Abstract
Purpose
Reduced port laparoscopic distal gastrectomy (RPLDG) using 3 ports is less invasive than conventional laparoscopic distal gastrectomy (CLDG) using 5 ports. Although RPLDG performed by expert surgeons is safe and feasible, novice surgeons have difficulty performing this procedure. This study evaluated the surgical outcomes and feasibility of RPLDG performed by a novice surgeon.
Materials and Methods
The records of 136 patients who underwent laparoscopic distal gastrectomy for gastric cancer performed by a single novice surgeon between May 2016 and December 2018 were retrospectively reviewed. Among these 136 patients, 52 underwent RPLDG and 84 underwent CLDG. The clinicopathological characteristics, operative outcomes, and short-term postoperative outcomes of the 2 groups were compared.
Results
The percentage of women was significantly higher in the RPLDG group than in the CLDG group (48.1% vs. 31%; P=0.045), but other baseline characteristics did not differ significantly between the groups. Billroth II anastomosis was performed significantly more frequent (90.4% vs. 73.8%, P=0.015) and operation time was significantly shorter (207.1±43.3 min vs. 225.5±44.6 min, P=0.020) in the RPLDG group than in the CLDG group. The time to first flatus, postoperative pain score, length of postoperative hospital stay, and incidence and severity of complications did not differ significantly between the groups. Analysis of the learning curve based on the operation time showed that performing RPLDG on 20–30 patients was required to achieve technical proficiency.
Conclusions
RPLDG is a safe and feasible surgical procedure for the treatment of gastric cancer, even when performed by a novice surgeon.
Keywords: Gastric cancer, Gastrectomy, Reduced port, Novice surgeon
INTRODUCTION
Randomized controlled trials in Asian countries have shown that laparoscopic distal gastrectomy is feasible and safe for the treatment of gastric cancer [1,2,3,4]. In conventional laparoscopic distal gastrectomy (CLDG), 5 ports are considered the standard. Accumulation of surgical experience and advances in surgical techniques have led to the development of reduced port laparoscopic distal gastrectomy (RPLDG), a less invasive alternative to CLDG [5,6,7]. Most studies have evaluated the performance of RPLDG by expert surgeons who have considerable experience with CLDG [8,9,10,11,12]. Although these studies have demonstrated that RPLDG performed by expert surgeons is feasible and safe, inexperienced novice surgeons have difficulty performing this procedure.
In our institution, one novice surgeon with limited experience with CLDG started performing RPLDG incidentally. The present study evaluated the surgical outcomes of RPLDG performed by this surgeon and investigated the feasibility and safety of RPLDG performed by a novice surgeon.
MATERIALS AND METHODS
Patients
The medical records of 136 patients who underwent laparoscopic distal gastrectomy for gastric cancer at Ulsan University Hospital between May 2016 and December 2018 were retrospectively reviewed. All operations were performed by a single surgeon who had previously performed <20 laparoscopic operations for gastric cancer and had been trained for 1 year in a high-volume center performing >800 laparoscopic operations per year for gastric cancer. Among these 136 patients, 52 underwent RPLDG and 84 underwent CLDG. The first RPLDG performed by this surgeon was his tenth laparoscopic distal gastrectomy.
Gastric cancer was diagnosed in all patients by endoscopic biopsy. In addition, all patients underwent abdominal computed tomography and laboratory tests before surgery, with the operative approach varying on a case-to-case basis. If an assistant and a scopist were present, CLDG was performed; if only a scopist was present, RPLDG was performed. The indications for laparoscopic gastrectomy for gastric cancer were clinical stage cT1 if the patient age was <70 years and clinical stage cT2 if the patient age was >70 years.
Lymph nodes were staged and classified according to the TNM classification [13] and dissected according to the Korean and Japanese gastric cancer treatment guidelines [14,15]. Patient data were collected by a review of medical records.
Surgical procedures for RPLDG
The surgical procedure used for RPLDG was similar to that previously described [8,10,11,12]. The patient was placed supine on a table in the reverse Trendelenburg position. The operator and scopist were positioned on the right side of the patient. A 12-mm trocar was inserted through an umbilical incision using the Hasson open technique [16]. After creating the pneumoperitoneum, a laparoscope was inserted through the umbilical trocar. One 5-mm trocar was inserted in the right subcostal margin and a 12-mm trocar was inserted in the right mid-abdomen under laparoscopic guidance (Fig. 1A).
Fig. 1. (A) Location of trocars during RPLDG. (B) A gauze is placed under the stomach to secure the field during dissection of lymph node No. 4sb. (C) The pancreas is compressed by forceps during suprapancreatic lymph node dissection. The gauze is placed under the caudate lobe.
RPLDG = reduced port laparoscopic distal gastrectomy.
The liver was retracted using a polypropylene monofilament suture. The suture was inserted at the epigastrium, the middle portion was placed in the gastrohepatic ligament with 2 plastic surgical clips, and both ends were fixed on the skin using mosquito forceps. During gastrectomy, all procedures were performed using conventional laparoscopic instruments, and no specific device was used for organ retraction.
Standard gastrectomy was initiated by dividing the greater omentum. During dissection of lymph node No. 4sb, a gauze was placed under the stomach to secure the field (Fig. 1B). The duodenum was transected after the lymph nodes along the greater curvature of the stomach were dissected. Suprapancreatic lymph node dissection was initiated by the dissection of lymph node No. 5. During suprapancreatic lymph node dissection, the tissue was pulled with forceps in the left hand, and the field was secured by compressing the pancreas by an instrument in the right hand (Fig. 1C). The gauze was placed under the caudate lobe of the liver to prevent injury to the inferior vena cava during dissection (Fig. 1C). After the completion of lymph node dissection, the stomach was transected with a laparoscopic linear stapler, and a specimen was extracted through the extended umbilical port site using a laparoscopic retrieval bag.
After assessing the frozen biopsy sample of the resection margin, reconstruction was performed in an intracorporeal fashion using a linear stapler. During the study period, patients underwent Billroth I, Billroth II, or Roux-en-Y reconstruction. Billroth I gastroduodenostomy was performed using a modified book-binding technique (MBBT) [17]. During Billroth I anastomosis, a camera was inserted through the right lower 12-mm trocar, and a linear stapler was introduced through the umbilical trocar.
Surgical procedures for conventional laparoscopic distal gastrectomy
CLDG involved placement of 5 trocars—two bilateral subcostal 5-mm trocars, one right mid-abdominal 12-mm trocar, one left mid-abdominal 5-mm trocar, and one umbilical 12-mm trocar. The procedures used for CLDG were similar to those used for RPLDG; however, in CLDG, the assistant was involved in traction or compression of tissue during dissection.
Postoperative care
All patients received intravenous patient-controlled analgesia after surgery and additional painkillers as needed. After the first flatus, the patients were allowed to drink sips of water. Their diets were gradually advanced in stages from liquids to a soft diet according to their condition. Patients who experienced no discomfort during diet advancement were discharged and followed up in the outpatient clinic. If pathological results indicated a need for adjuvant chemotherapy, the patient was referred to a medical oncologist.
Statistical analysis
Data were analyzed using SPSS statistical software, version 21 (IBM Inc., Armonk, NY, USA) and R software (version 4.0.2, R Foundation for Statistical Computing, Vienna, Austria). Intergroup differences in continuous variables were compared using Student's t-test, and differences in categorical variables were compared using the chi-square test. Probability (P) values <0.05 were considered statistically significant.
The learning curves for RPLDG and CLDG were evaluated using the cumulative sum (CUSUM) method. The CUSUM is the running total of the differences between each datum and the mean. The point at which the slope of the CUSUM curve gradually stabilizes is regarded as the breakpoint of the learning curve [10,18].
Ethics committee approval
This study was reviewed and approved by the Institutional Review Board (IRB) of Ulsan University Hospital (IRB No. 2020-04-024).
RESULTS
A comparison of the clinicopathological characteristics of patients in the RPLDG and CLDG groups (Table 1) showed that the percentage of male patients was significantly higher in the CLDG group and the percentage of histologically undifferentiated tumors was higher in the RPLDG group. Age, body mass index, American Society of Anesthesiologists physical status classification, and history of abdominal operation did not differ between the groups. In addition, there were no significant differences in tumor location, tumor size, T stage, N stage, TNM stage, and number of retrieved lymph nodes between the groups.
Table 1. Clinicopathological characteristics of patients who underwent CLDG and RPLDG.
| Characteristics | CLDG (n=84) | RPLDG (n=52) | P-value | |
|---|---|---|---|---|
| Age (yr) | 62±12.1 | 60.5±13.6 | 0.508 | |
| Sex | 0.045 | |||
| Male | 58 (69) | 27 (51.9) | ||
| Female | 26 (31) | 25 (48.1) | ||
| BMI (kg/m2) | 23.8±2.74 | 24.1±3.68 | 0.618 | |
| ASA classification | 0.265 | |||
| I | 13 (15.5) | 14 (26.9) | ||
| II | 64 (76.2) | 34 (65.4) | ||
| III | 7 (8.3) | 4 (7.7) | ||
| Prior abdominal surgery | 0.210 | |||
| No | 69 (82.1) | 38 (73.1) | ||
| Yes | 15 (17.9) | 14 (26.9) | ||
| Tumor location | 0.147 | |||
| Lower | 50 (59.5) | 22 (42.3) | ||
| Middle | 32 (38.1) | 28 (53.8) | ||
| Upper | 2 (2.4) | 2 (3.8) | ||
| Tumor size (cm) | 2.68±1.58 | 3.06±1.78 | 0.204 | |
| Differentiation | 0.045 | |||
| Differentiated | 37 (44) | 14 (26.9) | ||
| Undifferentiated | 47 (56) | 38 (73.1) | ||
| Depth of invasiona | 0.397 | |||
| pT1 | 67 (79.7) | 35 (67.3) | ||
| pT2 | 5 (6) | 7 (13.5) | ||
| pT3 | 8 (9.5) | 8 (15.4) | ||
| pT4 | 4 (4.8) | 2 (3.8) | ||
| Nodal metastasis* | 0.965 | |||
| pN0 | 62 (73.8) | 37 (71.2) | ||
| pN1 | 14 (16.7) | 10 (19.2) | ||
| pN2 | 4 (4.8) | 2 (3.8) | ||
| pN3 | 4 (4.8) | 3 (5.8) | ||
| pTNM stage* | 0.290 | |||
| I | 67 (79.8) | 36 (69.2) | ||
| II | 12 (14.3) | 13 (25) | ||
| III | 5 (6) | 3 (5.8) | ||
| No. of retrieved LNs | 33.01±10.56 | 31.85±7.50 | 0.488 | |
Data are reported as mean±standard deviation or number (%).
CLDG = conventional laparoscopic distal gastrectomy; RPLDG = reduced port laparoscopic distal gastrectomy; BMI = body mass index; ASA = American Society of Anesthesiologists; pTNM = pathological tumor, node, metastasis; LN = lymph node.
*According to the 8th edition of the American Joint Committee on Cancer.
The operative outcomes are summarized in Table 2. The most common type of reconstruction overall and in each group was Billroth II reconstruction, but Billroth I reconstruction was performed more frequently in the CLDG group. There were no differences in the rates of D2 lymph node dissection or combined resection between the groups. The mean operation time was significantly shorter in the RPLDG group than in the CLDG group (207.1±43.3 min vs. 225.5±44.6 min; P=0.020), but intraoperative estimated blood loss was similar in these groups.
Table 2. Operative outcomes of patients who underwent CLDG and RPLDG.
| Characteristics | CLDG (n=84) | RPLDG (n=52) | P-value | |
|---|---|---|---|---|
| Reconstruction | 0.015 | |||
| Billroth I | 22 (26.2) | 4 (7.7) | ||
| Billroth II | 62 (73.8) | 47 (90.4) | ||
| Roux-en-Y | 0 (0) | 1 (1.9) | ||
| LN dissection | 0.500 | |||
| D1+ | 11 (13.1) | 9 (17.3) | ||
| D2 | 73 (86.9) | 43 (82.7) | ||
| Combined resection | 0.765 | |||
| No | 68 (81) | 41 (78.8) | ||
| Gallbladder | 13 (15.5) | 10 (19.2) | ||
| Small bowel | 1 (1.2) | 1 (1.9) | ||
| Adrenal gland | 2 (2.4) | 0 (0) | ||
| Operative time (min) | 225.5±44.6 | 207.1±43.3 | 0.020 | |
| Estimated blood loss (mL) | 82.3±88.6 | 78.2±73.1 | 0.781 | |
| Open conversion | 1 (1.2) | 0 (0) | ||
| Additional port insertion | 0 (0) | 4 (7.7) | ||
Data are reported as mean±standard deviation or number (%).
CLDG = conventional laparoscopic distal gastrectomy; RPLDG = reduced port laparoscopic distal gastrectomy; LN = lymph node.
Among the 84 patients in the CLDG group, one patient required conversion to open surgery because of portal vein injury during dissection of lymph node No. 12a. Four of the 52 patients in the RPLDG group required insertion of an additional trocar, including 3 patients who required a fourth trocar because of obesity and a protruding pancreatic body and one patient who required an additional trocar for combined resection of the gallbladder (Table 3).
Table 3. Details of cases that required additional port insertion during reduced port laparoscopic distal gastrectomy.
| Patient | Sex | Age (yr) | BMI (kg/m2) | Prior abdominal surgery | No. of additional port | Combined resection |
|---|---|---|---|---|---|---|
| 1 | Male | 50 | 27.6 | No | 1 | No |
| 2 | Female | 65 | 26.4 | Yes | 1 | No |
| 3 | Female | 57 | 32.2 | No | 1 | No |
| 4 | Male | 58 | 20.2 | No | 1 | Gallbladder |
BMI = body mass index.
Table 4 summarizes the postoperative outcomes of these patients. There were no significant differences in the time to first flatus, sips of water, and soft diet or visual analogue scale (VAS) score of postoperative pain 1, 3, and 5 days after surgery. None of the patients died postoperatively, and there were no significant between-group differences in the incidence or severity of complications. Three patients in the CLDG group experienced severe complications with a Clavien–Dindo grade [19] of ≥III. Among these 3 patients, one patient experienced a wound complication requiring reoperation under local anesthesia (Clavien–Dindo IIIa), one patient experienced duodenal stump leakage requiring percutaneous drainage (Clavien–Dindo IIIa), and one patient required ventilator care because of aspiration pneumonia (Clavien–Dindo IVa). One patient in the RPLDG group experienced wound complications requiring reoperation under local anesthesia (Clavien–Dindo IIIa).
Table 4. Postoperative outcomes of patients who underwent CLDG and RPLDG.
| Characteristics | CLDG (n=84) | RPLDG (n=52) | P-value | |
|---|---|---|---|---|
| Time to first flatus (days) | 3.57±0.88 | 3.32±0.88 | 0.104 | |
| Time to sips of water (days) | 4.24±1.28 | 3.97±1.48 | 0.261 | |
| Time to soft diet (days) | 5.55±1.50 | 5.67±2.14 | 0.689 | |
| VAS of postoperative pain | ||||
| POD 1 | 6.17±1.17 | 6.13±0.99 | 0.870 | |
| POD 3 | 4.65±1.62 | 4.62±1.46 | 0.886 | |
| POD 5 | 2.99±1.49 | 3.33±1.34 | 0.184 | |
| Postoperative hospital stay (days) | 9.74±7.00 | 9.38±2.72 | 0.729 | |
| Complications | 0.812 | |||
| CD classification I | 9 (10.7) | 8 (15.4) | ||
| CD classification II | 6 (7.1) | 5 (9.6) | ||
| CD classification III | 2 (2.4) | 1 (1.9) | ||
| CD classification IV | 1 (1.2) | 0 (0) | ||
Data are reported as mean±standard deviation or number (%).
CLDG = conventional laparoscopic distal gastrectomy; RPLDG = reduced port laparoscopic distal gastrectomy; VAS = visual analogue scale; POD = postoperative day; CD = Clavien–Dindo.
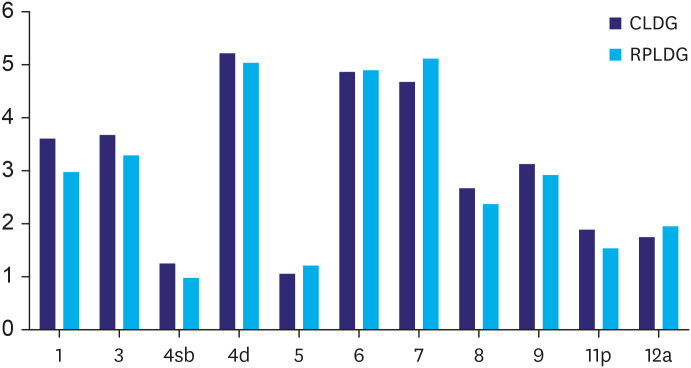
To determine whether there were any differences in the quality of lymph node dissection between the 2 groups, the number of lymph nodes retrieved per station was compared. No significant differences were observed between groups (Fig. 2).
Fig. 2. Comparison of lymph node dissection. No significant differences are observed in each lymph node station (P-value=LN1, 0.174; LN3, 0.467; LN4sb, 0.415; LN 4d, 0.772; LN5, 0.391; LN6, 0.943; LN7, 0.355; LN8, 0.352; LN9, 0.593; LN11p, 0.193; and LN12a, 0.543).
CLDG = conventional laparoscopic distal gastrectomy; RPLDG = reduced port laparoscopic distal gastrectomy.
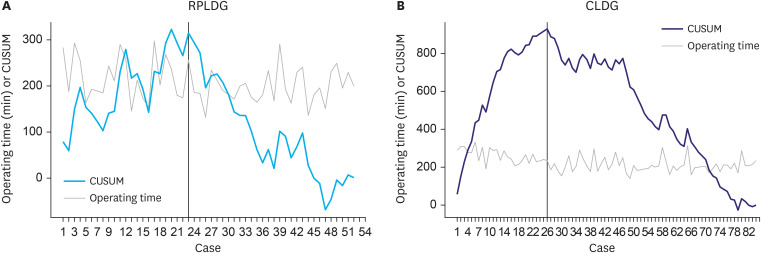
The CUSUM learning curves of this surgeon for RPLDG and CLDG based on operation time are shown in Fig. 3. The CUSUM curves of RPLDG and CLDG peaked at operations 24 and 26, respectively, and then gradually decreased.
Fig. 3. (A) Operative time and CUSUM curve of RPLDG. Black line indicates breakthrough point (24th case). (B) Operative time and CUSUM curve of CLDG. Black line indicates breakthrough point (26th case).
CUSUM = cumulative sum; RPLDG = reduced port laparoscopic distal gastrectomy; CLDG = conventional laparoscopic distal gastrectomy.
DISCUSSION
To our knowledge, this is the first study to analyze the surgical outcomes and learning curves of RPLDG and CLDG performed by a novice surgeon. The results of this study showed that the outcomes and learning curves of RPLDG and CLDG were similar.
In Korea, surgical specialties tend to be avoided [20]. This has not changed, despite the fact that residents are being restricted to 80 working hours per week by the “resident special law” and the reduction in surgical training period to 3 years [12,21]. Moreover, most residents are concentrated in major hospitals, making the lack of surgical manpower a more serious problem in local hospitals. Therefore, even novice surgeons in local hospitals are forced to perform reduced port operations. RPLDG requires only a scopist to solve the manpower shortage. In this study, a novice surgeon who had previously performed only 9 CLDG operations was forced to start preforming RPLDG.
Consistent with previous findings [8,12], the mean operation time required for this surgeon to perform RPLDG was shorter than that required for CLDG. However, the anastomosis methods differed in this study, with Billroth I gastroduodenostomy performed more frequently in patients who underwent CLDG than in those who underwent RPLDG. A new Billroth I gastroduodenostomy technique, called the MBBT, was introduced at our center during this period [17]. The MBBT requires approximately 50 minutes for complete anastomosis, whereas Billroth II anastomosis is a simpler technique, requiring lesser time than that for the MBBT. Thus, the longer operation time in the CLDG group may have been due to the more frequent performance of Billroth I anastomosis in this group. The mean operation time for RPLDG with Billroth II anastomosis was shorter than that for CLDG with Billroth II anastomosis, but the difference was not statistically significant (206±38.3 min vs. 215±37.3 min, P=0.258).
Moreover, there were more female patients in the RPLDG group than in the CLDG group. This can also affect the operation time. Therefore, propensity score matching was performed to adjust for variables that may affect the operation time. After propensity score matching, 46 patients were included in each group. The mean time for RPLDG was shorter than that for CLDG, but the difference was not statistically significant (208±41.6 min vs. 214±40.3 min, P=0.465).
Another possible reason for the shorter mean operation time in the RPLDG group than in the CLDG group may be the different periods in which the operations were performed. To confirm this, all cases were divided into 4 periods, and each period included 34 cases. The RPLDG group included 10 cases in period I, 16 cases in period II, 14 cases in period III, and 12 cases in period IV (Fig. 4), and there was no difference in the proportion of RPLDG in each period. (P=0.465).
Fig. 4. Distribution of cases according to the period. The white circle indicates RPLDG and black circle indicates CLDG.
RPLDG = reduced port laparoscopic distal gastrectomy; CLDG = conventional laparoscopic distal gastrectomy.
The quality of lymph node dissection is a critical factor in gastrectomy for gastric cancer. We found no significant differences in the number of retrieved lymph nodes at each station between the groups. In laparoscopic gastric cancer surgery, lymph node dissection in the suprapancreatic area is considered technically difficult, but it is more difficult in RPLDG than in CLDG. However, there were no significant between-group differences in the number of suprapancreatic lymph nodes retrieved at each station. In the present study, suprapancreatic lymph node dissection involved lifting the tissue to be removed with forceps in the left hand while compressing the upper border of the pancreas with the shaft of the energy device in the right hand. This allowed using the jaws of the energy device to resect the tissue being removed, a method similar to that described in other studies [10,12].
When performing RPLDG, the pancreas was compressed by an instrument by the operator (Fig. 1C). This can raise concerns regarding pancreatic injury. Therefore, the serum amylase levels on postoperative days (PODs) 1 and 3 after gastrectomy were compared between the 2 groups. The CLDG group had significantly higher serum amylase levels on both POD 1 (69.6±42.1 vs. 110±102 IU/L, P=0.009) and POD 3 (52.1±27.6 vs. 83±99.8 IU/L, P=0.036) than the RPLDG group. Even after propensity score matching, serum amylase levels on PODs 1 and 3 were higher in the CLDG group than in the RPLDG group. The difference in serum amylase levels on POD 1 between the groups was statistically significant (67.4±34.2 vs. 111±109 IU/L, P=0.013), but not on POD 3 (51.7±28.3 vs. 84.9±122 IU/L, P=0.097). In addition, one patient in the CLDG group experienced pancreatitis; however, no patients in the RPLDG who experienced pancreatitis. During this study period, most assistants were inexperienced assistants, such as junior residents or interns. Therefore, there was a risk of unnecessary compression or excessive force applied during suprapancreatic lymph node dissection. Conversely, there may have been a lesser risk of pancreatic injury when performing RPLDG because the operator carefully compressed the pancreas as needed. In the present study, the learning curve was evaluated using the CUSUM method. Other studies analyzing the learning curve of RPLDG reported that experience with approximately 30 such operations was required to attain technical expertise [10,22]. The CUSUM curve reached a peak at operation 24 and then entered the mastery phase. Although the number of patients was smaller in our study than in other studies that analyzed the learning curve of laparoscopic gastrectomy [10,18], our findings suggest that experience with approximately 20–30 operations is required to achieve technical proficiency with RPLDG. Further experience can result in more stable surgical outcomes [10,22].
In comparison, the CUSUM learning curve of CLDG reached a peak at operation 26 and plateaued until operation 50, suggesting that experience with approximately 25–30 operations is required to achieve proficiency in performing CLDG. Similarly, other studies assessing the CLDG learning curves reported that 20–40 operations were required to achieve technical proficiency [23,24]. Taken together, these findings indicate that the learning curves of RPLDG and CLDG are almost identical. Because our novice surgeon performed his first RPLDG after only 9 CLDG operations, he did not have sufficient experience to become accustomed to laparoscopic gastrectomy. This suggests that considerable experience with CLDG is not mandatory to achieve technical proficiency with RPLDG.
In the early period of laparoscopy-assisted distal gastrectomy (LADG), several studies showed that expert surgeons familiar with open gastrectomy master LADG faster than novice surgeons [25,26]. Previous studies included expert surgeons with considerable experience with CLDG to evaluate the learning curve for RPLDG [8,9,10,11,12]. In this study, in which the performance of RPLDG and CLDG by a novice surgeon was simultaneously evaluated, we found no difference between the learning curve for RPLDG and CLDG. As mentioned above, this suggests that even a novice surgeon with insufficient experience in performing CLDG can perform RPLDG. Therefore, novice surgeons can start performing RPLDG at the start of their career, depending on the clinical context.
Concerns have been raised about the outcomes when residents or fellows perform RPLDG. Similar findings have been reported for single-incision distal gastrectomy [18]. During laparoscopic surgery, most of the main procedures are performed by the operator, with the assistant holding only a camera or gripping tissue for retraction. This experience can accustom the assistant to laparoscopic equipment and movements. As surgery changes, so must the surgical training methods. Multimedia presentations, although providing an indirect experience, are available for observational learning. Trainees can begin learning about these surgical methods and practice with virtual reality training systems and dry laboratories. Under the supervision of an expert surgeon and without an assistant, these trainees can subsequently perform reduced port surgery, even RPLDG.
As previously described, to overcome the learning curve of laparoscopic gastrectomy for gastric cancer, approximately 20–40 cases are required to gain experience. However, in this study, the operator performed RPLDG, despite being a novice surgeon. This could potentially raise ethical concers, and the data will be described in detail. Before the first 20 cases, only one patient aged >70 years with cT2 disease was included. Before the first 40 cases, a total of 5 patients with cT2 disease were included, including 4 patients aged >70 years and one 52 years old female patient who strongly wanted to undergo laparoscopic surgery. The first RPLDG case of cT2 disease was the 31st case among all cases and the ninth RPLDG case. Therefore, most laparoscopic surgeries for patients with cT2 disease were performed after achieving technical proficiency.
The present study has several limitations, including its retrospective and non-randomized design. In addition, all operations were performed by a single surgeon, making it difficult to generalize the results. Furthermore, patients were followed up for a short period of time, precluding the determination of long-term outcomes. These limitations may be overcome in larger prospective randomized studies involving many novice surgeons with longer term follow-up.
To our knowledge, this is the first report of surgical outcomes of RPLDG performed by a novice surgeon, and it showed acceptable outcomes. These findings suggest that novice surgeons who are interested in this field can to perform RPLDG.
ACKNOWLEDGMENTS
The authors wish to express their sincere thanks to Nayoung Kim (Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, Seoul, South Korea) and Eun Ji Park (Medical Information Center, Ulsan University Hospital, Ulsan, South Korea) for assistance with the statistical analysis.
Footnotes
- Conceptualization: P.D.J., K.G.Y.
- Data curation: P.D.J., L.E.J.
- Formal analysis: P.D.J.
- Investigation: P.D.J., L.E.J.
- Methodology: P.D.J.
- Project administration: P.D.J.
- Resources: P.D.J.
- Software: P.D.J.
- Supervision: K.G.Y.
- Validation: P.D.J., L.E.J.
- Visualization: P.D.J.
- Writing - original draft: P.D.J.
- Writing - review & editing: P.D.J., K.G.Y.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131(Suppl):S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 2.Hiki N, Katai H, Mizusawa J, Nakamura K, Nakamori M, Yoshikawa T, et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG0703) Gastric Cancer. 2018;21:155–161. doi: 10.1007/s10120-016-0687-0. [DOI] [PubMed] [Google Scholar]
- 3.Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5:506–513. doi: 10.1001/jamaoncol.2018.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. 2019;321:1983–1992. doi: 10.1001/jama.2019.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inaki N. Reduced port laparoscopic gastrectomy: a review, techniques, and perspective. Asian J Endosc Surg. 2015;8:1–10. doi: 10.1111/ases.12163. [DOI] [PubMed] [Google Scholar]
- 6.Inaki N, Tsuji T, Doden K, Sakimura Y, Tawara H, Matsui R, et al. Reduced port laparoscopic gastrectomy for gastric cancer. Transl Gastroenterol Hepatol. 2016;1:38. doi: 10.21037/tgh.2016.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunisaki C, Makino H, Yamaguchi N, Izumisawa Y, Miyamato H, Sato K, et al. Surgical advantages of reduced-port laparoscopic gastrectomy in gastric cancer. Surg Endosc. 2016;30:5520–5528. doi: 10.1007/s00464-016-4916-8. [DOI] [PubMed] [Google Scholar]
- 8.Seo HS, Lee HH. Is the 5-ports approach necessary in laparoscopic gastrectomy? Feasibility of reduced-port totally laparoscopic gastrectomy for the treatment of gastric cancer: A Prospective Cohort Study. Int J Surg. 2016;29:118–122. doi: 10.1016/j.ijsu.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 9.Kunisaki C, Miyamoto H, Sato S, Tanaka Y, Sato K, Izumisawa Y, et al. Surgical outcomes of reduced-port laparoscopic gastrectomy versus conventional laparoscopic gastrectomy for gastric cancer: a propensity-matched retrospective cohort study. Ann Surg Oncol. 2018;25:3604–3612. doi: 10.1245/s10434-018-6733-x. [DOI] [PubMed] [Google Scholar]
- 10.Kim HG, Kim DY, Jeong O. Transition from conventional to reduced-port laparoscopic gastrectomy to treat gastric carcinoma: a single surgeon's experience from a small-volume center. J Gastric Cancer. 2018;18:172–181. doi: 10.5230/jgc.2018.18.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo HS, Jung YJ, Kim JH, Park CH, Lee HH. Three-port right-side approach-duet totally laparoscopic distal gastrectomy for uncut Roux-en-Y reconstruction. J Laparoendosc Adv Surg Tech A. 2018;28:1109–1114. doi: 10.1089/lap.2018.0331. [DOI] [PubMed] [Google Scholar]
- 12.Seo HS, Song KY, Jung YJ, Kim JH, Park CH, Lee HH. Right-side approach-duet totally laparoscopic distal gastrectomy (R-duet TLDG) using a three-port to treat gastric cancer. J Gastrointest Surg. 2018;22:578–586. doi: 10.1007/s11605-017-3575-y. [DOI] [PubMed] [Google Scholar]
- 13.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed. Chicago (IL): Springer; 2017. [Google Scholar]
- 14.Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019;19:1–48. doi: 10.5230/jgc.2019.19.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021:1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110:886–887. doi: 10.1016/0002-9378(71)90593-x. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Park EY, Park DJ, Kim GY. Modified book binding technique (MBBT) for intracorporeal gastroduodenostomy in totally laparoscopic distal gastrectomy: initial experience. J Gastric Cancer. 2019;19:355–364. doi: 10.5230/jgc.2019.19.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang SH, Cho YS, Min SH, Park YS, Ahn SH, Park DJ, et al. Early experience and learning curve of solo single-incision distal gastrectomy for gastric cancer: a review of consecutive 100 cases. Surg Endosc. 2019;33:3412–3418. doi: 10.1007/s00464-018-06638-1. [DOI] [PubMed] [Google Scholar]
- 19.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 20.Kim YY, Kim UN, Kim YS, Lee JS. Factors associated with the specialty choice of Korean medical students: a cross-sectional survey. Hum Resour Health. 2016;14:45. doi: 10.1186/s12960-016-0141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu HW, Choi JY, Park YS, Park HS, Choi Y, Ahn SH, et al. Implementation of a resident night float system in a surgery department in Korea for 6 months: electronic medical record-based big data analysis and medical staff survey. Ann Surg Treat Res. 2019;96:209–215. doi: 10.4174/astr.2019.96.5.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong O, Park YK, Ryu SY. Early experience of duet laparoscopic distal gastrectomy (duet-LDG) using three abdominal ports for gastric carcinoma: surgical technique and comparison with conventional laparoscopic distal gastrectomy. Surg Endosc. 2016;30:3559–3566. doi: 10.1007/s00464-015-4653-4. [DOI] [PubMed] [Google Scholar]
- 23.Chi F, Lan Y, Zhou S, Yang L, Chen M, Bi T. Learning curve of totally laparoscopic distal gastrectomy for gastric cancer: a single teaching hospital study. Wideochir Inne Tech Malo Inwazyjne. 2018;13:442–447. doi: 10.5114/wiitm.2018.78965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HG, Park JH, Jeong SH, Lee YJ, Ha WS, Choi SK, et al. Totally laparoscopic distal gastrectomy after learning curve completion: comparison with laparoscopy-assisted distal gastrectomy. J Gastric Cancer. 2013;13:26–33. doi: 10.5230/jgc.2013.13.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichikawa D, Hiki N, Fukunaga T, Tokunaga M, Komatsu S, Kuriu Y, et al. Usefulness of standardization in spreading of laparoscopy-assisted distal gastrectomy. Hepatogastroenterology. 2010;57:975–979. [PubMed] [Google Scholar]
- 26.Kang SY, Lee SY, Kim CY, Yang DH. Comparison of learning curves and clinical outcomes between laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Gastric Cancer. 2010;10:247–253. doi: 10.5230/jgc.2010.10.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]