Abstract
The current study was carried out to measure the basal ileal endogenous amino acid (EAA) flow in male broilers (Ross 308) at different ages (d 7, 14, 21, 28, 35, and 42), following the feeding of a nitrogen-free diet. Titanium dioxide (5 g/kg) was included as an indigestible marker. The nitrogen-free diet was offered for four days prior to ileal digesta collection to 6 replicate cages housing 14 (d 3–7), 12 (d 10–14), 10 (d 17–21), 8 (d 24–28), 8 (d 31–35), and 6 (d 38–42) birds per cage. The basal EAA flow was calculated as g/kg DM intake. The amino acid (AA) profile of endogenous protein, expressed as g/100 g protein, was also calculated. The basal endogenous flow of nitrogen and all individual and total AA decreased quadratically (P < 0.05 to 0.001), with flows being higher on d 7, then decreasing on d 14, plateauing until d 35 and decreasing further on d 42. The concentrations of Trp, Cys, and Gly in the endogenous protein increased linearly (P < 0.01 to 0.001) with advancing age, whereas a linear decrease (P < 0.001) was noted for Lys. A quadratic influence (P < 0.05 to 0.001) was observed for the concentrations of Ile, Leu, Met, Val, and Asp. These changes in the endogenous protein profile may be attributed to variations in the contribution of endogenous sources with age but delineating the exact contribution of different sources is complicated. Overall, the current findings suggest that the basal ileal EAA flow is influenced by broiler age and age-specific EAA flows may need to be considered to standardize the AA digestibility.
Key words: age, endogenous flow, amino acids, broilers
INTRODUCTION
Continuous flow of significant quantities of endogenous protein occurs during the process of digestion of ingested feed in poultry (Ravindran, 2021). The sources of endogenous protein are various digestive secretions (bile, gastric, pancreatic, and intestinal secretions), mucoproteins and desquamated epithelial cells lining the gastrointestinal tract (GIT). Accurate quantification of the flow of endogenous amino acids (EAA) is necessary to calculate the standardized amino acid (AA) digestibility of feed ingredients. These inevitable flows also contribute to the metabolic costs associated with protein synthesis and turnover in the GIT, and to the determination of protein and AA requirements by the factorial method. The measurement and a better understanding of the factors influencing the EAA flow is relevant in improving protein or AA utilization, and thereby reducing nitrogen (N) excretion into the environment (Cowieson et al., 2009).
Endogenous AA losses are influenced primarily by dry matter intake (DMI) and secondarily by the composition of the feed ingredient or diet. These 2 fractions are categorized as basal and specific EAA losses, respectively (Ravindran, 2016). Basal endogenous losses are defined as those closely associated with the metabolic functions of the animal and are independent of the diet type. These losses represent the minimum losses that can be expected under any feeding situation. Several methods are available to determine the basal EAA flows in poultry including the regression method, feeding a N-free diet (NFD) or a diet containing highly digestible protein, the peptide alimentation (enzyme hydrolyzed casein) method, or the use of fasted birds. Each of these approaches has its advantages and limitations (Ravindran and Bryden, 1999; Bloke et al., 2017; Parsons, 2020). Of these, basal ileal EAA estimates determined following the feeding of an NFD is now accepted as being valid for the correction of apparent AA digestibility values (Stein et al., 2007; Ravindran et al., 2017; Parsons, 2020).
The NFD is generally composed of starch or sugar (>80%), fortified with insoluble fiber (e.g., cellulose), minerals and vitamins. The assumption is that, since no protein is fed, all N and AA in the ileal digesta are of endogenous origin and represent the basal flows. Feeding an NFD is a simple method despite suffering from limitations of underestimation and nonphysiological feeding (Donkoh and Moughan, 1999; Stein et al., 2007).
A wide range of factors including the class of birds (broilers, layers, roosters; Ravindran and Hendriks, 2004), protein status, BW, health status, and DMI are reported to influence the basal EAA estimates (Lemme et al., 2004; Adedokun et al., 2007a). Possible age effect may be an important factor. However, no previous studies have compared the EAA flows throughout the growth cycle of broilers. To the author's knowledge, except those by Adedokun et al. (2007a,b), all previous studies (Ravindran et al., 2004; Golian et al., 2008; Soleimani et al., 2010; Kong and Adeola, 2013) have the measured EAA flow at a single age using broilers older than 21 d. The applicability of EAA data derived from one single age to all broiler ages is questionable and, therefore, the aim of present study was to determine the basal ileal endogenous N and AA flows at different ages of broilers.
MATERIALS AND METHODS
The experimental procedures were in accordance with the New Zealand Revised Code of Ethical Conduct for the use of live animals for research, testing, and teaching, approved by Massey University Animal Ethics Committee.
Diets
The composition of the NFD is shown in Table 1. Titanium dioxide was included as an indigestible marker.
Table 1.
Composition of the nitrogen-free diet (g/kg, as fed basis).
| Ingredients | g/kg |
|---|---|
| Corn starch | 842 |
| Cellulose1 | 50 |
| Soybean oil | 50 |
| Dicalcium phosphate | 19 |
| Limestone | 13 |
| Dipotassium phosphate | 12 |
| Titanium dioxide2 | 5.0 |
| Trace mineral premix3 | 3.0 |
| Vitamin premix3 | 2.0 |
| Sodium bicarbonate | 2.0 |
| Sodium chloride | 2.0 |
Ceolus, Microcrystalline Cellulose, Asahi Kasei Corporation, Tokyo, Japan.
Merck KGaA, Darmstadt, Germany.
Provided per kilogram of diet: antioxidant, 100 mg; biotin, 0.2 mg; calcium pantothenate, 12.8 mg; cholecalciferol, 0.06 mg; cyanocobalamin, 0.017 mg; folic acid, 5.2 mg; menadione, 4 mg; niacin, 35 mg; pyridoxine, 10 mg; transretinol, 3.33 mg; riboflavin, 12 mg; thiamine, 3.0 mg; dl-α-tocopheryl acetate, 60 mg; choline chloride, 638 mg; Co, 0.3 mg; Cu, 3.0 mg; Fe, 25 mg; I, 1 mg; Mn, 125 mg; Mo, 0.5 mg; Se, 0.2 mg; Zn, 60 mg.
Birds and Housing
A total of 500, day-old male broilers (Ross 308) were obtained from a commercial hatchery, raised in floor pens and fed a commercial crumbled broiler starter diet (AME, 2,900 kcal/kg; CP, 225 g/kg) from d 1 to 21 and a broiler finisher diet (AME, 3,030 kcal/kg; CP, 190 g/kg) in pelleted form from d 22 to 42 (Table 2). Of the 500, 348 healthy birds were used for the experiment. The starter, finisher and NFD diets, in mash form, were offered ad libitum and fresh drinking water was available at all times. During the first week, the average temperature was 32 ± 1°C and gradually reduced to 23°C by the end of the third week.
Table 2.
Composition and calculated analysis (g/kg, as fed basis) of broiler starter and finisher diets.
| Ingredients | Starter diet (0–21 d) | Finisher diet (22–42 d) |
|---|---|---|
| Corn | 574.2 | 660 |
| Soybean meal, 46% | 381.4 | 295.6 |
| Soybean oil | 8.8 | 13.6 |
| Limestone | 11.3 | 9.9 |
| Dicalcium phosphate | 10.7 | 8.2 |
| DL-methionine | 3.3 | 3.0 |
| L-lysine HCl | 2.0 | 1.9 |
| L-threonine | 1.0 | 0.7 |
| Sodium bicarbonate | 2.7 | 2.5 |
| Sodium chloride | 2.5 | 2.5 |
| Trace mineral premix1 | 1.0 | 1.0 |
| Vitamin premix1 | 1.0 | 1.0 |
| Phytase | 0.1 | 0.1 |
| Calculated analysis | ||
| AME (kcal/kg) | 2900 | 3030 |
| CP | 225 | 190 |
| Digestible lysine | 11.0 | 9.2 |
| Digestible methionine | 6.2 | 5.6 |
| Digestible methionine + cysteine | 9.2 | 8.3 |
| Digestible threonine | 7.2 | 6.0 |
| Crude fat | 32 | 39 |
| Crude fibre | 29.3 | 27.5 |
| Calcium | 9.8 | 8.5 |
| Available phosphorus | 4.9 | 4.2 |
| Sodium | 2.2 | 2.1 |
| Chloride | 2.3 | 2.3 |
| Potassium | 11.5 | 9.7 |
Provided per kilogram of diet: antioxidant, 100 mg; biotin, 0.2 mg; calcium pantothenate, 12.8 mg; cholecalciferol, 0.06 mg; cyanocobalamin, 0.017 mg; folic acid, 5.2 mg; menadione, 4 mg; niacin, 35 mg; pyridoxine, 10 mg; transretinol, 3.33 mg; riboflavin, 12 mg; thiamine, 3.0 mg; dl-α-tocopheryl acetate, 60 mg; choline chloride, 638 mg; Co, 0.3 mg; Cu, 3.0 mg; Fe, 25 mg; I, 1 mg; Mn, 125 mg; Mo, 0.5 mg; Se, 0.2 mg; Zn, 60 mg.
There were 6 groups of different ages (d 3–7, 10–14, 17–21, 24–28, 31–35, and 38–42) of broilers. On d 3, 84 birds were weighted individually and allocated to 6 battery brooders (n = 14 chicks per replicate) in such a way that the average bird weight per cage was similar. The remaining chicks (n = 264) were raised in floor pens until they were weighed and allocated to 6 replicate grower cages per age group on d 10 (n = 12 birds per cage), d 17 (n = 10 birds per cage), d 24 (n = 8 birds per cage), d 31 (n = 8 birds per cage), and d 38 (n = 6 birds per cage), respectively. In each age group, the NFD was fed for 4 d (d 3–7, 10–14, 17–21, 24–28, 31–35, and 38–42) prior to the ileal digesta collection.
The floor pens, battery brooder and grower cages were housed in an environmentally controlled room with 20 h of fluorescent illumination per day.
Determination of Ileal Nutrient Digestibility
The birds were euthanized by intravenus injection (1 mL per 2 kg body weight) of sodium pentobarbitone solution (Provet NZ Pty. Ltd., Auckland, New Zealand) on d 7, 14, 21, 28, 35, and 42. Digesta were collected from the lower half of the ileum, as described by Ravindran et al. (2005). The ileum was considered as the portion of the small intestine from Meckel's diverticulum to a point about ~40 mm proximal to the ileocecal junction. The ileal digesta were collected from all birds into plastic containers by gentle flushing by distilled water, pooled within a cage, immediately frozen, and subsequently freeze dried (Model 0610, Cuddon Engineering, Blenheim, New Zealand). The diet and freeze-died digesta samples were ground to pass through a 0.5-mm sieve. The samples were stored in airtight plastic containers at 4°C until the analysis of DM, titanium (Ti), N, and AA.
Chemical Analysis
The DM was determined using the standard procedure (Method 930.15; AOAC International, 2016). Titanium was measured on an ultraviolet spectrophotometer following the method of Short et al. (1996). Nitrogen was analyzed by combustion (Method 968.06; AOAC International, 2016) using a CNS-200 carbon, N, sulfur analyzer (LECO Corporation, St. Joseph, MI).
Amino acids were analyzed following the standard procedures (Method 994.12; AOAC International, 2011). In brief, the samples were hydrolyzed with 6 N HCl (containing phenol) for 24 h at 110 ± 2°C in glass tubes in an oven. The AA was measured using AA analyzer (ion exchange) with ninhydrin postcolumn derivatization. The chromatograms were integrated using dedicated software (Agilent Open Lab software, Waldbronn, Baden-Württemberg, Germany) with AA simultaneously detected at 570 and 440 nm. Cysteine and methionine were determined as cysteic acid and methionine sulphone, respectively, by oxidation with performic acid for 16 h at 0°C and neutralization with hydrobromic acid prior to hydrolysis.
For Trp analysis, the samples were saponified under alkaline conditions with barium hydroxide solution in the absence of air at 110°C for 20 h in an autoclave. Following alkaline hydrolysis, the internal standard α-methyl Trp was added to the mixture. After adjusting the hydrolysate to pH 3.0 and diluting with 30% methanol, Trp and the internal standard were separated by reverse phase chromatography on a HPLC column. Detection was selectively done by means of a fluorescence detector to prevent interference by other AA and constituents.
Calculations
All data were expressed on a DM basis for calculations. The basal EAA flow at the terminal ileum was calculated as g AA flow per kg DMI using the following formula (Moughan et al., 1992).
The AA profile of endogenous protein was calculated by expressing each AA as g/100 g of endogenous protein (N × 6.25), as indicated below.
Data Analysis
Data were analyzed by the GLM procedure of SAS (version 9.4; 2015; SAS Institute, Cary, NC). Orthogonal polynomial contrasts were performed to determine the linear and quadratic effects of broiler age. Cage served as the experimental unit. Statistical significance was declared at P < 0.05.
RESULTS
No evidence of intestinal abnormalities was observed when the abdominal cavity was opened following euthanasia.
Feed Intake
Feed intake (FI) increased (quadratic, P < 0.001) with advancing age of birds. The average daily FI of birds were 11.4 (d 7), 27.3 (d 14), 49.1 (d 21), 79.6 (d 28), 80.9 (d 35), 79.1 (d 42) g/bird, respectively.
Basal Endogenous Flow of Nitrogen and Amino Acids
The basal ileal endogenous flow of N and AA at different ages (d 7, 14, 21, 28, 35, and 42) of broiler, expressed as g/kg DMI, is presented in Table 3.
Table 3.
Basal ileal endogenous nitrogen (N) and amino acid flows1 (g/kg DM intake) at different ages of broilers.
| Age (days) |
Orthogonal polynomial contrasts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | Pooled SEM | Linear | Quadratic |
| N | 3.599 | 1.866 | 1.793 | 1.823 | 1.808 | 1.295 | 0.1943 | 0.001 | 0.001 |
| Indispensable amino acids | |||||||||
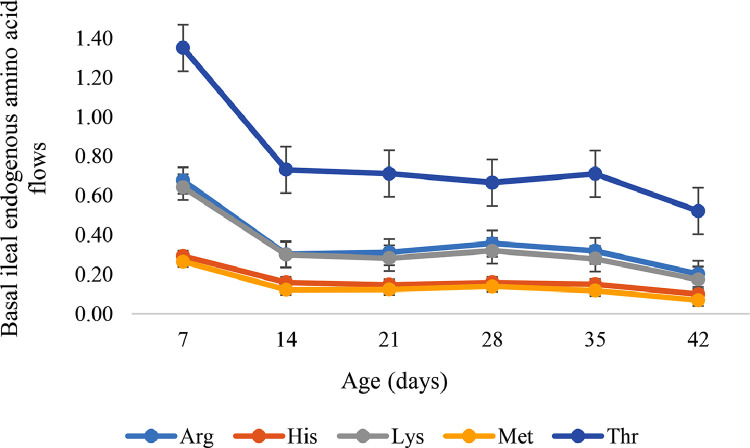
| Arg | 0.678 | 0.303 | 0.313 | 0.358 | 0.320 | 0.203 | 0.0411 | 0.001 | 0.007 |
| His | 0.294 | 0.160 | 0.148 | 0.160 | 0.150 | 0.100 | 0.0176 | 0.001 | 0.012 |
| Ile | 0.626 | 0.325 | 0.323 | 0.343 | 0.301 | 0.201 | 0.0352 | 0.001 | 0.013 |
| Leu | 0.974 | 0.485 | 0.490 | 0.545 | 0.485 | 0.314 | 0.0574 | 0.001 | 0.017 |
| Lys | 0.644 | 0.301 | 0.283 | 0.321 | 0.280 | 0.175 | 0.0388 | 0.001 | 0.004 |
| Met | 0.266 | 0.123 | 0.124 | 0.140 | 0.118 | 0.069 | 0.0157 | 0.001 | 0.013 |
| Thr | 1.352 | 0.733 | 0.713 | 0.667 | 0.712 | 0.523 | 0.0751 | 0.001 | 0.002 |
| Trp | 0.206 | 0.116 | 0.121 | 0.127 | 0.121 | 0.088 | 0.0129 | 0.001 | 0.048 |
| Val | 0.811 | 0.431 | 0.433 | 0.453 | 0.420 | 0.299 | 0.0458 | 0.001 | 0.009 |
| IAA | 5.852 | 2.975 | 2.948 | 3.114 | 2.906 | 1.973 | 0.3365 | 0.001 | 0.007 |
| Dispensable amino acids | |||||||||
| Ala | 0.745 | 0.366 | 0.361 | 0.408 | 0.370 | 0.248 | 0.0437 | 0.001 | 0.008 |
| Asp | 1.407 | 0.733 | 0.721 | 0.744 | 0.721 | 0.497 | 0.0785 | 0.001 | 0.005 |
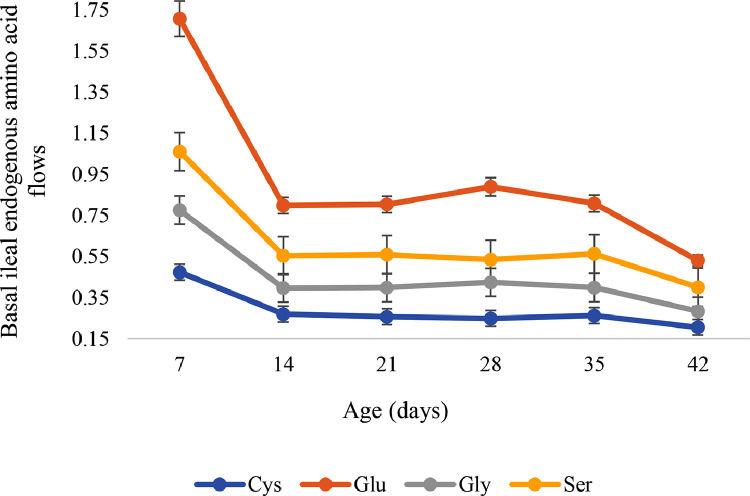
| Cys2 | 0.474 | 0.270 | 0.257 | 0.249 | 0.263 | 0.206 | 0.0251 | 0.001 | 0.001 |
| Glu | 1.707 | 0.799 | 0.804 | 0.890 | 0.809 | 0.531 | 0.0985 | 0.001 | 0.004 |
| Gly2 | 0.776 | 0.397 | 0.399 | 0.425 | 0.399 | 0.284 | 0.0437 | 0.001 | 0.005 |
| Pro | 0.911 | 0.479 | 0.481 | 0.476 | 0.473 | 0.341 | 0.0509 | 0.001 | 0.004 |
| Ser | 1.061 | 0.554 | 0.559 | 0.536 | 0.563 | 0.401 | 0.0579 | 0.001 | 0.002 |
| DAA | 7.081 | 3.599 | 3.583 | 3.729 | 3.559 | 2.508 | 0.3966 | 0.001 | 0.004 |
| TAA | 12.93 | 6.574 | 6.531 | 6.842 | 6.505 | 4.481 | 0.7328 | 0.001 | 0.005 |
Each value represents the mean of six replicates (14, 12, and 10 birds per replicate for 7, 14, and 21-d old birds, respectively; eight birds per replicate for 28, 35-d old birds; and six birds per replicate for 42-d old birds).
Semi-indispensable amino acids for poultry.
Abbreviations: DAA, total endogenous flow of dispensable amino acids; IAA, total endogenous flow of indispensable amino acids; TAA, total endogenous flow of all amino acids.
A quadratic influence (P < 0.05 to 0.001) was observed on the basal endogenous flow of N and all AA. The flows were higher on d 7, then decreased on d 14 and plateaued until d 35. After d 35, a further decrease to d 42 was observed. The highest and lowest values were recorded on d 7 and 42, respectively (Figure 1, Figure 2).
Figure 1.
Basal ileal endogenous amino acid flow of selected indispensable amino acids (bars represent means ± standard error) as influenced by broiler age (Quadratic effects, P < 0.05 to 0.01).
Figure 2.
Basal ileal endogenous amino acid flow of selected dispensable amino acids (bars represent means ± standard error) as influenced by broiler age (Quadratic effects, P < 0.05 to 0.01).
Ileal Digesta Concentrations of Nitrogen and Amino Acids
The N and AA concentrations in the ileal digesta of birds fed the NFD, expressed as g/100g digesta, are summarized in Table 4. The concentrations of N and all AA, except Trp and Cys, linearly decreased (P < 0.05 to 0.001) as the broilers grew older.
Table 4.
Amino acid concentrations1 (g/100 g of digesta) in the ileal digesta of broilers fed NFD at different ages.
| Age (days) |
Orthogonal polynomial contrasts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | Pooled SEM | Linear | Quadratic |
| N | 1.072 | 0.884 | 0.957 | 1.001 | 0.848 | 0.725 | 0.0663 | 0.003 | 0.344 |
| Indispensable amino acids | |||||||||
| Arg | 0.202 | 0.145 | 0.168 | 0.197 | 0.149 | 0.114 | 0.0157 | 0.005 | 0.234 |
| His | 0.088 | 0.076 | 0.079 | 0.088 | 0.069 | 0.056 | 0.0072 | 0.009 | 0.152 |
| Ile | 0.187 | 0.155 | 0.174 | 0.189 | 0.141 | 0.113 | 0.0144 | 0.003 | 0.066 |
| Leu | 0.289 | 0.233 | 0.263 | 0.299 | 0.227 | 0.176 | 0.0227 | 0.007 | 0.075 |
| Lys | 0.192 | 0.143 | 0.152 | 0.176 | 0.129 | 0.098 | 0.0146 | 0.001 | 0.326 |
| Met | 0.079 | 0.058 | 0.067 | 0.077 | 0.055 | 0.039 | 0.0059 | 0.001 | 0.078 |
| Thr | 0.404 | 0.349 | 0.381 | 0.366 | 0.335 | 0.294 | 0.0294 | 0.019 | 0.487 |
| Trp | 0.061 | 0.055 | 0.066 | 0.069 | 0.057 | 0.049 | 0.0055 | 0.279 | 0.059 |
| Val | 0.242 | 0.205 | 0.233 | 0.249 | 0.198 | 0.167 | 0.0183 | 0.019 | 0.100 |
| IAA | 1.743 | 1.418 | 1.583 | 1.711 | 1.359 | 1.105 | 0.1313 | 0.006 | 0.165 |
| Dispensable amino acids | |||||||||
| Ala | 0.222 | 0.174 | 0.194 | 0.224 | 0.172 | 0.139 | 0.0159 | 0.007 | 0.154 |
| Asp | 0.419 | 0.349 | 0.386 | 0.409 | 0.338 | 0.278 | 0.0293 | 0.007 | 0.166 |
| Cys2 | 0.141 | 0.128 | 0.137 | 0.137 | 0.124 | 0.115 | 0.0091 | 0.074 | 0.444 |
| Glu | 0.508 | 0.381 | 0.431 | 0.489 | 0.377 | 0.297 | 0.0368 | 0.003 | 0.229 |
| Gly2 | 0.231 | 0.189 | 0.214 | 0.233 | 0.188 | 0.159 | 0.0166 | 0.018 | 0.160 |
| Pro | 0.271 | 0.229 | 0.258 | 0.261 | 0.223 | 0.191 | 0.0204 | 0.021 | 0.255 |
| Ser | 0.317 | 0.264 | 0.299 | 0.295 | 0.264 | 0.225 | 0.0221 | 0.018 | 0.338 |
| DAA | 2.109 | 1.714 | 1.919 | 2.049 | 1.688 | 1.404 | 1.4881 | 0.009 | 0.220 |
| TAA | 3.853 | 3.132 | 3.502 | 3.760 | 3.047 | 2.509 | 0.2799 | 0.008 | 0.193 |
Each value represents the mean of six replicates (14, 12, and 10 birds per replicate for 7, 14, and 21-d old birds, respectively; eight birds per replicate for 28, 35-d old birds; and six birds per replicate for 42-d old birds).
Semi-indispensable amino acids for poultry.
Abbreviations: DAA, total endogenous flow of dispensable amino acids; IAA, total endogenous flow of indispensable amino acids; TAA, total endogenous flow of all amino acids.
Amino Acid Profile of Endogenous Protein
The AA profile of ileal endogenous protein, expressed as g/100 g protein, in broilers of different ages is shown in Table 5. The most abundant AA in the endogenous protein was Glu, Asp, Thr, Ser, Leu, Pro, Val, and Ala. The lowest concentration among all AA was recorded for Trp, followed by Met.
Table 5.
Amino acid composition of endogenous protein1 (g per 100 g crude protein) at different ages of broilers.
| Age (days) |
Orthogonal polynomial contrasts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | 7 | 14 | 21 | 28 | 35 | 42 | Pooled SEM | Linear | Quadratic |
| Indispensable amino acids | |||||||||
| Arg | 3.031 | 2.439 | 2.679 | 2.992 | 2.883 | 2.551 | 0.1069 | 0.415 | 0.922 |
| His | 1.304 | 1.359 | 1.243 | 1.367 | 1.348 | 1.232 | 0.0422 | 0.462 | 0.253 |
| Ile | 2.780 | 2.658 | 2.763 | 2.942 | 2.712 | 2.479 | 0.0401 | 0.004 | 0.001 |
| Leu | 4.335 | 3.971 | 4.178 | 4.622 | 4.367 | 3.910 | 0.1028 | 0.582 | 0.031 |
| Lys | 2.869 | 2.429 | 2.366 | 2.695 | 2.532 | 2.217 | 0.1139 | 0.018 | 0.832 |
| Met | 1.189 | 0.985 | 1.045 | 1.200 | 1.078 | 0.859 | 0.0357 | 0.001 | 0.031 |
| Thr | 5.952 | 6.284 | 6.234 | 5.874 | 6.287 | 6.353 | 0.1724 | 0.274 | 0.747 |
| Trp | 0.923 | 0.967 | 1.039 | 1.096 | 1.074 | 1.086 | 0.0343 | 0.001 | 0.115 |
| Val | 3.599 | 3.583 | 3.751 | 3.927 | 3.764 | 3.683 | 0.0569 | 0.035 | 0.008 |
| Dispensable amino acids | |||||||||
| Ala | 3.312 | 3.028 | 3.124 | 3.569 | 3.319 | 3.106 | 0.0803 | 0.676 | 0.189 |
| Asp | 6.244 | 6.093 | 6.331 | 6.489 | 6.428 | 6.169 | 0.0861 | 0.297 | 0.049 |
| Cys2 | 2.092 | 2.345 | 2.314 | 2.192 | 2.310 | 2.540 | 0.0572 | 0.001 | 0.379 |
| Glu | 7.616 | 6.504 | 6.889 | 7.547 | 7.279 | 6.613 | 0.1668 | 0.171 | 0.808 |
| Gly2 | 3.443 | 3.269 | 3.497 | 3.697 | 3.571 | 3.527 | 0.0455 | 0.002 | 0.091 |
| Pro | 4.043 | 4.068 | 4.227 | 4.111 | 4.212 | 4.179 | 0.1046 | 0.273 | 0.599 |
| Ser | 4.684 | 4.689 | 4.919 | 4.699 | 4.916 | 4.909 | 0.1097 | 0.109 | 0.914 |
Each value represents the mean of six replicates (14, 12, and 10 birds per replicate for 7, 14, and 21-d old birds, respectively; eight birds per replicate for 28, 35-d old birds; and six birds per replicate for 42-d old birds).
Semi-indispensable amino acids for poultry.
Abbreviations: IAA, total endogenous flow of indispensable amino acids; DAA, total endogenous flow of dispensable amino acids; AAA, total endogenous flow of all amino acids.
The concentration of Trp, Cys and Gly linearly (P < 0.01 to 0.001) increased with advancing age, whereas that for Lys linearly (P < 0.001) decreased. Quadratic age effects (P < 0.05 to 0.001) were observed for the concentration of Ile, Leu, Met, Val, and Asp, but the responses at different ages were variable and inconsistent. No age effect was observed for the concentrations of other AA.
DISCUSSION
The aim of the current study was to investigate whether the basal ileal endogenous flows of N and AA are influenced by the age of broilers. The results showed that the basal ileal endogenous N and AA flows were markedly higher on d 7 and, then declined on d 14 and plateaued until d 35. A further decrease was observed on d 42. Compared to d 7, the basal endogenous N flow declined by 48 and 64% on d 14 and d 42, respectively. The endogenous flow of all AA on d 7 was almost twice of the values from d 14 to 35, and three times higher than the value on d 42. In agreement with the current findings, following the feeding of a NFD to broilers, Adedokun et al. (2007a) recorded approximately 2 times higher ileal EAA flow on d 5 (8.69 g/kg DMI) compared to d 15 (3.73 g/kg DMI) and 21 (3.95 g/kg DMI), with no significant differences between the flows on d 15 and 21.
Possible explanations for the higher ileal EAA flows determined on d 7 and reduced flows with advancing age are intricate. A proportion of secreted endogenous proteins is digested, along with ingested dietary proteins, in the GIT and absorbed. The EAA values determined at the ileum therefore relate to the algebraic differences between that secreted and absorbed (Moughan, 2003; Ravindran et al., 2004). The source of endogenous proteins and the entry point into the GIT further complicate this dynamic (Ravindran, 2021). When digestive enzymes dominate the endogenous flow, the proteins pass through the duodenum and jejunum where there is greater opportunity for digestion and absorption. In contrast, if mucus secretion or desquamation is significant, particularly if they occur distal to the duodenum, then the opportunity for digestion is lower and there will be relatively higher endogenous losses at the ileal level. Owing to this complicated dynamic, it is difficult to differentiate the true contribution of each endogenous source.
Nevertheless, the ramification is that the higher EAA losses on d 7 may reflect increased secretion, reduced absorption, or both. The first week after hatch is the most critical period in the life of a broiler chicken. At hatching, the digestive system of the chick is still immature and there is the transition from yolk to oral nutrition. Substantial physical and functional development of the GIT and digestive organs take place during the first week. It is therefore clear that the capacity to digest the feed and, absorb and transport nutrients is limited during this period. Thus, reduced absorption, rather than increased secretion, is the likely reason for the greater ileal EAA losses determined at d 7. Against this background, several possibilities may be considered as discussed below.
First, the GIT of the newly hatched chick is immature and the bird places high priority on intestinal growth to ensure the development of nutrient supply functions, as evidenced by dramatic growth during the first week (Sklan, 2001). In the days following hatching, weights of proventriculus, gizzard, and small intestine increase more rapidly in relation to BW than other organs and tissues (Katanbaf et al., 1988; Sell et al., 1991). This enhanced growth is maximal in chicks between 4 and 8 d of age and thereafter there is a relative decline. The mass of the small intestine increases almost 600% within the first 7 d (Noy et al., 2001). The length of the small intestine and its individual component regions also increase with age. According to Iji et al. (2001), the relative weight of small intestine peaked between d 7 and 14. The rapid growth in intestinal size during the first week may lead to mechanical inefficiency in the passage and mixing of digesta, reducing nutrient digestion, and increasing the EAA flow.
Second, the function of the GIT is strictly related to its microscopic structure. The architecture of GIT is not well developed during the first week of life but rapidly develops with age. The dramatic post-hatch increases observed in the weight and length of small intestine is reported to be minor relative to the growth of gut mucosa (Dibner et al., 1996). Uni et al. (1995) found that the villus height and area in the chick increase rapidly at different rates in different intestinal segments, particularly in the jejunum and ileum from 4 to 10 d post-hatch. Crypt depth, which reflects enterocyte maturation rate, increased linearly in both the duodenum and jejunum until 10 d. A study of morphological development of GIT in broiler chicks by Uni et al. (1996) showed that the height and perimeter of villi increased by 34 to 100% in all small intestinal segments between 4 and 10 d after hatching. In addition, the crypt depth, the enterocytes number per longitudinal section of villi increased with increasing age. The poorly developed intestinal architecture will also lead to lower digestion during week 1.
Third, the secretion of digestive enzymes by pancreas and the brush border of the small intestine is low at the time of hatch (Sell, 1996), but increase after hatch although the rate of increase was different for different enzymes (Tarvid, 1995; Noy and Sklan, 1997). The relative activity of aminopeptidases in all intestinal segments reduces after hatching, reaches a low point on d 10 and increase on d 15 (Tarvid, 1992). The greatest activity of dipeptidase was found at hatching and decrease within 7 days by 25% (Tarvid, 1990). The specific activity of pancreatic trypsin is lower during the first 3 to 6 d post-hatch, increases afterwards by up to 20% on d 14 (Nitsan et al., 1991). Both the relative trypsin and chymotrypsin activities (unit per kg BW) in pancreas increases with age, reaching a maximum level on d 11. The activity of trypsin in small intestine contents increases 10-fold from hatching to d 14. In case of chymotrypsin, it increases 3-fold to a maximum at 20 d of age, suggesting that lower protease activities during the first week after hatch could limit the AA digestibility. However, after wk 1, the trypsin activity increases up to 21 d, with no noticeable changes afterward (Nitsan et al., 1991).
In summary, during wk 1, the GIT is in a maturation phase in terms of growth, morphology and, secreted amounts and activities of proteolytic enzymes. Taken together, these imply a compromised protein digestion increasing the endogenous AA recovery at the ileal level.
Some other relevant factors potentially contributing to the higher EAA flow at d 7 may be worth considering. Cell proliferation and cell turnover are greater during wk 1 (Iji et al., 2001) and desquamated cells may therefore be important contributors to the EAA flow at d 7. There is some evidence that, when an NFD is fed, the source of EAA is largely mucoproteins (Adedokun et al., 2007a). The intestinal mucous layer is a protective barrier against harmful intraluminal components and microflora (Gork et al., 1999). Mucin plays a significant role in filtering nutrients in GIT and thus affecting the absorption of nutrients (Smirnov et al., 2006). Mucin glycoproteins are rich in Thr, Ser, Pro, Glu, and Asp (Souffrant, 1991; Lien et al., 1997). Adedokun et al. (2007b) reported higher Thr and Ser contents in the ileal digesta of 5-day-old broiler chickens compared to d 15 and 21 following feeding an NFD, reflecting a higher concentration of intestinal mucin at early ages. Similarly, in the present study, higher mucin production in the newly hatched chick was manifested by the higher endogenous flows of Thr (102%), Asp (106%), Glu (123%), Pro (103%), and Ser (103%) on d 7 than the average of other ages (d 14–42). Intestinal cells and mucin are poorly digested, and this may explain the higher EAA recovery at d 7. Lower relative mucin secretion and, increased endogenous protein digestion and absorption with age (Nasset, 1972; Ravindran and Bryden, 1999) may account for the lower endogenous N and AA secretion in older birds.
Food transit time through the digestive tract is an important factor influencing the nutrient digestion by determining the available time for contact among nutrients, digestive enzymes, absorptive surfaces, and microbiome (Clemens et al., 1975; Mateos et al., 1982; Vergara et al., 1989). Longer digesta retention increases the absorption of nutrients by enhancing contact time with absorptive cells (Washburn, 1991). Several studies have recorded slower intestinal passage rate in adult birds (Hurwitz and Bar, 1966; Sklan et al., 1975; Noy and Sklan, 1995). Noy and Sklan (1995) reported a longer digesta passage time in broiler chicks on d 4 than on d 14 (160 vs. 110 min). Decreased passage rate with age allows the digesta to be exposed to the digestive and absorptive processes for a longer time that may improve nutrient digestibility, including AA, in older birds.
Since all N in the ileal digesta in birds fed the NFD diet come only from endogenous proteins, one may argue that the AA concentration in the ileal digesta, expressed as g/kg and not corrected for DMI, may give some representation of the dynamic in EAA flow with age. When DMI was excluded, the concentration of almost all AA in the ileal digesta decreased with advancing broiler age. Since the AA originated from endogenous sources, this observation may suggest increased re-absorption of endogenous protein with age. In present study, FI increased gradually from 11.4 g/bird on d 7 to 79.1 g/bird on d 42, which might account, to some extent, for the observed differences in basal EAA flows, expressed as g/kg DMI, among broiler ages. It must be noted, however, the primary aim of measuring EAA losses is to standardize the apparent digestibility values and this correction will not be possible when EAA values are not linked to the DMI.
The concentrations of EAA in the ileal digesta are within the range reported in the literature (Ravindran, 2021). The higher proportions of Glu, Asp, Thr, and Ser determined in the endogenous protein were as expected. Asp, Leu, Ser, and Glu are the major AA in the ileal endogenous secretions in chickens (Bielorai and Iosif, 1987; Siriwan et al., 1994; Adedokun et al. 2007b). Ravindran et al. (2004) reported that the major AA in endogenous protein in the ileal digesta of 35-day-old chickens fed an NFD was Thr, Asp, and Glu. Tarvener et al. (1981) speculated that the high proportions of Glu, Asp, Thr, Ser, and Leu in the endogenous protein may be due to their slower rate of re-absorption compared with other AA. The lower concentrations of Met and His can be attributed to the fact that these AA are absorbed in greatest proportions compared to others in the GIT (Webb, 1990).
It is generally assumed that the AA profile of endogenous protein is somewhat constant, although it is known that individual sources of endogenous protein have different AA composition (Ravindran, 2021). The current findings suggest that the composition of the AA flow was altered by broiler age, ostensibly reflecting changes in the contribution of endogenous protein components with age. The concentration of Cys, Gly, and Trp was linearly increased with age. Cys is found in relatively higher concentrations in the mucin (Lien et al., 1997). The Gly was previously assumed to be originating from biliary secretions (Ravindran and Bryden, 1999; Ravindran and Hendriks, 2004; Adedokun et al., 2011), but current evidence indicates that the bile acid conjugation in poultry is exclusively with taurine and not with Gly as in pigs (Ravindran, 2021). The inconsistent effects of age on Ile, Leu, Met, Val, and Asp are difficult to explain and, as mentioned earlier, underline the complexity of identifying the exact contribution of different endogenous components.
CONCLUSIONS
The current findings, for the first time, provide information on the basal endogenous AA flows in broilers from hatching to 42 d of age. The flow was higher on d 7, reduced on d 14 and remained constant until d 35. A further reduction was noticed on d 42. The higher EAA flow at d 7 is ostensibly reflective of the immature digestive system and the resultant low digestive capacity in the newly hatched broiler chick. Factors like longer digesta retention, improved AA digestibility and, relatively lower production and increased utilization of mucin may account for the lower basal EAA flows in older birds. Therefore, the use of a single EAA value for birds of all ages will underestimate the standardized ileal AA digestibility in early life and overestimate the digestibility in older birds. Application of age-specific values for EAA flow may benefit accurate standardization of amino acid digestibility and improve the precision of feed formulations.
Acknowledgments
ACKNOWLEDGMENTS
The authors wish to express their appreciation to the “AgriFutures Australian Chicken Meat Program” for funding the project, and to Preethi Ramesh, AMINOLab EMSEA, Singapore for AA analyses.
DISCLOSURES
Authors declare no conflict of interest.
REFERENCES
- Adedokun S.A., Adeola O., Parsons C.M., Lilburn M.S., Applegate T.J. Factors affecting endogenous amino acid flow in chickens and the need for consistency in methodology. Poult. Sci. 2011;90:1737–1748. doi: 10.3382/ps.2010-01245. [DOI] [PubMed] [Google Scholar]
- Adedokun S.A., Parsons C.M., Lilburn M.S., Adeola O., Applegate T.J. Endogenous amino acid flow in broiler chicks is affected by the age of birds and method of estimation. Poult. Sci. 2007;86:2590–2597. doi: 10.3382/ps.2007-00096. [DOI] [PubMed] [Google Scholar]
- Adedokun S.A., Utterback P., Parsons C.M., Adeola O., Lilburn M.S., Applegate T.J. Comparison of ileal endogenous amino acid flows in broiler chicks and turkey poults. Poult. Sci. 2007;86:1682–1689. doi: 10.1093/ps/86.8.1682. [DOI] [PubMed] [Google Scholar]
- AOAC International . 18th ed. Association of Official Analytical Chemists; Washington, DC: 2011. Official Methods of Analysis. [Google Scholar]
- AOAC International . 20th ed. Association of Official Analytical Chemists; Washington, DC: 2016. Official Methods of Analysis. [Google Scholar]
- Bielorai R., Iosif B. Amino acid absorption and endogenous amino acids in the lower ileum and excreta of chicks. J. Nutr. 1987;117:1459–1462. doi: 10.1093/jn/117.8.1459. [DOI] [PubMed] [Google Scholar]
- Blok M.C., Jansman A.J.M., Makkink C.A. Amount and amino acid composition of basal endogenous protein losses at the terminal ileum of broilers. Wageningen Livestock Res. (CVB Documentation report). 2017;60:8–20. [Google Scholar]
- Clemens E., Stevens C., Southworth M. Sites of organic acid production and pattern of digesta movement in the gastrointestinal tract of geese. J. Nutr. 1975;105:1341–1350. doi: 10.1093/jn/105.10.1341. [DOI] [PubMed] [Google Scholar]
- Cowieson A.J., Bedford M.R., Selle P.H., Ravindran V. Phytate and microbial phytase: implications for endogenous nitrogen losses and nutrient availability. Worlds Poult. Sci. J. 2009;65:401–418. [Google Scholar]
- Dibner J.J., Kitchell M.L., Atwell C.A., Ivey F.J. The effect of dietary ingredients and age on the microscopic structure of the gastrointestinal tract in poultry. J. Appl. Poult. Res. 1996;5:70–77. [Google Scholar]
- Donkoh A., Moughan P.J. Endogenous ileal nitrogen and amino acid flows in the growing pig receiving a protein-free diet and diets containing enzymically hydrolyzed casein or graded levels of meat and bone meal. Anim. Sci. J. 1999;68:511–518. [Google Scholar]
- Golian A., Guenter W., Hoehler D., Jahanian H., Nyachoti C.M. Comparison of various methods for endogenous ileal amino acid flow determination in broiler chickens. Poult. Sci. 2008;87:706–712. doi: 10.3382/ps.2007-00330. [DOI] [PubMed] [Google Scholar]
- Gork A.S., Usui N., Ceriati E., Drongowski R.A., Epstein M.D., Coran A.G., Harmon C.M. The effect of mucin on bacterial translocation in I-407 fetal and Caco-2 adult enterocyte cultured cell lines. Pediatr. Surg. Int. 1999;15:155–159. doi: 10.1007/s003830050544. [DOI] [PubMed] [Google Scholar]
- Hurwitz S., Bar A. Rate of passage of Calcium-45 and Yttrium-91 along the intestine, and calcium absorption in the laying fowl. J. Nutr. 1966;89:311–316. doi: 10.1093/jn/89.3.311. [DOI] [PubMed] [Google Scholar]
- Iji P., Hughes R.J., Choct M., Tivey D. Intestinal structure and function of broiler chickens on wheat-based diets supplemented with a microbial enzyme. Asian-Australas. J. Anim. Sci. 2001;14:54–60. [Google Scholar]
- Katanbaf M.N., Dunnington E.A., Siegel P.B. Allomorphic relationships from hatching to 56 days in parental lines an F1 crosses of chickens selected 27 generations for high or low body weight. Growth Dev. Aging. 1988;52:11–22. [PubMed] [Google Scholar]
- Kong C., Adeola O. Ileal endogenous amino acid flow response to nitrogen-free diets with differing ratios of corn starch to dextrose in broiler chickens. Poult. Sci. 2013;92:1276–1282. doi: 10.3382/ps.2012-02835. [DOI] [PubMed] [Google Scholar]
- Lemme A., Ravindran V., Bryden W.L. Ileal digestibility of amino acids in feed ingredients for broilers. Worlds Poult. Sci. J. 2004;60:423–438. [Google Scholar]
- Lien K.A., Sauer W.A., Fenton M. Mucin output in ileal digesta of pigs fed a protein-free diet. J. Anim. Sci. 1997;72:1737–1743. doi: 10.1007/BF01611398. [DOI] [PubMed] [Google Scholar]
- Mateos G.G., Sell J.L., Eastwood J.A. Rate of food passage (transit time) as influenced by level of supplemental fat. Poult. Sci. 1982;61:94–100. doi: 10.3382/ps.0610094. [DOI] [PubMed] [Google Scholar]
- Moughan P.J. Amino acid digestibility and availability in foods and feedstuffs. Proc. 9th Intl. Symp. on Dig. Physiol. in Pigs; Banff, Canada; 2003. [Google Scholar]
- Moughan P.J., Schuttert G., Leenaars M. Endogenous amino acid flow in the stomach and small intestine of the young growing pig. J. Sci. Food Agric. 1992;60:437–442. [Google Scholar]
- Nasset E.S. Amino acid homeostasis in the gut lumen and its nutritional significance. World Rev. Nutr. Dietetics. 1972;14:134–153. doi: 10.1159/000392735. [DOI] [PubMed] [Google Scholar]
- Nitsan Z., Ben-Avraham G., Zoref Z., Nir I. Growth and development of the digestive organs and some enzymes in broiler chicks after hatching. Br. Poult. Sci. 1991;32:515–523. doi: 10.1080/00071669108417376. [DOI] [PubMed] [Google Scholar]
- Noy Y., Geyra A., Sklan D. The effect of early feeding on growth and small intestinal development in the posthatch poult. Poult. Sci. 2001;80:912–919. doi: 10.1093/ps/80.7.912. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Digestion and absorption in the young chick. Poult. Sci. 1995;74:366–373. doi: 10.3382/ps.0740366. [DOI] [PubMed] [Google Scholar]
- Noy Y., Sklan D. Post-hatch development in poultry. J. Appl. Poult. Res. 1997;6:344–354. [Google Scholar]
- Parsons C.M. Unresolved issues for amino acid digestibility in poultry nutrition. J. Appl. Poult. Res. 2020;29:1–10. [Google Scholar]
- Ravindran V. Feed-induced specific ileal endogenous amino acid losses: Measurement and significance in the protein nutrition of monogastric animals. Anim. Feed Sci. Technol. 2016;221:304–313. [Google Scholar]
- Ravindran V. Progress in ileal endogenous amino acid flow research in poultry. J. Anim. Sci. Biotechnol. 2021;12:5. doi: 10.1186/s40104-020-00526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran V., Hendriks W.H. Endogenous amino acid flows at the terminal ileum of broilers, layers and adult roosters. Anim. Sci. J. 2004;79:265–271. [Google Scholar]
- Ravindran V., Bryden W.L. Amino acid availability in poultry in vitro and in vivo measurements. Aust. J. Agric. Res. 1999;50:889–908. [Google Scholar]
- Ravindran V., Hew L.I., Ravindran G., Bryden W.L. Endogenous amino acid flow in the avian ileum: quantification using three techniques. Br. J. Nutr. 2004;92:217–223. doi: 10.1079/BJN20041202. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Hew L.I., Ravindran G., Bryden W.L. Apparent ileal digestibility of amino acids in dietary ingredients for broiler chickens. Anim. Sci. J. 2005;81:85–97. [Google Scholar]
- Ravindran V., Adeola O., Rodehutscord M., Kluth H., van der Klis J.D., van Eerden E., Helmbrecht A. Determination of ileal digestibility of amino acids in raw for broiler chickens - Results of collaborative studies and assay recommendations. Anim. Feed Sci. Technol. 2017;225:62–72. [Google Scholar]
- Sell J. Physiological limitations and potential for improvement in gastrointestinal tract function of poultry. J. Appl. Poult. Res. 1996;5:96–101. [Google Scholar]
- Sell J., Angel C.R., Piquer F.J., Mallarino E.G., Albatshan H.A. Developmental patterns of selected characteristics of the gastrointestinal tract of young turkeys. Poult. Sci. 1991;70:1200–1205. doi: 10.3382/ps.0701200. [DOI] [PubMed] [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996;59:215–221. [Google Scholar]
- Siriwan P., Bryden W.L., Annison E.F. Use of guanidinated dietary protein to measure losses of endogenous amino acids in poultry. Br. J. Nutr. 1994;71:515–529. doi: 10.1079/bjn19940159. [DOI] [PubMed] [Google Scholar]
- Sklan D. Development of the digestive tract of poultry. World's Poult. Sci. J. 2001;57:415–428. [Google Scholar]
- Sklan D., Dubrov D., Eisner U., Hurwitz S. 51Cr-EDTA, 91Y and 141Ce as nonabsorbed reference substances in the gastrointestinal tract of the chicken. J. Nutr. 1975;105:1549–1552. doi: 10.1093/jn/105.12.1549. [DOI] [PubMed] [Google Scholar]
- Smirnov A., Tako E., Ferket P., Uni Z. Mucin gene expression and mucin content in the chicken intestinal goblet cells is affected by in ovo feeding of carbohydrates. Poult. Sci. 2006;85:669–673. doi: 10.1093/ps/85.4.669. [DOI] [PubMed] [Google Scholar]
- Soleimani A.F., Kasim A., Alimon A.R., Meimandipour A., Zulkifli I. Ileal endogenous amino acid flow of broiler chickens under high ambient temperature. J. Anim. Physiol. Anim. Nutr. 2010;94:641–647. doi: 10.1111/j.1439-0396.2009.00951.x. [DOI] [PubMed] [Google Scholar]
- Souffrant W.B. Endogenous nitrogen losses during digestion in pigs. Proc. 5th Intl. Symp. on Dig. Physiol. in Pigs; Wageningen; 1991. [Google Scholar]
- Stein H.H., Seve B., Fuller M.F., Moughan P.J., deLange C.F.M. Amino acid bioavailability and digestibility in pig feed ingredients: terminology and application. J. Anim. Sci. 2007;85:172–180. doi: 10.2527/jas.2005-742. [DOI] [PubMed] [Google Scholar]
- Tarvid I. Peptide digestion in poultry in early ontogenesis. In: Valman, A.R., editor. Assimilation of Organic and Inorganic Compounds in Animal Organisms. Institute of Biology, Latvian Academy of Science; Zinatne, Riga, Latvia: 1990. pp. 265–304. [Google Scholar]
- Tarvid I. Effect of early postnatal long-term fasting on the development of peptide hydrolysis in chicks. Comp. Biochem. Physiol. 1992;101A:161–166. doi: 10.1016/0300-9629(92)90645-7. [DOI] [PubMed] [Google Scholar]
- Tarvid I. The development of protein digestion in poultry. Avian Poultry Biol. Rev. 1995;6:35–54. [Google Scholar]
- Taverner M.R., Hume I.D., Farrell D.J. Availability to pigs of amino acids in cereal grains. 1. Endogenous levels of amino acids in ileal digesta and faeces of pigs given cereal diets. Br. J. Nutr. 1981;46:149–158. doi: 10.1079/bjn19810017. [DOI] [PubMed] [Google Scholar]
- Uni Z., Noy Y., Sklan D. Posthatch changes in morphology and function of the small intestines in heavy- and light-strain chicks. Poult. Sci. 1995;74:1622–1629. doi: 10.3382/ps.0741622. [DOI] [PubMed] [Google Scholar]
- Uni Z., Noy Y., Sklan D. Development of the small intestines in heavy and light strain chicks before and after hatching. Br. Poult. Sci. 1996;37:63–71. doi: 10.1080/00071669608417837. [DOI] [PubMed] [Google Scholar]
- Vergara P., Ferrando C., Jimenez M., Fernandez E., Gonalons E. Factors determining gastrointestinal transit-time of several markers in the domestic fowl. Q. J. Exp. Physiol. Cogn. Med. Sci. 1989;74:867–874. doi: 10.1113/expphysiol.1989.sp003357. [DOI] [PubMed] [Google Scholar]
- Washburn K.W. Efficiency of feed utilization and rate of feed passage through the digestive system. Poult. Sci. 1991;70:447–452. doi: 10.3382/ps.0700447. [DOI] [PubMed] [Google Scholar]
- Webb K.E. Intestinal absorption of protein hydrolysis products: a review. J. Anim. Sci. 1990;68:3011–3022. doi: 10.2527/1990.6893011x. [DOI] [PubMed] [Google Scholar]