Abstract
Avian coccidiosis continues to be one of the costliest diseases of commercial poultry. Understanding the epidemiology of Eimeria species in poultry flocks and the resistance profile to common anticoccidials is important to design effective disease prevention and control strategies. This study examined litter samples to estimate the prevalence and distribution of Eimeria species among broiler farms in 4 geographic regions of Colombia. A total of 245 litter samples were collected from 194 broiler farms across representative regions of poultry production between March and August 2019. The litter samples were processed for oocysts enumeration and speciation after sporulation. End-point polymerase chain reaction (PCR) analysis was conducted to confirm the presence of Eimeria species. Anticoccidial sensitivity was determined with 160 Ross AP males in 5 treatment groups: noninfected, nonmedicated control (NNC), infected, nonmedicated control (INC), infected salinomycin treated (SAL, dose: 66 ppm), infected diclazuril treated (DIC, dose: 1 ppm), and infected methylbenzocuate-Clopidol treated (MET.CLO, dose: 100 ppm), All birds were orally inoculated with 1 × 106 sporulated oocysts using a 1 mL syringe, except for the NNC- group who received 1ml of water.Eimeria spp. were found in 236 (96.3%) out of 245 individual houses, representing 180 (92.8%) out of 194 farms. Eimeria acervulina was the most prevalent species (35.0%) followed by Eimeria tenella (30.9%), Eimeria maxima (20.4%), and other Eimeria spp. (13.6%). However, mixed species infections were common, with the most prevalent combination being mixtures of E. acervulina, E. maxima, E. tenella, and other species in 31.4% of the Eimeria-positive samples. PCR analysis identified E. acervulina, E. maxima, E. tenella, Eimeria necatrix, Eimeria mitis, and Eimeria praecox with variable prevalence across farms and regions. Anticoccidial sensitivity testing of strains of Eimeria isolated from 1 region, no treatment difference (P > 0.05) was observed in final weight (BW), weight gain (BWG) or feed conversion (FCR). For the global resistance index (GI) classified SAL and MET.CLO as good efficacy (85.79 and 85.49, respectively) and DIC as limited efficacy (74.52%). These results demonstrate the ubiquitous nature of Eimeria spp. and identifies the current state of sensitivity to commonly used anticoccidials in a region of poultry importance for Colombia.
Key words: broiler chicken, Eimeria monitoring, oocyst, litter, anticoccidial sensitivity
INTRODUCTION
Coccidiosis is the costliest parasitic disease in commercial poultry. Global estimates of the economic loss caused by coccidiosis in chickens range from USD 3 billion in 1995 (Williams, 1999) to over USD 13 billion annually in 2016 (Blake et al., 2020). Such losses include decreased feed consumption and growth rate, increased feed conversion (Williams, 1999; Dalloul and Lillehoj, 2006), and the cost of prophylactic and therapeutic control of the disease (Blake and Tomley 2014; Blake et al., 2020). Currently, intensive chicken farming relies heavily on anticoccidial drugs and live vaccines to control coccidiosis (Jenkins et al., 2017a). Anticoccidial control programs can be optimized by knowing the severity and timing of challenge as well as the species present and their anticoccidial drug sensitivity profile (Jenkins et al., 2017a). The number of Eimeria oocysts per g of litter follows a general pattern throughout the life of a flock and these data can be used to evaluate coccidiosis control programs (Chapman et al., 2002, 2016; Williams, 2002). In addition, Eimeria can be identified and speciated by visual evaluation of species-specific intestinal lesions (Johnson and Reid, 1970) and through oocysts morphology as assessed by the microscopic evaluation of intestinal scrapings (Hadipour et al., 2011; Györke et al., 2013). However, under field conditions, the common occurrence of mixed infections makes it difficult to reach a specific diagnosis (Long and Joyner, 1984; Györke et al., 2013) that is essential for optimal prevention and control of coccidiosis. Molecular techniques such as the polymerase chain reaction (PCR) have proven to be effective tools for Eimeria diagnosis in broilers (Györke et al., 2013; Moraes et al., 2015). Molecular techniques reduce possible misdiagnosis between species with similar morphometric characteristics and can identify infections that might be missed through gross evaluation (Kučera, 1990). Nevertheless, PCR is not commonly used in field conditions.
Apart from accurately assessing the presence and severity of coccidia in the field, it can be helpful to understand the sensitivity of field isolates against commonly used anticoccidials (Abbas et al., 2011). Sensitivity testing usually consists of infecting groups of medicated and nonmedicated birds with Eimeria isolated from the litter of commercial farms (Chapman, 1998). Sensitivity tests can provide valuable information to help producers design effective Eimeria control strategies (Abbas et al., 2008).
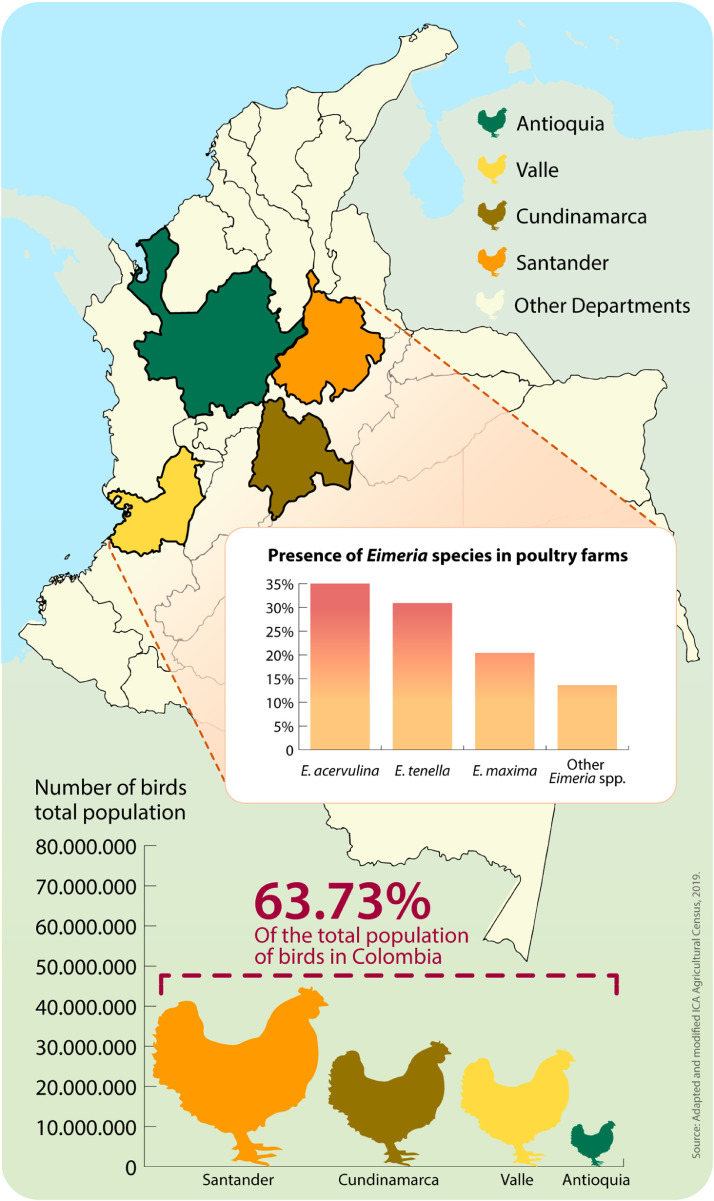
In Colombia the broiler and layer sectors have an annual growth rate of approximately 7 to 8%, as reported by the Colombian Agriculture Institute (ICA: Instituto Colombiano Agropecuario in spanish) (ICA, 2019), with a current population of 187.5 million birds, of which 104.8 million are broilers (ICA, 2019). The production of chicken meat in 2019 was 1.69 million tons (Fenavi, 2019). Poultry production is concentrated in 4 States: Santander (24.4%), Cundinamarca (17.06%), Valle del Cauca (15.98%) and Antioquia (6.25%) -. Each state has different climates, general management conditions and production environments. The ubiquitous nature of coccidia and concentrated poultry production can make disease control a challenge (Carvalho et al., 2011).
In Colombia there is no information on the incidence or prevalence of coccidiosis and only production companies know which anticoccidial drugs are used. In addition, vaccines are not commonly used to control coccidia (personal communications, March to October 2019). Having more information on Eimeria prevalence and the sensitivity of field isolates to commonly used anticoccidials can help producers make better treatment and control decisions. Therefore, the objective of this work is to survey the prevalence of Eimeria spp. on broiler farms in 4 important broiler-producing regions of Colombia and perform an anticoccidial sensitivity test with strains isolated from one of the surveyed areas.
MATERIALS AND METHODS
Ethics Committee Approval
The work was approved by the animal ethics committee of Universidad de Antioquia (Act No. 122 of February 5, 2019).
Sample Area and Farms
The study was conducted between March and August 2019. Using data from the MAPS-Fenavi - (2018) national database on poultry farms, the sample size was calculated (Grisales, 2011) for studies with known populations assuming an error of 10%. A total of 219 farms were sampled: 62 in Cundinamarca, 61 in Santander, 58 in Valle del Cauca and 38 in Antioquia. In each state, commercial farms that were in the finishing phase of the productive cycle (beyond d 21) were selected. A survey was used to collect additional information from each farm (e.g., location, size, number of birds, number of sheds, management practices and biosecurity).
Litter Sampling and Analysis of Eimeria spp.
Samples were collected from 194 of the 219 proposed farms, corresponding to 88.6% of the initial objective.
It was not possible to comply with the entire sampling because confidentiality of the farms. Litter samples were randomly collected from each shed while walking in a zigzag pattern (Goan, 2009). Depending on the size of the shed, between 6 and 12 grab samples of approximately 100g were taken, pooled, and homogenized. A 500g subsample was taken from each composite sample, placed in a hermetically sealed plastic bag, and transported to the laboratory where it were kept refrigerated at 4°C for 1 to 3 days. Oocysts per g of litter (OPG) was calculated by microscopic (Olympus CH30, Olympus Optical Co., Ltd., Tokyo, Japan) enumeration using a McMaster chamber (Conway and McKenzie, 2007). To identify the Eimeria species, positive litter samples were washed in tap water, kept at room temperature overnight and filtered through sieves (Endecotts, London, England) of different diameters in a descending manner: 500, 212, 180, 75 and 45 µm. The resulting liquid was diluted in a proportion 1:1 with 5% potassium dichromate (K2Cr2O7) [200 mL liquid in 200 mL potassium dichromate in an Erlenmeyer flask], and aerated with an aquarium pump (DG-302A, Aquarium air pump CE, China) at room temperature (approximately 27°C) for 1 wk. Oocysts were isolated in saturated saline using a flotation technique (López et al., 2018) and 100 sporulated oocysts were microscopically (100X objective. Olympus Optical Co., Ltd., Tokyo, Japan) speciated using morphometric measurements of length, width and shape index (Castañon et al., 2007; Haug et al., 2008).
Determination of Eimeria species by Polymerase Chain Reaction (PCR)
Sample Collection
Litter samples from each of the 4 geographic regions were processed as described previously except a saturated sugar solution (density 1.18−1.20 g/mL- Sheather) was used for oocyst flotation prior to the addition of 2.5% potassium dichromate (K2Cr2O7) for storage at 4°C until analysis. Prior to the DNA extraction, 500 µL of the final solution was washed 5 times with PBS and centrifuged at 847 RCF (3000 rpm) for 5 minutes (Hettich Mikro 120 centrifuge [Tuttlingen, Germany]), to remove the potassium dichromate.
DNA Extraction
DNA extraction was carried out for Antioquia, Santander, Cundinamarca and Valle del Cauca, taking 200 µL of the suspension containing 100 oocysts from each zone and using the commercial NucleoSpin Soil KIT (Macherey -Nagel, Düren, Germany). Following the methodology of López et al. (2018), the lysis time of the sample was modified by adjusting the agitation time in A-NucleoSpin Bead tubes (50 preps., Cat. No. 74078050, Macherey-Nagel GmbH & Co.KG, Düren, Germany) to 20 minutes. The quality of the DNA was verified and quantified in an Epoch-spectrophotometer using a nucleic acid quantification Take 3 plate (BioTek Instruments, Inc., Winooski, VT), with Gen5 2.04 software (BioTek Instruments, Inc., Winooski, VT).
PCR Analysis
PCR analysis was performed using a modified method of Moraes et al. (2015). The reaction was performed in a T3-Thermoblock thermocycler (Biometra GmbH, Gottingen, Germany), with initial denaturation at 96°C for 5 min, followed by 35 cycles of 1 minute at 94°C and 2 minutes at 55°C (50°C for Eimeria necatrix, Eimeria tenella, and Eimeria acervulina), an extension at 72°C for 30 seconds and a final extension at 72°C for 10 minutes. The PCR products were mixed with DNA-6X loading buffer (Thermo scientific, Waltham, MA) and separated on a 1% agarose gel with the electrophoresis technique, marking them with GelRed (Bioutium, SF). The specific size fragments were identified, using a molecular weight pattern with a 100 bp ladder under ultraviolet light (UV Transilluminator Model M-20, Upland, CA).
The PCR was performed for each Eimeria species, preparing a solution with 200 µM of dNTps, 2.5 mM of MgCl2; 5 U of Taq DNA polymerase (Thermo Scientific- Waltham, Massachusetts, USA), 10 pM Primer R, 10 pM Primer F and 10 ng of DNA. The solution for the species E. necatrix, Eimeria maxima, Eimeria praecox, and Eimeria mitis was brought to a final volume of 33 µL and for E. acervulina and E. tenella to a final volume of 20 µL. The primers used are shown in Table 1. As positive controls, Evant-Hipramunet vaccine (HIPRA-The Reference in Prevention for Animal Health) was used for E. acervulina, E. maxima, E. tenella, E. praecox, and E. mitis and Livacox Q vaccine (BIOPHARM, Research Institute of Biopharmacy and Veterinary Drugs) was used for E. necatrix. Eimeria brunetti is not reported since there was no positive control available.
Table 1.
Forward (F) and reverse (R) primers in end-point PCR for detection of 6 Eimeria species and size of generated amplicon (TA).1
| Name of primer | Primer sequence | Size (pb) |
|---|---|---|
| ac-A03-F | AGT CAG CCA CAC AAT AAT GGC AAA CAT G | 811 |
| ac-A03-R | AGT CAG CCA CAG CGA AAG ACG TAT GTG | |
| tn-K04-F | CCG CCC AAA CCA GGT GTC ACG | 539 |
| tn-K04-R | CCG CCC AAA CAT GCA AGA TGG C | |
| mt-A03-F | AGT CAG CCA CCA GTA GAG CCA ATA TTT | 460 |
| mt-A03-R | AGT CAG CCA CAA ACA AAT TCA AAC TCT AC | |
| pr-A03-F | AGT CAG CCA CCA CCA AAT AGA ACC TTG G | 354 |
| pr-A03-R | GCC TGC TTA CTA CAA ACT TGC AAG CCC T | |
| mx-A09-F | GGG TAA CGC CAA CTG CCG GGT ATG | 272 |
| mx-A09-R | AGC AAA CCG TAA AGG CCG AAG TCC TAG A | |
| nc-A18-F | TTC ATT TCG CTT AAC AAT ATT TGG CCT CA | 200 |
| nc-ENec-R | ACA ACG CCT CAT AAC CCC AAG AAA TTT TG |
Eimeria species: E. acervulina (ac), E. tenella (tn), E. mitis (mt), E. praecox (pr), E. maxima (mx), E. necatrix (nc). (Pb): base pair. Adapted from Moraes et al., 2015.
In vivo Anticoccidial Sensitivity Test With Field Strains Isolated from Cundinamarca (Colombia)
Eimeria Strain Isolation
Cundinamarca isolate was chosen for being one of the most important states between the higher poultry production states in Colombian. The 64 samples of litter containing Eimeria from Cundinamarca state was used to isolate oocysts for sensitivity testing. Litter were processed as described previously and oocysts were isolated by flotation in a saturated sugar solution (density 1.20 g/mL-Sheather). The saturated solution was removed by 5 serial washes with buffered water and centrifugation at 1211 RCF (2,500 rpm) for 10 minutes (Thermo Scientific IEC Centra GP8R, Needham Heights, MA). The oocysts of the 64 samples were mixed and deposited in 2.5% potassium dichromate, at room temperature and with constant stirring for 4 days to achieve at least 70% sporulation. Oocyst concentration, speciation, and sporulation rate was determined using a hemocytometer (Neubauer Chamber). Subsequently, the potassium dichromate was removed replacing it with buffered water, performing 5 centrifugations at 1211 RCF (2,500 rpm) X 10 minutes (Thermo Scientific IEC Centra GP8R, Needham Heights, MA), and the challenge inoculum was adjusted to 1 × 106 sporulated oocysts/mL (Gerhold, et al., 2011). The mixed inoculum had a similar composition of oocysts of E. acervulina, E. maxima and E. tenella, with approximately 33% of each of these species (333.000 oocyst for each Eimeria), differentiated on the basis of oocyst morphology (Stephan, et al., 1997).
The poultry farms in this study belong to different owners. They report to use anticoccidial drugs via feed, but do not know the type of anticoccidial. In direct communication with technical personnel in the area, they report that the basic anticoccidial plan for the area is the supply of ionophore for the starter period (D. 1−21) and a combination of ionophore with chemical for the growth period (D. 21−42).
Anticoccidial Sensitivity Test
The test was carried out following the guidelines for evaluation of anticoccidial efficacy by the World Association for the Advancement of Veterinary Parasitology (Holdsworth et al., 2004) and the methodology used by Stephan et al. (1997) and Thabet et al. (2017). The anticoccidial sensitivity test lasted 21 days and was carried out during October and November 2019, in an experimental farm located in the town of Rionegro (Antioquia, Colombia) at an altitude of 2,125 m, with an average temperature of 17°C.
A total of 160 one-day-old Ross AP males were used to test the sensitivity of the Eimeria spp. strain to 3 commonly used anticoccidial medications. Broiler chickens were sourced from a commercial hatchery, received a standard vaccination program, and were housed together in floor pens previously flamed and sanitized with Farm Fluid S (NEOGEN, Lansing, MI) with ad libitum access to water and starter feed without anticoccidials or antibiotic growth promoters. Feed was formulated to complying with Ross 308 AP nutrient recommendations (Aviagen, 2019). At 12 d of age, animals were weighed, randomized into 4 replicates of 8 birds each (Table 2) and placed in wire-floor cages previously randomized to treatment, without blocking by cage. The wire-floor cages had been thoroughly cleaned and fumigated with concentrated ammonia solution prior to use. Birds received experimental diets from 12 d of age to trial termination at 21 d of age. The following experimental groups were evaluated: Noninfected nonmedicated control (NNC), Infected nonmedicated control (INC) and 3 infected medicated treatments including Salinomycin (SAL, dose: 66 ppm), Diclazuril (DIC, dose: 1 ppm), and Methylbenzocuate Clopidol (MET.CLO, dose: 100 ppm).
Table 2.
Description of treatments for in vivo anticoccidial sensitivity test in Broilers from Colombia.
| Treatment1 | Number of replicates | Dose (ppm) |
|---|---|---|
| NNC | 4 | |
| INC | 4 | |
| SAL | 4 | 66 |
| DIC | 4 | 1 |
| MET.CLO | 4 | 100 |
Experimental group of 4 replicates with 8 individuals each. Each challenged bird was gavaged with 1 × 106 sporulated oocysts containing equal portions of E. acervulina, E. maxima, and E. tenella,333.000 oocysts each specie). Non-infected non-medicated control (NNC), Infected non-medicated control (INC) and 3 infected medicated treatments including Salinomycin (SAL-66 ppm), Diclazuril (DIC-1 ppm), and Methylbenzocuate Clopidol (MET.CLO -100 ppm).
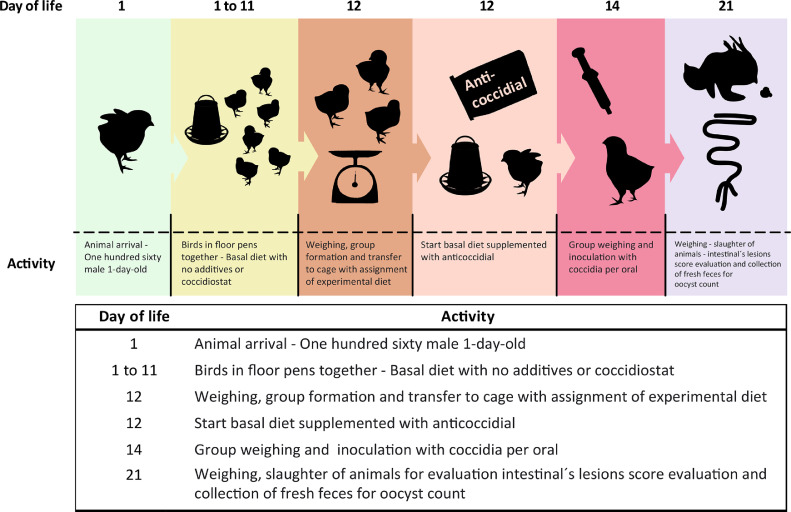
On day 14, each bird was weighed and orally inoculated with 1 × 106 sporulated oocysts using a 1 mL syringe. On day 21 birds were group weighed and 2 individuals per replicate were randomly selected, for a total of 8 individuals per experimental group, to evaluate intestinal lesion scores (Johnson and Reid 1970) and fresh feces were collected directly from the bird at death to perform the count of oocysts per gram of feces (OPG) (Figure 1; experimental test scheme).
Figure 1.
Experimental test scheme.
Calculation of the Global Resistance Index
The global resistance index (GI) for each experimental group was calculated using a modified version of the formula developed by Stephan et al. (1997) where oocyst index (OI) was obtained from the OPG count in fresh feces not from mucosa scrapings and used the following 0 to 5 categorical ranking: 0 = no, 1 = 1−10,000, 2 = 10,001−49,999, 3 = 50,000−79,999, 4 = 80,000−99,999, and 5 >99,999 OPG of feces.
Where, OI = Oocyst index (Score 0-5), BWGNNC = relative weight gain calculated as the percentage (%) gain of the NNC group, LS = Lesion score (score 0-4), FCR = feed conversion, MR = mortality (%), M = medicated, NNC = noninfected, nonmedicated control, and INC = infected, nonmedicated control.
In addition, the GI for each test group was calculated as a percentage of the GI for the NNC (Table 3).
Table 3.
In vivo sensitivity rating parameters.
| Efficacy | GINNC (%) |
|---|---|
| Very good | ≥90 |
| Good | 80−89 |
| Limited | 70−79 |
| Partial resistance | 50−69 |
| Resistance | <50 |
The global resistance index (GI). Non-infected non-medicated control (NNC).
Adapted from Thabet et al. (2017).
Histopathological Analysis
On the final day of the test, 3 birds per experimental group were randomly chosen for intestinal histopathology at approximately 4 cm from the duodenal loop, 5 cm after Meckel's diverticulum (ileum), and from the cecum. Tissue samples were fixed in 10% buffered formalin, routinely processed, embedded in paraffin, sectioned, and stained with hematoxilyn and eosin (H&E). Microscopic evaluation was conducted at 100X and 400X magnification after staining with hematoxylin and eosin and was performed in duplicate by an expert avian pathologist who was blinded to treatments. Histopathological lesions and the presence or absence of Eimeria spp were categorized (Table 4) and summed across birds and intestinal locations for each experimental group. Finally, the total score is presented, a higher score indicates a greater degree of damage and a greater amount of intestinal Eimeria.
Table 4.
Categorization histopathological of intestinal lesions and presence of Eimeria spp.
| Score | Intestinal microscopic-lesions | Presence of Eimeria |
|---|---|---|
| 0 | Without lesions | No presence |
| 1 | Mild lesion | Sparse - scattered |
| 2 | Moderate lesion | Moderate - medium |
| 3 | Severe lesion | Numerous |
Statistical Analysis
Survey data (OPG and Eimeria species present) were entered manually into Microsoft Excel (Microsoft Office 2016, Microsoft Corporation, Redmond, WA) to determine frequencies and prevalence of species by state. R version 4.0.2 was used to calculate a Pearson correlation coefficient between flock age and logarithmically transformed OPG. Results of the sensitivity test were analyzed using JMP 15 (SAS Institute Inc., Cary, NC). Growth performance data were analyzed as a randomized block (for high and low weights) design with cage as the experimental unit. For intestinal lesions and oocyst count the nonparametric Wilcoxon test was used, with bird as the experimental unit. The OPG data were transformed by (Ln + 1) (Oviedo et al., 2006) and evaluated by a mixed model with cage as the random factor. Histopathology results are presented descriptively.
RESULTS
Field Survey Study
The density of birds in most farms was between 12 to 14 birds/m2, with an average number of 5.5 (± 3.0) sheds per farm, an average flock size of approximately 80,000 birds (range 10,000−330,000), and an average slaughter age of 37.5 days (Table 5). The most common building design was traditional open-sided sheds with tarpaulin or curtains with concrete floors using rice hull or wood shaving litter. In regions with warmer temperatures, mechanical fans are used to regulate the climate inside the sheds. Very few farms had controlled environment systems in the sampling areas that coincide with the warmest areas in the departments of Santander and Valle del Cauca. The commercial broiler lines used in the 194 farms studied were: Ross AP 51.2% (83/160), Ross AP x Cobb 500 33.8% (54/160), Cobb 500 13.7% (21/160) and others 1.3 % (2/160) (Table 6). Except for farms in Santander, which milled their own feed, most of the farms used contract feed suppliers that manufacture diets for prestarter (hatching until day 10, starter from 11−21 days, and grower/finisher from 22 to 35−42 days). In Colombia, the use of anticoccidial vaccines is not very frequent and none of the farms included in the study were using coccidial vaccines at the time of sample collection. The use of recycled litter between flocks was only reported as a frequent practice in the region of Cundinamarca, where litter was typically reused for 3-5 cycles; 80% of the farms in the other sampling areas used new litter between each flock. At the end of each flock farms are chemically cleaned and disinfected, with an average down time of 10 to 15 days.
Table 5.
Management characteristics of broiler farms in 4 regions of Colombia during 2019.
| Department | Cundinamarca | Santander | Valle del Cauca | Antioquia |
|---|---|---|---|---|
| Sampled/registered farms1 | 37/803 | 72/648 | 57/379 | 28/86 |
| Annual average temperature (°C) | 24.2 | 26.5 | 24.5 | 23.2 |
| Altitude (masl) | 1,781 | 1,098 | 1,150 | 1,679 |
| Genetic line in number of sampled farms2 | ||||
| Ross AP | 14 | 45 | 11 | 13 |
| Ross 308 | 0 | 1 | 0 | 1 |
| Cobb 500 | 2 | 5 | 5 | 9 |
| Mix of multiple lines | 2 | 21 | 26 | 5 |
| Density, birds per m2 (min. to max.) | 14.0 (13.0−15.0) | 12.2 (8.8−17.0) | 12.6 (11.0−17.9) | 14.1 (13.5−14.5) |
| Litter composition | 44.4% WS | 100% RS | 56.1% WS | 92.9% WS |
| 55.6% RS | 43.9% RS | 7.1% M | ||
| Birds placed per farm (min.to max.) | 36.591 (10.200−162.000) | 56.346 (5.700−195.000) | 126.715 (9.180−330.000) | 83.672 (20.400−147.600) |
| Sheds per farm (min.to max.) | 2.7 (1−7) | 4.4 (1−14) | 6.4 (1 to 18) | 7.6 (3−14) |
| Average age of birds at sampling (min.to max.) | 29 (21−38) | 30 (21−39) | 31 (25−43) | 32 (22−41) |
Abbreviations: M, mix of wood shavings with rice hulls; RS, rice hulls; WS, wood shavings.
Register of farms by department - MAPS-Fenavi.
160 total surveys.
Table 6.
Frequency of Eimeria species and average (± SD) oocysts per gram (OPG) of farm litter in 4 regions of Colombia.
| Department | Cundinamarca | Santander | Valle del Cauca | Antioquia | Total |
|---|---|---|---|---|---|
| Number of litter samples analyzeda | 67 | 72 | 62 | 44 | 245 |
| Number of farms positive for Eimeria spp/total analyzed (%) | 29/37 (78.4%) | 69/72 (95.8%) | 55/57 (96.5%) | 27/28 (96.4%) | 180/194 (92.8%) |
| Average OPG values of positive samples | 1,592 ± 4,735 | 1,386 ± 3,185 | 1,018 ± 3,711 | 4,728 ± 9,827 | 1,931 ± 5,543 |
| (min. to max.) | (0−32,800) | (0−24,300) | (0−28,044) | (0−48,960) | (0−48,960) |
| Eimeria spp. (%) | |||||
| E. acervulina | 39.3 | 38.9 | 37.3 | 20.2 | 35.0 |
| E. maxima | 13.6 | 21.4 | 8.5 | 35.5 | 20.4 |
| E. tenella | 35.3 | 22.6 | 36.1 | 36.0 | 31.0 |
| Other spp. | 11.8 | 17.2 | 18.2 | 8.3 | 13.6 |
Percentage of diversity and distribution of species.
The overall prevalence of Eimeria spp. was 92.8% (180/194) and OPG of litter varied widely between farms and departments (Figure 2), with an average of 1,931 (±5,543) (Table 6). Eimeria species identified in descending order of frequency were: E. acervulina (35.0%), E. tenella (30.9%), E. maxima (20.4%), and other Eimeria spp. (13.6%). Infections of mixed species with 2, 3, and more species were common and the most frequently found combination was the mix of at least 4 Eimeria spp., of which 3 are E. acervulina, E. tenella, E. maxima, and others, in 74 of 236 positive samples (Table 7).
Figure 2.
Eimeria species distribution in 4 departments of Colombia.
Table 7.
Number of litter samples with different combinations of Eimeria species for each department sampled.
| Region | No. of farms | No. of litter | No. of Posit. | No. of Negat. | a+m+t+o | a+m+t | a+t+o | m+t+o | a+m | a+t | a+o | m+t | m+o | t+o | a | m | t | Not identified1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ant | 28 | 46 | 44 | 2 | 9 | 6 | 1 | 1 | 1 | 2 | 1 | 8 | 0 | 1 | 0 | 1 | 2 | 13 |
| San | 72 | 72 | 69 | 3 | 35 | 7 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 26 |
| Vall | 57 | 62 | 59 | 3 | 15 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 36 |
| Cun | 37 | 75 | 64 | 11 | 15 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 43 |
Abbreviations: a, E. acervulina; Ant, Antioquia; Cun, Cundinamarca; m, E. maxima; o, other spp.; San, Santander, t, E. tenella; Vall, Valle del Cauca.
Not identified: samples with an OPG ≤170 species identification was not possible.
No association was found between the age of the flock of the litter samples collected and the number of OPG found (R2 = 0.00 and P = 0.2227) (Data not shown).
Confirmation of the Presence of Eimeria Species by the PCR Technique in 4 Regions of Poultry Importance in Colombia
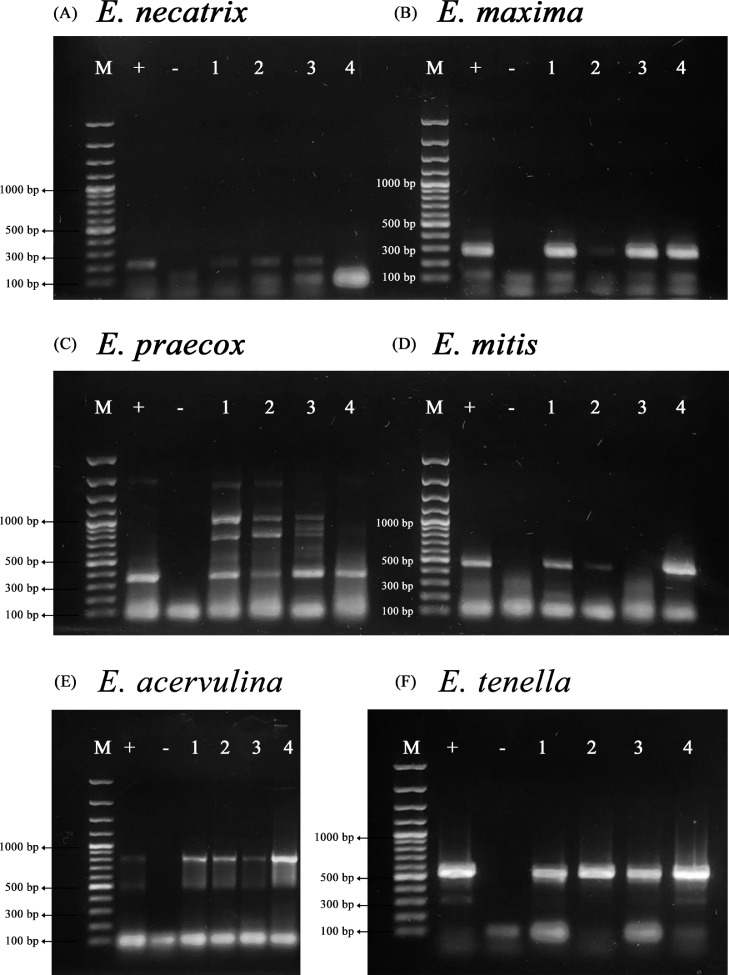
The end-point PCR results showed that Antioquia and Santander were positive for the 6 Eimeria species analyzed E. necatrix, E. maxima, E. praecox, E. mitis, E. acervulina, and E. tenella; Cundinamarca was positive for E. necatrix, E. maxima, E. praecox, E. acervulina, and E. tenella and negative for E. mitis while, Valle del Cauca was positive for E. maxima, E. praecox, E. mitis, E. acervulina, and E. tenella and negative for E. necatrix (Figures 3A–3F).
Figure 3.
Gel electrophoresis. PCR result: molecular weight (marker 100 base pairs (bp)) (A) Eimeria necatrix (amplicon size 200bp) + (positive control) ¥/*; - (negative control); 1 (Antioquia); 2 (Santander); 3 (Cundinamarca); 4 (Valle del Cauca). (B) Eimeria maxima (amplicon size 272bp). (C) Eimeria praecox (amplicon size 354bp). (D) Eimeria mitis (amplicon size 460bp). (E) Eimeria acervulina (amplicon size 811bp). (F) Eimeria tenella (amplicon size 539bp). ¥Positive control LIVACOX Q vaccine with E. necatrix. *Positive control EVANT-HIPRAMUNET with E. maximum, E. praecox, E. mitis, E. tenella, and E. acervulina.
In vivo Anticoccidial Resistance Test With Field Strains Isolated From Department of Cundinamarca (Colombia)
No treatment difference (P > 0.05) were observed in final weight (BW), weight gain (BWG) or feed conversion (FCR) (Table 8).
Table 8.
Summary of the zootechnical results for the In vivo Anticoccidial sensibility test.
| Group | BW | BWG | FCR |
|---|---|---|---|
| NNC | 760.48 ± 36.78 | 403.62 ± 35.24 | 1.33 ± 0.110 |
| INC | 746.28 ± 36.78 | 395.59 ± 35.24 | 1.38 ± 0.110 |
| SAL | 750.12 ± 36.78 | 398.25 ± 35.24 | 1.39 ± 0.110 |
| DIC | 731.60 ± 36.78 | 356.81 ± 35.24 | 1.59 ± 0.110 |
| MET.CLO | 732.53 ± 37.17 | 379.49 ± 35.90 | 1.44 ± 0.121 |
Measurements taken 7 days after the challenge with 1 × 106 sporulated oocysts/mL of field Eimeria spp.
Final weight (BW), weight gain (BWG) represented in grams, and feed conversion (g/g) (FCR) (Least squares mean ± standard error). Non-infected non-medicated control (NNC), Infected non-medicated control (INC) and 3 infected medicated treatments including Salinomycin (SAL-66 ppm), Diclazuril (DIC-1 ppm), and Methylbenzocuate Clopidol (MET.CLO-100 ppm).
No intestinal lesions, oocyst excretion, or mortality was observed in the NNC group (Table 9). The oocyst index presented a statistical difference between the SAL and MET.CLO groups. The only death recorded was in the DIC group but there were no treatment differences (P > 0.05) in mortality. SAL and MET.CLO treatments received a good efficacy with GI scores of 85.79 and 85.49, respectively. With a GI score of 74.52, the DIC treatment received a limited efficacy rating (Table 10).
Table 9.
Gross lesion score (LS) for E. acervulina, E. tenella and total mean lesion score (TMLS), oocyst index (OI) and mortality rate (MR).
| Group | LS - E. acervulina | LS - E. tenella | TMLS1 | OI | MR (%) |
|---|---|---|---|---|---|
| NNC | 0.00b | 0.00b | 0.00b | 0.0b | 0 |
| INC | 0.50 ± 0.53a | 1.37 ± 1.19a | 1.88 ± 1.46a | 2.2 ± 0.64a | 0 |
| SAL | 0.62 ± 0.52a | 1.37 ± 0.74a | 2.00 ± 1.07a | 2.8 ± 1.19c | 0 |
| DIC | 0.50 ± 0.53a | 0.62 ± 0.74a | 1.13 ± 1.55a | 3.4 ± 1.69a | 3.21 |
| MET.CLO | 0.37 ± 0.52a | 1.50 ± 1.31a | 1.88 ± 2.07d | 1.2 ± 1.13d | 0 |
Measurements taken 7 days after the challenge with 1 × 106 sporulated oocysts of field Eimeria spp.
Different letter indicates statistical significance (P < 0.05).
TMLS: Sumatory of the average score of Eimeria species intestinal lesions, 8 chickens per treated group. Non-infected non-medicated control (NNC), Infected non-medicated control (INC) and 3 infected medicated treatments including Salinomycin (SAL-66 ppm), Diclazuril (DIC- 1 ppm), and Methylbenzocuate Clopidol (MET.CLO- 100 ppm).
Table 10.
Relative weight gain (% BWGNNC), global resistance index (GI) and Global index of NNC (%), estimated average values (± standard deviation) for field Eimeria spp.
| Group | BWGNNC (%) | GI | Global index of NNC (%) | Status |
|---|---|---|---|---|
| INC | 98.01 ± 3.75 | |||
| SAL | 98.67 ± 5.09 | 97.27 | 85.79 | Good efficacy |
| DIC | 88.40 ± 25.79 | 84.49 | 74.52 | Limited efficacy |
| MET.CLO | 96.93 ± 9.02 | 96.93 | 85.49 | Good efficacy |
Histopathology Analysis
The NNC group showed a degree of inflammation categorized as enteritis and minimal eosinophilic typhlitis, but no presence of Eimeria (Score 18) (Table 11). The INC group showed evidence of intestinal damage, with tissue erosion and severe focal cecal hemorrhage and E. acervulina, E. maxima and E. tenella organisms were observed in each segment (Score 18); coccidial typhlitis was diagnosed. The groups medicated with SAL (Score 25) and with DIC (Score 23) showed mild to moderate hyperplasia of gut-associated lymphoid tissue (GALT) in the lamina propria, slight necrosis in some segments (ceca) and E. acervulina, E. maxima, and E. tenella organisms were identified. Eimeria necatrix was only found in 1 bird receiving DIC. In the DIC group, there were large oocysts that coincide with E. necatrix, where severe coccidial typhlitis was diagnosed. The group treated with MET.CLO (Score 33) showed moderate hyperplasia of the GALT in the lamina propria, moderate necrosis of the cecal tissue, and all the sections evaluated had coccidia: E. acervulina, E. maxima, and E. tenella. Unidentified Eimeria species were found in the ileum of all 3 anticoccidial treated groups but not in the infected control group. In the matters of histological score, the group with MET.CLO shows the highest score indicating greater intestinal damage and greater amount of coccidia.
Table 11.
Histological description of lesions and presence of Eimeria in 3 intestinal segments of individuals challenged with coccidia.
| Experimental group |
|||||
|---|---|---|---|---|---|
| Histopathological findings1 | NNC | INC | SAL66 ppm | DIC1 ppm | MET.CLO100 ppm |
| Hyperplasia GALT2 | 4 | 0 | 2 | 1 | 2 |
| Hyperplasia lamina propria | 3 | 0 | 2 | 1 | 2 |
| Eosinophils | 9 | 0 | 1 | 0 | 0 |
| Heterophiles | 0 | 0 | 0 | 0 | 1 |
| Lymphocytes | 1 | 0 | 0 | 0 | 0 |
| Fibrin | 0 | 0 | 1 | 1 | 1 |
| Necrosis | 1 | 0 | 1 | 0 | 2 |
| Erosion | 0 | 1 | 0 | 0 | 0 |
| Hemorrhage | 0 | 1 | 0 | 0 | 4 |
| E. acervuline | 0 | 2 | 4 | 4 | 4 |
| E. maxima | 0 | 7 | 6 | 6 | 7 |
| E. tenella | 0 | 7 | 6 | 6 | 8 |
| E. Necatrix | 0 | 0 | 0 | 1 | 0 |
| Other Eimerias | 0 | 0 | 2 | 3 | 2 |
| Total score | 18 | 18 | 25 | 23 | 33 |
Sum of 3 individuals and 3 intestinal segments (duodenum, ileum and cecum) per treatment. The table shows the number of individuals that presented different cell types in the different intestinal layers and segments; different type of lesions: necrosis, erosion, hemorrasge; and the presence of different Eimeria spp. stages.
GALT: gut-associated lymphoid tissue, NNC: non-infected non-medicated, INC: infected non-medicated, SAL: infected treated with Salinomycin, DIC: infected treated with Diclazuril, MET.CLO: infected treated with Methylbenzocuate Clopidol.
DISCUSSION
This is the first study we are aware of that reports the prevalence and anticoccidial sensitivity of Eimeria on commercial broiler farms in Colombia. In this study, litter samples were taken since it can be difficult to get fresh feces from commercial farms (Chapman et al., 2016). Chapman et al. (2016) reported that sampling built-up litter allows the evaluation of accumulated oocysts across several grow-outs and indirectly verifies the conditions of the litter to know whether the parasite sporulation process is taking place (Venkateswara et al., 2015). In this work at the time of sampling, it was not possible to sacrifice birds to identify scores of intestinal lesions by Eimerias spp, as in other reported studies (Gharekhanietal, 2014; Belal, 2017; Gazoni et al., 2020) to be able to complement the OPG found in litter with the intestinal health of the birds. Of the total litter sampled, 92.8% were positive for Eimeria spp. This high frequency of Eimeria spp. has also been reported in other studies using litter or fecal samples: 90% in Argentina (Mattiello et al., 2000); 92% in Romania (Györke et al., 2013), 79.4% in North India (Prakashbabu et al., 2017), 65.8% in East China (Sun et al., 2009) and 78.7% in South Korea (Lee et al., 2010); but different from the reports of Gharekhani et al., 2014 in Hamedan (Western Iran) and Kaboudi, et al., 2016 in Sidi Thabet, (Tunisia), where the general prevalence of coccidia was 31.8%. Additionally, 41.1% (74/180) had mixed Eimeria (E. acervulina, E. maxima, E. tenella and others) infections, although 1 species was always predominant in each farm. Results agreed with other studies in various regions of the world, such as Iran, India, Alegeria, Brazil (Gharekhanietal., 2014; Prakashbabu, et al., 2017; Debbou-Iouknane et al., 2018; Gazoni et al., 2021). This determination of the distribution of coccidial species is important because it depends not only on the level of oocysts ingested, but also on the species involved and the host's immune response (Yun et al., 2000). It is known that E. acervulina can develop resistance to anticoccidials at a faster rate, due to its high reproductive potential and short life cycle (Williams, 2001; Chapman, et al., 2010). This behavior could explain the higher frequency of E. acervulina (35.0%) in all sampling areas of this study, as well as in the study by Haug et al. (2008) in Norwey with 100%, Györke et al. (2013) in Romania with 91%, and in the work by Gazoni et al. (2015) in Brazil with 13.5%.
Others have reported that there is an increase in the number of oocysts in litter between 3 and 4 weeks after bird placement and OPG can be a good way to monitor the course of infection throughout the life of a flock (Chapman et al., 2016; Jenkins et al., 2017a). In this work, there was no evidence of a relationship between the age of the flock at the time of sampling and the oocyst count, possibly because only 1 sample was taken per farm. In contrast, Chapman et al. (2016) evaluated the course of infection at weekly intervals in 6 successive flock showing a bell-shaped curve with a oocyst peak around 21 to 33 d of age. The average OPG in the current study (1.9 × 103), similar to that reported in Hamedan (Western Iran) by Gharekhani et al. (2014) (1.8 × 103), was lower than the 52 × 103 (range 35.8 × 103 to 73.6 × 103) reported by Chapman et al. (2016), but relatively similar considering production difference between the US and Colombia and the fact we only sampled farms once and potentially missed the peak of oocyst production. Of the regions sampled, Antioquia presented the highest oocyst count (average 4.73 × 103, range 0−48.96 × 103), this result could be speculatively due to particular conditions of the area, such as handling a higher density of birds in the sheds (average 14.1 birds /m2), where 92.9% wood shavings are used as litter material and the climatic conditions of the area at the time when the sampling was carried out, coinciding with the rainy months of the year between March and May 2019. These results agree with the work of Balel (2017) in Bangladesh, showing a higher occurrence of coccidiosis in the rainy season, with higher relative humidity and temperatures. These conditions favor humidity, facilitating the spread of the parasite, reflecting in a higher oocyst count (Bachaya, et al., 2012, Lawal et al., 2016) from this sampling area.
As reported by other authors, coccidia is ubiquitous in poultry farms (Bachaya, et al., 2012; Debbou-Iouknane et al., 2018). The variation in terms of hygiene conditions, management and geographic location of the farms can be the main causes of the differences in the presence of the parasite in the field (Bachaya et al., 2012; Gharekhani et al., 2014), as reflected in the results of the OPG counts in the 4 sampling areas in this work. In addition, as a differential management practice, the use of recycled litters was reported more frequently in the Cundinamarca zone, a condition that can directly affect the amount of oocysts in litter (Chapman, 2016). Garcés et al (2018) in Ecuador, showed that the use of recycled litter more than twice, improves productive parameters on day 35 of the chickens' life, compared to the use of new litters between each batch. This results possibly due to the immunity generated by early exposure to oocysts in the litter, in addition to the effect of earlier colonization with microbiota that can act as a probiotic or direct feed microbial supplement, leading to better bird performance. Another condition that can alter the results of the oocyst counts is the anticoccidial treatment program implemented in each farm at the time of sampling, which was not possible to obtain due to the confidentiality claimed for the producer (Chapman and Jeffers, 2015; Garcés et al., 2018).
Epidemiological studies on the frequency of Eimeria spp. complemented with molecular analyzis are valuable tools for the prevention and control of coccidiosis (Haug et al., 2008; Ogedengbe et al., 2011; Györke et al., 2013; Jenkins, et al., 2019). They can identify in a short time and with precision, for example the viability of oocysts capable of causing infection (Jenkins, et al., 2019) and which species of Eimeria are compromising the productive parameters in poultry farms, so an effective control strategy can be developed. This study, like the study by Hamidinejat et al. (2010) and Moraes et al. (2015), used the PCR technique to identify the species of E. acervulina, E. maxima, E. praecox, E. mitis, E. necatrix and E. tenella. Despite the differences in the sampling locations and methods, both studies showed that it is common to find mixed infections which can be difficult to characterize through clinical signs alone which require more specialized training (Carvalho et al., 2011). Finally, in this work, due to morphological characteristics of the oocysts in order of prevalence, the species of E. acervulina (35.0%), E. tenella (31.1%) and E. maxima (20.4%) were identified, data similar to those presented by Debbou -Iouknane et al (2018) in Algeria, with prevalence of 32.05%, 26.92% and 11.53% and to the work of Gharekhani et al (2014) in Western Iran, with higher but consistent prevalence in the order of frequency 75.7%, 54.3 % and 20% for E. acervulina, E. tenella and E. maxima, respectively; species classified between moderate and high pathogenicity (Kaboudi et al., 2016). Additionally with the PCR E. praecox, E. mitis and E. necatrix were identified, the latter being highly pathogenic (Kaboudi et al., 2016). These findings are of great importance, since the species with moderate and high pathogenicity are possibly interfering with the productive response of the birds in the sampled farms (Debbou-Iouknane et al., 2018), in addition to this subclinical form (mild infections that do not show symptoms) of coccidiosis is responsible for causing a reduction in the feed conversion of birds (Gazoni et al., 2021) and taking into account that the feed account for approximately 70% of the cost of broiler chicken production, the economic impact is very high (Jordan et al., 2002; Molla & Ali 2015; Gazoni et al., 2021) only due to the reduction in this productive parameter.
In this work, the PCR technique complements the diagnosis of Eimeria species, which due to morphological characteristics of the oocysts was not easy to identify, for this reason the PCR technique is a methodology that recently has gained relevance for its rapid and accurate diagnosis (Brown et al., 2018).
In countries such as Norwey (Haug et al., 2008), Romania (Györke et al., 2013), Brazil (Moraes et al., 2015), and India (Brown et al., 2018), the PCR technique is frequently used for the identification and diagnosis of Eimeria. Additionally, data from PCR can complement control programs against coccidia, which, due to the reduced use of antibiotic feed additives in the European Union and other regions of the world (Regulation EC N° 1831/2003), have been focused on reinforcing biosecurity and hygiene practices in poultry farms, combined with the prophylactic use of live vaccines (Thabet et al., 2017) and natural feed additives (Peek and Landman, 2010).
Although there is a trend to reduced chemotherapy in poultry production, anticoccidial products are still used frequently (Thabet et al., 2017) and are considered critical for broiler production in many regions of the world (Chapman et al., 2010; Peek, 2010). Widespread use of anticoccidial products has led to the appearance of resistant strains, which is a serious challenge for the poultry industry in general (Quiroz-Castañeda and Dantán-González, 2015). In this study, representatives of 3 classes of anticoccidials were evaluated in a sensitivity test. For synthetic compounds popularly known as “chemicals” (Chapman and Jeffers, 2015), DIC was used. In the category of ionophores that are produced by fermentation (Noack et al., 2019), SAL was evaluated (Chapman and Jeffers, 2015). And MET.CLO was used as an example of a combined product (Peek, 2010; Noack et al., 2019). Strain sensitivity was measured using the global resistance index methodology, which considers several relevant parameters related to the pathogenic effects of coccidia and zootechnical performance (Thabet et al., 2017). This methodology considers oocyst excretion, lesion score, growth performance, and mortality, with weight gain and feed conversion weighted heaviest because of their economic impact (Stephan et al., 1997; Arabkhazaeli et al., 2013).
Oocyst excretion is influenced by various factors such as the reproductive potential of the various species, overcrowding, and the host's immune response (Fayer, 1980), therefore, as the only variable to measure anticoccidial efficacy, it can be misleading (Reid, 1975). Several publications have shown that oocyst production has little correlation with weight gain, lesion score and, in highly pathogenic species (E. tenella and E. necatrix), even with mortality (Fayer, 1980; Stephan et al., 1997). The intestinal lesion score is one of the most common methods for evaluating intestinal damage caused by coccidia, but it involves the evaluator's subjectivity and experience (Conway et al., 1990; Stephan et al., 1997; Li et al., 2004). Under field conditions for highly pathogenic species, the mortality variable is the strongest sign of resistance (Bedrnik, 1983; Stephan et al., 1997).
In this work, a modification was made in the IO calculation of the formula by Stephan et al. (1997), since the oocyst count was done from fresh feces and not from intestinal scrapings. However, they were categorized on a scale of 0 to 5 to be able to include them in the same way in the formula for sensitivity analysis. The DIC group presented the highest IO values (3.4 ± 1.69). However, it was not found to be statistically significant with the other treated groups, and although the DIC group had higher IO, poorest weight gain and feed conversion, it did not present the highest intestinal lesion scores. This result is similar to Chapman's (1998) report that high count oocyst is not always related to greater intestinal damage.
Similarly, in this study, it was not possible to find a statistical difference in the intestinal lesion score for any groups evaluated, which could possibly reflect a low pathogenicity of the field strains used (Thabet et al., 2017).
E. maxima is classified as a species with a moderate to high pathogenicity (Chapman, 2014, Quiroz-Castañeda and Dantán-González, 2015) and the infective dose used in this work was high compared to other reports such as Oviedo et al. (2006), (3.33 × 105 vs. 100 × 103 oocysts, respectively), but during the experimental period, lesions caused by E. maxima could not be observed. Although, in the evaluation of coprological analysis on the final day of the test, it was possible to oocysts count of this species, which, due to their large size, shape, and color, are difficult to confuse. To corroborate these findings, the histology analysis performed on different intestinal sections was of great help, as it showed the presence of Eimeria maxima and other species such as E. mitis and E. necatrix. Possibly, on the day of the final evaluation, this variety of Eimeria maxima, was just reaching its oocyst excretion peak, which, as reported by Jenkins et al. (2017b), can occur between 130 and 162 hours post inoculation. Another possibility is that this field strain of E. maxima was not pathogenic and had little replication capacity (Dalloul and Lillehoj, 2006) to show intestinal lesions. This is according to results reported by Schwarz et al. (2009), that indicated there are genetic variants with different pathogenic capacities among the same species of Eimeria maxima.
The values of the global index of resistance with respect to the NNC group were in the range of good efficacy for the groups medicated with SAL and MET.CLO (85.79% and 85.49% respectively) and the group treated with DIC reported a score of 74.52%, classified as limited efficacy. These data is consistent with the field results evaluated, since this last group was the only one that presented mortality caused by coccidia. According to Stephan et al. (1997), this is the greatest evidence of resistance problems, what generated a decrease in the value of the index of this group, down to the category of limited efficacy. The results in this work are alike with other results under similar evaluation conditions: strains from the field in a mixture of diverse species, where drugs such as DIC, for coccidia control reported limited efficacy to total resistance but differs for SAL and MET.CLO who report resistance (Stephan et al., 1997; Abbas et al., 2008; Arabkhazaeli et al., 2013; Lan et al; 2017; Thabet et al., 2017).
For future work, performing a dose titration to confirm the estimated level of challenge, include others anticoccidial treatments and make a comparison with different methodologies for evaluating anticoccidial sensitivity. For example: Anticoccidial index (ACI), percentage of optimum anticoccidial activity (POAA), Reduction of lesion scores (RLS) and Relative oocyst production (ROP) (Arabkhazaeli et al., 2013; Lan et al; 2017), that allow broadening the criteria to determine anticoccidial sensitivity.
This work is the first epidemiological report of coccidia in 4 regions responsible for 63.7% (ICA, 2019) of broiler production in Colombia. In addition, this study provides information on the identification of the most frequently identified coccidial species and their distribution, along with information on the sensitivity of field strains to some of the most commonly used anticoccidials in the region. More research is needed, but this is a great first step to provide more knowledge about the current state of coccidial challenge in Colombia.
ACKNOWLEDGEMENTS
This research was funded by CIBAV- the Strategy of Consolidation of Research Groups (CODI 2018-2019), Universidad de Antioquia, (UdeA), Medellín, Colombia and the company Solla S.A. Special thanks to the Veterinarian and Zootechnician Jorge Hernán Duque for his advice and support during the development of the proposal, to the poultry companies that voluntarily participated in the project and provided technical staff to take samples, to the technical staff of Solla S.A. and the Avilandia research farm for field support, and to all the staff of the CIBAV and Nutri-Solla. We sincerely acknowledge the contributions of Dr. Kevin Watkins who carried out an outstanding review of our manuscript.
Disclosures
The authors declare no conflict of interest
REFERENCES
- Abbas R.Z., Iqbal Z., Sindhu Z.F.D., Khan M.N., Arshad M. Identification of cross-resistance and multiple resistance in Eimeria tenella field isolates to commonly used anticoccidials in Pakistan. J. Appl. Poult. Res. 2008;17:361–368. [Google Scholar]
- Abbas R.Z., Iqbal Z., Blake D., Khan M.N., Saleemi M.K. Anticoccidial drug resistance in fowl coccidia: the state of play revisited. World's Poult. Sci. J. 2011;67:337–350. [Google Scholar]
- Arabkhazaeli F., Modrisanei M., Nabian S., Mansoori B., Madani A. Evaluating the resistance of Eimeria spp. Field isolates to anticoccidial drugs using three different indices. Iran J Parasitol. 2013;8:234–241. [PMC free article] [PubMed] [Google Scholar]
- Aviagen. 2019. Ross Nutrient Specifications. Accessed Jun. 2019.https://es.aviagen.com/assets/Tech_Center/BB_Foreign_Language_Docs/Spanish_TechDocs/RossBroilerNutritionSpecs2019-ES.pdf
- Bachaya H.A., Raza M.A., Khan M.N., Iqbal Z., Abbas R.Z., Murtaza S., Badar N. Predominance and detection of different Eimeria species causing coccidiosis in layer chickens. J Anim Plant Sci. 2012;22:597–600. [Google Scholar]
- Bedrnik P. Evaluation of sensitivity of coccidia to ionophores. Arch. Gefliigelk. 1983;47:129–133. [Google Scholar]
- Belal S.M.S.H. Prevalence of coccidiosis in Sonali birds in Sirajgonj district of Bangladesh. Bangladesh J. Vet. Med. 2017;15:107–111. [Google Scholar]
- Blake D.P., Tomley F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014;30:12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Blake D.P., Knox J., Dehaeck B., Huntington B., Rathinam T., Ravipati V., Raman M. Re-calculating the cost of coccidiosis in chickens. Vet. Res. 2020;51:1–14. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Jordan A., Blake D., Beard J., Beharry A., Serrette L., Soleyn A., Oura C. Molecular identification of Eimeria species in broiler chickens in Trinidad, West Indies. Vet. Sci. 2018;5:12. doi: 10.3390/vetsci5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho F.S., Wenceslau A.A., Teixeira M., Carneiro J.A.M., Melo A.D.B., Albuquerque G.R. Diagnosis of Eimeria species using traditional and molecular methods in field studies. Vet Parasitol. 2011;176:95–100. doi: 10.1016/j.vetpar.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Castañón C.A., Fraga J.S., Fernandez S., Gruber A., Costa L.D.F. Biological shape characterization for automatic image recognition and diagnosis of protozoan parasites of the genus Eimeria. Pattern Recognit. 2007;40:1899–1910. [Google Scholar]
- Chapman H.D. Evaluation of the efficacy of anticoccidial drugs against Eimeria species in the fowl. Int J Parasitol. 1998;28:1141–1144. doi: 10.1016/s0020-7519(98)00024-1. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Cherry T.E., Danforth H.D., Richards G., Shirley M.W., Williams R.B. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int J Parasitol. 2002;32:617–629. doi: 10.1016/s0020-7519(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Milestones in avian coccidiosis research: a review. Poult. Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Barta J.R., Hafeez M.A., Matsler P., Rathinam T., Raccoursier M. The epizootiology of Eimeria infections in commercial broiler chickens where anticoccidial drug programs were employed in six successive flocks to control coccidiosis. Poult Sci. 2016;95:1774–1778. doi: 10.3382/ps/pew091. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K. Restoration of sensitivity to salinomycin in Eimeria following 5 flocks of broiler chickens reared in floor-pens using drug programs and vaccination to control coccidiosis. Poult Sci. 2015;94:943–946. doi: 10.3382/ps/pev077. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Jeffers T.K., Williams R.B. Forty years of monesin for the control of coccidiosis in poultry. Poult. Sci. J. 2010;89:1788–1801. doi: 10.3382/ps.2010-00931. [DOI] [PubMed] [Google Scholar]
- Conway D.P., McKenzie M.E., Dayton A.D. Relationship of coccidial lesion scores and weight gain in infections of Eimeria acervulina, E. maxima and E. tenella in broilers. Avian Pathol. 1990;19:489–496. doi: 10.1080/03079459008418702. [DOI] [PubMed] [Google Scholar]
- Conway D.P., McKenzie M.E. Procedures. 3th ed. Blackwell Publishing; Ames, IA: 2007. Poultry Coccidiosis: Diagnostic and Testing. [Google Scholar]
- Dalloul R.A., Lillehoj H.S. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- Debbou-Iouknane N., Benbarek H., Ayad A. Prevalence and aetiology of coccidiosis in broiler chickens in Bejaia province, Algeria. Onderstepoort J. Vet. Res. 2018;85:1–6. doi: 10.4102/ojvr.v85i1.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R. Epidemiology of protozoan infections: the coccidia. Vet Parasitol. 1980;6:75–103. [Google Scholar]
- Fenavi . Producción de pollo (Ton); 2019. Estadísticas del Sector.https://fenavi.org/informacion-estadistica/ Accessed Sept. 2019. [Google Scholar]
- Garcés Gudiño J.A., Merino Guzmán R., Cevallos Gordón A.L. Litter reuse reduces Eimeria spp oocyst counts and improves the performance in broiler chickens reared in a tropical zone in Ecuador. Europ. Poult. Sci. 2018;82 [Google Scholar]
- Gazoni F.L., Adorno F.C., Lovato M., Dilkin P., Hermes S., Junior P.M., Tellez G. Coccidiosis prevalence and correlation with intestinal health of broilers in Brazilian agricultural industries between the years 2012 and 2014. Int J Poult Sci. 2015;14:511. [Google Scholar]
- Gazoni F.L., Adorno F.C., Matte F., Alves A.J., Campagnoni I.D.P., Urbano T., da Silva A.S. Correlation between intestinal health and coccidiosis prevalence in broilers in Brazilian agroindustries. Parasitol Int. 2020;76:102027. doi: 10.1016/j.parint.2019.102027. [DOI] [PubMed] [Google Scholar]
- Gazoni F., Matte F., Chiarelli-Adorno F., Mariely-Jaguezeski A., Tellez-Isaias G., Schafer-da-Silva A. Coccidiosis en pollos de engorda comerciales en Brazil entre 2012 y 2019: especies principales y grados de daño. Abanico vet. 2021;11:2020–2086. [Google Scholar]
- Gerhold R.W., Fuller A.L., Lollis L., Parr C., McDougald L.R. The efficacy of anticoccidial products against Eimeria spp. in northern bobwhites. Avian Dis. 2011;55:59–64. doi: 10.1637/9572-101310-Reg.1. [DOI] [PubMed] [Google Scholar]
- Gharekhani J., Sadeghi-Dehkordi Z., Bahrami M. Prevalence of coccidiosis in broiler chicken farms in Western Iran Medellín, Colombia. J. Vet. Med. 2014;2014:1–5. doi: 10.1155/2014/980604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goan C. The University of Tennessee, Agricultural Extension Service; 2009. Poultry Litter Sampling and Testing.https://extension.tennessee.edu/publications/Documents/SP563.pdf Accessed Feb. 2018. [Google Scholar]
- Grisales-Romero H. Universidad de Antioquia; Medellín, Colombia: 2011. Métodos Estadísticos. Capítulo 1-5. Facultad de Ciencias Agrarias. [Google Scholar]
- Györke A., Pop L., Cozma V. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite. 2013;20:50. doi: 10.1051/parasite/2013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadipour M.M., Olyaie A., Naderi M., Azad F., Nekouie O. Prevalence of Eimeria species in scavenging native chickens of Shiraz Iran. Afr J Microbiol Res. 2011;5:3296–3299. [Google Scholar]
- Hamidinejat H., Shapouri M.S., Mayahi M., Borujeni M.P. Characterization of Eimeria species in commercial broilers by PCR based on ITS1 regions of rDNA. Iran J Parasitol. 2010;5:48–54. [PMC free article] [PubMed] [Google Scholar]
- Haug A., Gjevre A.G., Thebo P., Mattsson J.G., Kaldhusdal M. Coccidial infections in commercial broilers: epidemiological aspects and comparison of Eimeria species identification by morphometric and polymerase chain reaction techniques. Avian Pathol. 2008;37:161–170. doi: 10.1080/03079450801915130. [DOI] [PubMed] [Google Scholar]
- Holdsworth P.A., Conway D.P., McKenzie M.E., Dayton A.D., Chapman H.D., Mathis G.F., Williams R.B. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet Parasitol. 2004;121:189–212. doi: 10.1016/j.vetpar.2004.03.006. [DOI] [PubMed] [Google Scholar]
- ICA-Instituto Colombiano Agropecuario . 2019. Censo Pecuario 2019.https://www.ica.gov.co/areas/pecuaria/servicios/epidemiologia-veterinaria/censos-2016/censo-2018.aspx Accessed Sept. 2019. [Google Scholar]
- Jenkins M.C., Parker C., Ritter D. Commercial broiler farms during anticoccidial drug or live Eimeria oocyst vaccine control programs. Avian Dis. 2017;61:214–220. doi: 10.1637/11578-010317-Reg.1. [DOI] [PubMed] [Google Scholar]
- Jenkins M.C., Dubey J.P., Miska K., Fetterer R. Differences in fecundity of Eimeria maxima strains exhibiting different levels of pathogenicity in its avian host. Vet Parasitol. 2017;236:1–6. doi: 10.1016/j.vetpar.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Jenkins M.C., Parker C.C., O'Brien C.N., Ritter D. Viable Eimeria oocysts in poultry house litter at the time of chick placement. Poult. Sci. 2019;98:3176–3180. doi: 10.3382/ps/pez147. [DOI] [PubMed] [Google Scholar]
- Jordan F., Pattison M., Alexander D., Faragher T. In PoultryDisease. 5th ed. W.B. Saunders; Hong Kong: 2002. Parasitic diseases; pp. 405–420. [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Kaboudi K., Umar S., Munir M.T. Prevalence of coccidiosis in free-range chicken in Sidi Thabet. Tunisia. Scientifica. 2016;2016:1–6. doi: 10.1155/2016/7075195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kučera J. Identification of Eimeria species in Czechoslovakia. Avian Pathol. 1990;19:59–66. doi: 10.1080/03079459008418656. [DOI] [PubMed] [Google Scholar]
- Lan L.H., Sun B.B., Zuo B.X.Z., Chen X.Q., Du A.F. Prevalence and drug resistance of avian Eimeria species in broiler chicken farms of Zhejiang province. China. Poult. Sci. 2017;96:2104–2109. doi: 10.3382/ps/pew499. [DOI] [PubMed] [Google Scholar]
- Lawal J.R., Gulani I.A., Ali A.M., Bello A.M., Abadam F.A., Mustapha M., Biu A.A. Dry season prevalence of avian Coccidia infection in domesticated chickens (Gallus domesticus) in Jere Council, Borno State, Nigeria. JASVM. 2016;9:653–659. [Google Scholar]
- Lee B., Kim W.H., Jeong J., Yoo J., Kwon Y.K., Jung B.Y., Kwon J.H., Lillehoj H.S., Min W. Prevalence and cross-immunity of Eimeria species on Korean chicken farms. J Vet Med Sci. 2010;72:985–989. doi: 10.1292/jvms.09-0517. [DOI] [PubMed] [Google Scholar]
- Li G.Q., Kanu S., Xiang F.Y., Xiao S.M., Zhang L., Chen H.W., Ye H.J. Isolation and selection of ionophore-tolerant Eimeria precocious lines: E. tenella, E. maxima and E. acervulina. Vet Parasitol. 2004;119:261–276. doi: 10.1016/j.vetpar.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Long P.L., Joyner L.P. Problems in the identification of species of Eimeria. J Parasitol. 1984;31:535–541. doi: 10.1111/j.1550-7408.1984.tb05498.x. [DOI] [PubMed] [Google Scholar]
- López S., Silva L.M., Taubert A., Chaparro-Gutiérrez J.J., Hermosilla C.R. Concomitant in vitro development of Eimeria zuernii-and Eimeria bovis-macromeronts in primary host endothelial cells. Parasitol Int. 2018;67:742–750. doi: 10.1016/j.parint.2018.07.009. [DOI] [PubMed] [Google Scholar]
- MAPS-Mapas Avícolas para la Productividad y Sostenibilidad. 2018. FENAVI. Accessed Dec. 2018. http://maps.fenavi.org/mapsfenavi/
- Mattiello R., Boviez J.D., McDougald L.R. Eimeria brunetti and Eimeria necatrix in chickens of Argentina and confirmation of seven species of Eimeria. Avian Dis. 2000;44:711–714. [PubMed] [Google Scholar]
- Molla B., Ali A. Epidemiological study on poultry coccidiosis: Prevalence, species identification and post mortem lesions in grower chicken in Kombolcha, North-Eastern Ethiopia. J. Vet. Med. Anim. Health. 2015;7:1–8. [Google Scholar]
- Moraes J.C., França M., Sartor A.A., Bellato V., de Moura A.B., Magalhães M.D.L.B., Miletti L.C. Prevalence of Eimeria spp. in broilers by multiplex PCR in the southern region of Brazil on two hundred and fifty farms. Avian Dis. 2015;59:277–281. doi: 10.1637/10989-112014-Reg. [DOI] [PubMed] [Google Scholar]
- Noack S., Chapman H.D., Selzer P.M. Anticoccidial drugs of the livestock industry. Parasitol Res. 2019;118:2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogedengbe J.D., Hunter D.B., Barta J.R. Molecular identification of Eimeria species infecting market-age meat chickens in commercial flocks in Ontario. Vet Parasitol. 2011;178:350–354. doi: 10.1016/j.vetpar.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Oviedo E.O., Clemente-Hernández S., Salvador F., Williams P., Losa R., Stephan F. Essential oils on mixed coccidia vaccination and infection in broilers. Int J Poult Sci. 2006;5:723–730. [Google Scholar]
- Peek H.W. Utrecht University; 2010. Resistance to Anticoccidial Drugs: Alternative Strategies to Control Coccidiosis in Broilers. Doctoral dissertation. [Google Scholar]
- Prakashbabu B.C., Thenmozhi V., Limon G., Kundu K., Kumar S., Garg R., Banerjee P.S. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet Parasitol. 2017;233:62–72. doi: 10.1016/j.vetpar.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz Castañeda R.E., Dantán González E. Control of avian coccidiosis: future and present natural alternatives. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/430610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid W.M. Relative value of oocyst counts in evaluating anticoccidial activity. Avian Dis. 1975;19:802–811. [PubMed] [Google Scholar]
- Schwarz R.S., Jenkins M.C., Klopp S., Miska K.B. Genomic analysis of Eimeria spp. populations in relation to performance levels of broiler chicken farms in Arkansas and North Carolina. J. Parasitol. 2009;95:871–888. doi: 10.1645/GE-1898.1. [DOI] [PubMed] [Google Scholar]
- Stephan B., Rommel M., Daugschies A., Haber Korn A. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet Parasitol. 1997;69:19–29. doi: 10.1016/s0304-4017(96)01096-5. [DOI] [PubMed] [Google Scholar]
- Sun X.M., Pang W., Jia T., Yan W.C., He G., Hao L.L., Bentue M., Suo X. Prevalence of Eimeria species in broilers with subclinical signs from fifty farms. Avian Dis. 2009;53:301–305. doi: 10.1637/8379-061708-Resnote.1. [DOI] [PubMed] [Google Scholar]
- Thabet A., Zhang R., Alnassan A.A., Daugschies A., Bangoura B. Anticoccidial efficacy testing: in vitro Eimeria tenella assays as replacement for animal experiments. Vet Parasitol. 2017;233:86–96. doi: 10.1016/j.vetpar.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Venkateswara, Rao P., Raman M., Gomathinayagam S. Sporulation dynamics of poultry Eimeria oocysts in Chennai. J Parasit Dis. 2015;39:689–692. doi: 10.1007/s12639-013-0403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.B. A compartmentalized model for the estimation of the cost of coccidiosis to the world´s chicken production industry. Int J Parasitol. 1999;29:1209–1229. doi: 10.1016/s0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl: its importance for experimental designs and the production of oocyst stocks. Int. J. Parasitol. 2001;31:1056–1069. doi: 10.1016/s0020-7519(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Anticoccidial vaccines for broiler chickens: pathways to success. Avian Pathol. 2002;31:317–353. doi: 10.1080/03079450220148988. [DOI] [PubMed] [Google Scholar]
- Yun C.H., Lillehoj H.S., Lillehoj E.P. Intestinal immune responses to coccidiosis. Dev. Comp. Immunol. 2000;24:303–324. doi: 10.1016/s0145-305x(99)00080-4. [DOI] [PubMed] [Google Scholar]