Abstract
Purpose
A defective epithelial barrier has been demonstrated in chronic rhinosinusitis with nasal polyps (CRSwNP). Lactobacilli are shown to restore epithelial barrier defects in gastrointestinal disorders, but their effect on the airway epithelial barrier is unknown. In this study, hence, we evaluated whether the nasopharyngeal isolates Lacticaseibacillus casei AMBR2 and Latilactobacillus sakei AMBR8 could restore nasal epithelial barrier integrity in CRSwNP.
Methods
Ex vivo trans-epithelial tissue resistance and fluorescein isothiocyanate-dextran 4 kDa (FD4) permeability of nasal mucosal explants were measured. The relative abundance of lactobacilli in the maxillary sinus of CRSwNP patients was analyzed by amplicon sequencing of the V4 region of the 16S rRNA gene. The effect of spray-dried L. casei AMBR2 and L. sakei AMBR8 on epithelial integrity was investigated in vitro in primary nasal epithelial cells (pNECs) from healthy controls and patients with CRSwNP as well as in vivo in a murine model of interleukin (IL)-4 induced barrier dysfunction. The activation of Toll-like receptor 2 (TLR2) was explored in vitro by using polyclonal antibodies.
Results
Patients with CRSwNP had a defective epithelial barrier which positively correlated with the relative abundance of lactobacilli-specific amplicons in the maxillary sinus. L. casei AMBR2, but not L. sakei AMBR8, increased the trans-epithelial electrical resistance (TEER) of pNECs from CRSwNP patients in a time-dependent manner. Treatment of epithelial cells with L. casei AMBR2 promoted the tight junction proteins occludin and zonula occludens-1 reorganization. Furthermore, L. casei AMBR2 prevented IL-4-induced nasal permeability in vivo and in vitro. Finally, the beneficial effect of L. casei AMBR2 on nasal epithelial cells in vitro was TLR2-dependent as blocking TLR2 receptors prevented the increase in TEER.
Conclusions
A defective epithelial barrier in CRSwNP may be associated with a decrease in relative abundance of lactobacilli-specific amplicons. L. casei AMBR2 would restore nasal epithelial integrity and can be a novel therapeutic strategy for CRSwNP.
Keywords: Lactobacillus casei, lactobacillus sakei, sinusitis, nasal polyps, epithelium, tight junctions, permeabilty, toll like receptor 2
INTRODUCTION
The nasal epithelial barrier is a pseudostratified epithelium that forms a physical and immunological barrier.1 The integrity of the nasal epithelium is maintained via intercellular junctions such as tight junctions (TJs), adherens junctions and desmosomes. TJ proteins regulate the mucosal transport of water, ions and certain molecules,1 and consist of different transmembrane proteins, including the claudin family and occludin, as well as cytoplasmic proteins such as the zonula occludens (ZO)-1, ZO-2 and ZO-3.1 Disturbance in the formation, expression or function of TJs can result in a temporal opening of the epithelium, facilitating the penetration of foreign molecules, such as allergens, to the submucosal region. Indeed, impaired epithelial barrier function as a result of a decreased expression of occludin and ZO-1 has been reported in different chronic airway diseases such as chronic rhinosinusitis (CRS).2 CRS is an immune disorder of the sinonasal mucosa, which is generally divided into two phenotypes: CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP).3 Moreover, bacterial translocation of Streptococcus pneumoniae and Haemophilus influenzae across the epithelium is demonstrated in mice along with a decrease in claudin-7 and -10 expression by activating Toll-like receptor (TLR)-2 and TLR4 signaling, respectively.4 Similarly, incubation of human nasal epithelial cells with Staphylococcus aureus V8 protease disrupted epithelial integrity via ZO-1 delocalization.5 In contrast, certain beneficial bacteria, such as specific strains of lactobacilli, appear to have the capacity to restore a defective epithelial barrier both in vitro as in vivo, especially in the gastrointestinal tract.6,7,8,9 The lactobacilli encompasses the strains, species and genera of Lactobacillaceae, one of the best studied groups of beneficial bacteria, recently reclassified in 23 genera.10 Lactobacillus plantarum MB452 (new genus name Lactiplantibacillus plantarum MB452) has been shown to promote the expression of occludin and ZO-1.11 Abreu et al.12 demonstrated a reduction in the relative abundance of taxa belonging to the order of Lactobacilliales in the maxillary sinus of CRS patients.12 Recently, we have also demonstrated that CRS patients have a reduced level of lactobacilli in the nose, with isolates such as Lacticaseibacillus casei AMBR2 having specific properties promoting its adaptation and survival in this human body site.13
In addition to the effects on epithelial integrity, lactobacilli also induce other health effects in the host, including modulation of local and systemic host immune responses.14 For this purpose, lactobacilli interact with pattern recognition receptors, such as TLRs present on the epithelium.15,16 Activation of TLRs can induce different signaling cascades that mount a proper immune response against the microorganisms detected.14 In vivo modulation of TLR9 expression by Lactobacillus reuteri ATCC 23272 (new taxonomy Limosilactobacillus reuteri ATCC 23272) resulted in reduced levels of interleukin (IL)-5 and IL-6 and attenuated allergic inflammation in the lungs of ovalbumin-sensitized mice.17 As for immunomodulatory effects of lactic acid bacteria such as lactobacilli, TLR2 has been studied in most detail.14,18. This receptor plays a pivotal role in maintaining immune homeostasis. More specifically, TLR2 either dimerizes with TLR1 or TLR6 to induce a pro-inflammatory or anti-inflammatory response, respectively.18,19,20 Changes in TLR expression have been reported in CRS patients.21,22 However, the interactions between lactobacilli and TLRs in the upper and/or lower airways are still underexplored.
The aim of the present study was to investigate the effect of the nasopharyngeal lactobacilli isolates L. casei AMBR2 and Latilactobacillus sakei AMBR8 on the epithelial barrier function of primary nasal epithelial cells (pNECs) of patients with CRSwNP and in a mouse model of IL-4 induced barrier dysfunction. Our results show that a decreased relative abundance of lactobacilli correlated with impaired epithelial barrier integrity in CRSwNP. Additionally, L. casei AMBR2 restores the nasal epithelial barrier in CRSwNP via TLR2 activation in vitro. Finally, L. casei AMBR2 prevents IL-4-induced barrier dysfunction in a mouse model.
MATERIALS AND METHODS
Illumina MiSeq V4 16S rRNA amplicon sequencing to determine the relative abundance of lactobacilli
This study was approved by the Institutional Review Board of University Hospitals of Leuven and Antwerp (study B300201524257). Nasal swabs from the maxillary sinus were obtained from CRS patients (n = 14) during functional endoscopic sinus surgery (FESS) as previously described previously.13,23 Samples were processed and analyzed by performing the dual-index paired-end sequencing on the V4 region of the 16S rRNA gene on the MiSeq Desktop sequencer (M00984; Illumina, Seoul, Korea) as previously described.13,23 The relative abundance of lactobacilli (family Lactobacilllaceae) was determined at the genus level. The sequencing data were deposited in ENA under accession number PRJEB30316.
Ussing chamber experiments for the evaluation of mucosal explant integrity
Sinus tissues from 14 non-smoking CRSwNP patients were collected during FESS, of whom 36% had an allergy, 43% had concomitant asthma and 36% underwent a previous FESS. The inferior turbinate was collected from 7 non-allergic, non-smoking, non-asthmatic healthy controls during esthetic rhinoplasty, of whom 14% underwent a previous esthetic and/or function rhinoplasty. Biopsies, in duplicate, were mounted in Ussing chambers to evaluate epithelial integrity ex vivo.24 Briefly, trans-epithelial tissue resistance (TER) was recorded for 2 hours and the average of all time points was calculated and are presented as Ω × cm2. Fluorescein isothiocyanate-dextran 4kDa (FD4, 1 mg/mL) (Sigma-Aldrich, St Louis, MO, USA) was added to the mucosal compartment. Serosal samples were collected every 30 minutes over 2 hours to evaluate fluorescence intensity with a fluorescence reader (FLUOstar Omega; BMG Labtech, Ortenberg, Germany). Patients' demographics are be found in Table 1.
Table 1. Patient demographics.
| Characteristics | Control | CRSwNP | P-value |
|---|---|---|---|
| No. of patients | 7 | 14 | - |
| Mean age with SD (yr) | 29 ± 11 | 44 ± 13 | 0.0195 |
| Sex (male/female) | 4/3 | 10/4 | - |
| Allergy (%) | 0 | 36 | - |
| Smoking (%) | 0 | 0 | - |
| Asthma (%) | 0 | 43 | - |
| Nasal polyps (%) | 0 | 100 | - |
| Prior surgical history (%) | 14 | 36 | - |
CRSwNP, chronic rhinosinusitis with nasal polyps; SD, standard devitaion.
Isolation of primary epithelial cells from nasal polyp tissue and inferior turbinate
Nasal polyp tissue from patients with CRSwNP and inferior turbinate of healthy controls were used to isolate pNECs. A highly purified epithelial cell population was obtained as previously reported.25 Isolated pNECs were grown in bronchial epithelial basal medium (Lonza BioWhittaker, Basel, Switzerland) supplemented with the SingleQuot Kit in a T75 culture flask at 37°C. Once cells reached 75%–80% confluency, cells were detached and seeded on Transwell inserts (Greiner Bio-One, Vilvoorde, Belgium) at a concentration of 110,000 cells/transwell. After 5–7 days, a confluent monolayer was obtained and medium was removed apically to allow further cell differentiation at air-liquid interphase (ALI). Then, pNECs were cultured in Dulbecco's modified Eagle's medium (DMEM/F12; Lonza BioWhittaker) supplemented with 100 U/mL penicillin, 100 g/mL streptomycin and 2% Ultroser G (Pall Life Sciences, Zaventem, Belgium). Medium was changed every other day.
Trans-epithelial electrical resistance (TEER) and paracellular flux measurements
The methodology for TEER and paracellular flux measurements of FD4 has previously been described.25 In brief, the epithelial integrity of ALI cultures was evaluated by measuring the TEER with an EVOM/Endohm (WPI, Sarasota, FL, USA) as described in the online data supplement. Paracellular flux measurements were made by applying FD4 (2 mg/mL) apically and measuring the FD4 intensity of the basolateral medium at different time points with a fluorescence reader (FLUOstar Omega; BMG LABTECH, Ortenberg, Germany). FD4 concentration was calculated and is expressed as pmols.
Spray drying process
L. casei AMBR2 and L. sakei AMBR8 were isolated from the nasopharynx of a healthy individual as part of the study B300201524257.13,23,26 The strains were grown at 37°C without agitation in the Man, Rogosa and Sharpe (MRS) broth (Difco, Erembodegem, Belgium). For spray-drying, the bacterial suspension was centrifuged at 3,983 × g for 12 minutes at 20°C after L. casei AMBR2 and L. sakei AMBR8 reached the stationary phase. The pH of the supernatant was measured as quality control. The bacterial pellet was re-suspended to its original volume in phosphate-buffered saline (PBS) or demineralized water. PBS medium contained 0.3 g/L NaH2PO4.2H2O, 1.54 g/L Na2HPO4.2H2O and 8.2g/L Sodium chloride (NaCl), and the pH was adjusted to 7.2. Sodium chloride (NaCl) was purchased from Carl Roth (Mühlburg, Germany), sodium dihydrogen phosphate dihydrate (NaH2PO4.2H2O) and disodium hydrogen phosphate dihydrate (Na2HPO4.2H2O) of analytical grade were acquired from Merck (Darmstadt, Germany). Spray drying was done using a laboratory-scale spray dryer (B-290; Büchi, Flawil, Switzerland). A co-current spray dryer configuration was used, and the feed was sprayed into the heated drying chamber using a 2-fluid nozzle (orifice diameter 1.4 mm). The spray drying parameters applied during the experiments were: inlet temperature of 135°C, feed rate of 25% (7.5 mL/min), atomization–spray flow rate of 45 mm (831 L/h), airflow rate of approximately 32.5 m3/h and outlet temperature of 55°C. The spray-dried powder was collected from a single cyclone separator, and stored in Eppendorf tubes (VWR International Europe, Leuven, Belgium), sealed with Parafilm®, and kept at 4°C.
Viability after spray drying, expressed in CFU/g, was evaluated via plate counting. The spray-dried powder was re-suspended in an appropriate medium (i.e., PBS or demineralized water) for several minutes at room temperature. Serial dilutions were prepared prior to plating onto MRS agar plates, followed by incubation at 37°C for 2 days.
In vitro stimulation experiments with spray-dried L. casei AMBR2 and L. sakei AMBR8
ALI cultures of healthy controls and CRSwNP patients were stimulated on day 21 with L. casei AMBR2 or L. sakei AMBR8 at 105 CFU/mL, 106 CFU/mL or 107 CFU/mL, at the apical site for 6 hours. Human TLR2 polyclonal antibody (10 µg/mL; R&D Systems, Abingdon, UK) was added apically 2 hours prior to stimulation with L. casei AMBR2 (107 CFU/mL) in ALI cultures of CRSwNP patients. At time points 0, 2, 4 and 6 hours, TEER was measured. The barrier restoring effects of L. casei AMBR2 were evaluated by adding IL-4 (10 ng/mL; R&D systems) 24 hours prior to stimulation with L. casei AMBR2 (107 CFU/mL) in ALI cultures of CRSwNP patients. TEER was measured for 6 hours.
TLR2-signaling assay
Human TLR2+TLR6/NF-κB/SEAP reporter HEK293 cells (InvivoGen, San Diego, CA, USA) and human TLR2+TLR1/NF-κB/SEAP reporter HEK293 cells (InvivoGen) were grown according to the manufacturer's recommendations. HEK cells were seeded on a 96 well plate when 70%–80% confluency was reached. After 48 hours, HEK cells were stimulated for 24 hours with spray-dried L. casei AMBR2 (107 CFU/mL), Pam2CSK4 (1 ng/mL) or Pam3CSK4 (1 µg/L). The induction of TLR2 signaling was assessed by measuring the pNPP in the supernatant of stimulated HEK cells. Therefore, 50 µL of the supernatant from stimulated HEK cells was added onto a 96 well plate and 100 µL of pNPP solution was added. After 20 minutes, absorbance was measured at 450 nm.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR) for TJ expression
The full methodology for mRNA isolation and RT-qPCR are described in the online data supplement. Primer sequences are found in Supplementary Table S1 in the online data supplement.
Immunofluorescence staining of TJs
ALI cultures were fixed with 4% paraformaldehyde (Sigma-Aldrich) after stimulation with L. casei AMBR2. Cultures were stained for occludin (mouse anti-occludin antibody; ThermoFisher, Waltham, MA, USA) which was detected with anti-mouse antibody Alexa Fluor 488 (ThermoFisher) and ZO-1 (rabbit: anti-ZO-1 antibody; ThermoFisher) which was detected with goat anti-rabbit antibody Alexa Fluor 555 (ThermoFisher). Finally, samples were stained with 4′-6-diamidino-2-phenylindole dihydrochloride (DAPI; ThermoFisher) and mounted on microscope slides. Images were recorded on a Zeiss LSM 780 – SP Mai Tai HP DS with Z-stacking (12 slides with a thickness of 11 µm for each section were used for creating the Z stack) (Cell and Tissue Imaging Cluster, Supported by Hercules AKUL/11/37 and FWO G.0929.15 to Pieter Vanden Berghe, University of Leuven). Images were processed using ImageJ (Java; NIH, Bethesda, MD, USA). The immunostainings were performed on 3 controls and 3 CRSwNP patients. From these controls and patients, several regions of interest were chosen, and a representative image of the specific condition was used in the figures.
Evaluation of the effect of spray-dried L. casei AMBR2 and L. sakei AMBR8 in a mouse model of IL-4-induced barrier dysfunction
Male BALB/c mice (6–8 weeks) were obtained from Envigo (Horst, The Netherlands) and were kept under conventional conditions. Experimental procedures were approved by the Ethical Committee for Animal Research at the KU Leuven (P015/2017). Mice were pretreated twice with 20 µL of spray-dried L. casei AMBR2 (107 CFU/mL), spray-dried L. sakei AMBR8 (107 CFU/mL) or PBS, 48 and 24 hours prior to IL-4 application. Next, the mice received 50 µL of IL-4 (250 ng/application) or PBS 3 times, at 1-hour intervals. Twenty-four hours after the last nasal application, 20 µL of FD4 (50 mg/mL) was applied endonasally for the evaluation of mucosal permeability. One hour after FD4 application, mice were sacrificed with an intraperitoneal injection of Dolethal (Vétoquinol S.A., Lure, France). Serum and nasal mucosae were collected for further analysis. The Levels of FD4 were determined in the serum by a fluorescence reader (FLUOstar Omega).
Statistical analysis
Data were analyzed using Graphpad Prism 7 (Graphpad, La Jolla, CA, USA). Normality was tested using the Shapiro-Wilk normality test. Differences between the 2 groups were analyzed using an Mann-Whitney test. Data are presented as means ± standard error of mean or medians with interquartile range. Differences between multiple groups were analyzed using One-way analysis of variance (ANOVA) with post hoc analysis. Two-way ANOVA with post hoc analysis was used to evaluate the effect of stimulation over time. Spearmen ρ measurements were used to determine correlations. Values were considered significantly different when P < 0.05.
RESULTS
Impaired barrier integrity in CRSwNP patients correlates with decreased relative abundance of lactobacilli
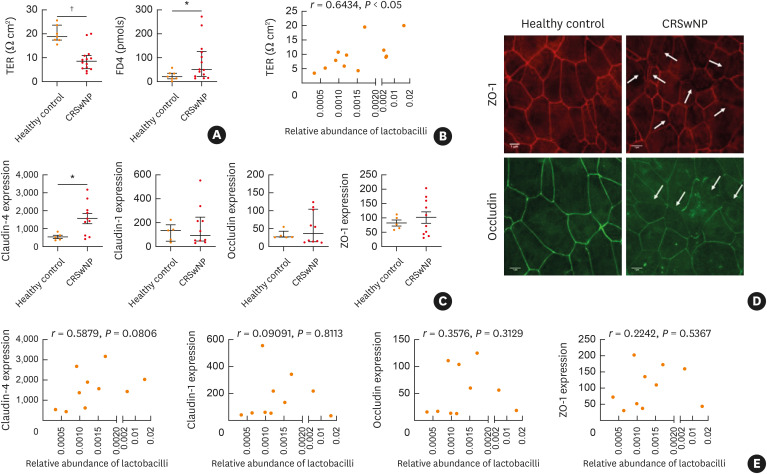
Previously, we have reported a lower relative abundance of lactobacilli in the anterior nares and nasopharynx of patients with CRSwNP and CRSsNP compared to healthy controls.13 Given the observation of an impaired epithelial barrier in CRS,2 we first investigated a possible correlation between nasal lactobacilli and nasal mucosal integrity. Therefore, mucosal biopsies were collected from 14 CRSwNP patients and 7 controls, and mucosal integrity was evaluated in Ussing chambers. A reduced TER, along with increased FD4 permeability, was found in the mucosal biopsy samples of CRSwNP patients compared to controls (Fig. 1A). Additionally, the relative abundance of lactobacilli in the maxillary sinuses of CRSwNP patients was determined by amplicon sequencing of V4 region of the 16S rRNA gene. The TER of the mucosal biopsy samples positively correlated with the relative abundance of lactobacilli (r = 0.6434, P < 0.05) in 12 CRSwNP patients, for which we obtained high-quality microbial profiles (Fig. 1B).
Fig. 1. Correlation between relative abundance of lactobacilli and mucosal integrity in patients with CRSwNP. (A) TER and FD4 permeability was measured on nasal biopsies collected from healthy controls and patients with CRSwNP. (B) Correlation between the relative abundance of lactobacilli and TER in patients with CRSwNP. (C) mRNA expression of claudin-4, claudin-1, occludin and ZO-1 in nasal biopsies of healthy controls (n = 5) and patients with CRSwNP (n = 10). Relative mRNA expression vs the housekeeping genes β-actin and β-2-microglobulin. (D) Representative immunofluorescence staining for occludin and ZO-1 in ALI cultures of healthy controls (n = 3) and CRSwNP patients (n = 3). White arrows indicate opening of the tight junctions (scale bar = 1 µm). (E) Correlation between the relative abundance of lactobacilli and TJ mRNA expression. Expression is relative to housekeeping genes β-actin and β-2-microglobulin. Data are presented as median with interquartile range for TER, FD4 measurements and the expression of occludin and claudin-1. Data are presented as mean ± SEM for claudin-4 and ZO-1 expression. Mann-Whitney test to analyze TER and FD4 passage in controls compared to CRSwNP patients. Spearman ρ for (C) and (D).
CRSwNP, chronic rhinosinusitis with nasal polyps; TER, trans-epithelial tissue resistance; FD4, fluorescein isothiocyanate-dextran 4 kDa; TJ, tight junction; SEM, standard error of mean; ZO-1, zonula occludens-1.
*P < 0.05; †P < 0.01.
To further explore the effect of lactobacilli on barrier integrity, we profiled the mRNA expression of different TJ proteins, i.e., occludin, ZO-1, claudin-1 and claudin-4, and correlated this with the relative abundance of lactobacilli of CRSwNP patients. We focused on these TJs, as their involvement in epithelial barrier dysfunction in CRSwNP has already been described.2 Interestingly, we observed an increased mRNA expression of claudin-4 in CRS patients compared to healthy controls (Fig. 1C). At the protein level, immunofluorescence staining for ZO-1 and occludin in ALI cultures of patients showed a decreased TJ architecture (Fig. 1D). Furthermore, we observed a positive, albeit insignificant, correlation between the claudin-4 mRNA expression and the relative abundance of lactobacilli (Fig. 1E).
L. casei AMBR2 increases TEER in patients with CRSwNP
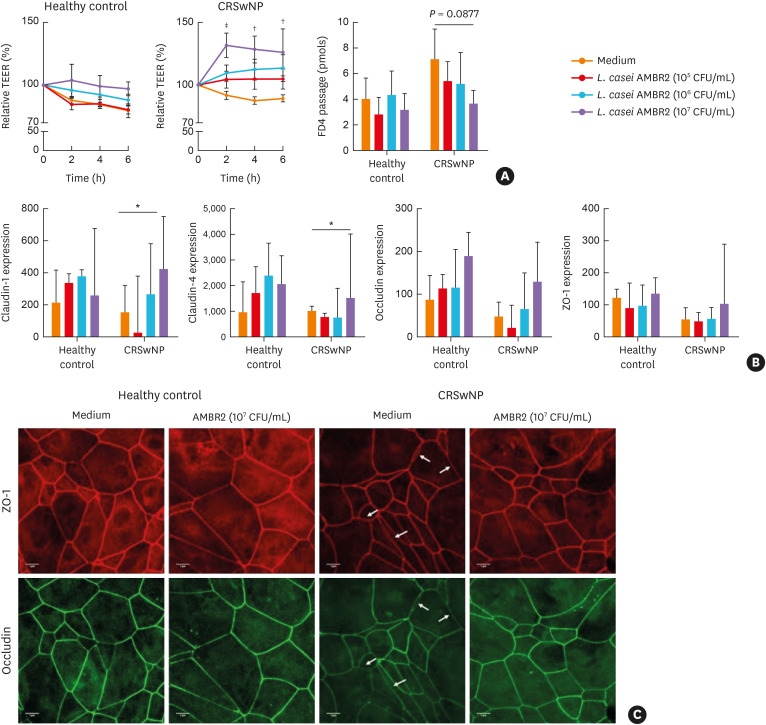
Since we observed an association between the relative abundance of lactobacilli and mucosal integrity in CRSwNP, we questioned whether lactobacilli could reconstitute a defective epithelial barrier. For this purpose, 2 spray-dried nasopharyngeal isolates, L. casei AMBR2 and L. sakei AMBR8, were used.13 ALI cultures of pNECs of healthy controls and patients with CRSwNP were stimulated for 6 hours with different concentrations of L. casei AMBR2 or L. sakei AMBR8. In ALI cultures of patients with CRSwNP, L. casei AMBR2 (107 CFU/mL) significantly increased TEER (Fig. 2A). FD4 permeability was also decreased after stimulation with L. casei AMBR2 (107 CFU/mL), though it did not reach significance (Fig. 2A). L. casei AMBR2 had no effect on TEER or FD4 permeability in ALI cultures of healthy controls (Fig. 2A). The isolate L. sakei AMBR8 did not alter TEER nor FD4 passage in ALI cultures of CRSwNP patients (Supplementary Fig. S1A and B).
Fig. 2. The effect of L. casei AMBR2 on nasal epithelial barrier function in vitro. Primary nasal epithelial cells from healthy controls and CRSwNP patients were grown at air-liquid interface for 21 days, and were stimulated with different concentrations of L. casei AMBR2 (both n = 6) for 6 hours. (A) Effect of different concentrations of L. casei AMBR2 on TEER and FD4 passage at time point six hours of epithelial cell cultures from healthy controls and patients with CRSwNP. (B) mRNA expression of occludin, ZO-1, claudin-1 and claudin-4 in nasal biopsies of healthy controls and patients with CRSwNP (both n = 5). Relative mRNA expression vs the housekeeping gene β-actin. (C) Representative immunofluorescence staining for occludin and ZO-1 in CRSwNP patients after treatment with L. casei AMBR2 (107 CFU/mL), compared to controls. White arrow indicates disrupted tight junction formation (scale bar = 1 μm). In (A), data are presented as mean ± SEM and in (B) data are presented as median with interquartile range. Two-way ANOVA with post hoc analysis for TER measurements in (A). One-way ANOVA with post hoc analysis for FD4 measurements in A and B.
CRSwNP, chronic rhinosinusitis with nasal polyps; TEER, trans-epithelial electrical resistance; FD4, fluorescein isothiocyanate-dextran 4 kDa; CFU, colony-forming unit; SEM, standard error of mean; ANOVA, analysis of variance; ZO-1, zonula occludens-1.
*P < 0.05; †P < 0.01; ‡P < 0.001.
Next, we evaluated the effect of L. casei AMBR2 on the mRNA expression of TJs in healthy controls and patients with CRSwNP. The effect of L. sakei AMBR8 on TJ expression and function was not investigated, as no effect on TEER and FD4 passage in ALI cultures of CRSwNP patients was found. RT-qPCR revealed a significant increase in the expression of claudin-1 and claudin-4 24 hours after stimulation of nasal explants from CRSwNP patients with 107 CFU/mL L. casei AMBR2 (Fig. 2B). At the protein level, immunofluorescence staining showed that L. casei AMBR2 promoted reorganization of occludin and ZO-1 after 6 hours in ALI cultures of CRSwNP patients (Fig. 2C).
L. casei AMBR2 restores nasal epithelial barrier integrity through activation of TLR2-TLR6
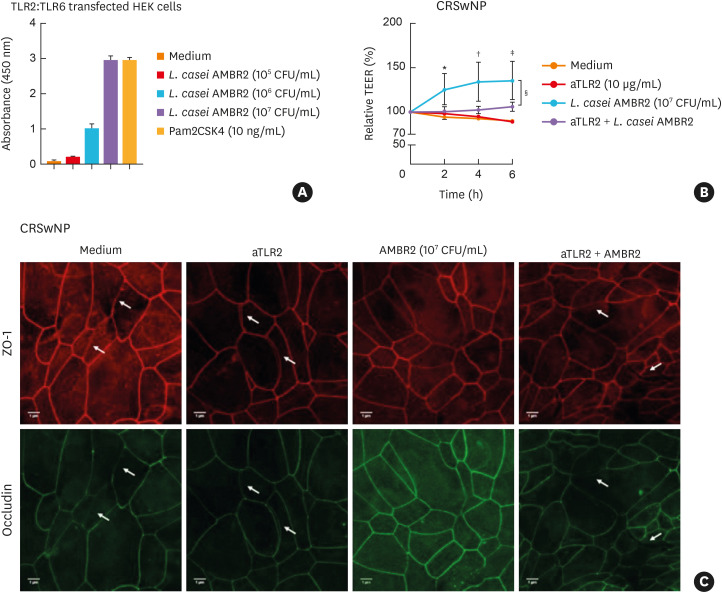
Lactobacilli modulate host immune responses via activation of TLR2 signaling.27 Given that L. casei AMBR2 reconstituted epithelial defects in CRSwNP, we questioned whether L. casei AMBR2 activates TLR2 to restore epithelial defects. Using TLR2-TRL6-transfected HEK293 cells, we verified a dose-dependent activation of TLR2/6 with L. casei AMBR2 (Fig. 3A), whereas TLR2-TLR1-transfected HEK293 cells were not activated (Supplementary Fig. S2). To validate the involvement of TLR2-activation in the L. casei AMBR2-mediated effect on epithelial integrity, ALI cultures from CRSwNP patients were pre-incubated with blocking anti-TLR2 polyclonal antibodies before stimulation with L. casei AMBR2 (107 CFU/mL). Pre-treatment with anti-TLR2 antibodies prevented L. casei AMBR2-mediated increase in TEER (Fig. 3B). At the protein level, pre-treatment with anti-TLR2 prevented occludin and ZO-1 reorganization in ALI cultures of CRSwNP patients (Fig. 3C).
Fig. 3. Role of TLR2 activation in L. casei AMBR2-induced epithelial barrier restoration in vitro. (A) Activation of TLR2:TLR6 receptor in transfected HEK293 cells by L. casei AMBR2. Pam2CSK4 and medium were used as positive and negative controls respectively. (B) Effect of 2 hours pre-treatment with human TLR2 polyclonal antibody before incubation with L. casei AMBR2 for 6 hours in ALI cultures of CRSwNP patients (n = 9). (C) Representative immunostainings for occludin and ZO-1 in ALI cultures from CRSwNP patients. White arrows indicate opening of tight junctions (scale bar = 1 μm). Data are presented as mean ± SEM. Two-way ANOVA with post hoc analysis for (B).
TLR, Toll-like receptor; TEER, trans-epithelial electrical resistance; CRSwNP, chronic rhinosinusitis with nasal polyps; ALI, air-liquid interphase; ANOVA, analysis of variance; CFU, colony-forming unit; ZO-1, zonula occludens-1.
*P < 0.05; †P < 0.01; ‡P < 0.001; §P < 0.05.
L. casei AMBR2 restores IL-4 induced barrier dysfunction
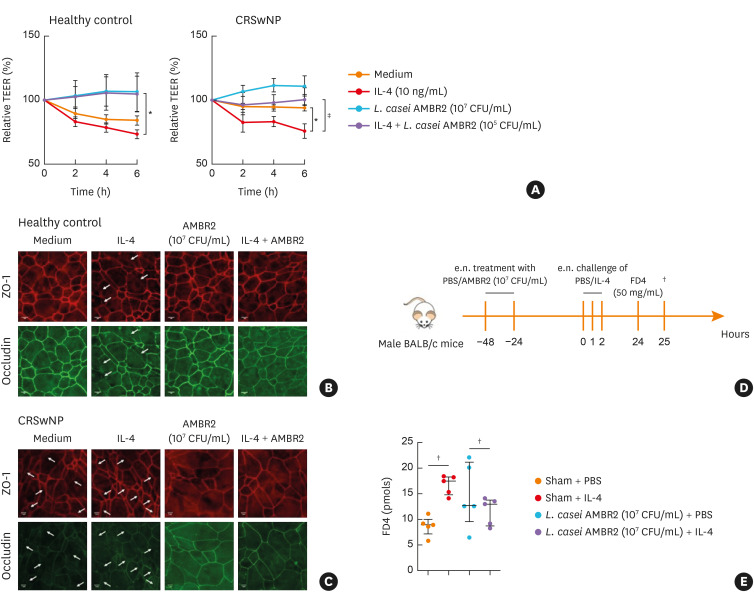
To confirm the barrier-restorative capacity of L. casei AMBR2 on barrier function, we explored whether L. casei AMBR2 could restore IL-4-induced barrier dysfunction in vitro. For this purpose, ALI cultures of controls and CRSwNP patients were stimulated with IL-4 for 24 hours. Next, L. casei AMBR2 (107 CFU/mL) was applied apically and TEER was measured for 6 hours. L. casei AMBR2 prevented further IL-4-mediated disruption of epithelial barrier integrity in ALI cultures of controls and CRSwNP patients (Fig. 4A). Immunofluorescence staining confirmed that L. casei AMBR2 restored ZO-1 and occludin protein reorganization (Fig. 4B and C).
Fig. 4. The effect of L. casei AMBR2 on IL-4 induced barrier dysfunction in vitro and in vivo. (A) Effect of L. casei AMBR2 with/without IL-4 on TEER of epithelial cell cultures of healthy controls (n = 5) and patients with CRSwNP patients (n = 6). (B, C) Representative immunostainings for occludin and ZO-1 from stimulated ALI cultures of healthy controls and patients with CRSwNP. White arrows indicate the opening of the tight junctions (scale bar = 1 μm). (D) Mice were endonasally pre-treated two times with L. casei AMBR2 (107 CFU/mL). Twenty-four hours later, IL-4 was applied three times with one hour interval. Twenty-four hours after the last IL-4 application, FD4 was applied endonasally to evaluate mucosal permeability in the serum of the treated mice. (E) Mucosal permeability for FD4. Data are presented as mean ± SEM. Two-way ANOVA with post hoc analysis for (A). Mann-Whitney test for (D).
CRSwNP, chronic rhinosinusitis with nasal polyps; TEER, trans-epithelial electrical resistance; IL, interleukin; FD4, fluorescein isothiocyanate-dextran 4 kDa; SEM, standard error of mean; ANOVA, analysis of variance; CFU, colony-forming unit; PBS, phosphate-buffered saline; ZO-1, zonula occludens-1.
*P < 0.05; †P < 0.01; ‡P < 0.001.
Finally, we evaluated the barrier preventive effect of L. casei AMBR2 in a mouse model of IL-4-induced barrier dysfunction.28 Mice were pre-treated endonasally with L. casei AMBR2 (107 CFU/mL) or L. sakei AMBR8 (107 CFU/mL) as controls, 48 and 24 hours prior to IL-4 application. FD4 passage was evaluated 24 hours after the last IL-4 instillation (Fig. 4D). Pre-treatment with L. casei AMBR2 prevented IL-4-induced barrier dysfunction (Fig. 4E). L. sakei AMBR8 could not prevent IL-4-induced barrier dysfunction in vivo (Supplementary Fig. S3).
DISCUSSION
Although not always present in high amounts, lactobacilli are commensals of the upper respiratory tract12,13 and are able to restore epithelial barrier integrity in chronic inflammatory disorders of the gut.7,11 However, little is known about the involvement of lactobacilli in maintaining an intact epithelial barrier in the airways. In this study, we investigated the possible correlations between the relative abundance of lactobacilli and the nasal epithelium in patients with CRSwNP.
Our data showed that patients with CRSwNP had a defective nasal epithelial barrier compared to healthy controls and confirmed previous findings.2 Furthermore, we observed that the relative abundance of lactobacilli in the maxillary sinuses positively correlated with mucosal integrity in these CRSwNP patients, suggesting that lactobacilli in the sinonasal cavity might play a role in maintaining an intact epithelial barrier, probably by modulating claudin-4 expression. In general, claudins are subdivided into 2 groups: pore-forming claudins like claudin-2 that increase epithelial permeability, and sealing claudins such as claudin-4 and claudin-5, which strengthen epithelial barrier function.29,30 Elevated claudin-4 expression might explain the strengthening of the epithelial barrier. Nevertheless, further research is warranted to confirm the involvement of lactobacilli in maintaining an intact epithelial barrier.
Considering the positive correlation between the relative abundance of lactobacilli and epithelial barrier function in CRSwNP, we next investigated whether administration of L. casei AMBR2 and L. sakei AMBR8 could improve barrier integrity. Our results showed that L. casei AMBR2 significantly increased epithelial integrity in ALI cultures of CRSwNP, mainly via the reorganization of TJ proteins. Interestingly, the barrier-enhancing effect of lactobacilli was only found with L. casei AMBR2, but not with L. sakei AMBR8, emphasizing bacterial species- and strain-specific actions such as differences in cell membrane components and/or production of metabolites. Similar positive effects of lactobacilli were seen on intestinal epithelial integrity and TJ expression with Lactiplantibacillus plantarum MB452 and Lacticaseibacillus rhamnosus GG.11 Of note, L. casei AMBR2 did not significantly alter epithelial integrity in ALI cultures of healthy individuals. We speculate that this discrepancy is related to a different inflammatory state of epithelial cells from healthy controls versus CRSwNP patients. More specifically, epithelial cells from healthy controls have an intact epithelial barrier,31 and have a lower expression of TLR2,21 and less production of pro-inflammatory cytokines IL-6 and IL-8.21
Lactobacilli interact with the epithelial barrier via TLRs to induce a proper immune response.32 As for, TLR2 has been studied in most detail.14 TLR2 signaling plays a pivotal role in maintaining immune homeostasis and an intact epithelial barrier, which depends on the dimerization with either TLR1 or TLR6.33,34,35,36 TLR2-TLR6 dimerization induces anti-inflammatory responses with the production of IL-10,18,19,20 whereas TLR2 and TLR1 dimerization results in a pro-inflammatory response with the production of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-8.18-20 As such, dysregulation of TLR2 function can lead to chronic inflammation. Indeed, in inflammatory bowel disease, distinctive changes in mucosal TLR expression in the intestinal epithelium have been demonstrated,37 with an inflammation-dependent induction of TLR2 and TLR4 expression in intestinal macrophages.38 In addition, changes in TLR expression have also been reported in CRS, which may be related to bacterial composition, diversity and abundance in the sinuses of these patients.21 We observed a dose-dependent effect of L. casei AMBR2 on TLR2 expression. Blocking TLR2 activation prevented the L. casei AMBR2-mediated restoration and reorganization of occludin and ZO-1 in ALI cultures of CRSwNP patients, suggesting a positive role for TLR2 in L. casei AMBR2 barrier promoting function. Moreover, pretreatment with anti-TLR2 antibodies prevented L. casei AMBR2 induced increase in TEER, confirming the involvement of TLR2 activation in the L. casei AMBR2-mediated effect on epithelial integrity. Additionally, stimulation of TLR2-TLR6-transfected HEK cells with L. casei AMBR2 suggests that activation of TLR2 by L. casei AMBR2 leads to dimerization between TLR2 and TLR6, which is in line with the result of a previous study on the interactions between different lactobacilli and TLRs.27 Nevertheless, further research is warranted to confirm if L. casei AMBR2 activates TLR2 directly by binding to the receptor or whether certain secreted metabolites are involved in TLR2 activation. We have preliminary data that L. casei AMBR2 has pili structures, which favors direct interaction, though we momentarily cannot exclude the contribution of metabolites.
CRSwNP is typically characterized by a type 2 immune response with increased levels of IL-5, IL-4 and IL-13.39,40 We and others have shown that IL-4 disrupts epithelial barrier integrity via decreasing the expression of TJs both in vitro as in vivo.28 As such, we evaluated the effect of L. casei AMBR2 on IL-4-induced barrier dysfunction. In vitro, L. casei AMBR2 restored IL-4-induced barrier dysfunction in ALI cultures of CRSwNP patients via reorganization of the TJ molecules occludin and ZO-1. In vivo, pre-treatment with L. casei AMBR2 prevented IL-4-induced increase in FD4 permeability. A possible mechanism could be that L. casei AMBR2 counteracts the effect of IL-4 on RhoGTPases. RhoGTPases are identified as major regulators of the NF-κB signaling pathway and play a role in modulating the actin cytoskeleton.41,42 Activation of the RhoGTPase signaling can lead to actinomyosin contractility or direct modification of the TJ transmembrane proteins by inducing phosphorylation and thereby resulting in increased permeability.41 IL-4 up-regulates RhoA protein expression, which promotes TJ breakdown. L. casei AMBR2, on the other hand, initiates the MyD88-dependent intracellular signaling pathway via TLR2. This pathway induces nuclear translocation of NF-κB to mediate several crucial cellular functions, including inflammatory cytokine production.14 There are some studies showing that TLR2 activation can modulate GTPases activity.43,44 As such, it is plausible that L. casei AMBR2 stimulates TLR2 which might lead to downregulation of RhoGTPase and in turn reorganization of the TJs, which counteracts the effect of IL-4 on the TJs.
In summary, our study demonstrates for the first time a positive correlation between epithelial barrier function and the relative abundance of lactobacilli in CRSwNP, and that lactobacilli maintain epithelial integrity by modulating TJ expression. Additionally, the nasopharynx isolates L. casei AMBR2 promotes nasal epithelial barrier function in patients with CRSwNP by reorganizing the expression of TJs, which is TLR2-TLR6 signaling-dependent. As such, this study highlights the potential of L. casei AMBR2 as a novel treatment strategy for CRSwNP.
ACKNOWLEDGMENTS
The authors would like to thank Professor Dr. Mark Jorissen and Professor Dr. Laura Van Gerven for their contribution in harvesting the sinonasal tissue for the cell cultures. We would also like to thank Ellen Dilissen for her insightful comments and technical advice, Dr. Camille Allonsius for her help with the TLR2-TLR6 transfected HEK cells and Stijn Wittouck for the help with processing and analyzing the Illumina sequencing data. Images were recorded on a Zeiss LSM 780 – SP Mai Tai HP DS (Cell and Tissue Imaging Cluster (CIC), supported by Hercules AKUL/11/37 and FWO G.0929.15 to Pieter Vanden Berghe, University of Leuven.
The author's laboratories are supported by grants from the Belgian Federal Government (IUAP P7/30), IWT (TBM project 130260) and the research council of the KU Leuven (GOA 14/011). P.W.H. is a recipient of a senior researcher fellowship from the Fund of Scientific Research (FWO), Flanders, Belgium. B.S. is a postdoctoral fellow of FWO. S.F.S. supported by the research council of KU Leuven (PDMK/14/189). S.L. currently holds a Starting Grant from the European Research Council, Lacto-Be (26850) on the ecology, evolutionary history and beneficial potential of lactobacilli.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Primer and probe sequences used for RT-qPCR
Effect of L. sakei AMBR8 on epithelial integrity in vitro. (A) Effect of different concentrations of L. sakei AMBR8 (106 and 105 CFU/mL) on TEER of epithelial cell cultures from healthy controls and patients with CRSwNP (both n = 4). (B) Effect of L. sakei AMBR8 on FD4 passage at time point 6 hours in ALI cultures of healthy controls and CRSwNP patients. Two-way ANOVA with post hoc analysis. Data are presented as mean ± SEM.
Role of TLR2 triggering in L. casei AMBR2 -induced epithelial barrier restoration in vitro. Effect of L. casei AMBR2 on TLR2/1 transfected reporter HEK cells. Pam3CSK4 was used as positive control. Pooled date from 2 experiments. Absorbance of pNPP was measured in the supernatant of HEK stimulated cells.
The effect of L. sakei AMBR8 on IL-4 induced barrier dysfunction in vivo. Mice were endonasally pre-treated 2 times with L. sakei AMBR8. Twenty-four hours later, IL-4 was applied 3 times with 1 hour interval. Twenty-four hours after the last IL-4 application, FD4 was applied endonasally to evaluate mucosal permeability in the serum of the treated mice. Mucosal permeability for FD4. Data are presented as mean ± SEM. One-way ANOVA with post hoc analysis.
References
- 1.Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. 2020;145:1499–1509. doi: 10.1016/j.jaci.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-γ and IL-4. J Allergy Clin Immunol. 2012;130:1087–1096.e10. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 3.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23:3 p preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 4.Clarke TB, Francella N, Huegel A, Weiser JN. Invasive bacterial pathogens exploit TLR-mediated downregulation of tight junction components to facilitate translocation across the epithelium. Cell Host Microbe. 2011;9:404–414. doi: 10.1016/j.chom.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy J, Ramezanpour M, Stach N, Dubin G, Psaltis AJ, Wormald PJ, et al. Staphylococcus aureus V8 protease disrupts the integrity of the airway epithelial barrier and impairs IL-6 production in vitro. Laryngoscope. 2018;128:E8–15. doi: 10.1002/lary.26949. [DOI] [PubMed] [Google Scholar]
- 6.Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52:827–833. doi: 10.1136/gut.52.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9:99–107. doi: 10.2174/1573401311309020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultana R, McBain AJ, O'Neill CA. Strain-dependent augmentation of tight-junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl Environ Microbiol. 2013;79:4887–4894. doi: 10.1128/AEM.00982-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298:G851–G859. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 10.Zheng J, Wittouck S, Salvetti E, Franz CM, Harris HM, Mattarelli P, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae . Int J Syst Evol Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 11.Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, et al. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10:316. doi: 10.1186/1471-2180-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abreu NA, Nagalingam NA, Song Y, Roediger FC, Pletcher SD, Goldberg AN, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med. 2012;4:151ra124. doi: 10.1126/scitranslmed.3003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Boeck I, van den Broek MFL, Allonsius CN, Spacova I, Wittouck S, Martens K, et al. Lactobacilli have a niche in the human nose. Cell Rep. 2020;37:107674. doi: 10.1016/j.celrep.2020.107674. [DOI] [PubMed] [Google Scholar]
- 14.Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 16.Macho Fernandez E, Valenti V, Rockel C, Hermann C, Pot B, Boneca IG, et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60:1050–1059. doi: 10.1136/gut.2010.232918. [DOI] [PubMed] [Google Scholar]
- 17.Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 2007;175:561–569. doi: 10.1164/rccm.200606-821OC. [DOI] [PubMed] [Google Scholar]
- 18.Netea MG, van de Veerdonk F, Verschueren I, van der Meer JW, Kullberg BJ. Role of TLR1 and TLR6 in the host defense against disseminated candidiasis. FEMS Immunol Med Microbiol. 2008;52:118–123. doi: 10.1111/j.1574-695X.2007.00353.x. [DOI] [PubMed] [Google Scholar]
- 19.DePaolo RW, Kamdar K, Khakpour S, Sugiura Y, Wang W, Jabri B. A specific role for TLR1 in protective TH17 immunity during mucosal infection. J Exp Med. 2012;209:1437–1444. doi: 10.1084/jem.20112339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DePaolo RW, Tang F, Kim I, Han M, Levin N, Ciletti N, et al. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 2008;4:350–361. doi: 10.1016/j.chom.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biswas K, Chang A, Hoggard M, Radcliff FJ, Jiang Y, Taylor MW, et al. Toll-like receptor activation by sino-nasal mucus in chronic rhinosinusitis. Rhinology. 2017;55:59–69. doi: 10.4193/Rhino16.201. [DOI] [PubMed] [Google Scholar]
- 22.Dong Z, Yang Z, Wang C. Expression of TLR2 and TLR4 messenger RNA in the epithelial cells of the nasal airway. Am J Rhinol. 2005;19:236–239. [PubMed] [Google Scholar]
- 23.De Boeck I, Wittouck S, Martens K, Claes J, Jorissen M, Steelant B, et al. Anterior nares diversity and pathobionts represent sinus microbiome in chronic rhinosinusitis. MSphere. 2019;4:e00532-19. doi: 10.1128/mSphere.00532-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanheel H, Vicario M, Vanuytsel T, Van Oudenhove L, Martinez C, Keita AV, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2014;63:262–271. doi: 10.1136/gutjnl-2012-303857. [DOI] [PubMed] [Google Scholar]
- 25.Steelant B, Farré R, Wawrzyniak P, Belmans J, Dekimpe E, Vanheel H, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016;137:1043–1053.e5. doi: 10.1016/j.jaci.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 26.De Boeck I, Wittouck S, Wuyts S, Oerlemans EF, van den Broek MF, Vandenheuvel D, et al. Comparing the healthy nose and nasopharynx microbiota reveals continuity as well as niche-specificity. Front Microbiol. 2017;8:2372. doi: 10.3389/fmicb.2017.02372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren C, Zhang Q, de Haan BJ, Zhang H, Faas MM, de Vos P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci Rep. 2016;6:34561. doi: 10.1038/srep34561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steelant B, Seys SF, Van Gerven L, Van Woensel M, Farré R, Wawrzyniak P, et al. Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol. 2018;141:951–963.e8. doi: 10.1016/j.jaci.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 29.Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol. 2006;22:85–89. doi: 10.1097/01.mog.0000203864.48255.4f. [DOI] [PubMed] [Google Scholar]
- 30.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–520. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steelant B. Epithelial dysfunction in chronic respiratory diseases, a shared endotype? Curr Opin Pulm Med. 2020;26:20–26. doi: 10.1097/MCP.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 32.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Podolsky DK, Gerken G, Eyking A, Cario E. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology. 2009;137:209–220. doi: 10.1053/j.gastro.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 37.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 39.De Greve G, Hellings PW, Fokkens WJ, Pugin B, Steelant B, Seys SF. Endotype-driven treatment in chronic upper airway diseases. Clin Transl Allergy. 2017;7:22. doi: 10.1186/s13601-017-0157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449–1456.e4. doi: 10.1016/j.jaci.2015.12.1324. [DOI] [PubMed] [Google Scholar]
- 41.Terry S, Nie M, Matter K, Balda MS. Rho signaling and tight junction functions. Physiology (Bethesda) 2010;25:16–26. doi: 10.1152/physiol.00034.2009. [DOI] [PubMed] [Google Scholar]
- 42.Montaner S, Perona R, Saniger L, Lacal JC. Multiple signalling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J Biol Chem. 1998;273:12779–12785. doi: 10.1074/jbc.273.21.12779. [DOI] [PubMed] [Google Scholar]
- 43.Shibolet O, Giallourakis C, Rosenberg I, Mueller T, Xavier RJ, Podolsky DK. AKAP13, a RhoA GTPase-specific guanine exchange factor, is a novel regulator of TLR2 signaling. J Biol Chem. 2007;282:35308–35317. doi: 10.1074/jbc.M704426200. [DOI] [PubMed] [Google Scholar]
- 44.Manukyan M, Nalbant P, Luxen S, Hahn KM, Knaus UG. RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-kappa B. J Immunol. 2009;182:3522–3529. doi: 10.4049/jimmunol.0802280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer and probe sequences used for RT-qPCR
Effect of L. sakei AMBR8 on epithelial integrity in vitro. (A) Effect of different concentrations of L. sakei AMBR8 (106 and 105 CFU/mL) on TEER of epithelial cell cultures from healthy controls and patients with CRSwNP (both n = 4). (B) Effect of L. sakei AMBR8 on FD4 passage at time point 6 hours in ALI cultures of healthy controls and CRSwNP patients. Two-way ANOVA with post hoc analysis. Data are presented as mean ± SEM.
Role of TLR2 triggering in L. casei AMBR2 -induced epithelial barrier restoration in vitro. Effect of L. casei AMBR2 on TLR2/1 transfected reporter HEK cells. Pam3CSK4 was used as positive control. Pooled date from 2 experiments. Absorbance of pNPP was measured in the supernatant of HEK stimulated cells.
The effect of L. sakei AMBR8 on IL-4 induced barrier dysfunction in vivo. Mice were endonasally pre-treated 2 times with L. sakei AMBR8. Twenty-four hours later, IL-4 was applied 3 times with 1 hour interval. Twenty-four hours after the last IL-4 application, FD4 was applied endonasally to evaluate mucosal permeability in the serum of the treated mice. Mucosal permeability for FD4. Data are presented as mean ± SEM. One-way ANOVA with post hoc analysis.