Abstract
Regulation of VDAC by α-Synuclein (αSyn) is a rich and instructive example of protein-protein interactions catalyzed by a lipid membrane surface. αSyn, a peripheral membrane protein involved in Parkinson’s disease pathology, is known to bind to membranes in a transient manner. αSyn’s negatively charged C-terminal domain is then available to be electromechanically trapped by the VDAC β-barrel, a process that is observed in vitro as the reversible reduction of ion flow through a single voltage-biased VDAC nanopore. Binding of αSyn to the lipid bilayer is a prerequisite of the channel-protein interaction; surprisingly, however, we find that the strength of αSyn binding to the membrane does not correlate in any simple way with its efficiency of blocking VDAC, suggesting that the lipid-dependent conformations of the membrane-bound αSyn control the interaction. Quantitative models of the free energy landscape governing the capture and release processes allow us to discriminate between several αSyn (sub-) conformations on the membrane surface. These results, combined with known structural features of αSyn on anionic lipid membranes, point to a model in which the lipid composition determines the fraction of αSyn molecules for which the charged C terminal domain is constrained to be close, but not tightly bound, to the membrane surface and thus readily captured by VDAC nanopore. We speculate that changes in the mitochondrial membrane lipid composition may be key regulators of the αSyn-VDAC interaction and consequently of VDAC-facilitated transport of ions and metabolites in and out of mitochondria and, therefore, mitochondrial metabolism.
Keywords: voltage-dependent anion channel, protein-lipid interaction, single-molecule measurement, energy landscape, mitochondrial lipids, a-hemolysin
Graphical Abstract

1. Introduction
The voltage-dependent anion channel (VDAC) is the most abundant protein at the mitochondrial outer membrane (MOM). Oxidative phosphorylation—the main function of mitochondria—requires efficient exchange of respiratory substrates, such as ATP, ADP, pyruvate, succinate, and inorganic phosphate, between mitochondria and the cytosol. The large share of the MOM’s permeation functions is executed by VDAC, which conducts and regulates fluxes of water-soluble metabolites and small ions in and out of mitochondria. It is VDAC’s unique location at the interface between mitochondria and the cytosol that ensures its central role in bioenergetics and cell metabolism by enabling its interaction with cytosolic proteins involved in multiple signaling and metabolic pathways [1, 2]. Indeed, VDAC was found associated with a plethora of different pro-survival and pro-apoptotic proteins, endogenous and synthetic steroids, and anti-cancer and neuroprotective drugs (for review see e.g., [3–5]) and was consequently reported to be implicated in various diseases from cancer to neurodegeneration [6, 7].
Despite its impressive multifunctionality, VDAC is relatively uncomplicated. It is a monomeric channel comprising 19 β-strands that define a 2.7 nm diameter pore. A broken α-helix that lies along one side of the channel wall, approximately equidistant from the two ends of the pore, reinforces the β-barrel [8] and creates a constriction zone of about 1.4 nm in the narrowest part [9–11] (Figure 1). There is some evidence that this N-terminal α-helix takes part in the gating process [12–15]. VDAC is a passive diffusion channel that allows the passage of non-charged polymers up to 2 – 5 kDa [16, 17] and disordered polypeptide chains [18]. When reconstituted into planar lipid membranes—so far the only available method to study this channel’s biophysical properties—VDAC forms large, weakly anion-selective channels with a conductivity of about 4 nS in 1 M KCl (M = mol/L). In 150 mM KCl, the conductance is about 0.7 nS [19] and the permeability ratio Cl−/K+ ~ 3 [16, 20, 21]. VDAC’s anion selectivity matches the negative charge of most mitochondrial metabolites, such as ATP and ADP.
Figure 1.
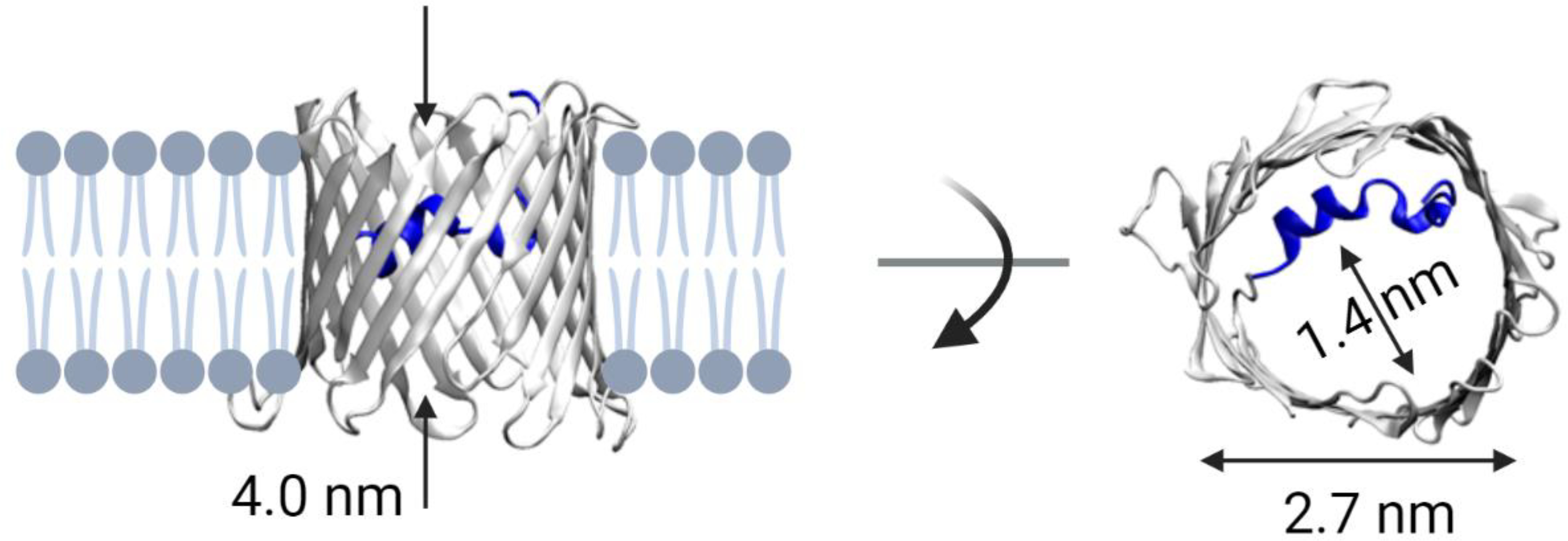
Structure of VDAC. Side and top view of mouse VDAC1 (mVDAC1, PDB ID: 3EMN). The elongated pore constriction is formed by the N-terminal α-helix (shown in dark blue) near the pore center. Created with Biorender.com.
VDAC’s name derives from its characteristic voltage gating behavior. Unlike VDAC’s structure, voltage gating is complicated, and, despite being known since 1976 when this mitochondrial channel was biophysically characterized first by Marco Colombini [22–24] and then by others [25–29], is still is not explained by a quantitative and comprehensive model (see, e.g. discussion in [30–32]. Gating, by definition, is VDAC’s ability to stochastically transition under transmembrane potentials of > 30 mV from a unique anion-selective high-conducting “open” state to a variety of less anion-selective, low-conducting “closed” states. Importantly, the open state is open for ATP transport and the closed states are essentially impermeable to ATP [33, 34], mostly due to the reduced anion selectivity of the closed states. The closed states still conduct small ions and appear to be significantly more permeable for calcium ions than for chloride ions [35]. These distinctive biophysical properties of VDAC’s different states suggest that by gating VDAC could regulate fluxes of metabolites and calcium in vivo [36]. Its open state facilitates ADP uptake and keeps calcium influx low, thus maintaining normal respiration, while its closedss states reduce metabolite transport but increase calcium uptake leading to respiration impairment. Therefore, VDAC gating, though observed so far only on reconstituted channels, is likely to be one of the mechanisms that control MOM permeability in cells [1, 33, 35–38]. The sensitivity of VDAC gating to the lipid environment is particularly intriguing [39–41] but remains poorly understood. Progress in computational methods and high-resolution spectroscopy may provide additional insight in the near future. Regardless, VDAC gating as a mechanism to control mitochondrial metabolism in vivo meets a serious obstacle: the uncertainty concerning the real value of the membrane potential across MOM (e.g., see discussions in [1, 37, 42])..
Considering all the above, the search for VDAC regulation then expands to include its interactions with cytosolic proteins. Of these, dimeric tubulin and alpha-synuclein (αSyn) have emerged as potent VDAC regulators [18, 43–47] due to their cytosolic abundance and the efficiency of their interactions with VDAC in vitro and in vivo. Here we focus on αSyn and discuss the possible mechanisms by which mitochondria may tune the efficiency of such regulation.
αSyn is an intrinsically disordered neuronal protein and a well-known pathological hallmark of Parkinson’s disease (PD) [48]. It was initially found in the fibrillar form in the so-called Lewy bodies of the post-mortem brains of PD patients [49]. In normal neurons, αSyn constitutes up to 1 % of the total cytosolic protein content [50] and exists mainly as monomers, not as the fibrillar form associated with pathology (Fig. 2A). While fibrillar forms of αSyn are undoubtedly linked to PD or other synucleinopathies, the physiological role of its monomeric form remains poorly understood. In mitochondria, αSyn in oligomeric and monomeric forms is found in association with both the inner and outer membranes [45, 51–53], causing typical mitochondrial dysfunctions, such as oxidative stress [54], impairment of the respiratory complexes [53, 55–57], or fission [58]. All these studies showed that when the αSyn expression level in a cell increases, αSyn enters the mitochondria and targets respiratory complexes at the mitochondrial inner membrane (MIM), inducing mitochondrial dysfunction. However, the molecular identity of the pathway for αSyn to cross the MOM has long been a mystery. Initially, Tom40, the channel component of the translocase of the outer membrane (TOM complex), was suggested as a possible candidate for such pathway [56]. Later, our group unambiguously proved that VDAC is indeed a gateway for αSyn entry into mitochondria. Using electrophysiological single-channel experiments with VDAC reconstituted into planar lipid membranes, we showed that αSyn effectively and reversibly blocks the VDAC pore at nanomolar concentrations; under some conditions, determined by the applied potential, lipid composition, or ionic strength of membrane-bathing solution, αSyn translocates through VDAC [18, 59, 60]. These results will be detailed throughout this review. By contrast, aSyn does not measurably affect the conductance of Tom40 reconstituted into planar membranes [61]. Further, using neuronally differentiated human cells overexpressing wild-type αSyn as an adequate cell model of PD, we showed that αSyn translocates into mitochondria, causing the loss of mitochondrial potential and consequent cell death, and that the VDAC1 isoform is required for αSyn translocation [45].
Figure 2.
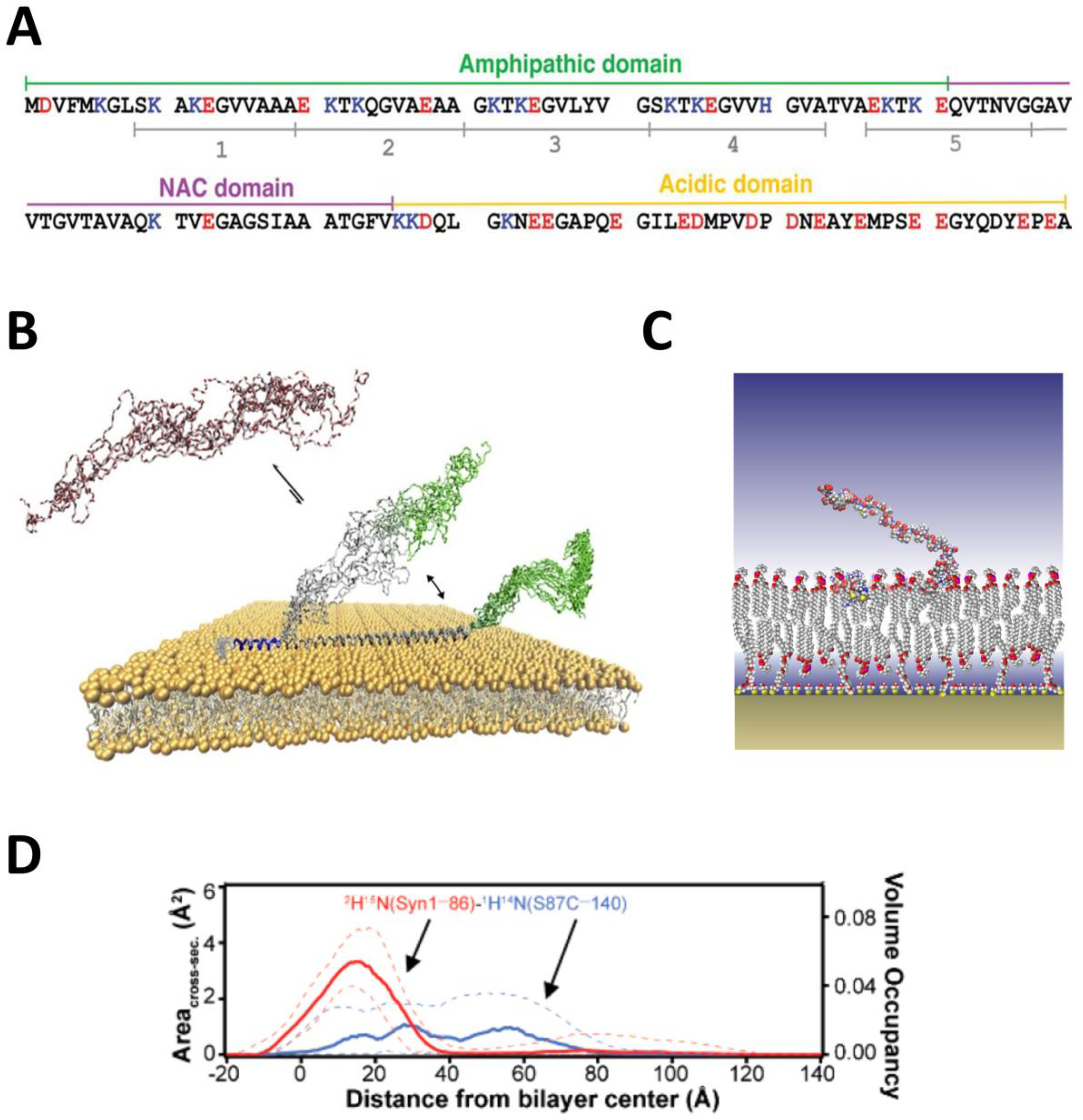
Membrane-bound structure of αSyn. (A) Amino acid sequence of αSyn. Adapted with permission from Robyn Croke et al, In: “Protein Science”, Wiley Online Library. V. 20 (2), pp 256–269, Copyright © 2010 The Protein Society. The N-terminal region responsible for membrane interaction comprises both the amphipathic and the central (NAC) domains. The polyanionic C-terminal tail is responsible for interaction with the VDAC nanopore. (B) Solution and solid-state NMR derived structure of αSyn on anionic bilayers showing three distinct regions: a persistent helical region (blue); a transient binding domain (gray), which is helical when bound and disordered when unbound; and the C-terminal tail (green), which is only weakly associated with the lipid surface. Adapted with permission from Fusco et al, Nat. Commun. (2014). Copyright 2017 Elsevier. (C) Low-resolution structure of αSyn embedded in POPC/POPA bilayers. (D) Volume occupancy and cross-section area of the deuterated residues 1−86 (red) and protiated residues 87−140 (blue). Segmental deuteration, in combination with contrast variation, provides a domain-level view of the protein-lipid complex. Dashed lines show 68% confidence intervals. Adapted with permission from Jiang et al, J. Phys. Chem. Lett. (2017). Copyright © 2016 American Chemical Society.
The immediate question is what are the endogenous factors that regulate the discovered αSyn-VDAC interaction in vivo? One would expect that the αSyn expression level [45, 56], its disease-related mutations [62], post-translational modifications (PTM) [63, 64], and different cytosolic factors such as pH [65] could affect this process. Here we address the question of how αSyn-VDAC interaction could be regulated by mitochondria. Leaving aside the role of transmembrane voltage briefly discussed above, we focus instead on the less obvious but no less important role of mitochondrial lipids in αSyn-VDAC interaction. We will show that mitochondria lipids turn out to be one of the tools for mitochondria to fulfill this task.
Mitochondrial lipids of both outer and inner membranes play important structural and functional roles in maintaining mitochondrial biogenesis. Changes in the phospholipid composition affect mitochondrial functions and dynamics and have been linked to a variety of human diseases such as Barth syndrome [66], heart failure [67], neurodegenerative diseases [68], and cancer [69]. Considering the substantial lipid homeostasis in mitochondria during morphological changes such as fusion and fission [70], interorganellar interaction through mitochondria-associated membranes [71], apoptotic stress [72], or lipid oxidation by the reactive oxygen species produced by mitochondria [73–75], lipids could be potent regulators of αSyn-VDAC interaction, thus controlling VDAC permeability in vivo.
In this review, we will show that single-molecule electrophysiology, using VDAC reconstituted into lipid bilayer membranes mimicking the mammalian MOM composition, reveals a strong dependence of the in vitro interaction of VDAC with αSyn on the detailed chemical composition of the lipid bilayer. We complement known structural properties of αSyn on lipid membranes with single-molecule interrogation using nanopores; this combination allows us to identify structural details of the distributions of conformations adopted by αSyn on lipid surfaces.
Throughout the discussion, VDAC will play a dual role. VDAC is a mitochondrial channel, a tunable transporter of polyvalent metabolites; as such, a target for αSyn. VDAC is also a nanopore, a passive ionic conductor, a source of electromechanical force, and a reporter on the presence and charge composition of polypeptide chains it is able to capture; as such, VDAC will be a probe for the αSyn presence and conformation at the lipid membrane. It is our contention that at the intersection of these dual roles—target vs. nanoprobe sensor—we gain the most insight into the biophysical chemistry of the αSyn-VDAC interaction, as catalyzed by the membrane surface. In other words, the use of VDAC, the very mitochondrial protein targeted by αSyn, as a nanopore probe provides unique mechanistic details of their interaction.
In Section 2, we will first briefly discuss the structure of αSyn on membrane surfaces and the role of lipid composition. Section 3 will introduce the principles of using a membrane-embedded nanopore as a local single-molecule probe for αSyn. Section 4 will detail the observations regarding the role of lipids in the interaction of aSyn with VDAC. Section 5 will relate these findings to the structural data reviewed in Section 2. Immediate physiological implications of the aSyn -VDAC interaction will be discussed in Section 6. Using a different, asymmetric nanopore of α-hemolysin, in Section 7 we will show that the nanopore-captured aSyn molecules are predominantly bound to membrane surfaces and then discuss the differences between the results obtained with this more traditional protein nanopore and VDAC.
2. Structure of membrane-bound aSyn
The structure of αSyn on lipid membranes is of significant contemporary interest, primarily due to the interplay between αSyn lipid membrane binding and its aggregate formation [76–80]. αSyn is disordered in solution but does have three distinct regions that respond differently to membrane surfaces and other αSyn molecules (Figure 2A) [81–84]. The amphipathic region is the membrane-binding domain, wheares the central nonpolar or so called NAC (non-amyloid b component) domain is involved in fibril formation [85]. While the first two regions obtain structural features, the polyanionic C-terminal tail (CTT) remains disordered.
Early NMR studies showed [86] that the membrane-binding region of αSyn adopts a broken amphipathic helical conformation when associated with anionic SDS micelles. There were also early hints pointing to conformational variability, as small populations that deviated from the majority conformation were also observed. Later NMR results [81] obtained with anionic small unilamellar vesicles also identified an extended amphipathic binding motif in the αSyn N-terminal domain (Figure 2B). The binding domain comprises two helical regions, one of which remains consistently membrane-associated. The other spans part of both the amphipathic and NAC domains and is thought to be in dynamic equilibrium between the helical, membrane-associated (“proximal”) and disordered, membrane-dissociated (“distal”) states (Figure 2B) [81]. In crosslinked mutants, the membrane binding energy differences among various conformers of αSyn on anionic lipid surfaces were shown to be minimal, suggesting that a variety of binding conformations are likely to be populated even on anionic surfaces [87].
Neutron reflectivity data with segmentally deuterated αSyn confirmed that on anionic lipid surfaces the membrane-binding region of αSyn resides at the interface between the lipid acyl chains and the headgroups, while the CTT is found above the lipid membrane (Figure 2C) [88, 89]. Complementary Trp fluorescence results showed that, when associated with these lipid surfaces, residue 94 appears to be held deeply buried into the lipid acyl chains [90], consistent with the results of Fusco et al. [81] for the membrane-associated state of the entire N-terminal domain.
The structure of αSyn on uncharged or weakly charged membranes is less extensively characterized. Circular dichroism reveals that αSyn remains unstructured in the presence of phosphatidylcholine (PC) vesicles [79]. αSyn does bind to PC lipids, however; high concentrations of αSyn can also cause tubulation of PC membranes [79], while binding is observed by Fluorescent Correlation Spectroscopy (FCS) and isothermal titration calorimetry [87, 91, 92].
3. Nanopore probes for surface-bound proteins
The use of nanopores—single perforations in a thin membrane that physically and electrically isolates two electrolyte-filled reservoirs—for single-molecule or single-particle analysis now has several decades of history. The principle is the same as that of a Coulter counter [93, 94]: the time-dependent modulation in ion transport through a voltage-biased nanopore, as revealed by the measured ionic current, reports on the dynamics of the internal state of the channel. Nanopores are effective in a number of applications, including measurement of chemical kinetics [95, 96], nucleic acid sequencing [97, 98], and more recently for protein sequencing [99], sensing of protein-protein interactions [100], detection of post-translational modifications [101], protein translocation [102, 103], and quantification of protein-lipid binding conformation and energetics [104].
In principle any channel is suitable as a nanopore, provided its internal dynamics can be separated from those that are caused by its interactions with analytes [105]. VDAC is one such channel; while its name derives from its characteristic gating response to voltage, under appropriate experimental conditions such as lipid composition [19, 25, 106], ionic strength [19, 25], or pH [107, 108], VDAC spends most of its time as a quiescent, passive transport channel.
Figure 3A shows a schematic of the sensing process of αSyn with a generic nanopore. Under an applied transmembrane potential, an ionic current is set up through the nanopore. When a charged polyelectrolyte, such as a nucleic acid or polypeptide, diffuses near the pore opening, it can be captured in the electric field and inserts into the channel. This process causes a modulation (reduction or increase, depending on the details of the bathing electrolyte and the captured molecule itself [109, 110]) of the ionic current through the nanopore (Figure 3B). After some time, the captured molecule leaves the nanopore, and the ionic current is restored to its original, analyte-free value. The entire process, known colloquially as an “event”, encodes information about the interaction of a single polyelectrolyte with a single pore. The event-specific observables are (1) the time elapsed before the event ton, (2) the duration toff, and, with sufficiently low noise at the required time resolution, (3) the time-dependent details of the ionic current modulation, ΔI(t), during the event. When many events are observed for the same nominal analyte-nanopore interaction, additional observables unique to single-molecule detection are in play, including detailed statistical analysis of the events and their correlations in time. Two of these multi-event observables are the mean time between events, τon =< ton >, and the average event duration, τoff =< toff >, where the brackets denote averages.
Figure 3.
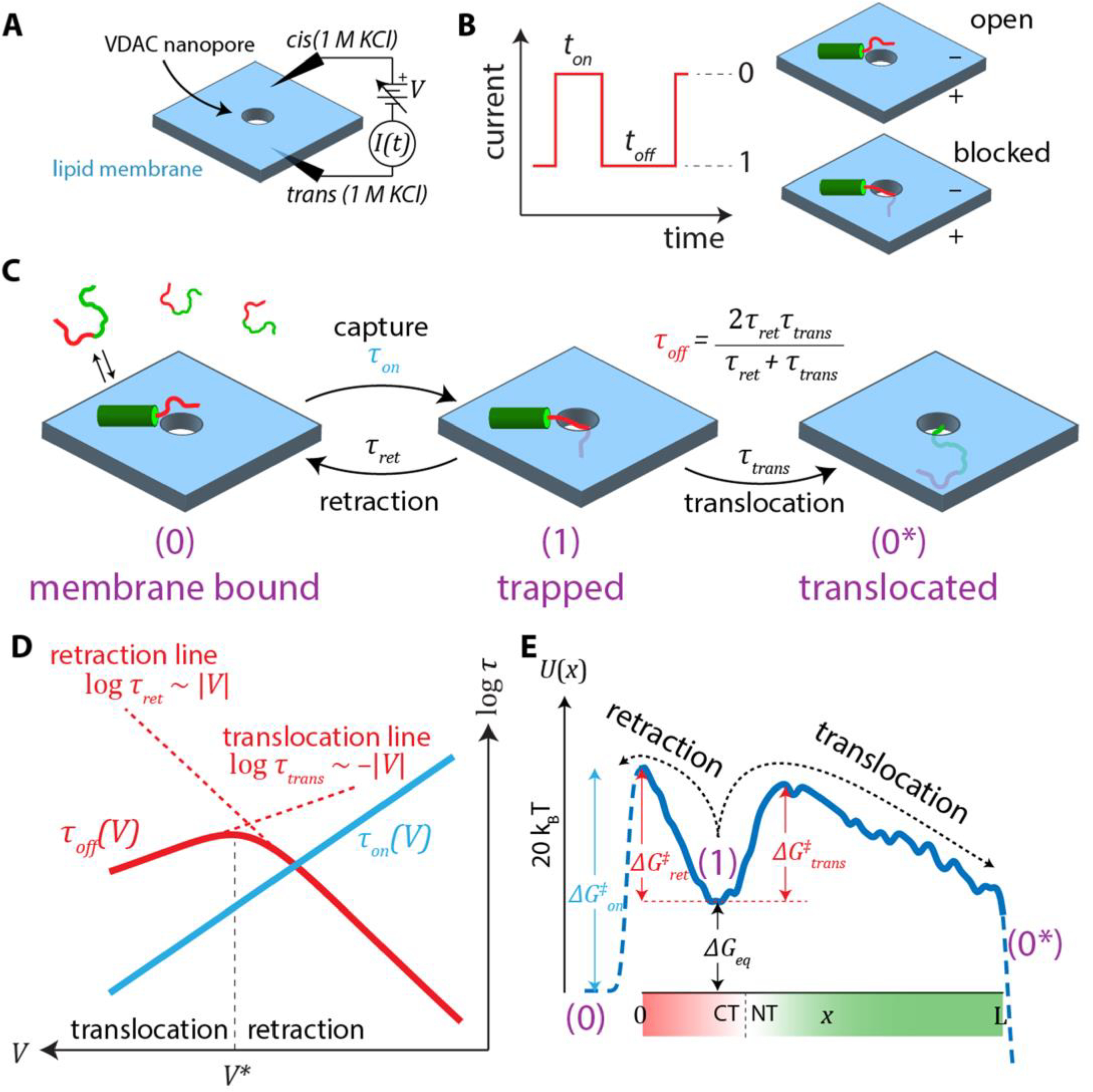
Nanopore-based analysis of surface-bound αSyn. (A) Schematic of a generic nanopore measurement. (B) An applied voltage produces an ionic current through the nanopore. If an analyte is drawn into the nanopore, it causes transient current blockages, or “events”, that are characterized by their onset time, ton, and duration, toff. (C) Reaction scheme of the nanopore-αSyn interaction. Once captured, the αSyn leaves the nanopore either by retraction or translocation, thus restoring the ionic current to its unblocked level. (D) Typical voltage dependences of the average rates. The average event duration, τoff, has a biphasic dependence on voltage that indicates the transition from primarily retraction events to primarily translocation events. (E) Free energy landscape governing the reaction scheme in (C). The “reaction coordinate” x denotes the length of αSyn that has threaded into the VDAC nanopore past the constriction. The trapped state (1) is a metastable state that is an electromechanical trap formed by the opposite action of the electric field on the charged C-terminal domain (CT; shown in red) and the N-terminal domain (NT; shown in green) lipid-associated anchor.
In single-channel experiments with VDAC reconstituted into a planar bilayer, nanomolar concentrations of αSyn added to either side of the bilayer induce characteristic blockage events, detectable only when a negative potential is applied to the side of addition [18] (Figure 4A). This observation and the fact that the rate of blockage events by modified αSyn with a half-truncated C-terminal domain is reduced by orders of magnitude in comparison with the wild-type, allowed us to suggest that the polyanionic 45-residue CTT of αSyn is the VDAC pore blocking domain [18].
Figure 4.
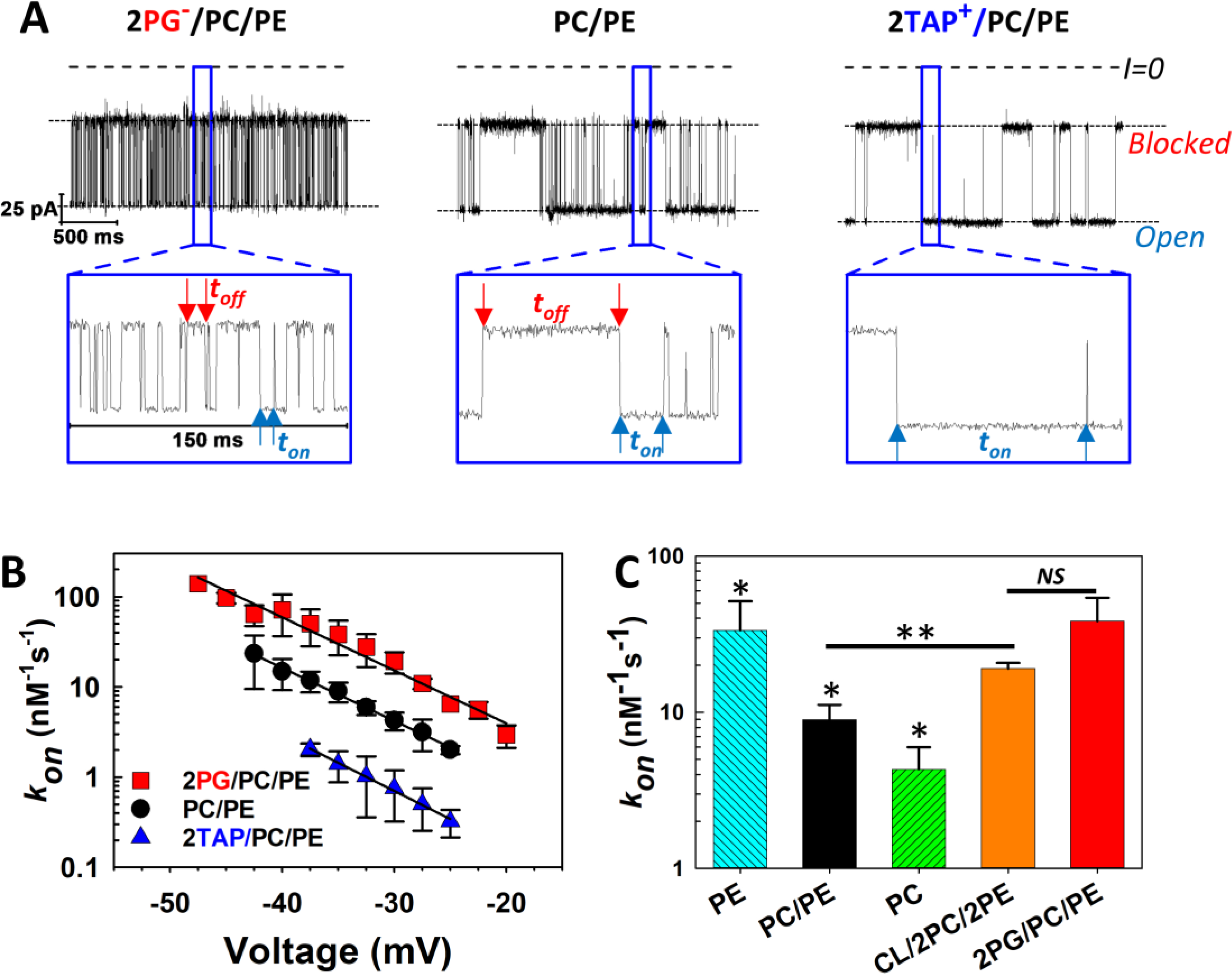
The kinetics of αSyn blockage of VDAC strongly depend on membrane lipid composition. (A) Records of ion currents through single VDAC1 channels reconstituted into planar bilayers formed from DOPG:DOPC:DOPE (2:1:1, mol:mol) (2PG/PC/PE) (left trace), DOPC:DOPE (1:1, mol:mol) (PC/PE) (middle traces), and DOTAP:DOPC:DOPE (2:1:1, mol:mol) (2TAP/PC/PE) (right trace) obtained at −35 mV applied voltage. Individual, time-resolved blockage events can be seen in the insets, which show fragments of current records at a finer time scale. Horizontal dotted lines indicate VDAC open and blocked states; dashed lines indicate zero current. Blue arrows indicate durations of the open state, ton, and red arrows show blocked state durations, toff. Both parameters of blockage events visibly depend on lipid composition. Current traces were digitally filtered using a 5 kHz lowpass Bessel filter for presentation. (B) Voltage dependences of the rate of capture, kon, obtained in three lipid compositions. The kon of the αSyn-VDAC interaction increases in the presence of anionic and nonlamellar lipids. Error bars show 68% confidence intervals. (C) Summary of results for kon obtained in DOPE (PE), PC/PE, DOPC (PC), Cardiolipin:DOPC:DOPE (1:2:2) (CL/2PC/2PE), and 2PG/PC/PE membranes at −35 mV applied voltage. All data were obtained in the presence of 10 nM of αSyn in the cis compartment in 1 M KCl at pH 7.4. Error bars show 68% confidence intervals. Adapted with permission from Jacobs et al. Sci. Reports (2019). Copyright © 2019 Springer Nature.
The results obtained with αSyn-VDAC interactions have led to a 3-step model (Figure 3C). In the first step, αSyn binds to the lipid membrane by its N-terminal domain [111]. In the second step, the polyanionic C-terminal domain, driven by the negative potential, is captured in the pore; the continued motion of αSyn is arrested by the N-terminal anchor and the αSyn molecule is electromechanically trapped. The third step is release of the C-terminal domain from the pore, either by the thermal motion-driven retraction from the nanopore or translocation of the whole αSyn molecule through the pore following unbinding of the N-terminal anchor from the membrane [18, 47]. The retraction process is characterized by an exponential increase of blockage time with voltage [18] (Figure 3D). Above a critical voltage—the “turnover” potential V*— the blockage time decreases with voltage. Direct measurements of the translocation probability (Figure 5) show that in this regime nearly all αSyn molecules translocate [18, 60].
Figure 5.

Determination of the relationship between the biphasic voltage dependence of τoff and the translocation probability. (A) The fraction of αSyn molecules that translocate through a VDAC pore transitions sharply from zero to unity near the turnover voltage V∗, where (B) the voltage-dependence of τoff reverses slope. The solid and dashed lines in (B) are the median and 95% confidence interval, respectively, of an energy landscape model optimized to the observed τoff; this model accurately predicts the translocation probability and its 95% confidence interval (solid and dashed lines, respectively) in (A). Error bars are 68% confidence intervals calculated using bootstrap resampling. Adapted with permission from Hoogerheide et al. Biophys. J. (2018). Copyright © 2018, Elsevier.
Figure 5 demonstrates that the processes depicted in Figure 3C can be quantitatively described by a free energy profile of the reaction [112]. An example of a free energy profile is shown in Figure 3E. The following subsections will discuss the two regions of the free energy profile governing capture (dashed lines on the lefts in Figure 3E) and release (solid line), and what can be learned from experimental observations of each. It is important to note that the essential features of the VDAC-αSyn interaction, such as the voltage-dependence of blockage durations and probability of αSyn translocation, can be described by a free energy profile incorporating the physical properties of the αSyn molecule, rather than those of VDAC [60, 101]. This constitutes conclusive evidence that the observed interaction mechanism is voltage-induced insertion of αSyn into the VDAC nanopore, rather than αSyn-induced gating of the VDAC channel.
3.1. Capture kinetics (the “on-rate”)
The rate of capture of molecules into a nanopore, , reveals important information about the underlying physical interaction. Indeed, in a diffusion-limited case, the nanopore captures with almost total efficiency each molecule that arrives in its vicinity. In this case one expects a weak dependence on the transmembrane potential. In a barrier-limited case, in which capture is inefficient due to the presence of an energetic barrier (e.g. the entropic cost of confinement), the capture rate is likely to depend exponentially on the applied transmembrane potential [113]. This is the regime that applies to the VDAC-αSyn interaction (Figure 3D, blue line). Using a Kramers-type formalism [114], the capture rate can be written:
| (1) |
Here, ω is a collision rate between αSyn and the nanopore that is usually proportional to the relevant concentration (surface or solution), and the exponential factor represents a success rate based on the height of the energy barrier to pore entry by the molecule. This energy barrier height has multiple contributions, e.g.
| (2) |
Here the first term represents the effect of the fraction of the transmembrane potential that extends beyond the nanopore into the bulk and can be characterized by an effective charge qon of the molecule; ΔGconfinement is the entropic cost to confine the molecule in the pore; and 𝛥Gconformation represents any energy required to rearrange the molecular conformation before capture by the pore. This region of the free energy profile, shown as a dashed line on the left in Figure 3E, can be directly probed with a nanopore only in very special circumstances [115], because in general the nanopore does not interrogate the αSyn molecule until it is already captured in the nanopore.
In sum, a panoply of effects can influence kon. The mentioned contributions to the exponent argument (Eq. 2) have the largest impact, though kon is also proportional to the concentration of the analyte. Note that the capture can occur either from solution (as is typically the case for nucleic acids), or from the surface of the membrane. In Section 7 of this review, we will show that α-hemolysin—a different, pronouncedly asymmetric β-barrel nanopore—reveals that more than 99.9 % of the αSyn molecules captured by the membrane-embedded nanopores are bound to the lipid surface. We will also see that the profound lipid dependence of the free energy profile further supports this conclusion and demonstrates that kon is sensitive not only to the total amount of membrane-bound αSyn, but also to the conformations αSyn adopts on the membrane surface.
3.2. Release kinetics (the “off-rate”)
Compared to the on-rate, the physics of the off-rate distribution of a polyelectrolyte has a more quantifiable theoretical basis and can be directly interrogated by the VDAC nanopore. The theory relies on the key insight that the forces acting on the polyelectrolyte can be expressed as the gradient of a one-dimensional potential function U(x), where the spatial dimension x is the linear progress of the polyelectrolyte through the nanopore. Thus, each boundary of the interval x corresponds to one or the other end of the linear polyelectrolyte chain in the nanopore (Figure 3E). The three-dimensional variability in the conformations explored by the captured polyelectrolytes can also be expressed as a one-dimensional entropic free energy [116].
An example of U(x) is shown as the solid line in Figure 3E. The local free energy minimum, labeled (1), is formed by two major free energy terms: the electrostatic interaction with the transmembrane potential, and the binding energy to the membrane. In the Kramers picture, the former is primarily responsible for the free energy barrier to retraction, , while the latter is primarily responsible for the free energy barrier to translocation,. Put simply, to retract the charged CT domain, αSyn must work against the electrodynamic forces that captured it; while to translocate, the αSyn molecule must unbind from the lipid membrane surface. The difference in these two free energies determines the partitioning of the population of the captured αSyn molecules between retraction and translocation, and hence the translocation probability Ptrans.
Because U(x) can be written as the sum of physically relevant terms, however, we do not have to rely on a Kramers-like description. U(x) is directly related to the measured distribution of off-rates via the adjoint of the Smoluchowski equation [117] and can be solved by a variety of numerical methods. The first moment of the off-rate distribution is τoff which can be determined by intra-pore diffusion constant 𝐷 and direct integration for an initial position x0 as:
| (3) |
In many cases, U(x) can be parameterized by a very few quantities, each of which has a well-defined physical interpretation [118]. A minimum set of these, which was previously validated on long DNA molecules in solid-state nanopores [119], includes two parameters describing the modification of the bare charge density on the polyelectrolyte due to electroosmotic flow in the nanopore [120–122] and any parameters required to describe the entropy of confinement of the polyelectrolyte in the nanopore [123]. Additional terms can be added to deal with particular cases, such as post-translational modifications [101] or association with the membrane[104, 112].
For determining the effect of membrane lipids on αSyn binding strength and conformation, one of the more important terms in U(x) is the membrane binding energy. This is modeled as an error function barrier with three parameters: the energy barrier height Eb, the position at which half the energy barrier has been overcome, xb, and the width of the error function wb, which is a measure of how gradually the unbinding process occurs. Eb is most closely related to the binding energy of a single molecule on the lipid membrane surface, while xb is related to the accessibility of the C-terminal tail for capture into the VDAC nanopore and will be of particular importance in distinguishing among surface binding conformations of αSyn. The effect of Eb and xb on the free energy profile is detailed in Figure 6 of [104]. Typical values of Eb are 15 kBT, which are largely consistent with the binding constants determined by fluorescence correlation spectroscopy (FCS) measurements of αSyn binding to large unilamellar liposome membranes [91].
Figure 6.
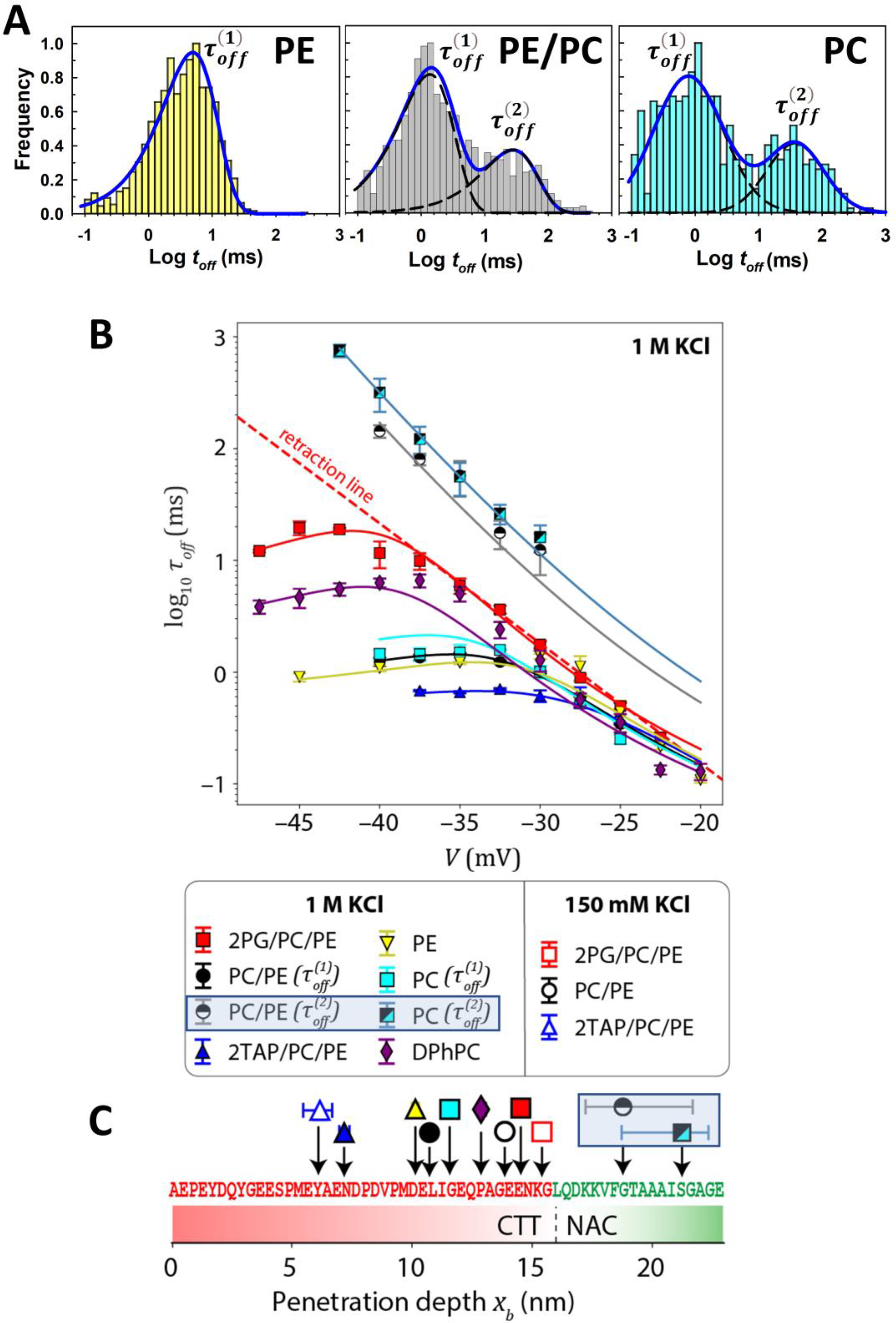
Lipid-dependent features of τoff. (A) Distributions of toff at V = −35 mV shows a single characteristic time scale, , for DOPE lipids. At higher potentials, a second, slower time scale, , is apparent in PC/PE and pure DOPC membranes. (B) For all lipid compositions, shows the characteristic transition from retraction to translocation; this transition is not observed for in PC/PE and DOPC lipids. The solid lines are model predictions with the optimized energy landscapes, U(x), with only two free parameters per curve. Error bars represent 68% confidence intervals. (C) Penetration depth obtained from the optimization for different lipid compositions. The depth represents the furthest residue that can penetrate the nanopore before deeper penetration is prevented by the membrane anchor. Error bars represent 95% confidence intervals and where not visible are smaller than the size of the data marker. Adapted with permission from Hoogerheide et al. ACS Nano (2021). Copyright © 2021, American Chemical Society.
It should be noted that while the theory of the off-rate distribution appears to be independent of the nanopore used, the nanopore geometry affects the hydrodynamic resistance encountered by a polypeptide moving through the nanopore, and hence the intra-pore diffusion constant. The effects of the bulk vs. intra-pore diffusivities on the dynamics of molecule capture and release by a single nanopore were recently analyzed in a series of theoretical studies [124–126].The geometry and the charge distribution on the walls of the pore also influence the magnitude of electroosmosis, which affects the effective charge density of the polypeptide. Additionally, one might imagine a position-dependent term for the interaction of the polypeptide charge density and that of the nanopore; the magnitude of this term can be estimated from the partition coefficient of charged vs. uncharged regions of αSyn in the VDAC nanopore [60] and is found to be weakly voltage-dependent and on the order of 1 kBT at typical experimental voltages. This is a small correction to the electrical and membrane binding forces and is neglected in this discussion.
4. Effect of lipids on αSyn-VDAC binding kinetics
αSyn added to the planar membrane containing VDAC nanopore produces characteristic current fluctuations in milliseconds range at application of negative voltage from the side of αSyn addition in a lipid-dependent way. Examples of three typical experiments with VDAC1 reconstituted into the planar membranes made of three different lipid compositions are shown in Figure 4A. Two lipid mixtures have been chosen to mimic rat liver MOM composition where DOPC (PC) and DOPE (PE) make up 54 and 29% of the total lipid content, respectively [127] and the negatively charged DOPG (PG) represents a relatively high content (up to 20%) of the negatively charged lipid to which αSyn preferentially binds [84, 111]. The positively charged synthetic lipid DOTAP (TAP) was chosen as an oppositely charged lipid. 50% (mol/mol) of DOPG or DOTAP have been used in PC:PE (1:1, mol/mol) (PC/PE) mixtures. All lipids in these experiments have the same dioleoyl acyl chains to discriminate the effect of the lipid headgroup chemistry and charge on αSyn-VDAC interaction.
The lipid composition does not measurably affect the conductance of either the open or αSyn-blocked states (Figure 4A). By contrast, the differences in and the distributions of toff are striking and can be seen in three current traces obtained on single channels in the presence 10 nM of αSyn (Figure 4A and Insets). The kon obtained in the negatively charged PG:PC:PE (2:1:1, mol:mol) (2PG/PC/PE) membranes is >10 times higher than in the positively charged TAP:PC:PE (2:1:1, mol/mol) (2TAP/PC/PE), with PC/PE membranes in between them (Figure 4B). The type of the lipid headgroup also affects the on-rate: the kon in pure DOPE is ~10 times higher than in pure DOPC (Figure 4C). Cardiolipin (CL), a signature lipid of the MIM and found in residual amounts in the MOM [127], almost doubles the kon when added at 20 mol% to the PC/PE mixture (Figure 4C), which could be accounted for by its negative charge and nonlamellar feature, but does not support the earlier proposed specificity of α-syn-CL binding [128].
The dependence of blockage times toff (indicated by red arrows in Figure 4A) on lipid composition is visible even in the current traces (insets), but can be best seen by the toff distributions in Figure 6A. Distributions of toff obtained on the membranes with highest αSyn kon—anionic, pure DOPE [92] (Figure 6A), or diphytanoylphosphatidylcholine (DPhPC) [18]—are well described by single exponential functions at all applied voltages [104]. In the membranes made of the lipids with low αSyn kon—neutral DOPC, an equimolar PC/PE mixture (Figure 6A) and cationic 2TAP/PC/PE [92]—the toff distributions cannot be described by a single exponent [104]. In PC/PE and pure DOPC, there are two well defined populations of toff, separated by a factor of 20, such that the whole toff distribution can be satisfactory described by a sum of two single exponents with characteristic times and (Figure 6A). In cationic 2TAP/PC/PE membranes, the long-lasting toff distribution is broad and requires more than two exponents to describe [104]. The occurrence of long-lasting increases with applied voltage from a minor, 10–20%, to a significant 50% fraction, depending on lipid composition [104] (Table 1).
Table 1.
Summary of results of VDAC nanopore-based analysis of αSyn-lipid interactions. Except for DPhPC, all lipids contained dioleoyl acyl chains.
| Lipid composition | 2PG/PC/PE | DPhPCa | PE | PC/PE | PC | 2TAP/PC/PE | |||
|---|---|---|---|---|---|---|---|---|---|
| Ionic strength (mM) | 150 | 1000 | 1000 | 1000 | 150 | 1000 | 1000 | 150 | 1000 |
| Kon(nM−1s−1) (−35 mV) | 108 | 38 | 29 | 33 | 99 | 8.9 | 4.3 | 5.65 | 1.4 |
| Fraction (%) | < 10 | < 10 | <10 | < 10 | < 10 | 32 | 36 | 14 | 37 |
| xb (nm) for | 15.4 | 14.5 | 12.9 | 10.1 | 13.9 | 10.7 | 11.6 | 6.2 | 7.2 |
| xb (nm) for | --- | --- | --- | --- | >17.2 | >18.7 | b | b | |
| Eb/kBT for | 35.7 | 19.0 | 18.3 | 15.0 | 29.3 | 15.0 | 15.8 | 18.4 | 14.8 |
| kd (μM)b | 47 | --- | --- | --- | 1500 | --- | --- | 116 | --- |
5. Model of membrane-bound αSyn conformations
The results of the VDAC nanopore-based analysis of αSyn-lipid interactions are summarized in Table 1 [92, 104]. To characterize kon, the capture rate at a single voltage (V = −35 mV) is chosen. This is justified because the slope of log10 kon with voltage is the same for all lipid compositions (Figure 4B). Using energy landscape modeling (section 3.2), the complex behavior of τoff can be reduced to membrane binding energy and the single geometric parameter, the penetration depth xb. This is shown for each lipid composition in Figure 6C. Note that the penetration depth informs us of the residue in the pore center at the furthest penetration of the CTT into the nanopore. Due to the thickness of the membrane, the residues closest to xb that could be responsible for membrane anchoring are at about +2 nm from the penetration depth. Thus, it is quite reasonable that αSyn residue F94 (at x = 18.4 nm) is membrane-associated on anionic membranes (xb ≈ 15 nm), as discussed in section 2.
Table 1 and Figure 6C show that the population of long-lived events, , is characterized by a much larger penetration depth xb. A physical model corresponding to this observation is shown in Figure 7. A shorter penetration depth corresponds to a membrane anchoring point that is near the CTT (starred positions in Figure 7), such that the CTT cannot fully penetrate the VDAC nanopore. This leads to faster retractions , but also allows increases in the applied voltage to destabilize membrane binding, leading to mostly translocation events at higher voltages. By contrast, a larger penetration depth suggests that the membrane anchoring point is further from the CTT. Under the applied voltage, the entire CTT can pass through the nanopore; the weakly charged region of αSyn is in the nanopore, the force acting on the trapped molecule is weaker, and membrane binding is not destabilized with increased voltage. At the voltages reported in the measurements, a translocation regime is not observed.
Figure 7.
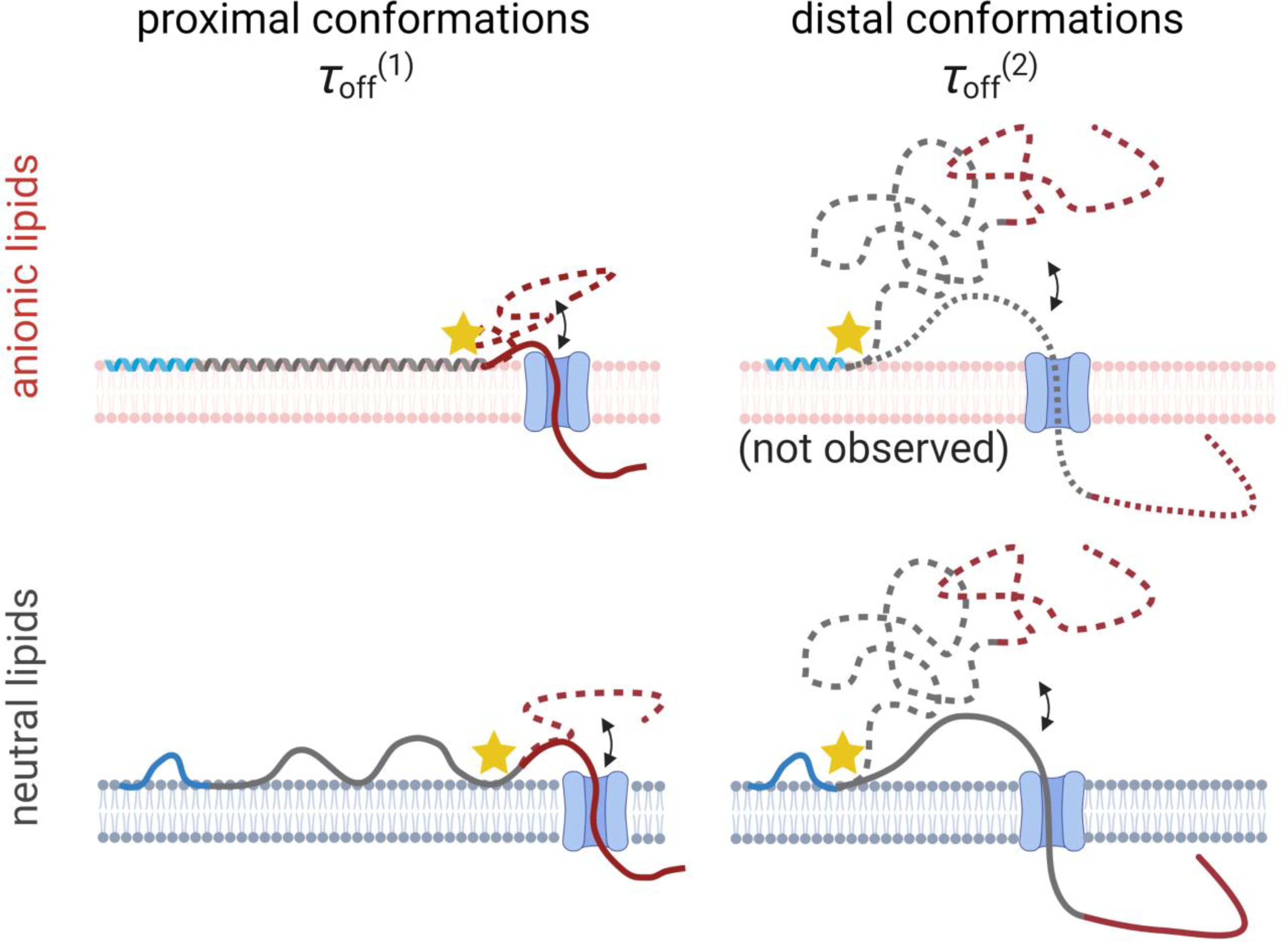
Model of the possible αSyn conformations responsible for the different observed τoff. The blue region of αSyn corresponds to the persistently membrane-bound domain identified by Fusco et al. [81] (Fig. 2B); the dynamic center region is shown in gray; and the CTT is shown in red. In each conformation, the position closest to the CTT that is pinned to the lipid membrane is shown with a star. For “proximal” membrane-bound conformations, where the pinning region extends near the CTT, which is constrained to be close to the membrane surface, is observed. For “distal” conformations, where the pinning region is further away from the CTT, allowing the CTT to float further from the membrane surface but also to penetrate entirely through the VDAC nanopore once captured, is observed. Created with Biorender.com.
NMR measurements suggest [81] the presence of a distal binding conformation on anionic lipids such as that shown in the upper right panel of Figure 7. In the VDAC nanopore measurements, however, is not observed at significant levels. A clue to the reason of this absence arises from the strong correlation between low kon and the presence of (Table 1). This leads to the natural conclusion that proximal conformations, for which the CTT is pinned close to the membrane surface (but not bound to the surface, as for the cationic lipids (Figure 8B)), are more likely to be captured by the VDAC nanopore. In other words, the incidence of each molecular conformation observed by the VDAC nanopore is strongly biased toward molecular conformations that lead account for . This bias is likely to be particularly significant on anionic lipids, for which electrostatic repulsion of the CTT from the membrane surface is strong; distal conformations may be still present, but their observation by the VDAC pore may be strongly repressed, so that the probability of capture is low. In the terms of Eq. (2), distal conformations add an additional term to that represents the free energy required to bring the polyanionic CTT close to the anionic lipid surface. As a corollary, for the lipid compositions in which is present in a measurable fraction, it is likely that a substantial majority of the surface-bound molecules are in the corresponding distal conformations, even if accounting for a minority of observed events. Future studies with engineered proteins may be required to quantify the efficiency of capture for different molecular conformations.
Figure 8.
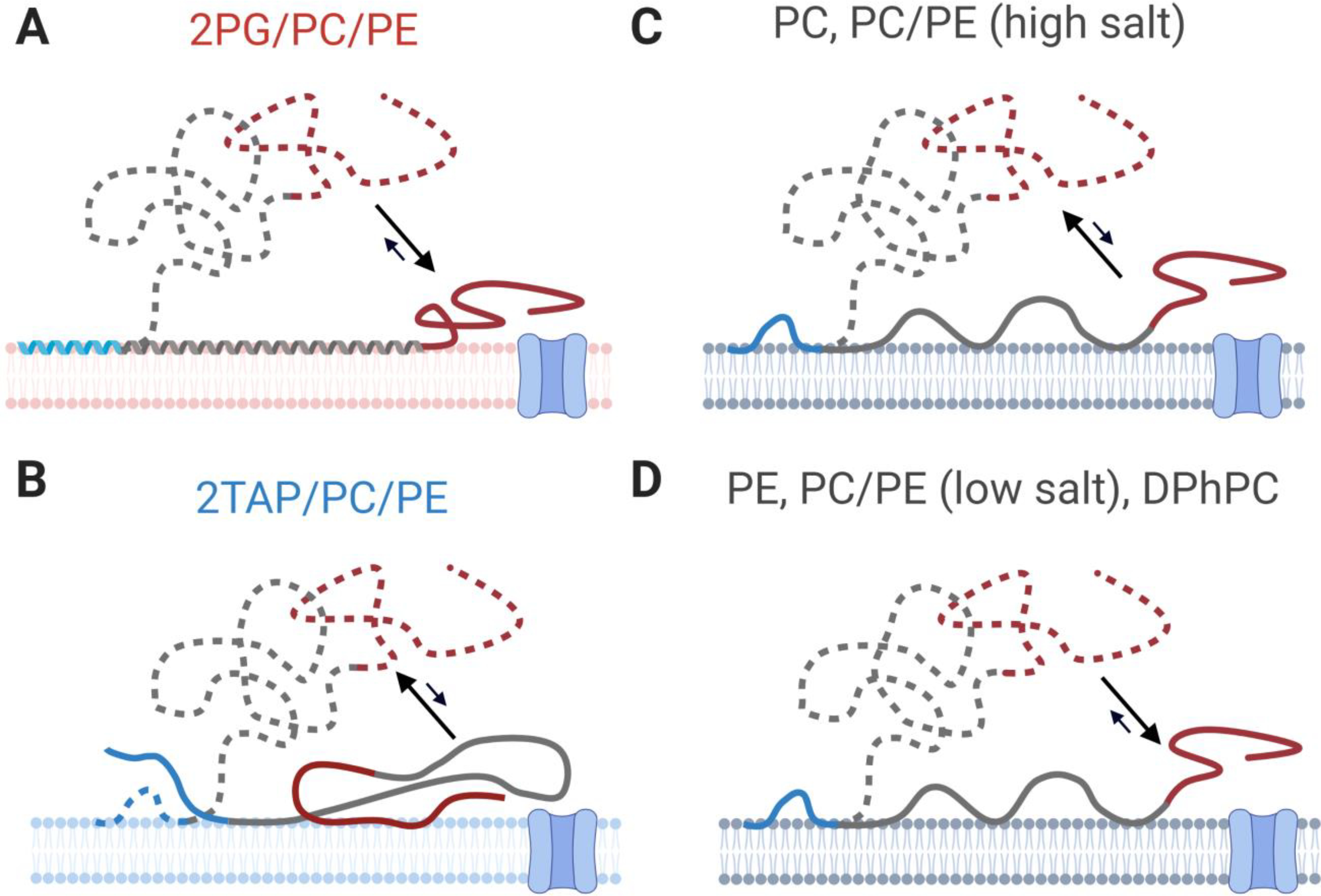
Model of binding conformations adopted by αSyn on membranes of different lipid compositions. Proximal conformations, which are readily captured and lead to short blockages of VDAC (), are shown in solid lines; distal conformations, which are less available to the VDAC pore but have longer lifetimes (), are shown by dashed lines. Arrows reflect upon relative populations rather than dynamic equilibrium. (A) On anionic lipids, VDAC nanopore-based measurements are consistent with binding conformations observed by NMR. Distal conformations, however, are not observed. (B) In the proximal conformations on cationic membranes, the C-terminal domain is closely associated with the lipid surface. Distal conformations, however, account for a significant fraction of observed events. For neutral lipids (C-D), PC lipids and PC/PE mixtures at high salt concentrations favor distal conformations (C), while (D) PE lipids, PC/PE mixtures at low salt concentrations, and DPhPC lipids favor proximal conformations. Created with Biorender.com.
A complete model for different lipid types is shown in Figure 8; proximal and distal conformations are shown by solid and dashed lines, respectively. For nominally neutral lipids with zwitterionic headgroups, the propensity of αSyn to adopt primarily proximal or distal conformations depends on the headgroup species and the salt concentration. For PC/PE membranes at low salt, proximal conformations are favored. This is likely due to electrostatic interactions between the lysines of the membrane-binding domain and the membrane surface, which is slightly anionic [129–132]. At high salt concentrations, electrostatic interactions are reduced and distal conformations are favored. Interestingly, unlike DOPC lipids, DOPE lipids appear to produce proximal conformations, possibly because the smaller PE lipid headgroup allows stronger hydrophobic interactions between the valine-rich αSyn central domain and the membrane interior (Figure 8D). DPhPC behaves similarly to DOPE [101, 112], presumably due to the increased lipid spacing introduced by the acyl chain methylation. Distal conformations, by contrast, appear to be favored on cationic lipids and DOPC (Figure 8C, B). Note that crowding on the membrane surface may also favor distal conformations.
Remarkably, Table 1 shows that the binding energy of αSyn to the lipid surface, as measured either by energy landscape modeling or FCS, is not strongly predictive of the conformational landscape and hence of the propensity of αSyn to block VDAC. This highlights an important predictive limitation of dissociation constant measurements in this system.
6. Role of mitochondrial lipids in αSyn-VDAC interaction: physiological implications
In the preceding sections, we have reviewed the nanopore-based evidence for the effect of lipid composition on the ensemble of conformations adopted by αSyn bound to membranes. These results confirm and complement a variety of previous studies indicating the interdependence of membrane composition and morphology for function and pathology of αSyn, as reviewed in [88]. The specific lipid content of the cytosolic membranes with which αSyn is found associated—plasma membrane [133], synaptic vesicles [134], or mitochondria [45, 52, 65, 135]—can modulate not only the quantity of membrane-bound αSyn but also its conformation. While a majority of studies are understandably focused on the effect of model and cell membranes on αSyn fibrillation potency, there is emerging interest in the conformations of lipid-associated αSyn monomers [81, 136, 137]. In our view, understanding how the conformation of αSyn molecules on membrane surfaces is related to function and pathologies of cell organelles is of great biological importance.
For the specific case of mitochondrial membranes, the lipid composition may play an additional role in modulating the αSyn-VDAC interaction and thus in regulation of metabolite fluxes through the VDAC channel (see discussion in [47]). Dynamic lipid exchange occurs at the contact sites between the two mitochondrial membranes and at the points of tight contact between mitochondria and other organelle membranes, such as endoplasmic or sarcoplasmic reticulum, or lysosomes that are known as mitochondrial associated membranes. The mitochondrial lipid composition is especially dynamic under oxidative stress [73–75] or apoptosis [72]. It is thus natural to speculate that the MOM lipid composition affects the ratio between αSyn populations in proximal and distal conformations at the MOM surface (Figure 7). In other words, lipid composition could affect the ratio between αSyn molecules in proximal conformations, which produce short-lived blockages but have a higher probability of entering mitochondria through the VDAC, and αSyn in distal conformations that produce longer-lived blockages and thus potentially could control metabolite flux through the VDAC more effectively (Figure 7) [104].
According to our working model, in normal mitochondrial physiology, αSyn regulates ATP/ADP fluxes through VDAC by dynamically blocking the pore [18]. In pathology such as αSyn overexpression, αSyn could enter mitochondria through VDAC and target electron transport complexes in the MIM causing their impairment. According to this model, an increase of anionic lipid content in the MOM could increase the population of αSyn in a membrane-proximal conformation, leading to higher translocation probability through VDAC (Figure 8A) and potentially to mitochondrial dysfunction. It is suggestive that under stress conditions such as apoptosis, the content of highly anionic and non-lamellar cardiolipin increases in the MOM [73] whereas it normally accounts for less than 1% of the total MOM lipid content [127]. Future experiments in vitro and in vivo will be required to determine if this model is relevant physiologically.
We have shown that the VDAC nanopore is sensitive to the effect of mitochondrial lipids on its cytosolic protein partners. By extension, this mitochondrial nanopore may be found to be useful for sensing the interactions of various cytosolic proteins such as glycolytic enzymes, hexokinase, cytoskeletal, neuronal, and Bcl-2 family pro- and anti-apoptotic proteins with the MOM [43, 138–144]. Notably, most of these cytosolic proteins, which execute their biological and pathological functions at the MOM platform, are weakly and transiently bound to the membrane.
7. Capture from the bulk versus capture from the membrane surface: experiments with α-Hemolysin nanopore
The idea of VDAC as a nanopore sensor is somewhat counterintuitive because under some conditions VDAC starts moving to its closed states at the applied transmembrane potentials as small as 30 mV. This should be compared with potentials of 100 mV or more necessary to gate many other β-barrel channels, especially those that find more regular use as sensors [145–149]. In addition, it was shown that changes in VDAC’s environment, such as application of osmotic pressure [150], altering membrane lipid type [41], or decreasing solution pH [107], are able to increase the propensity of VDAC to gate under applied voltage. Even more importantly in the present context, it was demonstrated that the presence of certain negatively charged polymers that do not permeate VDAC can greatly increase the probability of gating transitions and thus VDAC’s sensitivity to voltage [151]. For that reason, experiments with different nanopores as sensors for membrane-bound αSyn could be of great value.
Such experiments have been performed with another β-barrel channel, α-Hemolysin (αHL), where the crucial involvement of the membrane surface in αSyn interactions with membrane-imbedded nanopores was first demonstrated [152]. This extensively studied, prototypical nanopore [153–155] is formed as the result of self-assembly of seven αHL monomers into a mushroom-shaped structure (Figure 9). The nanopore, about 10 nm in length, is slightly anion-selective in 1M KCl solutions at neutral pH [156] and is characterized by a diameter which varies along the channel length, with the narrowest constriction of about 1.4 nm [153, 157]. Most notably, the αHL nanopore has a pronounced and functionally important asymmetry (Figure 9). The cap side of the nanopore, corresponding to the side of αHL addition in a reconstitution experiment (cis-side), is elevated by about 5 nm above the surface of the bilayer, while the opening on the other, stem side (trans-side), is flush with the membrane surface. The asymmetry, which is absent in the structure of membrane-embedded VDAC (Figure 1), makes the αHL nanopore an ideal probe for the discrimination between the bulk and membrane-surface-catalyzed processes.
Figure 9.
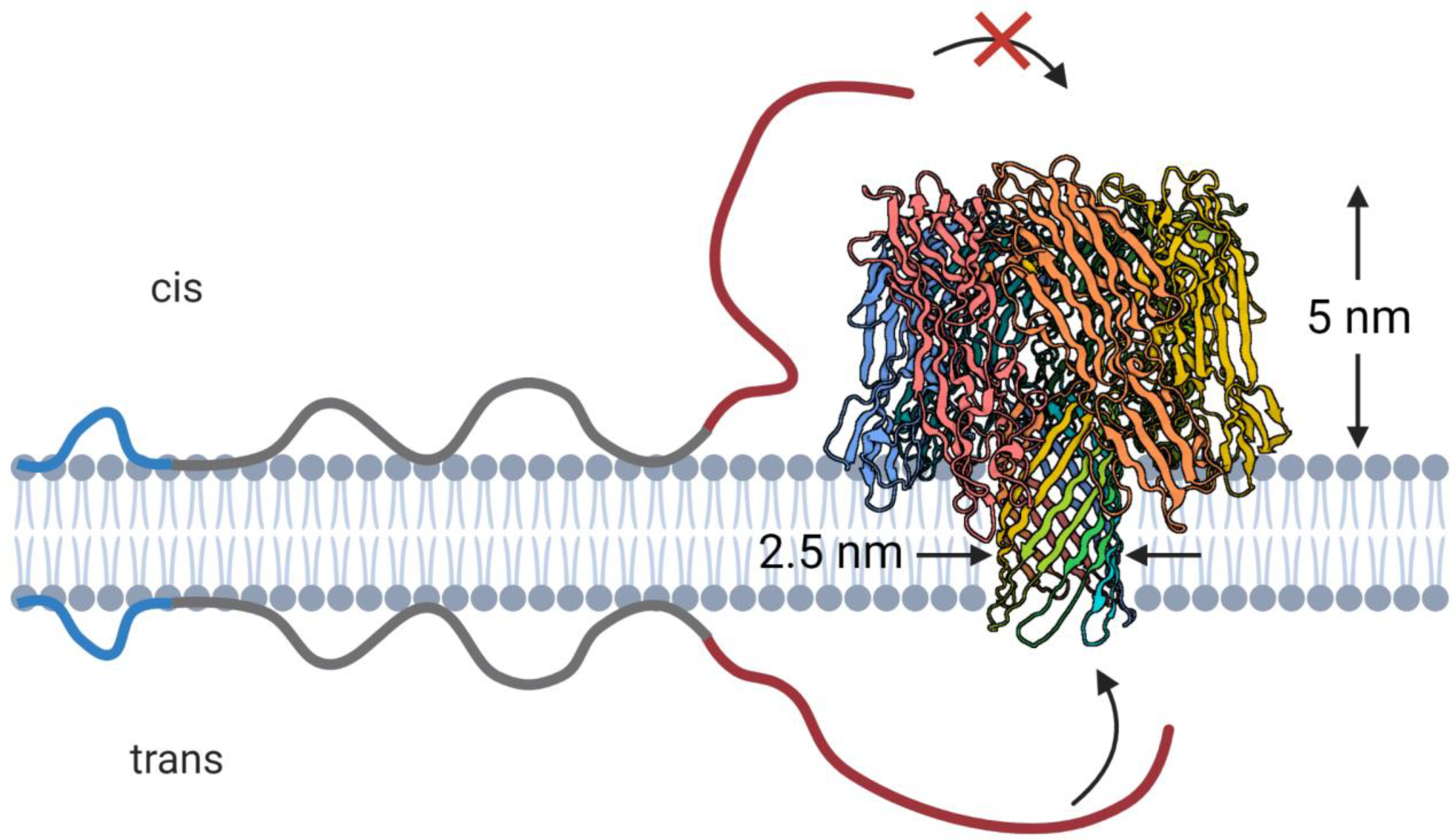
The asymmetric structure of α-hemolysin (PDB ID: 3ANZ) prevents capture of membrane-bound αSyn from the cap (cis) side but allows capture from the stem side. Created with Biorender.com.
One of the key findings reported by Gurnev et al. [152] was that addition of αSyn to the stem (trans in Figure 9) side of the nanopore results in an orders of magnitude greater on-rate of αSyn capture than that described in the initial publications on the interactions between αSyn and αHL [158, 159], where αSyn was added to the cap side of the pore. Indeed, capture events were readily observable when 50 nM αSyn was added to the trans-side of the membrane under transmembrane voltages of 40 mV relative to the side of αSyn addition, with the on-rate increasing by about 10-fold for a 20 mV increase in the applied voltage. For the cis-side addition of 50 nM αSyn, events were not observed at any voltages of either polarity; observation of rare events required a significantly higher αSyn concentration and voltages of 100 mV or more. This vast difference in the on-rates, exceeding three orders of magnitude if recalculated to the same voltages and concentrations, suggests that the protruding cap side of the channel cannot capture αSyn molecules from the membrane surface (Figure 9), but instead captures them from the bulk. In the context of equations (1) and (2), the value of is considerably larger for the membrane-bound αSyn molecules that must stretch away from the membrane surface to reach the cap side of the channel, while the collision rate is much smaller for the molecules that do not adhere to the membrane surface.
The second finding of the study was that the on-rate for the surface-bound αSyn depended on the lipid species used for membrane formation, varying by more than 100-fold for the lipid compositions tested [152]. Similar to the findings with VDAC, the on-rate was shown to grow exponentially with the applied voltage (at least for relatively small voltages) and, in the range of small αSyn concentrations, to scale linearly with the concentration. At small αSyn concentrations it was found that for DPhPC membranes the on-rate constant was about 20 times higher than that for bilayers formed from the soybean polar lipid extract (PLE), with palmitoyl-phosphatidylcholine (POPC) bilayers demonstrating a 10-fold further reduction in the on-rate constant relative to that for PLE. Comparison of the capture rate dependences on αSyn concentration obtained from DPhPC and PLE bilayers revealed that, while they differ significantly, they both saturate at similar concentrations [152]. The dependences were best fit using simple binding isotherms with the characteristic concentrations of 30 and 32 nM for DPhPC and PLE, respectively. This observation led the authors to speculate that both DPhPC and PLE membranes bind αSyn in similar amounts, but PLE-bound molecules are somehow mostly incapacitated regarding their interaction with the channel. Indeed, the conjecture of the existence of different lipid-dependent conformations of αSyn, which differ in their ability to be trapped by a β-barrel nanopore, was, as described above, proved by further studies with the VDAC nanopore [92, 104].
The nanopore structures of VDAC and αHL are drastically different by the overall architecture, chemistry of the residues, and number of β-strands (19 and 14, respectively) involved in barrel formation (Figures 1 and 9). The stem side of the αHL nanopore, however, is similar to VDAC in that in both cases the pore opening is flush with the membrane surface. Besides, both nanopores have slight anion-selectivity in 1 M KCl and, while different in the total channel length, have similar dimensions of their narrowest constrictions and similar diameters of the trans-side opening. Given these similarities, it is instructive to compare the rates of αSyn capture by the αHL and VDAC nanopores. Such comparison suggests that the VDAC nanopore is orders-of-magnitude more proficient. Indeed, the most catalyzing membrane surface for the reaction of αHL with αSyn was found to be that of DPhPC bilayers, where the on-rate constant is about 8.10−3 s−1 nM−1, measured at 40 mV applied voltage in 1 M KCl solutions at neutral pH (Figure 3C in[152]). This value is about 5.103 times lower than that for VDAC in DPhPC, other experimental conditions being the same [18]. Even for the least catalyzing lipid composition explored for VDAC, the 2TAP/PC/PE mixture, the on-rate constant is more than two orders of magnitude higher than the highest for αHL [92]. Specifically, extrapolated to 40 mV from the exponential fit of the data in the 25 mV to 37.5 mV range (Figure 1C in [92]), it is about 2.7 s−1 nM−1. This huge difference in the ability of the two nanopores to capture membrane-bound αSyn molecules reflects structural and electrostatic dissimilarities in the nanopore architectures. The main reason is probably in the channel conductance. VDAC, being nearly five times more conductive, upon application of the transmembrane voltage is expected to create five times stronger fields in the vicinity of its entrance. These fields act to capture αSyn molecules more efficiently. In the formalism of equations (1) and (2), the change in the on-rate is due to the change in the contribution of the first term in the right-hand side of Eq. (2). An additional change in may also originate from the differences in the structures of the channel openings, which result in substantially different free energy barriers for the entry of the C-terminal tail of αSyn, accounted for by the second and third terms in right-hand side of Eq. (2). This conjecture is strongly supported by the two orders of magnitude difference in the on-rate of aSyn capture found for VDAC1 and VDAC3 isoforms [160].
In summary, experiments with αHL clearly demonstrated the crucial role of the membrane surface in the chain of events leading to αSyn interaction with β-barrel nanopores. Observation of the orders-of-magnitude lower probability of αSyn capture by the cap side of the αHL channel, which extends several nanometers into the bulk, compared to that of the capture by the stem side of the channel from the membrane surface, explicates the powerful catalyzing effect of the membrane interface. The data also support the notion of different conformations of membrane-bound αSyn, which are characterized by drastically different accessibility of its negatively charged C-terminus for the interaction with a membrane-embedded nanopore. Finally, comparison of the data obtained with VDAC and αHL suggests the importance of the fine structural and electrostatic features of a nanopore, including those that change electric field and its distributions at the nanopore entrance.
8. Conclusions
One of the important peculiarities of disordered proteins is their ability to undergo structural rearrangements upon binding/association with cell membranes. Moreover, their structure on the membrane surface strongly depends on membrane lipid composition, that is, on the nature of the acyl chains and the phospholipid headgroup charge, size, and hydration. These factors define membrane electrostatics and mechanical properties, such as membrane fluidity, bending and compressibility moduli, lipid spontaneous curvature, and, therefore, lipid packing stress. There are significant experimental limitations to identifying the structure of proteins that are weakly or transiently bound to the membrane, or that populate many different binding conformations. In the present review we demonstrate that a single-molecule nanopore-based approach is particularly useful in this case. Importantly, here the nanopore of our choice, VDAC, plays an unusual dual role: on the one hand, it is a nanoscale electromechanical device for probing the fine structural features of membrane-bound αSyn; on the other, it is the natural target of this cytosolic protein in mitochondria. VDAC as a nanopore probe turns out to be sensitive to the distribution of conformations adopted by different αSyn molecules on the same lipid surfaces. We show that the strength of αSyn binding to the membrane does not correlate in any simple way with its rates of capture or release by VDAC. Among many other membrane-specific effects, we indicate that the lipid-dependent association of the central domain of αSyn with the membrane surface appears to determine the availability of αSyn for capture by the VDAC nanopore and thus governs the overall interaction of αSyn with VDAC. We conclude that in addition to advocating VDAC as a promising nanopore sensor, our results have straightforward implications for the potential role of mitochondrial lipids in regulation of bioenergetics, stress response, and apoptosis.
Highlights.
The voltage-dependent anion channel (VDAC) acts as both ion channel and nanopore
As an ion channel VDAC is regulated by membrane-bound α-Synuclein (αSyn)
As a nanopore VDAC allows electro-mechanical probing of membrane-bound proteins
Lipid composition determines αSyn’s surface density and conformational ensembles
αSyn surface conformation defines rates of insertion into and release from VDAC
Acknowledgments
The authors acknowledge contributions from Daniel Jacobs and Philip Gurnev to the experimental work, and thank Jennifer Lee for the kind gift of αSyn. Certain commercial materials, equipment, and instruments are identified in this work to describe the experimental procedure as completely as possible. In no case does such an identification imply a recommendation or endorsement by NIST, nor does it imply that the materials, equipment, or instrument identified are necessarily the best available for the purpose.
Funding sources
T.K.R. and S.M.B. were supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH).
Footnotes
Conflict of Interests
The authors declare that they have no competing interests with the contents of this article.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Colombini M, VDAC: The channel at the interface between mitochondria and the cytosol, Mol Cell Biochem 256(1–2) (2004) 107–115. [DOI] [PubMed] [Google Scholar]
- [2].Lemasters JJ, Holmuhamedov E, Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box, Biochimica et biophysica acta 1762(2) (2006) 181–90. [DOI] [PubMed] [Google Scholar]
- [3].Magri A, Messina A, Interactions of VDAC with Proteins Involved in Neurodegenerative Aggregation: An Opportunity for Advancement on Therapeutic Molecules, Curr Med Chem 24(40) (2017) 4470–4487. [DOI] [PubMed] [Google Scholar]
- [4].Reina S, De Pinto V, Anti-Cancer Compounds Targeted to VDAC: Potential and Perspectives, Curr Med Chem 24(40) (2017) 4447–4469. [DOI] [PubMed] [Google Scholar]
- [5].Leanza L, Checchetto V, Biasutto L, Rossa A, Costa R, Bachmann M, Zoratti M, Szabo I, Pharmacological modulation of mitochondrial ion channels, Br J Pharmacol 176(22) (2019) 4258–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shoshan-Barmatz V, Ben-Hail D, VDAC, a multi-functional mitochondrial protein as a pharmacological target, Mitochondrion 12(1) (2012) 24–34. [DOI] [PubMed] [Google Scholar]
- [7].Shoshan-Barmatz V, Nahon-Crystal E, Shteinfer-Kuzmine A, Gupta R, VDAC1, mitochondrial dysfunction, and Alzheimer’s disease, Pharmacol Res 131 (2018) 87–101. [DOI] [PubMed] [Google Scholar]
- [8].Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping PP, Abramson J, The crystal structure of mouse VDAC1 at 2.3 angstrom resolution reveals mechanistic insights into metabolite gating, Proc Natl Acad Sci USA 105(46) (2008) 17742–17747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G, Solution structure of the integral human membrane protein VDAC-1 in detergent micelles, Science 321(5893) (2008) 1206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, Vonrhein C, Griesinger C, Zweckstetter M, Zeth K, Structure of the human voltage-dependent anion channel, Proc Natl Acad Sci U S A 105(40) (2008) 15370–15375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, Ping PP, Abramson J, The crystal structure of mouse VDAC1 at 2.3 angstrom resolution reveals mechanistic insights into metabolite gating, Proc Natl Acad Sci U S A 105(46) (2008) 17742–17747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Song JM, Midson C, Blachly-Dyson E, Forte M, Colombini M, The sensor regions of VDAC are translocated from within the membrane to the surface during the gating processes, Biophys J 74(6) (1998) 2926–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thomas L, Blachly-Dyson E, Colombini M, Forte M, Mapping of residues forming the voltage sensor of the voltage-dependent anion-selective channel., Proceedings of the National Academy of Sciences 90(12) (1993) 5446–5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Popp B, Court DA, Benz R, Neupert W, Lill R, The Role of the N and C Termini of Recombinant Neurospora Mitochondrial Porin in Channel Formation and Voltage-dependent Gating, J Biol Chem 271(23) (1996) 13593–13599. [DOI] [PubMed] [Google Scholar]
- [15].Zachariae U, Schneider R, Briones R, Gattin Z, Demers J-P, Giller K, Maier E, Zweckstetter M, Griesinger C, Becker S, Benz R, Groot D, Bert L, Lange A, β-Barrel Mobility Underlies Closure of the Voltage-Dependent Anion Channel, Structure 20(9) (2012) 1540–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gurnev PA, Rostovtseva TK, Bezrukov SM, Tubulin-blocked state of VDAC studied by polymer and ATP partitioning, FEBS Lett. 585(14) (2011) 2363–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Colombini M, Pore-Size and Properties of Channels from Mitochondria Isolated from Neurospora-Crassa, J Membrane Biol 53(2) (1980) 79–84. [Google Scholar]
- [18].Rostovtseva TK, Gurnev PA, Protchenko O, Hoogerheide DP, Yap TL, Philpott CC, Lee JC, Bezrukov SM, alpha-Synuclein Shows High Affinity Interaction with Voltage-dependent Anion Channel, Suggesting Mechanisms of Mitochondrial Regulation and Toxicity in Parkinson Disease, J Biol Chem 290(30) (2015) 18467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Queralt-Martin M, Bergdoll L, Jacobs D, Bezrukov SM, Abramson J, Rostovtseva TK, Assessing the role of residue E73 and lipid headgroup charge in VDAC1 voltage gating, Biochim Biophys Acta Bioenerg 1860(1) (2019) 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zambrowicz EB, Colombini M, Zero-current potentials in a large membrane channel: a simple theory accounts for complex behavior, Biophys J 65(3) (1993) 1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Krammer EM, Saidani H, Prevost M, Homble F, Origin of ion selectivity in Phaseolus coccineus mitochondrial VDAC, Mitochondrion 19 Pt B (2014) 206–13. [DOI] [PubMed] [Google Scholar]
- [22].Schein SJ, Colombini M, Finkelstein A, Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria, J Membr Biol 30(2) (1976) 99–120. [DOI] [PubMed] [Google Scholar]
- [23].Colombini M, Blachly-Dyson E, Forte M, VDAC, a channel in the outer mitochondrial membrane, Ion Channels 4 (1996) 169–202. [DOI] [PubMed] [Google Scholar]
- [24].Colombini M, Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane, Ann N Y Acad Sci 341 (1980) 552–63. [DOI] [PubMed] [Google Scholar]
- [25].Mlayeh L, Chatkaew S, Leonetti M, Homble F, Modulation of plant mitochondrial VDAC by phytosterols, Biophys J 99(7) (2010) 2097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gincel D, Zaid H, Shoshan-Barmatz V, Calcium binding and translocation by the voltage-dependent anion channel: a possible regulatory mechanism in mitochondrial function, Biochem J 358(Pt 1) (2001) 147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carbonara F, Popp B, Schmid A, Iacobazzi V, Genchi G, Palmieri F, Benz R, The role of sterols in the functional reconstitution of water-soluble mitochondrial porins from plants, J Bioenerg Biomembr 28(2) (1996) 181–9. [DOI] [PubMed] [Google Scholar]
- [28].Ermishkin LN, Mirzabekov TA, Redistribution of the electric field within the pore contributes to the voltage-dependence of mitochondrial porin channel, Biochim Biophys Acta 1021(2) (1990) 161–8. [DOI] [PubMed] [Google Scholar]
- [29].Rostovtseva TK, Bezrukov SM, ATP transport through a single mitochondrial channel, VDAC, studied by current fluctuation analysis, Biophys J 74(5) (1998) 2365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Noskov SY, Rostovtseva TK, Chamberlin AC, Teijido O, Jiang W, Bezrukov SM, Current state of theoretical and experimental studies of the voltage-dependent anion channel (VDAC), Biochimica et biophysica acta 1858(7 Pt B) (2016) 1778–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rappaport SM, Teijido O, Hoogerheide DP, Rostovtseva TK, Berezhkovskii AM, Bezrukov SM, Conductance hysteresis in the voltage-dependent anion channel, Eur Biophys J 44(6) (2015) 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Queralt-Martin M, Hoogerheide DP, Noskov SY, Berezhkovskii AM, Rostovtseva TK, Bezrukov SM, VDAC Gating Thermodynamics, but Not Gating Kinetics, Are Virtually Temperature Independent, Biophys J 119(12) (2020) 2584–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rostovtseva T, Colombini M, ATP flux is controlled by a voltage-gated channel from the mitochondrial outer membrane, J Biol Chem 271(45) (1996) 28006–8. [DOI] [PubMed] [Google Scholar]
- [34].Noskov SY, Rostovtseva TK, Bezrukov SM, ATP Transport through VDAC and the VDAC-Tubulin Complex Probed by Equilibrium and Nonequilibrium MD Simulations, Biochemistry-Us 52(51) (2013) 9246–9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tan W, Colombini M, VDAC closure increases calcium ion flux, Biochimica et biophysica acta 1768(10) (2007) 2510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rosencrans WM, Rajendran M, Bezrukov SM, Rostovtseva TK, VDAC regulation of mitochondrial calcium flux: From channel biophysics to disease, Cell Calcium 94 (2021) 102356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lemeshko VV, Electrical control of the cell energy metabolism at the level of mitochondrial outer membrane, Biochim Biophys Acta Biomembr 1863(1) (2021) 183493. [DOI] [PubMed] [Google Scholar]
- [38].Rostovtseva TK, Tan W, Colombini M, On the role of VDAC in apoptosis: fact and fiction, J Bioenerg Biomembr 37(3) (2005) 129–42. [DOI] [PubMed] [Google Scholar]
- [39].Mlayeh L, Krammer E-M, Léonetti M, Prévost M, Homblé F, The mitochondrial VDAC of bean seeds recruits phosphatidylethanolamine lipids for its proper functioning, Biochim Biophys Acta - Bioenergetics 1858(9) (2017) 786–794. [DOI] [PubMed] [Google Scholar]
- [40].Queralt-Martín M, Bergdoll L, Jacobs D, Bezrukov SM, Abramson J, Rostovtseva TK, Assessing the role of residue E73 and lipid headgroup charge in VDAC1 voltage gating, Biochim Biophys Acta - Bioenergetics 1860(1) (2019) 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rostovtseva TK, Kazemi N, Weinrich M, Bezrukov SM, Voltage gating of VDAC is regulated by nonlamellar lipids of mitochondrial membranes, J Biol Chem 281(49) (2006) 37496–506. [DOI] [PubMed] [Google Scholar]
- [42].Rostovtseva TK, Bezrukov SM, VDAC regulation: role of cytosolic proteins and mitochondrial lipids, J Bioenerg Biomembr 40(3) (2008) 163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL, Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration, Proceedings of the National Academy of Sciences 105(48) (2008) 18746–18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maldonado EN, Patnaik J, Mullins MR, Lemasters JJ, Free Tubulin Modulates Mitochondrial Membrane Potential in Cancer Cells, Cancer Res. 70(24) (2011) 10192–10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rovini A, Gurnev PA, Beilina A, Queralt-Martin M, Rosencrans W, Cookson MR, Bezrukov SM, Rostovtseva TK, Molecular mechanism of olesoxime-mediated neuroprotection through targeting alpha-synuclein interaction with mitochondrial VDAC, Cell Mol Life Sci (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Monge C, Beraud N, Kuznetsov AV, Rostovtseva T, Sackett D, Schlattner U, Vendelin M, Saks VA, Regulation of respiration in brain mitochondria and synaptosomes: restrictions of ADP diffusion in situ, roles of tubulin, and mitochondrial creatine kinase, Mol Cell Biochem 318(1–2) (2008) 147–65. [DOI] [PubMed] [Google Scholar]
- [47].Rostovtseva TK, Hoogerheide DP, Rovini A, Bezrukov SM, Lipids in Regulation of the Mitochondrial Outer Membrane Permeability, Bioenergetics, and Metabolism, in: Rostovtseva TK (Ed.), Molecular Basis for Mitochondrial Signaling, Springer International Publishing, Cham, 2017, pp. 185–215. [Google Scholar]
- [48].Goedert M, Jakes R, Spillantini MG, The Synucleinopathies: Twenty Years On, J Parkinsons Dis 7(s1) (2017) S53–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M, Alpha-synuclein in Lewy bodies, Nature 388(6645) (1997) 839–40. [DOI] [PubMed] [Google Scholar]
- [50].Kruger R, Muller T, Riess O, Involvement of alpha-synuclein in Parkinson’s disease and other neurodegenerative disorders, J Neural Transm (Vienna) 107(1) (2000) 31–40. [DOI] [PubMed] [Google Scholar]
- [51].Robotta M, Gerding HR, Vogel A, Hauser K, Schildknecht S, Karreman C, Leist M, Subramaniam V, Drescher M, Alpha-synuclein binds to the inner membrane of mitochondria in an alpha-helical conformation, Chembiochem 15(17) (2014) 2499–502. [DOI] [PubMed] [Google Scholar]
- [52].Li WW, Yang R, Guo JC, Ren HM, Zha XL, Cheng JS, Cai DF, Localization of alpha-synuclein to mitochondria within midbrain of mice, Neuroreport 18(15) (2007) 1543–6. [DOI] [PubMed] [Google Scholar]
- [53].Ludtmann MHR, Angelova PR, Horrocks MH, Choi ML, Rodrigues M, Baev AY, Berezhnov AV, Yao Z, Little D, Banushi B, Al-Menhali AS, Ranasinghe RT, Whiten DR, Yapom R, Dolt KS, Devine MJ, Gissen P, Kunath T, Jaganjac M, Pavlov EV, Klenerman D, Abramov AY, Gandhi S, alpha-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease, Nat Commun 9(1) (2018) 2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P, Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells, Int J Biochem Cell Biol 41(10) (2009) 2015–24. [DOI] [PubMed] [Google Scholar]
- [55].Elkon H, Don J, Melamed E, Ziv I, Shirvan A, Offen D, Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome C oxidase, J Mol Neurosci 18(3) (2002) 229–38. [DOI] [PubMed] [Google Scholar]
- [56].Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK, Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain, J Biol Chem 283(14) (2008) 9089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chinta SJ, Mallajosyula JK, Rane A, Andersen JK, Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo, Neurosci Lett 486(3) (2010) 235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, Sesaki H, Cheng Y, Finkbeiner S, Nussbaum RL, Masliah E, Edwards RH, Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein, J Biol Chem 286(23) (2011) 20710–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hoogerheide DP, Gurnev PA, Rostovtseva TK, Bezrukov SM, Mechanism of alpha-synuclein translocation through a VDAC nanopore revealed by energy landscape modeling of escape time distributions, Nanoscale (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hoogerheide DP, Gurnev PA, Rostovtseva TK, Bezrukov SM, Real-Time Nanopore-Based Recognition of Protein Translocation Success, Biophys J 114(4) (2018) 772–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rovini A, Gurnev PA, Beilina A, Queralt-Martin M, Rosencrans W, Cookson MR, Bezrukov SM, Rostovtseva TK, Molecular mechanism of olesoxime-mediated neuroprotection through targeting alpha-synuclein interaction with mitochondrial VDAC, Cell Mol Life Sci 77(18) (2020) 3611–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nussbaum RL, Genetics of Synucleinopathies, Cold Spring Harb Perspect Med 8(6) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Barrett PJ, Timothy Greenamyre J, Post-translational modification of alpha-synuclein in Parkinson’s disease, Brain Res 1628(Pt B) (2015) 247–253. [DOI] [PubMed] [Google Scholar]
- [64].Zhang J, Li X, Li JD, The Roles of Post-translational Modifications on alpha-Synuclein in the Pathogenesis of Parkinson’s Diseases, Front Neurosci 13 (2019) 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cole NB, Dieuliis D, Leo P, Mitchell DC, Nussbaum RL, Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification, Exp Cell Res 314(10) (2008) 2076–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Schlame M, Greenberg ML, Biosynthesis, remodeling and turnover of mitochondrial cardiolipin, Biochim Biophys Acta Mol Cell Biol Lipids 1862(1) (2017) 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL, Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure, Journal of lipid research 48(7) (2007) 1559–70. [DOI] [PubMed] [Google Scholar]
- [68].Aufschnaiter A, Kohler V, Diessl J, Peselj C, Carmona-Gutierrez D, Keller W, Buttner S, Mitochondrial lipids in neurodegeneration, Cell and tissue research (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ribas V, Garcia-Ruiz C, Fernandez-Checa JC, Mitochondria, cholesterol and cancer cell metabolism, Clinical and translational medicine 5(1) (2016) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Furt F, Moreau P, Importance of lipid metabolism for intracellular and mitochondrial membrane fusion/fission processes, Int. J. Biochem. Cell. B 41(10) (2009) 1828–1836. [DOI] [PubMed] [Google Scholar]
- [71].Murley A, Nunnari J, The Emerging Network of Mitochondria-Organelle Contacts, Mol Cell 61(5) (2016) 648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Crimi M, Esposti MD, Apoptosis-induced changes in mitochondrial lipids, Biochim. Biophys. Acta 1813(4) (2011) 551–557. [DOI] [PubMed] [Google Scholar]
- [73].Kagan VE, Borisenko GG, Tyurina YY, Tyurin VA, Jiang JF, Potapovich AI, Kini V, Amoscato AA, Fujii Y, Oxidative lipidomics of apoptosis: Redox catalytic interactions of cytochrome C with cardiolipin and phosphatidylserine, Free Radical Bio. Med. 37(12) (2004) 1963–1985. [DOI] [PubMed] [Google Scholar]
- [74].Pamplona R, Membrane phospholipids, lipoxidative damage and molecular integrity: A causal role in aging and longevity, Biochim. Biophys. Acta 1777(10) (2008) 1249–1262. [DOI] [PubMed] [Google Scholar]
- [75].Paradies G, Petrosillo G, Paradies V, Ruggiero FM, Mitochondrial dysfunction in brain aging: Role of oxidative stress and cardiolipin, Neurochem. Int. 58(4) (2011) 447–457. [DOI] [PubMed] [Google Scholar]
- [76].Zhu M, Li J, Fink AL, The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation, J Biol Chem 278(41) (2003) 40186–97. [DOI] [PubMed] [Google Scholar]
- [77].Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S, Acceleration of α-Synuclein Aggregation by Exosomes*, J Biol Chem 290(5) (2015) 2969–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Braun AR, Lacy MM, Ducas VC, Rhoades E, Sachs JN, alpha-Synuclein-induced membrane remodeling is driven by binding affinity, partition depth, and interleaflet order asymmetry, J Am Chem Soc 136(28) (2014) 9962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jiang Z, de Messieres M, Lee JC, Membrane remodeling by alpha-synuclein and effects on amyloid formation, J Am Chem Soc 135(43) (2013) 15970–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Jiang Z, Flynn JD, Teague WE, Gawrisch K, Lee JC, Stimulation of α-synuclein amyloid formation by phosphatidylglycerol micellar tubules, Biochimica et Biophysica Acta (BBA) - Biomembranes 1860(9) (2018) 1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Fusco G, De Simone A, Gopinath T, Vostrikov V, Vendruscolo M, Dobson CM, Veglia G, Direct observation of the three regions in alpha-synuclein that determine its membrane-bound behaviour, Nat Commun 5 (2014) 3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ulmer TS, Bax A, Comparison of structure and dynamics of micelle-bound human alpha-synuclein and Parkinson disease variants, J Biol Chem 280(52) (2005) 43179–87. [DOI] [PubMed] [Google Scholar]
- [83].Eliezer D, Kutluay E, Bussell R Jr., Browne G, Conformational properties of alpha-synuclein in its free and lipid-associated states, J Mol Biol 307(4) (2001) 1061–73. [DOI] [PubMed] [Google Scholar]
- [84].Pfefferkorn CM, Jiang Z, Lee JC, Biophysics of alpha-synuclein membrane interactions, Biochim Biophys Acta 1818(2) (2012) 162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].El-Agnaf OM, Bodles AM, Guthrie DJ, Harriott P, Irvine GB, The N-terminal region of non-A beta component of Alzheimer’s disease amyloid is responsible for its tendency to assume beta-sheet and aggregate to form fibrils, Eur J Biochem 258(1) (1998) 157–63. [DOI] [PubMed] [Google Scholar]
- [86].Ulmer TS, Bax A, Cole NB, Nussbaum RL, Structure and dynamics of micelle-bound human alpha-synuclein, J Biol Chem 280(10) (2005) 9595–603. [DOI] [PubMed] [Google Scholar]
- [87].Lokappa SB, Ulmer TS, α-Synuclein Populates Both Elongated and Broken Helix States on Small Unilamellar Vesicles, J Biol Chem 286(24) (2011) 21450–21457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kaur U, Lee JC, Membrane Interactions of α-Synuclein Probed by Neutrons and Photons, Accounts of Chemical Research (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Jiang Z, Heinrich F, McGlinchey RP, Gruschus JM, Lee JC, Segmental Deuteration of alpha-Synuclein for Neutron Reflectometry on Tethered Bilayers, J Phys Chem Lett 8(1) (2017) 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Jiang Z, Hess SK, Heinrich F, Lee JC, Molecular Details of α-Synuclein Membrane Association Revealed by Neutrons and Photons, The Journal of Physical Chemistry B 119(14) (2015) 4812–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Middleton ER, Rhoades E, Effects of curvature and composition on alpha-synuclein binding to lipid vesicles, Biophys J 99(7) (2010) 2279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Jacobs D, Hoogerheide DP, Rovini A, Jiang Z, Lee JC, Rostovtseva TK, Bezrukov SM, Probing Membrane Association of alpha-Synuclein Domains with VDAC Nanopore Reveals Unexpected Binding Pattern, Sci Rep 9(1) (2019) 4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Bezrukov SM, Vodyanoy I, Parsegian VA, Counting polymers moving through a single ion channel, Nature 370(6487) (1994) 279–281. [DOI] [PubMed] [Google Scholar]
- [94].Coulter WA, Means for counting particles suspended in a fluid, US, 1953. [Google Scholar]
- [95].Hoogerheide DP, Garaj S, Golovchenko JA, Probing Surface Charge Fluctuations with Solid-State Nanopores, Phys Rev Lett 102(25) (2009) 256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kasianowicz JJ, Bezrukov SM, Protonation dynamics of the alpha-toxin ion channel from spectral analysis of pH-dependent current fluctuations, Biophys J 69(1) (1995) 94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Deamer D, Akeson M, Branton D, Three decades of nanopore sequencing, Nat Biotechnol 34(5) (2016) 518–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kasianowicz JJ, Bezrukov SM, On ‘three decades of nanopore sequencing’, Nat Biotechnol 34(5) (2016) 481–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ouldali H, Sarthak K, Ensslen T, Piguet F, Manivet P, Pelta J, Behrends JC, Aksimentiev A, Oukhaled A, Electrical recognition of the twenty proteinogenic amino acids using an aerolysin nanopore, Nat Biotechnol 38(2) (2020) 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Thakur AK, Movileanu L, Real-time measurement of protein–protein interactions at single-molecule resolution using a biological nanopore, Nat Biotechnol 37(1) (2019) 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hoogerheide DP, Gurnev PA, Rostovtseva TK, Bezrukov SM, Effect of a post-translational modification mimic on protein translocation through a nanopore, Nanoscale 12(20) (2020) 11070–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Finkelstein A, Proton-coupled protein transport through the anthrax toxin channel, Philosophical Transactions of the Royal Society B: Biological Sciences 364(1514) (2009) 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Schiffmiller A, Anderson D, Finkelstein A, Ion selectivity of the anthrax toxin channel and its effect on protein translocation, Journal of General Physiology 146(2) (2015) 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hoogerheide DP, Rostovtseva TK, Jacobs D, Gurnev PA, Bezrukov SM, Tunable Electromechanical Nanopore Trap Reveals Populations of Peripheral Membrane Protein Binding Conformations, ACS Nano 15(1) (2021) 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Willems K, Meervelt VV, Wloka C, Maglia G, Single-molecule nanopore enzymology, Philosophical Transactions of the Royal Society B: Biological Sciences 372(1726) (2017) 20160230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mlayeh L, Krammer EM, Leonetti M, Prevost M, Homble F, The mitochondrial VDAC of bean seeds recruits phosphatidylethanolamine lipids for its proper functioning, Biochim Biophys Acta Bioenerg 1858(9) (2017) 786–794. [DOI] [PubMed] [Google Scholar]
- [107].Teijido O, Rappaport SM, Chamberlin A, Noskov SY, Aguilella VM, Rostovtseva TK, Bezrukov SM, Acidification asymmetrically affects voltage-dependent anion channel implicating the involvement of salt bridges, J Biol Chem 289(34) (2014) 23670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bowen KA, Tam K, Colombini M, Evidence for titratable gating charges controlling the voltage dependence of the outer mitochondrial membrane channel, VDAC, J Membr Biol 86(1) (1985) 51–9. [DOI] [PubMed] [Google Scholar]
- [109].Bezrukov SM, Ion Channels as Molecular Coulter Counters to Probe Metabolite Transport, J Membrane Biol 174(1) (2000) 1–13. [DOI] [PubMed] [Google Scholar]
- [110].Smeets RMM, Keyser UF, Krapf D, Wu M-Y, Dekker NH, Dekker C, Salt Dependence of Ion Transport and DNA Translocation through Solid-State Nanopores, Nano Lett 6(1) (2006) 89–95. [DOI] [PubMed] [Google Scholar]
- [111].Davidson WS, Jonas A, Clayton DF, George JM, Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes, J Biol Chem 273(16) (1998) 9443–9. [DOI] [PubMed] [Google Scholar]
- [112].Hoogerheide DP, Gurnev PA, Rostovtseva TK, Bezrukov SM, Mechanism of alpha-synuclein translocation through a VDAC nanopore revealed by energy landscape modeling of escape time distributions, Nanoscale 9(1) (2017) 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Muthukumar M, Theory of capture rate in polymer translocation, J Chem Phys 132(19) (2010) 195101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Kramers HA, Brownian motion in a field of force and the diffusion model of chemical reactions, Physica 7(4) (1940) 284–304. [Google Scholar]
- [115].Hoogerheide DP, Lu B, Golovchenko JA, Pressure-Voltage Trap for DNA near a Solid-State Nanopore, Acs Nano 8(7) (2014) 7384–7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chuang J, Kantor Y, Kardar M, Anomalous dynamics of translocation, Phys Rev E 65(1) (2001). [DOI] [PubMed] [Google Scholar]
- [117].van Kampen NG, Stochastic processes in physics and chemistry, 3rd ed., Elsevier, Amsterdam; Boston, 2007. [Google Scholar]
- [118].Hoogerheide DP, PPDiffuse: A Quantitative Prediction Tool for Diffusion of Charged Polymers in a Nanopore, J Res Natl Inst Stan 125 (2020) 125018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Hoogerheide DP, Albertorio F, Golovchenko JA, Escape of DNA from a Weakly Biased Thin Nanopore: Experimental Evidence for a Universal Diffusive Behavior, Phys Rev Lett 111(24) (2013) 248301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Keyser UF, Koeleman BN, van Dorp S, Krapf D, Smeets RMM, Lemay SG, Dekker NH, Dekker C, Direct force measurements on DNA in a solid-state nanopore, Nat Phys 2(7) (2006) 473–477. [Google Scholar]
- [121].Lu B, Hoogerheide DP, Zhao Q, Yu DP, Effective driving force applied on DNA inside a solid-state nanopore, Phys Rev E 86(1) (2012). [DOI] [PubMed] [Google Scholar]
- [122].van Dorp S, Keyser UF, Dekker NH, Dekker C, Lemay SG, Origin of the electrophoretic force on DNA in solid-state nanopores, Nature Physics 5(5) (2009) 347–351. [Google Scholar]
- [123].Rostovtseva TK, Gurnev PA, Hoogerheide DP, Rovini A, Sirajuddin M, Bezrukov SM, Sequence diversity of tubulin isotypes in regulation of the mitochondrial voltage-dependent anion channel, J Biol Chem 293(28) (2018) 10949–10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Berezhkovskii AM, Bezrukov SM, Blocker escape kinetics from a membrane channel analyzed by mapping blocker diffusive dynamics onto a two-site model, J Chem Phys 150(19) (2019) 194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Berezhkovskii AM, Bezrukov SM, Capturing single molecules by nanopores: measured times and thermodynamics, Phys Chem Chem Phys 23(2) (2021) 1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Berezhkovskii AM, Dagdug L, Bezrukov SM, Two-site versus continuum diffusion model of blocker dynamics in a membrane channel: Comparative analysis of escape kinetics, J Chem Phys 151 (2019) 054113. [Google Scholar]
- [127].Horvath SE, Daum G, Lipids of mitochondria, Prog Lipid Res 52(4) (2013) 590–614. [DOI] [PubMed] [Google Scholar]
- [128].Zigoneanu IG, Yang YJ, Krois AS, Haque E, Pielak GJ, Interaction of alpha-synuclein with vesicles that mimic mitochondrial membranes, Biochim Biophys Acta 1818(3) (2012) 512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Chibowski E, Szczes A, Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme, Adsorption 22(4–6) (2016) 755–765. [Google Scholar]
- [130].Morini MA, Sierra MS, Pedroni VI, Alarcon LM, Appignanesi GA, Disalvo EA, Influence of temperature, anions and size distribution on the zeta potential of DMPC, DPPC and DMPE lipid vesicles, Colloid Surf. B-Biointerfaces 131 (2015) 54–58. [DOI] [PubMed] [Google Scholar]
- [131].Smith MC, Crist RM, Clogston JD, McNeil SE, Zeta potential: a case study of cationic, anionic, and neutral liposomes, Anal Bioanal Chem 409(24) (2017) 5779–5787. [DOI] [PubMed] [Google Scholar]
- [132].Michalak DJ, Lösche M, Hoogerheide DP, Charge Effects Provide Ångström-Level Control of Lipid Bilayer Morphology on Titanium Dioxide Surfaces, Langmuir 37(13) (2021) 3970–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Kaur U, Lee JC, Unroofing site-specific alpha-synuclein-lipid interactions at the plasma membrane, Proc Natl Acad Sci U S A 117(32) (2020) 18977–18983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Clayton DF, George JM, The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease, Trends Neurosci 21(6) (1998) 249–54. [DOI] [PubMed] [Google Scholar]
- [135].Nakamura K, Nemani VM, Wallender EK, Kaehlcke K, Ott M, Edwards RH, Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria, The Journal of neuroscience : the official journal of the Society for Neuroscience 28(47) (2008) 12305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kaur U, Lee JC, Membrane Interactions of alpha-Synuclein Probed by Neutrons and Photons, Acc Chem Res 54(2) (2021) 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Theillet FX, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, Verzini S, Lorenz D, van Rossum M, Goldfarb D, Selenko P, Structural disorder of monomeric alpha-synuclein persists in mammalian cells, Nature 530(7588) (2016) 45–50. [DOI] [PubMed] [Google Scholar]
- [138].Al Jamal JA, Involvement of porin N, N-dicyclohexylcarbodiimide-reactive domain in hexokinase binding to the outer mitochondrial membrane, Protein J 24 (2005) 1–8. [DOI] [PubMed] [Google Scholar]
- [139].Magri A, Belfiore R, Reina S, Tomasello MF, Di Rosa MC, Guarino F, Leggio L, De Pinto V, Messina A, Hexokinase I N-terminal based peptide prevents the VDAC1-SOD1 G93A interaction and re-establishes ALS cell viability, Sci Rep 6 (2016) 34802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Gouarne C, Giraudon-Paoli M, Seimandi M, Biscarrat C, Tardif G, Pruss RM, Bordet T, Olesoxime protects embryonic cortical neurons from camptothecin intoxication by a mechanism distinct from BDNF, Br J Pharmacol 168(8) (2013) 1975–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, Petit PX, Vaz FM, Gottlieb E, Cardiolipin provides an essential activating platform for caspase-8 on mitochondria, J Cell Biol 183(4) (2008) 681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Yethon JA, Epand RF, Leber B, Epand RM, Andrews DW, Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis, The Journal of biological chemistry 278(49) (2003) 48935–41. [DOI] [PubMed] [Google Scholar]
- [143].McNally MA, Soane L, Roelofs BA, Hartman AL, Hardwick JM, The N-terminal helix of Bcl-xL targets mitochondria, Mitochondrion 13(2) (2013) 119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Renault TT, Teijido O, Antonsson B, Dejean LM, Manon S, Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x(L): keep your friends close but your enemies closer, Int J Biochem Cell Biol 45(1) (2013) 64–7. [DOI] [PubMed] [Google Scholar]
- [145].Van Gelder P, Saint N, Phale P, Eppens EF, Prilipov A, van Boxtel R, Rosenbusch JP, Tommassen J, Voltage sensing in the PhoE and OmpF outer membrane porins of Escherichia coli: role of charged residues, Journal of molecular biology 269(4) (1997) 468–72. [DOI] [PubMed] [Google Scholar]
- [146].Bainbridge G, Gokce I, Lakey JH, Voltage gating is a fundamental feature of porin and toxin beta-barrel membrane channels, FEBS Lett 431(3) (1998) 305–8. [DOI] [PubMed] [Google Scholar]
- [147].Derrington IM, Butler TZ, Collins MD, Manrao E, Pavlenok M, Niederweis M, Gundlach JH, Nanopore DNA sequencing with MspA, Proc Natl Acad Sci U S A 107(37) (2010) 16060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Mohammad MM, Iyer R, Howard KR, McPike MP, Borer PN, Movileanu L, Engineering a rigid protein tunnel for biomolecular detection, J Am Chem Soc 134(22) (2012) 9521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Bogard A, Abatchev G, Hutchinson Z, Ward J, Finn PW, McKinney F, Fologea D, Lysenin Channels as Sensors for Ions and Molecules, Sensors (Basel) 20(21) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zimmerberg J, Parsegian VA, Polymer Inaccessible Volume Changes during Opening and Closing of a Voltage-Dependent Ionic Channel, Nature 323(6083) (1986) 36–39. [DOI] [PubMed] [Google Scholar]
- [151].Mangan PS, Colombini M, Ultrasteep voltage dependence in a membrane channel, Proc Natl Acad Sci U S A 84(14) (1987) 4896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Gurnev PA, Yap TL, Pfefferkorn CM, Rostovtseva TK, Berezhkovskii AM, Lee JC, Parsegian VA, Bezrukov SM, Alpha-synuclein lipid-dependent membrane binding and translocation through the alpha-hemolysin channel, Biophys J 106(3) (2014) 556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Song L, Hobaugh MR, Shustak C, Cheley S, Bayley H, Gouaux JE, Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore, Science 274(5294) (1996) 1859–66. [DOI] [PubMed] [Google Scholar]
- [154].Gurnev PA, Nestorovich EM, Channel-forming bacterial toxins in biosensing and macromolecule delivery, Toxins (Basel) 6(8) (2014) 2483–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Gu LQ, Braha O, Conlan S, Cheley S, Bayley H, Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter, Nature 398(6729) (1999) 686–90. [DOI] [PubMed] [Google Scholar]
- [156].Krasilnikov OV, Sabirov RZ, Ion transport through channels formed in lipid bilayers by Staphylococcus aureus alpha-toxin, Gen Physiol Biophys 8(3) (1989) 213–22. [PubMed] [Google Scholar]
- [157].Merzlyak PG, Yuldasheva LN, Rodrigues CG, Carneiro CM, Krasilnikov OV, Bezrukov SM, Polymeric nonelectrolytes to probe pore geometry: application to the alpha-toxin transmembrane channel, Biophys J 77(6) (1999) 3023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Madampage C, Tavassoly O, Christensen C, Kumari M, Lee JS, Nanopore analysis: An emerging technique for studying the folding and misfolding of proteins, Prion 6(2) (2012) 116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wang HY, Gu Z, Cao C, Wang J, Long YT, Analysis of a single alpha-synuclein fibrillation by the interaction with a protein nanopore, Anal Chem 85(17) (2013) 8254–61. [DOI] [PubMed] [Google Scholar]
- [160].Queralt-Martin M, Bergdoll L, Teijido O, Munshi N, Jacobs D, Kuszak AJ, Protchenko O, Reina S, Magri A, De Pinto V, Bezrukov SM, Abramson J, Rostovtseva TK, A lower affinity to cytosolic proteins reveals VDAC3 isoform-specific role in mitochondrial biology, J Gen Physiol 152(2) (2020) e201912501. [DOI] [PMC free article] [PubMed] [Google Scholar]


