Abstract
Purpose:
The purpose of this study was to assess for any differences in brain maturation, structure and morphometry in the fetus exposed to opioids in utero, compared to non-opioid exposed fetuses on fetal MRI.
Methods:
We performed a prospective study in pregnant women using opioids and healthy pregnant women without prenatal opioid use. We evaluated brain maturation, structure, and morphometry on second trimester and third trimester fetal MRI and assessed group differences.
Results:
28 pregnant women were enrolled, 12 with opioid exposure (average gestational age 33.67, range 28–39w), 9 of whom also smoked, and 16 without opioid exposure (average gestational age 32.53, range 27–38w). There was a significant difference in the anteroposterior diameter of the fetal cerebellar vermis in the opioid exposed fetuses compared to non-opioid exposed fetuses (p=0.004). There was no significant difference in brain biparietal diameter, fronto-occipital diameter, transverse cerebellar diameter and anteroposterior dimension of the pons in opioid exposed fetuses compared to non-opioid exposed fetuses. There were no abnormalities in brain maturation and no major brain structural abnormalities in the opioid exposed fetuses.
Conclusion
Smaller fetal anteroposterior cerebellar vermian dimension was associated with in utero opioid exposure. There were no abnormalities in brain maturation or major structural abnormalities in fetuses exposed to opioids.
Introduction
There is increasing opioid use in the general population and in pregnant women, which is of growing social concern. As a result, there are increasing numbers of infants born exposed to opioids in utero [1–3]. Prenatal opioid exposure has been associated with high risk of poor neurodevelopmental, cognitive and behavioral outcomes in children [4–7]. It is not yet clear what effects opioids have on the developing brain, and to what extent presence of other maternal comorbidities, and socioeconomic, genetic and environmental factors influence developmental outcomes in these children.
Preclinical studies suggest that fetal brain development can be influenced by maternal intake of opioids because of their effect on the maturation of developing oligodendrocytes and neurons [8–11]. Direct effects of opioids on the central nervous system are thought to be through the specific mu, delta, and kappa opioid receptors and their endogenous ligands [12–14]. There is differential expression of the different opioid receptors in the developing fetal brain, which may partly explain the effects of opioids on specific regions of the developing brain [15–17].
So far, a few studies in small samples have examined the effect of prenatal exposure to opioids on the infant brain [18]. It has been shown that prenatal exposure to opioids is associated with decreased head circumference and decreased total brain volumes, decreased basal ganglia volume and cerebellar volumes [19]. No major morphological or structural abnormalities were identified in neonates with prenatal opioid exposure, [20, 21] but abnormalities in white matter microstructure are reported in prenatal opioid exposed infants on diffusion tensor imaging [22, 23]. Resting state functional MRI (rs-fMRI) in early infancy has shown alterations in amygdala-cortical connectivity in opioid exposed infants compared to non-opioid exposed controls [24]. However, since these studies are all performed postnatally, the effects of the perinatal environment, including opioid withdrawal symptoms and their management may also have influenced these findings. We have identified a single prenatal ultrasound study in fetuses exposed to opioids, showing enlargement of the cross-sectional area of the thalami on fetal ultrasound at 18–22 weeks’ gestation associated with prenatal opioid exposure [25]. With the higher resolution of fetal MRI in assessing in utero brain development, our aim in this study was to assess for any alterations in fetal brain growth, brain structure and morphometry when exposed to opioids in utero on fetal MRI. Based on existing postnatal brain studies in infants with prenatal opioid exposure, we hypothesized that there would be lower brain measurements in prenatal opioid exposed infants.
Methods
Patient recruitment
This prospective study was performed with Indiana University Institutional Board approval as a part of an ongoing larger study in mothers and infants with prenatal opioid exposure and healthy mothers and infants. All pregnant women using opioids were recruited from prenatal centering groups and were on opioid maintenance therapy at time of enrollment. All opioid using pregnant women had started using opioids prior to pregnancy. All control healthy pregnant women were recruited from the routine prenatal care clinics at the same facilities. Control pregnant women had healthy pregnancies, were not using opioid and non-opioid illicit drugs, and did not have any other chronic medical illness. All pregnant women were recruited in the second trimester of pregnancy. Written informed consent was obtained from all pregnant women.
Imaging review
Fetal MRI was performed on a 3-tesla (T) Skyra MR scanner (Siemens Healthineers, Erlangen, Germany). Women were positioned supine and/or lateral decubitus. Two sets of T2 weighted images of the fetal brain were obtained in all 3 planes. These were the half-acquisition single-shot fast spin-echo (SSFSE; typical parameters - varied slightly due to gestational age of fetus, fetal position, and fetal motion - slice thickness 3mm, slice gap 0, TR 1100 ms, TE 100 ms, flip angle 150°, FOV 30 × 30 cm, acquisition matrix 256 × 256) and steady-state free precession (SSFP; typical parameters - slice thickness 5mm, slice gap 0, TR 1300 ms, TE 2.5 ms, flip angle 79°, FOV 32 × 32 cm, acquisition matrix 230 × 230). Other sequences of the brain included sagittal T1-W Volumetric Interpolated Breath-hold Examination (VIBE) sequence (typical parameters - slice thickness 1.2 mm, slice gap 0, TR 4 ms, TE 2.5 ms, flip angle 9°, FOV 30 × 30 cm, acquisition matrix 230 × 230, axial diffusion-weighted (typical parameters - slice thickness 5mm, slice gap 0, TR 3500 ms, TE 50 ms, flip angle 90°, FOV 32 × 32 cm, acquisition matrix 72 × 72), and axial gradient echo (GRE; typical parameters - slice thickness 6 mm, slice gap 0.9 mm, TR 400 ms, TE 10 ms, flip angle 25°, FOV 34 × 34 cm, acquisition matrix 115 × 115). Images were repeated if motion degraded and all scans were checked for adequacy by a radiologist before completion.
Imaging Review
Brain maturation and presence of structural abnormalities including brain malformations, significant ventriculomegaly, germinal matrix hemorrhage and diffusion abnormalities were assessed by a blinded reviewer (BB) with 7 years of experience in interpreting fetal MRI. All imaging sequences were reviewed. Brain maturation was considered advanced or delayed if there was a greater than two week difference between the estimated gestational age based on published timing of brain neuronal migration and sulcation [26] and the actual gestational age. This blinded assessment of structural maturation was confirmed by a second blinded reviewer (RR).
Brain morphometry was performed on T2 weighted images. Since the SSFSE were thinner slice images, this sequence was typically chosen for morphometry, however SSFP sequence was also used if they provided better definition of brain anatomy in standard planes. Measurements were performed by a single blinded reviewer (RR) with 7 years of experience in interpreting fetal MRI. Measurements were performed twice with an interval of one month between measurements. During each measurement session, all images were re-reviewed and independently measured in best non-oblique standard planes and on non-motion degraded images. Average of the two blinded measurements were used for analysis. Accuracy of these blinded brain morphometric measurements were confirmed by a second reviewer (BB). The intrarater agreement for brain measurements was assessed using the intraclass correlation coefficient (ICC) method described by Koo and Li [27]. ICC values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 were considered to be indicative of poor, moderate, good, and excellent reliability, respectively [27]. Brain morphometric indices for the fronto-occipital diameter, brain biparietal diameter, transverse cerebellar diameter, anteroposterior (AP) diameter and craniocaudal (CC) diameter of the cerebellar vermis and the AP dimension of the pons were obtained in a manner described previously [28].
Statistical analysis
Linear brain measurements were converted into gestational age corrected z-scores using previously published normative data [26]. To ensure that comparisons were made in similar geographic populations, we used the gestational age corrected z-score values in the prospectively recruited control subjects in our study. The mean z-score values between the two groups were compared using t-test. Additionally, for morphometric indices that were significantly different between the two groups, ANCOVA analysis was used to test for the effect of other covariates including gestational age, fetal sex, maternal race, maternal age, and any clinically diagnosed maternal anxiety, depression or stress disorder on mean centered z-score values. In addition, we also tested for correlations of the significantly different brain morphometric measurements between the groups with dose of opioid replacement therapy used. To account for multiple simultaneous comparisons of brain morphometric measurements, we used Bonferroni correction to assume statistical significance for a p-value of <0.008 for group comparisons.
Results
There were 12 fetuses (9 male) with in utero opioid exposure and 16 fetuses (5 male) without in utero opioid exposure. Demographic information is provided in Table 1. There were no structural brain malformations in either group identified on fetal MRI. There was no significant ventriculomegaly, abnormalities of sulcation and maturation or intracranial hemorrhage or diffusion restriction in infants with in-utero opioid exposure. Five out of the six brain morphometric measurements had excellent intra-rater agreement (ICC 0.91–0.99) except for the brain biparietal diameter, which had moderate intra-rater agreement (ICC 0.62).
Table 1:
Demographic information
| Opioid exposed N=12 | Opioid non-exposed N=16 | P value | |
|---|---|---|---|
| Maternal age in years mean (SD) | 27.4 (3.7) | 30.7 (4.3) | 0.02* |
| Maternal Race White: Non-White (Black or Mixed) | 11:1 | 12:4 | 0.4† |
| Prenatal Stress, Anxiety, Depression or PTSD | 7 | 3 | 0.05† |
| College graduate mothers | 0 | 10 | NA |
| Number with smoking/tobacco use | 9 | 0 | NA |
| Gestational age in weeks. mean (SD) | 33.67 (3.4) | 32.25 (3.3) | 0.14* |
| Fetal sex Male:Female | 9:3 | 5:11 | 0.054† |
Fisher test
T-test
The AP diameter of the cerebellar vermis in opioid exposed fetuses was significantly smaller (p=0.004) compared to controls.(Table 2) Scatter plots of the actual fetal vermian measurements in the opioid exposed and control fetuses are provided in figure 2. There was no significant difference between the prenatal opioid exposed and control groups in the gestational age corrected z-scores for the fronto-occipital diameter, brain biparietal diameter, transverse cerebellar diameter craniocaudal cerebellar vermian dimension and anteroposterior dimension of the pons although the mean scores for the opioid exposed group were smaller than the control group for all these measures (Table 2). Post hoc analysis of the AP diameter of the cerebellar vermis using covariates of gestational age, fetal sex, maternal race, maternal age, smoking and maternal stress, anxiety or depression, showed persistent significant group differences in the AP diameter of the cerebellar vermis. There was no significant correlation between the AP diameter of the cerebellar vermis and the dose of buprenorphine therapy used at the time of MRI.
Table 2.
Brain morphometry in fetuses with in utero opioid exposure and controls
| Measurement | Opioid Exposed Gestational age corrected Z-score (mean) | Controls Gestational age corrected Z-score (mean) | P-value of t-test |
|---|---|---|---|
| Fronto-occipital diameter | 0.32 | 0.94 | 0.17 |
| Brain biparietal diameter | −1.4 | 0.24 | 0.09 |
| Transverse cerebellar diameter | 0.11 | 0.59 | 0.13 |
| Craniocaudal cerebellar vermis dimension | −0.67 | −0.32 | 0.12 |
| Anteroposterior dimension of the pons | −0.8 | 0.31 | 0.09 |
| Anteroposterior dimension of the cerebellar vermis | −1.42 | −0.27 | 0.004* |
Figure 2:

Scatter plot of the AP dimension of the cerebellar vermis on fetal MRI in fetuses with in utero opioid exposure versus non drug exposed control fetuses.
Of the 12 fetuses with in utero opioid exposure, 6 had variable exposure to multiple other drugs during pregnancy including cannabinoids, benzodiazepines, cocaine, methamphetamine and alcohol; however there were no significant differences in gestational age corrected brain morphometric scores between those fetuses exposed to multiple drugs versus those exposed to opioids alone.
Discussion
In this study, we show morphometric differences in the fetal cerebellar vermis with in-utero opioid exposure. Previous studies have shown decreased cerebellar volume after birth in infants with prenatal opioid exposure, with decreased cerebellar volumes correlating with exposure to more than one type of opioid prenatally [19]. Some, but not all studies show persistent differences in cerebellar and other regional brain volumes in older children with a history of prenatal opioid exposure [29–31], suggesting the potential role of neuroplasticity in the developing infant brain. Since the method of postnatal brain quantification and evaluation of the cerebellar vermis is not performed in the same way as fetal brain MRI, it is possible that vermian alterations that we recognize in our study may persist postnatally but be unrecognized.
Although all measured brain morphometric values in our study were smaller in the opioid exposed fetuses, the antero-posterior vermian dimension was the only significantly different measurement between the two groups. This difference persisted after including other covariates in the analysis, suggesting a potential greater susceptibility of the cerebellar vermis to prenatal opioids. A large proportion of the opioid using pregnant women also smoked (75%), while none of the healthy control pregnant women smoked; therefore, it is possible that smoking may have also contributed to these alterations in brain morphometric values.
Similar to other postnatal imaging studies, we did not find any other macrostructural or maturational abnormalities in our fetuses with in-utero opioid exposure [20, 21]. A minor fusion anomaly – namely septo-preoptic fusion abnormality has been described postnatally in 2 of the 20 infants with prenatal opioid exposure which cannot be explained by the mechanisms of opioid mediated neurotoxicity and is suggested to be an incidental finding [21]. In our fetal cohort, we did not identify any midline segmentation abnormalities.
Although the long-term consequence of this difference in cerebellar vermian dimension needs to be explored, it should be noted that the functional profile of the cerebellar vermis is different from the cerebellar hemispheres in developmental outcomes and addiction related behaviors [32–36]. While the cerebellar hemispheres are thought to play a predominant role in cognitive function, the vermis is thought to play a role in emotional processes related to addiction [37]. Regardless of this distinction, the cerebellar hemispheres and the cerebellar vermis maintain a homeostatic balance of the drug related addictive behavioral regions through their connections to the cortico-mesolimbic neuronal circuitry [38]. Functional abnormalities associated with the cerebellum are identified in prenatal opioid exposed adolescents, where decreased activation in the culmen of the cerebellum was noted with visuospatial working memory task when compared to healthy adolescents [39]. Lin et al. showed reduced volume in the cerebellar gray matter, as well as the cerebellar vermis, in adults with opioid use correlating with depression scores [40]. Opioid using adults who had been abstinent also showed enhanced cerebellar hemispheric activity and vermian activity elicited by heroin versus neutral pictures [34]. Cerebellar vermian activation is also seen in other drug injury in animal studies with cocaine [41]. In adult opioid abstinent individuals, cerebellar vermian activation was also shown in an adult study to be a marker of relapse [36], providing further evidence for the role of the vermis in addiction related behaviors. Therefore, cerebellar vermian developmental alterations could be important in the understanding of future addiction related behaviors.
The mu, delta and kappa opioid receptors have varying distribution in the brain and varied effects related to opioid ligand binding. Most of the effects of buprenorphine or methadone, (drugs that are used for medication assisted treatment in opioid use disorder) are through the mu opioid receptors. In adult humans, the mu opioid receptor concentrations are found to be highest in the caudate nucleus, anterior cingulate cortex, thalamus, and pituitary gland; the delta opioid receptor distributed throughout the neocortex; and the kappa opioid receptor expressed in the insular cortex, lateral frontal cortex and amygdala [42]. Opioid receptors are also shown to be present in the cerebellar vermis in addition to the cerebellar cortex and dentate nuclei [43, 42]. While the distribution of opioid receptors in different stages of fetal life and in the developing human brain is not known, preclinical studies suggest that there may be redistribution of opioid receptors during development, and therefore the potential to influence regional brain development in the setting of opioid abuse [44, 45, 16, 17, 46]. For example, exposure to heroin prenatally in mice is shown to result in postnatal memory deficits due to neuronal apoptosis[47]. Preclinical studies also show that modulation of opioid receptors expressed by oligodendroglia and their precursors can induce mitogenesis and differentiation of these cells, indicating a crucial role of the opioid system in myelination [48]. However, these findings cannot be directly translated to human fetuses, and much work needs to be done in understanding the developmental profile of opioid receptors in the human fetus and their contribution to development and maturation of the CNS.
One of the limitations of our study is the small sample size, and therefore possible decreased power to identify significant differences in morphometry of other brain regions. Moreover, the cerebellar vermis may also be influenced by other fetal or maternal conditions. For example, in our cohort, the educational level of opioid using pregnant women was lower compared to control women. Therefore, we cannot statistically assess the contribution of maternal education to fetal cerebellar vermian dimension. As mentioned earlier, the influence of smoking on the fetal brain in the presence of opioid exposure could also not be distinguished in this pilot study. Smoking has been shown to be associated with smaller overall brain volumes in the fetus [49] and smaller regional brain volumes including cerebellar volume in the preterm infant at term gestational age [50]. Future studies should therefore be aimed at comparable recruitment of drug using and control populations to better understand the effects of other social and environmental covariates on brain development. Other authors have shown specifically that postnatal cerebellar volumes in infants is decreased with multiple opioid drug exposures [19] and while our study may be underpowered to identify this difference, or it is possible that cerebellar growth would be affected more with greater duration of in utero drug exposure and therefore most obvious after birth. We have used linear measurements of the brain that are commonly used in clinical practice to measure fetal brain growth, and results of our pilot study suggest that future studies of fetal brain development in larger cohorts should also include specifically cerebellar vermian assessment.
Conclusion
There were no major structural or maturational abnormalities on fetal MRI with in utero opioid exposure. Decreased fetal cerebellar vermian dimensions were associated with in-utero opioid exposure. Future larger studies should be aimed at replicating these results and understanding the neurodevelopmental and behavioral significance of alteration in growth of the cerebellar vermis in prenatal drug exposures.
Figure 1:
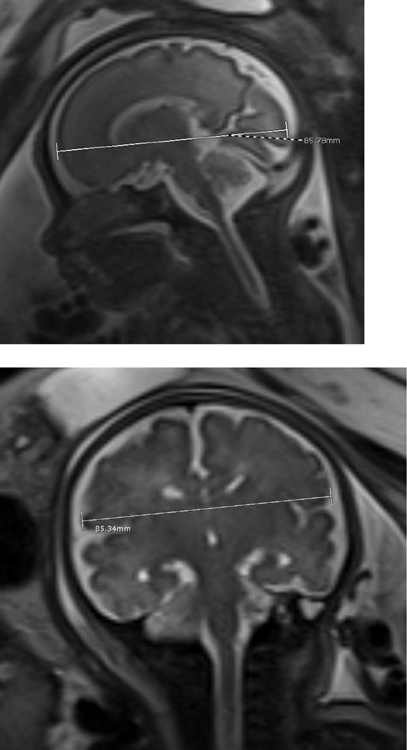

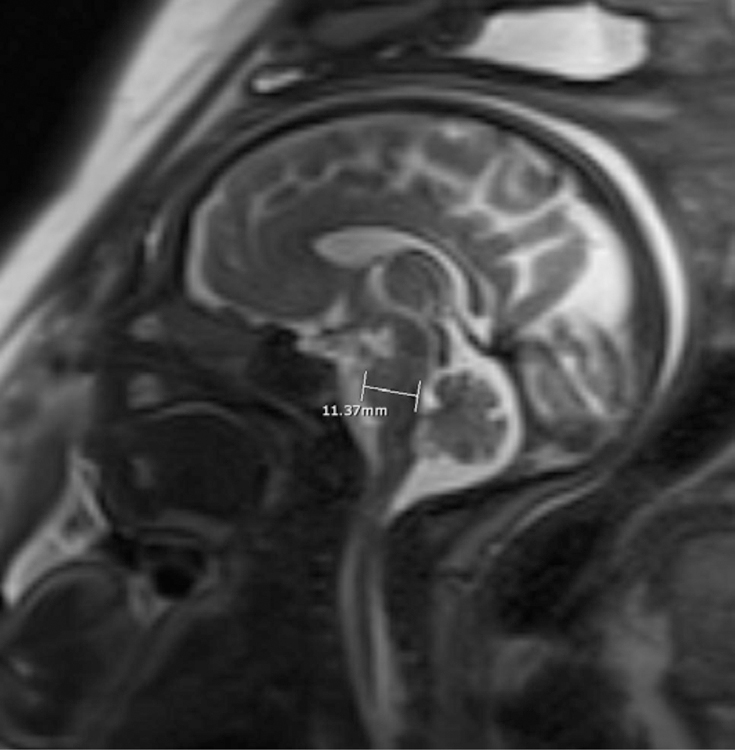
Sagittal SSFSP T2w image of the brain in a 28 week-gestation fetus with calipers indicating frontooccipital diameter (a); Coronal SSFSP T2w images of the brain in a 35-week gestation fetus with calipers showing measurement of the biparietal diameter (b) and transverse cerebellar diameter (c). Sagittal SSFSP T2w images of the brain in another 35 week-gestation fetus with calipers showing measurement of the anteroposterior and craniocaudal diameter of the cerebellar vermis (e) and the anteroposterior diameter of the pons (f).
Highlights.
No macrostructural or maturational alterations on Fetal brain MRI in prenatal opioid exposure
Significantly smaller vermian dimension on MRI in prenatal opioid exposed fetuses
Differences in vermian dimensions persist after correcting for gestational age and maternal factors
Funding Sources:
RR was supported by the American Roentgen Ray Scholarship Award 2018 and Radiological Society of North America Seed Grant 2018. SS and DH were supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award, R01HD096800 (PI: Sadhasivam and Haas). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
References
- 1.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012. May 9;307(18):1934–40. [DOI] [PubMed] [Google Scholar]
- 2.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014. May;123(5):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. Journal of perinatology : official journal of the California Perinatal Association. 2015. Aug;35(8):650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross EJ, Graham DL, Money KM, Stanwood GD. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015. Jan;40(1):61–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nygaard E, Slinning K, Moe V, Due-Tonnessen P, Fjell A, Walhovd KB. Neuroanatomical characteristics of youths with prenatal opioid and poly-drug exposure. Neurotoxicol Teratol. 2018. Jul – Aug;68:13–26. [DOI] [PubMed] [Google Scholar]
- 6.Romanowicz M, Vande Voort JL, Shekunov J, Oesterle TS, Thusius NJ, Rummans TA, et al. The effects of parental opioid use on the parent-child relationship and children’s developmental and behavioral outcomes: a systematic review of published reports. Child Adol Psych Men. 2019. Jan 12;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen JM, Høiseth G, Nygaard E. Prenatal exposure to methadone or buprenorphine and long-term outcomes: A meta-analysis. Early Human Development. 2020. 2020/04/01/;143:104997. [DOI] [PubMed] [Google Scholar]
- 8.Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: mu and kappa opioid receptors mediate differential mitogenic and growth responses. Glia. 1998. Feb;22(2):189–201. [DOI] [PubMed] [Google Scholar]
- 9.Stiene-Martin A, Knapp PE, Martin K, Gurwell JA, Ryan S, Thornton SR, et al. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia. 2001. Oct;36(1):78–88. [PMC free article] [PubMed] [Google Scholar]
- 10.Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002. May;42(6):829–36. [DOI] [PubMed] [Google Scholar]
- 11.Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci. 2014;36(5):409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009. Oct;89(4):1379–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets. 2012. Feb;13(2):230–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callaghan CK, Rouine J, O’Mara SM. Potential roles for opioid receptors in motivation and major depressive disorder. Prog Brain Res. 2018;239:89–119. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Pasulka P, Perry B, Pizzi WJ, Schnoll SH. Effect of perinatal exposure to methadone on brain opioid and alpha 2-adrenergic receptors. Neurobehavioral toxicology and teratology. 1986. Jul-Aug;8(4):399–402. [PubMed] [Google Scholar]
- 16.Slamberova R, Rimanoczy A, Bar N, Schindler CJ, Vathy I. Density of mu-opioid receptors in the hippocampus of adult male and female rats is altered by prenatal morphine exposure and gonadal hormone treatment. Hippocampus. 2003;13(4):461–71. [DOI] [PubMed] [Google Scholar]
- 17.Vathy I, Slamberova R, Rimanoczy A, Riley MA, Bar N. Autoradiographic evidence that prenatal morphine exposure sex-dependently alters mu-opioid receptor densities in brain regions that are involved in the control of drug abuse and other motivated behaviors. Progress in neuro-psychopharmacology & biological psychiatry. 2003. May;27(3):381–93. [DOI] [PubMed] [Google Scholar]
- 18.Radhakrishnan R, Grecco G, Stolze K, Atwood B, Jennings SG, Lien IZ, et al. Neuroimaging in infants with prenatal opioid exposure: Current evidence, recent developments and targets for future research. J Neuroradiol. 2020. Oct 13. [DOI] [PMC free article] [PubMed]
- 19.Yuan Q, Rubic M, Seah J, Rae C, Wright IM, Kaltenbach K, et al. Do maternal opioids reduce neonatal regional brain volumes? A pilot study. J Perinatol. 2014. Dec;34(12):909–13. [DOI] [PubMed] [Google Scholar]
- 20.Kahila H, Kivitie-Kallio S, Halmesmaki E, Valanne L, Autti T. Brain magnetic resonance imaging of infants exposed prenatally to buprenorphine. Acta Radiol. 2007. Mar;48(2):228–31. [DOI] [PubMed] [Google Scholar]
- 21.Merhar SL, Parikh NA, Braimah A, Poindexter BB, Tkach J, Kline-Fath B. White Matter Injury and Structural Anomalies in Infants with Prenatal Opioid Exposure. AJNR Am J Neuroradiol. 2019. Dec;40(12):2161–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walhovd KB, Watts R, Amlien I, Woodward LJ. Neural tract development of infants born to methadone-maintained mothers. Pediatr Neurol. 2012. Jul;47(1):1–6. [DOI] [PubMed] [Google Scholar]
- 23.Monnelly VJ, Anblagan D, Quigley A, Cabez MB, Cooper ES, Mactier H, et al. Prenatal methadone exposure is associated with altered neonatal brain development. NeuroImage Clinical. 2018;18:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radhakrishnan R, Elsaid NMH, Sadhasivam S, Reher TA, Hines AC, Yoder KK, et al. Resting state functional MRI in infants with prenatal opioid exposure-a pilot study. Neuroradiology. 2020. Sep 26. [DOI] [PMC free article] [PubMed]
- 25.Schulson M, Liu A, Bjorkman T, Quinton A, Mann KP, Benzie R, et al. Mid-Gestational Enlargement of Fetal Thalami in Women Exposed to Methadone during Pregnancy. Front Surg. 2014;1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kline-Fath B, Bulas D, R B-S>, editors. Fundamentals and Advanced Fetal Imaging. 2015.
- 27.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016. Jun;15(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radhakrishnan R, Merhar SL, Burns P, Zhang B, Lim FY, Kline-Fath BM. Fetal brain morphometry on prenatal magnetic resonance imaging in congenital diaphragmatic hernia. Pediatr Radiol. 2019. Feb;49(2):217–23. [DOI] [PubMed] [Google Scholar]
- 29.Walhovd KB, Moe V, Slinning K, Due-Tonnessen P, Bjornerud A, Dale AM, et al. Volumetric cerebral characteristics of children exposed to opiates and other substances in utero. NeuroImage. 2007. Jul 15;36(4):1331–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walhovd KB, Bjornebekk A, Haabrekke K, Siqveland T, Slinning K, Nygaard E, et al. Child neuroanatomical, neurocognitive, and visual acuity outcomes with maternal opioid and polysubstance detoxification. Pediatr Neurol. 2015. Mar;52(3):326–32 e1–3. [DOI] [PubMed] [Google Scholar]
- 31.Sirnes E, Oltedal L, Bartsch H, Eide GE, Elgen IB, Aukland SM. Brain morphology in school-aged children with prenatal opioid exposure: A structural MRI study. Early human development. 2017. Mar – Apr;106–107:33–39. [DOI] [PubMed] [Google Scholar]
- 32.Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000. Aug 1;60(2):207–16. [DOI] [PubMed] [Google Scholar]
- 33.Xiao Z, Lee T, Zhang JX, Wu Q, Wu R, Weng X, et al. Thirsty heroin addicts show different fMRI activations when exposed to water-related and drug-related cues. Drug Alcohol Depend. 2006. Jun 28;83(2):157–62. [DOI] [PubMed] [Google Scholar]
- 34.Li Q, Wang Y, Zhang Y, Li W, Yang W, Zhu J, et al. Craving correlates with mesolimbic responses to heroin-related cues in short-term abstinence from heroin: an event-related fMRI study. Brain Res. 2012. Aug 21;1469:63–72. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Zhu J, Li Q, Li W, Wu N, Zheng Y, et al. Altered fronto-striatal and frontocerebellar circuits in heroin-dependent individuals: a resting-state FMRI study. PLoS One. 2013;8(3):e58098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Li W, Wang H, Wang Y, Zhang Y, Zhu J, et al. Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: an event-related functional magnetic resonance imaging study. Addict Biol. 2015. Sep;20(5):968–78. [DOI] [PubMed] [Google Scholar]
- 37.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009. Jan 15;44(2):489–501. [DOI] [PubMed] [Google Scholar]
- 38.Moulton EA, Elman I, Becerra LR, Goldstein RZ, Borsook D. The cerebellum and addiction: insights gained from neuroimaging research. Addict Biol. 2014. May;19(3):317–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schweitzer JB, Riggins T, Liang X, Gallen C, Kurup PK, Ross TJ, et al. Prenatal drug exposure to illicit drugs alters working memory-related brain activity and underlying network properties in adolescence. Neurotoxicol Teratol. 2015. Mar-Apr;48:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin WC, Chou KH, Chen HL, Huang CC, Lu CH, Li SH, et al. Structural deficits in the emotion circuit and cerebellum are associated with depression, anxiety and cognitive dysfunction in methadone maintenance patients: a voxel-based morphometric study. Psychiatry Res. 2012. Feb 28;201(2):89–97. [DOI] [PubMed] [Google Scholar]
- 41.Carbo-Gas M, Moreno-Rius J, Guarque-Chabrera J, Vazquez-Sanroman D, Gil-Miravet I, Carulli D, et al. Cerebellar perineuronal nets in cocaine-induced pavlovian memory: Site matters. Neuropharmacology. 2017. Oct;125:166–80. [DOI] [PubMed] [Google Scholar]
- 42.Cumming P, Marton J, Lilius TO, Olberg DE, Rominger A. A Survey of Molecular Imaging of Opioid Receptors. Molecules. 2019. Nov 19;24(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schadrack J, Willoch F, Platzer S, Bartenstein P, Mahal B, Dworzak D, et al. Opioid receptors in the human cerebellum: evidence from [11C]diprenorphine PET, mRNA expression and autoradiography. NeuroReport. 1999;10(3):619–24. [DOI] [PubMed] [Google Scholar]
- 44.Kornblum HI, Hurlbut DE, Leslie FM. Postnatal development of multiple opioid receptors in rat brain. Brain Res. 1987. Dec 15;465(1–2):21–41. [DOI] [PubMed] [Google Scholar]
- 45.Rius RA, Barg J, Bem WT, Coscia CJ, Loh YP. The prenatal development profile of expression of opioid peptides and receptors in the mouse brain. Brain Res Dev Brain Res. 1991;58(2):237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kivell BM, Day DJ, McDonald FJ, Miller JH. Developmental expression of mu and delta opioid receptors in the rat brainstem: evidence for a postnatal switch in mu isoform expression. Brain Res Dev Brain Res. 2004. Feb 20;148(2):185–96. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Han TZ. Prenatal exposure to heroin in mice elicits memory deficits that can be attributed to neuronal apoptosis. Neuroscience. 2009. 04/January;160:330–8. [DOI] [PubMed] [Google Scholar]
- 48.Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C. The Opioid System and Brain Development: Effects of Methadone on the Oligodendrocyte Lineage and the Early Stages of Myelination. Developmental Neuroscience. 2014;36(5):409–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anblagan D, Jones NW, Costigan C, Parker AJ, Allcock K, Aleong R, et al. Maternal smoking during pregnancy and fetal organ growth: a magnetic resonance imaging study. PLoS One. 2013;8(7):e67223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekblad M, Korkeila J, Parkkola R, Lapinleimu H, Haataja L, Lehtonen L, et al. Maternal smoking during pregnancy and regional brain volumes in preterm infants. J Pediatr. 2010. Feb;156(2):185–90 e1. [DOI] [PubMed] [Google Scholar]


