Abstract
Introduction: Nephrolithiasis is common after malabsorptive bariatric surgery; however, the comparative risk of stone formation after different bariatric surgeries remains unclear. We seek to compare the risk of stone diagnosis and stone procedure after gastric banding (GB), sleeve gastrectomy (SG), short-limb Roux-en-Y (SLRY), long-limb Roux-en-Y (LLRY), and biliopancreatic diversion with duodenal switch (BPDDS).
Patients and Methods: Using an administrative database, we retrospectively identified 116,304 patients in the United States, who received bariatric surgery between 2007 and 2014, did not have a known kidney stone diagnosis before surgery, and were enrolled in the database for at least 1 year before and after their bariatric surgery. We used diagnosis and procedural codes to identify comorbidities and events of interest. Our primary analysis was performed with extended Cox proportional hazards models using time to stone diagnosis and time to stone procedure as outcomes.
Results: The adjusted hazard ratio of new stone diagnosis from 1 to 36 months, compared to GB, was 4.54 for BPDDS (95% confidence interval [CI] 3.66–5.62), 2.12 for LLRY (95% CI 1.74–2.58), 2.15 for SLRY (95% CI 2.02–2.29), and 1.35 for SG (95% CI 1.25–1.46). Similar results were observed for risk of stone diagnosis from 36 to 60 months, and for risk of stone removal procedure. Male sex was associated with an overall 1.63-fold increased risk of new stone diagnosis (95% CI 1.55–1.72).
Conclusions: BPDDS was associated with a greater risk of stone diagnosis and stone procedures than SLRY and LLRY, which were associated with a greater risk than restrictive procedures. Nephrolithiasis is more common after more malabsorptive bariatric surgeries, with a much greater risk observed after BPDDS and for male patients.
Keywords: bariatric surgery, endourology, epidemiology, kidney stones, nephrolithiasis, urolithiasis
Introduction
The incidence of obesity and its associated morbidity continues to rise.1 A variety of different bariatric surgeries are currently used to induce weight loss in this population. These surgeries achieve weight loss and correct associated metabolic comorbidities, such as diabetes mellitus and hypertension, through a combination of complex mechanisms that include malabsorption and restriction. They are also associated with a variety of adverse sequelae, including the well-known and long-term complication of nephrolithiasis.2 Malabsorptive bariatric surgery increases urinary oxalate levels and predisposes patients to kidney stone formation.3 However, bariatric surgeries that are primarily restrictive, that is, gastric banding (GB), have been largely shown to be free from this association.4,5 Nevertheless, bariatric surgery exists on a spectrum of malabsorption and restriction, and the comparative risks of nephrolithiasis across the spectrum of modern bariatric surgery remain unclear.
Sleeve gastrectomy (SG) and GB are restrictive bariatric surgeries that constrain the gastric lumen, either through resection or through adjustable banding. Alternatively, biliopancreatic diversion with duodenal switch (BPDDS) is the most malabsorptive modern bariatric surgery whereby much of the small bowel is bypassed leaving a long biliopancreatic limb and a short residual common channel. Intermediary between BPDDS and restrictive surgery is the Roux-en-Y gastric bypass wherein the length of the “Roux” limb can be lengthened to yield a shorter common channel and a more malabsorptive procedure, that is, the long-limb Roux-en-Y (LLRY) is more malabsorptive than short-limb Roux-en-Y (SLRY).
There are limited data assessing the rates of nephrolithiasis associated with the distinct types of bariatric surgeries. Herein, we seek to evaluate the association between the different types of bariatric surgeries and subsequent kidney stone diagnoses and stone removal procedures. In doing so, we attempt to test whether the risk of kidney stones is greater in procedures that are more malabsorptive. We hypothesize that GB and SG, as restrictive procedures, will have similarly low rates of nephrolithiasis, and that SLRY, LLRY, and BPDDS will have increasingly higher rates of nephrolithiasis. By clarifying the varying rates of diagnosed and treated kidney stones among the different bariatric procedures, we will allow bariatric surgeons and other providers caring for this population to better counsel patients on a prominent long-term complication of these surgeries.
Patients and Methods
Patient selection and variable identification
This is a retrospective administrative data analysis using the Truven Health MarketScan® Commercial Database that received Institutional Review Board Waiver at our institution. This database includes longitudinal inpatient and outpatient medical and prescription drug claims from numerous employer-sponsored and other commercial insurance plans in the United States. Using this database, we identified patients 18 to 64 years of age, who underwent bariatric surgery (GB, SG, SLRY, LLRY, and BPDDS) between 2007 and 2014.
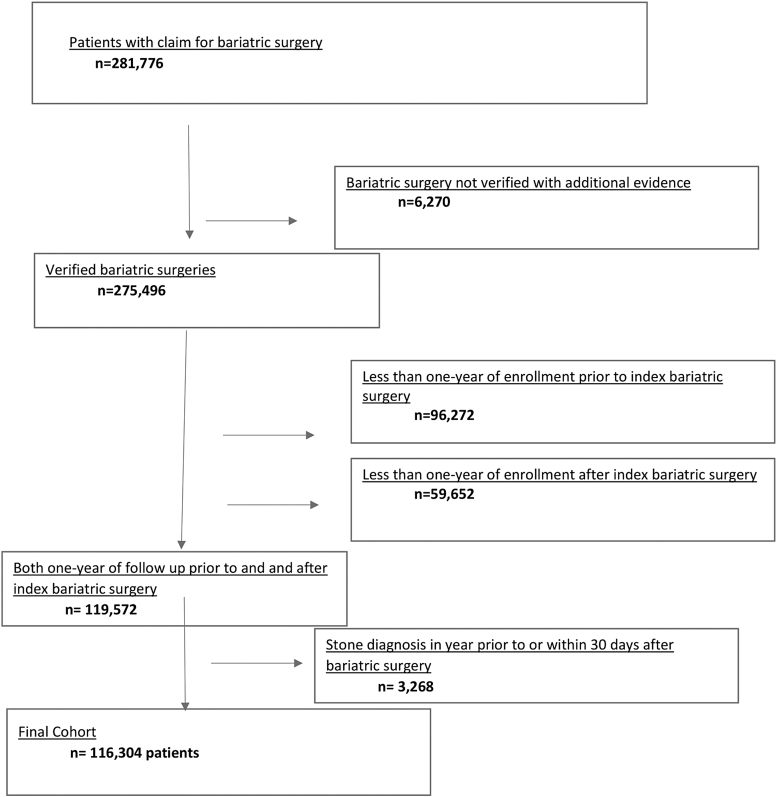
Exclusion criteria included patients without at least 1 year of health insurance enrollment before their index bariatric surgery, to adequately identify underlying comorbidities; patients without at least 1 year of follow-up insurance enrollment after their index bariatric surgery, to ensure adequate follow-up; and patients with any kidney stone diagnosis within 1 year before or within 30 days after bariatric surgery, suggestive of preexisting stone disease (Fig. 1).
FIG. 1.
Cohort selection flow chart.
Bariatric and stone procedures were identified using Current Procedural Terminology, Fourth Edition (CPT) codes (Supplementary Table S1). All procedures required supporting information to confirm that the procedure was performed. Information used to confirm surgical procedures included procedure claims from both a provider and facility (either CPT-4 or International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes for inpatient surgeries), or a procedure claim for surgery plus either a provider anesthesia claim or facility revenue codes for operating room or anesthesia services. Distinction was not made between bariatric procedures performed laparoscopically versus open as this likely does not affect stone outcomes. CPT coding defines LLRY as a nonabsorptive alimentary limb greater than 150 cm and SLRY as a limb less than 150 cm. Stone procedures included shockwave lithotripsy, ureteroscopy, percutaneous nephrolithotomy, and open stone procedures.
ICD-9-CM diagnosis codes were used to identify comorbidities, as defined by Elixhauser.6,7 New stone diagnoses were identified with a coding definition previously validated by Semins et al.8 See Supplementary Table S1 for a full list of the variable coding definitions. Comorbidities were captured within the year preceding and during the bariatric surgery admission, and required either two outpatient claims coded at least 30 days apart or one inpatient claim, as we have described previously.9
Statistical analyses
Descriptive statistics were performed using chi-square, Student's t-test, and Mann-Whitney U tests, as appropriate. We performed two analyses using Cox proportional hazards models. In the first, we used time to first coding of stone diagnosis as the event of interest, and in the second, we used time to first stone procedure as the event of interest. By performing these analyses in parallel, with separately coded events, we sought to overcome some of the possible limitations of miscoding in our administrative dataset. In both analyses, patients were censored at the first of a second bariatric procedure, loss of health insurance enrollment, and inpatient death. Kaplan-Meier curves were started at 30 days after bariatric surgery since a stone event within 30 days of bariatric surgery was an exclusion criterion.
Univariable and multivariable Cox models were used to examine the hazards associated with the different bariatric surgeries and other known associated risk factors including age, diabetes mellitus,10 male sex,11 hypertension,12 and geographical region.13 GB was used as the reference bariatric surgery for analysis as available literature suggests it is not associated with increased stone formation.4 Although certain variables, like paralysis, are known risk factors for stone formation, they were not included in our final model because the numbers of these patients were very small in this cohort. Age was grouped in discrete 10-year increments.
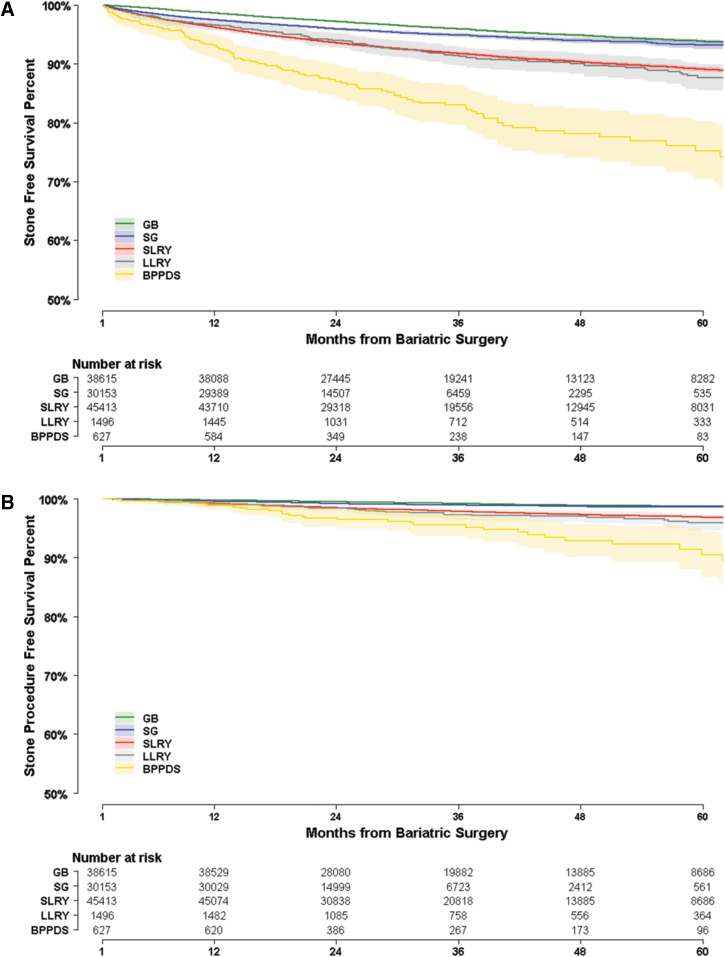
On graphical assessment of the proportional hazard's assumption, the Kaplan-Meier plots demonstrated changing slopes of the survival curves for stone diagnosis and stone procedure with respect to the referent GB. For this reason, an extended Cox model was implemented, partitioning the time axis into two time intervals using a Heaviside function.14 For diagnoses, a time interval was implemented at 36 months where the slopes of the GB and SG curves where observed to converge (Fig. 2A). For procedures, a time interval was implemented at 18 months where slopes began to diverge (Fig. 2B). Graphical assessment of the proportional hazards' assumption for other covariates with Kaplan-Meier plots was otherwise unremarkable.
FIG. 2.
(A, B) Kaplan-Meier survival curves for time to stone diagnosis and stone procedure. Shading indicates 95% confidence interval. Number at risk is listed at yearly increments.
Post hoc comparisons were performed on the multivariable Cox models using a Bonferroni correction for multiple comparisons with the adjusted α set at 0.00625.
Results
We identified 281,776 patients from 18 to 64 years of age coded for bariatric surgery between 2006 and 2015. Around 116,304 met all inclusion criteria and were included for final analysis with a median follow-up of 31.6 months (minimum 12.0 months and maximum 108 months) (Fig. 1).
Demographics and clinical characteristics of the bariatric surgery groups are shown in Table 1. The largest number of patients in our cohort underwent SLRY (n = 45,413), followed by GB (n = 38,615) and SG (n = 30,153). A smaller number of patients underwent LLRY (n = 1,496) and BPDDS (n = 627). Female sex predominated in all groups and geographic variation was observed in type of bariatric surgery patients received (both p < 0.001), with highest utilization of GB in the South and SG in the Northeast.
Table 1.
Demographic Characteristics and Underlying Comorbidities of Patients Who Underwent Bariatric Surgery from 2007 to 2014
| Variable (%) | GB (n = 38,615) | SG (n = 30,153) | SLRY (n = 45,413) | LLRY (n = 1,496) | BPDDS (n = 627) |
|---|---|---|---|---|---|
| Age [SD] | 44.1 [10.4] | 44.0 [10.3] | 44.8 [10.3] | 44.7 [10.4] | 43.9 [10.2] |
| Female sex | 30,552 (79.1) | 23,406 (77.6) | 35,387 (77.9) | 1153 (77.1) | 454 (72.4) |
| Region of the United States | |||||
| North East | 5870 (15.2) | 5361 (17.8) | 7251 (16.0) | 181 (12.1) | 89 (14.2) |
| North Central | 7688 (19.9) | 5703 (18.9) | 10,031 (22.1) | 822 (54.9) | 164 (26.2) |
| South | 17,776 (46.0) | 14,044 (46.6) | 18,432 (40.6) | 341 (22.8) | 186 (29.7) |
| West | 6798 (17.6) | 4797 (15.9) | 9042 (19.9) | 72 (4.8) | 179 (28.5) |
| Unknown | 483 (1.3) | 248 (0.8) | 657 (1.4) | 80 (5.3) | 9 (1.4) |
| Coronary artery disease | 1085 (2.8) | 804 (2.7) | 1529 (3.4) | 46 (3.1) | 24 (3.8) |
| Chronic pulmonary disease | 3214 (8.3) | 2726 (9.0) | 4183 (9.2) | 144 (9.6) | 52 (8.3) |
| Diabetes mellitus | 8550 (22.1) | 6886 (22.8) | 14,844 (32.7) | 429 (28.7) | 175 (27.9) |
| Fluid and electrolyte disorders | 333 (0.9) | 343 (1.1) | 519 (1.1) | 11 (0.7) | 11 (1.8) |
| Hypertension | 17,092 (44.3) | 140,141 (46.6) | 21,925 (48.3) | 670 (44.8) | 306 (48.8) |
| Hypothyroidism | 3248 (8.4) | 2985 (9.9) | 4136 (9.1) | 118 (7.9) | 59 (9.4) |
| Paralysis | 26 (0.1) | 20 (0.1) | 26 (0.1) | 2 (0.1) | 0 (0.0) |
| Chronic renal failure | 211 (0.5) | 262 (0.9) | 394 (0.9) | 8 (0.5) | 6 (1.0) |
| Rheumatoid arthritis | 617 (1.6) | 604 (2.0) | 830 (1.8) | 17 (1.1) | 8 (1.3) |
| Depression | 2751 (7.1) | 2713 (9.0) | 3612 (8.0) | 123 (8.2) | 61 (9.7) |
BPDDS = biliopancreatic diversion with duodenal switch; GB = gastric banding; LLRY = long-limb Roux-en-Y; SD = standard deviation; SG = sleeve gastrectomy; SLRY = short-limb Roux-en-Y.
Kaplan-Meier plots for time to stone diagnosis and time to stone procedure after the four types of bariatric procedures are shown in Figure 2A and B. The highest incidence of new stone diagnosis and stone procedures was observed for BPDDS, followed by LLRY and SLRY, and then SG and GB.
The comparative incidence of new stone diagnosis and stone procedure per person-year of observation for all patients, and separately for male and female patients are shown in Table 2. For patients undergoing malabsorptive surgery, a nearly twofold increase in new stone diagnosis and stone procedures was observed for males compared to females.
Table 2.
Incidence of New Stone Diagnosis and Stone Procedure After Bariatric Surgery in All Patients and Stratified by Sex
| Bariatric procedure | New stone diagnosis and procedure incidences per 100 person-years |
|||||
|---|---|---|---|---|---|---|
| All patients |
Female patients |
Male patients |
||||
| Stone diagnosis | Stone procedure | Stone diagnosis | Stone procedure | Stone diagnosis | Stone procedure | |
| GB (n = 38,615) | 1.3 | 0.3 | 1.2 | 0.2 | 1.9 | 0.4 |
| SG (n = 30,153) | 1.9 | 0.4 | 1.7 | 0.3 | 4.1 | 0.9 |
| SLRY (n = 45,413) | 2.8 | 0.7 | 2.4 | 0.6 | 4.7 | 1.9 |
| LLRY (n = 1496) | 2.8 | 0.8 | 2.3 | 0.5 | 21.3 | 8.4 |
| BPDDS (n = 627) | 6.4 | 1.7 | 5.0 | 1.3 | 10.4 | 2.9 |
The results from the multivariable extended Cox proportional hazards models are shown in Table 3 (univariable results are shown in Supplementary Table S2). The independent risk of new stone diagnosis from 1 to 36 months, compared to GB, was 4.54 for BPDDS (95% confidence interval [CI] 3.66–5.62), 2.12 for LLRY (95% CI 1.74–2.58), 2.15 for SLRY (95% CI 2.02–2.29), and 1.35 for SG (95% CI 1.25–1.46). From 36 to 60 months, the risk of new stone diagnosis was 4.65 for BPDDS (95% CI 2.85–7.57), 1.56 for LLRY (95% CI 0.99–2.46), 1.29 for SLRY (95% CI 1.12–1.50), and 0.77 for SG (95% CI 0.57–1.03).
Table 3.
Results of Multivariable Extended Cox Proportional Hazards Models Comparing Time to Stone Diagnosis and Time to Stone Procedure After Bariatric Surgery
| Variable | Time to stone diagnosis |
|
Time to stone procedure |
||
|---|---|---|---|---|---|
| Adjusted hazard ratio (95% CI) | p | Variable | Adjusted hazard ratio (95% CI) | p | |
| Age <30 (referent) | 1.00 | Age <30 (referent) | 1.00 | ||
| 30–40 | 1.15 (1.03–1.28) | 0.009 | 30–40 | 1.57 (1.19–2.06) | 0.001 |
| 40–50 | 1.23 (1.1–1.37) | <0.001 | 40–50 | 2.03 (1.55–2.65) | <0.001 |
| 50–60 | 1.21 (1.08–1.34) | <0.001 | 50–60 | 2.13 (1.62–2.78) | <0.001 |
| >60 | 1.21 (1.05–1.39) | 0.008 | >60 | 2.37 (1.73–3.26) | <0.001 |
| Males | 1.63 (1.55–1.72) | <0.001 | Males | 1.53 (1.37–1.70) | <0.001 |
| GB (referent) | 1.00 | GB (referent) | 1.00 | ||
| SG (1–36 months) | 1.35 (1.25–1.46) | <0.001 | SG (1–18 months) | 1.71 (1.36–2.14) | <0.001 |
| SLRY (1–36 months) | 2.15 (2.02–2.29) | <0.001 | SLRY (1–18 months) | 3.23 (2.66–3.91) | <0.001 |
| LLRY (1–36 months) | 2.12 (1.74–2.58) | <0.001 | LLRY (1–18 months) | 3.23 (1.99–5.24) | <0.001 |
| BPDDS (1–36 months) | 4.54 (3.66–5.62) | <0.001 | BPDDS (1–18 months) | 5.61 (3.11–10.13) | <0.001 |
| SG (36–60 months) | 0.77 (0.57–1.03) | 0.080 | SG (18–36 months) | 0.98 (0.75–1.29) | 0.909 |
| SLRY (36–60 months) | 1.29 (1.12–1.50) | <0.001 | SLRY (18–36 months) | 2.11 (1.78–2.5) | <0.001 |
| LLRY (36–60 months) | 1.56 (0.99–2.46) | 0.053 | LLRY (18–36 months) | 2.35 (1.49–3.7) | <0.001 |
| BPDDS (36–60 months) | 4.65 (2.85–7.57) | <0.001 | BPDDS (18–36 months) | 7.50 (4.73–11.89) | <0.001 |
| Comorbidities | Comorbidities | ||||
| Coronary artery disease | 1.17 (1.03–1.32) | 0.014 | Coronary artery disease | 1.16 (0.92–1.48) | 0.215 |
| Diabetes mellitus | 1.07 (1.01–1.13) | 0.014 | Diabetes mellitus | 1.12 (1–1.25) | 0.045 |
| Depression | 1.22 (1.12–1.33) | <0.001 | Depression | 1.25 (1.05–1.49) | 0.013 |
| Hypertension | 1.04 (0.99–1.10) | 0.098 | Hypertension | 0.92 (0.83–1.02) | 0.124 |
| Region of the United States | Region of the United States | ||||
| North East (referent) | 1.00 | North East (referent) | 1.00 | ||
| North Central | 1.13 (1.04–1.23) | 0.002 | North Central | 1.61 (1.36–1.90) | <0.001 |
| South | 1.14 (1.06–1.22) | <0.001 | South | 1.35 (1.15–1.58) | <0.001 |
| West | 0.98 (0.90–1.07) | 0.718 | West | 0.93 (0.77–1.13) | 0.483 |
| Unknown | 0.99 (0.78–1.24) | 0.906 | Unknown | 1.13 (0.69–1.83) | 0.629 |
CI = confidence interval.
The independent risk of stone procedure from 1 to 18 months, compared to GB, was 5.61 for BPDDS (95% CI 3.11–10.13), 3.23 for LLRY (95% CI 1.99–5.24), 3.23 for SLRY (95% CI 2.66–3.91), and 1.71 for SG (95% CI 1.36–2.14). From 18 to 60 months, the risk of new stone procedures was 7.50 for BPDDS (95% CI 4.73–11.89), 2.35 for LLRY (95% CI 1.49–3.70), 2.11 for SLRY (95% CI 1.78–2.50), and 0.98 for SG (95% CI 0.75–1.29).
Male sex was associated with 1.63-fold increased risk of stone diagnosis (95% CI 1.55–1.72) and 1.53-fold increased risk of stone procedure (95% CI 1.37–1.70). Supplementary Figure 1A–D show Kaplan-Meier plots for time to new stone diagnosis and time to stone procedure stratified by sex.
Post hoc analyses comparing the risk of stone diagnosis and stone procedure are shown in Table 4. For stone diagnosis risk, no difference was observed between LLRY and SLRY in either time period (p ≥ 0.407). BPDDS was associated with significantly greater risk of stone diagnosis than all other procedures (p < 0.001), and SG was associated with significantly less risk of stone diagnosis risk than SLRY (p < 0.001).
Table 4.
Post Hoc Comparisons of the Risk of Stone Diagnosis and Stone Procedures Between SG, SLRY, LLRY, and BPDDS
| Stone diagnosis comparisons |
pb | Stone procedure comparisons |
pb |
|---|---|---|---|
| Contrastsa | Contrastsa | ||
| BPDDS vs SLRY (1–36 months) | <0.001 | BPDDS vs SLRY (1–18 months) | 0.058 |
| BPDDS vs SG (1–36 months) | <0.001 | BPDDS vs SG (1–18 months) | <0.001 |
| LLRY vs SLRY (1–36 months) | 0.884 | LLRY vs SLRY (1–18 months) | 0.99i4 |
| SLRY vs SG (1–36 months) | <0.001 | SLRY vs SG (1–18 months) | <0.001 |
| BPDDS vs SLRY (36–60 months) | <0.001 | BPDDS vs SLRY (18–60 months) | <0.001 |
| BPDDS vs SG (36–60 months) | <0.001 | BPDDS vs SG (18–60 months) | <0.001 |
| LLRY vs SLRY (36–60 months) | 0.407 | LLRY vs SLRY (18–60 months) | 0.636 |
| SLRY vs SG (36–60 months) | <0.001 | SLRY vs SG (18–60 months) | <0.001 |
Comparisons with GB were made in the primary Cox analysis.
Bonferroni-corrected α set at 0.00625.
Similar post hoc results were observed for stone procedure risk where BPDDS was associated with significantly greater risk than other procedures (p < 0.001), except for 1–18 months when very few stone procedures were performed, and no significant difference was observed between BPDDS and SLRY (p = 0.058). As in stone diagnosis, SG was associated with less stone procedure risk than SLRY (p < 0.001) and no difference was observed between SLRY and LLRY (p ≥ 0.636).
Discussion
In this study, we demonstrate that kidney stone diagnosis and kidney stone removal after bariatric surgery exist on a spectrum, with higher risk of stone diagnosis and stone procedures in patients undergoing more malabsorptive procedures. BPDDS patients were at higher risk than LLRY and SLRY patients, all of whom were at higher risk than patients undergoing SG and GB.
Prior studies on kidney stone formation after bariatric surgery support these results, but lack our study's granularity. Most studies have looked at one type of bariatric surgery and do not compare between the different types of bariatric surgeries. Several studies have demonstrated increased stone formation and oxaluria after Roux-en-Y gastric bypass compared to obese controls,3,15,16 whereas other studies have demonstrated no difference in stone formation and oxaluria among restrictive bariatric surgery patients (primarily GB) compared to obese controls.4,5 Lieske et al. retrospectively compared a number of “restrictive” patients and “malabsorptive” patients (undergoing BPDDS or very LLRY) to patients receiving SLRY, but no statistically significant difference was observed as the study was underpowered for this comparison.3 The focused investigations of these other studies highlight the significance of our results where the common bariatric surgeries were compared among themselves.
Our results mostly align with our hypothesis that SG and GB would have the lowest risk of nephrolithiasis with increasing risk for SLRY, LLRY, and BPDDS, respectively. However, two main results differed from this hypothesis.
First, we did not observe a significant difference between LLRY and SLRY in risk of stone diagnosis or stone procedure, despite LLRY being a more malabsorptive surgery. This may reflect the simplification of a continuous variable to a binary; CPT coding defines LLRY as a nonabsorptive alimentary limb greater than 150 cm and SLRY as less than 150 cm. One hundred fifty centimeter may not represent the most discerning threshold to differentiate levels of malabsorption. To our knowledge, no investigation has evaluated the relationship between limb length as a continuous variable and oxalate levels or stone incidence.
Second, a significantly greater risk of stone diagnosis and stone procedure was observed in SG compared to GB, despite both being restrictive procedures thought not to increase stone risk.5 This increased risk was observed in the time period immediately after SG, with no significant difference in diagnosis risk after 18 months and no significant difference in procedure risk after 36 months. The underlying mechanisms in restrictive procedures are not fully understood, and thus an unknown physiologic difference between SG and GB may partially explain this difference in stone risk. This finding is supported by a study that found an increased incidence of stone formation after SG compared to GB; however, this study lacked any statistical comparison.17 Regardless, the comparative risk of stones for SG versus GB was much smaller than for the other bariatric surgeries we examined and likely less clinically significant.
Although BPDDS is not as common as the other bariatric surgeries compared in this study, it remains relevant because of its greater prominence in certain countries and its greater success in addressing obesity-related outcomes.18–20 In addition to previously reported adverse events, our results suggest that BPDDS patients are also at greatly increased risk for stones.20
Reflecting reported trends in patients undergoing bariatric surgery, our study from 2007 to 2014 had a much higher number of female patients than males.21 Male patients undergoing bariatric surgery had a higher risk of stone diagnosis and procedures, especially after malabsorptive surgery, suggesting this group is uniquely at risk. Males are known to be at higher risk of stone formation,11 an association that persists and is seemingly accentuated after bariatric surgery in our study. Possibly, the predilection of male patients to form oxalate stones may be exacerbated by the oxaluria inherent to malabsorptive bariatric surgery.22
The mean age for patients undergoing each bariatric surgery in our cohort was ∼44 years. The median follow-up of 31.6 months reflects only a small proportion of a patient's remaining life expectancy.23 Long-term adverse sequelae in bariatric surgeries are of special relevance owing to the middle age of these patients, who may be potentially burdened by complications for decades to come.
This study has several limitations. First, obesity alone is associated with kidney stone formation.24 Although all subjects in this study can be assumed to be obese, there are likely variations in the degree of obesity between patients receiving different procedures. Given the inability to calculate body mass index using administrative data, we chose not to attempt to control for this potential confounder.25 Nevertheless, the risks of stone formation associated with increasing obesity reported in the literature are small in comparison to the risks we observed in stone diagnosis and stone procedure among different surgeries.24 In addition, we controlled for obesity-related comorbidities, including diabetes mellitus, hypertension, and coronary artery disease, to minimize residual confounding.
Second, baseline characteristics differed between cohorts receiving different bariatric surgeries. Patients receiving more malabsorptive surgeries tended to have more obesity-related comorbidities such as diabetes mellitus, hypertension, and coronary artery disease. These were controlled for in multivariable analysis when these comorbidities had a literature-supported relationship to stone formation.
Finally, this administrative cohort is limited by its retrospective nature and the inaccuracies of coding. Given that kidney stones are a long-term complication of bariatric surgery, recent prospective trials on bariatric surgery are unlikely to be sufficiently powered or have long enough follow-up to adequately observe this outcome. Furthermore, by using stone diagnosis and stone procedures in parallel analyses, we attempted to overcome miscoding of stone diagnosis.
Conclusions
BPDDS is associated with greater stone risk compared to all other bariatric surgeries, whereas LLRY and SLRY are associated with a greater risk than the restrictive procedures, GB and SG. This work suggests that with greater malabsorption comes greater stone risk. We further observe that male patients may be at unique risk for kidney stones after bariatric surgery.
Supplementary Material
Abbreviations Used
- BPDDS
biliopancreatic diversion with duodenal switch
- CI
confidence interval
- CPT
Current Procedural Terminology
- GB
gastric banding
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- LLRY
long-limb Roux-en-Y
- SD
standard deviation
- SG
sleeve gastrectomy
- SLRY
short-limb Roux-en-Y
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The Center for Administrative Data Research is supported, in part, by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and grant no. R24 HS19455 through the Agency for Health care Research and Quality (AHRQ).
Supplementary Material
References
- 1. The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tarplin S, Ganesan V, Monga M. Stone formation and management after bariatric surgery. Nat Rev Urol 2015;12:263–270 [DOI] [PubMed] [Google Scholar]
- 3. Lieske JC, Mehta RA, Milliner DS, Rule AD, Bergstralh EJ, Sarr MG. Kidney stones are common after bariatric surgery. Kidney Int 2015;87:839–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Penniston KL, Kaplon DM, Gould JC, Nakada SY. Gastric band placement for obesity is not associated with increased urinary risk of urolithiasis compared to bypass. J Urol 2009;182:2340–2346 [DOI] [PubMed] [Google Scholar]
- 5. Semins MJ, Asplin JR, Steele K, et al. The effect of restrictive bariatric surgery on urinary stone risk factors. Urology 2010;76:826–829 [DOI] [PubMed] [Google Scholar]
- 6. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27 [DOI] [PubMed] [Google Scholar]
- 7. Healthcare Cost and Utilization Project (HCUP). HCUP Elixhauser Comorbidity Software. Agency for Healthcare Research and Quality. 2017. www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp (accessed January6, 2018) [PubMed]
- 8. Semins MJ, Trock BJ, Matlaga BR. Validity of administrative coding in identifying patients with upper urinary tract calculi. J Urol 2010;184:190–192 [DOI] [PubMed] [Google Scholar]
- 9. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–1267 [DOI] [PubMed] [Google Scholar]
- 10. Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int 2005;68:1230–1235 [DOI] [PubMed] [Google Scholar]
- 11. Rule AD, Lieske JC, Li X, Melton LJ, Krambeck AE, Bergstralh EJ. The ROKS nomogram for predicting a second symptomatic stone episode. J Am Soc Nephrol 2014;25:2878–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: A review. Am J Hypertens 2008;21:257–264 [DOI] [PubMed] [Google Scholar]
- 13. Curhan GC, Rimm EB, Willett WC, Stampfer MJ. Regional variation in nephrolithiasis incidence and prevalence among United States men. J Urol 1994;151:838–841 [DOI] [PubMed] [Google Scholar]
- 14. Kleinbaum DG, Klein M.. Extension of thecox proportional Hazards Model for time-dependent variables. In: Krickeberg K, Gail M, eds. Survival Analysis, A Self-Learning Text. (Third Edition). Springer; 2012 [Google Scholar]
- 15. Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol 2009;181:2573–2577 [DOI] [PubMed] [Google Scholar]
- 16. Duffey BG, Pedro RN, Makhlouf A, et al. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J Am Coll Surg 2008;206:1145–1153 [DOI] [PubMed] [Google Scholar]
- 17. Chen T, Godebu E, Horgan S, Mirheydar HS, Sur RL. The effect of restrictive bariatric surgery on urolithiasis. J Endourol 2013;27:242–244 [DOI] [PubMed] [Google Scholar]
- 18. Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg 2017;27:2279–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skogar ML, Sundbom M. Duodenal switch is superior to gastric bypass in patients with super obesity when evaluated with the bariatric analysis and reporting outcome system (BAROS). Obes Surg 2017;27:2308–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Risstad H, Søvik TT, Engström M, et al. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60. JAMA Surg 2015;150:352. [DOI] [PubMed] [Google Scholar]
- 21. Young MT, Phelan MJ, Nguyen NT. A decade analysis of trends and outcomes of male vs female patients who underwent bariatric surgery. J Am Coll Surg 2016;222:226–231 [DOI] [PubMed] [Google Scholar]
- 22. Lieske JC, Rule AD, Krambeck AE, et al. Stone composition as a function of age and sex. Clin J Am Soc Nephrol 2014;9:2141–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim J, Eisenberg D, Azagury D, Rogers A, Campos GM. ASMBS Guidelines/Statements American Society for Metabolic and Bariatric Surgery position statement on long-term survival benefit after metabolic and bariatric surgery. Surg Obes Relat Dis 2016;12:453–459 [DOI] [PubMed] [Google Scholar]
- 24. Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA 2005;293:455. [DOI] [PubMed] [Google Scholar]
- 25. Golinvaux NS, Bohl DD, Basques BA, Fu MC, Gardner EC, Grauer JN. Limitations of administrative databases in spine research: A study in obesity. Spine J 2014;14:2923–2928 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.