Abstract
Introduction
The therapy to reduce urinary oxalate excretion in primary hyperoxaluria type 1 is still required.
Case presentation
A 37‐year‐old hemodialyzed man suffered from systemic oxalosis secondary to primary hyperoxaluria type 1 exhibited a drastic plasma oxalate decrease from 110 to 22 µmol/L two months after adjunction of lanthanum carbonate to classical treatment (intensive hemodialysis with pyridoxine). A 34‐year‐old woman with normal kidney function presented 10 years of bilateral kidney stones due to primary hyperoxaluria type 1 [hyperoxaluria (109.2 mg/24 h), plasma oxalate (56.0 µmol/L)]. The oxalate level remained uncontrolled despite of low oxalate‐normal calcium diet, pyridoxine and increased water intake though the lanthanum carbonate adjunction resulted in significant decrease in plasma oxalate and oxaluria.
Conclusion
We report the lanthanum efficacy in reducing circulating and urinary oxalate levels in type 1 primary hyperoxaluria. Possible mechanism of observed falls in oxalate concentration would be a decrease in the intestinal absorption of oxalate.
Keywords: kidney stones, lanthanum carbonate, nephrocalcinosis, oxalate, primary hyperoxaluria type 1
Abbreviations & Acronyms
- AGT
alanine:glyoxylate aminotransferase
- CaOx
calcium Ox
- COM
CaOx monohydrate
- EMA
European Medicine Agency
- ESRD
end stage renal disease
- LaC
lanthanum carbonate
- Ox
oxalate
- PN
pirydoxine
- siRNA
small‐interfering RNA
Keynote message.
The therapy to reduce urinary oxalate excretion in primary hyperoxaluria type 1 is still required. We report lanthanum carbonate (LaC) encouraging effects on plasma and urine oxalate in two patients with primary hyperoxaluria type 1. The possible mechanism of observed falls in plasma oxalate concentration would be a decrease in the intestinal absorption of oxalate and/or increase in intestinal oxalate excretion.
Introduction
PH1 is a rare autosomal recessive inborn disorder leading to overproduction of Ox by impairing activity of the liver enzyme AGT involved in glyoxylate metabolism. 1 As an end product, Ox is mainly eliminated by the kidney and prompt the formation of type Ic kidney stones and intratubular precipitation of insoluble COM (whewellite). The heavy, chronic crystalluria induces tubulointerstitial damages that lead to progressive renal function decline associated with the risk of ESRD. 2 , 3
Once the capacity of urinary excretion of Ox is overloaded, 4 CaOx precipitates in all vital organs resulting in fine in irreversible systemic oxalosis. 5 The main goals in the management of PH1 patients consist in strict control of plasma oxalate level and oxaluria by reducing: (i) urinary Ox saturation, (ii) exogenous source of Ox, (iii) endogenous synthesis of Ox, and/or (iv) intestinal CaOx availability. 6 , 7 Targeting endogenous synthesis and exogenous source of Ox in PH1 seems to be the best further therapeutic approach. 5 , 8 , 9 Specific inhibition of hepatic glycolate oxidase or lactate dehydrogenase impairing endogenous Ox synthesis is under clinical investigations. Despite of Oxalobacter formigenes based therapy control exogenous oxaluria in short term, its long‐term efficacy has been reported unsuccessful. 10 Lanthanum is a rare earth metal with high Ox‐binding capacity in rat model of secondary hyperoxaluria. We report LaC encouraging effects on plasma and urine oxalate in two patients with PH1.
Case description
Case 1
A 37‐year‐old Tunisian man with PH1 (AGXT mutation has been confirmed by genetic testing), hemodialyzed because of anuric ESRD was admitted for dyspnea and right lateral thoracic pain. At his physical examination, we noticed marked cachexia and general amyotrophia, and total abolishment of sound at right lung. Blood tests demonstrated: hemoglobin: 7.2 (N 13.0–18.0 g/dL), urea and creatinine: 91 and 6.13 (N 13–47 and 0.72–1.17 mg/dL, respectively), GFR (CKD‐EPI): 11 ml/min/1.73 m2, bicarbonate: 21 (N 22–29 mmol/L), phosphorus: 2.04 (N 0.81–1.45 mmol/L), calcidiol: 22.4 (N 30–80 µg/L), and normal parathyroid hormone level.
The imaging explorations demonstrated calcified granulomas of the right lung upper lobe and right pleural effusion with passive atelectasis, right nephrocalcinosis (left kidney has been removed because of recurrent infections in the past) and severe oxalate osteopathy (Fig. 1a–c). We found diffuse CaOx deposits and areas of bone formation on pleural biopsy (Fig. 1d). Therefore, we intensified hemodialysis (4h, 5 times weekly) and introduced PN (250 mg, twice daily) to control oxalemia. As a phosphate levels remained uncontrolled, we introduced LaC (1000 mg, three times daily). Under this combination we observed normalization of oxalemia (Fig. 1e,f) but we were unable to evaluate the effect of LaC on oxaluria considering anuric stage of ESRD. He came back to his country and we have no follow‐up data.
Fig. 1.
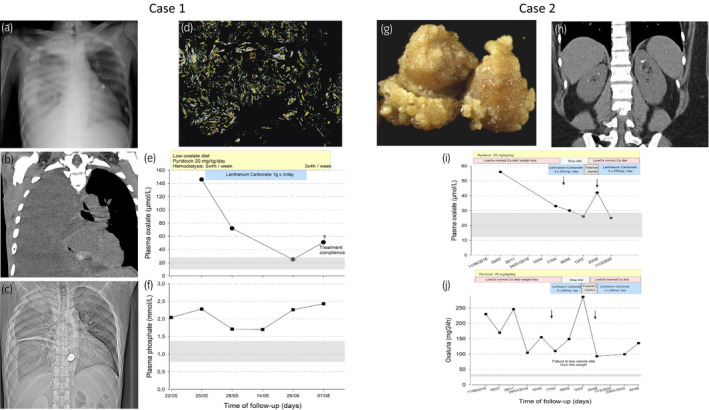
Representative images of chest radiography (a and c) and chest tomography (b) right pleural effusion and marked oxalate osteopathy. (d) Diffuse oxalate calcium depositions within the pleural biopsy (bipolarized microscopy). Graphical representation of plasma oxalate and phosphoremia (e and f, respectively) time course during the follow up of patient 1. Photography of kidney stones with typical futures of type Ic: budding surface with light cream to pale yellow, sometimes whitish areas (g) and abdominal tomography with bilateral kidney stones (h). Graphical representation of plasma oxalate and urinary oxalate excretion (i and j, respectively) time course during the follow up of patient 2
Case 2
A 34‐year‐old woman with normal kidney function presented 10‐years history of severe bilateral kidney stone disease secondary to PH1 (AGXT mutation has been confirmed by genetic testing and morphoconstitutional analysis by stereomicroscopy for stone typing (here type Ic suggestive for PH1) and sequential analysis by Fourier transform infrared spectroscopy from the core to the surface. Infrared analysis provided a spectrum of pure whewellite (100% calcium oxalate) (Fig. 1g–j). She reported constant gravel elimination and recurrent bilateral acute nephritic colic 2–3 per month. Her physical examination was marked by abdominal obesity and sensitivity of both flanks. Her 24‐h metabolic work‐up demonstrated Urine volume 2000 ml/24 h, uricuria 628 mg/24 h (31.4 mg/dL), natriuresis 259 mg/24 h (129 mmol/L), chloruria 269 mmol/24 h (135 mmol/L), calciuria2.2 mmol/24 h (1.11 mmol/L), magnesuria 4.1 mmol/24 h (2.03 mmol/L), citraturia 1824 µmol/24 h (1569 µmol/L), oxalaturia 109.2 mg/24 (Oxalate/creatinine ratio 0.11) and hyperoxalemia (56.0 µmol/L). Her whewellite crystalluria (447 crystals/µl, volume 12.644 µm3/µL) disappeared under low Ox‐normal Ca diet, increased water intake, PN and potassium citrate supplementation. However, circulating Ox level remained uncontrolled. Therefore, we introduced LaC based on our previous observation. Considering the normal renal function, we advised 250 mg three times daily to avoid hypophosphatemia. The LaC was well tolerated. No hypophosphatemia was observed during the 6 months of follow‐up. She stopped the LaC during few weeks, the circulating and urinary Ox levels increased. Once LaC reintroduced, we noticed normalization of circulating Ox level and nearly 50% decrease in urinary oxalate excretion rate (Fig. 1i, j).
Discussion
To the best of our knowledge, we report for the first time the effect of LaC on circulating and urinary Ox in a patient with PH1 at anuric ESRD stage with systemic oxalosis as well as in a patient with normal kidney function. We recognize following limitations of our observations: (i) we intensified extrarenal Ox removal in the first case and (ii) reported follow‐up is short. However, in both patients Ox remained uncontrolled under recommended supportive therapy. We achieved even lower levels of plasma Ox that actually advised target (30 to 50 µmol/L) 11 , 12 only after deferred adjunction of LaC. 8
The mechanism of Ox synthesis being incompletely understood and the main source of Ox in PH1 patients remain to be clarified. 13 On the contrary, the contribution of intestinal absorption of dietary Ox to urinary excretion of Ox and kidney stones formation is well recognized in secondary hyperoxaluria 1 , 2 , 14 but not obvious in PH1. 14
Recently, U.S. Food and Drug Administration and EMA approved the first RNA interference drug “lumasiran,” a siRNA double stranded that target specifically the 3’ untranslated region of hydroxyacid oxidase‐1 RNA and inhibit the synthesis of glycolate oxidase in the liver therefore decrease endogenous oxalate production. Compared to lumasiran, LaC operates by different mechanism. The rationale for the use of LaC to manage PH1 patients is solid. Its long‐term safety profile is positive. 15 , 16 In vitro, LaC has a high affinity for Ox across the entire pH range encountered in the gastrointestinal tract and reduce oxalate intestinal absorption. 17 Possible mechanism of observed falls in plasma oxalate concentration would be a decrease in the intestinal absorption of oxalate and/or increase in intestinal oxalate excretion. 18
In vivo, LaC reduces serum Ox concentration and urinary Ox excretion in a rat model of hyperoxaluria induced by ethylene glycol intoxication. 17 Additionally, LaC do not affects calciuria in rats with normal renal function and in healthy volunteers. 19 Importantly, citrate (current kidney stones recurrence preventive therapy) does not have any effect on lanthanum absorption. The bioavailability of La is very low (0.00127 ± 0.0008%) and the urinary excretion is less than 1% because >99% of La is excreted by the bile. The main reason for use in patients with ESRD at anuric stage. 15
Given that no treatment modality has yet been reported to affect urinary Ox excretion in PH1 patients by lowering intestinal Ox absorption, the significant fall in plasma Ox concentration in our patient with PH1 is intriguing. Our data suggest that LaC by binding Ox in the intestinal lumen reduces the Ox absorption throughout gastrointestinal tract and simultaneously Ox transport might be directed towards Ox secretion from plasma to intestinal lumen rather than absorption thus contributing to the decrease in plasma Ox (Fig. 2b).
Fig. 2.
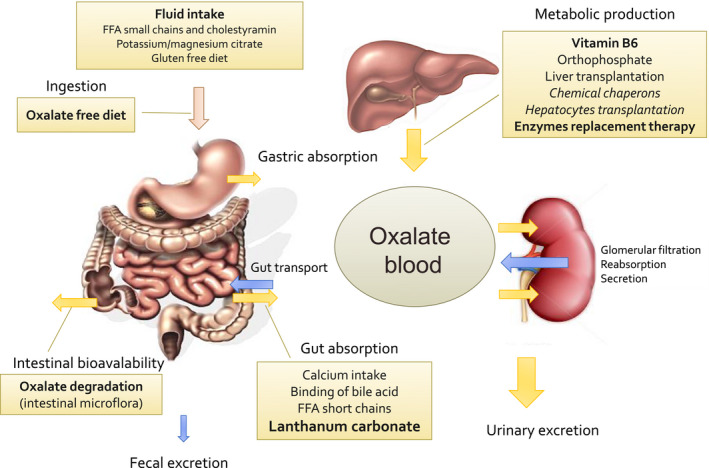
Homeostasis of oxalate, current and further therapy to control circulating and urinary excretion of oxalate by reduction of intestinal absorption (exogenous source – dietary oxalate) and endogenous production (liver synthesis) of oxalate
Future controlled studies are needed to unravel the value of LaC for treatment in Ox burden in PH1.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
This project was funded by the Brugmann Foundation.
Pozdzik A, David C, Vekeman J, Tielens F, Daudon M. Lanthanum carbonate to control plasma and urinary oxalate level in type 1 primary hyperoxaluria?. IJU Case Rep. 2021; 4: 235–238.
References
- 1. Cellini B, Oppici E, Paiardini A, Montioli R. Molecular insights into primary hyperoxaluria type 1 pathogenesis. Front. Biosci. 2012; 17: 621–34. [DOI] [PubMed] [Google Scholar]
- 2. Vervaet BA, Verhulst A, De Broe ME, D'Haese PC. The tubular epithelium in the initiation and course of intratubular nephrocalcinosis. Urol. Res. 2010; 38: 249–56. [DOI] [PubMed] [Google Scholar]
- 3. Martin‐Higueras C, Ludwig‐Portugall I, Hoppe B, Kurts C. Targeting kidney inflammation as a new therapy for primary hyperoxaluria? Nephrol. Dial. Transplant. 2019; 34: 908–14. [DOI] [PubMed] [Google Scholar]
- 4. Beck BB, Hoyer‐Kuhn H, Gobel H, Habbig S, Hoppe B. Hyperoxaluria and systemic oxalosis: an update on current therapy and future directions. Expert Opin. Investig. Drugs 2013; 22: 117–29. [DOI] [PubMed] [Google Scholar]
- 5. Cochat P, Liutkus A, Fargue S, Basmaison O, Ranchin B, Rolland MO. Primary hyperoxaluria type 1: still challenging!. Pediatr. Nephrol. 2006; 21: 1075–81. [DOI] [PubMed] [Google Scholar]
- 6. Cochat P, Hulton S‐A, Acquaviva C et al. Primary hyperoxaluria Type 1: indications for screening and guidance for diagnosis and treatment. Nephrol. Dial. Transplant. 2012; 27: 1729–36. [DOI] [PubMed] [Google Scholar]
- 7. Oppici E, Fargue S, Reid ES et al. Pyridoxamine and pyridoxal are more effective than pyridoxine in rescuing folding‐defective variants of human alanine:glyoxylate aminotransferase causing primary hyperoxaluria type I. Hum. Mol. Genet. 2015; 24: 5500–11. [DOI] [PubMed] [Google Scholar]
- 8. Burns Z, Knight J, Fargue S, Holmes R, Assimos D, Wood K. Future treatments for hyperoxaluria. Curr. Opin. Urol. 2020; 30: 171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robijn S, Hoppe B, Vervaet BA, D'Haese PC, Verhulst A. Hyperoxaluria: a gut‐kidney axis? Kidney Int. 2011; 80: 1146–58. [DOI] [PubMed] [Google Scholar]
- 10. Milliner D, Hoppe B, Groothoff J. A randomised Phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 2018; 46: 313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cochat P, Rumsby G. Primary hyperoxaluria. N. Engl. J. Med. 2013; 369: 649–58. [DOI] [PubMed] [Google Scholar]
- 12. Illies F, Bonzel KE, Wingen AM, Latta K, Hoyer PF. Clearance and removal of oxalate in children on intensified dialysis for primary hyperoxaluria type 1. Kidney Int. 2006; 70: 1642–8. [DOI] [PubMed] [Google Scholar]
- 13. Fargue S, Milliner DS, Knight J, Olson JB, Lowther WT, Holmes RP. Hydroxyproline metabolism and oxalate synthesis in primary hyperoxaluria. J. Am. Soc. Nephrol. 2018; 29: 1615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sikora P, von Unruh GE, Beck B et al. [13C2]oxalate absorption in children with idiopathic calcium oxalate urolithiasis or primary hyperoxaluria. Kidney Int. 2008; 73: 1181–6. [DOI] [PubMed] [Google Scholar]
- 15. Hutchison AJ, Wilson RJ, Garafola S, Copley JB. Lanthanum carbonate: safety data after 10 years. Nephrology 2016; 21: 987–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dion M, Ankawi G, Chew B et al. CUA guideline on the evaluation and medical management of the kidney stone patient ‐ 2016 update. Can. Urol. Assoc. J. 2016; 10: E347–E358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robijn S, Vervaet BA, Hoppe B, D'Haese PC, Verhulst A. Lanthanum carbonate inhibits intestinal oxalate absorption and prevents nephrocalcinosis after oxalate loading in rats. J. Urol. 2013; 189: 1960–6. [DOI] [PubMed] [Google Scholar]
- 18. Pozdzik AA, Verhulst A, Nortier JL, D’Haese P, Roumeguère T, Daudon M. Lanthanum carbonate – a new therapeutic intervention in management of uncontrolled hyperoxaluria? J. Nephrol. Ther. 5: 215. [Google Scholar]
- 19. Behets GJ, Dams G, Damment SJ, Martin P, De Broe ME, D'Haese PC. Differences in gastrointestinal calcium absorption after the ingestion of calcium‐free phosphate binders. Am. J. Physiol. Renal Physiol. 2014; 306: F61–F67. [DOI] [PubMed] [Google Scholar]


