Abstract
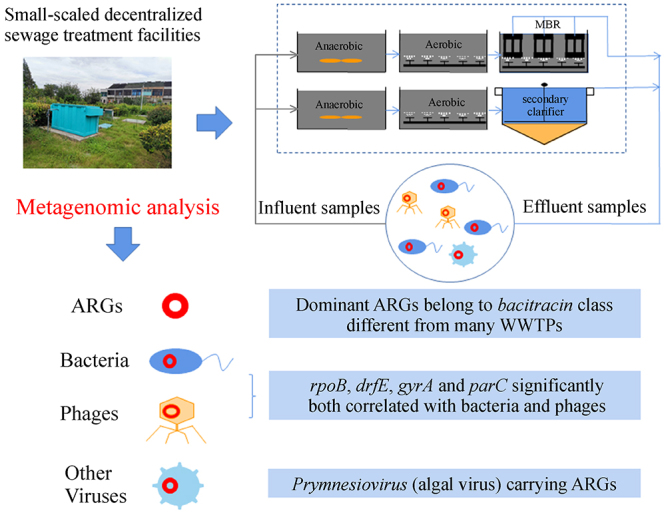
The distribution of antibiotic resistance genes (ARGs) has been intensively studied in large-scale wastewater treatment plants and livestock sources. However, small-scale decentralized sewage treatment facilities must also be explored due to their possible direct exposure to residents. In this study, six wastewater treatment facilities in developed rural areas in eastern China were investigated to understand their risks of spreading ARGs. Using metagenomics and network analysis tools, ARGs and bacterial and viral communities were identified in the influent (INF) and effluent (EFF) samples. The dominant ARGs belonged to the bacitracin class, which are different from most of municipal wastewater treatment plants (WWTPs). The dominant hosts of ARGs are Acidovorax in bacterial communities and Prymnesiovirus in viral communities. Furthermore, a positive relationship was found between ARGs and phages. The ARGs significantly correlated with phages were all hosted by specific genera of bacteria, indicating that phages had contributed to the ARG’s proliferation in sewage treatment facilities. Paying significant concern on the possible enhanced risks caused by bacteria, viruses and their related ARGs in decentralized sewage treatment facilities is necessary.
Electronic Supplementary Material
Supplementary material is available in the online version of this article at 10.1007/s11783-021-1469-4 and is accessible for authorized users.
Keywords: Decentralized sewage treatment facilities, Antibiotic resistance genes, Virus, Metagenomics, Network analysis
Acknowledgements
This work was supported by the Major Science and Technology Program for Water Pollution Control and Treatment (2017ZX07202-003), National Natural Science Foundation of China (51778325), the Fundamental Research Fund for the Central Universities (FRF-TP-20-011A) and the Research Fund Program of Guangdong Provincial Key Laboratory of Environmental Pollution Control and Remediation Technology(2020B1212060022).
Supporting Information
Distribution of antibiotic resistance genes and their association with bacteria and viruses in decentralized sewage treatment facilities
Footnotes
Highlights
• Distribution of ARGs in decentralized sewage facilities were investigated.
• Bacitracin-ARGs were most predominant ARGs in rural wastewater.
• ARGs were identified in bacterial and viral community.
• ARGs of rpoB, drfE, gyrA and parC were both correlated with bacteria and phages.
• More attention should be paid to the risk of spreading ARG by phages.
Contributor Information
Bing Li, Email: libing@ustb.edu.cn.
Yong Qiu, Email: qiuyong@tsinghua.edu.cn.
References
- Ahmed W, Angel N, Edson J, Bibby K, Bivins A, O’Brien J W, Choi P M, Kitajima M, Simpson S L, Li J, Tscharke B, Verhagen R, Smith W J M, Zaugg J, Dierens L, Hugenholtz P, Thomas K V, Mueller J F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Science of the Total Environment. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcázar J L. How do bacteriophages promote antibiotic resistance in the environment? Clinical Microbiology and Infection. 2018;24(5):447–449. doi: 10.1016/j.cmi.2017.10.010. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 1995;57(1):289–300. [Google Scholar]
- Blair J M A, Webber M A, Baylay A J, Ogbolu D O, Piddock L J V. Molecular mechanisms of antibiotic resistance. Nature Reviews. Microbiology. 2015;13(1):42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- Calero-Cáceres W, Muniesa M. Persistence of naturally occurring antibiotic resistance genes in the bacteria and bacteriophage fractions of wastewater. Water Research. 2016;95:11–18. doi: 10.1016/j.watres.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Calero-Cáceres W, Ye M, Balcázar J L. Bacteriophages as environmental reservoirs of antibiotic resistance. Trends in Microbiology. 2019;27(7):570–577. doi: 10.1016/j.tim.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Chen H, Bai X, Li Y, Jing L, Chen R, Teng Y. Source identification of antibiotic resistance genes in a peri-urban river using novel crAssphage marker genes and metagenomic signatures. Water Research. 2019;167:115098. doi: 10.1016/j.watres.2019.115098. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang M. Occurrence and removal of antibiotic resistance genes in municipal wastewater and rural domestic sewage treatment systems in eastern China. Environment International. 2013;55:9–14. doi: 10.1016/j.envint.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Chopyk J, Nasko D J, Allard S, Bui A, Treangen T, Pop M, Mongodin E F, Sapkota A R. Comparative metagenomic analysis of microbial taxonomic and functional variations in untreated surface and reclaimed waters used in irrigation applications. Water Research. 2020;169:115250. doi: 10.1016/j.watres.2019.115250. [DOI] [PubMed] [Google Scholar]
- Collignon P C, Conly J M, Andremont A, McEwen S A, Aidara-Kane A. World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clinical Infectious Diseases. 2016;63(8):1087–1093. doi: 10.1093/cid/ciw475. [DOI] [PubMed] [Google Scholar]
- Debroas D, Siguret C. Viruses as key reservoirs of antibiotic resistance genes in the environment. ISME Journal. 2019;13(11):2856–2867. doi: 10.1038/s41396-019-0478-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enault F, Briet A, Bouteille L, Roux S, Sullivan M B, Petit M-A (2016). Phages rarely encode antibiotic resistance genes: A cautionary tale for virome analyses. BioRxiv: 053025 [DOI] [PMC free article] [PubMed]
- Forsberg K J, Patel S, Gibson M K, Lauber C L, Knight R, Fierer N, Dantas G. Bacterial phylogeny structures soil resistomes across habitats. Nature. 2014;509(7502):612–616. doi: 10.1038/nature13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg K J, Reyes A, Wang B, Selleck E M, Sommer M O A, Dantas G. The shared antibiotic resistome of soil bacteria and human pathogens. Science. 2012;337(6098):1107–1111. doi: 10.1126/science.1220761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J A. Marine viruses and their biogeochemical and ecological effects. Nature. 1999;399(6736):541–548. doi: 10.1038/21119. [DOI] [PubMed] [Google Scholar]
- Gunathilaka G U, Tahlan V, Mafiz A I, Polur M, Zhang Y. Phages in urban wastewater have the potential to disseminate antibiotic resistance. International Journal of Antimicrobial Agents. 2017;50(5):678–683. doi: 10.1016/j.ijantimicag.2017.08.013. [DOI] [PubMed] [Google Scholar]
- Guo J, Li J, Chen H, Bond P L, Yuan Z. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Research. 2017;123:468–478. doi: 10.1016/j.watres.2017.07.002. [DOI] [PubMed] [Google Scholar]
- Haaber J, Leisner J J, Cohn M T, Catalan-Moreno A, Nielsen J B, Westh H, Penades J R, Ingmer H. Bacterial viruses enable their host to acquire antibiotic resistance genes from neighbouring cells. Nature Communications. 2016;7(1):13333. doi: 10.1038/ncomms13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journal of Cleaner Production. 2019.
- Hu Q, Zhang X, Jia S, Huang K, Tang J, Shi P, Ye L, Ren H. Metagenomic insights into ultraviolet disinfection effects on antibiotic resistome in biologically treated wastewater. Water Research. 2016;101:309–317. doi: 10.1016/j.watres.2016.05.092. [DOI] [PubMed] [Google Scholar]
- Hu Y, Yang X, Qin J, Lu N, Cheng G, Wu N, Pan Y, Li J, Zhu L, Wang X, Meng Z, Zhao F, Liu D, Ma J, Qin N, Xiang C, Xiao Y, Li L, Yang H, Wang J, Yang R, Gao G F, Wang J, Zhu B. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nature Communications. 2013;4(1):2151. doi: 10.1038/ncomms3151. [DOI] [PubMed] [Google Scholar]
- Ji M, Sun K, Li Z, Fan X, Li Q. Bacteriophages in water pollution control: Advantages and limitations. Frontiers of Environment Science & Engineering. 2021;15(5):84. doi: 10.1007/s11783-020-1378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B, Raphenya A R, Alcock B, Waglechner N, Guo P, Tsang K K, Lago B A, Dave B M, Pereira S, Sharma A N, Doshi S, Courtot M, Lo R, Williams L E, Frye J G, Elsayegh T, Sardar D, Westman E L, Pawlowski A C, Johnson T A, Brinkman F S L, Wright G D, McArthur A G. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Research. 2017;45(D1):D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju F, Beck K, Yin X, Maccagnan A, McArdell C S, Singer H P, Johnson D R, Zhang T, Burgmann H. Wastewater treatment plant resistomes are shaped by bacterial composition, genetic exchange, and upregulated expression in the effluent microbiomes. ISME Journal. 2019;13(2):346–360. doi: 10.1038/s41396-018-0277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Park E, Roh S W, Bae J. Diversity and abundance of single-stranded DNA viruses in human feces. Applied and Environmental Microbiology. 2011;77(22):8062–8070. doi: 10.1128/AEM.06331-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones S J, Marra M A. Circos: An information aesthetic for comparative genomics. Genome Research. 2009;19(9):1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C E, Wu S, Bhattacharjee A S, Hamilton J J, McMahon K D, Goel R, Noguera D R. Metabolic network analysis reveals microbial community interactions in anammox granules. Nature Communications. 2017;8(1):15416. doi: 10.1038/ncomms15416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Yang Y, Ma L, Ju F, Guo F, Tiedje J M, Zhang T. Metagenomic and network analysis reveal wide distribution and cooccurrence of environmental antibiotic resistance genes. ISME Journal. 2015;9(11):2490–2502. doi: 10.1038/ismej.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang Z, Duan J, Li N, Li B, Song T, Sardar M F, Lv X, Zhu C. Electrochemical disinfection of secondary effluent from a wastewater treatment plant: Removal efficiency of ARGs and variation of antibiotic resistance in surviving bacteria. Chemical Engineering Journal. 2020;392:123674. doi: 10.1016/j.cej.2019.123674. [DOI] [Google Scholar]
- Li R, Zhu H, Ruan J, Qian W, Fang X, Shi Z, Li Y, Li S, Shan G, Kristiansen K, Li S, Yang H, Wang J, Wang J. De novo assembly of human genomes with massively parallel short read sequencing. Genome Research. 2010;20(2):265–272. doi: 10.1101/gr.097261.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Pop M. ARDB-Antibiotic Resistance Genes Database. Nucleic Acids Research. 2009;37(SI):D443–D447. doi: 10.1093/nar/gkn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu X, Gao L, Xu S, Chen X, Tian H, Kang X. Performance and microbial community of a novel combined anaerobic bioreactor integrating anaerobic baffling and anaerobic filtration process for low-strength rural wastewater treatment. Environmental Science and Pollution Research International. 2020;27(15):18743–18756. doi: 10.1007/s11356-020-08263-9. [DOI] [PubMed] [Google Scholar]
- Ma L, Li B, Jiang X, Wang Y, Xia Y, Li A, Zhang T. Catalogue of antibiotic resistome and host-tracking in drinking water deciphered by a large scale survey. Microbiome. 2017;5(1):154. doi: 10.1186/s40168-017-0369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J L. Antibiotics and antibiotic resistance genes in natural environments. Science. 2008;321(5887):365–367. doi: 10.1126/science.1159483. [DOI] [PubMed] [Google Scholar]
- Medema G, Heijnen L, Elsinga G, Italiaander R, Brouwer A (2020). Presence of SARS-Coronavirus-2 in sewage. MedRxiv: 2020.2003.2029.20045880. [DOI] [PubMed]
- Moon K, Jeon J H, Kang I, Park K S, Lee K, Cha C, Lee S H, Cho J. Freshwater viral metagenome reveals novel and functional phage-borne antibiotic resistance genes. Microbiome. 2020;8(1):75. doi: 10.1186/s40168-020-00863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C, Tan B, Jiang X, Gu X, Chen H, Schmitz B W, Haller L, Charles F R, Zhang T, Gin K. Metagenomic and resistome analysis of a full-scale municipal wastewater treatment plant in Singapore containing membrane bioreactors. Frontiers in Microbiology. 2019;10:172. doi: 10.3389/fmicb.2019.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osińska A, Korzeniewska E, Harnisz M, Felis E, Bajkacz S, Jachimowicz P, Niestepski S, Konopka I. Small-scale wastewater treatment plants as a source of the dissemination of antibiotic resistance genes in the aquatic environment. Journal of Hazardous Materials. 2020;381:121221. doi: 10.1016/j.jhazmat.2019.121221. [DOI] [PubMed] [Google Scholar]
- Pazda M, Kumirska J, Stepnowski P, Mulkiewicz E. Antibiotic resistance genes identified in wastewater treatment plant systems: A review. Science of the Total Environment. 2019;697:134023. doi: 10.1016/j.scitotenv.2019.134023. [DOI] [PubMed] [Google Scholar]
- Peng S, Feng Y, Wang Y, Guo X, Chu H, Lin X. Prevalence of antibiotic resistance genes in soils after continually applied with different manure for 30 years. Journal of Hazardous Materials. 2017;340:16–25. doi: 10.1016/j.jhazmat.2017.06.059. [DOI] [PubMed] [Google Scholar]
- Petrovich M L, Zilberman A, Kaplan A, Eliraz G R, Wang Y, Langenfeld K, Duhaime M, Wigginton K, Poretsky R, Avisar D, Wells G F. Microbial and viral communities and their antibiotic resistance genes throughout a hospital wastewater treatment system. Frontiers in Microbiology. 2020;11:153. doi: 10.3389/fmicb.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G (2020). SARS-CoV-2 RNA titers in wastewater anticipated COVID-19 occurrence in a low prevalence area. MedRxiv: 2020.2004.2022.20075200. [DOI] [PMC free article] [PubMed]
- Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy M C, Michael I, Fatta-Kassinos D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Science of the Total Environment. 2013;447:345–360. doi: 10.1016/j.scitotenv.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Sharma V K, Yu X, Mcdonald T J, Jinadatha C, Dionysiou D D, Feng M. Elimination of antibiotic resistance genes and control of horizontal transfer risk by UV-based treatment of drinking water: A mini review. Frontiers of Environment Science & Engineering. 2019;13(3):37. doi: 10.1007/s11783-019-1122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy S M, Huang J, Gogarten J P. Horizontal gene transfer: building the web of life. Nature Reviews. Genetics. 2015;16(8):472–482. doi: 10.1038/nrg3962. [DOI] [PubMed] [Google Scholar]
- Su J, An X, Li B, Chen Q, Gillings M R, Chen H, Zhang T, Zhu Y. Metagenomics of urban sewage identifies an extensively shared antibiotic resistome in China. Microbiome. 2017;5(1):84. doi: 10.1186/s40168-017-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subirats J, Sànchez-Melsió A, Borrego C M, Balcázar J L, Simonet P. Metagenomic analysis reveals that bacteriophages are reservoirs of antibiotic resistance genes. International Journal of Antimicrobial Agents. 2016;48(2):163–167. doi: 10.1016/j.ijantimicag.2016.04.028. [DOI] [PubMed] [Google Scholar]
- Tang J, Bu Y, Zhang X, Huang K, He X, Ye L, Shan Z, Ren H. Metagenomic analysis of bacterial community composition and antibiotic resistance genes in a wastewater treatment plant and its receiving surface water. Ecotoxicology and Environmental Safety. 2016;132:260–269. doi: 10.1016/j.ecoenv.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Tong J, Tang A, Wang H, Liu X, Huang Z, Wang Z, Zhang J, Wei Y, Su Y, Zhang Y. Microbial community evolution and fate of antibiotic resistance genes along six different full-scale municipal wastewater treatment processes. Bioresource Technology. 2019;272:489–500. doi: 10.1016/j.biortech.2018.10.079. [DOI] [PubMed] [Google Scholar]
- Van Etten J L, Dunigan D D. Chloroviruses: Not your everyday plant virus. Trends in Plant Science. 2012;17(1):1–8. doi: 10.1016/j.tplants.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chu L, Wojnárovits L, Takács E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Science of the Total Environment. 2020;744:140997. doi: 10.1016/j.scitotenv.2020.140997. [DOI] [PubMed] [Google Scholar]
- Wang M, Liu P, Zhou Q, Tao W, Sun Y, Zeng Z. Estimating the contribution of bacteriophage to the dissemination of antibiotic resistance genes in pig feces. Environmental Pollution. 2018;238(5):291–298. doi: 10.1016/j.envpol.2018.03.024. [DOI] [PubMed] [Google Scholar]
- Wang M, Xiong W, Liu P, Xie X, Zeng J, Sun Y, Zeng Z. Metagenomic Insights Into the Contribution of Phages to Antibiotic Resistance in Water Samples Related to Swine Feedlot Wastewater Treatment. Frontiers in Microbiology. 2018;9:2474. doi: 10.3389/fmicb.2018.02474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li B, Zou S, Fang H H P, Zhang T. Fate of antibiotic resistance genes in sewage treatment plant revealed by metagenomic approach. Water Research. 2014;62:97–106. doi: 10.1016/j.watres.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shi W, Lu S, Liu J, Liang H, Yang Y, Duan G, Li Y, Wang H, Zhang A. Prevalence of antibiotic resistance genes in bacteriophage DNA fraction from Funan River water in Sichuan, China. Science of the Total Environment. 2018;626:835–841. doi: 10.1016/j.scitotenv.2018.01.148. [DOI] [PubMed] [Google Scholar]
- Yu K, Li P, Chen Y, Zhang B, Huang Y, Huang F, He Y. Antibiotic resistome associated with microbial communities in an integrated wastewater reclamation system. Water Research. 2020;173:115541. doi: 10.1016/j.watres.2020.115541. [DOI] [PubMed] [Google Scholar]
- Zhang G, Guan Y, Zhao R, Feng J, Huang J, Ma L, Li B. Metagenomic and network analyses decipher profiles and cooccurrence patterns of antibiotic resistome and bacterial taxa in the reclaimed wastewater distribution system. Journal of Hazardous Materials. 2020;400:123170. doi: 10.1016/j.jhazmat.2020.123170. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chang F, Shi P, Ye L, Zhou Q, Pan Y, Li A. Antibiotic resistome alteration by different disinfection strategies in a full-scale drinking water treatment plant deciphered by metagenomic assembly. Environmental Science & Technology. 2019;53(4):2141–2150. doi: 10.1021/acs.est.8b05907. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tang W, Chen Y, Yin W. Disinfection threatens aquatic ecosystems. Science. 2020;368(6487):146–147. doi: 10.1126/science.abb8905. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hu H, Chen Q, Singh B K, Yan H, Chen D, He J. Transfer of antibiotic resistance from manure-amended soils to vegetable microbiomes. Environment International. 2019;130:104912. doi: 10.1016/j.envint.2019.104912. [DOI] [PubMed] [Google Scholar]
- Zhao R, Feng J, Yin X, Liu J, Fu W, Berendonk T U, Zhang T, Li X, Li B. Antibiotic resistome in landfill leachate from different cities of China deciphered by metagenomic analysis. Water Research. 2018;134:126–139. doi: 10.1016/j.watres.2018.01.063. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang X, Zhao Z, Duan C, Chen H, Wang M, Ren H, Yin Y, Ye L. Metagenomic analysis revealed the prevalence of antibiotic resistance genes in the gut and living environment of freshwater shrimp. Journal of Hazardous Materials. 2018;350:10–18. doi: 10.1016/j.jhazmat.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zheng J, Wei Y, Chen T, Dahlgren R A, Shang X, Chen H. Antibiotic resistance genes in an urban river as impacted by bacterial community and physicochemical parameters. Environmental Science and Pollution Research International. 2017;24(30):23753–23762. doi: 10.1007/s11356-017-0032-0. [DOI] [PubMed] [Google Scholar]


