Abstract
Vasectomy is a simple, safe, effective, and economical method used worldwide for long-term male contraception. As a surgical operation, it has short-term and long-term complications such as hematoma formation, infection, sterilization failure, sperm granulomas, short-term postoperative pain (nodal pain, scrotal pain, and ejaculation pain), and chronic pain syndrome. Whether it increases the risk of autoimmune disease, cardiovascular disease, testicular cancer, or prostate cancer is still controversial. Changes in plasma concentrations of luteinizing hormone, follicle-stimulating hormone, and testosterone after vasectomy have also been studied, as well as the relation between vasectomy and sexual function. Sperm quality decreases very slowly after vasectomy, and vasovasostomy and intracytoplasmic sperm injection could help a couple achieve a pregnancy if they change their minds at any point. We include a follow-up strategy and suggestions for follow-up care at the end of this review.
Keywords: Complications, Contraception, Safety, Vasectomy
INTRODUCTION
Vasectomy, as a widely accepted method of long-term male sterilization in both developed and developing countries, has the advantages of simplicity, safety, effectiveness, and economy. Nearly 43 million males worldwide have undergone a vasectomy until 2006 [1]. The highest rates of vasectomy are in Oceania, North America, parts of Asia, and Western Europe, especially in Bhutan (14%), Canada (15%), New Zealand (19%), and the United Kingdom (20%) [2].
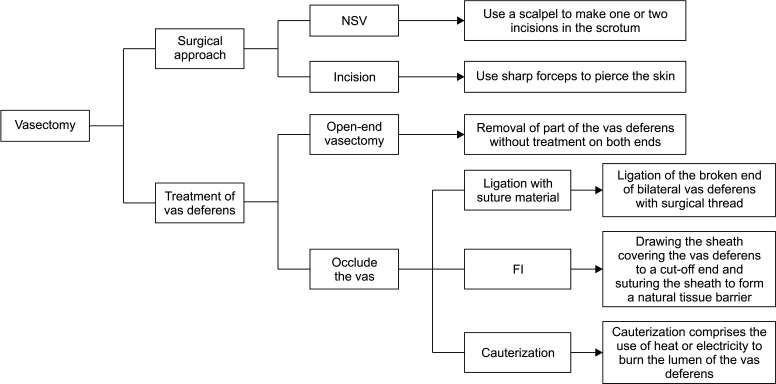
At present, the two commonest surgical techniques used in vasectomy are incision and no-scalpel vasectomy (NSV). Traditional incision requires a scalpel to open one or two incisions in the scrotum, whereas NSV uses sharp forceps to pierce the skin. In comparison with traditional incision techniques, the risk of clinical complications (e.g., hematoma, hemorrhage, and infection) after NSV surgery is markedly reduced, the operation duration is shorter, and sexual activity can be resumed faster [3]. The early postoperative complications of NSV were less than those of conventional vasectomy [4]. The World Health Organization (WHO) recommends NSV, which is becoming the global standard [5].
The techniques used to seal the vas deferens include open-end vasectomy, ligation, fascial interposition (FI), and cauterization (Fig. 1). FI is a technique that involves drawing the sheath covering the vas deferens to a cut-off end and suturing the sheath to form a natural tissue barrier. Ligation and resection plus FI are more effective than ligation and resection alone [6]. A mid-term analysis of 552 men showed that FI markedly reduced the failure rate of vasectomy (from 12.7% to 5.9%) [7]. Cauterization comprises the use of heat or electricity to burn the lumen of the vas deferens. Thermal cautery is more popular than electrocautery because it reduces the incidence of granulomas and nodular thickening [8].
Fig. 1. The main surgical techniques of vasectomy. NSV: no-scalpel vasectomy, FI: fascial interposition.
Complications of vasectomy include hematoma formation, infection, sterilization failure, sperm granulomas, short-term postoperative pain (nodal pain, scrotal pain, and ejaculation pain), and chronic pain syndrome [9]. The long-term safety of vasectomy is mainly threatened by cardiovascular disease, testicular or prostate cancer, long-term loss of sexual function after the operation, and the formation of antisperm antibodies (AsAbs) [10].
The frequency of complications of vasectomy is low. Specific complications are listed in Table 1 [11,12,13,14].
Table 1. Common complications and postoperative management after vasectomy.
| Complication | Incidence rate | Notes |
|---|---|---|
| Infection and hematoma | 0.2%–1.5%/4%–22% | Usually, infections are mild and limited to the incision site. Rare complications such as Fournier gangrene, endocarditis, arteriovenous fistulas, and angiocutaneous fistulas have been reported in very few patients [11,12]. Hematoma appear shortly after surgery [11]. |
| Post-vasectomy pain syndrome | 1%–14% | Usually light. Some cases have a negative impact on quality of life and sometimes require pain management or surgery [13]. |
| Sperm granulomas | 40% | It may occur 2–3 weeks after surgery at the site of vasectomy or in the epididymis or testicular reticulum [14]. |
| Antisperm antibodies (autoimmune disease) | No increased risk | In men who had undergone a vasectomy, the risk of several immune system-related diseases did not increase for a long time [11]. |
| Prostate cancer | No increased risk | The correlation is weak, and there is no convincing biological mechanism [11]. |
| Sexual dysfunction | No increased risk | Most studies have thus far shown that vasectomy does not affect sexual function or can even improve it. |
| Cardiovascular disease | No increased risk | Including BMI, cholesterol, triglycerides, protein, albumin, HDL, and globulin ratios [11]. |
| Reproductive hormones | No increased risk | There is no significant changes after vasectomy. |
| Sperm injury and options for future pregnancy | - | The extent of sperm damage after vasectomy is related to the time since vasectomy; Vasovasostomy and assisted reproductive technology are two main options for couples who want to achieve a pregnancy after vasectomy. |
| Semen analysis and follow-up care after vasectomy | - | 8–16 weeks is an appropriate timeframe; Rest for 24 hours, avoid cycling for 7 days for patients after vasectomy. |
BMI: body mass index, HDL: high-density lipoprotein.
The purpose of this paper is to review the literature on short-term and long-term complications of vasectomy, including rare complications, and to discuss the current understanding of health risks that have been controversial in the past.
METHODS
We searched the PubMed, MEDLINE, EMBASE, CNKI, and Wan Fang databases for relevant literature. The search terms used were vasectomy, sterilization, male contraception, vasectomy+prostate cancer, vasectomy+sexual function, vasectomy+cardiovascular disease, and vasectomy+antisperm antibody. The article publication date range was up to march, 2020.
RESULTS
1. Infection and hematoma
Infection and hematoma are the most frequently reported complications of vasectomy [9]. In general, the incidence rate of infection is between 3% and 4% [11,13], although in individual reports it has reached 30% [15]. The incidence rate of hematoma mainly varies from 0% to 29%, and an acceptable rate is 2% [13,15]. The incidence of vasectomy complications varies and depends on the number of vasectomies performed each year by the practitioner and the surgical technique [16]. A national survey in the United States reported that the incidence rate of hematoma for physicians who performed vasectomies 1–10 times annually was 4.6%, whereas the rates for those who performed vasectomies 11–50 times and more than 50 times annually were 2.4% and 1.6%, respectively [17].
It has been confirmed that the surgical technique has a marked effect on the incidence of infection and hematoma. Currently, NSV is widely recognized and accepted worldwide because of its low incidence of complications (especially hematoma and infection) [18].
Thus far, the report have compared the incidence of complications between NSV and incisional vasectomy [15]. The results showed that the rates of hematoma formation and infection caused by NSV were low. Furthermore, a Cochrane review in 2014 confirmed this conclusion [19]. In addition, cautery of the vas lumen and/or FI have been recommended as ways to increase the effectiveness of occlusion [20]. However, the incidence rates of hematoma and infection were higher in a cautery group than in a clipping group (1.6% versus 0.5%, odds ratio=3.4, 95% confidence interval=1.6–6.9, p=0.000) [10].
Prophylactic antimicrobials are not indicated for routine vasectomy unless the patient presents a high risk of infection [13]. The treatment of infection is the same as that used for other parts of the body, most infections are local. However, rare infectious complications have been reported, mostly in case reports. These include vas deferens abscess, vesicular gland abscess, endocarditis [11], scrotal skin necrosis, and Fournier gangrene. The treatment of these rare infectious conditions is more complicated. Relevant experts should be asked to assist in their diagnosis and treatment, if necessary. Most hematomas are minor and can resolve without therapeutic intervention.
The screening of indications for surgery, the assessment of local and systemic diseases, the prevention and control of infection, and consultation are essential to ensure the safety of the operation. Standard preoperative and postoperative management could reduce the risk of infection and hematoma. As in the case of other body parts, the risk of infection can be minimized by depilating the surgical area and limiting skin damage. Scrotal elevation and compression can minimize the risk of bleeding and hematoma by providing a tamponade effect. Scrotal support while the patient is active can also reduce the risk of delayed bleeding.
2. Post-vasectomy pain syndrome
Post-vasectomy pain syndrome (PVPS) comprises persistent or intermittent scrotal discomfort/pain that lasts for at least 3 months without definite epididymitis or other obvious pathological features [21]. As one form of chronic scrotal pain, PVPS is the commonest late-stage complication of vasectomy. Retrospective case series and prospective observational and follow-up studies suggest that chronic pain follows vasectomy in 1%–15% of men [11], but only about 1%–2% of men noted that it affected their quality of life [13]. The average time until the onset of PVPS is 7–24 months. Demographic characteristics (age, socioeconomic status, and ethnicity) and surgical techniques have not been shown to be associated with the occurrence of PVPS [22].
The pathophysiology of PVPS remains elusive. The etiological hypotheses have been proposed as follows [23]. The expansion and obstruction of the epididymal duct leads to the development of interstitial fibrosis, according to one hypothesis, whereas the other hypothesis proposes that rupture of the epididymal tube leads to peripheral fibrosis, which is accompanied by the extravasation of sperm to the epididymal tubules and vas deferens.
Treatments for PVPS include conservative interventions and surgical treatment. The former include the use of nonsteroidal anti-inflammatory drugs (NSAIDs), tricyclic antidepressants (TCAs), gabapentin, local or regional nerve blocks, nonspecific pain medication, acupuncture, and other complementary approaches. Drug therapy usually begins with NSAIDs for 4–6 weeks, with TCAs or gabapentin as the recommended second-line drug if pain remains unrelieved [24]. After these avenues have been exhausted, willing patients may consider operative intervention. The main surgical options for PVPS include the resection of vascular nodules, vasectomy reversal (VR), epididymectomy, and microsurgical denervation of the spermatic cord (MDSC).
The incidence rate of long-term pain requiring surgical treatment has been estimated to be approximately 0.1% [23]. Many studies reported that patients with PVPS were pain-free postoperatively and were satisfied with the outcome of epididymectomy or VR [22,24]. However, there was no significant difference between epididymectomy and VR groups in the degree of pain relief and patients' satisfaction with the outcome of surgery (The difference in the mean preoperative and postoperative VAPS scores was 6.00±1.34 in the epididymectomy group and 5.50±1.03 in the VR group) [25]. MDSC is another important surgical method with many reports of its effectiveness [26]. The last option for patients with intractable pain is orchiectomy, but it has been reported that 27% of patients still suffer from pain after surgery [8].
A multidisciplinary team could be used in severe cases such as psychiatric assessments for emotional disorders and assessments of the severity of pain by anesthesiologists.
3. Sperm granulomas
Sperm granulomas are a common phenomenon after vasectomy. Sperm granuloma is a granulomatous lesion that constitutes a foreign-body giant cell reaction to the extravasated sperm [27]. The infiltration of germ cells into the epididymal stroma after injury to the epididymal epithelium is one of the main reasons for the pathogenesis of sperm granulomas. From animal studies, there is a general consensus that the penetration of germ cells into the epididymis or the space of the vas deferens will produce autoimmune and/or inflammatory reactions, which will lead to the formation of a sperm granuloma [28]. These opalescent granulomatous nodules appear at the end of the vas deferens or epididymis and consist of a central part of degenerated sperm, which is surrounded by a layer of epithelioid macrophages and, in turn, by loose connective tissue rich in lymphocytes and plasma cells [29].
A sperm granuloma may occur 2–3 weeks after surgery at the site of vasectomy or in the epididymis or testicular reticulum [14]. Histological examination is the gold standard for the diagnosis of sperm granuloma. Histology confirmed that there was a sperm core in the centre of the interstitium surrounded by inflammatory cells such as macrophages and lymphocytes, apoptotic cells and fibrous tissues in sperm granuloma. Empty tubules with vacuolation and cellular debris adjacent to the granuloma were also observed [28]. It needs to be associated with foreign body granuloma, inflammatory nodules in the vas deferens, nodular vasculitis (characterized by local hyperplasia of ductal structures after injury to the vas deferens) and supernumerary testis, and differentiated from neurofibromas and fibrosis. Sperm granuloma containing blood masquerading as a supernumerary testis showed by a case report, which histopathological examination of the right paratesticular mass revealed suture granuloma, sperm granuloma, vasitis nodosa, and fibrosis near the prior vasectomy site [27]. A study by Seppan and Krishnaswamy [30] suggests that in Macaca radiata the expansion of the epididymis and vas deferens is inversely proportional to the size of sperm granulomas. In the short term (6 months after the operation), the expansion was obvious, whereas sperm granulomas were small. In contrast, in the long term (2 years after the operation), the degree of expansion was relatively small, whereas sperm granulomas were large.
Sperm granulomas are usually not painful, and most are asymptomatic. Rayala and Viera [31] believed that the formation of a sperm granuloma is protective because it can prevent obstruction of the epididymis and testicles. Sperm granulomas play a role in PVPS and recanalization after failed vasectomy. Multiple epithelialized microtubules can form in sperm granulomas and can connect the two stumps of the vas deferens and reconstruct a channel for sperm, which leads to failure of the operation [11].
For vasectomy patients, refraining from ejaculation for 1 week after surgery can reduce the risk of formation of sperm granulomas [32]. Dutta et al [28] believed that testosterone deficiency may play an important role in the development of sperm granulomas, and testosterone supplementation can reduce inflammation and complications related to sperm granuloma. If the symptoms persist and conservative treatment is ineffective, surgical resection of the nodule where the pain is localized and burning and ligation of the stumps of the vas deferens after inflammation is controlled can often alleviate the pain and avoid its recurrence [33].
4. Antisperm antibodies
Vasectomy may lead to the exposure of sperm antigens to the immune system, which will stimulate an antisperm autoantibody reaction. Animal studies have found that AsAbs can lead to sperm agglutination and the activation of a complement cascade reaction, and immune complexes are thereby formed and deposited in the basement membrane [34]. At present, researcher believes that the reproductive duct is blocked after vasectomy, which will inevitably lead to semen deposition, an increase in pressure in the reproductive duct, expansion of epididymal tubules, and the formation of sperm granulomas due to sperm leakage or stimulation of epididymal endothelial cells [35]. This results in an increase in absorption of sperm by endothelial cells and an enhancement of phagocytosis, and stimulation of the sensitive immune system thus produces an immune response [35].
More than 60% of men develop circulating AsAbs within 6–8 weeks after vasectomy [36]. About 7%–30% of vasectomy patients also have AsAbs in the epididymis, which is due to destruction of the blood-testis barrier [36].
AsAbs have been found to affect sperm motility, the acrosome reaction, penetration of the cervical mucus, the binding of sperm to the zona pellucida, and sperm-egg fusion [37,38]. According to data from the WHO, about 15%–30% of the etiological classes of male infertility worldwide may be caused by immune factors, such as AsAbs in serum or seminal plasma, and the associated detection rate is about 20%–30% [39]. AsAbs can also decrease the pregnancy rate after VR. Existing data show that AsAbs found after vasectomy do not seem to increase the risk of immune system-related diseases, such as lupus erythematosus, scleroderma, and rheumatoid arthritis [40]. A study with an average follow-up period of 13 years also showed that in men who had undergone a vasectomy the risk of several immune system-related diseases did not increase for a long time [41]. These diseases included ankylosing spondylitis, asthma, diabetes mellitus, inflammatory bowel disease, multiple sclerosis, myasthenia gravis, rheumatoid arthritis, and thyrotoxicosis [41].
5. Vasectomy and prostate cancer
Many patients expressed concern that vasectomy may increase the risk of prostate cancer. Such reports have been discovered in the early 1990s [42], and data anomalies caused by various risks of bias have not been ruled out [42,43]. It was mainly focused on high-grade/low-grade cancer and lethality that increased overall risk of prostate cancer which reported by literatures (Table 2) [44,45,46,47]. It has also been reported that the incidence of prostate cancer after vasectomy exhibits a slight increase, but mortality after vasectomy has been markedly reduced [48]. The reason may be that men who choose vasectomy are more willing to undergo regular testing for prostate-specific antigen so as to exclude prostate cancer, which is also related to a healthier lifestyle.
Table 2. Studies of possible correlation between vasectomy and prostate cancer.
| Study | Study design | Time range (year) | Total subjects/subjects with prostate cancer (n) | Relative risk (95% confidence interval) | |
|---|---|---|---|---|---|
| Correlation found | Millard [42] | Review | 1985–1996 | 14 reports | 1.23 (1.01–1.49) |
| Dennis et al [43] | Meta-analysis | 1996–2001 | 24 reports | 1.37 (1.15–1.62) | |
| Siddiqui et al [44] | Prospective cohort | 1986–2010 | 49,405/6,023 | Overall risk 1.10 (1.04–1.17) | |
| Risk of high-grade disease 1.22 (1.03–1.45) | |||||
| Risk of fatal disease 1.19 (1.00–1.43) | |||||
| Davenport et al [45] | Prospective cohort | 1995–2011 | 111,914/13,885 | 1.05 (1.01–1.11) | |
| Husby et al [46] | Retrospective cohort | 1977–2014 | 2,150,162/26,238 | 1.15 (1.10–1.20) | |
| Rohrmann et al [47] | Prospective cohort | 1996–2004 | 3,373/78 | Overall risk 2.03 (1.24–3.32) | |
| Risk of low-grade disease 2.87 (1.49–5.54) | |||||
| Seikkula et al [48] | Prospective cohort | 1987–2014 | 38,124/413 | 1.15 (1.04–1.27) | |
| No correlation found | Liu et al [49] | Prospective cohort | Before 2014 | 1,127,096/7,539 | 1.08 (0.87–1.34) |
| Zhang et al [50] | Meta-analysis | 1966–2013 | 331,436/1,245 | 1.07 (0.79–1.46) | |
| Jacobs et al [51] | Prospective cohort | 1982–2012 | 363,726/7,451 | 1.01 (0.93–1.10) | |
| Nayan et al [52] | Matched cohort study | 1994–2012 | 326,607/3,462 | 1.02 (0.95–1.09) | |
| Shang et al [53] | Meta-analysis | 1980–2015 | 429,914/7,027 | 1.11 (0.98–1.27) | |
| Bhindi et al [54] | Systematic review | Before 2017 | 2,563,519/44,536 | 1.05 (1.02–1.09) | |
| Randall et al [55] | Systematic review | - | 684,660/9,754 | 0.92 (0.70–1.21) | |
| Smith et al [56] | Prospective investigation | 1992–2000 | 84,753/4,377 | 1.05 (0.96–1.15) | |
| DeAntoni et al [57] | Cross-sectional study | 1993–1995 | 95,961/2,530 | 0.93 (0.77–1.14) | |
| Ferrís-I-Tortajada et al [58] | Retrospective study | 1985–2010 | 42,425 | 1.1 (0.9–1.4) | |
| Bernal-Delgado et al [59] | Systematic review | 1985–1996 | 14 reports | 1.23 (1.01–1.49) | |
| Holt et al [60] | Retrospective study | 2002–2005 | 1,001 | 1.0 (0.8–1.2) |
However, results reported in recent years from a large number of cohort studies, systematic reviews, prospective studies, and cross-sectional studies show that vasectomy does not increase the risk of prostate cancer (Table 2) [49,50,51,52,53,54,55,56,57,58,59,60]. Interestingly, six medical institutions in Yichang city in China carried out a retrospective study on 3186 patients, which found that vasectomy can reduce the incidence of prostate cancer in the elderly according to their age groups [61].
In studies of a possible correlation between vasectomy and prostate cancer, most positive results showed that any correlation was weak and there was no convincing biological mechanism. Some studies believe that the physiological changes after vasectomy may explain its relationship with prostate cancer, such as immune changes (AsAbs), endocrine changes (changing levels of circulating androgens), local growth factor production (epidermal growth factor), etc. [62]. Therefore, according to our review, vasectomy has no direct correlation with the risk of prostate cancer and will hence not increase the risk of prostate cancer, and it can still be regarded as a safe method of contraception.
6. Sexual dysfunction
Many patients are concerned about the association between vasectomy and sexual function and worry that the quality of their sexual lives might be affected after surgery. Fortunately, most studies have thus far shown that vasectomy does not affect sexual function or can even improve it. Study carried out in the 1980s showed that vasectomy had a positive psychological effect on patients, improving their sexual lives, harmony between couples, and sexual desire and increasing the frequency of sexual intercourse [63]. The male psychologic response to vasectomy suggested two main responses: a decreased anxiety of unplanned pregnancy and a desire to compensate for the perceived “demascu-linization” of the vasectomy procedure. Several studies undertaken in recent years have also confirmed that men after undergoing a vasectomy experience markedly improved erectile function, orgasms, and sexual satisfaction and feel safer and more confident in their sexual lives after surgery [64,65]. Their female partners reported marked improvements in terms of sexual arousal, satisfaction, and orgasm, as well as lubrication and libido [65]. The mechanisms underlying these favorable effects are most likely the disappearance of the reproductive burden and the fear of unwanted pregnancies. Thereafter, female partners are able to have a more relaxed approach to sexual activity. In addition, Guo et al [66] showed that men who had undergone a vasectomy experienced more instances of sexual contact per month than men who had not undergone a vasectomy.
Relatively report has shown that men are more likely to develop symptoms of depression and anxiety after vasectomy [67]. Performance anxiety can be caused by several factors, such as stress and fear, which result in the release of the neurohormones adrenaline and noradrenaline, with consequent contraction of the smooth muscles in the corpus cavernosum that results in detumescence and thus the inability to maintain an erection long enough to complete sexual intercourse [65].
7. Cardiovascular disease after surgery
In the 1980s, Clarkson and Alexander [68] reported that vasectomy can accelerate the development of diet-induced arteriosclerosis in monkeys, An association between vasectomy and cardiovascular disease was first proposed. Bhatia et al [69] reported substantial increases in serum levels of cholesterol and triglycerides in rabbits that had undergone a vasectomy. However, a large number of studies have confirmed (including Clarkson's research) that there is no association between vasectomy and cardiovascular disease or related factors (even if the time after surgery exceeds 20 years) (Table 3) [40,68,70,71,72,73,74,75,76,77]. These factors include body mass index, cholesterol, triglycerides, protein, albumin and high-density lipoprotein levels, and the ratio of globulin.
Table 3. Studies of possible correlation between vasectomy and risk factors for cardiovascular disease.
| Authors | Study design | Age range (y) | Total subject | Relative risk (95% CI) | Correlation |
|---|---|---|---|---|---|
| Coady et al [70] | Prospective study | 45–64 | 3,957 | CVD 1.1 (0.8–1.5) | No |
| Zhao et al [73] | Case-control study | 40–59 | 485 | BMI 0.53 (0.16–0.90) | No |
| Goldacre et al [74] | Retrospective study | 20–59 | 24,773 | Coronary heart disease 0.95 (0.88–1.02); >20 years after vasectomy 0.98 (0.80–1.19) | No |
| Xiong et al [75] | Case-control study | ≥40 | 261 | TG 0.041 (−0.111–0.301); TCH 0.015 (−0.184 to 0.253); LDL −0.063 (−0.242 to 0.050); HDL −0.236 (−0.258 to 0.119) | No |
| Guo et al [76] | Systematic review | - | 299,436 | CVD 0.90 (0.81–1.00); myocardial infarction 0.95 (0.88–1.02); coronary heart disease 0.94 (0.88–1.01) | No |
CI: confidence interval, CVD: cardiovascular disease, BMI: body mass index, TG: total triglycerides, TCH: total cholesterol, LDL: low-density lipoprotein, HDL: high-density lipoprotein.
It was speculated that an AsAb immune complex that forms as a result of vasectomy may exacerbate atherosclerosis [68]. However, the potential biological mechanisms underlying the observed association between vasectomy and CVD risk remain unclear. Overall, a substantial amount of evidence demonstrates that there is no association between atherosclerotic coronary heart disease, hyperlipidemia-related factors, and vasectomy.
8. Reproductive hormones
We should pay attention to the change of testoster-one level after vasectomy, because it will affect the testicular spermatogenesis function. Early animal experiments showed that testosterone levels temporarily decreased [78,79,80,81] within a short period of time (about three months) after vasectomy, possibly due to the disruption of the dynamic balance of the blood-testis barrier after vasectomy and the epididymal. The structure is impaired and its function impaired, which leads to an increase in local interleukin-1 (IL-1) levels. After IL-1 levels increase, the effect of human chorionic gonadotropin on the secretion of testosterone from Leydig cells is inhibited, resulting in a brief decrease in blood testosterone levels [82].
However, previous long-term evidence from research on animals and humans suggests that vasectomy does not affect reproductive hormones [83]. Results from recent years show that the endocrine function of the testis was not affected in the short term after vasectomy in adult rats, and there were no significant changes in plasma concentrations of luteinizing hormone (LH), follicle-stimulating hormone, and testosterone after vasectomy [28]. A survey in China of 485 men who had undergone a vasectomy and 1,940 men who had not undergone a vasectomy showed that the levels of LH and free testosterone and the testosterone secretion index were not significantly different but the level of total testosterone was significantly different (p=0.02) between the two groups [73]. These results also show that vasectomy does not increase the risk of developing late-onset hypogonadism.
Therefore, we conclude that testicular reproductive hormones will decrease in a short time after vasectomy, but will slowly return to normal levels, which is good news for patients who want to get pregnant again.
9. Sperm injury and options for future pregnancy
The effect of vasectomy on sperm quality is positive. The sperm motility and number were decreased, sperm DNA fragmentation was increased, sperm production was inhibited, germ cell apoptosis increased after vasectomy, and sperm granuloma and abnormal sperm formation were induced [84,85]. There is no correlation between the Y chromosome microdeletion which causes abnormal sperm quality and vasectomy [86]. Endothelial nitric oxide synthase and inducible nitric oxide synthase may play a key role in apoptosis of germ cells after vasectomy [87]. The extent of sperm damage after vasectomy is related to the time since vasectomy. The longer is the duration since vasectomy, the greater are the negative effects on semen quality and fertility [88].
The extent of sperm damage after vasectomy is related to the time since vasectomy. The longer is the duration since vasectomy, the greater are the negative effects on semen quality and fertility [87]. O'Neill et al [85] reported that yields of sperm from men who had been vasectomized for more than 5 years were markedly reduced in comparison with those from fertile men (as observed from biopsies) and that sperm after vasectomy displayed an increase in DNA fragmentation [88].
Belker et al [89] reported a negative correlation between time since vasectomy and the probability of postoperative pregnancy. Such results also appeared in studies of intracytoplasmic sperm injection (ICSI) with testicular sperm extraction [90], in which the success rate of assisted conception was affected 10 years after vasectomy [86]. However, research has shown that the age of the woman rather than the time since vasectomy is the main determinant of success in in vitro fertilization (IVF)–embryo transfer and ICSI in cases of obstructive azoospermia after vasectomy [90]. A recent report also showed that there is no correlation between obstructive azoospermia caused by vasectomy and the success of ICSI with sperm retrieval [91].
Vasovasostomy (VV) and assisted reproductive technology (ART) are two main options for couples who want to achieve a pregnancy after vasectomy. VV can be the first step, and if a couple do not achieve a pregnancy naturally within 18 months after VV, ART can be employed to increase the chance of pregnancy [92]. Conversely, if the female partner is over 35 years old it may be more prudent to consider IVF/ICSI [92]. The cumulative pregnancy rate for VV is 28%–40%, and that for ICSI is between 60% and 80%, whereas the rate for a single cycle is 29%–41% [93].
10. Semen analysis and follow-up care after vasectomy
The best time for post-vasectomy semen analysis (PVSA) has for a long time been a topic of debate. PVSA is an important part of patient follow-up after vasectomy. A study has shown that as many as 33% of patients have no sperm when semen is tested 12 weeks after vasectomy [94]. The American Urological Association guideline (2012) recommends that 8–16 weeks is an appropriate timeframe for performing PVSA [13]. Griffin et al [95] believed that PVSA should be given priority within 3 months after surgery and that there should be a sufficient number of ejaculations (at least 20) in this period. Some studies suggested that PVSA at 12 weeks is more reliable than after more than 20 ejaculations. The proportion of men who cannot release sperm at 12 weeks is 20% higher than that among men who have ejaculated more than 20 times [96].
The presence of motile sperm in semen at 3–6 months after surgery has been defined as early recanalization, which has a probability of 0.36%. If the length of the vas deferens excised is in the range of 5–20 mm, it is not related to the risk of recanalization. Recanalization is basically impossible if the excised length is ≥40 mm [96]. If no sperm are found or a small number of immotile sperm are present within 3 months, no further semen analysis is required. If motile sperm are found or the sperm concentration exceeds 100,000/mL, testing should be repeated at intervals of 6 weeks until no more sperm are found or immotile sperm are obtained with a sperm concentration of <100,000/mL [97]. Dohle et al [14] recommended repeat vasectomy if motile sperm persist after 6 months of follow-up.
Eighty percent of vasectomy patients resume normal activity within 1 week. After the operation, patients are advised to rest for 24 hours, avoid cycling for 7 days, and wear tight underwear for 48 hours [98]. Men are encouraged to apply ice to the scrotum continuously for 24–48 hours and minimize their amount of exercise for one week [99].
CONCLUSIONS
Numerous reports have confirmed that vasectomy is a safe, reliable, and low-complication method for male birth control. Although the short-term complications of vasectomy cannot be ignored, such as hematoma, pain, and infection, especially postoperative pain that a small number of patients may suffer from it for life; meanwhile, there is no increased risk with vasectomy and autoimmune disease, cardiovascular disease, prostate cancer and sexual dysfunction. But long-term observation is still needed to obtain more evidence. It is important for clinicians to disseminate this information for education to reduce the risk of vasectomy and encourage vasectomy for male sterilization.
ACKNOWLEDGEMENTS
This research was supported by the National Natural Science Foundation of China (Grant no. 81673808 and no.81973647); and the Chengdu University of Traditional Chinese Medicine Foundation (Grant no. 2018yky12; 2017-EL-23; 2017-EL-21).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: FY, XY.
- Data curation: FY.
- Methodology: XY, LD.
- Supervision: DC, XL.
- Writing — original draft: FY, KT, JL.
- Writing — review & editing: PZ, XH.
References
- 1.Barone MA, Hutchinson PL, Johnson CH, Hsia J, Wheeler J. Vasectomy in the United States, 2002. J Urol. 2006;176:232–236. doi: 10.1016/S0022-5347(06)00507-6. discussion 236. [DOI] [PubMed] [Google Scholar]
- 2.World contraceptive use 2007. New York: United Nations; 2008. [Google Scholar]
- 3.Cook LA, Van Vliet H, Lopez LM, Pun A, Gallo MF. Vasectomy occlusion techniques for male sterilization. Cochrane Database Syst Rev. 2007;(2):CD003991. doi: 10.1002/14651858.CD003991.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Cook LA, Pun A, van Vliet H, Gallo MF, Lopez LM. Scalpel versus no-scalpel incision for vasectomy. Cochrane Database Syst Rev. 2007;(2):CD004112. doi: 10.1002/14651858.CD004112.pub3. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Department of Reproductive Health and Research (WHO/RHR) and Johns Hopkins Bloomberg School of Public Health/Center for Communication Programs (CCP), INFO Project. Family planning: a global handbook for providers. Baltimore and Geneva: CCP and WHO; 2007. pp. 183–198. [Google Scholar]
- 6.Sokal D, Irsula B, Hays M, Chen-Mok M, Barone MA Investigator Study Group. Vasectomy by ligation and excision, with or without fascial interposition: a randomized controlled trial. BMC Med. 2004;2:6. doi: 10.1186/1741-7015-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen-Mok M, Bangdiwala SI, Dominik R, Hays M, Irsula B, Sokal DC. Termination of a randomized controlled trial of two vasectomy techniques. Control Clin Trials. 2003;24:78–84. doi: 10.1016/s0197-2456(02)00267-2. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt SS, Minckler TM. The vas after vasectomy: comparison of cauterization methods. Urology. 1992;40:468–470. doi: 10.1016/0090-4295(92)90468-c. [DOI] [PubMed] [Google Scholar]
- 9.Pant PR, Sharma J, Subba S. Scrotal haematoma: the most common complication of no-scalpel vasectomy. Kathmandu Univ Med J (KUMJ) 2007;5:279–280. [PubMed] [Google Scholar]
- 10.Labrecque M, Nazerali H, Mondor M, Fortin V, Nasution M. Effectiveness and complications associated with 2 vasectomy occlusion techniques. J Urol. 2002;168:2495–2498. doi: 10.1016/S0022-5347(05)64176-6. discussion 2498. [DOI] [PubMed] [Google Scholar]
- 11.Awsare NS, Krishnan J, Boustead GB, Hanbury DC, McNicholas TA. Complications of vasectomy. Ann R Coll Surg Engl. 2005;87:406–410. doi: 10.1308/003588405X71054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook LA, Van Vliet HA, Pun A, Gallo MF. Vasectomy techniques for male sterilization: systematic Cochrane review of randomized controlled trials and controlled clinical trials. Hum Reprod. 2004;19:2431–2438. doi: 10.1093/humrep/deh484. [DOI] [PubMed] [Google Scholar]
- 13.Sharlip ID, Belker AM, Honig S, Labrecque M, Marmar JL, Ross LS, et al. Vasectomy: AUA guideline. J Urol. 2012;188(6 Suppl):2482–2491. doi: 10.1016/j.juro.2012.09.080. [DOI] [PubMed] [Google Scholar]
- 14.Dohle GR, Diemer T, Kopa Z, Krausz C, Giwercman A, Jungwirth A Grupo de Trabajo de la Asociación Europea de Urología sobre la Infertilidad Masculina. [European Association of Urology guidelines on vasectomy] Actas Urol Esp. 2012;36:276–281. doi: 10.1016/j.acuro.2012.01.005. Spanish. [DOI] [PubMed] [Google Scholar]
- 15.Benger JR, Swami SK, Gingell JC. Persistent spermatozoa after vasectomy: a survey of British urologists. Br J Urol. 1995;76:376–379. [PubMed] [Google Scholar]
- 16.Nirapathpongporn A, Huber DH, Krieger JN. No-scalpel vasectomy at the King's birthday vasectomy festival. Lancet. 1990;335:894–895. doi: 10.1016/0140-6736(90)90487-p. [DOI] [PubMed] [Google Scholar]
- 17.Forste R, Tanfer K, Tedrow L. Sterilization among currently married men in the United States, 1991. Fam Plann Perspect. 1995;27:100–107. [PubMed] [Google Scholar]
- 18.Kols A, Lande R. Vasectomy: reaching out to new users. Baltimore: Johns Hopkins Bloomberg School of Public Health Center for Communication Programs, INFO Project; 2008. [Google Scholar]
- 19.Cook LA, Pun A, Gallo MF, Lopez LM, Van Vliet HA. Scalpel versus no-scalpel incision for vasectomy. Cochrane Database Syst Rev. 2014;2014:CD004112. doi: 10.1002/14651858.CD004112.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein M. Surgical management of male infertility and other scrotal disorders. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ, editors. Campbell's urology vol. 2. 7th ed. Philadelphia: W. B. Saunders Co.; 1998. p. 6. [Google Scholar]
- 21.Tan WP, Levine LA. An overview of the management of post-vasectomy pain syndrome. Asian J Androl. 2016;18:332–337. doi: 10.4103/1008-682X.175090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabanegh ES, Thomas AJ. Microsurgical treatment of male infertility. In: Lipshultz L, Howards SS, Niederberger CS, editors. Infertility in the male. 4th ed. Cambridge: Cambridge University Press; 2008. pp. 392–406. [Google Scholar]
- 23.Tandon S, Sabanegh E., Jr Chronic pain after vasectomy: a diagnostic and treatment dilemma. BJU Int. 2008;102:166–169. doi: 10.1111/j.1464-410X.2008.07602.x. [DOI] [PubMed] [Google Scholar]
- 24.Sinha V, Ramasamy R. Post-vasectomy pain syndrome: diagnosis, management and treatment options. Transl Androl Urol. 2017;6(Suppl 1):S44–S47. doi: 10.21037/tau.2017.05.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JY, Cho KS, Lee SH, Cho HJ, Cho JM, Oh CY, et al. A comparison of epididymectomy with vasectomy reversal for the surgical treatment of postvasectomy pain syndrome. Int Urol Nephrol. 2014;46:531–537. doi: 10.1007/s11255-013-0517-9. [DOI] [PubMed] [Google Scholar]
- 26.Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol. 2008;180:949–953. doi: 10.1016/j.juro.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Su JS, Farber NJ, Feldman MK, Vij SC. Sperm granuloma masquerading as a supernumerary testis. Urol Case Rep. 2019;29:101080. doi: 10.1016/j.eucr.2019.101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta D, Park I, Guililat H, Sang S, Talapatra A, Hanson L, et al. Ethylene dimethane sulfonate (EDS) ablation of Leydig cells in adult rat depletes testosterone resulting in epididymal sperm granuloma: testosterone replacement prevents granuloma formation. Reprod Biol. 2019;19:89–99. doi: 10.1016/j.repbio.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Pienkos EJ. The use of testosterone in the treatment of chronic postvasectomy pain syndrome: case report and review of the literature. Mil Med. 2007;172:676–679. doi: 10.7205/milmed.172.6.676. [DOI] [PubMed] [Google Scholar]
- 30.Seppan P, Krishnaswamy K. Long-term study of vasectomy in Macaca radiata--histological and ultrasonographic analysis of and duct system. Syst Biol Reprod Med. 2014;60:151–160. doi: 10.3109/19396368.2014.896957. [DOI] [PubMed] [Google Scholar]
- 31.Rayala BZ, Viera AJ. Common questions about vasectomy. Am Fam Physician. 2013;88:757–761. [PubMed] [Google Scholar]
- 32.Lowe G. Optimizing outcomes in vasectomy: How to ensure sterility and prevent complications. Transl Androl Urol. 2016;5:176–180. doi: 10.21037/tau.2016.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu XZ, Li SQ. Complications and long-term safety of vasectomy. J Int Reprod Health Fam Plan. 2009;28:390–393. [Google Scholar]
- 34.Alexander NJ. Vasectomy: long-term effects in the rhesus monkey. J Reprod Fertil. 1972;31:399–406. doi: 10.1530/jrf.0.0310399. [DOI] [PubMed] [Google Scholar]
- 35.McLachlan RI. Basis, diagnosis and treatment of immunological infertility in men. J Reprod Immunol. 2002;57:35–45. doi: 10.1016/s0165-0378(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 36.Chehval MJ, Doshi R, Kidd CF, Winkelmann T, Chehval V. Antisperm autoantibody response after unilateral vas deferens ligation in rats: When does it develop? J Androl. 2002;23:669–673. [PubMed] [Google Scholar]
- 37.Barratt CL, Havelock LM, Harrison PE, Cooke ID. Antisperm antibodies are more prevalent in men with low sperm motility. Int J Androl. 1989;12:110–116. doi: 10.1111/j.1365-2605.1989.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 38.Rajah SV, Parslow JM, Howell RJ, Hendry WF. The effects on in-vitro fertilization of autoantibodies to spermatozoa in subfertile men. Hum Reprod. 1993;8:1079–1082. doi: 10.1093/oxfordjournals.humrep.a138196. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. WHO Manual for the standardized investigation, diagnosis and management of the infertile male. Cambrige: Cambirge University Press; 2006. pp. 11–46. [Google Scholar]
- 40.Massey FJ, Jr, Bernstein GS, O'Fallon WM, Schuman LM, Coulson AH, Crozier R, et al. Vasectomy and health. Results from a large cohort study. JAMA. 1984;252:1023–1029. doi: 10.1001/jama.252.8.1023. [DOI] [PubMed] [Google Scholar]
- 41.Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Immune-related disease before and after vasectomy: an epidemiological database study. Hum Reprod. 2007;22:1273–1278. doi: 10.1093/humrep/dem010. [DOI] [PubMed] [Google Scholar]
- 42.Millard PS. Review: Bias may contribute to association of vasectomy with prostate cancer. West J Med. 1999;171:91. [PMC free article] [PubMed] [Google Scholar]
- 43.Dennis LK, Dawson DV, Resnick MI. Vasectomy and the risk of prostate cancer: a meta-analysis examining vasectomy status, age at vasectomy, and time since vasectomy. Prostate Cancer Prostatic Dis. 2002;5:193–203. doi: 10.1038/sj.pcan.4500586. [DOI] [PubMed] [Google Scholar]
- 44.Siddiqui MM, Wilson KM, Epstein MM, Rider JR, Martin NE, Stampfer MJ, et al. Vasectomy and risk of aggressive prostate cancer: a 24-year follow-up study. J Clin Oncol. 2014;32:3033–3038. doi: 10.1200/JCO.2013.54.8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davenport MT, Zhang CA, Leppert JT, Brooks JD, Eisenberg ML. Vasectomy and the risk of prostate cancer in a prospective US Cohort: data from the NIH-AARP Diet and Health Study. Andrology. 2019;7:178–183. doi: 10.1111/andr.12570. [DOI] [PubMed] [Google Scholar]
- 46.Husby A, Wohlfahrt J, Melbye M. Vasectomy and prostate cancer risk: a 38-year nationwide cohort study. J Natl Cancer Inst. 2020;112:71–77. doi: 10.1093/jnci/djz099. [DOI] [PubMed] [Google Scholar]
- 47.Rohrmann S, Paltoo DN, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Association of vasectomy and prostate cancer among men in a Maryland cohort. Cancer Causes Control. 2005;16:1189–1194. doi: 10.1007/s10552-005-0304-8. [DOI] [PubMed] [Google Scholar]
- 48.Seikkula H, Kaipia A, Hirvonen E, Rantanen M, Pitkäniemi J, Malila N, et al. Vasectomy and the risk of prostate cancer in a Finnish nationwide population-based cohort. Cancer Epidemiol. 2020;64:101631. doi: 10.1016/j.canep.2019.101631. [DOI] [PubMed] [Google Scholar]
- 49.Liu LH, Kang R, He J, Zhao SK, Li FT, Wan SP, et al. Vasectomy and risk of prostate cancer: a systematic review and meta-analysis of cohort studies. Andrology. 2015;3:643–649. doi: 10.1111/andr.12040. [DOI] [PubMed] [Google Scholar]
- 50.Zhang XL, Yan JJ, Pan SH, Pan JG, Ying XR, Zhang GF. Vasectomy and the risk of prostate cancer: a meta-analysis of cohort studies. Int J Clin Exp Med. 2015;8:17977–17985. [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobs EJ, Anderson RL, Stevens VL, Newton CC, Gansler T, Gapstur SM. Vasectomy and prostate cancer incidence and mortality in a large US cohort. J Clin Oncol. 2016;34:3880–3885. doi: 10.1200/JCO.2015.66.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nayan M, Hamilton RJ, Macdonald EM, Li Q, Mamdani MM, Earle CC, et al. Vasectomy and risk of prostate cancer: population based matched cohort study. BMJ. 2016;355:i5546. doi: 10.1136/bmj.i5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shang Y, Han G, Li J, Zhao J, Cui D, Liu C, et al. Vasectomy and prostate cancer risk: a meta-analysis of cohort studies. Sci Rep. 2015;5:9920. doi: 10.1038/srep09920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhindi B, Wallis CJD, Nayan M, Farrell AM, Trost LW, Hamilton RJ, et al. The association between vasectomy and prostate cancer: a systematic review and meta-analysis. JAMA Intern Med. 2017;177:1273–1286. doi: 10.1001/jamainternmed.2017.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Randall S, Boyd J, Fuller E, Brooks C, Morris C, Earle CC, et al. The effect of vasectomy reversal on prostate cancer risk: international meta-analysis of 684,660 vasectomized men. J Urol. 2018;200:121–125. doi: 10.1016/j.juro.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Smith K, Byrne, Castaño JM, Chirlaque MD, Lilja H, Agudo A, et al. Vasectomy and prostate cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) J Clin Oncol. 2017;35:1297–1303. doi: 10.1200/JCO.2016.70.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeAntoni EP, Göktaş S, Stenner J, O'Donnell C, Crawford ED. A cross-sectional study of vasectomy, time since vasectomy and prostate cancer. Prostate Cancer Prostatic Dis. 1997;1:73–78. doi: 10.1038/sj.pcan.4500209. [DOI] [PubMed] [Google Scholar]
- 58.Ferrís-I-Tortajada J, Berbel-Tornero O, Garcia-I-Castell J, López-Andreu JA, Sobrino-Najul E, Ortega-García JA. [Non dietetic environmental risk factors in prostate cancer] Actas Urol Esp. 2011;35:289–295. doi: 10.1016/j.acuro.2010.12.010. Spanish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernal-Delgado E, Latour-Pérez J, Pradas-Arnal F, Gómez-López LI. The association between vasectomy and prostate cancer: a systematic review of the literature. Fertil Steril. 1998;70:191–200. doi: 10.1016/s0015-0282(98)00142-3. [DOI] [PubMed] [Google Scholar]
- 60.Holt SK, Salinas CA, Stanford JL. Vasectomy and the risk of prostate cancer. J Urol. 2008;180:2565–2567. doi: 10.1016/j.juro.2008.08.042. discussion 2567–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nie Y, Liu CR, Guo XK, Yan KJ, Du D, Gao WY, et al. [Clinical study of long term effects of vasectomy on the incidence rate of prostate cancer] J Clin Urol. 2008;24:424–427. Chinese. [Google Scholar]
- 62.Pereira S, Martinez M, Martinez FE, Júnior WM. Repercussions of castration and vasectomy on the ductal system of the rat ventral prostate. Cell Biol Int. 2006;30:169–174. doi: 10.1016/j.cellbi.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 63.Leavesley JH. A study of vasectomized men and their wives. Aust Fam Physician. 1980;9:8–10. [PubMed] [Google Scholar]
- 64.Engl T, Hallmen S, Beecken WD, Rubenwolf P, Gerharz EW, Vallo S. Impact of vasectomy on the sexual satisfaction of couples: experience from a specialized clinic. Cent European J Urol. 2017;70:275–279. doi: 10.5173/ceju.2017.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertero E, Hallak J, Gromatzky C, Lucon AM, Arap S. Assessment of sexual function in patients undergoing vasectomy using the international index of erectile function. Int Braz J Urol. 2005;31:452–458. doi: 10.1590/s1677-55382005000500006. [DOI] [PubMed] [Google Scholar]
- 66.Guo DP, Lamberts RW, Eisenberg ML. Relationship between vasectomy and sexual frequency. J Sex Med. 2015;12:1905–1910. doi: 10.1111/jsm.12962. [DOI] [PubMed] [Google Scholar]
- 67.Shaik S, Rajkumar RP. Post-vasectomy depression: a case report and literature review. Ment Illn. 2014;6:5494. doi: 10.4081/mi.2014.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarkson TB, Alexander NJ. Long-term vasectomy: effects on the occurrence and extent of atherosclerosis in rhesus monkeys. J Clin Invest. 1980;65:15–25. doi: 10.1172/JCI109645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhatia ND, Chakravarti RN, Majumdar S. Immune response to spermatozoal antigens in vasectomized normal and atherogenic diet fed rabbits. Indian J Med Res. 1981;73:910–915. [Google Scholar]
- 70.Coady SA, Sharrett AR, Zheng ZJ, Evans GW, Heiss G. Vasectomy, inflammation, atherosclerosis and long-term followup for cardiovascular diseases: no associations in the atherosclerosis risk in communities study. J Urol. 2002;167:204–207. [PubMed] [Google Scholar]
- 71.Chi IC, Ko UR, Wilkens LR, Chang HK, Nam JJ. Vasectomy and non-fatal acute myocardial infarction: a hospital-based case-control study in Seoul, Korea. Int J Epidemiol. 1990;19:32–41. doi: 10.1093/ije/19.1.32. [DOI] [PubMed] [Google Scholar]
- 72.Mullooly JP, Wiest WM, Alexander NJ, Greenlick MR, Fulgham DL. Vasectomy, serum assays, and coronary heart disease symptoms and risk factors. J Clin Epidemiol. 1993;46:101–109. doi: 10.1016/0895-4356(93)90014-r. [DOI] [PubMed] [Google Scholar]
- 73.Zhao K, Wu L, Kong X, Chen Y, Li H, Gu Y, et al. Long-term safety, health and mental status in men with vasectomy. Sci Rep. 2018;8:15703. doi: 10.1038/s41598-018-33989-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldacre MJ, Wotton CJ, Seagroatt V, Yeates D. Cancer and cardiovascular disease after vasectomy: an epidemiological database study. Fertil Steril. 2005;84:1438–1443. doi: 10.1016/j.fertnstert.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 75.Xiong SM, Zhou Y, Liu F, Shi Y, Yu Q, Dai L, et al. Long-term effects of vasectomy on lipid metabolism in aging males. Chin J Reprod Contracept. 2018;38:44–48. [Google Scholar]
- 76.Guo ZL, Xu JL, Lai RK, Wang SS. Vasectomy and cardiovascular disease risk: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7852. doi: 10.1097/MD.0000000000007852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guan HT, Qing XR, Zhang HP, Zhao K, Kong XG, Chen YP, et al. Effect of vasectomy on serum lipids, renal and hepatic function in middle-aged and elderly men. Chin J Fam Plan Gynecotokol. 2014;6:5–8. [Google Scholar]
- 78.Zhu CC, Tang B, Su J, Zhao H, Bu X, Li Z, et al. Abnormal accumulation of collagen type I due to the loss of discoidin domain receptor 2 (Ddr2) promotes testicular interstitial dysfunction. PLoS One. 2015;10:e0131947. doi: 10.1371/journal.pone.0131947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duru FI, Ajayi S, Azu OO. The effect of unilateral vasectomy on testosterone and testicular parameters in the adult male African giant rat (Cricetomys gambianus) Afr Health Sci. 2013;13:483–489. doi: 10.4314/ahs.v13i2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hinz S, Rais-Bahrami S, Kempkensteffen C, Weiske WH, Miller K, Magheli A. Effect of obesity on sex hormone levels, antisperm antibodies, and fertility after vasectomy reversal. Urology. 2010;76:851–856. doi: 10.1016/j.urology.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 81.Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril. 2008;90:619–626. doi: 10.1016/j.fertnstert.2007.07.1292. [DOI] [PubMed] [Google Scholar]
- 82.Zhou Y. Ultrasound diagnosis of epididymosis. J Med Imaging. 2010;20:197. [Google Scholar]
- 83.Alexander NJ, Free MJ, Paulsen CA, Buschbom R, Fulgham DL. A comparison of blood chemistry, reproductive hormones and the development of antisperm antibodies after vasectomy in men. J Androl. 1980;1:40–50. [Google Scholar]
- 84.Cook LA, Van Vliet HA, Lopez LM, Pun A, Gallo MF. Vasectomy occlusion techniques for male sterilization. Cochrane Database Syst Rev. 2014;2014:CD003991. doi: 10.1002/14651858.CD003991.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O'Neill DA, McVicar CM, McClure N, Maxwell P, Cooke I, Pogue KM, et al. Reduced sperm yield from testicular biopsies of vasectomized men is due to increased apoptosis. Fertil Steril. 2007;87:834–841. doi: 10.1016/j.fertnstert.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 86.Ghirelli-Filho M, Marchi PL, Mafra FA, Cavalcanti V, Christofolini DM, Barbosa CP, et al. Incidence of Y-chromosome microdeletions in children whose fathers underwent vasectomy reversal or in vitro fertilization with epididymal sperm aspiration: a case-control study. Einstein (Sao Paulo) 2016;14:534–540. doi: 10.1590/S1679-45082016AO3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McVicar CM, O'Neill DA, McClure N, Clements B, McCullough S, Lewis SE. Effects of vasectomy on spermatogenesis and fertility outcome after testicular sperm extraction combined with ICSI. Hum Reprod. 2005;20:2795–2800. doi: 10.1093/humrep/dei138. [DOI] [PubMed] [Google Scholar]
- 88.Urry RL, Heaton JB, Moore M, Middleton RG. A fifteen-year study of alterations in semen quality occurring after vasectomy reversal. Fertil Steril. 1990;53:341–345. doi: 10.1016/s0015-0282(16)53292-0. [DOI] [PubMed] [Google Scholar]
- 89.Belker AM, Thomas AJ, Jr, Fuchs EF, Konnak JW, Sharlip ID. Results of 1,469 microsurgical vasectomy reversals by the Vasovasostomy Study Group. J Urol. 1991;145:505–511. doi: 10.1016/s0022-5347(17)38381-7. [DOI] [PubMed] [Google Scholar]
- 90.Nicopoullos JD, Gilling-Smith C, Almeida PA, Ramsay JW. Effect of time since vasectomy and maternal age on intracytoplasmic sperm injection success in men with obstructive azoospermia after vasectomy. Fertil Steril. 2004;82:367–373. doi: 10.1016/j.fertnstert.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 91.Blok JM, van Roekel C, Oude Ophuis RJA, Lock TMTW. Open epididymal spermatozoa aspiration for obstructive azoospermia. Andrologia. 2019;51:e13218. doi: 10.1111/and.13218. [DOI] [PubMed] [Google Scholar]
- 92.Valerie U, De Brucker S, De Brucker M, Vloeberghs V, Drakopoulos P, Santos-Ribeiro S, et al. Pregnancy after vasectomy: surgical reversal or assisted reproduction? Hum Reprod. 2018;33:1218–1227. doi: 10.1093/humrep/dey101. [DOI] [PubMed] [Google Scholar]
- 93.Mroue S, Delaloye JF, Wunder D. [New child wish after vasectomy: vasovasostomy or assisted reproductive medicine?] Rev Med Suisse. 2010;6:2030–2032. [PubMed] [Google Scholar]
- 94.Smith AG, Crooks J, Singh NP, Scott R, Lloyd SN. Is the timing of post-vasectomy seminal analysis important? Br J Urol. 1998;81:458–460. doi: 10.1046/j.1464-410x.1998.00563.x. [DOI] [PubMed] [Google Scholar]
- 95.Griffin T, Tooher R, Nowakowski K, Lloyd M, Maddern G. How little is enough? The evidence for post-vasectomy testing. J Urol. 2005;174:29–36. doi: 10.1097/01.ju.0000161595.82642.fc. [DOI] [PubMed] [Google Scholar]
- 96.Liu XZ, Li SQ. Progress in clinical application of vasectomy. Chin J Androl. 2006;(10):1–3. [Google Scholar]
- 97.Korthorst RA, Consten D, van Roijen JH. Clearance after vasectomy with a single semen sample containing < than 100 000 immotile sperm/mL: analysis of 1073 patients. BJU Int. 2010;105:1572–1575. doi: 10.1111/j.1464-410X.2009.09074.x. [DOI] [PubMed] [Google Scholar]
- 98.Bhuyan K, Ali I, Barua SJ. Role of no scalpel vasectomy in male sterilization. Indian J Surg. 2012;74:284–287. doi: 10.1007/s12262-011-0401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johnson D, Sandlow JI. Vasectomy: tips and tricks. Transl Androl Urol. 2017;6:704–709. doi: 10.21037/tau.2017.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]