congenital hyperinsulinism. Semin Pediatr Surg. 2011;20:23–7. doi: 10.1053/j.sempedsurg.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Koopmans KP, Neels ON, Kema IP, Elsinga PH, Links TP, de Vries EG, Jager PL. Molecular imaging in neuroendocrine tumors: molecular uptake mechanisms and clinical results. Crit Rev Oncol Hematol. 2009;71:199–213. doi: 10.1016/j.critrevonc.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Minn H, Kauhanen S, Seppänen M, Nuutila P. 18F-FDOPA: a multiple-target molecule. J Nucl Med. 2009;50:1915–8. doi: 10.2967/jnumed.109.065664. [DOI] [PubMed] [Google Scholar]
- 30.Imperiale A, Sebag F, Vix M, Castinetti F, Kessler L, Moreau F, Bachellier P, Guillet B, Namer IJ, Mundler O, Taïeb D. 18F-FDOPA PET/CT imaging of insulinoma revisited. Eur J Nucl Med Mol Imaging. 2015;42:409–18. doi: 10.1007/s00259-014-2943-z. [DOI] [PubMed] [Google Scholar]
- 31.Leroy-Freschini B, Amodru V, Addeo P, Sebag F, Vix M, Brunaud L, Klein M, Bahougne T, Bachellier P, Castinetti F, Goichot B, Chevalier E, Taieb D, Imperiale A. Early (18)F-FDOPA PET/CT imaging after carbidopa premedication as a valuable diagnostic option in patients with insulinoma. Eur J Nucl Med Mol Imaging. 2019;46:686–695. doi: 10.1007/s00259-018-4245-3. [DOI] [PubMed] [Google Scholar]
- 32.Imperiale A, Bahougne T, Goichot B, Bachellier P, Taïeb D, Namer IJ. Dynamic 18F-FDOPA PET findings after carbidopa premedication in 2 adult patients with insulinoma-related hyperinsulinemic hypoglycemia. Clin Nucl Med. 2015;40:682–4. doi: 10.1097/RLU.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 33.Nakuz TS, Berger E, El-Rabadi K, Wadsak W, Haug A, Hacker M, Karanikas G. Clinical value of (18)F-FDOPA PET/CT with contrast enhancement and without carbidopa premedication in patients with insulinoma. Anticancer Res. 2018;38:353–358. doi: 10.21873/anticanres.12229. [DOI] [PubMed] [Google Scholar]
- 34.Schonbrunn A, Tashjian H Jr. Characterization of functional receptors for somatostatin in rat pituitary cells in culture. J Biol Chem. 1978;253:6473–83. [PubMed] [Google Scholar]
- 35.Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O’Carroll AM, Patel YC, Schonbrunn A, Taylor JE, et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16:86–8. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- 36.Filippi L, Pizzichini P, Bagni O, Scopinaro F. Somatostatin receptor analogs (68Ga-DOTATOC, 68Ga-DOTANOC, 68Ga-DOTATATE), in radiopharmaceuticals. Cham: Springer; 2020. [Google Scholar]
- 37.Pauwels E, Cleeren F, Bormans G, Deroose CM. Somatostatin receptor PET ligands - the next generation for clinical practice. Am J Nucl Med Mol Imaging. 2018;8:311–331. [PMC free article] [PubMed] [Google Scholar]
- 38.Pattison DA, Hicks RJ. Molecular imaging in the investigation of hypoglycaemic syndromes and their management. Endocr Relat Cancer. 2017;24:R203–R221. doi: 10.1530/ERC-17-0005. [DOI] [PubMed] [Google Scholar]
- 39.Prasad V, Sainz-Esteban A, Arsenic R, Plöckinger U, Denecke T, Pape UF, Pascher A, Kühnen P, Pavel M, Blankenstein O. Role of (68)Ga somatostatin receptor PET/CT in the detection of endogenous hyperinsulinaemic focus: an explorative study. Eur J Nucl Med Mol Imaging. 2016;43:1593–600. doi: 10.1007/s00259-016-3331-7. [DOI] [PubMed] [Google Scholar]


