Abstract
Objective:
To study genetic variants and their function within genes coding for complement receptors in preeclampsia.
Design:
A case-control study.
Setting:
Preeclampsia is a common vascular disease of pregnancy. The clearance of placenta-derived material is one of the functions of the complement system in pregnancy.
Population:
We genotyped 500 women with preeclamptic pregnancies and 190 non-preeclamptic controls FINNPEC cohort and 122 preeclamptic women and 1905 controls from the national FINRISK cohort.
Methods:
The functional consequences of genotypes discovered targeted exomic sequencing were explored by analyzing the binding of the main ligand iC3b to mutated CR3 or CR4, which were transiently expressed on the surface of COS-1 cells.
Main Outcome Measures:
Allele frequencies were compared between preeclamptic cases and controls in genetic studies. The functional consequences of selected variants were measured by binding assays.
Results:
The most significantly preeclampsia-linked CR3 variant M441K (p=4.27E-4, OR=1.401, CI95=1.167–1.682) displayed a trend of increased adhesion to iC3b (p=0.051). CR4 variant A251T was found to enhance the adhesion of CR4 to iC3b, while W48R resulted in a decrease of the binding of CR4 to iC3b.
Conclusions:
Results suggest that changes in complement-facilitated phagocytosis are associated with preeclampsia. Further studies are needed to ascertain, whether aberrant CR3 and CR4 activity leads to altered pro- and anti-inflammatory cytokine responses in individuals carrying the associating variants and the role of these receptors in preeclampsia pathogenesis.
Funding:
This study was supported by Alfred Kordelin and by Jane and Aatos Erkko foundations, The Academy of Finland (121196 and 278941), Sigrid Jusélius Foundation and National Institutes of Health grants U54 HL112303, and R01 GM099111–20A1 (JPA).
Keywords: Preeclampsia, Pregnancy, Complement system, Beta2-integrins, Genetic association, Complement receptors
Tweetable abstract:
Genetic variants of complement receptors CR3 and CR4 have functional consequences and associate to preeclampsia.
Introduction
Preeclampsia is a common pregnancy-specific vascular disorder that affects 3% of pregnancies1. It accounts for over 50,000 maternal and 900,000 perinatal deaths annually2,3. The clinical characteristics are diverse, and the course of the disease is unpredictable.
Epidemiological evidence indicates that preeclampsia is partially inherited4–9. Among immunological pathways, abnormalities in the complement system have recently been found to be one of the contributing mechanisms in preeclampsia10–12. The complement system discriminates between self- and nonself-structures and causes inflammation, cell death, tissue destruction and initiates adaptive immune responses. Inadequate regulation of the complement system may result in poor placentation and predispose to preeclampsia13,14. Three pathways of complement activation lead to the common end-point, activation of C3 and of the terminal pathway, and membrane attack complex (MAC) formation. Genetic polymorphisms within genes coding for components of the complement system have been linked to preeclampsia susceptibility15,16. The levels of C3 are increased in inflammation but decreased or overactivated in immune diseases such as systemic lupus erythematosus (SLE), which is known to predispose to preeclampsia
When complement is activated, the generated opsonins C3b and C4b bind covalently to the targets. Since activation of C3 is a critical step in complement activation, it must be controlled carefully. An important outcome of C3 inactivation is the generation of iC3b molecules, because they are recognized on phagocytic cells by the complement receptors type 3 (CR3, CD11b/18, Mac-1, or integrin αMβ2) and 4 (CR4, CD11c/18, p150.95, or integrin αXβ2). CR3 and CR4 are β2-integrins that function as complement receptors and specifically recognize iC3b17,18. Microbes and particles coated with iC3b are efficiently phagocytosed and eradicated by neutrophils and macrophages. In the absence of alarm signals indicating infection or major tissue injury, the iC3b-mediated phagocytosis occurs in a relatively silent fashion as a basic homeostatic clearance process.
Due to the rapid growth of the placenta and direct exposure to maternal blood, it releases microparticles into the maternal circulation. We have hypothesized that in preeclampsia, a major disorder of pregnancy, the clearance of the placental particles, cells, or their remnants could be abnormal. Therefore, we have studied the genetic associations of genes coding for receptors of the complement system with preeclampsia in a large patient cohort. β2-integrins, may play an important role in mediating phagocytosis of particles e.g. from damaged endothelium or placenta, as well as because of their ability to control the inflammatory balance of the immune response.
Materials and Methods
Subjects and sequencing protocol
Briefly, we studied 500 non-obese (body mass index <30 kg/m2) women with preeclamptic pregnancies and 190 non-preeclamptic controls from the The Finnish Genetics of Preeclampsia Consortium (FINNPEC) cohort19. Data was combined with additional patients and controls from the national FINRISK study cohort to comprise altogether 609 cases and 2,092 controls for association analyses. See Table S1 in supplementary material for details. Patients were not involved in the research effort. A predefined core outcome set was not used in the study design.
In a previously described custom-made targeted exomic sequencing protocol20, we combined an improved Illumina sequencing library for capture and sequencing with Nimblegen sequence capture to study variants within exons of eleven genes coding for receptors of the complement system: ITGAM (coding for subunit of CR3), ITGAX (coding for subunit of CR4), CD93, C3AR1, ITGB2, C5AR1, C5AR2, C1qR, CR2, CR1, and CR1L. Detailed description of the study materials and methods are available elsewhere20
Functional studies
To study how the preeclampsia related variants in the CR3- and CR4-receptors affect cell adhesion to iC3b, we transiently transfected COS-1 cells with either the wild type beta2-integrin (wt-CR3 or wt-CR4) or one of the mutant variants (M441K-CR3, T1000N-CR3 or W48R-CR4, A251T-CR4). Expression was established by flow cytometry and adhesion analyses were conducted according to a previously published protocol21 (see Supplemental Methods in Appendix S1 for details).
In silico studies
Loss of function vulnerability score per gene and PolyPhen and Sift functional in silico analyses per variant were conducted in Variant effect predictor (VEP; https://www.ensembl.org/Tools/VEP) online with annotations as follows: LoF score < 0.2 = probably damaging, LoF score 0.2–0.7 = possibly damaging, LoF score < 0.7 = benign.
Modelling of CD11b structure
The CR3 structure was modelled on the basis of the previously solved CR4 structure (PDB: 3K71, chain G,22) using the PRIME module of Schrödinger suite version 2018–4, (Schrödinger LLC. 23,24 Protein structure function Bioinformatics) via the Maestro interface. The model of CD11b generated was manually checked especially in the non-similar residues with CR4. The model was also verified looking into its Ramachandran diagram.
Statistical methods
The genes with variants associated to preeclampsia are listed in Table 1. Data were analyzed in PlinkSeq, Plink25 and R programs. The significance of the allelic associations was analyzed by the Fisher’s exact test. Analysis was divided to low-frequency (minor allele frequency MAF < 10%) and common (MAF >10%) variants. Kaviar26 and VEP Build 37 were used for additional annotations27. In addition to the appropriate statistical probability test, odds ratios (OR) with 95% confidence intervals (CI95) were calculated for all variants. The Student’s t test was used for statistical analysis of the functional assays, probability value smaller than 0.05 was considered significant.
Table 1.
Associating variants within genes coding for complement receptors in preeclampsia. LoF – loss of function tool from Variant effect predictor (https://www.ensembl.org/Tools/VEP) indicates the per-gene susceptibility to disease based on the ratio of loss-of-function to synonymous mutations in ExAC data. MAF=minor allele frequency.
| RSID | Gene name | P-value | OR (95% confidence interval) | MAF (preeclampsia) | MAF (controls) | HWE | Consequence (distance from exon, base pairs) | LoFtool* |
|---|---|---|---|---|---|---|---|---|
| rs2230428 | ITGAX | 3.47E-5 | 1.416 (1.205 – 1.664) | 0.209 | 0.157 | 1 | missense variant, A251T | 0.441 |
| rs1143680 | ITGAM | 4.27E-4 | 1.401 (1.167 – 1.682) | 0.154 | 0.115 | 0.645 | missense variant, M441K | 0.543 |
| rs2230424 | ITGAX | 2.76E-4 | 1.414 (1.177 – 1.698) | 0.153 | 0.113 | 0.643 | missense variant, W48R | 0.441 |
| rs55865320 | ITGB2 | 0.019 | 1.256 (1.041 – 1.516) | 0.141 | 0.116 | 1 | intronic variant (−11) | 0.033 |
| rs201176761 | ITGAX | 0.019 | 5.634 (1.094 – 36.328) | 0.004 | <0.001 | 1 | intronic variant (−13) | 0.441 |
| rs61734513 | CR1 | 0.027 | 2.824 (1.032 – 7.515) | 0.007 | 0.003 | 1 | synonymous variant, C1907C | na |
| rs17853815 | ITGAX | 0.032 | 4.311 (0.926 – 21.758) | 0.004 | <0.001 | 1 | missense variant, E547K | 0.441 |
| rs2230528 | ITGB2 | 0.036 | 1.192 (1.013 – 1.403) | 0.200 | 0.170 | 0.904 | synonymous variant, G273G | 0.033 |
| rs41321249 | ITGAM | 0.044 | 1.639 (1.024 – 2.626) | 0.022 | 0.013 | 0.348 | missense variant, T1000N | 0.543 |
| rs41258244 | CD46 | 0.047 | 1.368 (0.995 – 1.863) | 0.051 | 0.038 | 0.083 | intronic variant (−41) | 0.983 |
| rs201463658 | CR2 | 0.050 | 4.596 (0.777 – 31.418) | 0.003 | <0.001 | 1 | missense variant, S381R | 0.952 |
LoF score < 0.2 = probably damaging, LoF score 0.2–0.7 = possibly damaging, LoF score > 0.7 = benign
Funding
This study was supported by Alfred Kordelin, Oskar Öflund, Maud Kuistila (A.I.L.) foundations and by Jane and Aatos Erkko Foundation (HL), The Academy of Finland (121196 and 278941, HL), Sigrid Jusélius Foundation and National Institutes of Health grants U54 HL112303 (JPA), and R01 GM099111–20A1 (JPA). Finnish Medical Foundation (HL), University of Helsinki Funds (HL), Special State Subsidy for Health Research (EVO funding, HL), Sakari and Päivikki Sohlberg Foundation (HL), Novo Nordisk Foundation, Signe and Ane Gyllenberg Foundation, and Foundation for Pediatric Research contributed to FINNPEC study. The funders have not influenced the design or data analysis of the study or contributed to the manuscript.
Results
Two predisposing variants in ITGAM coding for the alpha-chain of the complement receptor type 3 (CR3, CD11b) rs41321249; T1000N (p-value = 0.044) and rs1143680; M441K (p-value = 4.27E-4) were found to be associated to preeclampsia susceptibility by unadjusted association analyses. As shown in Table 1, the more significant of theses alleles, M441K, was found in 15.4% of the preeclamptic women as opposed to 11.5% of the controls.
For ITGAX coding for the alpha-chain of the complement receptor type 4 (CR4, CD11c), three predisposing variants: rs17853815; E547K (p-value = 0.032), rs2230424; W48R (p-value = 2.76E-4,) and rs2230428; A251T (p-value = 3.47E-5) were found to be associated with preeclampsia susceptibility. Of these, A251T was found in 20.9 % of patients vs. 15.7% of controls and W48R in 15.3% of patients and 11.5% of controls.
Another missense functional variant, rs201463658, was found in CR2 coding for the complement receptor type 2 (CD21) (p-value = 0.050, possibly predisposing). This causes the change S381R, which was found to be probably damaging by PolyPhen and deleterious by Sift in the in silico functional analyses. All significant results of the association analyses are listed in Table 1. All associated variants conformed to the proportions of Hardy-Weinberg equilibrium (HWE, p>0.05). With mid-range LoFtool scores of 0.543 and 0.441 in the ‘possibly damaging’ –category, the ITGAM and ITGAX genes were deemed to be somewhat resistant to deleterious effects of variants.
The tail of the p-value distribution of benign variants was as expected, suggesting that the overall study design and quality control were successful. Half of the associated variants are located in genes coding the β2-integrins, and most promising of these variants were subjected to functional studies.
Functional analyses of β2-integrin missense variants related to preeclampsia
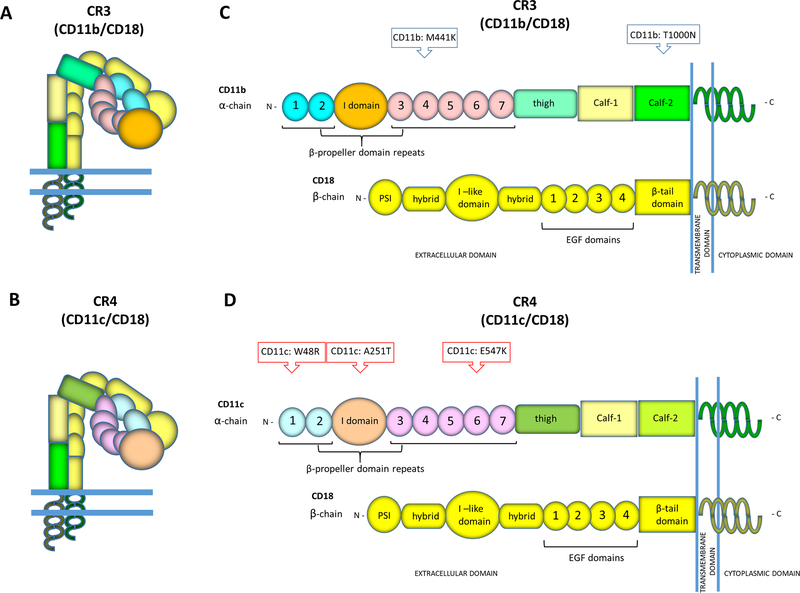
The CR3 and CR4-integrins are both heterodimeric receptors consisting of a common beta-chain (β2- or CD18) pairing with different alpha-chains: CD11b for CR3 and CD11c for CR4, respectively (Figure 1A,B). The primary protein domains of CR3 and CR4 and the relative locations of the PE-associated missense variants are shown in schematic pictures in Figures 1C and 1D, whilst the locations of the studied mutations in the structures of CR3 and CR4 are shown in Figures 2A and 2B.
Figure 1. The relative locations of the preeclampsia associated missense variants in CR3 or CR4.
The complement receptors CR3 and CR4 are heterodimeric integrin-type receptors comprising of alpha-chains, CD11b for CR3 or CD11c for CR4, that are associated non-covalently with a common beta chain CD18. In this drawing (A) CR3 and (B) CR4 are shown in their inactive and bent forms. The schematic pictures of the primary protein domains in the alpha-chains of the β2-integrins show (C) the locations of CR3-related mutations (M441K, T1000N) and (D) the CR4-related mutations (W48R, A251T, E547K).
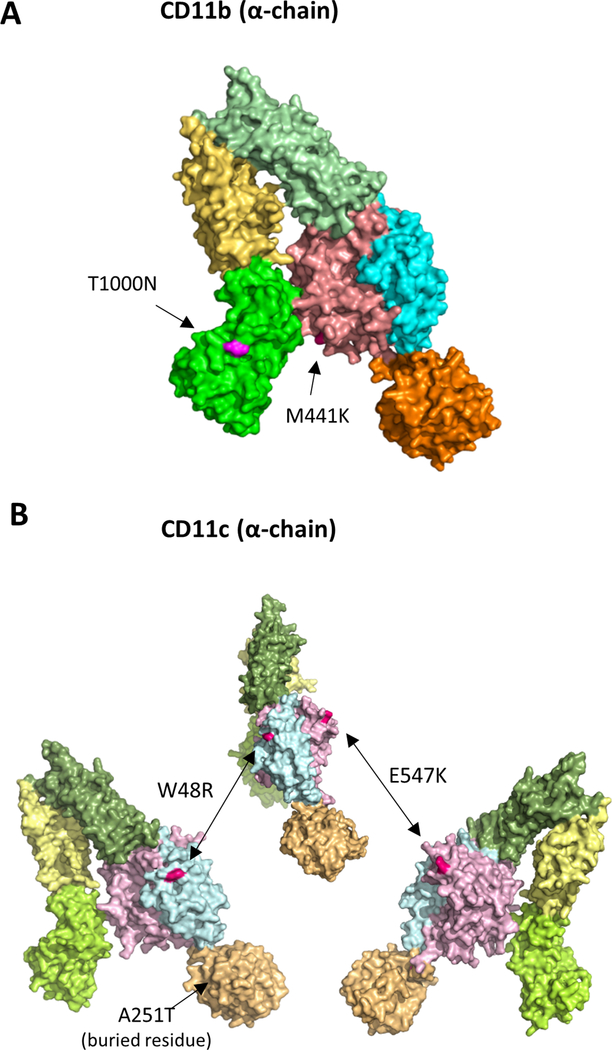
Figure 2. The locations of the preeclampsia associated missense variants shown in three-dimensional structural models of CR3 and CR4.
Structural models of CD11b (E) and CD11c (F) indicate the locations of preeclampsia-related variants (based on the crystal structure of CR422). CR4 is presented from three angles to illustrate the locations of W48R and E547K variants on opposite sides of the β-propeller domain.
Small arrows point to the positions of the mutations.
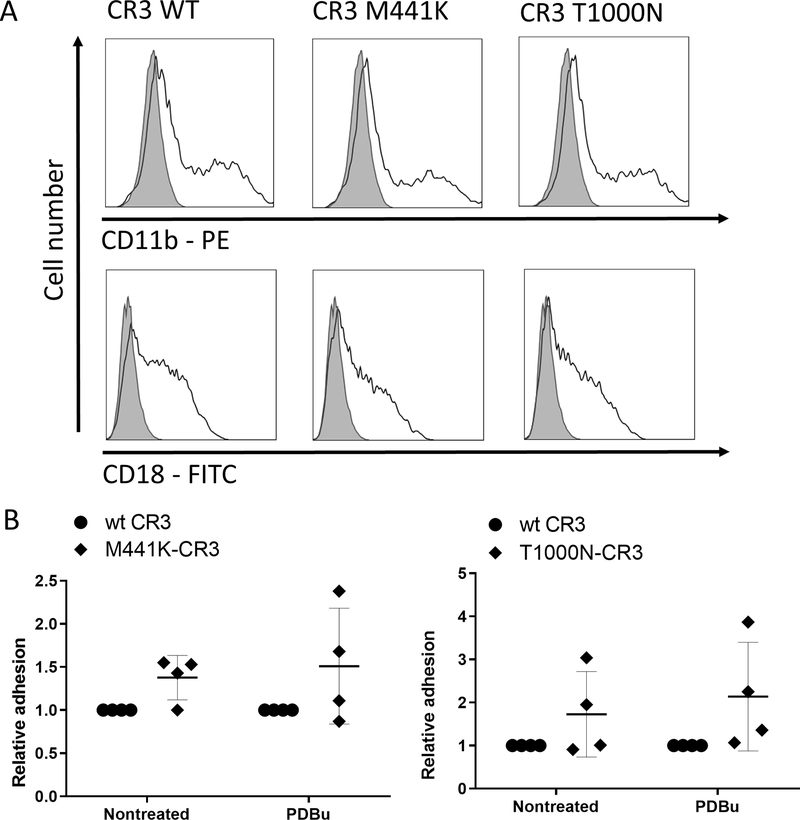
To analyze the functional effects of the preeclampsia associated CR3 and CR4 variants, COS-1 cells were transiently transfected with the variant and wild-type integrins. As analyzed by FACS, all the CR3 and CR4 variants were found to be expressed at equal quantities on the surface of COS-1 cells (Figures 3A and 4A). According to this result, none of these preeclampsia related CR3- and CR4- missense variants altered the integrin epitopes in such a way that it would prevent the binding of the β2-integrin-antibodies used in our FACS analysis. Subsequently, COS-1-transfectants were allowed to adhere to the iC3b-ligand coated on plastic. Cell binding of the mutant variants and wild type CR3 or CR4 to iC3b is shown in Figures 2B and 3B. The change of methionine 441 to lysine in CD11b resulted in a trend of increased cell adhesion of unstimulated and PDBu-stimulated M441K-CR3 transfected COS-1 cells to iC3b. A similar trend was observed in the iC3b adhesion of threonine to asparagine mutation at position 1000 (T1000N) in the Calf-2 domain of CR3 (Figure 3B).
Figure 3. The CR3 –mutant M441K, located in the β-propeller domain, adhered more strongly to iC3b than the wild type CR3-integrin, while no difference in binding between the CR3-mutant T1000N and the wild-type was observed. The latter residue is located in the membrane proximal area.
A. Representative flow cytometry histograms show an equal expression of CD11b and CD18 between the wild-type and mutant-CR3-transfected COS-1 cells. Shaded line – nontransfected cells, solid line – transfected cells.
B. Non-treated or PDBu-activated COS-1 cells transfected with wt-CD11b/CD18 or M441K-CD11b/CD18 or T1000N-CD11b/CD18 were allowed to bind to iC3b coated on plastic, and bound cells were detected enzymatically. Cell binding is reported relative to wt-CD11b/CD18 transfectant binding, the wild-type values defined as 1. Data are from 3–4 independent experiments. Error bars represent SD, horizontal lines show Mean. *p < 0.05
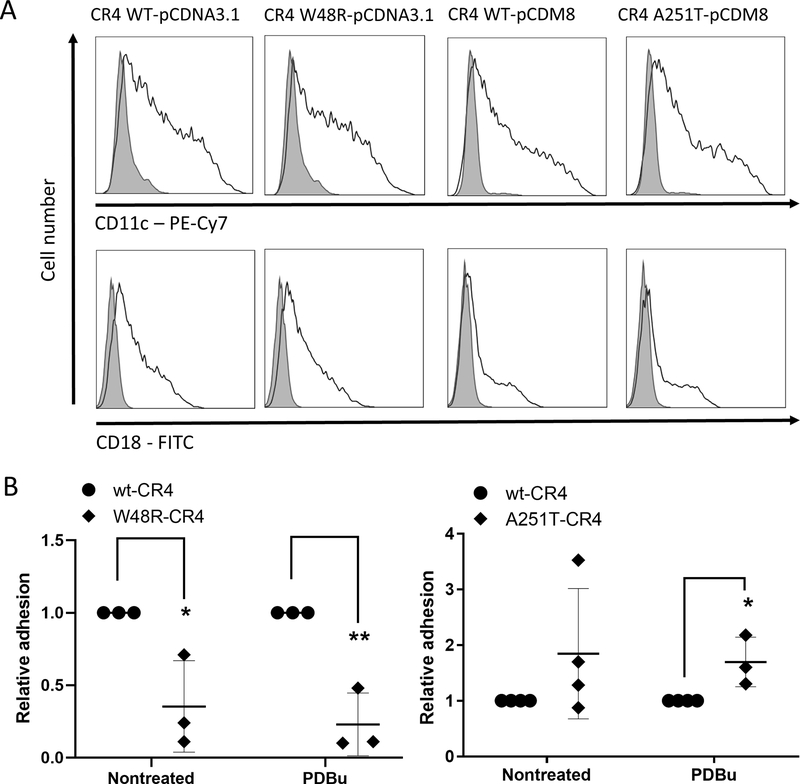
Figure 4. W48R -mutation in the β-propeller area decreases and A251T -mutation in the I-domain increases the adhesion of CR4-integrin to complement component iC3b.
A. Representative histograms of flow cytometry analysis show an equal expression of CD11c/CD18 between the wt -and mutant-CR4-transfected COS-1 cells. Shaded line – nontransfected cells, solid line – transfected cells.
B. Non-treated or PDBu-activated COS-1 cells transfected with wt-CD11c/CD18 or W48R-CD11c/CD18 or A251T-CD11c/CD18 were allowed to bind to iC3b coated on plastic, and bound cells were detected enzymatically. Cell binding is reported relative to wild-type CD11c/CD18 transfectant binding, the wild-type values defined as 1. Data are from 3–4 independent experiments. Error bars represent SD horizontal lines show Mean. *p < 0.05
The mutation of the tryptophan 48 to arginine in CR4 (W48R) decreased the binding of either untreated or PDBu-activated CR4-transfected COS-1 cells to iC3b (Figure 4B). In contrast, the mutation of alanine 251 to threonine (A251T) in the CD11c-chain increased the binding of PDBu-activated COS-1 cells to iC3b compared to wt-CD11c (Figure 4B). For unstimulated cells, the difference in binding between the mutant and wild-type CR4 was not significant.
Discussion
Main findings: Amino acid variations in CR3 and CR4 affect iC3b binding
We have discovered that two missense variants in ITGAM coding for CR3 and three missense variants in ITGAX coding for CR4 predispose to preeclampsia. The two variants with strongest association to preeclampsia in each gene were found to have varying functional effects on the respective protein.
The M441K variant in the fourth blade of β-propeller domain seems to lead to more adhering form of the integrin to iC3b, although this result was not statistically significant and thus warrants further investigation. The M441K is a common variant, with a minor allele frequency (MAF) of approximately 15%, which is strongly predisposing to preeclampsia. Another CR3 variant, M441T (rs1143680) was shown not to affect phagocytosis of iC3b-coated sheep RBC by CR3-expressing COS-7 cells28. This may reflect the requirement for a positively charged amino acid at this position for the increased adhesion to iC3b.
In accordance with M441K-CR3, the variant A251T located in the iC3b-binding I-domain of CR4 was found to increase adhesion to iC3b (Figure 4B) 18. A common variant (MAF=20%), the 251T is associated to preeclampsia with the most robust probability value among the discovered associations in complement receptor genes. The stronger adhesion of 251T-CR4 to iC3b, like 441K-CR3, may result in a shift to a more inflammatory CR4-mediated response.
In contrast to the above-mentioned variants, the variant W48R of CR4, located in the first blade of the β-propeller domain, resulted in a decrease in its binding capacity to iC3b. As the binding site for iC3b in CR4 appears to be in the I-domain, and no binding sites in the beta-propeller have been described18, the W48R effect on adhesion to iC3b is probably indirect, and may affect the binding of several ligands. Similar diminished adhesion to iC3b as observed with W48R on CR4 has been previously shown with the R77H-substituted CR3, which is associated to SLE29. The R77H mutation is located in the second blade of the β-propeller domain of CR3. In a pregnant woman, SLE is known to increase the risk of preeclampsia 2- to 4- fold 30.
Variants were observed as heterozygous mutations with the exception of one individual, who was found to be homozygous for the 1000N variant in ITGAM coding for CR3. She was diagnosed with severe late onset preeclampsia complicated by HELLP-syndrome (hemolysis, elevated liver enzymes and low platelets) postpartum.
We found that the T1000N predisposing variant of CR3 adhered to iC3b similarly as the wild type CR3 integrin or may possibly have an increasing effect. The implications of T1000N are discussed in the online supplementary Appendix S2. The I-domain in the α-chains of CR3 and CR4 has been reported to be the major binding site for iC3b31–33. Furthermore, the β-propeller region of CR3 and especially the 4th blade have been suggested to play a role in iC3b binding18,34. These results together with our earlier observations on C3 suggest a contribution of abnormal interactions between iC3b and its receptors to preeclampsia.
Strengths and limitations
The FINNPEC cohort is exhaustively characterized and allows for specific clinical phenotyping with severe disease, which is a strength in our study. None of the observed variants were unique to the patients and they are therefore unlikely to be the only causative factor of preeclampsia in our cohort. While iC3b is the main ligand for β2-integrins, the interactions with other ligands were not studied.
Interpretations
The important phagocytic complement receptors CR3 and CR4 belong to the family of β2-integrins,surface receptors of leukocytes that play critical roles in innate and adaptive immune responses. Human CR3 and CR4 share significant similarity; they are 87% homologous according to the sequence analysis of their encoding cDNAs35,36. However, they bind to distinct sites on iC3b18. CR3 and CR4 are predominantly expressed on myeloid cells including neutrophilic granulocytes, monocytes, macrophages and dendritic cells but are also found on B- and T-lymphocytes and lymphoid natural killer (NK) cells37. Although both integrins are expressed by similar cell types, their patterns and functions can be different38–40. CR3 and CR4 havebeen reported to bind many of the same ligands, including iC3b, ICAM-1 and fibrinogen37,41.
We propose that the genetic variants in the CR3 and CR4 may affect the ability of the maternal system to respond to placental or endothelial injury or influence other interactions that these receptors may have during pregnancy. For example, changes in the ability to clear placental debris from the maternal circulation may alter the waste removal and consequent inflammatory or coagulation system homeostasis during pregnancy. A key causative mechanism of early onset preeclampsia is suggested to be the shedding of fragmented syncytiotrophoblast particles from the placenta into the maternal circulation42. Accumulating material in blood vessels and kidney capillaries could thus increase vascular resistance and lead to increased blood pressure and kidney dysfunction with consequent proteinuria43,44. A correlation of complement activation and antiangiogenic activity has been established, although it remains unclear whether complement activation is the cause or the consequence of the antiangiogenic balance observed in preeclampsia 45.
The phagocytosis of placental material is assisted by blood opsonins, such as the complement component iC3b, which is recognized by the CR3 and CR4 receptors on phagocytic cells46,47,48. Opsonization helps the body to maintain homeostasis and avoid further tissue damage. Apoptotic cells opsonized by iC3b can be ingested by macrophages and dendritic cells through CR3 and CR4 without triggering inflammatory responses such as release of oxygen radicals or proinflammatory cytokines49–52. β2-integrins also mediate the interactions between immune cells, including the formation of the immunological synapse between the T-cell and the antigen-presenting cell. β2-integrins also influence pro-and anti-inflammatory signaling pathways of leukocytes and affect cytokine production40. β2-integrins promote leukocyte extravasation by facilitating interactions between the leukocytes and endothelium. Leukocyte-endothelial interaction is increased in preeclampsia53.
The I-domain in the α-chains of CR3 and CR4 has been reported to be the major binding site for iC3b31–33. Furthermore, the β-propeller region of CR3 and especially the 4th blade have been suggested to play a role in iC3b binding18,34. These results together with our earlier observations on C3 suggest a contribution of abnormal interactions between iC3b and its receptors to preeclampsia.
The engagement of CR3 by iC3b on macrophages may result in an immune-inhibitory responses, such as production of the anti-inflammatory cytokines IL-10 and TGF-beta that may block the function of NF-κB, the transcription factor needed for promotion of the transcription of many pro-inflammatory genes54. Increased serum levels of IL-10 have been reported in the third trimester of preeclamptic pregnancies55. However, CR3 can also have strong inflammatory effects in cells neutrophils and monocytes40,56,57,58.
Women with small for gestational age neonates have a significantly higher expression of CR3 in their peripheral blood granulocytes and monocytes than normal pregnant women. Interestingly, slightly elevated expression levels of CR3 have been observed in granulocytes in preeclamptic women59. Furthermore, a significantly higher median mean channel brightness (MCB) of CR3 and lower MCB of CD62L in granulocytes and high CR3 in monocytes in association with oxidative burst and basal iROS concentration have been observed in preeclamptic women60. In contrast, CR4-expressing monocytes have been observed in normal amounts in the peripheral blood of preeclamptic women61. CR4 contributes to proinflammatory functions of monocytes, macrophages and dendritic cells.
Conclusion
It is likely that preeclampsia develops as a result of multiple genetic and/or environmental factors. Our results reflect the complex roles of CR3 and CR4 function in downstream signalling and leukocyte function, because β-integrins are clearly involved in both pro-inflammatory and anti-inflammatory effects. Future research will decipher, whether disturbances in the functions of integrins, such as those reported here, contribute to a shift towards a more pro-inflammatory immune response and if the phenomenon is observed in non-severe as well as severe preeclampsia. On the other hand, in discordant cases a disruption in the function of these key receptors may result in dysregulation of inflammation in early pregnancy, or perhaps more importantly, clearance of placental particles or trophoblast cells during late pregnancy.
Supplementary Material
Acknowledgements
The Finnish Genetics of Pre-eclampsia Consortium (FINNPEC) Core Investigator Group
Principal investigator Hannele Laivuori, Members: Seppo Heinonen, Obstetrics and Gynecology, University of Helsinki and Helsinki University Hospital; Eero Kajantie, PEDEGO Research Unit, Medical Research Center Oulu, Oulu University Hospital and University of Oulu Public. Health Promotion Unit, National Institute for Health and Welfare; Juha Kere, Department of Biosciences and Nutrition, Karolinska Institutet, Huddinge, Sweden. Folkhälsan Research Center, Helsinki, Finland School of Basic & Medical Biosciences, King’s College London, London, United Kingdom; Katja Kivinen, Institute for Molecular Medicine Finland, Helsinki Institute of Life Science, University of Helsinki, Helsinki, Finland; Anneli Pouta, Department of Government Services, National Institute for Health and Welfare, Helsinki
Funding
This study was supported by Alfred Kordelin, Oskar Öflund, Maud Kuistila (A.I.L.) foundations and by Jane and Aatos Erkko Foundation (HL), The Academy of Finland (121196 and 278941, HL), Sigrid Jusélius Foundation and National Institutes of Health grants U54 HL112303 (JPA), and R01 GM099111-20A1 (JPA). Finnish Medical Foundation (HL), University of Helsinki Funds (HL), Special State Subsidy for Health Research (EVO funding, HL), Sakari and Päivikki Sohlberg Foundation (HL), Novo Nordisk Foundation, Signe and Ane Gyllenberg Foundation, and Foundation for Pediatric Research contributed to FINNPEC study. The funders have not influenced the design or data analysis of the study or contributed to the manuscript.
Footnotes
Disclosure of interests
Dr. Lokki reports personal fees from Alexion, outside the submitted work Dr.; Salmon reports personal fees from ReAlta Life Sciences, grants from UCB, personal fees from UCB, outside the submitted work. The remaining authors have no disclosures. Completed disclosure of interest forms are available to view online as supporting information.
Ethics statement
All subjects provided a written informed consent and the FINNPEC study protocol was approved by the coordinating Ethics Committee of the Hospital District of Helsinki and Uusimaa (permit number 149/E0/07). The National Finrisk cohort was accessed by FINRISK licence # 8/2016. National FINRISK Study description and ethical approvals are available online: https://www.thl.fi/documents/10531/1921702/2015+FINRISK+description_for_researchers_final.pdf/fc952cba-86f6-4ef5-8ef2-fa13c23173c3.
This article has a Video Abstract presented by A. Inkeri Lokki.
• Vimeo URL (https://vimeo.com/503496322)
• Shareable link to the Video
References
- 1.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013. December;209(6):544.e1–544.e12. [DOI] [PubMed] [Google Scholar]
- 2.Van Lerberghe W, Manuel A, Matthews Z, Cathy W. The World Health Report 2005 - make every mother and child count. World Health Organization; 2005. [Google Scholar]
- 3.Duley L The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009. June;33(3):130–7. [DOI] [PubMed] [Google Scholar]
- 4.Harrison GA, Humphrey KE, Jones N, Badenhop R, Guo G, Elakis G, et al. A genomewide linkage study of preeclampsia/eclampsia reveals evidence for a candidate region on 4q. Am J Hum Genet. 1997. May;60(5):1158–67. [PMC free article] [PubMed] [Google Scholar]
- 5.Arngrimsson R, Siguroardottir S, Frigge ML, Bjarnadottir RI, Jonsson T, Stefansson H, et al. A genome-wide scan reveals a maternal susceptibility locus for pre-eclampsia on chromosome 2p13. Hum Mol Genet. 1999. September;8(9):1799–805. [DOI] [PubMed] [Google Scholar]
- 6.Salonen Ros H, Lichtenstein P, Lipworth L, Cnattingius S. Genetic effects on the liability of developing pre-eclampsia and gestational hypertension. Am J Med Genet. 2000;91(4):256–60. [PubMed] [Google Scholar]
- 7.Lachmeijer AM, Arngrimsson R, Bastiaans EJ, Frigge ML, Pals G, Sigurdardottir S, et al. A genome-wide scan for preeclampsia in the Netherlands. Eur J Hum Genet. 2001. October;9(10):758–64. [DOI] [PubMed] [Google Scholar]
- 8.Moses EK, Lade JA, Guo G, Wilton AN, Grehan M, Freed K, et al. A genome scan in families from Australia and New Zealand confirms the presence of a maternal susceptibility locus for pre-eclampsia, on chromosome 2. Am J Hum Genet. 2000. December;67(6):1581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laivuori H, Lahermo P, Ollikainen V, Widen E, Haiva-Mallinen L, Sundstrom H, et al. Susceptibility loci for preeclampsia on chromosomes 2p25 and 9p13 in Finnish families. Am J Hum Genet. 2003. January;72(1):168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girardi G Complement activation, a threat to pregnancy. Semin Immunopathol. 2017. September 12; 40(1):103–111. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Kong L-R, Ge Q, Lu Y-Y, Hong M-N, Zhang Y, et al. Complement 5a-mediated trophoblasts dysfunction is involved in the development of pre-eclampsia. J Cell Mol Med. 2018. November 23;22(2):1034–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lokki AI, Heikkinen-Eloranta JK, Laivuori H. The Immunogenetic Conundrum of Preeclampsia. Front Immunol. 2018. November 13;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lokki AI, Heikkinen-Eloranta J, Jarva H, Saisto T, Lokki M-L, Laivuori H, et al. Complement activation and regulation in preeclamptic placenta. Front Immunol. 2014;5July;5:312.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmon JE, Heuser C, Triebwasser M, Liszewski MK, Kavanagh D, Roumenina L, et al. Mutations in Complement Regulatory Proteins Predispose to Preeclampsia: A Genetic Analysis of the PROMISSE Cohort. PLoS Med. 2011;8(3):e1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lokki AI, Kaartokallio T, Holmberg V, Onkamo P, Koskinen LLE, Saavalainen P, et al. Analysis of Complement C3 Gene Reveals Susceptibility to Severe Preeclampsia. Front Immunol. 2017;8:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banadakoppa M, Balakrishnan M, Yallampalli C. Common variants of fetal and maternal complement genes in preeclampsia: pregnancy specific complotype. Sci Rep. 2020. December 1;10(1): 4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bajic G, Yatime L, Sim RB, Vorup-Jensen T, Andersen GR. Structural insight on the recognition of surface-bound opsonins by the integrin I domain of complement receptor 3. Proc Natl Acad Sci. 2013. October 8;110(41):16426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu S, Wang J, Wang J-H, Springer TA. Distinct recognition of complement iC3b by integrins α X β 2 and α M β 2. Proc Natl Acad Sci. 2017. March 28;114(13):3403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jääskeläinen T, Heinonen S, Kajantie E, Kere J, Kivinen K, Pouta A, et al. Cohort profile: the Finnish Genetics of Pre-eclampsia Consortium (FINNPEC). BMJ Open. 2016;6:e013148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inkeri Lokki A, Daly E, Triebwasser M, Kurki MI, Roberson EDO, Häppölä P, et al. Protective Low-Frequency Variants for Preeclampsia in the Fms Related Tyrosine Kinase 1 Gene in the Finnish Population. Hypertension. 2017;70(2): 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uotila LM, Aatonen M, Gahmberg CG. Integrin CD11c/CD18 α-chain phosphorylation is functionally important. J Biol Chem. 2013. November 15;288(46):33494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie C, Zhu J, Chen X, Mi L, Nishida N, Springer TA. Structure of an integrin with an αI domain, complement receptor type 4. EMBO J. 2010. February 3;29(3):666–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson MP, Friesner RA, Xiang Z, Honig B. On the role of the crystal environment in determining protein side-chain conformations. J Mol Biol. 2002; July 12;320(3):597–608. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson MP, Pincus DL, Rapp CS, Day TJ, Honig B, Shaw DE, et al. A hierarchical approach to all-atom protein loop prediction\rAccurate and efficient loop selections by the DFIRE-based all-atom statistical potential\rModeling structurally variable regions in homologous proteins with rosetta. Proteins. 2004; May;55(2):351–367. [DOI] [PubMed] [Google Scholar]
- 25.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007. September;81(3):559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glusman G, Caballero J, Mauldin DE, Hood L, Roach JC. Kaviar: an accessible system for testing SNV novelty. Bioinformatics. 2011. November 15;27(22):3216–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, Cunningham F. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010. August 15;26(16):2069–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts AL, Thomas ER, Bhosle S, Game L, Obraztsova O, Aitman TJ, et al. Resequencing the susceptibility gene, ITGAM, identifies two functionally deleterious rare variants in systemic lupus erythematosus cases. Arthritis Res Ther. 2014. May 21;16(3):R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacPherson M, Lek HS, Prescott A, Fagerholm SC. A systemic lupus erythematosus-associated R77H substitution in the CD11b chain of the Mac-1 integrin compromises leukocyte adhesion and phagocytosis. J Biol Chem. 2011. May 13;286(19):17303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clowse MEB, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol. 2008. August;199(2):127.e1–127.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilsland CA, Diamond MS, Springer TA. The leukocyte integrin p150,95 (CD11c/CD18) as a receptor for iC3b. Activation by a heterologous beta subunit and localization of a ligand recognition site to the I domain. J Immunol. 1994. May 1;152(9):4582–9. [PubMed] [Google Scholar]
- 32.Diamond MS, Garcia-Aguilar J, Bickford JK, Corbi AL, Springer TA. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993. February;120(4):1031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Yu Y, Mi L-Z, Walz T, Springer TA. Molecular basis for complement recognition by integrin αXβ2. Proc Natl Acad Sci U S A. 2012. March 20;109(12):4586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Zhang L. The fourth blade within the beta-propeller is involved specifically in C3bi recognition by integrin alpha M beta 2. J Biol Chem. 2003. September 5;278(36):34395–402. [DOI] [PubMed] [Google Scholar]
- 35.Corbi AL, Miller LJ, O’Connor K, Larson RS, Springer TA. cDNA cloning and complete primary structure of the alpha subunit of a leukocyte adhesion glycoprotein, p150,95. EMBO J. 1987. December 20;6(13):4023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbi AL, Kishimoto TK, Miller LJ, Springer TA. The human leukocyte adhesion glycoprotein Mac-1 (complement receptor type 3, CD11b) alpha subunit. Cloning, primary structure, and relation to the integrins, von Willebrand factor and factor B. J Biol Chem. 1988. September 5;263(25):12403–11. [PubMed] [Google Scholar]
- 37.Arnaout MA. Biology and structure of leukocyte β 2 integrins and their role in inflammation. F1000Research. 2016. October 4;5:2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jawhara S, Pluskota E, Cao W, Plow EF, Soloviev DA. Distinct Effects of Integrins αXβ2 and αMβ2 on Leukocyte Subpopulations during Inflammation and Antimicrobial Responses. Deepe GS, editor. Infect Immun. 2017. January;85(1) e00644–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erdei A, Lukácsi S, Mácsik-Valent B, Nagy-Baló Z, Kurucz I, Bajtay Z. Non-identical twins: Different faces of CR3 and CR4 in myeloid and lymphoid cells of mice and men. Semin Cell Dev Biol. 2019;85:110–21. [DOI] [PubMed] [Google Scholar]
- 40.Schittenhelm L, Hilkens CM, Morrison VL. β2 Integrins As Regulators of Dendritic Cell, Monocyte, and Macrophage Function. Front Immunol. 2017. December 20;8:1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Podolnikova NP, Podolnikov A V., Haas TA, Lishko VK, Ugarova TP. Ligand Recognition Specificity of Leukocyte Integrin α M β 2 (Mac-1, CD11b/CD18) and Its Functional Consequences. Biochemistry. 2015. February 17;54(6):1408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redman CWG, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009. March;30 Suppl A:S38–42. [DOI] [PubMed] [Google Scholar]
- 43.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003. March;111(5):649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugimoto H, Hamanog Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, et al. Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem. 2003; April 11;278(15):12605–8. [DOI] [PubMed] [Google Scholar]
- 45.Yonekura Collier AR, Zsengeller Z, Pernicone E, Salahuddin S, Khankin E V, Karumanchi SA. Placental sFLT1 is associated with complement activation and syncytiotrophoblast damage in preeclampsia. Hypertens pregnancy. 2019;38(3):193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hart SP, Smith JR, Dransfield I. Phagocytosis of opsonized apoptotic cells: roles for “old-fashioned” receptors for antibody and complement. Clin Exp Immunol. 2004. February;135(2):181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupuy AG, Caron E. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J Cell Sci. 2008. June 1;121(11):1773–83. [DOI] [PubMed] [Google Scholar]
- 48.Morelli AE, Larregina AT, Shufesky WJ, Zahorchak AF, Logar AJ, Papworth GD, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003. January 15;101(2):611–20. [DOI] [PubMed] [Google Scholar]
- 49.Wright SD, Silverstein SC. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983. December 1;158(6):2016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aderem AA, Wright SD, Silverstein SC, Cohn ZA. Ligated complement receptors do not activate the arachidonic acid cascade in resident peritoneal macrophages. J Exp Med. 1985. March 1;161(3):617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997. June 2;185(11):1987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skoberne M, Somersan S, Almodovar W, Truong T, Petrova K, Henson PM, et al. The apoptotic-cell receptor CR3, but not vbeta5, is a regulator of human dendritic-cell immunostimulatory function. Blood. 2006. August 1;108(3):947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension. 2002; 39:155–160. [DOI] [PubMed] [Google Scholar]
- 54.Amarilyo G, Verbovetski I, Atallah M, Grau A, Wiser G, Gil O, et al. iC3b-opsonized apoptotic cells mediate a distinct anti-inflammatory response and transcriptional NF-κB-dependent blockade. Eur J Immunol. 2010. March;40(3):699–709. [DOI] [PubMed] [Google Scholar]
- 55.Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, et al. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol (New York, NY 1989). 2013. November;70(5):412–27. [DOI] [PubMed] [Google Scholar]
- 56.Rezzonico R, Chicheportiche R, Imbert V, Dayer JM. Engagement of CD11b and CD11c beta2 integrin by antibodies or soluble CD23 induces IL-1beta production on primary human monocytes through mitogen-activated protein kinase-dependent pathways. Blood. 2000; June 15;95(12):3868–77. [PubMed] [Google Scholar]
- 57.Fagerholm SC, Guenther C, Llort Asens M, Savinko T, Uotila LM. Beta2-Integrins and Interacting Proteins in Leukocyte Trafficking, Immune Suppression, and Immunodeficiency Disease. Front Immunol. 2019. February 19;10:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, et al. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996. December;5(6):653–66. [DOI] [PubMed] [Google Scholar]
- 59.Oggé G, Romero R, Chaiworapongsa T, Gervasi MT, Pacora P, Erez O, et al. Leukocytes of pregnant women with small-for-gestational age neonates have a different phenotypic and metabolic activity from those of women with preeclampsia. J Matern Neonatal Med. 2010; June;23(6):476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. In: American Journal of Obstetrics and Gynecology. 2001. October;185(4):792–7. [DOI] [PubMed] [Google Scholar]
- 61.Bajnok A, Ivanova M, Rigó J, Toldi G. The Distribution of Activation Markers and Selectins on Peripheral T Lymphocytes in Preeclampsia. Mediators Inflamm. 2017; 8045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.