Abstract
Negativity bias is a core feature of depression that is associated with dysfunctional frontoamygdalar connectivity; this pathway is associated with emotion regulation and sensitive to neurobiological change during puberty. We used a valence bias task (ratings of emotional ambiguity) as a potential early indicator of depression risk and differences in frontoamygdalar connectivity. Previous work using this task demonstrated that children normatively have a negative bias that attenuates with maturation. Here, we test the hypothesis that persistence of this negativity bias as maturation ensues may reveal differences in emotion regulation development, and may be associated with increased risk for depression. In children aged 6–13 years, we tested the moderating role of puberty on relationships between valence bias, depressive symptoms, and frontoamygdalar connectivity. A negative bias was associated with increased depressive symptoms for those at more advanced pubertal stages (within this sample) and less regulatory frontoamygdalar connectivity, whereas a more positive bias was associated with more regulatory connectivity patterns. These data suggest that with maturation, individual differences in positivity biases and associated emotion regulation circuitry confer a differential risk for depression. Longitudinal work is necessary to determine the directionality of these effects and explore the influence of early life events.
Keywords: amygdala, emotion, medial prefrontal cortex, negativity bias, puberty
1 |. INTRODUCTION
Individual differences in internalizing problems, like depression, are associated with amygdala-prefrontal cortex (PFC) circuitry (Gee, Gabard-Durnam, et al., 2013; Pezawas et al., 2005; Wang et al., 2009), and have been shown to emerge during the transition from childhood to adulthood (Emslie et al., 2005; Hankin et al., 1998; Kessler et al., 2001). Notably, a number of studies have linked the emergence of these individual differences to neurobiological changes during pubertal development (Andersen & Teicher, 2008; Angold & Costello, 2006; Paus et al., 2008). In particular, the medial prefrontal cortex (mPFC) strengthens connections with the amygdala during the years of puberty (Tottenham & Gabard-Durnam, 2017), a change that is intimately tied to the release of gonadal hormones (Lebron-Milad & Milad, 2012; van Wingen et al., 2011). These brain changes accompany profound changes in emotional behavior during this period of development that support functional social behavior (Denham, 1998; Eisenberg et al., 1993; John & Gross, 2004; Saarni, 1984).
In adulthood, connections between amygdala and a network of subregions in the PFC are understood to underpin emotion regulation mechanisms (Banks et al., 2007; Phillips et al., 2008; Wager et al., 2008) vis-a-vis downregulation of amygdala activity during emotional processing (Hare et al., 2008; Hariri et al., 2003; Pezawas et al., 2005). In particular, the mPFC and amygdala, in addition to sharing numerous structural connections (Ghashghaei et al., 2007), show inverse functional connectivity during explicit emotion regulation (Urry, 2006) that is suggestive of a downregulation of amygdala (Gee, Humphreys, et al., 2013). Further, adults compared to children show greater inverse amygdala-mPFC connectivity (Gabard-Durnam et al., 2014; Gee, Humphreys, et al., 2013; Perlman & Pelphrey, 2011) in addition to relatively decreased amygdala activation (Guyer et al., 2008; Hare et al., 2008; Monk et al., 2003; Swartz et al., 2014; but see Moore et al., 2012) in response to negative emotional information. Indeed, younger children show a non-regulatory positive amygdala-mPFC connectivity pattern, but older children and adolescents show an inverse regulatory connectivity (Gee, Humphreys, et al., 2013). Moreover in adults, this more positive amygdala-mPFC connectivity has been implicated in the development of mental health disorders (Das et al., 2007; Gee, Gabard-Durnam, et al., 2013; Phillips et al., 2008). Taken together, this work suggests that although emotion regulation relies on a broad network of regions, the connections between the mPFC and the amygdala lie at the core of emotion regulation abilities in adulthood.
One notable feature of internalizing symptoms is a negativity bias, or an enhanced attention to and memory for negative information (Browning et al., 2010; Roy et al., 2008; Vasey et al., 1995), which is central to a variety of mood and anxiety disorders (e.g., depression; Beck, 1976; Williams et al., 2007). Interestingly, negativity bias is associated with more positive amygdala-mPFC connectivity (i.e., a non-regulatory pattern) (Etkin et al., 2009; Roy et al., 2013; see also Kim et al., 2011). Given that this same circuitry is sensitive to pubertal changes (Blakemore et al., 2010; Goddings et al., 2014; Herting et al., 2014; Mills et al., 2014; Vijayakumar et al., 2018, 2019) and is related to mental health disorders which emerge during puberty (Burghy et al., 2012; Hulvershorn et al., 2011; Kessler et al., 2005; Lee et al., 2014; Roy et al., 2013), understanding how this circuitry is associated with individual differences in negativity bias across puberty is essential to understanding the development of depression.
Because the release of gonadal hormones which drive neurobiological changes can begin as early as 8 years (Blakemore et al., 2010; Dorn et al., 2006), whereas depression tends to onset at around 14 years of age (Burke, 1991; Lewinsohn et al., 1994), the construction of this system may begin in relatively early pubertal stages prior to the onset of internalizing symptoms. That is, pubertal transitions may act as a developmental “prism”, revealing individual differences in emotion regulation behaviors. In particular, the period from early to middle puberty is met with structural (Blanton et al., 2012; Goddings et al., 2014; Hu et al., 2013; Pfefferbaum et al., 2016) and functional (Clark & Beck, 2010; Forbes et al., 2011; Moore et al., 2012; Pfeifer et al., 2013; Vijayakumar et al., 2018, 2019) changes within both the amygdala and mPFC, as well as in their connectivity (Asato et al., 2010; Herting et al., 2014; Menzies et al., 2015; see also Gee, Humphreys, et al., 2013). This evidence is consistent with detailed animal models which find that the onset of puberty triggers reorganization within both the mPFC and its white matter fibers shared with the amygdala (Juraska & Willing, 2017; Zimmermann et al., 2019). The neurobiological processes associated with these relatively early pubertal stages may produce measureable individual differences in frontoamygdalar function critical to mental health outcomes.
Reliably measuring emotional biases in children during these early pubertal stages poses a methodological challege. For example, extant literature on negativity bias, which includes subclinical symptomology (Pagliaccio et al., 2014), tends to measure emotional biases using stimuli that rely on developmentally advanced cognitive/linguistic abilities not ideally suited for younger children. An emerging alternative approach has measured emotional biases using valence ratings of facial expressions, which are reliably identified in early childhood (Bruce et al., 2000; Widen & Russell, 2008). Although some expressions (e.g., happy or angry) signal clear valence information about the emotions and intentions of others, other expressions (e.g., surprise) are ambiguous because they signal both positive (e.g., an unexpected gift) and negative events (e.g., witnessing an accident). Notably, children compared to adults tend to rate surprised expressions as having a more negative meaning (i.e., more negative valence bias; Tottenham et al., 2013). Given that surprised expressions may be reliably identified across all ages and their ratings track developmental changes in emotional behavior, the ambiguity conveyed through surprised expressions is ideally suited to probe differences in negativity bias at early pubertal stages.
Neuroimaging work suggests that normative developmental shifts in emotional biases might reveal how pubertal changes correlate with variability in the neurobiology of depression. For instance, in adults, positive ratings of surprised faces depend upon slower and more deliberate processing (Kaffenberger et al., 2010; Neta et al., 2011; Neta & Tong, 2016), and appear to rely on emotion regulation mechanisms (Kim et al., 2003; Petro et al., 2018). This work suggests that the development of a more positive valence bias and a more mature (inverse) amygdala-mPFC connectivity during puberty can powerfully impact the emergence of internalizing symptoms during this developmentally sensitive period. For example, maintaining a negative valence bias and a more immature (positive) amygdala-mPFC connectivity into adulthood may be a risk factor for the onset of depression. However, no study has yet used neuroimaging to explore valence bias in youth populations. Thus, measuring normative shifts in valence bias and its associated amygdala-mPFC connectivity is an innovative approach that could reveal how pubertal changes correlate with variability in the neurobiology of depression.
Using a cross-sectional design, we explored the relationships between valence bias, depressive symptoms, and emotion regulation circuitry within an age range demonstrated to show a more negative valence bias (ages 6–13 years; Tottenham et al., 2013), a developing regulatory circuitry (Gabard-Durnam et al., 2014; Gee, Humphreys, et al., 2013; Perlman & Pelphrey, 2011; Silvers et al., 2017), and prior to the typical emergence of depression (age 14 years; Burke, 1991; Lewinsohn et al., 1994). This methodological choice enabled us to explore how relatively early pubertal changes contribute to the emergence of internalizing problems in depression. We examined functional brain connectivity while viewing facial expressions during magnetic resonance imaging (MRI). We used pubertal scores as our proxy for maturity because (a) puberty is more closely tied than age to neurobiological changes in brain structure and function (Blakemore et al., 2010; Goddings et al., 2012) and especially amygdala-mPFC circuitry (Gabard-Durnam et al., 2014; Gee, Humphreys, et al., 2013; Herting et al., 2014; Perlman & Pelphrey, 2011; Silvers et al., 2017), and (b) age represents a different stage in maturation for males and females (Schuiling & Likis, 2016). For our exploratory analysis, we predict that with increasing maturation (within this relatively immature sample), a more negative valence bias and a more immature amygdala-mPFC connectivity pattern may pose an increased risk for depression.
2 |. MATERIALS AND METHODS
2.1 |. Participants
We collected data from 61 participants (29 female; ages 6–13 years, mean(SD) age = 9.18(2.13)). All participants and their parent reported that the participant had no history of neurological or psychiatric disorders, nor were any taking psychotropic medications. All protocols were approved by the University of Nebraska Committee for the Protection of Human Subjects. The participants and their parent were informed of all procedures prior to the child’s participation, and a parent of each participant gave written informed consent prior to testing in accordance with the Declaration of Helsinki.
Six participants did not complete the neuroimaging portion of the task. An additional fourteen participants were excluded for failing to accurately rate the clearly valenced angry (N = 4) and happy (N = 10) expressions on at least 60% of trials, an accuracy threshold used for adult participants (Brown et al., 2017; Neta et al., 2009, 2013, 2018; Neta & Tong, 2016). The exclusion criteria for accuracy is particularly important because, if participants are not accurately rating angry faces as negative and happy faces as positive, then it is difficult to discern the specific valence interpretations of emotional ambiguity (i.e., valence bias). The final sample consisted of 41 participants (23 female; ages 6–13 years, mean(SD) age = 9.46(2.12)) that did not differ in age from those excluded (t59 = 1.44, p = .16, d = 0.38; range = 6–13; mean(SD) age = 8.60(2.06)), and who identified as either Caucasian (N = 38), Black/African-American (N = 1), Asian (N = 1), or Unknown (N = 1).
Albeit relatively small given the analysis of individual differences (Dubois & Adolphs, 2016), this sample size allowed us to explore the potential relationship between pubertal development, depressive symptoms, valence bias, and amygdala-mPFC functional connectivity. Further, given that no study has combined this valence bias task with neuroimaging in childhood, this exploration aims to serve as a first step in developing a testable model of the development of depressive symptoms and emotion regulation circuitry as measured by the valence bias task.
2.1.1 |. Individual difference measures
We assessed puberty using the Petersen Puberty Development Scale (PDS; Petersen et al., 1988), and calculated scores as the average of each of the five items assessing physical development (out of the items numbered 1–7, where the five items included varied based on the participant’s sex; each with a possible response of 1–4), as described by Petersen and colleagues (possible range = 1–4). Because PDS scores tend to deviate from 1 only after the age of 8 (Hu et al., 2013), all participants under the age of 8 were assigned the lowest score of 1 (i.e., prior to the onset of puberty). The data for each of these variables were submitted to a Shapiro-Wilk test to determine normality. The PDS score (Shapiro-Wilk: W = 0.90, p = .001), age (Shapiro-Wilk: W = 0.94, p = .03), and depressive symptoms (see below; Shapiro-Wilk: W = 0.78, p < .001) were non-normally distributed and valence bias trended toward a non-normal distribution (W = 0.95, p = .07). Because of the non-normality of these distributions, robust regression was used for all analyses of these variables.
As expected, given our targeted age range, the current sample subtended to relatively low PDS scores (range = 1–2.8, mean(SD) = 1.67(0.56), skewness(SD) = 0.71(0.37), kurtosis(SD) = −0.54(0.72)) rather than representing the full spectrum of possible scores. This questionnaire was completed by the child with instruction from the experimenter and is comprised of questions related to secondary sex characteristics (e.g., growth of body hair, deepening of voice), the appearance of which are a marker of gonadal hormone release during gonadarche (Susman & Rogol, 2013). The PDS score was not related to sex (Figure 1a; t39 = 0.16, p = .87, d = 0.05), but was positively corelated with age (Figure 1b; t39 = 6.68, p < .001, d = 2.14). The average full-scale intelligence quotient of the sample was within normal range as assessed by the two subset Wechsler Abbreviated Scale of Intelligence (WASI; range = 88–142; mean(SD) = 114.18(14.53), however there were missing data for one participant; Wechsler, 1999), and was not related to age (t38 = −0.92, p = .36, d = −0.30) or PDS score (t38 = −0.66, p = .52, d = −0.21).
FIGURE 1.
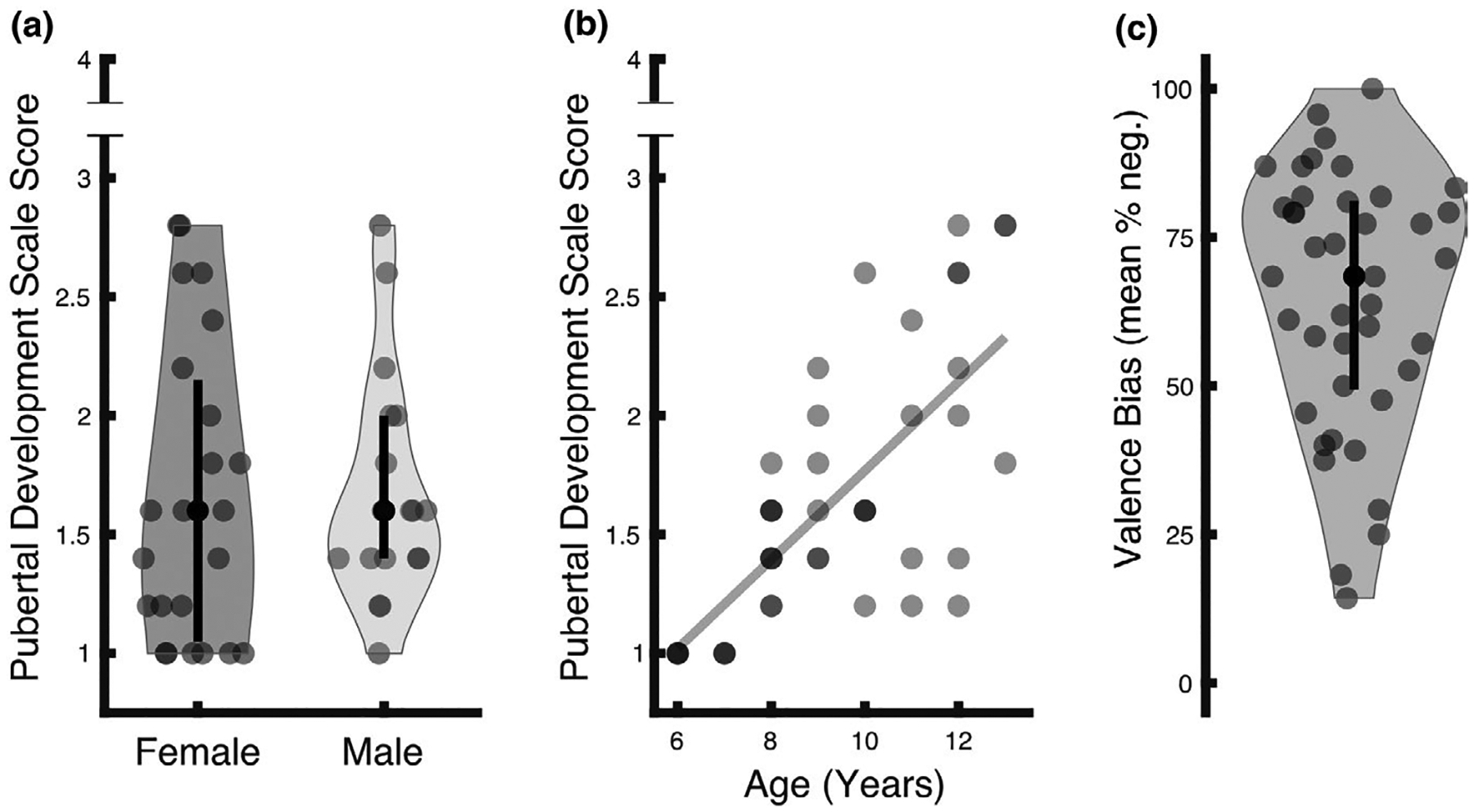
Descriptive illustration of measures. (a) The sample’s PDS scores ranged from 1 to 2.8, out of a highest possible score of 4. These scores subtend to a low range (mean(SD) = 1.67(0.56), skewness(SD) = 0.71(0.37), kurtosis(SD) = −0.54(0.72)), consistent with the fact that the age range (6–13 years) of the sample subtended to relatively low PDS scores. Females (N = 23) and males (N = 18) did not differ as a function of PDS score (t39 = 0.16, p = .87). In each violin plot, the black circle indicates the median (females = 1.60, males = 1.60), and the lower and upper ends of the black line indicate the first (females = 1.05, males = 1.40) and third (females = 2.15, males = 2.00) quartile cut-off, respectively. (b) Age was positively related to PDS score (t39 = 6.68, p < .001). Darker dots represent an overlap of participants with identical age/PDS values. (c) Valence bias scores, computed as the percent of negative ratings of surprised expressions, ranged from 14.29% to 100% with a mean of 64.66%. The black circle indicates the median (68.42%), and the lower and upper ends of the black line indicate the first (49.41%) and third (81.12%) quartile cut-offs, respectively
Depressive symptoms were quantified using the major depression subscale of the Revised Child Anxiety and Depression Scale (RCADS; Chorpita et al., 2000) administered to the child’s parent to maintain consistency across the sample given the young ages. The non-normality of these data (range = 0–12, possible range = 0–30, mean(SD) = 2.20(2.40), skewness(SD) = 2.06(0.37), kurtosis(SD) = 5.95(0.72)) was characterized by a skewness toward to the lower end of the RCADS scale. This scale has been validated in ages as young as 3 years (Ebesutani et al., 2015). However, because our age range (6–13 years) extends below the minimum of standardized T scores (grades 3–12), raw scores of the major depression subscale were used in the analyses, consistent with a recent study (Vantieghem et al., 2017). One participant reported a major depression score of 12 which, while the age of this participant (7 years) was below the range included in the published T scores, was associated with a T score in the youngest standardized sample (3rd grade) of 74, exceeding the cut-off associated with clinical diagnoses (T = 70; Chorpita et al., 2000). However, the inclusion of this participant did not qualitatively affect the reported results (see Sections 3.1.2 And 3.2.1).
2.2 |. Procedure
2.2.1 |. Session 1: behavioral
All experimental stimuli were presented on E-Prime software (version 2.0.10; Psychology Software Tools, Pittsburgh, PA). In Session 1 (Figure 2), participants performed a task to assess their baseline valence bias in which they viewed positive (e.g., happy), negative (e.g., angry), and ambiguous (e.g., surprised) images on a black background. For each image, participants were asked to make a two-alternative, forced-choice decision (via keyboard press) as quickly and as accurately as possible, indicating whether each image “felt good or felt bad”; this language and stimuli have been in previous work within similar age groups (Kestenbaum, 1992; Tottenham et al., 2013). This included a set of 48 faces, 24 with an ambiguous valence (surprised expression), and 24 with a clear valence (12 angry and 12 happy expressions). Of the 48 faces, 34 discrete face identities were used (17 males, 17 females) posing angry, happy, and surprised expressions. Fourteen identities (seven females, ages 21–30 years) were taken from the NimStim Set of Facial Expressions (Tottenham et al., 2009) and 20 (10 female, age 20–30 years) from the Karolinska Directed Emotional Faces database (Goeleven et al., 2008). All identities were European/European American. In separate but alternating blocks, scenes from the International Affective Picture System (IAPS; Lang et al., 1997) were presented, consisting of 24 scenes with an ambiguous valence and 24 with a clear valence (12 negative and 12 positive). The purpose of rating the IAPS scenes was outside the scope of the current report, and did not contribute to the valence bias score nor were they included in any analysis. All blocks of stimuli included 24 images (12 ambiguous, six positive, and six negative) presented in a pseudorandom order (see Figure 2 for a depiction of tasks). Each stimulus was presented for 1,500 ms followed by a fixation cross for either 200 or 1,900 ms.
FIGURE 2.
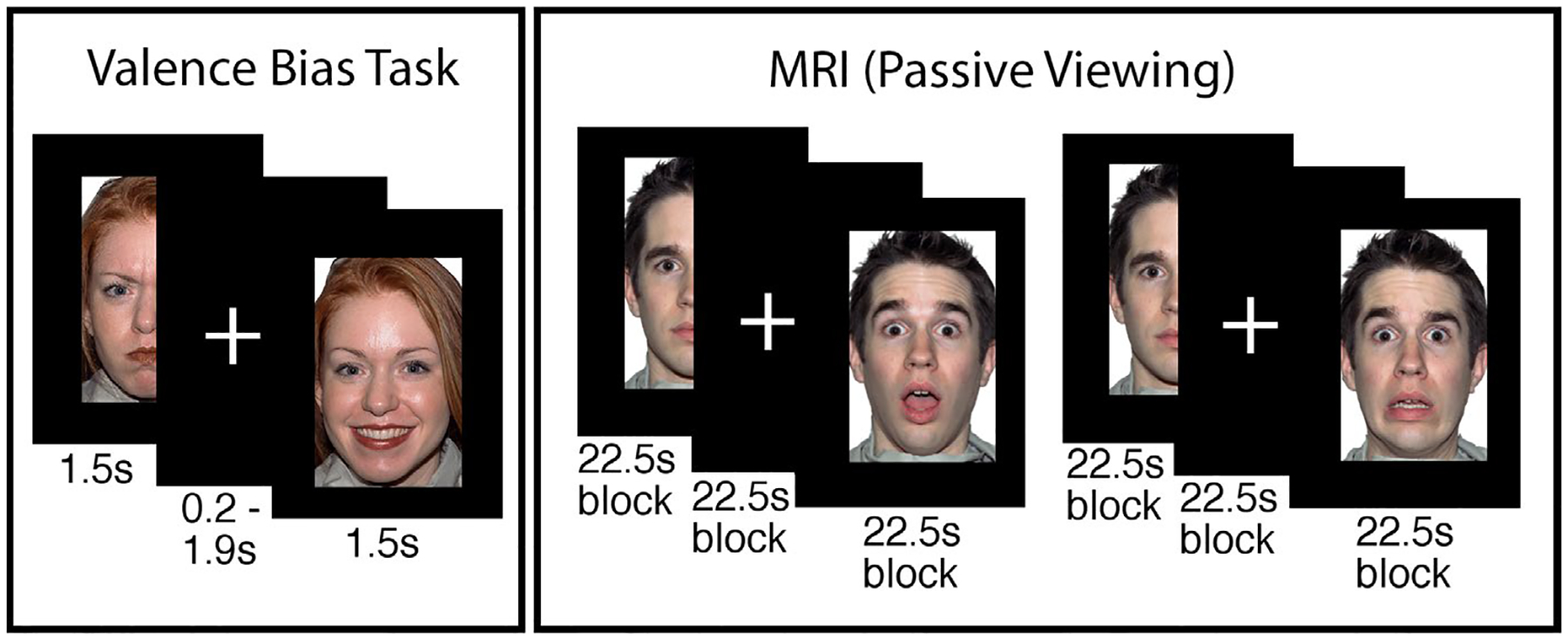
Depiction of procedure. In the valence bias task, participants viewed happy, angry, and surprised faces, and indicated whether each image “felt good or felt bad.” In the MRI, participants passively viewed new faces (i.e., not overlapping with the valence bias task) during two runs with blocks of surprised and neutral faces. Two runs with blocks of fearful and neutral faces followed, but were not included in the present analysis. Faces shown here are from the NimStim Set of Facial Expressions (Tottenham et al., 2009)
We calculated the valence bias for each participant as the percentage of times that a participant indicated that a particular surprised face felt bad (e.g., a valence bias was 100% if that participant rated surprised faces as bad on all trials). Note that only the ratings of the surprised faces were used to calculate each valence bias score. Indeed, ratings of angry and happy faces serve primarily as anchors that support the validity of the valence bias measure. For this reason, we excluded participants whose ratings of angry and happy faces were below 60% accuracy. Thus, the variability in ratings for angry and happy faces is necessarily restricted and would not be a useful measure to include when calculating valence bias. Perhaps more importantly, ratings of surprised faces are the best representation of one’s tendency to interpret dual-valence ambiguity as having a positive or negative meaning, which is indeed what the valence bias measure is intended to capture. Participants and parents then completed surveys (see above) and participants completed a mock scan (see below).
2.2.2 |. Session 2: MRI
Session 2 followed Session 1 by approximately 9 days (mean(SD) days = 9.34(6.69), range 1–46 days; Table 1). Days between sessions was unrelated to the behavioral (see Section 3.1.2) and neuroimaging results (see Section 3.2.1). Participants viewed a new set of European/European American faces from the Umeå University Database of Facial Expressions (Samuelsson et al., 2012) across four experimental runs while positioned in a MRI scanner (Figure 2). Prior to entering the MRI, all participants underwent a mock scanning session to acclimate to the environment and practice instructions to remain still during scanning. Padding was used to secure the participants’ head in a comfortable, static position. The experimenters provided feedback and reminders to remain still throughout the session.
TABLE 1.
Descriptive statistics for behavioral variables
| Variables | Mean (SD) | Min | Max | Min possible | Max possible |
|---|---|---|---|---|---|
| Valence bias (mean % negative) | 64.66% (21.76%) | 14.29% | 100% | 0% | 100% |
| Depressive Symptoms (RCADS - Major Depression; Chorpita et al., 2000) | 2.20 (2.40) | 0 | 12 | 0 | 30 |
| Petersen Pubertal Development Scale (PDS) score (Petersen et al., 1988) | 1.67 (0.56) | 1 | 2.8 | 1 | 4 |
| Days between sessions | 9.34 (6.69) | 1 | 46 | – | – |
Each experimental run consisted of four blocks of 15 image presentations. Faces were presented for 500 ms and separated by a fixed interstimulus interval of 1,000 ms. The first two runs consisted of two blocks of surprised faces and two blocks of neutral faces. Then, two additional runs followed which contained fearful instead of surprised faces, but their analysis was outside the scope of the present report. For the purpose of monitoring participants’ attention to the stimuli, participants were instructed to make a button response each time a flower appeared (instead of a face) on the screen; within each block, three images of flowers were pseudo-randomly presented amid the presentation of 12 face stimuli. All stimuli during this session were 500 × 750 pixels with a black background. Note that, consistent with extant work measuring valence bias, we used angry and happy faces in the behavioral task because they are clearly negative and positive and also perceptually distinct from surprised faces. However, for the MRI session, we used fearful and surprised faces because both recruit a similar dorsal region of the amygdala due to their increased uncertainty (Whalen, 2007) and because fearful faces are commonly used in studies measuring amygdala reactivity in development (Gee, Gabard-Durnam, et al., 2013; Gee, Humphreys, et al., 2013; Perlman & Pelphrey, 2011). However the scope of the current report is focused on the neural responses to surprised faces given their dual-valence ambiguity and use in the valence bias task.
2.3 |. MRI Parameters
2.3.1 |. MRI data acquisition parameters
The MRI data were collected at the University of Nebraska-Lincoln, Center for Brain, Biology, & Behavior, on a Siemens 3T Skyra scanner using a 32-channel head coil. Structural images were acquired using a T1-weighted MPRAGE sequence (TR = 2.20 s, TE = 3.37 ms, slices = 192 interleaved, voxel size = 1.0 × 1.0 × 1.0 mm matrix = 256 × 256, FOV = 256 mm, flip angle = 7°, total acquisition time = 5:07). Blood oxygen level-dependent (BOLD) activity was measured using an EPI scanning sequence (TR = 2.50 s, TE = 30 ms, slides = 42 interleaved, voxel size = 2.50 × 2.50 × 2.80 mm, matrix = 88 × 88 mm, FOV = 220 mm, flip angle = 80°, total acquisition time = 3:14 per block) in which slices were acquired parallel to the intercommissural plane and the volume positioned to cover the extent of the entire brain.
2.3.2 |. Preprocessing
Preprocessing of MR images was conducted using the Analysis of Functional Neuroimages (AFNI) program suite (Cox, 1996). The first four TRs of each run were excluded to allow for scanner stabilization. Voxel time-series were first despiked by removing values with outlying data in each separate voxel’s time-series. Then, slice timing correction was accomplished by re-referencing each scan to the first slice. These slice time corrected volumes were realigned to the minimum outlier image. Each volume was then aligned to the anatomical image before being warped to the Talairach template atlas (Talairach & Tournoux, 1988) provided by AFNI. Functional volumes were then spatially smoothed using a 6 mm3 full width at half maximum kernel. The BOLD time-series, in each voxel separately, was normalized by dividing each time point by its average across all time points and then multiplying each time point by 100. Any images containing movements >0.9 mm3, as determined by the motion parameters calculated during spatial realignment, were censored frame-wise from further analysis. No participants showed excessive movements in more than 16% of their scans (mean(SD) = 2.47%(4.37%)), consistent with previous work (Gee, Humphreys, et al., 2013). The number of censored scans did not differ between surprise and neutral trials (t40 = 0.74, p = .46, d = 0.23), and was not related to participant age (t39 = −1.50, p = .14, d = −0.48).
2.4 |. Statistical analysis
2.4.1 |. Behavioral
See Table 1 for descriptive information of analyzed behavioral variables. Although this study aimed to explore a puberty-moderated link between valence bias and both depressive symptoms and amygdala-mPFC connectivity, the cross-sectional design limited the possibility of inferring directional associations between these variables (e.g., valence bias impacts depressive symptoms or depressive symptoms impacts valence bias). We conducted two separate moderation analyses, first within the behavioral data, and second related to the MRI data (see section 2.4.4).
First, to determine whether a more negative valence bias was related to higher depressive symptoms, the two measures were submitted to a robust bivariate regression (calculated using the fitlm command in Matlab) in which valence bias was a predictor of depression. Then, the moderating effect of pubertal score on the relationship between valence bias and depressive symptoms was tested in a robust regression with valence bias as the outcome variable; this model had a constant term and four predictors: (a) depressive symptoms, (b) PDS score, (c) the interaction between depressive symptoms × PDS score, and (d) age, which was included in the model as a covariate. The interaction term coefficient represented the moderation effect. Importantly, the covariate of age was included to assess the effect of maturity as measured by PDS score, above and beyond the effect of age (Wierenga et al., 2014).
Previous studies have shown that differences in relative pubertal timing, or the relative individual differences in pubertal score at each age, are related to differences in emotion regulation and mental health outcomes (Gee, Gabard-Durnam, et al., 2013; Vantieghem et al., 2017). One possibility is that a moderation effect including valence bias and puberty could reflect a difference in relative pubertal timing, such that those who have a more negative valence bias are those who are either more or less developmentally mature than is typical for that age. To test for this possibility, the relative pubertal timing scores were submitted to a robust regression with valence bias.
2.4.2 |. BOLD reactivity
To identify brain regions in which BOLD activity related to each stimulus condition, the BOLD data were submitted to a general linear model (GLM) in which surprise, fear, and neutral blocks were modeled separately for the four runs of the experiment. These regressors included a boxcar function modeling the onset and duration of each block that was convolved with the hemodynamic response function. Each run included a constant term and nuisance regressors: six motion parameters (three rotational and three translational vectors) calculated during realignment, and both a linear and cubic polynomial trend model to control for BOLD signal drifts, consistent with AFNI guidelines given each runs’ 172.5 s duration.
2.4.3 |. BOLD context-dependent connectivity
To assess the functional connectivity between the amygdala and the rest of the brain specific to each condition, a context-dependent connectivity analysis (i.e., psychophysiological interaction or PPI) was conducted using AFNI commands. Given that previous research has shown that a dorsal region of the amygdala—which is not typically captured in a structural amygdala region of interest—is particularly sensitive to the ambiguity conveyed by surprised faces (Kim, Somerville, McLean, et al., 2003; Whalen et al., 2009), a functional, rather than structural, amygdala region was used as the seed. This functional amygdala seed region was defined as the voxels showing activation while viewing surprised expressions compared to baseline (the fixation period between blocks). This contrast was used to identify all voxels functionally related to the surprised expressions. The resulting amygdala clusters were considered significant if exceeding a corrected threshold (FWE: p < .05) based on Gaussian Random Field theory (Friston et al., 1994; Hayasaka & Nichols, 2003) that avoids the spatial autocorrelation issue raised by Eklund and colleagues (Eklund et al., 2016). This threshold consisted of both a cluster-forming (p < .001) and cluster-extent (k > 21) threshold. One voxel cluster, which thus served as the seed region in the context-dependent connectivity analysis, showed peak activation in the right basal forebrain (peak-t40 = 7.01, p < .001, d = 2.22; k = 86; x = 16, y = −4, z = −11) and extended into the dorsal amygdala (Figure 3a). Highlighting the importance of using this functionally defined amygdala region, this seed region overlapped in only 10 voxels with a structural amygdala region in an atlas from Faria et al. (2012).
FIGURE 3.
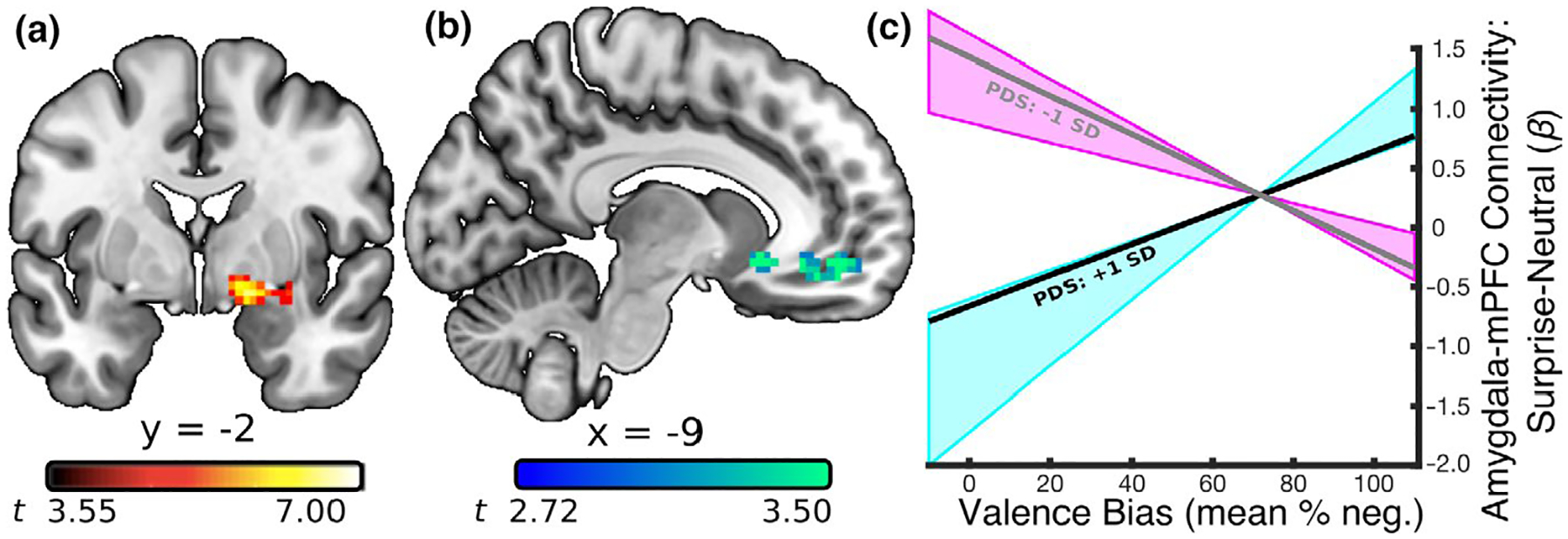
Relationship between amygdala-mPFC connectivity and negative valence bias as a function of PDS score. (a) A seed region in the right amygdala was defined using the contrast of surprised facial expressions versus baseline (p < .0005). The dorsal position of this cluster within the amygdala is consistent with previous work demonstrating that content conveying ambiguous valence recruits the amygdala/substantia innominata in particular (Kim, Somerville, McLean, et al., 2003; Whalen et al., 2009). (b) A PPI analysis based on surprise > neutral activity in the amygdala seed revealed a relationship between amygdala connectivity and valence bias that was moderated by PDS score in the mPFC (peak-t36 = 4.50, p = .00007). (c) The estimated regression slopes between valence bias and the surprise > neutral amygdala-mPFC connectivity betas, at a lower (gray line; 1 standard deviation below the mean PDS score; t36 = −4.89, p = .00002) and relatively higher (black line; 1 standard deviation above the mean PDS score; t36 = 3.52, p = .001) PDS scores. More mature children (within this relatively immature sample) that have a mature (inverse) connectivity pattern were more likely to have a positive valence bias while those that have the less mature (positive) connectivity pattern were more likely to show a negative valence bias; this relationship reached significance for PDS scores between 2.0 and 2.8 (blue shaded area). Lower PDS scores predicted the opposite relationship between valence bias and amygdala-mPFC connectivity; this relationship reached significance for PDS scores between 1.0 and 1.4 (pink shaded area)
To model the face-evoked BOLD activity in this amygdala region, the BOLD activity from this dorsal amygdala region was first deconvolved with a hemodynamic response function, and then multiplied with boxcar functions modeling stimulus onsets and durations separately for each condition, resulting in four condition-specific models of amygdala activity. Lastly, these condition-specific amygdala regressors were convolved with a hemodynamic response function. These regressors entered a GLM with a constant term, task onset regressors, and a model of the amygdala activity across the whole duration of the experiment. Thus, the beta values associated with the condition-specific amygdala regressors reflect changes in amygdala connectivity evoked by the blocked stimulus presentation. As in the previously described analysis of BOLD reactivity, nuisance regressors consisted of the six motion models to control for movement artifacts and, because the BOLD activity was analyzed across all runs continuously (690 s), five polynomial trends to control for BOLD signal drifts, consistent with AFNI guidelines.
2.4.4 |. mPFC-amygdala BOLD connectivity and valence bias
Connectivity between the amygdala and mPFC during emotional processing changes across development, such that there is a shift toward more inverse connectivity around age 10 years, thought to support age-related changes in emotion regulatory behaviors (Gee, Humphreys, et al., 2013). The amygdala seed region used in this connectivity analysis was defined as voxels showing significant activation for surprise relative to baseline, a criterion chosen in order to include as many voxels sensitive to the surprised face expressions as possible. For comparison with valence bias and puberty, the connectivity beta differences for surprise relative to neutral were used in order to isolate amygdala connectivity specific to the ambiguity conveyed through the surprised expressions rather than a general response to facial expressions. Thus, these surprise > neutral connectivity beta differences for each participant were submitted to a robust multiple regression (calculated using the fitlm command in Matlab), separately at each voxel, with a constant term and four predictors: (a) valence bias, (b) PDS score, (c) the interaction between valence bias × PDS score, and (d) age, which was included in the model as a covariate. To determine whether puberty moderated the relationship between valence bias and amygdala connectivity in any mPFC region, the interaction term coefficients, computed at each voxel separately, were passed through a cluster-forming (p < .01) and -extent threshold (k > 75) according to Guassian Random Field theory guidelines for multiple comparison correction (Friston et al., 1994; Hayasaka & Nichols, 2003).
2.4.5 |. Individual differences between BOLD, age, and puberty
Lastly, we examined the direct relationship between the BOLD measures (amygdala activation and connectivity with mPFC) and age and puberty. Specifically, we calculated the average betas values, separately for each participant, for (a) the surprise > neutral BOLD activation for all voxels in the amygdala seed (see Section 2.4.3) and (b) the surprise > neutral amygdala connectivity for all voxels in the mPFC (see Section 3.2.1). These average beta values were submitted to a robust regression with both age and PDS score, yielding four separate beta coefficients.
3 |. RESULTS
3.1 |. Behavioral
3.1.1 |. Valence ratings - Characterizing valence bias
Participants rated angry faces as negative (mean(SD) % negative = 85.91(11.75); range = 62–100), and happy faces as positive (mean(SD) % negative = 10.18(9.10); range = 0–30). In contrast, ratings of surprised faces showed greater variability (Figure 1c; mean(SD) % negative = 64.66(21.75); range = 14.29–100), and represented the valence bias for each individual, such that higher scores indicated a more negative bias. Within this age range, which subtended to relatively low PDS scores, valence bias was not significantly related to age (t39 = −0.48, p = .63, d = −0.15) or PDS score (t39 = −0.42, p = .68, d = −0.14).
3.1.2 |. Depressive symptoms and valence bias moderated by puberty
Table 1 lists the descriptive statistics for the behavioral variables. A bivariate robust regression revealed that valence bias was positively related to depressive symptoms (t39 = 2.29, p = .03, d = 0.73), such that those with a more negative bias had higher depressive symptoms. For the moderation analysis, the interaction between depressive symptoms and PDS score was significant (t36 = 2.48, p = .02, d = 0.83; Figure 4) indicating that puberty, when controlling for age, moderated the relationship between depression and valence bias. As an additional means to address the concern of sample size in this model, we also conducted the moderation analyses in Bayesian models and found moderate support for the hypothesis that puberty moderates the relationship between depression and valence bias compared to the null hypothesis of no moderation (see Supporting Information).
FIGURE 4.
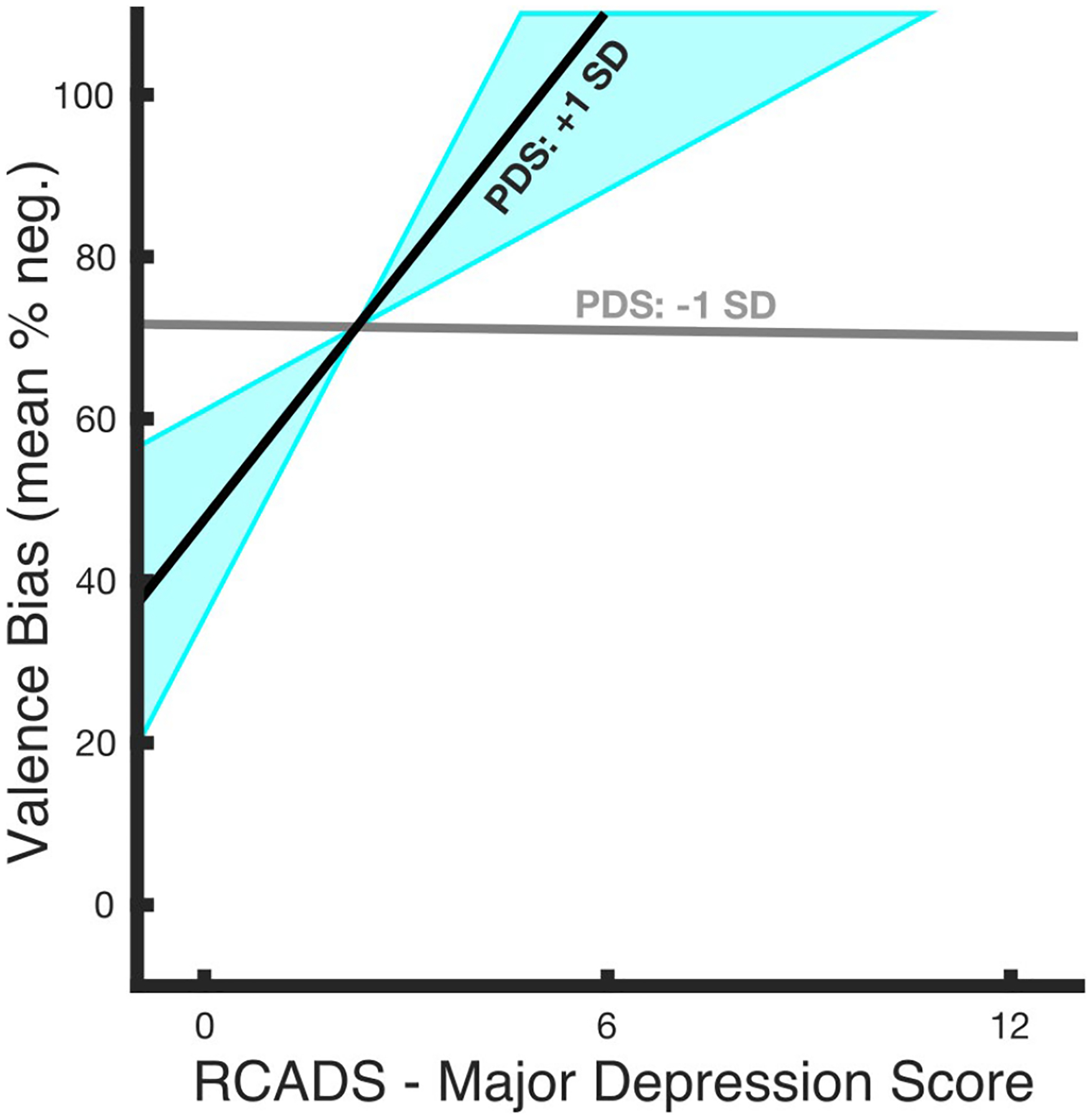
Relationship between valence bias and depressive symptoms as a function of PDS score. The relationship between depressive symptoms and valence bias was moderated by PDS score (t36 = 2.48, p = .02) such that valence bias and depressive symptoms shared a stronger positive relationship at relatively higher PDS scores. The estimated regression slopes for the relationship between valence bias and depressive symptoms at different PDS scores illustrate that a lower PDS score (gray line; 1 standard deviation below the mean PDS score; t36 = −0.06, p = .95) predicted no relationship, whereas a higher PDS score (black line; 1 standard deviation above the mean pubertal score; t36 = 2.63, p = .01) predicted a positive relationship; this relationship reached significance for PDS scores between 1.6 and 2.8 (blue shaded area). At no point did lower PDS scores predict a significant relationship between valence bias and depressive symptoms, therefore no shaded area (region of significance) is illustrated around the −1 SD line
To explore this moderation, the relationship between valence bias and depressive symptoms was calculated for each PDS score. This relationship became significant between a PDS score of 1.6 and 2.8 (out of the sample’s range of 1.0–2.8; Figure 4, blue shaded area). To test for potential confounding effects, we ran similar moderation analyses that included sex (male or female), or number of days between sessions 1 and 2, or excluded the participant with a supra-threshold clinical major depression score, or excluded participants who did not identify as Caucasian. Notably, the effects were qualitatively the same with each of these modifications, suggesting these variables did not impact the reported findings.
As an additional analysis we tested whether a difference in relative pubertal timing is associated with valence bias, which may potentially confound the moderation effects reported above. Relative pubertal timing was operationalized as the residuals of the relationship between PDS score and age. These relative pubertal timing scores were not related to valence bias across all participants (t39 = −0.17, p = .86, d = −0.05).
3.2 |. MRI
3.2.1 |. Context-dependent amygdala-mPFC connectivity and valence bias
The relationship between surprise > neutral amygdala connectivity and valence bias, when controlling for age, was moderated by puberty in two clusters (Table 2). In other words, those with a higher PDS score (within this relatively immature sample) showed an effect whereby a more positive valence bias was associated with a more inverse (mature, i.e., suggestive of emotion regulation) connectivity in these regions. The first cluster showed peak activation in the right subcollosal gyrus (peak-t36 = 5.52, p = .000003, d = 1.84; k = 170; Talaraich (x, y, z) coordinates: 11, 11, −14) and extended into the right rectal gyrus and right anterior cingulate. The second cluster showed peak activation in the left medial frontal gyrus (Figure 3b; peak-t36 = 4.50, p = .00007, d = 1.50; k = 92; Talaraich (x, y, z) coordinates: −11, 49, −11) and extended into the left anterior cingulate, consistent with reports of amygdala connectivity with the mPFC (Kim, Somerville, Johnstone, et al., 2003). Again, as an additional means to address the concern of sample size in this model, we conducted the moderation analyses in Bayesian models and found strong support for the hypothesis that puberty moderates the relationship between valence bias and amygdala-mPFC connectivity compared to the null hypothesis of no moderation (see Supporting Information).
TABLE 2.
Clusters of significant BOLD surprise > neutral amygdala connectivity whose relationship with valence bias was moderated by pubertal score
| Region | x | y | z | Peak-t | k |
|---|---|---|---|---|---|
| R Subcollosal Gyrus | 11 | 11 | −14 | 5.52 | 170 |
| L Medial Frontal Gyrus | −11 | 49 | −11 | 4.50 | 92 |
To explore the moderation within this latter mPFC cluster, the conditional effects of puberty were calculated from the intercept and slope of the relationship between valence bias and the voxel averaged amygdala-mPFC connectivity beta values; this was done separately at each PDS score in this sample (range = 1.0–2.8). These conditional effects (Figure 3c) indicated that for those with a relatively higher PDS score (2.0–2.8; i.e., the highest scores in this sample), the relationship between valence bias and amygdala-mPFC connectivity was more positive (Figure 3c, blue shaded area). In other words, children with both a higher PDS score and a less mature (positive) connectivity pattern had a more negative valence bias. In contrast, children with both a higher PDS score and a more mature (inverse) connectivity pattern had a more positive valence bias. The opposite effect (i.e., a more negative valence bias predicted more inverse amygdala-mPFC connectivity) was significant at PDS scores 1.0–1.4 (Figure 3c, pink shaded area).
As with the behavioral results (see Section 3.1.2), we tested for potential confounding effects by rerunning the moderation analyses and including sex, or number of days between sessions 1 and 2, or excluding the participant with a supra-threshold clinical major depression score, or excluding participants who did not identify as Caucasian. In addition, although surprise > neutral amygdala activation was not related to amygdala connectivity (t39 = −1.10, p = .28), we ran another moderation which included amygdala activation as a covariate to test whether the connectivity moderation effect was confounded by the level of BOLD activation. Notably, the effects were qualitatively the same with each of these modifications, suggesting these variables did not impact the reported findings.
It is also important to note that the BOLD signal within the amygdala is sensitive to signal dropout (Krasnow et al., 2003). Because the PPI analysis consists of correlations between BOLD signals in different brain regions, the diminished signal may lead to false-positive or false-negative correlations related to noise. To rule out the possibility that the current results were driven by signal dropout, we performed the same PPI and moderation analysis, but applied an intensity-based mask, calculated as described by Peer et al. (2016) and implemented using these authors’ Matlab code as provided on their webpage (Computational Neuropsychiatry Lab - Intensity Based Masking (IBM) Tool, n.d.) to remove amygdala voxels with low signal in each individual participant. The results of this analysis were qualitatively identical to the moderation analysis reported above.
3.2.2 |. Individual differences between BOLD, age, and puberty
To describe the basic relationship between the BOLD measures and age and puberty, the surprise > neutral amygdala BOLD activation betas and the amygdala-mPFC connectivity betas were submitted to a robust regression with age and PDS score. Neither amygdala activation nor connectivity were related to age (activation: t39 = 0.68, p = .50, d = 0.22; connectivity: t39 = −0.19, p = .85, d = −0.06) or PDS score (activation: t39 = 0.08, p = .94, d = 0.03; connectivity: t39 = −0.37, p = .72, d = −0.12).
Finally, we used a Cox test (Greene, 2003) to compare the fit of the model that measures the moderating effect of puberty (see section 3.2.1) with a second model in which puberty is replaced by age as the moderating variable. This analysis found that the model including puberty compared to the model including age explained more variance in amygdala-mPFC connectivity (z = −3.72, p = .0002; but note that both models explained significant variance in depression symptomology). In other words, as expected, brain activity was more sensitive to the biological changes that occur during puberty, as there does appear to be a unique role for pubertal variability in moderating these overarching effects.
4 |. DISCUSSION
Within this relatively pubertally immature sample, higher PDS scores coupled with a more negative valence bias were associated with more depressive symptoms and less inverse amygdala-mPFC connectivity (suggestive of weaker emotion regulation). Broadly, this pattern of exploratory results is consistent with the notion that internalizing problems, such as depression, arise from dysfunctional emotion regulation circuitry (Banks et al., 2007; Phillips et al., 2008) and, more specifically, that this link arises from developmental differences (Gee, Gabard-Durnam, et al., 2013; Gee, Humphreys, et al., 2013; Hare et al., 2008; Perlman & Pelphrey, 2011).
Given that this amygdala-mPFC circuit is intimately tied to biological changes during puberty (Andersen & Teicher, 2008; Angold & Costello, 2006; Paus et al., 2008), the early pubertal period explored in this study may be critical in the construction of healthy emotion regulation mechanisms. These exploratory results support a model for future research which predicts that, while negative valence bias in early childhood is normative, negative bias in later development is putatively maintained by the failure to develop a more mature, regulatory frontoamygdalar circuitry, which may increase the risk for depression.
These findings are consistent with our “initial negativity hypothesis” that posits that the initial or default interpretation of surprise is more negative (Neta et al., 2011; Neta & Tong, 2016; Neta & Whalen, 2010). In contrast, positive ratings depend upon slower and more elaborate emotion regulation processes which override the initial negativity and putatively downregulate the amygdala response (Kaffenberger et al., 2010; Kim, Somerville, Johnstone, et al., 2003; Neta et al., 2011; Neta & Tong, 2016; Neta & Whalen, 2010; Petro et al., 2018), processes that are likely compromised in depression and anxiety (Beck, 1976; Reef et al., 2011; Williams et al., 2007). Indeed, age-related differences in this emotion regulation circuity (i.e., amygdala-mPFC connectivity; Gee, Humphreys, et al., 2013) are associated with mental health risk factors in adults (Hare et al., 2008). The current results extend this “initial negativity hypothesis” by suggesting that individual differences in valence bias may originate during pubertal development, alongside the development of this emotion regulatory circuitry that putatively overrides the default, or initial negativity.
The utility of surprised faces in tracking individual differences in negativity bias and emotion regulation brain circuits is broadly consistent with a functional—contextual account of facial displays (Crivelli & Fridlund, 2018), which predicts that an expression’s emotional value depends on its social, environmental, and/or cultural context (Barrett & Kensinger, 2010). Whereas happy and angry expressions signal predominantly positive or negative social outcomes, respectively, surprised faces signal multiple possible outcomes spanning positive and negative valence (i.e., dual-valence ambiguity). Thus, the valence assigned to surprised expressions presented without context is more pliable to interpretation. The use of stimuli with dual-valence ambiguity represents a methodological advance in conceptualizing negativity bias in that it side-steps limitations present in extant literature. For instance, negativity bias is often measured via either an attentional bias toward clearly negative or away from clearly positive stimuli (see Fales et al., 2008) or by examining responses to ambiguous stimuli with alternate meanings that could be either negative or neutral, but not positive (e.g., “lie”; Mathews et al., 1989). These findings not only rely on cognitive/linguistic abilities not developmentally appropriate for children, but by not examining responses to stimuli with a dual-valence representation (i.e., negative and positive possible interpretations), these earlier findings are skewed toward the extremes of the valence spectrum. As such, this earlier work is limited in its ability to identify individual differences in responses to emotional stimuli during sensitive periods of development, and thus has more limited findings regarding the developmental origins of negativity bias. Future work will benefit from incorporating our valence bias task to examine individual differences in emotion reactivity and regulation, particularly in young ages.
These findings make a meaningful first step toward establishing the developmental origins of negativity bias. One caveat is the relatively small sample size with somewhat limited variance in depressive symptoms, and that the PDS was only administered to children ages 8 years and older. Future work should replicate our exploratory findings in larger samples with a wider range of depressive symptoms and also determine the extent to which these findings, which use parent-reported subclinical depression (see; Muris et al., 2003), generalize to those with clinically diagnosed or self-reported depression. Further, future work should also explore the extent to which the effects generalize to the range of mental health disorders in which internalizing problems manifest. Finally, although there was no explicit emotion regulation task, a more inverse frontoamygdalar connectivity is thought to represent emotion regulation (Gee, Humphreys, et al., 2013; Ochsner et al., 2004). Indeed, the connectivity in these children spanned both positive and negative values, consistent with previous studies (Gee, Humphreys, et al., 2013; Hare et al., 2008) which suggest that inverse connectivity (rather than a lack of positivity connectivity) may be associated with a more adult-like regulatory circuitry. While this study treated connectivity as a continuous measure in order to test its relationship to valence bias and puberty, future work with larger sample sizes in normative adults should aim to identify the point at which negative frontoamygdalar connectivity defines an emotion regulatory process.
Whether or not long-term mental health trajectories are impacted by environmental factors may also be explored in future research. For instance, early life stress is a risk factor for mental health disorders (Tottenham et al., 2011) and is associated with developmental differences in regulatory circuitry (Cohodes et al., 2020; Heim & Binder, 2012; Lupien et al., 2009). Recent work suggests that positive affect (Sewart et al., 2019) and even more specifically, an earlier developmental shift toward a more positive valence bias (Gee, Gabard-Durnam, et al., 2013; Vantieghem et al., 2017) may serve as a buffer from the development of depressive symptoms. This suggests that resiliency, or the ability to find a positive outlook in potentially negative situations (Gross & John, 2003), may implicate the same emotion regulation mechanism explored in this study (Tugade & Fredrickson, 2004).
The moderating role of puberty on the relationship between valence bias and depressive symptoms occurred earlier in maturation (starting at a PDS score of 1.6 and up to the highest score in this sample of 2.8) than the relationship between valence bias and brain connectivity (starting at a PDS score of 2 and up to the highest score in this sample of 2.8). Although cross-sectional, these findings provide preliminary evidence that a change toward a more positive valence bias and away from depressive symptoms may have downstream effects on developing more adult-like brain connectivity patterns. Future longitudinal work should extend our exploratory work and aim to (a) test the prediction that the maintenance of negative valence bias is associated with both an increase in depressive symptoms and a slower development of (inverse) amygdala-mPFC connectivity, and (b) establish the directionality these relationships. The opposite relationship between valence bias and amygdala-mPFC connectivity was also observed at a PDS score of 1.4 and below. However, our predictions about the effects in those at the earliest stages of puberty were less clear, particularly before the point at which puberty moderates the link between valence bias and depressive symptoms (below a PDS score of 1.6) and given the assumed scores in children ages 6–7 years.
Because females and males show different age onsets in puberty (Schuiling & Likis, 2016), maturity was measured via a scale of pubertal development. While sex differences in depression tend to emerge after the age of 13 (i.e., outside of this sample’s range; Ferguson et al., 1999; Hankin & Abramson, 2001), other work has shown that there are important sex differences in the emergence of depression (Graber, 2013; Hankin et al., 1998). If negative valence bias is a risk factor for depression, then sex may moderate the relationship between these two variables. Relatedly, early pubertal timing is also a risk factor for depression in females in particular (Hankin & Abramson, 2001). Given that this study was underpowered to test potential sex differences, continued work should explore, in wider ranges of age and PDS, whether sex differences in the relationships between valence bias, depression, and emotion regulation circuitry emerge in older ages. Further, this future work could also explore whether or not normative pubertal timing differences predict differences in valence bias and emotion regulation skills.
Future longitudinal work may also hold broad implications for treating mental health. For instance, the effects reported here may pinpoint developmental periods most sensitive to long-term mental health outcomes. Such information will be critical for informing potential interventions (e.g., mindfulness), which can improve emotion regulation success and decrease negativity bias (Goldin & Gross, 2010; Jazaieri et al., 2014). These types of training may be particularly useful for individuals that putatively maintain a negativity bias beyond a normative developmental stage such that this bias interferes with normal, healthy functioning.
Finally, there are a few limitations worth noting. First, although the face stimuli used here were taken from previous work examining valence bias (Neta et al., 2009) even using a developmental sample (Tottenham et al., 2013), one potential limitation of this work is that the face stimuli were exclusively adult facial expressions. Indeed, previous research has shown better facial identity recognition and memory for own-age faces (Anastasi & Rhodes, 2005; Denkinger & Kinn, 2018). Having said that, children appear to perform equally well at identifying facial expressions (Vetter et al., 2018) of children versus adult faces. In addition, given that the participants range from 6 to 13 years of age, the use of appropriately age-matched actors would introduce a new challenge of providing different stimuli to participants of different ages. Future studies comparing valence bias across child and adult facial expressions in a developmental sample will be useful for identifying the impact of this methodological choice. Second, the present findings did not replicate the age-related decrease in amygdala activity (Gee, Humphreys, et al., 2013; Guyer et al., 2008; Hare et al., 2008). This discrepancy may have arisen because the current sample was aged 6–13 years, which is on the low end of pubertal maturation and thus may not capture the full range of biological changes which affect the amygdala (Blakemore et al., 2010; Goddings et al., 2014; Vijayakumar et al., 2019). Indeed, previous work showing an age-related decrease in amygdala activity reported such an effect only after adolescence (Gee, Humphreys, et al., 2013; Hare et al., 2008). Third, we note that the reported effect sizes may be inflated, given recent work showing that lower sample sizes are susceptible to biased and unstable effect sizes (Marek et al., 2020). Lastly, the pattern of results reported here was achieved using two separate moderation analyses and did not directly test the relationship between depression and brain activity, given there was insufficient power to relate these measures in the context of both valence bias and puberty. In other words, additional work may test the degree to which developmental differences in depression are linked with emotion regulation circuitry, and if valence bias is a risk factor for these developmental differences.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (NIMH111640; PI: Neta), and by Nebraska Tobacco Settlement Biomedical Research Enhancement Funds. We thank Rebecca L. Brock for consultation regarding statistical analyses. We thank Lisa Crockett for consultation regarding the use and interpretation of the Pubertal Development Scale. We thank R. James Blair and Leah H. Somerville for helpful comments on the manuscript. We also thank Ruby Basyouni, Kayla Clark, Daniel J. Henley, and Tien T. Tong for assistance in data collection and management.
Funding information
National Institute of Mental Health, Grant/Award Number: NIMH111640; Nebraska Tobacco Settlement Biomedical Research Enhancement Fund
Footnotes
CONFLIC TS OF INTEREST
The authors declare that they have no competing interests.
DATA AVAIL ABILIT Y STATEMENT
The data relevant to this manuscript are available on the NIH National Database Archive.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- Anastasi JS, & Rhodes MG (2005). An own-age bias in face recognition for children and older adults. Psychonomic Bulletin & Review, 12(6), 1043–1047. 10.3758/BF03206441 [DOI] [PubMed] [Google Scholar]
- Andersen SL, & Teicher MH (2008). Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences, 31(4), 183–191. 10.1016/j.tins.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Angold A, & Costello EJ (2006). Puberty and depression. Child and Adolescent Psychiatric Clinics of North America, 15(4), 919–937. 10.1016/j.chc.2006.05.013 [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, & Luna B (2010). White matter development in adolescence: A DTI study. Cerebral Cortex, 20(9), 2122–2131. 10.1093/cercor/bhp282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, & Phan KL (2007). Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience, 2(4), 303–312. 10.1093/scan/nsm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, & Kensinger EA (2010). Context is routinely encoded during emotion perception. Psychological Science, 21(4), 595–599. 10.1177/0956797610363547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT (1976). Cognitive therapy and the emotional disorders. International Universities Press. [Google Scholar]
- Blakemore S-J, Burnett S, & Dahl RE (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31(6), 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton RE, Cooney RE, Joormann J, Eugène F, Glover GH, & Gotlib IH (2012). Pubertal stage and brain anatomy in girls. Neuroscience, 217, 105–112. 10.1016/j.neuroscience.2012.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CC, Raio CM, & Neta M (2017). Cortisol responses enhance negative valence perception for ambiguous facial expressions. Scientific Reports, 7. 10.1038/s41598-017-14846-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M, Holmes EA, & Harmer CJ (2010). The modification of attentional bias to emotional information: A review of the techniques, mechanisms, and relevance to emotional disorders. Cognitive, Affective, & Behavioral Neuroscience, 10(1), 8–20. 10.3758/CABN.10.1.8 [DOI] [PubMed] [Google Scholar]
- Bruce V, Campbell RN, Doherty-Sneddon G, Langton S, McAuley S, & Wright R (2000). Testing face processing skills in children. British Journal of Developmental Psychology, 18(3), 319–333. 10.1348/026151000165715 [DOI] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, & Birn RM (2012). Developmental pathways to amygdala-pre-frontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15(12), 1736–1741. 10.1038/nn.3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke KC (1991). Comparing age at onset of major depression and other psychiatric disorders by birth cohorts in five US community populations. Archives of General Psychiatry, 48(9), 789. 10.1001/archpsyc.1991.01810330013002 [DOI] [PubMed] [Google Scholar]
- Chorpita BF, Yim L, Moffitt C, Umemoto LA, & Francis SE (2000). Assessment of symptoms of DSM-IV anxiety and depression in children: A revised child anxiety and depression scale. Behaviour Research and Therapy, 38(8), 835–855. 10.1016/S0005-7967(99)00130-8 [DOI] [PubMed] [Google Scholar]
- Clark DA, & Beck AT (2010). Cognitive theory and therapy of anxiety and depression: Convergence with neurobiological findings. Trends in Cognitive Sciences, 14(9), 418–424. 10.1016/j.tics.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Cohodes EM, Kitt ER, Baskin-Sommers A, & Gee DG (2020). Influences of early-life stress on frontolimbic circuitry: Harnessing a dimensional approach to elucidate the effects of heterogeneity in stress exposure. Developmental Psychobiology. 10.1002/dev.21969 [DOI] [PubMed] [Google Scholar]
- Computational Neuropsychiatry Lab. (n.d.). Computational Neuropsychiatry Lab—Intensity based masking (IBM) tool. Retrieved from http://mind.huji.ac.il/intensity_based_masking.aspx
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Crivelli C, & Fridlund AJ (2018). Facial displays are tools for social influence. Trends in Cognitive Sciences, 22(5), 388–399. 10.1016/j.tics.2018.02.006 [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Flynn G, Harris AWF, Liddell BJ, Whitford TJ, Peduto A, Gordon E, & Williams LM (2007). Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophrenia Research, 90(1), 284–294. 10.1016/j.schres.2006.11.023 [DOI] [PubMed] [Google Scholar]
- Denham SA (1998). Emotional development in young children. Guilford Press. [Google Scholar]
- Denkinger B, & Kinn M (2018). Own-age bias and positivity effects in facial recognition. Experimental Aging Research, 44(5), 411–426. 10.1080/0361073X.2018.1521493 [DOI] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, & Biro F (2006). Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science, 10(1), 30–56. 10.1207/s1532480xads1001_3 [DOI] [Google Scholar]
- Dubois J, & Adolphs R (2016). Building a science of individual differences from fMRI. Trends in Cognitive Sciences, 20(6), 425–443. 10.1016/j.tics.2016.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebesutani C, Tottenham N, & Chorpita B (2015). The revised child anxiety and depression scale - Parent version: Extended applicability and validity for use with younger youth and children with histories of early-life caregiver neglect. Journal of Psychopathology and Behavioral Assessment, 37(4), 705–718. 10.1007/s10862-015-9494-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Bernzweig J, Karbon M, Poulin R, & Hanish L (1993). The relations of emotionality and regulation to preschoolers’ social skills and sociometric status. Child Development, 64(5), 1418–1438. 10.2307/1131543 [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ, Mayes TL, & Ruberu M (2005). Continuation and maintenance therapy of early-onset major depressive disorder. Pediatric Drugs, 7(4), 203–217. 10.2165/00148581-200507040-00001 [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, & Greicius MD (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry, 66(12), 1361. 10.1001/archgenpsychiatry.2009.104 [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Mathews J, & Sheline YI (2008). Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry, 63(4), 377–384. 10.1016/j.biopsych.2007.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AV, Joel SE, Zhang Y, Oishi K, van Zjil PCM, Miller MI, Pekar JJ, & Mori S (2012). Atlas-based analysis of resting-state functional connectivity: Evaluation for reproducibility and multi-modal anatomy-function correlation studies. NeuroImage, 61(3), 613–621. 10.1016/j.neuroimage.2012.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson TJ, Stegge H, Miller ER, & Olsen ME (1999). Guilt, shame, and symptoms in children. Developmental Psychology, 35(2), 347–357. 10.1037/0012-1649.35.2.347 [DOI] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, & Dahl RE (2011). Neural systems of threat processing in adolescents: Role of pubertal maturation and relation to measures of negative affect. Developmental Neuropsychology, 36(4), 429–452. 10.1080/87565641.2010.550178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RS, Mazziotta JC, & Evans AC (1994). Assessing the significance of focal activations using their spatial extent. Human Brain Mapping, 1(3), 210–220. 10.1002/hbm.460010306 [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, & Tottenham N (2014). The development of human amygdala functional connectivity at rest from 4 to 23 years: A cross-sectional study. NeuroImage, 95, 193–207. 10.1016/j.neuroimage.2014.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D, Gabard-Durnam L, Flannery J, Goff B, Humphreys K, Telzer E, Hare T, Bookheimer S, & Tottenham N (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America, 110(39), 15638–15643. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D, Humphreys K, Flannery J, Goff B, Telzer EH, Shapiro M, Hare T, Bookheimer S, & Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala-pre-frontal circuitry. Journal of Neuroscience, 33(10), 4584–4593. 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, & Barbas H (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage, 34(3), 905–923. 10.1016/j.neuroimage.2006.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A, Heyes SB, Bird G, Viner RM, & Blakemore SJ (2012). The relationship between puberty and social emotion processing. Developmental Science, 15(6), 801–811. 10.1111/j.1467-7687.2012.01174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A-L, Mills KL, Clasen LS, Giedd JN, Viner RM, & Blakemore S-J (2014). The influence of puberty on subcortical brain development. NeuroImage, 88, 242–251. 10.1016/j.neuroimage.2013.09.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Leyman L, & Verschuere B (2008). The karolinska directed emotional faces: A validation study. Cognition and Emotion, 22(6), 1094–1118. 10.1080/02699930701626582 [DOI] [Google Scholar]
- Goldin PR, & Gross JJ (2010). Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion, 10(1), 83–91. 10.1037/a0018441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA (2013). Pubertal timing and the development of psychopathology in adolescence and beyond. Hormones and Behavior, 64(2), 262–269. 10.1016/j.yhbeh.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Greene WH (2003). Econometric analysis (5th ed.). Prentice Hall. [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–362. 10.1037/0022-3514.85.2.348 [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, & Ernst M (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20(9), 1565–1582. 10.1162/jocn.2008.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, & Abramson LY (2001). Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin, 127(6), 773–796. 10.1037/0033-2909.127.6.773 [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, & Angell KE (1998). Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology, 107(1), 128–140. 10.1037/0021-843X.107.1.128 [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, & Casey BJ (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63(10), 927–934. 10.1016/j.biopsych.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, & Weinberger DR (2003). Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry, 53(6), 494–501. 10.1016/S0006-3223(02)01786-9 [DOI] [PubMed] [Google Scholar]
- Hayasaka S, & Nichols TE (2003). Validating cluster size inference: Random field and permutation methods. NeuroImage, 20(4), 2343–2356. 10.1016/j.neuroimage.2003.08.003 [DOI] [PubMed] [Google Scholar]
- Heim C, & Binder EB (2012). Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Experimental Neurology, 233(1), 102–111. 10.1016/j.expneurol.2011.10.032 [DOI] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Kan E, Dahl RE, & Sowell ER (2014). The role of testosterone and estradiol in brain volume changes across adolescence: A longitudinal structural MRI study: Pubertal Hormones and Brain Volume. Human Brain Mapping, 35(11), 5633–5645. 10.1002/hbm.22575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Pruessner JC, Coupé P, & Collins DL (2013). Volumetric analysis of medial temporal lobe structures in brain development from childhood to adolescence. NeuroImage, 74, 276–287. 10.1016/j.neuroimage.2013.02.032 [DOI] [PubMed] [Google Scholar]
- Hulvershorn LA, Cullen K, & Anand A (2011). Toward dysfunctional connectivity: A review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging and Behavior, 5(4), 307–328. 10.1007/s11682-011-9134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazaieri H, McGonigal K, Jinpa T, Doty JR, Gross JJ, & Goldin PR (2014). A randomized controlled trial of compassion cultivation training: Effects on mindfulness, affect, and emotion regulation. Motivation and Emotion, 38(1), 23–35. 10.1007/s11031-013-9368-z [DOI] [Google Scholar]
- John OP, & Gross JJ (2004). Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. Journal of Personality, 72(6), 1301–1333. 10.1111/j.1467-6494.2004.00298.x [DOI] [PubMed] [Google Scholar]
- Juraska JM, & Willing J (2017). Pubertal onset as a critical transition for neural development and cognition. Brain Research, 1654, 87–94. 10.1016/j.brainres.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffenberger T, Brühl AB, Baumgartner T, Jäncke L, & Herwig U (2010). Negative bias of processing ambiguously cued emotional stimuli. NeuroReport, 21(9), 601–605. 10.1097/WNR.0b013e328337ff18 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, & Ries Merikangas K (2001). Mood disorders in children and adolescents: An epidemiologic perspective. Biological Psychiatry, 49(12), 1002–1014. 10.1016/S0006-3223(01)01129-5 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62(6), 593–602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kestenbaum R (1992). Feeling happy versus feeling good: The processing of discrete and global categories of emotional expressions by children and adults. Developmental Psychology, 28(6), 1132–1142. 10.1037/0012-1649.28.6.1132 [DOI] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Alexander AL, & Whalen PJ (2003). Inverse amygdala and medial prefrontal cortex responses to surprised faces. NeuroReport, 14(18), 2317. 10.1097/00001756-200312190-00006 [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, McLean AA, Johnstone T, Shin LM, & Whalen PJ (2003). Functional MRI responses of the human dorsal amygdala/substantia innominata region to facial expressions of emotion. Annals of the New York Academy of Sciences, 985(1), 533–535. 10.1111/j.1749-6632.2003.tb07120.x [DOI] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, & Whalen PJ (2011). The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioural Brain Research, 223(2), 403–410. 10.1016/j.bbr.2011.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow B, Tamm L, Greicius MD, Yang TT, Glover GH, Reiss AL, & Menon V (2003). Comparison of fMRI activation at 3 and 1.5 T during perceptual, cognitive, and affective processing. NeuroImage, 18(4), 813–826. 10.1016/S1053-8119(03)00002-8 [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1997). International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention, 39–58. [Google Scholar]
- Lebron-Milad K, & Milad MR (2012). Sex differences, gonadal hormones and the fear extinction network: Implications for anxiety disorders. Biology of Mood & Anxiety Disorders, 2(1), 10.1186/2045-5380-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, estan N, Weinberger DR, & Casey BJ (2014). Adolescent mental health—Opportunity and obligation. Science, 346(6209), 547–549. 10.1126/science.1260497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Clarke GN, Seeley JR, & Rohde P (1994). Major depression in community adolescents: Age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child & Adolescent Psychiatry, 33(6), 809–818. 10.1097/00004583-199407000-00006 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, Donohue MR, Foran W, Miller RL, Feczko E, Miranda-Dominguez O, Graham AM, Earl EA, Perrone AJ, Cordova M, Doyle O, Moore LA, Conan G, Uriarte J, … Dosenbach NUF (2020). Towards Reproducible Brain-Wide Association Studies [Preprint]. Neuroscience, 10.1101/2020.08.21.257758 [DOI] [Google Scholar]
- Mathews A, Richards A, & Eysenck M (1989). Interpretation of homophones related to threat in anxiety states. Journal of Abnormal Psychology, 98(1), 31–34. 10.1037/0021-843X.98.1.31 [DOI] [PubMed] [Google Scholar]
- Menzies L, Goddings A-L, Whitaker KJ, Blakemore S-J, & Viner RM (2015). The effects of puberty on white matter development in boys. Developmental Cognitive Neuroscience, 11, 116–128. 10.1016/j.dcn.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings A-L, Clasen LS, Giedd JN, & Blakemore S-J (2014). The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience, 36(3–4), 147–160. 10.1159/000362328 [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, & Pine DS (2003). Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage, 20(1), 420–428. 10.1016/S1053-8119(03)00355-0 [DOI] [PubMed] [Google Scholar]
- Moore WE, Pfeifer JH, Masten CL, Mazziotta JC, Iacoboni M, & Dapretto M (2012). Facing puberty: Associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience, 7(1), 35–43. 10.1093/scan/nsr066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Meesters C, & Fijen P (2003). The self-perception profile for children: Further evidence for its factor structure, reliability, and validity. Personality and Individual Differences, 35(8), 1791–1802. 10.1016/S0191-8869(03)00004-7 [DOI] [Google Scholar]
- Neta M, Davis FC, & Whalen PJ (2011). Valence resolution of facial expressions using an emotional oddball task. Emotion, 11(6), 1425–1433. 10.1037/a0022993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Kelley WM, & Whalen PJ (2013). Neural responses to ambiguity involve domain-general and domain-specific emotion processing systems. Journal of Cognitive Neuroscience, 25(4), 547–557. 10.1162/jocn_a_00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Norris CJ, & Whalen PJ (2009). Corrugator muscle responses are associated with individual differences in positivity-negativity bias. Emotion, 9(5), 640–648. 10.1037/a0016819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, & Tong TT (2016). Don’t like what you see? Give it time: Longer reaction times associated with increased positive affect. Emotion, 16(5), 730–739. 10.1037/emo0000181 [DOI] [PubMed] [Google Scholar]
- Neta M, Tong TT, & Henley DJ (2018). It’s a matter of time (perspectives): Shifting valence responses to emotional ambiguity. Motivation and Emotion, 42(2), 258–266. 10.1007/s11031-018-9665-7 [DOI] [Google Scholar]
- Neta M, & Whalen PJ (2010). The primacy of negative interpretations when resolving the valence of ambiguous facial expressions. Psychological Science, 21(7), 901–907. 10.1177/0956797610373934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, & Gross JJ (2004). For better or for worse: Neural systems supporting the cognitive down-and up-regulation of negative emotion. NeuroImage, 23(2), 483–499. 10.1016/j.neuroimage.2004.06.030 [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Luking KR, Belden AC, & Barch DM (2014). Brain-behavior relationships in the experience and regulation of negative emotion in healthy children: Implications for risk for childhood depression. Development and Psychopathology, 26(4), 1289–1303. 10.1017/S0954579414001035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, & Giedd JN (2008). Why do many psychiatric disorders emerge during adolescence? Nature Reviews Neuroscience, 9(12), 947–957. 10.1038/nrn2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer M, Abboud S, Hertz U, Amedi A, & Arzy S (2016). Intensity-based masking: A tool to improve functional connectivity results of resting-state fMRI: Intensity-Based Masking. Human Brain Mapping, 37(7), 2407–2418. 10.1002/hbm.23182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, & Pelphrey KA (2011). Developing connections for affective regulation: Age-related changes in emotional brain connectivity. Journal of Experimental Child Psychology, 108(3), 607–620. 10.1016/j.jecp.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Petro NM, Tong TT, Henley DJ, & Neta M (2018). Individual differences in valence bias: FMRI evidence of the initial negativity hypothesis. Social Cognitive and Affective Neuroscience, 13(7), 687–698. 10.1093/scan/nsy049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, & Weinberger DR (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience, 8(6), 828–834. 10.1038/nn1463 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, Nolan Nichols B, Brown SA, Tapert SF, Cummins K, Thompson WK, Brumback TY, Meloy MJ, Jernigan TL, Dale A, Colrain IM, Baker FC, Prouty D, De Bellis MD, … Sullivan EV (2016). Adolescent development of cortical and white matter structure in the NCANDA sample: Role of sex, ethnicity, puberty, and alcohol drinking. Cerebral Cortex, 26(10), 4101–4121. 10.1093/cercor/bhv205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Kahn LE, Merchant JS, Peake SJ, Veroude K, Masten CL, Lieberman MD, Mazziotta JC, & Dapretto M (2013). Longitudinal change in the neural bases of adolescent social self-evaluations: Effects of age and pubertal development. Journal of Neuroscience, 33(17), 7415–7419. 10.1523/JNEUROSCI.4074-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, & Drevets WC (2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 833–857. 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reef J, Diamantopoulou S, van Meurs I, Verhulst FC, & van der Ende J (2011). Developmental trajectories of child to adolescent externalizing behavior and adult DSM-IV disorder: Results of a 24-year longitudinal study. Social Psychiatry and Psychiatric Epidemiology, 46(12), 1233–1241. 10.1007/s00127-010-0297-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Fudge JL, Kelly C, Perry JSA, Daniele T, Carlisi C, Benson B, Xavier Castellanos F, Milham MP, Pine DS, & Ernst M (2013). Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 52(3), 290–299. e2. 10.1016/j.jaac.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Vasa RA, Bruck M, Mogg K, Bradley BP, Sweeney M, Bergman RL, McClure-Tone EB, & Pine DS (2008). Attention bias toward threat in pediatric anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 47(10), 1189–1196. 10.1097/CHI.0b013e3181825ace [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarni C (1984). An observational study of children’s attempts to monitor their expressive behavior. Child Development, 55(4), 1504–1513. 10.2307/1130020 [DOI] [Google Scholar]
- Samuelsson H, Jarnvik K, Henningsson H, Andersson J, & Carlbring P (2012). The Umeå university database of facial expressions: A validation study. Journal of Medical Internet Research, 14(5), e136. 10.2196/jmir.2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuiling KD, & Likis FE (2016). Women’s gynecologic health. Jones & Bartlett Learning. [Google Scholar]
- Sewart AR, Zbozinek TD, Hammen C, Zinbarg RE, Mineka S, & Craske MG (2019). Positive affect as a buffer between chronic stress and symptom severity of emotional disorders. Clinical Psychological Science, 7(5), 914–927. 10.1177/2167702619834576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, Weber J, Mischel W, Casey BJ, & Ochsner KN (2017). VlPFC-vmPFC-amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cerebral Cortex, 27(7), 3502–3514. 10.1093/cercor/bhw073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, & Rogol A (2013). Puberty and psychological development. In Lerner RM & Steinberg L (Eds.), Handbook of adolescent psychology (pp. 15–44). John Wiley & Sons, Inc. 10.1002/9780471726746.ch2 [DOI] [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, Thomason ME, & Monk CS (2014). Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: A multi-modal imaging approach. NeuroImage, 86, 212–220. 10.1016/j.neuroimage.2013.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach P, & Tournoux J (1988). Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. Thieme Medical. [Google Scholar]
- Tottenham N, & Gabard-Durnam LJ (2017). The developing amygdala: a student of the world and a teacher of the cortex. Current Opinion in Psychology, 17, 55–60. 10.1016/j.copsyc.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin J, & Casey BJ (2011). Elevated amygdala response to faces following early deprivation. Developmental Science, 14(2), 190–204. 10.1111/j.1467-7687.2010.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Phuong J, Flannery J, Gabard-Durnam L, & Goff B (2013). A negativity bias for ambiguous facial expression valence during childhood: Converging evidence from behavior and facial corrugator muscle responses. Emotion, 13(1), 92–103. 10.1037/a0029431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey B, & Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugade MM, & Fredrickson BL (2004). Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology, 86(2), 320–333. 10.1037/0022-3514.86.2.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 26(16), 4415–4425. 10.1523/jneurosci.3215-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, Ossewaarde L, Bäckström T, Hermans EJ, & Fernández G (2011). Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience, 191, 38–45. 10.1016/j.neuroscience.2011.04.042 [DOI] [PubMed] [Google Scholar]
- Vantieghem MR, Gabard-Durnam L, Goff B, Flannery J, Humphreys KL, Telzer EH, Caldera C, Louie JY, Shapiro M, Bolger N, & Tottenham N (2017). Positive valence bias and parent-child relationship security moderate the association between early institutional caregiving and internalizing symptoms. Development and Psychopathology, 29(2), 519–533. 10.1017/S0954579417000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasey MW, Daleiden EL, Williams LL, & Brown LM (1995). Biased attention in childhood anxiety disorders: A preliminary study. Journal of Abnormal Child Psychology, 23(2), 267–279. 10.1007/BF01447092 [DOI] [PubMed] [Google Scholar]
- Vetter NC, Drauschke M, Thieme J, & Altgassen M (2018). Adolescent basic facial emotion recognition is not influenced by puberty or own-age bias. Frontiers in Psychology, 9, 956. 10.3389/fpsyg.2018.00956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N, Op de Macks Z, Shirtcliff EA, & Pfeifer JH (2018). Puberty and the human brain: Insights into adolescent development. Neuroscience & Biobehavioral Reviews, 92, 417–436. 10.1016/j.neubiorev.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N, Pfeifer JH, Flournoy JC, Hernandez LM, & Dapretto M (2019). Affective reactivity during adolescence: Associations with age, puberty and testosterone. Cortex, 117, 336–350. 10.1016/j.cortex.2019.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, & Ochsner KN (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59(6), 1037–1050. 10.1016/j.neuron.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, Tie K, Gong G, Shah MP, Jones M, Uderman J, Constable RT, & Blumberg HP (2009). Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biological Psychiatry, 66(5), 516–521. 10.1016/j.biopsych.2009.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler abbreviated scale of intelligence. The Psychological Corporation. [Google Scholar]
- Whalen PJ (2007). The uncertainty of it all. Trends in Cognitive Sciences, 11(12), 499–500. 10.1016/j.tics.2007.08.016 [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Davis FC, Oler JA, Kim H, Kim MJ, & Neta M (2009). Human amygdala responses to facial expressions of emotion. In The human amygdala (pp. 265–288). Guilford Press. [Google Scholar]
- Widen SC, & Russell JA (2008). Children acquire emotion categories gradually. Cognitive Development, 23(2), 291–312. 10.1016/j.cogdev.2008.01.002 [DOI] [Google Scholar]
- Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, & Durston S (2014). Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. NeuroImage, 96, 67–72. 10.1016/j.neuroimage.2014.03.072 [DOI] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Liddell BJ, Palmer DM, & Bryant RA (2007). Neural biases to convert and overt signals of fear: Dissociation by trait anxiety and depression. Journal of Cognitive Neuroscience, 19(10), 1595–1608. 10.1162/jocn.2007.19.10.1595 [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Richardson R, & Baker K (2019). Maturational changes in prefrontal and amygdala circuits in adolescence: Implications for understanding fear inhibition during a vulnerable period of development. Brain Sciences, 9(3), 65. 10.3390/brainsci9030065 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


