Abstract
Humidifier disinfectants (HDs) exposure has now been associated with acute lung injury and pulmonary fibrosis; polyhexamethylene guanidine (PHMG) has been confirmed to cause severe lung inflammation and fibrosis in mice. Recent evidence also indicates that HDs exposure increases the asthma risk in children, but the underlying mechanisms remain unclear. We aimed to investigate the effects of PHMG exposure on asthma in mice and the potential underlying mechanisms. BALB/c mice were intranasally administered PHMG (0.1 mg/kg/day; 5 days per week) during 2 episodes of ovalbumin (OVA) sensitization and were then challenged with 1% OVA by inhalation. Bronchial hyperresponsiveness (BHR), inflammatory cell influx into bronchoalveolar lavage (BAL) fluid, serum total and OVA-specific immunoglobulin (Ig) E levels, and histopathological changes in the lung were analyzed. The levels of asthma-related cytokines and chemokines were assayed in the lung tissues to evaluate possible mechanisms. Exposure to PHMG following OVA sensitization and challenge significantly enhanced BHR, inflammatory cell counts in BAL fluid, airway inflammation, and total serum IgE levels in the asthma mouse model. In addition, the levels of chemokine ligand (CCL) 11 and serpine F1/pigment epithelium-derived factor (SERPINF1) were significantly elevated in the lungs of these mice compared to those in the control and OVA-treated only groups. Our findings suggest that PHMG can enhance the development of allergic responses and lung inflammation via CCL11- and SERPINF1-induced signaling in a mouse model of asthma.
Keywords: Humidifiers, disinfectants, polyhexamethyleneguanidine, asthma, bronchial hyperreactivity, mice, chemokine CCL11, Serpins
INTRODUCTION
Polyhexamethylene guanidine (PHMG) has been widely used as a biocide and was incorporated into humidifier disinfectants (HDs) because of its potent bactericidal activity and low toxicity.1 However, the Korea Center for Disease Control and Prevention and the Ministry of Health and Welfare reported in 2011 that an outbreak of pulmonary disease of unknown cause may have originated from HDs containing PHMG as the main ingredient.2 HDs are dispersed into the air via the humidifier's aerosolizer as nanoparticles, which can easily reach the distal airways, resulting in the development of acute interstitial pneumonia and pulmonary fibrosis.3,4 In addition, PHMG causes severe lung inflammation, fibrosis, and thymic atrophy-via generation of reactive oxygen species and induction of fibrotic inflammatory cytokine release in mice.5,6 Additional evidence has shown that HDs exposure increases the risk of asthma in children.7 Despite the association between PHMG exposure and lung disease, however, mechanisms underlying the development of asthma through actions of PHMG have remained unclear.
Asthma is a chronic respiratory disorder of the lungs characterized by airway obstruction, wheezing and bronchial hyperresponsiveness (BHR).8 Chronic inflammation causes airway remodeling and specific changes manifesting as sub-epithelial fibrosis.9,10,11 Due to its high susceptibility to environmental exposure, the development of asthma is known to be strongly linked to a number of environmental factors including microbes, allergens, tobacco smoke, pollutants and chemicals.12,13,14,15,16 Most notably, exposure to pollutants and chemicals accounts for the majority of known triggers of obstructive events in asthma.17,18 Experimental studies have provided evidence of a biological basis for particulates and chemicals as risk factors for asthma, as indicated by the enhanced airway inflammation and immunoglobulin (Ig) E production that accompany this phenomenon.19,20,21,22,23 Hence, the inadvertent inhalation of pollutants and chemicals is a human health concern, particularly for susceptible individuals with underlying disease such as asthma.
There has been no direct evidence to date that PHMG can cause asthma. We investigated in this current study, therefore, whether PHMG causes asthma-related consequences in a BALB/c mice model.
MATERIALS AND METHODS
Animal experiments
Female BALB/c mice (5-week-old, n = 5 per group) were purchased from OrientBio (Seongnam, Korea) and housed under controlled humidity (40%) and temperature (22°C ± 2°C) conditions, with a 12 hour- light/dark cycle. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of Asan Medical Center. The mouse model of asthma was generated using a previously established protocol.24 Briefly, on days 0 and 7 the animals were sensitized by intraperitoneal administration of ovalbumin (OVA 10 µg, grade V, Sigma-Aldrich, St. Louis, MO, USA) and alum (Imject®, 2.25 mg, Thermo Fisher Scientific, Waltham, MA, USA). One week after the final sensitization, the mice were administrated 1% OVA by inhalation using an ultrasonic sprayer (Nescosonic UN-511, Alfresa, Osaka, Japan) for 30 minutes on 3 successive days (OVA challenge). During the sensitization phase, the PHMG-exposure groups received 0.1 mg/kg/day PHMG intranasally, 5 days per week, over 2 weeks. The animals were euthanized 24 hours after the final OVA challenge.
Measurement of BHR and cell number in bronchoalveolar lavage fluid (BALF) and histopathological analysis of the lung
Twenty-four hours after the final OVA challenge, BHR was measured in conscious unrestrained mice using a barometric whole-body plethysmograph (All Medicus, Anyang, Korea). BALF was obtained through the airway and the number of nucleated cells was counted using a hemocytometer. For histological evaluations, fixed left lung tissue was stained with hematoxylin and eosin as previously described.24
Quantitation of serum IgE
Total and OVA-specific IgE levels in serum were measured by Enzyme-Linked Immunosorbent Assay. Details are provided in the ‘Supplementary Methods’ section in the Online Repository.
Real-time reverse transcription polymerase chain reaction (qPCR) and western blotting
The relative expression of the C-C motif chemokine ligand (CCL) 11, CCL17, and T helper (Th) 2-related cytokines (interleukin [IL]-4, IL-5 and IL-13) was measured using qPCR. Each signal was normalized against GAPDH signals in the same sample. Total protein was extracted from lung and resolved on 10% SDS-PAGE. Anti-serpineF1/pigment epithelium-derived factor (SERPINF1/PEDF) antibody was procured from R&D Systems (Minneapolis, MN, USA) and anti-β-actin from Sigma-Aldrich. Blots were analyzed using Image J (National Institutes of Health, Rockville, MD, USA). Details are provided in the ‘Supplementary Methods’ section in the Online Repository.
Statistical analysis
Significance was determined by performing one-way analysis of variance with Tukey's multiple comparison test using GraphPad Prism 5.0 (San Diego, CA, USA). A P value of <0.05 was considered significant.
Supplementary methods
Further information on detailed methods is provided in the Supplementary Methods section in this article's Online Repository.
RESULTS
Exposure to PHMG exacerbates characteristics of asthma in a mouse model
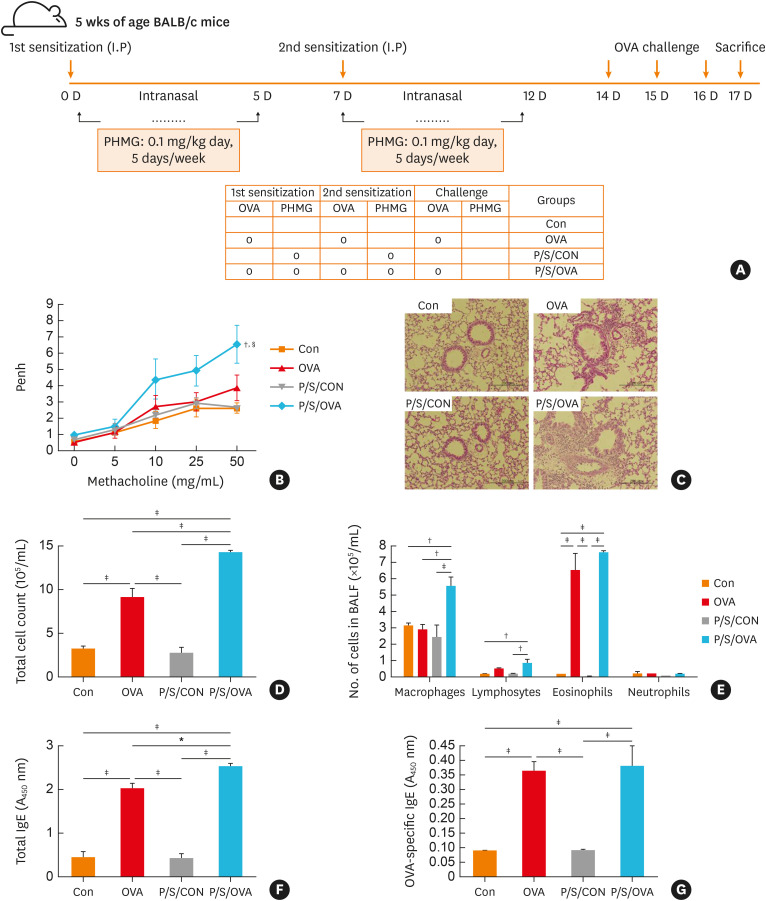
We first investigated whether PHMG exposure during allergen sensitization in mice enhances asthma-related consequences. The animals were intranasally exposed to PHMG during the OVA sensitization phase (Fig. 1A), which significantly elevated BHR compared to the control group (Fig. 1B). We then observed increased inflammatory cell infiltration in the peribronchial and perivascular areas of these PHMG-exposed mice compared to the control and OVA-treated only groups (Fig. 1C). Concordantly, the number of total cells, eosinophils and macrophages in BALF were significantly higher following exposure to PHMG and OVA (Fig. 1D and E), as were the total and OVA-specific IgE levels in the serum (Fig. 1F and G). However, mice exposed to PHMG plus OVA did not exhibit significant differences in OVA-specific IgE in the serum compared to those exposed to OVA alone (Fig. 1G).
Fig. 1. PHMG enhances allergic airway inflammation in a mouse model of asthma. (A) Experimental protocol for the intranasal administration of PHMG. BALB/c mice were either exposed or not to PHMG during OVA sensitization and then subjected to a further OVA challenge. (B) Measurement of BHR in the mice in response to inhaled methacholine. (C) hematoxylin and eosin-stained lung tissue. (D) Total and (E) differential cell counts in the bronchoalveolar lavage fluid. (F) Total- and (G) Serum levels of OVA-specific IgE.
Con, control; OVA, ovalbumin; P/S/CON, control group exposed to PHMG during sensitization; P/S/OVA, OVA group exposed to PHMG during sensitization; Ig, immunoglobulin; PHMG, polyhexamethyleneguanidine; BHR, bronchial hyperresponsiveness.
Significance was determined using one-way analysis of variance, *P < 0.05, †P < 0.01, ‡P < 0.001. In the significance of BHR, §P < 0.01 was compared to the control group, and ∥P < 0.05 was compared to the OVA group.
The increased allergic response caused by PHMG is mediated via upregulation of CCL11 and SERPINF1
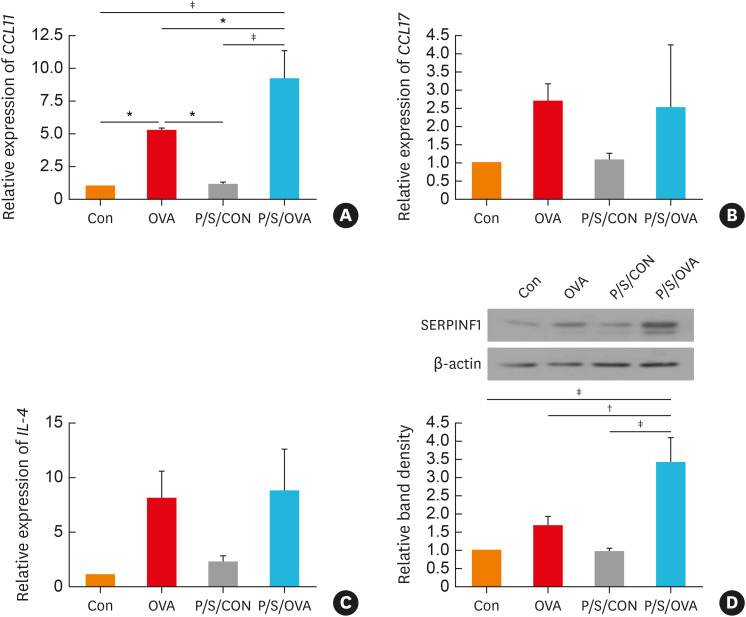
To elucidate mechanisms by which PHMG during OVA sensitization exacerbate asthma, we assayed various asthma-related chemokines (CCL11 and CCL17) and Th2 related cytokines (IL-4, IL-5, and IL-13) in the lungs. However, there were no detectable IL-5 and IL-13 transcripts in the lung (data not shown). PHMG exposure with OVA produced a greater increase in CCL11 expression compared to the control or OVA-treated only groups (Fig. 2A). However, no greater increase was observed in the IL-4 or CCL17 following PHMG exposure with OVA (Fig. 2B and C). We also observed higher SERPINF1 expression in the PHMG-exposed animals (Fig. 2D).
Fig. 2. The expression of chemokine ligand (CCL) 11 and serpine F1/pigment epithelium-derived factor (SERPINF1/PEDF) in the mice lungs. PHMG enhances mRNA expressions of (A) CCL11, (B) CCL17 and (C) interleukin (IL)-4 in the mouse lung tissue. (D) Western blotting analysis shows increased expressions of SERPINF1 and β-actin in the lung tissue.
Con, control; OVA, ovalbumin; P/S/CON, control group exposed to PHMG during sensitization; P/S/OVA, OVA group exposed to PHMG during sensitization; IL, interleukin; PHMG, polyhexamethyleneguanidine; BHR, bronchial hyperresponsiveness.
Significance was determined using one-way analysis of variance, *P < 0.05, †P < 0.01, ‡P < 0.001.
DISCUSSION
Our present findings demonstrated that intranasally administered PHMG during OVA sensitization enhanced the pathological aspects of allergic airway disease upon OVA challenge. We further observed that PHMG aggravated allergen-related airway inflammation by increasing CCL11 and SERPINF1 expression in the lungs of asthma. Therefore, the key finding of our current study is that PHMG acted an adjuvant that aggravates allergic airway inflammation via CCL11- and SERPINF1-induced signaling in the lung.
Particulate matter (PM) and pollutants are known to have adjuvant effects on allergic sensitization and induce inflammation upon exposure to typical allergens.25 Our finding that PHMG exposure during allergen sensitization enhanced the allergic response to subsequent allergen challenge indicates that it may exhibit adjuvant-like characteristics during the development of allergic airway disease. It is notable that PHMG alone did not induce inflammation in low dose exposure but when combined with OVA challenge caused significant inflammation, a marked degree of BHR, and an increase in the total serum IgE level. Similarly, it has previously been reported that exposure to carbon nanoparticles aggravates allergic inflammation and mucus hypersecretion, especially in the presence of antigen.20,21,22 Moreover, another study has demonstrated that exposure to PM causes airway inflammation and allergic sensitization after an OVA challenge in mice, and has immune adjuvant activity.23 Hence, our current results and previously reported evidence suggest that PHMG as a chemical acts as an adjuvant effector for OVA in the induction of airway inflammation.
Our results also indicated that CCL11 expression, but not CCL17 or IL-4, in the mouse lungs was significantly upregulated following PHMG exposure during OVA sensitization. CCL11 and CCL17 are known to play important roles in orchestrating inflammatory responses in asthma through different routes.26 Generally, CCL11 functions via C-C chemokine receptor (CCR) 3 to promote eosinophil infiltration of the lung, which in turn stimulates the release of more of these CCR3-binding chemokines. However, CCL17 induces a Th2 response and Th2 cell migration through induction of IL-4 expression. Importantly, CCL11 is a biomarker of prolonged eosinophilia after allergen exposure and plays a key role in mediating chronic inflammatory responses in asthma, bronchitis, and chronic obstructive pulmonary disease (COPD) by recruiting, activating, and promoting eosinophils in the respiratory tract.27,28,29 Furthermore, CCL11 stimulates macrophage and eosinophil migration by binding to CCR3.30 Macrophages are known to contribute to the development of chronic inflammation and persistent macrophage activation further damages the airways.31 Here, we observed a higher number of macrophages and eosinophils, in the PHMG with OVA group compared to the OVA group (Fig. 1). It is thus possible that in mice, PHMG enhances allergic asthma-related consequences via CCL11-related responses rather than through CCL17, in the background of exposure to another allergen.
A recent report has indicated that CCL11 contributes to profibrogenic activity and airway remodeling in asthma through the recruitment and migration of circulating fibrocytes.32,33 Furthermore, high expression of CCL11 enhances lung fibrosis and profibrotic cytokine production in bleomycin-stimulated mice.34 Our current analyses have revealed that SERPINF1 levels were increased in the lung following exposure to PHMG in the background of OVA challenge. SERPINF1, also known as PEDF, is an important regulator of pulmonary fibrosis35 and is expressed in human pulmonary epithelia, pulmonary fibroblasts/myofibroblasts, and alveolar interstitium.36 SERPINF1 levels have been shown to be significantly elevated in the lungs of patients with COPD or idiopathic pulmonary fibrosis.36,37 SERPINF1 may contribute to the induction of endothelial cell apoptosis and the subsequent destruction of alveolar lung tissue as SERPINF1 is an angiogenesis inhibitor.38,39 SERPINF1 may also contribute to fibrotic scaring through direct binding to collagen-1 as evidenced by the colocalization of SERPINF1 with collagen-1.40 As our data revealed a significantly high level of CCL11 in response to PHMG administration with OVA challenge, it is possible that the effects of PHMG are mediated through CCL11, which affects the profibrogenic activity of lung fibroblasts during airway remodeling in asthma.32 Although the expression of IL-4 significantly increased in the OVA group and the PHMG-treated OVA group compared to PBS controls, the expression of IL-5 and IL-13 mRNAs was not detected in the lungs. It is likely that IL-4 and IL-13 (or IL-5) act on different cell types and IL-4 drives IL-13 (or IL-5)-independent inflammation in various settings and over time.41,42,43,44,45,46 However, this phenomenon should be further investigated in future studies to elucidate its underlying mechanisms. Nevertheless, the results of this study suggest an association between PHMG exposure and asthma, and that the underlying mechanism involves the profibrotic process rather than a typical allergic Th2 pathway.
It must be noted that as of yet, we do not know the mechanism by which PHMG-induced increase in CCL11 directly results in enhanced profibrogenic activity. Importantly, a direct relationship between CCL11 and SERPINF1 has not been identified to date. Further studies will therefore be needed to confirm this relationship and more precisely elucidate the biological mechanism. Additionally, our results revealed that PHMG treatment without OVA sensitization or challenge did not significantly impact on pulmonary inflammation or damage and showed an outcome similar to that observed in the control group. This seems to be related to the lower concentration of PHMG (0.1 mg/kg) used in our experiments compared to other studies (0.3-1.5 mg/kg). PHMG exposure can increase the risk of lung injury, depending on its duration and dose and can affect lung health several years after exposure.5,7,47,48 Importantly, when present at low concentrations (0.3 mg/kg), PHMG is not removed from the lungs and continuously stimulates immune and/or epithelial cells, thereby causing delayed onset of inflammation.5 Here, no inflammation was observed at the end of the experiment, probably as a result of delayed inflammatory response.
In conclusion, the exposure of the airways to PHMG results in aggravated BHR and lung inflammation via CCL11- and SERPINF1-induced signaling in mice, suggesting that PHMG exposure exacerbates asthma. However, even healthy people may develop lung diseases including asthma, a phenomenon that warrants further investigation in future studies.
ACKNOWLEDGMENTS
This study was supported by a grant from the National Institute of Environment Research (NIER) funded by the Ministry of Environment (MOE) of the Republic of Korea (NIER-SP2020-007).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIAL
References
- 1.Aleshina EY, Yudanova TN, Skokova IF. Production and properties of polyvinyl alcohol spinning solutions containing protease C and polyhexamethylene guanidine. Fibre Chem. 2001;33:421–423. [Google Scholar]
- 2.Korea Centers for Disease Control and Prevention. Interim report of epidemiological investigation on lung injury with unknown cause in Korea. Public Health Wkly Rep. 2011;4:817–832. [Google Scholar]
- 3.Suda T, Sato A, Ida M, Gemma H, Hayakawa H, Chida K. Hypersensitivity pneumonitis associated with home ultrasonic humidifiers. Chest. 1995;107:711–717. doi: 10.1378/chest.107.3.711. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Kim YH, Kwon JH. Fatal misuse of humidifier disinfectants in Korea: importance of screening risk assessment and implications for management of chemicals in consumer products. Environ Sci Technol. 2012;46:2498–2500. doi: 10.1021/es300567j. [DOI] [PubMed] [Google Scholar]
- 5.Song JA, Park HJ, Yang MJ, Jung KJ, Yang HS, Song CW, et al. Polyhexamethyleneguanidine phosphate induces severe lung inflammation, fibrosis, and thymic atrophy. Food Chem Toxicol. 2014;69:267–275. doi: 10.1016/j.fct.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Kim HR, Lee K, Park CW, Song JA, Shin DY, Park YJ, et al. Polyhexamethylene guanidine phosphate aerosol particles induce pulmonary inflammatory and fibrotic responses. Arch Toxicol. 2016;90:617–632. doi: 10.1007/s00204-015-1486-9. [DOI] [PubMed] [Google Scholar]
- 7.Yoon J, Lee SY, Lee SH, Kim EM, Jung S, Cho HJ, et al. Exposure to humidifier disinfectants increases the risk for asthma in children. Am J Respir Crit Care Med. 2018;198:1583–1586. doi: 10.1164/rccm.201805-0840LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy DM, O'Byrne PM. Recent advances in the pathophysiology of asthma. Chest. 2010;137:1417–1426. doi: 10.1378/chest.09-1895. [DOI] [PubMed] [Google Scholar]
- 9.Bergeron C, Tulic MK, Hamid Q. Airway remodelling in asthma: from benchside to clinical practice. Can Respir J. 2010;17:e85–93. doi: 10.1155/2010/318029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folli C, Descalzi D, Scordamaglia F, Riccio AM, Gamalero C, Canonica GW. New insights into airway remodelling in asthma and its possible modulation. Curr Opin Allergy Clin Immunol. 2008;8:367–375. doi: 10.1097/ACI.0b013e32830a7086. [DOI] [PubMed] [Google Scholar]
- 11.Walters EH, Soltani A, Reid DW, Ward C. Vascular remodelling in asthma. Curr Opin Allergy Clin Immunol. 2008;8:39–43. doi: 10.1097/ACI.0b013e3282f42696. [DOI] [PubMed] [Google Scholar]
- 12.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–709. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 13.Acevedo N, Zakzuk J, Caraballo L. House dust mite allergy under changing environments. Allergy Asthma Immunol Res. 2019;11:450–469. doi: 10.4168/aair.2019.11.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaakkola JJ, Knight TL. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis. Environ Health Perspect. 2008;116:845–853. doi: 10.1289/ehp.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E, Song DJ, Kim WK, Suh DI, Baek HS, Shin M, et al. Associated factors for asthma severity in Korean children: a Korean childhood asthma study. Allergy Asthma Immunol Res. 2020;12:86–98. doi: 10.4168/aair.2020.12.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin X, Ren X, Xiao X, Yang Z, Yao S, Wong GW, et al. Important role of immunological responses to environmental exposure in the development of allergic asthma. Allergy Asthma Immunol Res. 2020;12:934–948. doi: 10.4168/aair.2020.12.6.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donaldson K, Gilmour MI, MacNee W. Asthma and PM10. Respir Res. 2000;1:12–15. doi: 10.1186/rr5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gowers AM, Cullinan P, Ayres JG, Anderson HR, Strachan DP, Holgate ST, et al. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence; a review. Respirology. 2012;17:887–898. doi: 10.1111/j.1440-1843.2012.02195.x. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Takano H, Yanagisawa R, Sakurai M, Ichinose T, Sadakane K, et al. Effects of nano particles on antigen-related airway inflammation in mice. Respir Res. 2005;6:106. doi: 10.1186/1465-9921-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue K, Takano H, Yanagisawa R, Ichinose T, Sakurai M, Yoshikawa T. Effects of nano particles on cytokine expression in murine lung in the absence or presence of allergen. Arch Toxicol. 2006;80:614–619. doi: 10.1007/s00204-006-0075-3. [DOI] [PubMed] [Google Scholar]
- 21.Koike E, Takano H, Inoue KI, Yanagisawa R, Sakurai M, Aoyagi H, et al. Pulmonary exposure to carbon black nanoparticles increases the number of antigen-presenting cells in murine lung. Int J Immunopathol Pharmacol. 2008;21:35–42. doi: 10.1177/039463200802100105. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Takano H, Yanagisawa R, Sakurai M, Abe S, Yoshino S, et al. Effects of nanoparticles on lung physiology in the presence or absence of antigen. Int J Immunopathol Pharmacol. 2007;20:737–744. doi: 10.1177/039463200702000409. [DOI] [PubMed] [Google Scholar]
- 23.de Haar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy. 2006;36:1469–1479. doi: 10.1111/j.1365-2222.2006.02586.x. [DOI] [PubMed] [Google Scholar]
- 24.Jang SO, Kim HJ, Kim YJ, Kang MJ, Kwon JW, Seo JH, et al. Asthma prevention by Lactobacillus rhamnosus in a mouse model is associated with CD4(+)CD25(+)Foxp3(+) T cells. Allergy Asthma Immunol Res. 2012;4:150–156. doi: 10.4168/aair.2012.4.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson AJ, Shields MD, Patterson CC. Acute asthma exacerbations and air pollutants in children living in Belfast, Northern Ireland. Arch Environ Health. 2001;56:234–241. doi: 10.1080/00039890109604447. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118:3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, et al. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J Immunol. 1999;163:6321–6329. [PubMed] [Google Scholar]
- 28.Dorman SC, Babirad I, Post J, Watson RM, Foley R, Jones GL, et al. Progenitor egress from the bone marrow after allergen challenge: role of stromal cell-derived factor 1alpha and eotaxin. J Allergy Clin Immunol. 2005;115:501–507. doi: 10.1016/j.jaci.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Bocchino V, Bertorelli G, Bertrand CP, Ponath PD, Newman W, Franco C, et al. Eotaxin and CCR3 are upregulated in exacerbations of chronic bronchitis. Allergy. 2002;57:17–22. [PubMed] [Google Scholar]
- 30.Kindstedt E, Holm CK, Sulniute R, Martinez-Carrasco I, Lundmark R, Lundberg P. CCL11, a novel mediator of inflammatory bone resorption. Sci Rep. 2017;7:5334. doi: 10.1038/s41598-017-05654-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Draijer C, Peters-Golden M. Alveolar macrophages in allergic asthma: the forgotten cell awakes. Curr Allergy Asthma Rep. 2017;17:12. doi: 10.1007/s11882-017-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puxeddu I, Bader R, Piliponsky AM, Reich R, Levi-Schaffer F, Berkman N. The CC chemokine eotaxin/CCL11 has a selective profibrogenic effect on human lung fibroblasts. J Allergy Clin Immunol. 2006;117:103–110. doi: 10.1016/j.jaci.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 33.Isgrò M, Bianchetti L, Marini MA, Bellini A, Schmidt M, Mattoli S. The C-C motif chemokine ligands CCL5, CCL11, and CCL24 induce the migration of circulating fibrocytes from patients with severe asthma. Mucosal Immunol. 2013;6:718–727. doi: 10.1038/mi.2012.109. [DOI] [PubMed] [Google Scholar]
- 34.Huaux F, Gharaee-Kermani M, Liu T, Morel V, McGarry B, Ullenbruch M, et al. Role of eotaxin-1 (CCL11) and CC chemokine receptor 3 (CCR3) in bleomycin-induced lung injury and fibrosis. Am J Pathol. 2005;167:1485–1496. doi: 10.1016/S0002-9440(10)61235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin ES, Sorenson CM, Sheibani N. PEDF expression regulates the proangiogenic and proinflammatory phenotype of the lung endothelium. Am J Physiol Lung Cell Mol Physiol. 2014;306:L620–34. doi: 10.1152/ajplung.00188.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosgrove GP, Brown KK, Schiemann WP, Serls AE, Parr JE, Geraci MW, et al. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170:242–251. doi: 10.1164/rccm.200308-1151OC. [DOI] [PubMed] [Google Scholar]
- 37.Shaw JG, Vaughan A, Dent AG, O'Hare PE, Goh F, Bowman RV, et al. Biomarkers of progression of chronic obstructive pulmonary disease (COPD) J Thorac Dis. 2014;6:1532–1547. doi: 10.3978/j.issn.2072-1439.2014.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spira A, Beane J, Pinto-Plata V, Kadar A, Liu G, Shah V, et al. Gene expression profiling of human lung tissue from smokers with severe emphysema. Am J Respir Cell Mol Biol. 2004;31:601–610. doi: 10.1165/rcmb.2004-0273OC. [DOI] [PubMed] [Google Scholar]
- 40.Meyer C, Notari L, Becerra SP. Mapping the type I collagen-binding site on pigment epithelium-derived factor. Implications for its antiangiogenic activity. J Biol Chem. 2002;277:45400–45407. doi: 10.1074/jbc.M208339200. [DOI] [PubMed] [Google Scholar]
- 41.Bao K, Reinhardt RL. The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine. 2015;75:25–37. doi: 10.1016/j.cyto.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mentink-Kane MM, Wynn TA. Opposing roles for IL-13 and IL-13 receptor alpha 2 in health and disease. Immunol Rev. 2004;202:191–202. doi: 10.1111/j.0105-2896.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 43.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forbes E, van Panhuys N, Min B, Le Gros G. Differential requirements for IL-4/STAT6 signalling in CD4 T-cell fate determination and Th2-immune effector responses. Immunol Cell Biol. 2010;88:240–243. doi: 10.1038/icb.2009.101. [DOI] [PubMed] [Google Scholar]
- 45.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–456. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- 46.Junttila IS, Mizukami K, Dickensheets H, Meier-Schellersheim M, Yamane H, Donnelly RP, et al. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Ralpha, IL-13Ralpha1, and gammac regulates relative cytokine sensitivity. J Exp Med. 2008;205:2595–2608. doi: 10.1084/jem.20080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park JH, Kim HJ, Kwon GY, Gwack J, Park YJ, Youn SK, et al. Humidifier disinfectants are a cause of lung injury among adults in South Korea: a community-based case-control study. PLoS One. 2016;11:e0151849. doi: 10.1371/journal.pone.0151849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park DU, Ryu SH, Roh HS, Lee E, Cho HJ, Yoon J, et al. Association of high-level humidifier disinfectant exposure with lung injury in preschool children. Sci Total Environ. 2018;616-617:855–862. doi: 10.1016/j.scitotenv.2017.10.237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.