Abstract
Cognitive impairment is a core aspect of psychotic disorders and difficult to treat. Atypical antipsychotics (AAs) might have differential effects on cognitive impairment, but rigid study designs and selective sampling limit the generalizability of existing findings. This pragmatic, semi-randomized, industry-independent study aimed to investigate and compare the effect of amisulpride, aripiprazole and olanzapine on cognitive performance in psychosis over a 12-month period controlling for diagnostic group.
This sub study of the BeSt InTro study recruited adults with ongoing psychosis in the schizophrenia spectrum of disorders (ICD-10 diagnoses F20-F23, F25, F28 or F29; n = 104) from Bergen and Stavanger, Norway; and Innsbruck, Austria. Participants were randomized to amisulpride, aripiprazole, or olanzapine and they completed neuropsychological assessments at baseline, 6 weeks, 6 and 12 months. The test battery targeted working memory, verbal ability, and processing speed. We used Longitudinal mixed effect (LME) models to assess cognitive change for intention to treat (ITT) and per protocol (PP) medication groups, as well as comparing cognitive performance between F20 and non-F20 participants.
The sample baseline global cognitive performance t-score was 42.20. Global performance improved significantly to every follow-up, including for the F20 group. There were however no significant differences in cognitive change over time between neither ITT nor PP medication groups.
Keywords: Antipsychotic medication, Psychosis, Neurocognition, Cognitive, Schizophrenia, RCT
1. Introduction
Cognitive impairment is a core symptom dimension of schizophrenia (Kahn and Keefe, 2013) and also occurs in other psychotic disorders (Bortolato et al., 2015). Cognitive impairment in first-episode psychosis (FEP) is consistently related to poor social and vocational functioning, and is a stronger predictor of halted recovery than are baseline positive and negative symptoms (Santesteban-Echarri et al., 2017). This makes cognitive challenges an essential treatment target in psychosis.
Although effective against positive symptoms, antipsychotic medication effects on cognitive impairment are uncertain. The advent of atypical antipsychotics (AA) in the 90s gave rise to optimism, with studies finding domain specific drug effects, e.g. olanzapine for verbal fluency problems (Meltzer and McGurk, 1999) and clozapine for attention (Sharma and Mockler, 1998). Early claims of differential effects have however failed to find more recent support in chronic schizophrenia (Keefe et al., 2007a) or FEP (Malla et al., 2004) samples.
Reasons for this discrepancy are unclear. However, early studies were to a large extent industry funded and recruited severely ill or chronic patient groups, e.g. only chronic schizophrenia patients (Bagnall et al., 2003), which may have affected the ecological validity of their findings. Although such designs demonstrate pre-marketing drug efficacy, findings may not be generalizable to every-day clinical practice. Clinical guidelines call for antipsychotic drug treatment to be initiated e.g. during acute first-episode psychosis (Barnes et al., 2019; NICE, 2014; Norwegian Directorate of Health, 2013), when exact diagnoses are hard to determine. Follow-up periods have frequently been short, e.g. two months (Riedel et al., 2010), while clinically, AAs are used over time. Recent reviews however note that early findings on atypical antipsychotics effects might have been related to participants changing from high doses of cognition impairing first-generation antipsychotics, and that although atypical antipsychotics s outperform first-generation antipsychotics for cognitive symptoms, effects sizes have been small (Désaméricq et al., 2014). One comprehensive meta-analysis concluded that there were no differences in effect on overall cognitive functioning between first- nor second-generation APs at all (Nielsen et al., 2015), though a more recent network meta-analysis found olanzapine to have a superior effect on overall score as well as most sub-domains, whereas haloperidol ranked below every other drug on every domain ranking (Baldez et al., 2021). To further complicate matters, although positive symptoms may rapidly abate at the initiation of APs, cognitive impairment might take longer to respond (Désaméricq et al., 2014). This leaves a need for independent studies focusing on samples more representative of the wide range of patients receiving these drugs in clinical settings.
All current APs, including AAs, are functional striatal dopamine D2 antagonists. The current dopamine theory of schizophrenia postulates that positive symptoms arise by excessive mesostriatal dopamine signalling causing aberrant salience (Howes and Nour, 2016) while negative symptoms are associated with low prefrontal dopamine and D1 receptor dysfunction (Howes and Kapur, 2009). Cognitive processes like attention, language processing and working memory all depend on dopamine-prefrontal interactions for organization and temporal planning of problem solving strategies (Barch and Ceaser, 2012). Although psychosocial stressors, cognitive reserve (Vance et al., 2010), brain structural (Jirsaraie et al., 2018) and connectivity (Giraldo-Chica et al., 2018) factors all affect cognitive performance in psychosis, any disturbance to dopaminergic signalling is also likely to impair a range of cognitive functions.
Despite all APs displaying functional D2 antagonism, AAs differ in their pharmacological profiles and might thus differentially affect brain function, both positively and negatively. The new line of partial D2 agonists, of which aripiprazole is the first, may be of particular relevance. Whereas amisulpride and olanzapine are strict D2 blockers aiming to counteract striatal hyperdopaminergia, the partial D2 agonists in theory also address prefrontal hypodopaminergia, thus targeting both aspects of the dopaminergic paradox (Howes and Kapur, 2009). In areas of excessive dopamine signalling the partial agonist will compete with endogenous dopamine for postsynaptic D2 receptors, thereby mediating functional D2 antagonism. In dopamine deficient prefrontal areas, a partial agonist may, contrary to the antagonists, increase dopamine signalling. For this reason, D2 partial agonists often are referred to as dopamine stabilizers, and might be expected to positively affect cognition. Indeed, animal models of aripiprazole have indicated improved prefrontal dopaminergic function (Li et al., 2004).
The AAs under investigation also differ in extra-dopaminergic receptor affinities. Amisulpride primarily targets D2 and D3 receptors with minimal effect on other neurotransmitter systems. Its cognitive effects are not well explored but one study found it to outperform high 5HT2 affinity AAs for sustained attention (Tyson et al., 2006). Olanzapine has a broad affinity profile in addition to dopaminergic antagonism, affecting serotonergic (5HT2) and adrenergic receptors as well as being a muscarinic acetylcholine (M3) and histaminergic (H1) receptor antagonist. H1 antagonists are known to impair psychomotor speed and memory scanning (Van Ruitenbeek et al., 2010), while anticholinergic effects may negatively affect verbal learning and visual memory (Chew et al., 2006). In sum, AAs might be expected to have differential positive and negative effects on cognitive functioning (Steen et al., 2017), with aripiprazole ostensibly displaying the most promising pro-cognitive receptor profile. Examining between-drug differences for cognition might thus yield clinically important information, as this would prevent the singular effects of each drug from drowning each other out at the stage of analysis.
In sum, there is a need for updated knowledge on differential effects of AA on cognition, based on clinically representative samples followed up over a sufficient period of time. It would also be interesting to compare effects in schizophrenia vs non-schizophrenia groups given that the cognitive trajectory is thought to be less favourable and responsive in schizophrenia. The aim of this study was therefore to compare the effectiveness on cognition of the three AAs aripiprazole, olanzapine and amisulpride over a 12-month period, in a clinically representative sample. Study drugs were specifically selected for their differing pharmacological and receptor affinity profiles, and based on previous efficacy comparison studies (Leucht et al., 2013). This is the first direct head-to-head comparison of the effect of these antipsychotic drugs on cognition in psychotic disorders. We also aimed to compare differences in cognitive change between schizophrenia and other psychotic disorders, in order to investigate whether there is less change in participants with schizophrenia.
2. Methods
2.1. Design and duration
Participants were part of the Bergen-Stavanger-Innsbruck-Trondheim (BeSt InTro) study, a semi-randomized, rater-blinded multisite comparison of amisulpride, aripiprazole, and olanzapine. Participants for the current sub-study were recruited from Bergen and Stavanger in Norway and from Innsbruck in Austria. Participants were followed for a year, corresponding with the minimum recommended maintenance period of drug therapy after an acute psychotic episode. They completed a brief neuropsychological assessment at baseline, 6 weeks, and 6 and 12 months.
2.2. Study population
The BeSt InTro inclusion criteria were age >18, a score ≥4 on either of the Positive And Negative Symptoms Scale (PANSS) scale (Kay et al., 1987) items for delusions (P1), hallucinatory behaviour (P3), grandiosity (P5), suspiciousness/persecution (P6) or unusual thought content (G9), and ability to understand and speak the site native language. Exclusion criteria were psychoactive drug use or neurological or endocrine disorders likely to have caused the presented psychotic symptoms. Trained clinicians administered the Structured Clinical Interview for the DSM-IV Axis I Disorders (SCID I) (Gibbon et al., 1997) to determine DSM-IV diagnoses. We converted these to ICD-10 (World Health Organization, 2009) diagnoses, with schizophrenia (F20), schizotypal disorder (F21), delusional disorder (F22), acute psychotic disorders (F23), schizoaffective disorder (F25), other non-organic psychotic disorders (F28), and unspecified nonorganic psychosis (F29) eligible for inclusion. Participants gave their written informed consent for participation and had to be eligible for oral antipsychotic drug treatment as determined by their attending clinician. Inclusion in the current sub-study also required completion of at least one neuropsychological assessment.
The enrolment flow is displayed in Fig. 1. Out of the 144 patients randomized, 104 completed baseline neuropsychological assessment. A further 11 had completed at least one non-baseline assessment. These could not be included in the baseline analyses but were included in the LME model development in order to strengthen the model. Inability to complete baseline neuropsychological testing was not quantitatively assessed, but a common reason was that the person was too unstable or unwell to be tested.
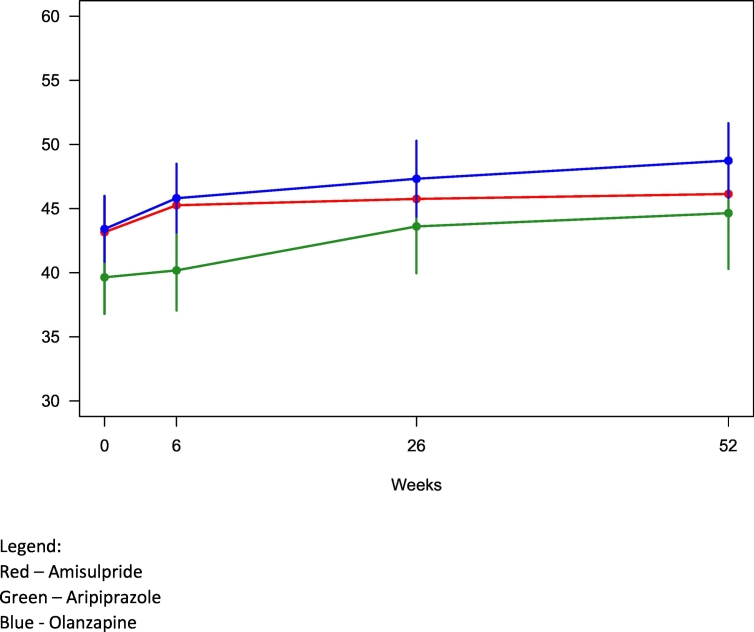
Fig. 1.
Change in overall cognitive performance t-scores over time per drug.
Legend:
Red – Amisulpride
Green – Aripiprazole
Blue - Olanzapine.
2.3. Study medication
Study medication consisted of oral tablets administered according to Summary of Product Characteristics for each drug with dosing intervals as follows: Amisulpride 50–1200 mg/day; aripiprazole 5–30 mg/day; and olanzapine 2.5-20 mg/day.
2.4. Open randomization
Study drug allocation was open to participants and clinical treatment teams, while research assessment teams remained blinded. Study-independent statisticians at the University of Bergen made computer generated consecutively numbered drug randomization envelopes, each containing all three study drugs listed in random order. Each participant's attending physician would open the envelope and offer the topmost listed drug. If the primary allocation was unacceptable for example due to previous negative experiences with the study drug, the second listed drug was offered, while noting the reason for unselecting the first. This procedure was repeated if the second listed drug was also rejected. Initiation and dosage of the chosen medication as well as any subsequent changes were left to the clinician's discretion.
Intention-to-treat (ITT) groups for analysis were based on the first study drug listed in participants' randomization envelope. Per protocol (PP) analysis groups were based on the study drug actually chosen.
2.5. Concomitant medications
In line with common clinical practice, we allowed use of concomitant medications. In line with available knowledge and treatment guidelines (Barnes et al., 2019; NICE, 2014; Norwegian Directorate of Health, 2013), we strongly discouraged antipsychotic polypharmacy, while allowing limited periods of cross-titration during antipsychotic drug switches.
2.6. Outcome measures
The brief neurocognitive test battery was administered by trained staff, took 45–60 min to complete and was designed to target working memory, selective attention, executive functioning, verbal learning, and processing speed. These high-level functions rely on frontal lobe function and are likely to be affected by drug-related changes in dopaminergic activity. The battery consisted of Dichotic Listening, Trail Making A (TMA) and Trail Making B (TMB) from the Halsted-Reitan test set (Reitan, 1958), The Hopkins Verbal Learning Test (HVLT-R) (Benedict et al., 1998), WAIS-IV letter number sequencing (Wechsler, 1997), and the D-KEFS FAS verbal fluency tests and Symbol Coding (Delis et al., 2004), administered in that order. We used alternative versions of the HVLT-R for each follow-up, and overall chose tests less vulnerable to learning effects. Raw test scores were converted to t-scores by way of their respective norm based scoring manuals. We calculated average t-scores for a global cognitive measure based on all subtests as well as three subdomain t-scores based on scores from respective subtests: Working memory and attention (WAIS Letter Number Sequencing, Trail making B), verbal learning and reasoning (FAS, HVLT-R) and speed of processing (Trail Making A, WAIS Coding).
We also collected Global Assessment of Functioning (GAF) scores (Pedersen et al., 2007), using the split version to separately score symptom levels and functioning. We used the AUDIT (Bush et al., 1998) and DUDIT (Berman et al., 2007) scales to record alcohol and drug use.
2.7. Data analysis and statistical methods
We used SPSS to calculate the percentage of participants with cognitive impairment at baseline and each follow-up. The cut-off for impairment was set at an overall cognitive performance t-score of t < 35, i.e. > 1.5 SD below the mean in line with Heaton et al. (1991).
2.8. Cognitive change analyses
Longitudinal mixed effect (LME) models in R were used to assess global cognitive change during the 12-month follow-up period. We chose this analysis both for its ability to handle missing data, given the commonly high drop-out rate in antipsychotic drug studies, and to account for dependencies in the data arising from repeated measurements of the same individuals. For the LME analyses, we assumed that missing data were missing at random.
The first model examined global cognitive score change over time for participants as a whole. Time was entered as a fixed effect, while a random intercept was included as a random effect to account for intra-individual correlation. We also calculated change over time in the three cognitive domains working memory, verbal learning and reasoning, and speed of processing, for the full sample and for the medication groups.
Two further models, based on ITT and PP groups respectively, were designed to examine any differences in effects of aripiprazole, olanzapine and amisulpride on cognitive change over 12 months. Fixed effects were time and medication as well as the interaction between these. Amisulpride was used as a reference drug in the analyses, with a random intercept included like previously.
A fourth model was designed to compare cognitive change between participants with schizophrenia, and those with other psychotic disorders. The model was analogous to the medication analyses, with diagnosis entered as a fixed effect in place of medication.
We used one-way ANOVAs to compare DDD as well as antipsychotic polypharmacy between the three medication groups (amisulpride, aripiprazole and olanzapine), for both ITT and PP analyses. We also performed a post-hoc power analysis to investigate the likelihood of our data set discovering significant differences in cognitive performance assuming it was representative of population data.
3. Results
3.1. Participant characteristics and antipsychotic use
Table I lists baseline demographic and clinical characteristics for participants (n = 104) as a whole and for the ITT groups. Ethnic minority individuals made up 6.7% of the sample, relatively close to the Stavanger (8.8%) and Bergen (7.0%) population proportion of non-European ethnicities (Statistisk Sentralbyrå, 2019). There were no significant differences between ITT groups in demographic variables.
Table I.
Demographic and clinical data.
| ITT group | All (n = 104) | Amisulpride (n = 33) |
Aripiprazole (n = 32) |
Olanzapine (n = 39) |
|---|---|---|---|---|
| Age mean (SD) | 31.3 (12.2) | 32.3 (11.8) | 28.2 (10.2) | 31.3 (12.2) |
| Female (%) | 37.5 | 42.4 | 31.3 | 38.5 |
| Years of education (SD) | 12.5 (2.7) | 13.1 (3.1) | 11.7 (2.2) | 12.7 (2.7) |
| Clinical information | ||||
| Medication naïve (%) | 42.3 | 30.3 | 59.4 | 38.5 |
| Age of onset (SD) (n = 71) | 23.3 (8.9) | 25.0 (10.2) | 20.3 (6.4) | 24.1 (9.1) |
| PANSSa Positive (SD) | 20.7 (4.7) | 20.6 (4.3) | 20.8(5.2) | 20.6 (4.7) |
| PANSSa Negative (SD) | 17.6 (5.7) | 16.7 (5.6) | 17.5(5.9) | 18.4 (5.6) |
| PANSSa General (SD) | 38.2 (7.7) | 38.3 (8.5) | 37.1 (6.1) | 39.0 (8.2) |
| PANSSa Total (SD) | 76.4 (14.3) | 75.6 (14.6) | 75.4 (12.5) | 78.0 (15.6) |
| GAFb Function (SD) | 36.8 (8.2) | 37.6 (7.5) | 34.4 (6.7) | 38.0 (9.6) |
| GAFb Symptoms (SD) | 33.8 (5.9) | 34.3 (5.5) | 32.7 (5.8) | 34.3 (6.5) |
| Diagnoses % (n) | ||||
| F20 schizophrenia | 57.7 (60) | 63.6 (21) | 53.1 (17) | 56.4 (22) |
| F21 schizotypal | 1.9 (2) | 3.0 (1) | 0 (0) | 2.6 (1) |
| F22 delusional | 15.4 (16) | 12.1 (4) | 15.6(5) | 17.9 (7) |
| F23 brief | 10.6 (11) | 12.1 (4) | 9.4 (3) | 10.3 (4) |
| F25 schizoaffective | 5.8 (6) | 9.1 (3) | 6.3 (2) | 2.6 (1) |
| F28 other | 1.0 (1) | 0 (0) | 3.1 (1) | 0 (0) |
| F29 unspecified | 7.7 (8) | 0 (0) | 12.5 (4) | 10.3 (4) |
| Drugs and alcohol use | ||||
| Misuse/dep. alcohol (%) | 5.8 | 3.0 | 9.7 | 5.1 |
| Misuse/dep. drugs (%) | 16.5 | 18.2 | 12.9 | 17.9 |
Note: There were no significant between-group differences in any of the demographic variables.
Positive and Negative Symptoms Scale.
Global Assessment of Functioning, split version.
For the ITT groups overall, 82.7% (n = 86) accepted the #1 allocated drug, while 17.3% switched. The ITT group percentage to reject the #1 drug was 12.1 (n = 4) for amisulpride, 9.4 (n = 3) for aripiprazole and 28.2 (n = 11) for olanzapine. A Chi square test showed no significant differences between groups (X2 = 5.26, df = 2, p = .074) as to #1 drug acceptance.
3.2. Study drug doses and polypharmacy
For the ITT group analysis, respective mean study drug doses and standard deviations to Visit 2 were 250.00 (170.11) mg for amisulpride, 12.35 (5.36) mg for aripiprazole and 10.83 (3.73) mg for olanzapine. Corresponding Visit 2 Defined Daily Doses (DDD) were 0.62 (0.42) for amisulpride; 0.82 (0.36) for aripiprazole; and 1.12 (0.41) for olanzapine. Table II shows dosing and DDD details for follow-ups with neurocognitive testing, with mean serum levels overall and per visit displayed in Table III. One-way ANOVAS found no significant differences in DDD for any of these follow-ups.
Table II.
Antipsychotic drug doses (SD) and Defined Daily Doses (DDD).
| ITT group |
Amisulpride |
Aripiprazole |
Olanzapine |
|||
|---|---|---|---|---|---|---|
| Week | mg (SD) | DDD (SD) | mg (SD) | DDD (SD) | mg (SD) | DDD (SD) |
| 6 | 401.08 (243.52) | 1.00 (0.61) | 17.86 (9.31) | 1.19 (0.62) | 13.55 (4.51) | 1.35 (0.45) |
| 26 | 449.41 (285.49) | 1.12 (0.71) | 14.76 (11.48) | 0.98 (0.77) | 10.91 (5.03) | 1.09 (0.50) |
| 52 | 337.50 (197.86) | 0.84 (0.49) | 16.32 (10.48) | 1.09 (0.70) | 10.00 (3.54) | 1.00 (0.35) |
| Mean | 358.68 (202.21) | 0.89 (0.50) | 14.31 (6.94) | 0.95 (0.46) | 11.86 (3.34) | 1.26 (0.39)⁎ |
Note: Although DDD is 10 mg for olanzapine, in clinical settings the dose is often 20 mg.
Table III.
Mean study drug serum levels with standard deviations.
| Week |
Ami |
Ari |
Ola |
|---|---|---|---|
| Reference range | 100–1500 NMOL/La | 200–1300 NMOL/La | 30–200 NMOL/La |
| Bergen and Stavanger (Norway) | |||
| 1 | 436.36 (460.46) | 321.93 (144.82) | 77.78 (34.87) |
| 6 | 777.43 (715.14) | 779.43 (661.81) | 101.19 (58.01) |
| 26 | 906.50 (697.60) | 740.40 (416.37) | 107.13 (78.76) |
| 52 | 696.67 (531.69) | 321.00 (325.19) | 98.20 (42.52) |
| Mean | 621.87 (506.96) (n = 28) | 553.99 (378.21) (n = 25) |
91.95 (47.36) (n = 21) |
| Innsbruck (Austria) | |||
| 1 | 91.00 (55.36) | 314.00 (337.76) | 64.75 (15.97) |
| 6 | 148.20 (113.58) | 529.0 (−) | 88.00 (11.31) |
| 26 | 299.67 (241.91) | – | 54.0 (−) |
| 52 | 211.00 (155.52) | – | 67.0 (−) |
| Mean | 244.02 (160.17) (n = 6) |
216.83 (146.45) (n = 3) |
204.13 (307.40) (n = 5) |
Note: Serum analyses are given separately for Norwegian and Austrian sites due to international differences in reference ranges and different laboratories performing analyses.
Nanomoles per litre.
Groups did not differ in use of antipsychotics ahead of BeSt InTro inclusion. At baseline, 38.5% (n = 40) of participants received some form of additional APs. For ITT groups, the percentage receiving additional APs at baseline were 39.4 (n = 13) for amisulpride, 34.4 (n = 11) for aripiprazole and 41.0 (n = 16) for olanzapine. One-way ANOVAs showed no significant difference between ITT groups for the proportion receiving additional APs at baseline nor any other visit. Baseline PP group percentages receiving additional APs were 46.2 (n = 18) for amisulpride, 38.2 (n = 13) for aripiprazole, and 29.0 (n = 9) for olanzapine. A one-way ANOVA found significant between-group differences (SS = 63.80, df = 91, p = .026). Post-hoc pairwise t-tests found a Bonferroni corrected significant (p = .022) difference between the olanzapine and amisulpride groups in terms of the percentage of participants receiving non-study APs at baseline. There were no further significant differences between PP groups for later follow-ups. ANOVAs/Fisher's exact tests found no significant differences in additional drugs use for neither ITT nor PP groups for any visit. Details on additional medication for ITT and PP groups at each follow-up time is displayed in Table IV.
Table IV.
Additional psychotropic drugs for ITT and PP groups.
| Week | Amisulpride |
Aripiprazole |
Olanzapine |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADa |
MSb |
Opic |
Bzdd |
Ache |
ADHf |
ADa |
MSb |
Opic |
Bzdd |
Ache |
ADHf |
ADa |
MSb |
Opic |
Bzdd |
Ache |
ADHf |
|
| N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
N (%) |
|
| ITT groups | ||||||||||||||||||
| Baseline | 2 (6.3) | 1 (3.1) | 1 (3.1) | 12 (37.5) | 1 (3.0) | 0 (0.0) | 3 (9.4) | 1 (3.1) | 0 (0.0) | 9 (28.1) | 0 (0.0) | 1 (2.1) | 6 (15.4) | 0 (0.0) | 0 (0.0) | 9 (23.1) | 0 (0.0) | 0 (0.0) |
| 6 | 4 (12.5) | 2 (6.3) | 1 (3.1) | 6 (18.8) | 2 (6.3) | 1 (3.1) | 4 (12.5) | 1 (3.1) | 0 (0.0) | 14 (29.2) | 1 (3.1) | 0 (0.0) | 5 (12.8) | 0 (0.0) | 0 (0.0) | 8 (20.5) | 0 (0.0) | 0 (0.0) |
| 26 | 2 (6.3) | 3 (9.4) | 0 (0.0) | 2 (6.3) | 0 (0.0) | 1 (3.1) | 4 (12.9) | 0 (0.0) | 0 (0.0) | 2 (6.5) | 0 (0.0) | 0 (0.0) | 4 (10.3) | 0 (0.0) | 0 (0.0) | 3 (7.7) | 0 (0.0) | 0 (0.0) |
| 52 | 5 (15.6) | 1 (3.1) | 0 (0.0) | 1 (3.1) | 0 (0.0) | 1 (3.1) | 3 (10.0) | 0 (0.0) | 0 (0.0) | 2 (6.7) | 1 (3.3) | 0 (0.0) | 4 (10.3) | 1 (2.6) | 0 (0.0) | 3 (7.7) | 1 (2.6) | 0 (0.0) |
| PP groups | ||||||||||||||||||
| Baseline | 4 (10.5) | 0 (0.0) | 1 (2.6) |
15 (39.5) | 1 (2.6) |
0 (0.0) |
3 (8.8) | 2 (5.9) |
0 (0.0) |
11 (32.4) | 1 (2.0) |
0 (0.0) |
4 (12.9) | 0 (0.0) |
0 (0.0) |
4 (12.9) | 0 (0.0) |
0 (0.0) |
| 6 | 7 (21.2) | 1 (3.0) |
1 (3.0) |
9 (27.3) | 2 (6.1) |
1 (3.0) |
3 (16.7) | 2 (11.1) | 0 (0.0) |
6 (33.3) | 0 (0.0) |
0 (0.0) |
1 (5.3) |
0 (0.0) |
0 (0.0) |
2 (10.5) | 0 (0.0) |
0 (0.0) |
| 26 | 3 (16.7) | 2 (11.1) | 0 (0.0) | 3 (16.7) | 0 (0.0) | 0 (0.0) | 2 (28.6) | 1 (14.3) |
0 (0.0) | 1 (14.3) |
0 (0.0) | 0 (0.0) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 52 | 3 (21.4) | 1 (7.1) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 1 (7.1) |
2 (33.3) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Antidepressants.
Mood stabilizers.
Opiates.
Benzodiazepines and related drugs.
Anticholinergic drugs.
Drugs used to treat ADHD.
3.3. Overall cognitive performance and change scores
The initial mean cognitive test performance t-score across the sample was 42.20, increasing to 46.39 at week 52. The participant group as a whole saw significant improvement in average overall t-scores for cognitive performance from baseline to each follow-up, i.e. at 6 (Δ2.0; p = .002), 26 (Δ3.4; p < .001) and 52 (Δ4.2; p < .001) weeks, with a respective average change per day of 0.05, 0.02 and 0.01 per day to respective visits. This corresponds to a Cohen's d of 0.53, or a medium effect size. Table V shows overall cognitive test and composite scores for baseline and each follow-up, while Table VI shows change over time for ITT and PP groups. At baseline, 27.9% (n = 29) showed cognitive impairment, defined as scoring 1.5 SD below the population mean (t < 35). For 6, 26 and 52 week follow-ups, 23.3%, 8.3%, and 17.0% scored at t < 35 respectively.
Table V.
Mean cognitive t-scores for total sample.
| BL mean (SD) t-scores (n = 104) | Wk 6 mean (SD) t-scores (n = 86) | Wk 26 mean (SD) t-scores (n = 48) | Wk 52 mean (SD) t-scores (n = 47) | |
|---|---|---|---|---|
| Composite t-scores | ||||
| Overall cognitive performance | 42.61 (7.58) | 44.17 (8.71) | 45.23 (8.25) | 47.99 (10.53) |
| Verbal learning and reasoninga | 43.01 (9.53) | 44.17 (8.71) | 45.23 (8.25) | 47.99 (10.53) |
| Working memoryb | 42.50 (8.17) | 44.42 (9.16) | 49.08 (9.27) | 47.59 (9.57) |
| Processing speedc | 39.38 (10.99) | 42.07 (9.94) | 45.51 (9.69) | 46.63 (8.14) |
| Single test t-scores | ||||
| FASd | 44.90 (12.16) | 47.55 (11.51) | 48.82 (11.80) | 49.55 (14.12) |
| TMAe | 43.50 (12.06) | 46.82 (10.35) | 52.23 (12.66) | 51.30 (11.97) |
| TMBf | 42.53 (10.88) | 45.17 (12.31) | 51.77 (12.58) | 48.76 (13.45) |
| LNSg | 42.08 (9.18) | 43.62 (9.85) | 46.38 (10.21) | 45.56 (9.45) |
| HVLT-Rh | 40.84 (11.38) | 40.67 (9.94) | 41.11 (11.10) | 46.72 (14.05) |
| SCi | 35.46 (13.44) | 36.93 (12.39) | 38.15 (11.25) | 41.63 (13.66) |
Composite of HVLT-R, FAS.
Composite of LNS, TMB.
Composite of TMA, SC.
DKEFS Verbal fluency.
Halsted Reitan Trailmaking A.
Halsted Reitan Trailmaking B.
WAIS Letter-number sequencing.
Hopkins verbal learning test revised.
DKEFS symbol coding.
Table VI.
Cognitive performance change over time for ITT and PP groups.
| Baseline t-score mean (SD) | P | Predicted Δ to 6 weeks (SD) | Daily change to 6 wks | P | Predicted Δ to 26 wks (SD) |
Daily change to 26 wks | P | Predicted Δ to 52 weeks (SD) |
Daily change to 52 wks | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ITT groups | |||||||||||
| Amisulpride (n = 33) | 43.16 (1.35) | N/A | 2.10 (1.31) | 0.005 | .039a | 2.59 (1.48) | 0.014 | .032a | 2.98 (1.50) | 0.008 | .015a |
| Aripiprazole (n = 32) | 39.64 (1.43) | .077b | 0.54 (1.57) | 0.013 | .342b | 3.96 (1.84) | 0.022 | .481b | 5.00 (2.19) | 0.014 | .378b |
| Olanzapine (n = 39) | 43.40 (1.30) | .895b | 2.40 (1.35) | 0.057 | .837b | 3.92 (1.49) | 0.022 | .441b | 5.33 (1.47) | 0.015 | .170b |
| PP groups | |||||||||||
| Amisulpride (n = 39) | 42.42 (1.27) | N/A | 2.13 (1.24) | 0.051 | .032a | 3.48 (1.39) | 0.020 | .003a | 4.14 (1.39) | 0.011 | <.001a |
| Aripiprazole (n = 34) | 39.88 (1.34) | .173b | 1.10 (1.44) | 0.026 | .492b | 3.06 (1.68) | 0.018 | .818b | 2.05 (2.02) | 0.006 | .324b |
| Olanzapine (n = 31) | 44.39 (1.44) | .307b | 2.47 (1.54) | 0.059 | .836b | 3.80 (1.69) | 0.022 | .860b | 5.83 (1.66) | 0.016 | .348b |
For the Amisulpride group, p values pertain to predicted change over time for that drug.
For the Aripiprazole and Olanzapine groups, p values pertain to predicted effect of time and drug, i.e. difference in effect of cognition over time as compared with the reference drug Amisulpride.
3.4. Primary outcome: cognitive change per medication group over time
Fig. 1 shows cognitive change over time with amisulpride being compared to aripiprazole and olanzapine. There were no significant differences between ITT medication groups for the primary outcome measure of overall cognitive change. Overall cognitive performance t-scores in the model increased by 2.98 points for the amisulpride group, i.e. a mean improvement of 6.7% over 12 months, with corresponding increases of 5.00 (12.6%) for aripiprazole and 5.33 (12.2%) for olanzapine. Per-protocol (PP) analyses yielded similar results with no significant between-group differences. There were no significant baseline cognitive performance differences between neither ITT nor PP groups. The post-hoc power analysis found that power for the respective variables were 70% for overall cognitive performance, 80% for verbal abilities, 20% for processing speed and 30% for working memory. The percentages indicate the stipulated chance of detecting significant differences if assuming that our data are indeed representative of population scores.
3.5. Secondary outcome: cognitive change in schizophrenia vs. other psychotic disorders
When comparing the 57.7% (n = 60) participants with a diagnosis of schizophrenia (F20) to other participants, there were no significant differences in cognitive impairment at baseline or cognitive change over time between the F20 group and the group with other psychotic disorders.
4. Discussion
This pragmatic randomized trial of atypical antipsychotics effects on cognitive test performance over 12 months found no significant differences between the groups receiving amisulpride, aripiprazole and olanzapine. This is contrary to a major meta-analysis which ranked olanzapine as superior to amisulpride (Désaméricq et al., 2014). However, our findings are in line with two other major AA comparison studies, the CATIE (Keefe et al., 2007b) and EUFEST (Davidson et al., 2009) projects, neither of which found differential effects of AAs on neurocognitive functioning.
A possible explanation for the non-significant difference between drug groups is that our study was underpowered to detect smaller, yet clinically meaningful differences in performance. A post-hoc power analysis indicated adequate power to detect differences in overall cognitive performance. However, this should be interpreted with caution, given that if the study was underpowered, the results of the post-hoc power analysis are subsequently also less reliable.
Of 144 patients enrolled in BeSt InTro overall, only 104 completed neuropsychological assessment. A commonly recorded reason for non-testing was insufficient level of general functioning. If assuming poor functioning in a disproportionately large fraction of those not tested, relatively higher levels of functioning in the current sample might have created ceiling effects in either test results or cognitive change. However, the low group baseline mean indicates that this would not on its own explain the lack of differences.
Despite the non-significant difference between groups, there was a notable trend for the aripiprazole group performing worse, especially during the first six weeks but also across the follow-up period. This appears mainly driven by weaker scores in the sub-scale for speed of processing. Our finding is surprising given the purported positive effect on cognition of aripiprazole, found to boost prefrontal DA function in rodent studies (Li et al., 2004). The effect of aripiprazole on attention, especially in previously medicated subjects, should be more closely examined in the future. It is also possible that despite the randomization procedure and no statistically significant differences between ITT groups, the aripiprazole group in some way differed from the other participants.
It should also be noted that the BeSt InTro project, of which the current paper is a sub-study, was designed to differentiate between drug effects primarily on overall psychotic symptoms load, based partly on meta-analysis results indicating the superior effects of olanzapine and amisulpride (Leucht et al., 2013). A design based on meta-analysis of cognitive symptom effects might be a fruitful future avenue. Future avenues to identify the best drugs for cognitive symptoms should also include exploration of new agents with novel mechanisms of action, as well as non-medication avenues like cognitive remediation training.
Participants' overall cognitive performance improved significantly during the 12-month follow-up period, from about one standard deviation below the population mean at baseline, to a t-score of 46.39 at 12 months. This supports earlier findings from our research group that cognitive functioning does improve significantly during antipsychotic treatment (Johnsen et al., 2013), including for non-FEP participants, even if some impairment still remains. It also supports our finding that positive cognitive change can and does happen in psychosis (Anda et al., 2016).
The F20 subgroup of our sample also saw significant cognitive improvement across the follow-up period, with no significant differences to other participants in terms of change. This is in contrast e.g. to the CATIE trial, whose saw limited overall improvement in participants with established schizophrenia. Our F20 group represents a mix of recently diagnosed individuals, some medication naïve, and others with a longer duration of illness. It is possible that a greater amount of change happened in the former subgroup, as indicated by our previous study (Anda et al., 2019), and that cognitive recovery from an acute psychotic episode is more likely to happen during early psychotic episodes regardless of diagnosis. It might also be argued that although the between-group difference was about 10% away from statistical significance, it still represents a clinically meaningful difference.
4.1. Strengths and limitations
A major strength of the current study is the industry-independent funding and a pragmatic design. The broad inclusion criteria capture neurocognitive variability in this patient group. The open randomization process may have been vulnerable to systematic differences in AA choice between clinicians or sites, given that prior experience with study drugs might have unpredictably affected patient and clinician drug choices. However, there were no indications of differences between drug groups in the proportion accepting the first drug offered. Open randomization is also more acceptable to both patients and prescribing physicians than a fully blinded study, yielding a more representative sample and more generalizable results.
The considerable attrition rate is unfortunately common in antipsychotic drug trials. This was a main motivation for selecting a mixed effects analysis in order to handle missing data. If missing cognitive change data are systematically related to other variables this may however affect the validity of findings. a missing at random assumption as with LME modelling yields a more robust result despite missing data, as compared to a listwise method assuming missing completely at random. Due to consent restrictions we were unfortunately unable to re-contact dropped-out participants to gather further data from them to directly assess reasons for missingness. However, missing analyses supported an assumption of missing at random: Medication groups did not differ in attrition rates, nor was attrition related to any known baseline characteristics. Subjects lost to follow-up were included in the LME model in order to strengthen its stability, but no data were imputed for this group for the longitudinal part of the dataset. Due to this high attrition, slopes may be less certain in the latter part of the follow-up period. It should also be noted that a common informally reported reason for non-participation in neurocognitive testing was that the person was “too unwell” to be tested. It is thus possible that clinical instability may be a reason for drop-out from neurocognitive testing throughout the follow-up period. Our results may therefore be of limited generalizability to those with extremely high psychotic symptom loads. However, any such effect is likely to have equally affected every medication group, and should thus not detract from our main comparison findings. Missingness in BeSt InTro has also been thoroughly assessed elsewhere, with findings supporting an assumption of missing at random (Johnsen et al., 2020).
The present study did not use a comprehensive test battery to assess cognitive change. This might have contributed to masking any differences between drugs, as suggested by EUFEST authors (Davidson et al., 2009). However, we did test four times during the 12-month follow-up period, with special attention paid to the early weeks when cognitive recovery is most likely to happen.
A final limitation to the current findings is that repeated-measures studies of cognition are vulnerable to practice effects, i.e. improved scores at re-test measurement points due to repeated testing. However, the tests we used, including HVLT-R, trail making, coding and letter-number sequencing were recently found resistant to such effects (Rodriguez-Toscano et al., 2019). Any learning effects are also likely be randomly distributed between medication groups and should thus not deter us from concluding about any between-drug differences in change trajectory.
Declaration of competing interest
No interests or activities of the authors are considered as having potentially influenced the research.
Acknowledgments
Acknowledgements
We would like to thank all the participants who gave their time and information to this study. Thank you also to the research staff at Haukeland University Hospital, especially to Lena Stabell who has supported the quality assurance of our data throughout. Last, our gratitude to the early intervention team in Stavanger and other clinicians at the recruitment sites who have supported recruitment and follow-up.
Funding
The study fully publicly funded, by the Research Council of Norway (RCN #213727), the Western Norway Regional Health Trust (#911820 and #911679), and participating hospitals and universities.
References
- Anda L., Brønnick K.S., Johnsen E., Kroken R.A., Jørgensen H., Løberg E.-M. The course of neurocognitive changes in acute psychosis: relation to symptomatic improvement. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda L., Brønnick K.K., Johannessen J.O., Joa I., Kroken R.A., Johnsen E., Rettenbacher M., Fathian F., Løberg E.-M. Cognitive profile in ultra high risk for psychosis and schizophrenia: a comparison using coordinated norms. Front. Psych. 2019;10(695) doi: 10.3389/fpsyt.2019.00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall A.-M., Jones L., Ginnelly L., Lewis R., Glanville J., Gilbody S., Davies L., Torgerson D., Kleijnen J. A systematic review of atypical antipsychotic drugs in schizophrenia, NIHR Health technology assessment programme: executive summaries. NIHR J. Libr. 2003;7(13):1–193. doi: 10.3310/hta7130. [DOI] [PubMed] [Google Scholar]
- Baldez D.P., Biazus T.B., Rabelo-da-Ponte F.D., Nogaro G.P., Martins D.S., Kunz M., Czepielewski L.S. The effect of antipsychotics on the cognitive performance of individuals with psychotic disorders: network meta-analyses of randomized controlled trials. Neurosci. Biobehav. Rev. 2021;126:265–275. doi: 10.1016/j.neubiorev.2021.03.028. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn. Sci. 2012;16(1):27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes T.R.E., Drake R., Paton C., Cooper S.J., Deakin B., Ferrier I.N., Gregory C.J., Haddad P.M., Howes O.D., Jones I., Joyce E.M., Lewis S., Lingford-Hughes A., MacCabe J.H., Owens D.C., Patel M.X., Sinclair J.M.A., Stone J.M., Talbot P.S., Upthegrove R., Wieck A., Yung A.R. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2019;30(6) doi: 10.1177/0269881119889296. 495-553. [DOI] [PubMed] [Google Scholar]
- Benedict R.H., Schretlen D., Groninger L., Brandt J. Hopkins verbal learning test–revised: normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- Berman A.H., Palmstierna T., Källmén H., Bergman H. The self-report drug use disorders identification test—extended (DUDIT-E): reliability, validity, and motivational index. J. Subst. Abus. Treat. 2007;32(4):357–369. doi: 10.1016/j.jsat.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Bortolato B., Miskowiak K.W., Köhler C.A., Vieta E., Carvalho A.F. Cognitive dysfunction in bipolar disorder and schizophrenia: a systematic review of meta-analyses. Neuropsychiatr. Dis. Treat. 2015;11:3111–3125. doi: 10.2147/NDT.S76700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch. Intern. Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Chew M.L., Mulsant B.H., Pollock B.G., Lehman M.E., Greenspan A., Kirshner M.A., Bies R.R., Kapur S., Gharabawi G. A model of anticholinergic activity of atypical antipsychotic medications. Schizophr. Res. 2006;88(1):63–72. doi: 10.1016/j.schres.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Davidson M., Galderisi S., Weiser M., Werbeloff N., Fleischhacker W.W., Keefe R.S.E., Boter H., Keet I.P., Prelipceanu D., Rybakowski J.K. Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST) Am. J. Psychiatr. 2009;166(6):675–682. doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- Delis D., Kramer J.H., Kaplan E., Holdnack J. Reliability and validity of the Delis-Kaplan executive function system: an update. J. Int. Neuropsychol. Soc. 2004;10(2):301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Désaméricq G., Schurhoff F., Meary A., Szöke A., Macquin-Mavier I., Bachoud-Lévi A., Maison P. Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur. J. Clin. Pharmacol. 2014;70(2):127–134. doi: 10.1007/s00228-013-1600-y. [DOI] [PubMed] [Google Scholar]
- Gibbon M., Spitzer R.L., Williams J.B., Benjamin L.S., First M.B. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II) Am Psych Pub. 1997 [Google Scholar]
- Giraldo-Chica M., Rogers B.P., Damon S.M., Landman B.A., Woodward N.D. Prefrontal-thalamic anatomical connectivity and executive cognitive function in schizophrenia. Biol. Psychiatry. 2018;83(6):509–517. doi: 10.1016/j.biopsych.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Grant I., Matthews C.G. Comprehensive norms for an expanded Halstead-Reitan battery: demographic corrections, research findings, and clinical applications; with a supplement for the Wechsler Adult Intelligence Scale-Revised (WAIS-R) Psychol. Assess. Resour. 1991 [Google Scholar]
- Howes O.D., Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr. Bull. 2009;35(3):549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O.D., Nour M.M. Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry. 2016;15(1):3–4. doi: 10.1002/wps.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsaraie R.J., Sheffield J.M., Barch D.M. Neural correlates of global and specific cognitive deficits in schizophrenia. Schizophr. Res. 2018;201:237–242. doi: 10.1016/j.schres.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen E., Jørgensen H.A., Kroken R.A., Løberg E.M. Neurocognitive effectiveness of quetiapine, olanzapine, risperidone, and ziprasidone: a pragmatic, randomized trial. Eur. Psychiatry. 2013;28(3):174–184. doi: 10.1016/j.eurpsy.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Johnsen E., Kroken R.A., Løberg E.-M., Rettenbacher M., Joa I., Larsen T.K., Reitan S.K., Walla B., Alisauskiene R., Anda L.G. Amisulpride, aripiprazole, and olanzapine in patients with schizophrenia-spectrum disorders (BeSt InTro): a pragmatic, rater-blind, semi-randomised trial. Lancet Psychiatry. 2020;7(11):945–954. doi: 10.1016/S2215-0366(20)30341-2. [DOI] [PubMed] [Google Scholar]
- Kahn R.S., Keefe R.S.E. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiat. 2013;70(10):1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe R.S.E., Bilder R.M., Davis S.M. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch. Gen. Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Keefe R.S.E., Sweeney J.A., Gu H., Hamer R.M., Perkins D.O., McEvoy J.P., Lieberman J.A. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am. J. Psychiatr. 2007;164(7):1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- Leucht S., Cipriani A., Spineli L., Mavridis D., Örey D., Richter F., Samara M., Barbui C., Engel R.R., Geddes J.R., Kissling W., Stapf M.P., Lässig B., Salanti G., Davis J.M. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- Li Z., Ichikawa J., Dai J., Meltzer H.Y. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur. J. Pharmacol. 2004;493(1–3):75–83. doi: 10.1016/j.ejphar.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Malla A., Norman R., Scholten D., Townsend L., Manchanda R., Takhar J., Haricharan R. A comparison of two novel antipsychotics in first episode non-affective psychosis: one-year outcome on symptoms, motor side effects and cognition. Psychiatry Res. 2004;129(2):159–169. doi: 10.1016/j.psychres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Meltzer H.Y., McGurk S.R. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr. Bull. 1999;25(2):233–256. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Sharma T., Mockler D. The cognitive efficacy of atypical antipsychotics in schizophrenia. J. Clin. Psychopharmacol. 1998;18(2):12S–19S. doi: 10.1097/00004714-199804001-00004. [DOI] [PubMed] [Google Scholar]
- NICE . National Collaborating Centre for Mental Health; 2014. Psychosis and Schizophrenia in Adults. [Google Scholar]
- Nielsen R.E., Levander S., Kjaersdam Telléus G., Jensen S.O.W., Østergaard Christensen T., Leucht S. Second-generation antipsychotic effect on cognition in patients with schizophrenia—a meta-analysis of randomized clinical trials. Acta Psychiatr. Scand. 2015;131(3):185–196. doi: 10.1111/acps.12374. [DOI] [PubMed] [Google Scholar]
- Norwegian Directorate of Health . In: Utredning, behandling og oppfølging av personer med psykoselidelser. Health N.D.o., editor. Norwegian Directorate of Health; Oslo: 2013. [Google Scholar]
- Pedersen G., Hagtvet K.A., Karterud S. Generalizability studies of the global assessment of functioning–split version. Compr. Psychiatry. 2007;48(1):88–94. doi: 10.1016/j.comppsych.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Reitan R.M. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Skills. 1958;8(3):271–276. [Google Scholar]
- Riedel M., Spellmann I., Schennach-Wolff R., Musil R., Dehning S., Cerovecki A., Opgen-Rhein M., Matz J., Seemüller F., Obermeier M., Severus E., Engel R.R., Müller N., Möller H.J. Effect of aripiprazole on cognition in the treatment of patients with schizophrenia. Pharmacopsychiatry. 2010;43(02):50–57. doi: 10.1055/s-0029-1239539. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Toscano E., López G., Mayoral M., Lewis S., Lees J., Drake R., Arango C., Rapado-Castro M. A longitudinal comparison of two neurocognitive test batteries in patients with schizophrenia and healthy volunteers: time effects on neuropsychological performance and their relation to functional outcome. Schizophr. Res. 2019;216:347–356. doi: 10.1016/j.schres.2019.11.018. [DOI] [PubMed] [Google Scholar]
- Santesteban-Echarri O., Paino M., Rice S., González-Blanch C., McGorry P., Gleeson J., Alvarez-Jimenez M. Predictors of functional recovery in first-episode psychosis: a systematic review and meta-analysis of longitudinal studies. Clin. Psychol. Rev. 2017;58:59–75. doi: 10.1016/j.cpr.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Statistisk Sentralbyrå . 2019. 09817: Innvandrere og norskfødte med innvandrerforeldre, etter landbakgrunn, år, statistikkvariabel, innvandringskategori og region - for Stavanger og Bergen og Trondheim. [Google Scholar]
- Steen N.E., Aas M., Simonsen C., Dieset I., Tesli M., Nerhus M., Gardsjord E., Mørch R., Agartz I., Melle I., Ueland T., Spigset O., Andreassen O.A. Serum levels of second-generation antipsychotics are associated with cognitive function in psychotic disorders. World J. Biol. Psychiatry. 2017;18(6):471–482. doi: 10.1080/15622975.2016.1245441. [DOI] [PubMed] [Google Scholar]
- Tyson P.J., Laws K.R., Flowers K.A., Tyson A., Mortimer A.M. Cognitive function and social abilities in patients with schizophrenia: relationship with atypical antipsychotics. Psychiatry Clin. Neurosci. 2006;60(4):473–479. doi: 10.1111/j.1440-1819.2006.01534.x. [DOI] [PubMed] [Google Scholar]
- Van Ruitenbeek P., Vermeeren A., Riedel W. Histamine H1 receptor antagonist cetirizine impairs working memory processing speed, but not episodic memory. Br. J. Pharmacol. 2010;161(2):456–466. doi: 10.1111/j.1476-5381.2010.00907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D.E., Roberson A.J., McGuinness T.M., Fazeli P.L. How neuroplasticity and cognitive reserve protect cognitive functioning. J. Psychosoc. Nurs. Ment. Health Serv. 2010;48(4):23–30. doi: 10.3928/02793695-20100302-01. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1997. Wechsler adult intelligence scale-III. [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2009. ICD 10: International Statistical Classification of Diseases and Related Health Problems. [Google Scholar]