Abstract
Background:
Opioid agonist treatment (OAT) reduces many of the harms associated with opioid dependence. We use mathematical modelling to comprehensively evaluate the overall health benefits of OAT among people who inject drugs (PWID) in Kentucky (USA), Kyiv (Ukraine), and Tehran (Iran).
Methods:
We developed a dynamic model of HIV and HCV transmission, incarceration, and mortality through overdose, injury, suicide, disease-related or other causes. The model was calibrated to site-specific data using Bayesian methods. We evaluated ‘preventable drug-related deaths’ (‘pDRD’: HIV/HCV/overdose/suicide/injury) averted over 2020-2040 for four scenarios, added incrementally, compared to a scenario without OAT: existing OAT coverage (setting dependent; community: 4-11%; prison: 0-40%); scaling-up community OAT to 40% coverage; increasing average OAT duration from 4-14 months to 2 years; and scaling-up prison-based OAT.
Outcomes:
Drug-related harms contribute differentially to mortality across settings: overdose contributes 27-47% (range of median projections) of pDRDs over 2020-2040, suicide 6-17%, injury 3-17%, HIV 0-59% and HCV 2-18%. Existing OAT coverage in Tehran (31%) could have substantial impact, averting 13% pDRDs, but will have negligible impact (<2%) in Kyiv and Kentucky due to low OAT coverage (<4%). Scaling-up community OAT to 40% could avert 12-24% pDRDs, including 13-19% of overdose deaths, with greater impact in settings with significant HIV mortality (Tehran and Kyiv). Improving OAT retention and providing prison-based OAT would have significant additional impact, averting 27-48% pDRDs.
Interpretation:
OAT can substantially reduce drug-related harms, particularly in settings with HIV epidemics among PWID. Maximising these impacts requires research and investment into achieving higher coverage, longer retention and provision of OAT in prisons and the community.
Funding:
UK NIHR, NIDA
Introduction
Injecting drug use (IDU) causes multiple health harms including overdose death1 and blood borne virus transmission.1,2 Due to the criminalisation of illicit opioid use, many people who inject drugs (PWID) become incarcerated2 which causes further detrimental health effects.3,4
Globally, 82% of PWID inject opioids.2 Effective treatment for opioid dependence reduces the harms associated with IDU.5 Opioid agonist treatment (OAT) reduces injecting risk behaviour5, the risk of HIV and HCV transmission6,7, all-cause8 and overdose mortality in the community8, prison9 and following release from prison5 and increases engagement in the HIV10 and HCV5 cascades of care. Other causes of mortality may also be reduced during OAT, including suicide and other injuries9,11, and OAT may reduce re-incarceration.12
Both methadone and buprenorphine are on the World Health Organization’s (WHO) list of essential medicines. OAT is also an important component of a comprehensive package of interventions supported by WHO and UNAIDS for preventing HIV transmission among PWID and reducing drug-related harms. However, global coverage of OAT remains low (16%), with 90% of PWID living in countries with OAT coverage below the WHO minimum recommended coverage of 20%.13
In this study, we use a previously published model5 of HIV and HCV transmission, disease-related mortality, incarceration and mortality due to overdose, suicide and other injuries to evaluate the health benefits of scaling-up OAT in three global settings: Kyiv (Ukraine), Tehran (Iran) and Perry County (Appalachian Kentucky, USA). These settings were chosen because of data availability and differences across multiple factors: HIV and HCV prevalence, HIV treatment and OAT coverage and history (Table 1).
Table 1:
Summary of the modelled settings.
| Appalachian Kentucky |
Kyiv | Tehran | |
|---|---|---|---|
| PWID population size | 700 | 33,700 | 130,000 |
| HIV prevalence among Community PWID | 0.0% | 26% | 15% |
| HCV prevalence among PWID | 54% | 77% | 56% |
| % PWID ever incarcerated | 86% | 54% | 61-83% |
| Year OAT started | 1972 | 2004 | 2002* |
| OAT coverage among community PWID | 5% | 5% | 11% |
| OAT coverage among incarcerated PWID | 0% | 0% | 40% |
| Average duration of OAT | 4 months | 14 months | 7 months |
| ART coverage among PWID | N/A | 26% | 15% |
| Overdose crude mortality rate (/100py) among PWID | 0.2 | 0.9 | 2.6 |
| HIV crude mortality rate (/100py) among PWID and ex-PWID | N/A | 1.4 | 0.9 |
| Overall crude mortality rate (/100py) among PWID | 1.0 | 6.2 | 4.2 |
OAT was reintroduced in Iran in 2002, after being introduced in the 1970s but then prohibited since 1979.
Methods
Model Description
The model (full details in appendix to 5) captures mortality through drug overdose, injury or trauma (e.g. homicide or accidents), suicide, HIV- and HCV-disease, and other causes (i.e. not causes listed). The model incorporates injecting transmission of HIV and HCV and sexual transmission of HIV among PWID, and tracks individuals following injecting cessation of drug use to fully capture HIV/HCV related mortality and life years lived. We do not model temporary cessation of injecting and relapse, instead injecting cessation in the model is assumed to be permanent and to occur at a constant rate. The model also includes stratification by HCV and HIV infection disease stage, incarceration status and OAT status.
The model incorporates transitions on and off OAT, with some PWID dying when they start or cease OAT, to simulate the excess overdose risk in the first 4-weeks on and off OAT8. While on OAT, PWID have reduced mortality risk through overdose, suicide, other injuries and other non-disease related causes, varying by incarceration status.8,9,11 They are also assumed to have reduced HIV and HCV transmission risk through injecting compared to those not on OAT.6,7 We conservatively assumed that OAT does not increase injecting cessation because whilst there is evidence for an association with short-term/temporary cessation14, the evidence for improvements in longer-term cessation is uncertain.15
PWID are incarcerated or re-incarcerated at different rates and released from prison (term used for any criminal justice facility) at a constant rate. The rate of (re-)incarceration is reduced if PWID are on OAT.12 During incarceration, PWID have different mortality rates compared to PWID in the community9, but ART recruitment and lost-to-follow-up are assumed to remain the same. Depending upon their OAT status5, some PWID released from prison die due to the excess overdose risk in the first 4-weeks following release.4 The model assumes constant sexual HIV transmission risk in the community but none whilst incarcerated. The injecting HIV/HCV transmission risk is elevated among recently released PWID (last 6 months) compared to those who have never been incarcerated or have been previously incarcerated but not recently3, and may be higher or lower among currently incarcerated PWID (i.e. can vary freely during model calibration).
PWID with latent HIV infection or AIDS can be recruited onto ART, which reduces HIV infectiousness, progression and mortality, dependent on levels of viral suppression, which is higher amongst those receiving OAT10. OAT also increases ART recruitment and retention.10
Model Parameterisation and Calibration
For Kentucky, the model is parameterised using data from the Social Networks Among Appalachian People study.16 The data included deaths (rate 0.95/100py) and their causes; determined through coroner’s reports. Based on study data, we model an increasing PWID population in Appalachian Kentucky.17,18 For Kyiv, the model is primarily parameterised using three surveys; the 2015 and 2017 Alliance for Public Health Integrated Bio-Behavioural Assessment (IBBA) surveys19,20 and the 2015 Expanding Medication-Assisted Therapy (ExMAT) bio-behavioural survey.21 Follow-up data from the control arm of HIV Prevention Trials 07422 are used to parameterise mortality rates (5.6/100py) and their causes in Kyiv; determined by two infectious disease physicians with discordant categorisations settled by a third. For Tehran, the model was parameterised using recent IBBAs and a synthesis of published estimates. Two studies provided mortality data among PWID; 4.8./100py in Tehran23 and 4.1/100py in Darab.24 Data on causes of death was only available for the Darab study (only for overdoses; determined by family members) and so we assumed that a similar rate of overdose deaths occurred in Tehran. Table 1 summarises key parameters/calibration data for each setting.
We calibrated the model using an approximate Bayesian computation sequential Monte Carlo scheme (see appendix and 5) to setting specific data on: HIV/HCV sero-prevalence among PWID by incarceration status; difference in HIV/HCV sero-prevalence between previously incarcerated and never incarcerated PWID; difference in HCV sero-prevalence between HIV-positive and HIV-negative PWID; proportion of PWID who have ever been incarcerated, or incarcerated twice or more; proportion of PWID currently or ever on OAT (all providers) or ART; all-cause mortality rates amongst PWID; and proportion of deaths due to different causes. Through this, we estimate HCV/HIV transmission rates (injecting and sexual), mortality rates due to different causes, (re-)incarceration rates and OAT and ART enrolment rates.
A simplified model without ex-PWID and HCV disease progression is used for model calibration, assuming that HCV deaths will mainly occur in ex-PWID. After calibration, for each model run we sample parameters related to HCV disease progression and mortality, and mortality rates among ex-PWID.
In each setting, the same estimates were used for the effects of OAT (Table 2) on overdose mortality in the community, HIV and HCV transmission risk and HIV treatment outcomes, based on data from systematic reviews. Other effects of OAT (Table 2) are parameterised using single estimates from Australia. Full parameter tables and calibration data are in 5.
Table 2:
Effects of OAT modelled
| Parameter | Parameter Value | Source |
|---|---|---|
| Effects of OAT: Mortality* | ||
| Relative risk of overdose mortality if on OAT outside of prison | 0.25 (95%CI: 0.18-0.36) | 5 |
| Relative risk of suicide mortality if on OAT outside of prison | 0.48 (95%CI: 0.39-0.59) | 11 |
| Relative risk of other injury mortality if on OAT outside of Prison | 0.40 (95%CI: 0.34-0.46) | 11 |
| Relative risk of other causes of mortality if on OAT in and outside of prison | 0.86 (95%CI:0.75-0.99) | 11 |
| Relative risk of suicide, injury and overdose mortality if on OAT in prison | 0.13 (95%CI: 0.05-0.35) | 9 |
| Relative risk of overdose in first 4 weeks after prison release if on OAT at time of release (independent of whether retained on release). | 0.25 (95%CI: 0.14-0.45) | 5 |
| Relative risk of overdose in first 4 weeks of OAT vs rest of time on OAT | 1.85 (95%CI: 0.93-3.66) | 8,25 |
| Relative risk of overdose in first 4 weeks off OAT vs rest of time off OAT | 1.98 (95%CI: 1.24-3.15) | 8,25 |
| Effects of OAT: HIV/HCV transmission* | ||
| Relative risk of HIV transmission due to IDU if on OAT | 0.46 (95%CI: 0.32-0.67) | 6 |
| Relative risk of HCV transmission due to IDU if on OAT | 0.50 (95%CI: 0.40-0.63) | 7 |
| Effects of OAT: HIV treatment* | ||
| Relative rate of ART recruitment if on OAT | 1.87 (95%CI: 1.50-2.33) | 10 |
| Relative rate of ART loss to follow up if on OAT | 0.77 (95%CI: 0.63-0.95) | 10 |
| Increase in odds of viral suppression if on OAT | 1.45 (95%CI: 1.21-1.73) | 10 |
| Effects of OAT: Incarceration* | ||
| Relative rate of re/incarceration if on OAT | 0.79 (95%CI: 0.70–0.89) | 12 |
All compared to risk if not on OAT in that setting unless stated; IDU: injecting drug use
Model Analyses
For each setting, we estimated the number of deaths over 20 years (2020-2040) through specific ‘preventable drug-related causes’ (HIV or HCV disease related or overdose, suicide, and other injuries) and premature mortality from all causes. Mortality rates were compared to those among the general population with similar age and sex profiles as PWID in each country.
We then considered the impact of OAT in terms of ‘preventable drug-related deaths’ (pDRDs) averted for 4 OAT scenarios starting in 2020:
Current OAT coverage (‘Status quo’)
Scaling-up OAT to 40% coverage amongst community PWID
Scenario 2 plus increasing the average length of OAT to 2 years
Scenario 3 with equivalent provision of OAT (same OAT recruitment rates and retention as for community) in prison as community, with all PWID on OAT being retained on OAT upon incarceration or release from prison
It is important to note that Scenarios 3 and 4 are modelled by first increasing OAT recruitment rates in the community to give 40% coverage and then increasing retention (scenarios 3 and 4) and then adding OAT provision in prisons (scenario 4 only). In this way, OAT coverage among community PWID will increase above 40% for these incremental changes in the model.
For each scenario, we estimated the contribution that different health harms have to mortality. The impact of current and scaled-up OAT coverages were estimated as the proportion of pDRDs averted compared to a counterfactual with no OAT from 2020. To account for differences in PWID population size across each setting (Table 1), model projections are presented per 1,000 PWID person-years. For scenario 4, we also estimated the impact of additionally scaling-up ART to UNAIDS targets (Scenario 5: 81% of HIV-positive PWID on ART, with 90% being virally suppressed) and evaluated how each effect of OAT contributes to the resulting impact. Cessation of PWID is only tracked from 2020 to capture the benefits of scaling-up OAT among PWID.
Uncertainty analysis
A linear regression analysis of covariance (ANCOVA) was undertaken to determine which parameter uncertainties contribute most to variability in the projected impact of scenario 4. The proportion of each model outcome's sum-of-squares contributed by each parameter was calculated to estimate the importance of individual parameters to overall uncertainty.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
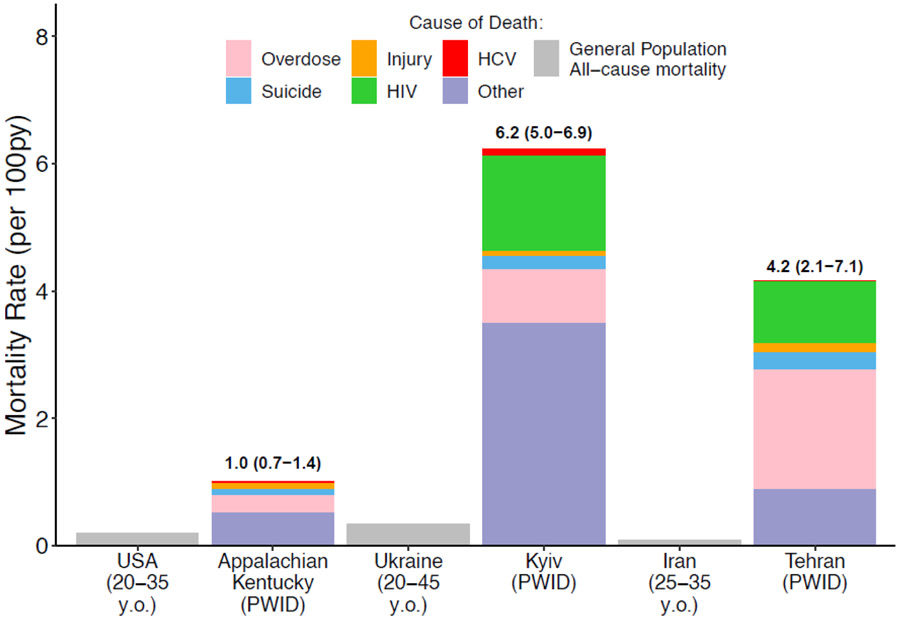
Status quo mortality rates among current PWID vary considerably between the three settings (Figure 1); 1.0 (95% credibility interval (CrI): 0.7-1.4), 6.2 (95%CrI: 5.0-6.9) and 4.2 (95%CrI: 2.1-7.1) per 100py in Appalachian Kentucky, Kyiv and Tehran, respectively. These rates are 5.1 (95%CrI: 3.6-7.1), 18.3 (95%CrI: 14.6-20.3), and 46.7 (95%CrI: 23.3-78.0) times higher than general population mortality rates in each setting with similar age/sex profiles (Figure 1).
Figure 1:
Mortality rates among PWID and the general population for each country setting over 2020-2040. Figure shows the median mortality rate among PWID over 2020-2040 in status quo projections and estimates of the national mortality rates among those of a similar age and sex profile as PWID population. Shading within the bars of the mortality rate among PWID show the median proportion of deaths due to overdose, suicide, injury, HIV, HCV or other causes. Labels above bars show median mortality rates with 95%CrI intervals.
Over the next 20 years, status quo projections show that IDU results in 5.9 (95%CrI: 4.0-8.4), 31.7 (95%CrI: 26.6-36.9) and 46.8 (95%CrI: 25.9-75.3) pDRDS occurring per 1,000 PWID person-years in Appalachian Kentucky, Kyiv and Tehran, respectively. These drug-related deaths account for 48.6% (95%CrI: 35.6-62.4), 43.8% (95%CrI: 37.8-57.2) and 78.2% (95%CrI: 61.8-93.1) of all deaths among PWID in Appalachian Kentucky, Kyiv and Tehran, and 3.5% (95%CrI: 1.4-7.8), 28.1% (95%CrI: 22.6-34.1) and 29.2% (95%CrI: 13.9-41.9) among ex-PWID. The varied harms associated with drug use contribute differentially in each setting (Figures 1 and 2), with the primary drug-related cause of death being overdose in Appalachian Kentucky (46.5% of pDRDS, 95%CrI: 27.3-66.2) and HIV in Kyiv (58.9%, 95%CrI: 48.7-73.4) and Tehran (48.0%, 95%CrI: 18.0-75.3). Across settings, fatal overdose contributes 27.1-46.5% of pDRDS, suicide 5.6-17.2%, injury 2.7-16.5%, HIV 48.0-58.9% (Kyiv and Tehran) and HCV 2.3-17.9%.
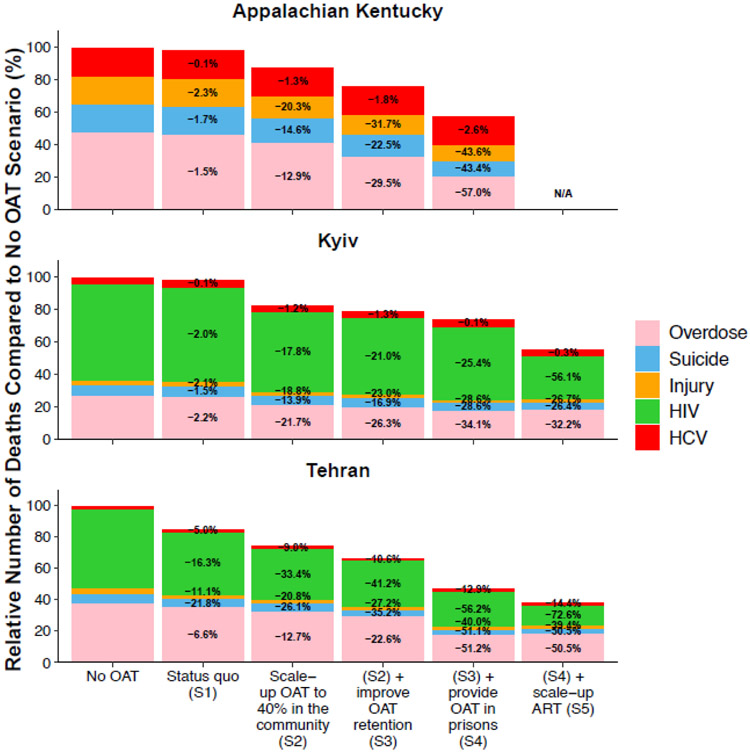
Figure 2:
Relative number of preventable drug-related deaths (overdose, suicide, injury, HIV, or HCV) among PWID and ex-PWID over 2020-2040 for different OAT scenarios, all compared to if there was no OAT in each setting. Scenarios are: no OAT from 2020; Scenario 1: Status quo OAT coverages; Scenario 2: OAT is scaled-up to 40% coverage among PWID in the community; Scenario 3: ‘Scenario 2’ followed by improved OAT retention such that the average duration of OAT is increased to 2 years; Scenario 4: ‘Scenario 3’ followed by also enrolling incarcerated PWID onto OAT at the same rate as the community and PWID retained on OAT upon incarceration and release; Scenario 5: ‘Scenario 4’ followed by also scaling-up ART to UNAIDS targets. Deaths from other causes which account for 52%, 54% and 82% of deaths in Tehran, Kyiv and Appalachian Kentucky in Status Quo projections, respectively, are not included.
Current levels of OAT have negligible impact on mortality in Appalachian Kentucky and Kyiv (Figure 2) but moderate impact in Tehran. Compared to no OAT, the model projects that current OAT provision will avert 0.1 (95%CrI: 0.05-0.2), 0.6 (95%CrI:0.4-0.9) and 6.3 (95%CrI: 2.1-13.9) deaths per 1,000 PWID person-years or 1.4% (95%CrI: 0.7-1.9), 2.0% (95%CrI: 1.3-2.8) and 12.6% (95%CrI: 5.0-28.1) of pDRDs over the next 20 years in Appalachian Kentucky, Kyiv and Tehran, respectively. Greater impact is projected in Tehran because of high OAT coverage in prisons (39.9%; 95%CrI: 35.2-44.7).
Scaling-up OAT in the community to 40% coverage (Scenario 2; Figure 3) increases overall OAT coverage to 30.2% (95%CrI: 28.0-33.9), 32.3% (95%CrI: 30.7-33.6) and 40.1% (95%CrI: 37.0-43.2) in Appalachian Kentucky, Kyiv and Tehran, respectively. This increases the impact of OAT on pDRDs in Appalachian Kentucky and Kyiv by nearly 10-times, and 2-times in Tehran, with 12.3% (95%CrI: 6.6-15.3), 17.6% (95%CrI: 14.4-21.3) and 24.4% (95%CrI: 11.4-43.7) of pDRDs being averted over the next 20 years in each setting, respectively (Figure 2). This is largely driven by reductions in overdose deaths in Appalachian Kentucky and reductions in HIV deaths in Kyiv and Tehran.
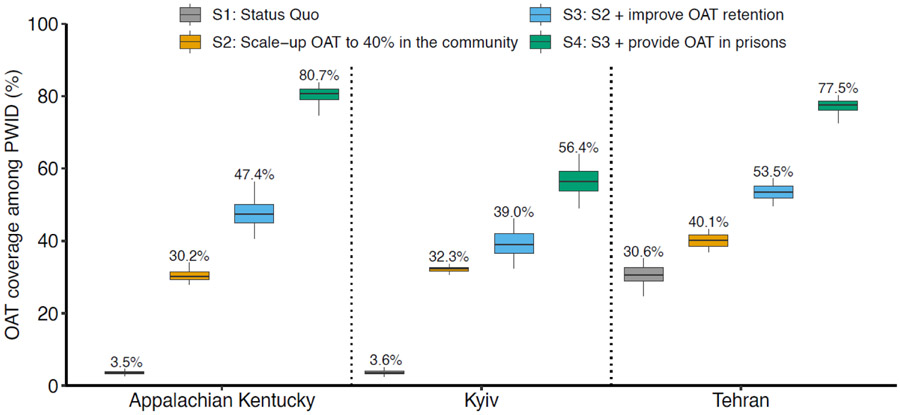
Figure 3:
OAT coverage among all PWID in 2040 for different OAT scenarios. The Scenarios are: Scenario 1: Status quo OAT coverages; Scenario 2: OAT is scaled-up to 40% coverage among PWID in the community; Scenario 3: ‘Scenario 2’ followed by improving OAT retention such that the average duration of OAT is increased to 2 years; Scenario 4: ‘Scenario 3’ followed by also enrolling incarcerated PWID onto OAT at the same rate as the community and retaining PWID on OAT upon incarceration and release. The middle line is the median, limits of boxes are the 25% and 75% percentiles, and whiskers are 2.5% and 97.5% percentile range. Labels at the end of the whiskers give the median.
If OAT retention was also improved to an average 2-year treatment duration (scenario 3) then OAT coverage in community PWID would increase by 25-50%, with the overall coverage reaching 47.4% (95%CrI: 40.7-56.4), 39.0% (95%CrI: 32.4-46.1) and 53.5% (95%CrI: 49.7-57.2) in Appalachian Kentucky, Kyiv and Tehran, respectively. These OAT coverages could avert 23.5% (95%CrI: 18.4-29.6), 21.2% (95%CrI: 16.8-27.1) and 32.5% (95%CrI: 18.4-52.3) of pDRDs in Appalachian Kentucky, Kyiv and Tehran, respectively. As well as increasing OAT coverage, the increase in OAT retention also reduces overdose deaths. Indeed, even if OAT retention increased to two years but OAT coverage remained at 40% then 52.4-149.0% more overdose deaths are averted than in scenario 2.
If access to OAT is also made equitable in prisons (same recruitment rate as community) with retention upon incarceration and release (Scenario 4), then overall OAT coverage increases by 45-70% compared to scenario 3, to 80.7% (95%CrI: 74.7-83.8), 56.4% (95%CrI: 49.2-64.0) and 77.5% (95%CrI: 72.6-80.3) in Appalachian Kentucky, Kyiv and Tehran, respectively. This results in significant reductions in mortality, most notably HIV and overdose, with 42.3% (95%CrI: 33.4-50.1), 26.8% (95%CrI: 22.0-32.8) and 51.3% (95%CrI: 34.6-66.5) of pDRDs averted over the next 20 years in Appalachian Kentucky, Kyiv and Tehran, respectively. This increases the impact achieved by the previous scenario by 26-80%.
Further reductions in mortality occur if ART is also scaled-up to UNAIDS targets (Scenario 5; Figure 2), especially in Kyiv where 65% greater impact on pDRDs is achieved (compared to scenario 4). This results in 56.1% (95%CrI: 50.7-62.3) and 72.6% (95%CrI: 63.5-84.2) of HIV deaths being averted in Kyiv and Tehran, respectively. However, mortality will still remain high, with rates of 0.5 (95%CrI: 0.3-0.7), 2.5 (95%CrI: 1.7-3.1) and 0.8 (95%CrI: 0.3-1.7) per 100py among current PWID in Appalachian Kentucky, Kyiv and Tehran, respectively; mostly (68.7-88.7%) due to other causes (i.e. not HIV, HCV, injury, overdose or suicide).
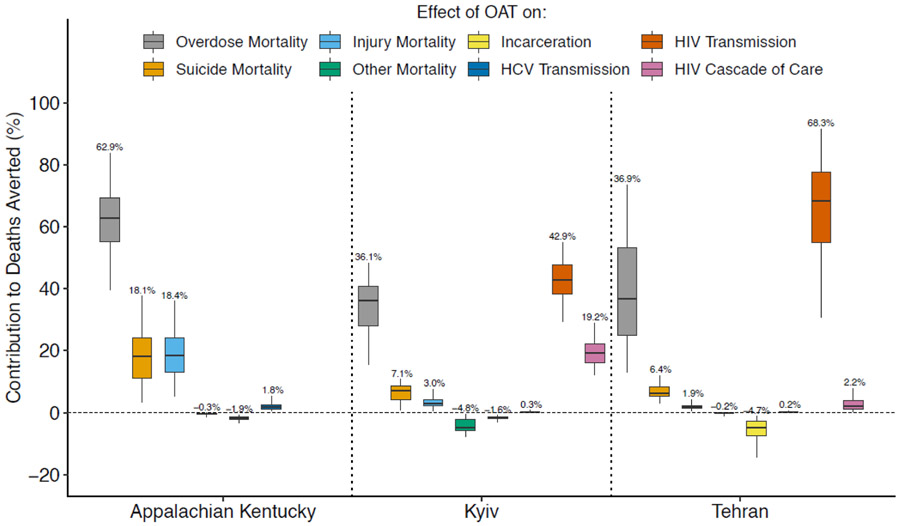
In each setting, the various effects of OAT contributed differently to its overall impact in scenario 4 because of variations in the incidence of different health harms (Figure 4). For example, the effect of OAT on reducing HIV transmission caused the greatest reduction in mortality in Kyiv and Tehran, accounting for 43-68% of deaths averted, whilst in Appalachian Kentucky, the effect of OAT on overdose was most important, accounting for over half of deaths averted. For settings with HIV, the effects of OAT on improving HIV treatment outcomes can also be important, accounting for 19.2% (95%CrI: 12.2-29.0) of pDRDs averted in Kyiv but only 2.2% (95%CrI: 0.4-7.9) in Tehran, largely due to greater HIV mortality in Kyiv than Tehran.
Figure 4:
The contribution of each effect of OAT on preventable drug-related deaths averted in ‘OAT Scenario 4’ where OAT is scaled-up to 40% coverage among community PWID followed by increasing the average duration of OAT to 2 years and providing OAT within prisons with retention upon incarceration and release. The box plots signify the % reduction in the number of deaths averted by OAT due to different benefits of OAT (evaluated by turning off each effect individually). The middle line is the median, limits of boxes are the 25% and 75% percentiles, and whiskers are 2.5% and 97.5% percentile range. Labels at the end of the whiskers give the median.
The analysis of covariance indicated that uncertainty in the rates of HCV disease progression and mortality (54.9% of variability) and overdose mortality (20.0%) contributed most to the variability in the impact of OAT in Appalachian Kentucky for scenario 4. In Kyiv, uncertainty in the rates of ART enrolment (13.6%) and sexual HIV transmission (14.1%), and the effect of OAT on HIV transmission (7.1%) contributed most to variability. In Tehran, uncertainty in the rate of sexual HIV transmission (43.3%), average duration of injecting (12.2%) and effect of OAT on HIV transmission (6.4%) contributed most to variability. All other model parameters contributed less than 5% to the variability.
Discussion
In three global settings, we found that substantial health harms caused by IDU can be markedly reduced through scaling-up OAT. Scaling-up OAT to 40% coverage in the community could avert up to a quarter of pDRDs over the next 20 years compared to a scenario where there is no OAT. Our findings also demonstrate the importance of improving retention on OAT and providing prison-based OAT. Increasing the average duration of OAT to 2 years and improving OAT access in prisons resulted in dramatic increases in OAT coverage and population benefits of OAT, especially for prevention of overdose and HIV mortality. Furthermore, in HIV epidemic settings the benefits of OAT on reducing HIV transmission and improving ART outcomes may result in the greatest reductions in mortality from scaling-up OAT. These findings illustrate how the epidemiology of diseases and characteristics of PWID populations can dramatically affect the impact that OAT can have in different settings; which should be accounted for when planning interventions to address drug-related harms.
Our results are consistent with previous modelling for Russia26 that showed scaling-up OAT can significantly reduce HIV and overdose deaths, particularly if OAT retention is increased. Our analyses add additional insights by considering additional benefits of OAT on reducing other causes of deaths, HCV transmission and incarceration. Our results are also consistent with previous modelling that showed OAT can improve the prevention benefit of ART through increasing HIV treatment initiation, retention and viral suppression27, with our analyses also suggesting significant improvements in HIV mortality. Previous modelling for Ukraine28,29 and Kentucky17,18 have shown OAT could have significant impact and be cost-effective. However, these studies generally focussed on one effect of OAT, primarily its impact on HIV or HCV transmission. Our findings suggest this will underestimate the impact of OAT, adding further evidence for the benefits of OAT. Lastly, previous modelling for Ukraine suggested that prison-based OAT may be more important for reducing HIV transmission among PWID than community-based OAT.29 Our findings also show that prison-based OAT is important for reducing mortality and expanding OAT because incarceration can limit its scale-up.
To our knowledge, this is the most comprehensive modelling study of the impact of OAT, considering the many benefits of OAT in three diverse settings. However, there are limitations, including those of the studies used to parameterise and calibrate the model. This includes the mortality data which was generally based on small samples.
Firstly, there was uncertainty in parameter values. Specifically, whilst the model was parameterised primarily using setting-specific data, this was limited for some parameters in Tehran. To address this, we used data from other sites in Iran and incorporated added uncertainty in these parameters. For all sites, there was uncertainty in causes of death. The model calibration accounted for this and other parameter uncertainty, with any uncertainties being incorporated into all model projections. There was also wide variation in HCV prevalence estimates for Tehran, due to variations in study characteristics. To account for this variation, we used a likelihood approach to calibrate the model which accounted for the sample size of each datapoint and heterogeneity in HCV prevalence estimates. There is also uncertainty in how drug use patterns and behaviours may evolve over time. Although model projections assume opioids will remain the main drug injected by PWID, even with relatively large shifts away from opioids, our modelled OAT scenarios should still be possible except possibly our highest coverage scenario.
Secondly, whilst effects of OAT were primarily based on global systematic reviews, this evidence is dominated by high-income settings. It is uncertain whether the same effects can be realised in all settings. In addition, the evidence is largely derived from observational studies with no trials or natural experiments evaluating the impact of scaling-up or increasing OAT retention.5 The projected impacts of scaling-up OAT also primarily reflect high quality OAT, and so may be over-estimated for some settings. Indeed, OAT dosing varies globally, with sub-optimal dosing being common. This affects retention on OAT5 and could affect other outcomes. Currently there is a lack of review level evidence on the impact of OAT on suicide and injury, and so we relied upon studies from Australia.9,11 We are undertaking reviews to strengthen this evidence.30 These effects only had significant impact in Kentucky where many averted deaths were suicides and other injuries, but even then uncertainty in these parameters did not contribute much to variability in our results. Also, we did not model the different modes of OAT (methadone vs. buprenorphine). Although there is evidence that effects may differ by modality8,25, OAT effect estimates are primarily based on methadone, which is the main form of OAT in our settings and globally.13
Lastly, we focused on the impact of OAT on mortality, not quality of life or other social harms, meaning we may have underestimated the overall benefits of OAT in terms of improved HIV/HCV morbidity and other aspects of quality of life. Also, focussing on PWID and ex-PWID means the model did not capture onwards benefits of reducing HIV infections in non-injecting populations in each setting. Despite this, our findings still emphasise the range of benefits of OAT for reducing mortality among PWID, something previous analyses have not done.5
People with problematic opioid use experience many adverse health outcomes, including overdose, suicide, other injuries and infectious diseases including HIV and HCV.5 Our modelling demonstrates that scaling-up OAT among PWID can substantially reduce mortality in three global settings, as can improving OAT retention and providing OAT in prisons. We acknowledge, though, that numerous barriers limit access to OAT in the community and prison, which would need to be overcome to achieve these benefits. Although some sites clearly achieve longer durations of OAT, there is also considerable heterogeneity and weak evidence on interventions that improve retention.27 As for studies of OAT provision8,25, our model projections highlight that duration of OAT matters, and so there is an urgent need for more research and investment into policies and interventions that improve OAT retention.
Whilst we have demonstrated that OAT can substantially reduce deaths, our findings also demonstrate the importance of scaling-up HIV treatment among PWID. Furthermore, given mortality rates are still high even after scaling-up OAT and HIV treatment, mostly due to chronic diseases, other interventions are also needed to reduce premature deaths among PWID. However, as ~90% of PWID live in countries with low OAT coverage (<20%)13, scale-up of OAT remains a high priority for reducing drug-related harms.
Supplementary Material
Research in Context.
Evidence before the study
We searched PubMed for published studies that modelled the impact of Opioid agonist treatment (OAT) among people who inject drugs (PWID), with the terms ("substitution therapy" OR "medically assisted treatment" OR "methadone" OR "buprenorphine" OR "Suboxone" OR "subutex" OR "naltrexone" OR "agonist treatment" OR "agonist therapy" OR "substitution treatment" OR "medically assisted therapy" OR "OST" OR "OAT" OR "MMT" OR "MAT") AND (model*) AND ("IDU" OR "IVDU" OR "injection drug" OR "injecting drug" OR "intravenous drug" OR "people who inject drugs"). We identified 38 mathematical modelling studies, of which 19 studies measured the impact of OAT on mortality. The remaining 19 studies measured the impact of OAT on HIV and/or HCV transmission among PWID in terms of reductions in incidence or prevalence or infections averted. Most (17) of the 19 studies that measured the impact of OAT on mortality considered only reductions due to HIV, HCV or all-cause mortality, with only two studies considering reductions in overdose deaths. No studies considered reductions in mortality due to suicide or other injuries. Only three studies considered improvements in HIV treatment access and outcomes for PWID on OAT, whilst none considered the effect of OAT in reducing incarceration.
Added value of the study
Our study is the most comprehensive modelling analysis of the potential impact of OAT on reducing mortality among PWID. We model both direct and indirect effects of OAT on overdose deaths, suicide deaths, deaths through other injuries, HIV and HCV disease related deaths and deaths from all other causes. Our results for three global diverse settings show that the substantial public health burden caused by injecting drug use can be markedly reduced through scaling-up OAT. This impact is greatest in settings with significant HIV mortality and can be maximised by improving retention in OAT and providing prison-based OAT. Our findings suggest that previous studies will have underestimated the impact and cost-effectiveness of OAT on reducing mortality due to only considering a limited range of benefits of OAT.
Implications of all the available evidence
OAT is a critical intervention which has been shown to be highly cost-effective through substantially reducing the health and social harms associated with opioid dependence and injecting drug use. The impact of OAT on mortality varies between settings based on the epidemiology of disease, particularly HIV. With low coverage of OAT among PWID globally and many countries declaring public health emergencies related to the rising number of overdose deaths, scaling-up OAT remains a priority for improving the health of PWID.
Acknowledgements
This study was funded by the NIHR Health Protection Research Unit in Behavioural Science and Evaluation at University of Bristol, in partnership with Public Health England (PHE). The views expressed are those of the author and not necessarily those of the NIHR, the Department of Health and Social Care, or PHE. JS, PV and LD acknowledge funding from NIAID/NIDA (R01 AI147490). JS, FLA and PV acknowledge support from NIDA (R01 DA033679). JS, MH and LD acknowledge support from NHMRC (APP1150078). MH is an NIHR senior investigator and acknowledges NIHR School of Public Health Research. PV acknowledges support from the NIHR HPRU in Blood Borne and Sexually Transmitted Infections at University College London, NIDA (grant number R01 DA037773 and R01 DA047952). LD and SL are supported by NHMRC Fellowships and National Institute of Health (NIH) grants National Institute on Drug Abuse (NIDA) (R01DA1104470). The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. FLA is supported on research related to this grant from NIDA (R01 DA043125, R01 DA029910, U01 DA045384, R21 DA047902, R01 DA030768, K24 DA017072, R01 DA025943, R01 DA030762, R21 DA041953). JRH, AMY, and the Social Networks among Appalachian People (SNAP) study were funded by grants from the National Institute on Drug Abuse (R01 DA033862, R01 DA024598). We wish to acknowledge Hamid Sharifi and Alireza Noroozi for providing information for Tehran.
Footnotes
Declaration of interests
JS reports grants from UK National Institute of Health Research, during the conduct of the study. LD reports grants from Indivior, Seqirus, outside the submitted work. JG reports grants and personal fees from Abbvie, grants and personal fees from Gilead Sciences, grants and personal fees from Merck, grants and personal fees from Cepheid, grants from Hologic, grants from Indivior, outside the submitted work. SL reports grants from Indivior, outside the submitted work. FLA reports grants paid to his organization for which he is PI: NIH, NIDA, FIC, HRSA, SAMHSA, Gilead Foundation, Merck and personal fees for consulting, lectures, and speakers bureau: PracticePoint Communications, Clinical Care Options, Merck, Abbvie, Gilead Sciences. JRH reports grants from Indivior, outside the submitted work. WCM reports grants from National Institutes of Health, during the conduct of the study. MH reports personal fees from MSD, Gillead, outside the submitted work. PV reports grants from UK National Institute of Health Research, during the conduct of the study. All other authors declare no conflicts of interest.
Data Sharing Statement
Model code will be made available immediately following publication. The code will be shared with researchers who provide a methodologically sound proposal approved by Dr Jack Stone and Professor Peter Vickerman. Proposals should be directed to jack.stone@bristol.ac.uk and Peter.vickerman@bristol.ac.uk; requesters will need to sign a data access agreement.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. The Lancet. 2012;379(9810):55–70. [DOI] [PubMed] [Google Scholar]
- 2.Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health. 2017;5(12):e1192–e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone J, Fraser H, Lim AG, et al. Incarceration history and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Infect Dis. 2018;18(12):1397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merrall EL, Kariminia A, Binswanger IA, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105(9):1545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet. 2019;394(10208):1560–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345(October03 3):e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9:CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larney S, Gisev N, Farrell M, et al. Opioid substitution therapy as a strategy to reduce deaths in prison: retrospective cohort study. BMJ Open. 2014;4(4):e004666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low AJ, Mburu G, Welton NJ, et al. Impact of Opioid Substitution Therapy on Antiretroviral Therapy Outcomes: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degenhardt L, Randall D, Hall W, Law M, Butler T, Burns L. Mortality among clients of a state-wide opioid pharmacotherapy program over 20 years: risk factors and lives saved. Drug and alcohol dependence. 2009;105(1-2):9–15. [DOI] [PubMed] [Google Scholar]
- 12.Larney S, Toson B, Burns L, Dolan K. Effect of prison-based opioid substitution treatment and post-release retention in treatment on risk of re-incarceration. Addiction. 2012;107(2):372–80. [DOI] [PubMed] [Google Scholar]
- 13.Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Global Health. 2017;5(12):E1208–E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia Y, Seaman S, Hickman M, et al. Factors affecting repeated cessations of injecting drug use and relapses during the entire injecting career among the Edinburgh Addiction Cohort. Drug Alcohol Depend. 2015;151:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimber J, Copeland L, Hickman M, et al. Survival and cessation in injecting drug users: prospective observational study of outcomes and effect of opiate substitution treatment. BMJ. 2010;341:c3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young AM, Havens JR. Transition from first illicit drug use to first injection drug use among rural Appalachian drug users: a cross-sectional comparison and retrospective survival analysis. Addiction. 2012;107(3):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone J, Fraser H, Young AM, Havens JR, Vickerman P. Modeling the role of incarceration in HCV transmission and prevention amongst people who inject drugs in rural Kentucky. Int J Drug Policy. 2020:102707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser H, Vellozzi C, Hoerger TJ, et al. Scaling Up Hepatitis C Prevention and Treatment Interventions for Achieving Elimination in the United States: A Rural and Urban Comparison. Am J Epidemiol. 2019;188(8):1539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International HIV/AIDS Alliance in Ukraine. Integrated Behavioral and Biological Assessment 2015 - National survey of PWID. Unpublished.
- 20.International HIV/AIDS Alliance in Ukraine. Integrated Behavioral and Biological Assessment 2017 - National survey of PWID. Unpublished.
- 21.Zelenev A, Shea P, Mazhnaya A, et al. Estimating HIV and HCV prevalence among people who inject drugs in 5 Ukrainian cities using stratification-based respondent driven and random sampling. Int J Drug Policy. 2019;67:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller WC, Hoffman IF, Hanscom BS, et al. A scalable, integrated intervention to engage people who inject drugs in HIV care and medication-assisted treatment (HPTN 074): a randomised, controlled phase 3 feasibility and efficacy study. Lancet. 2018;392(10149):747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahimi-Movahgar A, Khastoo G, Razzaghi E, Saberi-Zafarghandi M, Noroozi A, Jar-Siah R. Compulsory methadone maintenance treatment of severe cases of drug addiction in a residential setting in Tehran, Iran: outcome evaluation in two and six-month follow-up. Payesh Health Monitor. 2011;10(4):505–14. [Google Scholar]
- 24.Jafari S, Rahimi-Movaghar A, Craib KJ, Baharlou S, Mathias R. A follow-up study of drug users in Southern Iran. Addiction Research & Theory. 2010;18(1):59–70. [Google Scholar]
- 25.Hickman M, Steer C, Tilling K, et al. The impact of buprenorphine and methadone on mortality: a primary care cohort study in the United Kingdom. Addiction. 2018;113(8):1461–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cepeda JA, Eritsyan K, Vickerman P, et al. Potential impact of implementing and scaling up harm reduction and antiretroviral therapy on HIV prevalence and mortality and overdose deaths among people who inject drugs in two Russian cities: a modelling study. Lancet HIV. 2018;5(10):e578–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukandavire C, Low A, Mburu G, et al. Impact of opioid substitution therapy on the HIV prevention benefit of antiretroviral therapy for people who inject drugs. AIDS. 2017;31(8):1181–90. [DOI] [PubMed] [Google Scholar]
- 28.Alistar SS, Owens DK, Brandeau ML. Effectiveness and cost effectiveness of expanding harm reduction and antiretroviral therapy in a mixed HIV epidemic: a modeling analysis for Ukraine. PLoS Med. 2011;8(3):e1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altice FL, Azbel L, Stone J, et al. The perfect storm: incarceration and the high-risk environment perpetuating transmission of HIV, hepatitis C virus, and tuberculosis in Eastern Europe and Central Asia. Lancet. 2016;388(10050):1228–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Degenhardt Louisa, Grebely Jason, Hickman Matthew, Campbell Gabrielle, Tran Lucy, Santo Thomas. Examining the effect of opioid agonist treatment on all-cause and cause-specific mortality. PROSPERO; 2020. CRD42020171949 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020171949. Last Accessed: 20 Nov 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.