Abstract
Androgen deprivation therapy (ADT) is the gold standard treatment in patients with locally advanced or metastatic prostate cancer (PC). Emerging evidence has documented a tight association between ADT and body composition, along with metabolic profile impairment. These alterations might underpin the observed ADT-related increase in cardiovascular (CV) and thromboembolic (venous thromboembolism, VTE) mortality and morbidity. However, the specific mechanisms underlying these associations have not yet been completely elucidated. In the present review we summarize and discussed the available evidence linking ADT to increased cardio-metabolic risk, using both preclinical and clinical data. When possible, meta-analytic studies were preferred. Preclinical evidence, using a rabbit model of gonadotrophin-releasing hormone analogue-induced hypogonadism, indicates that the induced condition is associated with a dramatic increase in visceral adiposity and with an impairment of acetylcholine induced vascular relaxation, along with an increased propensity towards fatty liver. This suggests a direct role of ADT in inducing a worsened metabolic profile. In contrast, available clinical data are not sufficient to clarify a direct pathogeniclink between reduced testosterone (T) and altered metabolism. In fact, although T deprivation is associated with an altered metabolism, it is possible that the association between ADT and CV or VTE risk could simply be the result of a selection bias, related to the poor health status of patients with advanced PC. Despite the aforementioned considerations, all patients who are candidatesfor ADT should be screened for CV risk factors at baseline and monitored during the therapy. Life-style modifications and physical exercise are strongly encouraged.
Keywords: Androgen deprivation therapy, Cardiovascular risk, Diabetes, Hypogonadism, Prostate cancer, Testosterone
INTRODUCTION
According to the Global Cancer Observatory database, which includes the epidemiological records from 36 cancers in 185 countries, in 2018 prostate cancer (PC) was reported as the second most common worldwide tumor in men (after lung cancer) [1]. In particular, at that time, PC counted for 1,276,106 new cases and 358,989 deaths (3.8% of all deaths caused by cancer in men) [1]. In addition, PC is considered the most common cancer in elderly men with an estimated 1,017,712 new cases by 2040 [1]. Besides age, other important risk factors for PC are ethnicity and family history. In particular, African Americans have the highest incidence of PC, whereas Asians, Pacific Islanders, Native Americans and Hispanic men the lowest one, when compared to white men [1]. Several environmental factors including western diet, obesity and metabolic syndrome (MetS) have been associated with a higher risk of PC. However, the current evidence is poor and no specific preventing or dietary measures can be suggested to reduce the risk of forthcoming PC [2].
After the introduction of prostate-specific antigen (PSA) screening in the late 1980s, the incidence of PC increased rapidly, peaking in 1992 and decreasing thereafter. PC mortally showed the same trend [2]. Early detection of the disease along with the improvement of the available medical and non medical options for both early-and late-stage PC can explain, at least partially, the observed epidemiological data [3].
Surgical or radiotherapeutic treatments represent the gold standard options in patients with intermediate or high-risk localized PC, whereas active surveillance can be considered an alternative choice in patients with low-risk disease [2]. Conversely, androgen deprivation therapy (ADT) represents the cornerstone in the treatment of advanced and metastatic PC [2,4,5]. However, it should be recognized that the latter treatment has been frequently misused and abused. A large observational study performed in Italy, including 1,075 patients with PC, showed that in more than 25% of the subjects, ADT was not correctly prescribed [6]. Similar results have been reported by other authors [7,8]. It is important to recognize that ADT is able to improve disease-free and overall survival only under two conditions: 1) in combination with primary radiation for locally advanced or high-risk cancer and 2) as an adjuvant therapy for positive lymph node (pN+) cancers after prostatectomy [2]. Hence, ADT abuse can only increase its related side effects without any advantage for the patients [2].
Male hypogonadism has been frequently associated with an increased cardiovascular (CV) risk and with a worse metabolic profile [8,9,10,11]. However, it is important to recognize that the latter association is still the subject of an intense debate. In particular, low testosterone (T) observed in patients with an increased CV risk can represent an adaptive protective mechanism, turning off T-dependent functions (such as reproduction and/or physical and sexual activity) not required when the physical condition is impaired [12,13,14,15]. Accordingly, a large body of evidence has documented that acute or chronic illness might interfere with the hypothalamic-pituitary-testis axis leading to the development of primary or, more frequently, secondary hypogonadism [16].
An association between worse body composition and metabolic profile in men undergoing ADT is also frequently reported [17]. However, the relationship between ADT and an increased CV risk is still conflicting as described in the general population for low T [11]. The aim of the present paper is to summarize discuss and better clarify available evidence regarding the possible association between ADT and CV risk. Both clinical and preclinical data will be considered.
METHODS
A comprehensive narrative review was performed using MEDLINE, Embase, and Cochrane search and including the following words: (“androgens”[Pharmacological Action] OR “androgens”[MeSH Terms] OR “androgens”[All Fields] OR “androgen”[All Fields]) AND deprivation[All Fields] AND (“therapy”[Subheading] OR “therapy”[All Fields] OR “therapeutics”[MeSH Terms] OR “therapeutics”[All Fields]) AND (“cardiovascular system”[MeSH Terms] OR (“cardiovascular”[All Fields] AND “system”[All Fields]) OR “cardiovascular system”[All Fields] OR “cardiovascular”[All Fields]) AND (“risk”[MeSH Terms] OR “risk”[All Fields]). Publications from January 1, 1969 up to March 31st, 2020 were included. When available, meta-analytic data were preferred. Preclinical data were obtained from previously published series of rabbits treated with a gonadotrophin-releasing hormone (GnRH) analogue with and without T supplementation.
ANDROGEN DEPRIVATION THERAPY
The analysis and discussion of specific therapeutic options for ADT is beyond the aim of the present paper and has been revised elsewhere [2]. Data from either surgical or pharmacological castration were obtained. The latter can be reached with the use of GnRH agonists and antagonists. However, after castration, a residual androgen synthesis in the adrenal gland is present (about 10% of the total androgen pool) and more potent androgen receptor (AR) ligands can be sensitized directly in the PC (non-gonadal androgens). Hence, a combination with AR competitive antagonists (anti-androgen, AA) is frequently used to obtain a stronger decline of circulating T levels. In addition, more recent new compounds, such as abiterone acetate, a CYP-17 inhibitor, able to block all steroid synthesis and acting at adrenal, testis as well as at PC cell level, are available for the treatment of more advanced and androgen resistant PC tumors [2].
ANDROGEN DEPRIVATION THERAPY AND CARDIO-METABOLIC CONSEQUENCES
Between 2006 and 2008, three different studies emphasized a possible increased risk of metabolic disturbances and CV diseases in men treated with ADT [18,19]. In 2006, Keating et al [18], in an observational study – including a population of 73,196 subjects with loco-regional PC, evaluated from 1992 to 1999 and observed up to 2001 – reported that GnRH agonist increased the risk of incident diabetes (type 2 diabetes, T2DM; 44%), coronary heart disease (CHD; 16%), acute myocardial infarction (AMI; 11%), and sudden cardiac death (16%). In line with these data, in 2007, Tsai et al [19] – by analyzing the data from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) – found that ADT was associated with a 2.6-fold increase in CV death among 3,262 patients treated with radical prostatectomy and among 1,630 patients treated with external beam radiation. Finally, in a post hoc pooled analysis of three randomized controlled trials (RCTs) with radiation therapy, with or without ADT, it was observed that ADT resulted in shorter time to the occurrence of a fatal AMI in men older than 65 [20]. On the basis of these and other studies, in 2010 the American Heart Association, along with the American Cancer Society, and the American Urological Association endorsed by the American Society for Radiation Oncology, recognized the possible increased CV mortality and morbidity related to ADT [21].
During the last 10 years both pre-clinical and clinical studies have better clarified the underlying mechanisms related to ADT increased cardio-metabolic risk. In the following sections, the most important available data will be summarized and discussed.
1. Preclinical data
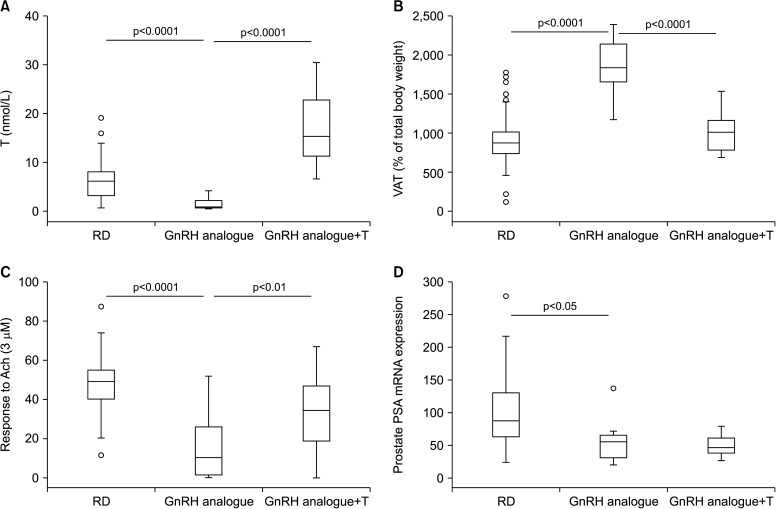
To better understand the metabolic effects of androgen deprivation upon treatment with a GnRH analogue, we re-analyzed data previously published in a rabbit model of GnRH analogue-induced hypogonadotropic hypogonadism [22,23,24,25]. In those studies, rabbits (n=10) were treated for 8 weeks with a single dose of the long-lasting GnRH analogue triptorelinpomoate (2.9 mg/kg) with or without supplementation with a weekly injection of T enanthate (30 mg/kg, n=10). Untreated rabbits were used as the control. As previously described [22,23,24,25], circulating T levels significantly decreased in GnRH analogue-treated rabbits and was fully restored upon T supplementation (Fig. 1A). Androgen deprivation was associated with a doubling of visceral fat (Fig. 1B) and with a significant increase in triglyceride levels (126.8±21 vs. control=81±4 mg/dL, mean±standard error, p=0.05) [24]. Numbers of MetS components were computed in five categories [1,2,3,4,5], as previously described [23], reflecting the alterations observed in the human phenotype: glucose intolerance, dyslipidemia (cholesterol and triglyceride), hypertension and visceral fat accumulation. Although GnRH analogue administration did not induce the full spectrum of the MetS phenotype (equal to, or more than, three components), as was the case with high fat diet [23], it doubled the average number of MetS components (1.46±0.2 vs. control=0.66±0.11, p=0.004). T administration completely normalized all the aforementioned differences (Fig. 1B). We did not observe any significant difference in blood pressure among groups [19], however, vascular reactivity was significantly affected. In fact, responsiveness to maximal concentration (3 µM) acetylcholine was depressed in penile cavernous strips by androgen ablation (Fig. 1C) [23] and normalized upon T administration. As expected, prostatic expression of the PSA gene was significantly decreased (Fig. 1D). However, it was not normalized by T, at least after two months of treatment (Fig. 1D).
Fig. 1. Effect of two-month administration to wild type rabbits of a gonadotrophin-releasing hormone (GnRH) analogue (leuroplide) with or without testosterone (T) administration on biological and experimental parameters, including circulating T (A), visceral fat accumulation (B), prostate-specific antigen (PSA) gene expression within the prostate (D), and in vitro penile responsiveness to a maximal (3 µM) acetylcholine (Ach) concentration (C), expressed as area under the curve. Untreated rabbit were used as a control. Results are derived from data from previous studies [23,24,25,26] and from unpublished observations. RD: regular diet, VAT: visceral adipose tissue.
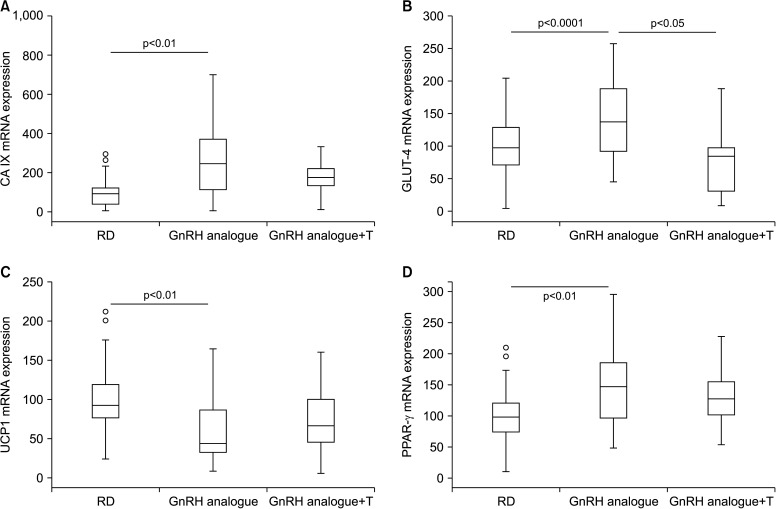
Concerning visceral fat, the aforementioned two-fold increase in the relative mass (Fig. 1B) was associated with an increase in carbonic anhydrase IX (CA IX) (Fig. 2A), a gene known to be up regulated in hypoxic tissue, having a hypoxia responsive element in the promoter that binds HIF1. Interestingly, hypoxia is one of the first alterations in dysfunctional visceral adipose tissue [26]. Changes in mature adipocyte mass are also reflected by an increased expression of peroxisome proliferator-activated receptor gamma an active modulator of lipid metabolism and insulin sensitivity (Fig. 2D). In humans, activation of this receptor is associated with an increase in weight gain and visceral obesity. In addition, the presence of dysfunctional fat is also indicated by the downregulation of uncoupling protein 1 (UCP1) (Fig. 2C), a protein involved in uncoupling the mitochondrial respiratory chain to produce heat, thus contributing greatly to energy expenditure and adaptive thermogenesis [27].
Fig. 2. Effect of two-month administration to wild type rabbits of a gonadotrophin-releasing hormone (GnRH) analogue (leuroplide) with or without testosterone (T) administration on gene expression in visceral fat homogenates. In particular, this graphs show effects on (A) carbonic anhydrase IX (CA IX), (B) glucose transporter type 4 (GLUT-4), (C) on uncoupling protein 1 (UCP1), and (D) peroxisome proliferator-activated receptor gamma (PPAR-γ). Untreated rabbit were used as a control. Results are derived from data from previous studies [23,24,25,26] and from unpublished observations. RD: regular diet.
In GnRH-induced hypogonadism, glucose metabolism was only marginally affected, with no significant change of glycaemia [24] and with a numerical, but not significant, increase in glucose intolerance, as demonstrated by oral glucose tolerance test (OGTT area under the curve: control=142.8±4, GnRH=160.6±11, p=0.121). In visceral fat, we noticed a significant increase in the expression in the insulin-regulated glucose transporter GLUT-4, leading to an increased glucose uptake and therefore to more fat storedby adipocytes (Fig. 2B). All the aforementioned alterations in visceral fat were counteracted by T supplementation, which, at least, restored altered gene expressions to the control level.
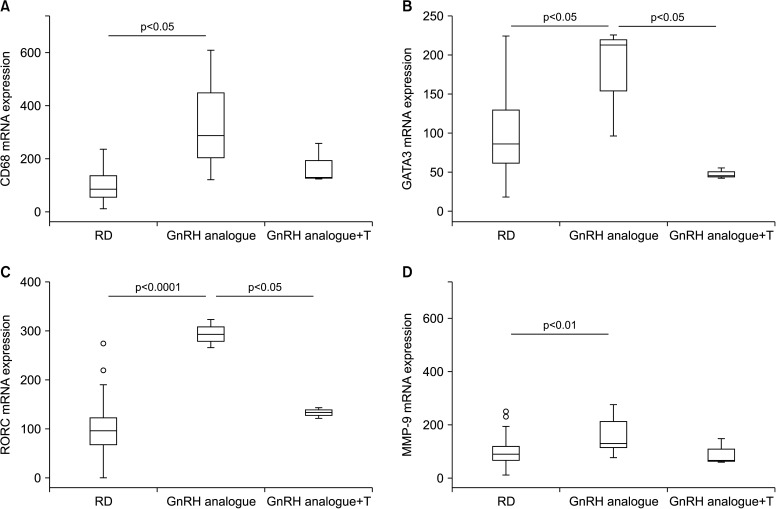
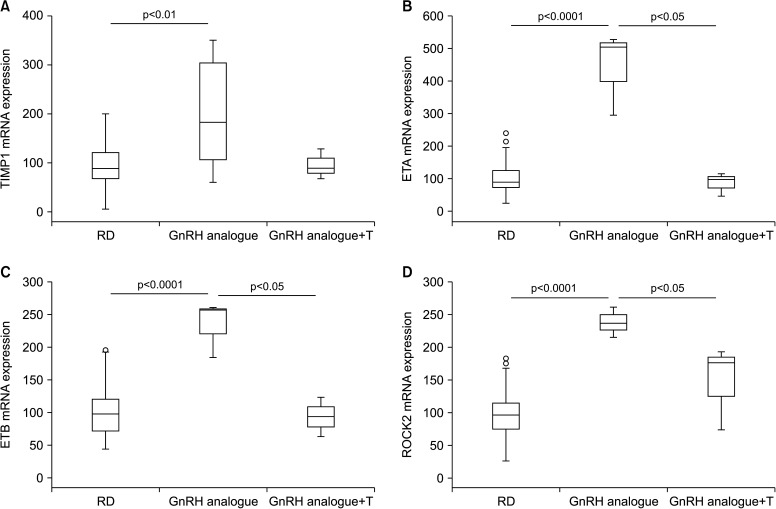
Non-alcoholic fatty liver disease, and its inflammatory correlate non-alcoholic steatohepatitis, are liver alterations nowadays considered as additional components of MetS or as directly connected to the syndrome. By analyzing gene expression in the liver of GnRH-treated animals, we found a significant increase in the expression of CD68, a well-accepted marker of activate macrophage lineage, including Kupffer cells (Fig. 3A). Moreover, we observed a clear up-regulation of GATA3 and RAR-related orphan receptor gamma mRNA, transcription factors associated with Th2 and Th17 immune response, respectively (Fig. 3B, 3C). These results are in some way in keeping with our previous research in a rabbit model of MetS [28], suggesting that treating hypogonadism with T could blunt high fat diet-induced liver inflammation. Similar data were provided by other preclinical and clinical studies [22]. Here we have demonstrated that inducing hypogonadism stimulates liver inflammation. Interestingly, reversing hypogonadism with T administration was able to restore the inflammatory response. Hence, all this evidence indicates that T deficiency has a causal effect in inducing liver inflammation. We did not observed only hepatic inflammation in the liver of GnRH treated rabbits. In fact, markers of activation of stellate cells (ROCK2, ETA, ETB) (Fig. 4B–4D) and fibrosis (MMP-9 and TIMP1) (Fig. 3D, Fig. 4A) were all increased by androgen deprivation and normalized by T administration.
Fig. 3. Effect of two-month administration to wild type rabbits of a gonadotrophin-releasing hormone (GnRH) analogue (leuroplide) with or without testosterone (T) administration on gene expression in liver homogenates. In particular, this graphs show effects on (A) Cluster of Differentiation 68 (CD68), (B) GATA3, (C) gene encoding for RAR-related orphan receptor gamma (RORC), and (D) matrix metallopeptidase 9 (MMP-9). Untreated rabbit were used as a control. Results are derived from data from previous studies [23,24,25,26] and from unpublished observations. RD: regular diet.
Fig. 4. Effect of two-month administration to wild type rabbits of a gonadotrophin-releasing hormone (GnRH) analogue (leuroplide) with or without testosterone (T) administration on gene expression in liver homogenates. In particular, this graphs show effects on (A) TIMP metallopeptidase inhibitor 1, (B) endothelin receptor A (ETA), (C) endothelin receptor B (ETB), and (D) Rho associated coiled-coil containing protein kinase 2 (ROCK2). Untreated rabbit were used as a control. Results are derived from data from previous studies [23,24,25,26] and from unpublished observations. RD: regular diet.
In conclusion, short-term (two months) androgen deprivation through GnRH analogue is associated with distinct alterations in visceral fat and liver as well as with impairment in endothelium-dependent vascular reactivity in small artery vessels, as in the penis. In particular, visceral adiposity increased by a factor of two upon GnRH analogue administration. In the liver, several markers related to inflammation and fibrosis were up-regulated. Accordingly, GnRH-treated rabbits have more MetS components than control ones. Nonetheless, all these alterations did not lead to the full MetS phenotype. It is possible that the short-term treatment does not allow for the full manifestation of the syndrome, including hypertension and glucose intolerance. An alternative explanation is that T deficiency per se only increases the risk of developing MetS but other environmental and lifestyle factors could play an additive role that might be crucial for the full MetS manifestation.
2. Clinical data
1) Metabolic and body composition impact
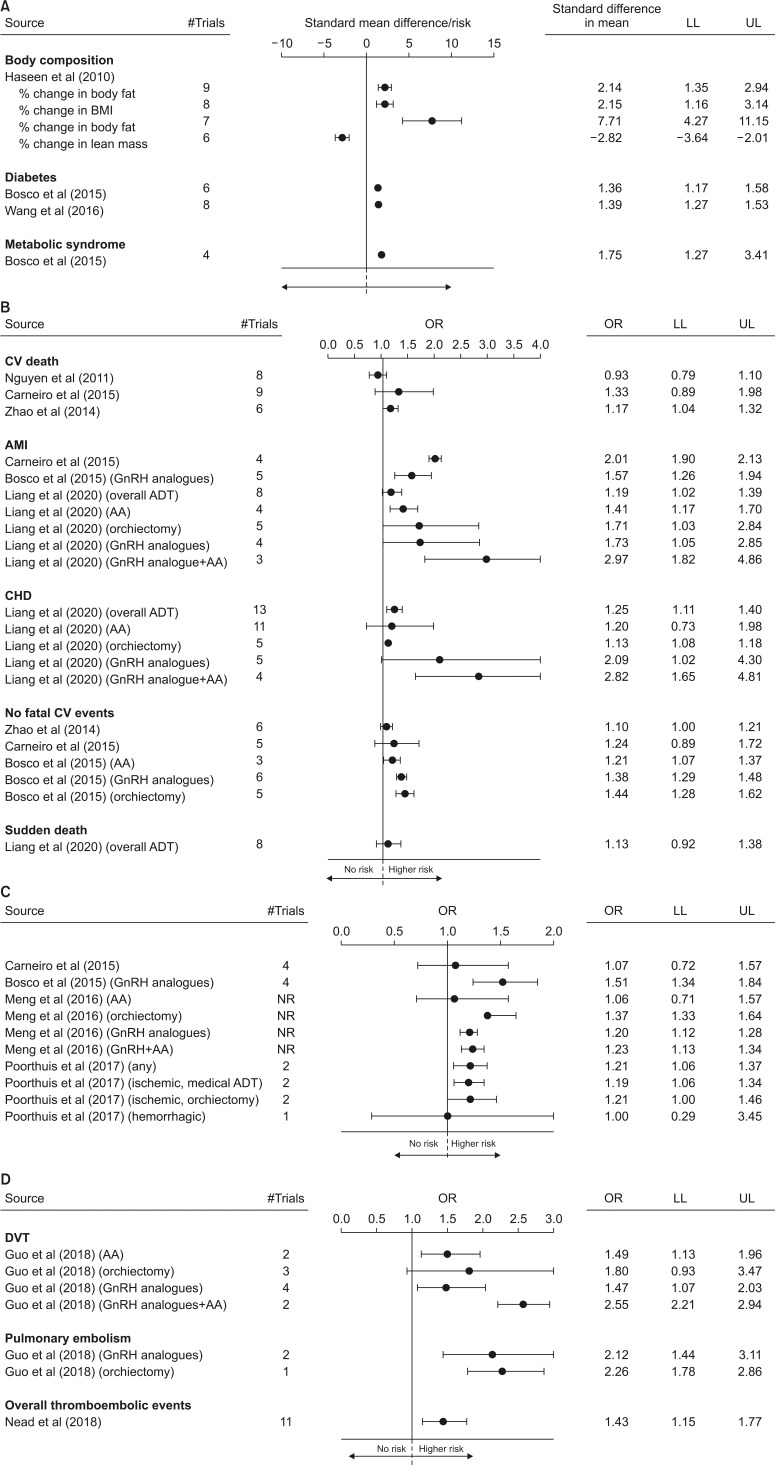
Three systematic meta-analyses have analyzed the impact of ADT on body composition and glyco-metabolic profile (Table 1). The first study included 16 trials (14 observational and 2 RCTs) and 573 subjects, with a period of treatment varying from one to 12 months [29]. ADT resulted in a significant increase of body mass index (BMI), body fat and fat mass as well as in a reduction of lean mass, as compared to the baseline (Fig. 5A). Unfortunately, no comparisons with control groups of patients not receiving ADT were available [29]. The other studies investigated the relationship between ADT and the risk of forthcoming T2DM and MetS (Table 1) [30,31]. The number of the included trials ranges from eight to nine and the patients under ADT from almost 6,000 to more than 61,000 (Table 1). In line with what has been previously reported, ADT was associated with a 36%–39% and 75% increased risk of T2DM and MetS, respectively (Fig. 5A).
Table 1. Comparisons of the available meta-analyses evaluating the relationship between androgen deprivation therapy and body composition and metabolic profile.
| Inclusion criteria | Haseen et al (2010) [29] | Bosco et al (2015) [30] | Wang et al (2016) [31] | ||||
|---|---|---|---|---|---|---|---|
| No. of trials included | 16 | 9 | 8 | ||||
| No. of patients analyzed | 573 | 61,830 | 65,695 | ||||
| No. of controls analyzed | NR | 65,944 | 92,893 | ||||
| Outcomes evaluated | Yes | No | Yes | No | Yes | No | |
| % change in body fat | × | × | × | ||||
| % change in BMI | × | × | × | ||||
| % change in body fat | × | × | × | ||||
| % change in lean mass | × | × | × | ||||
| MetS | × | × | × | ||||
| Diabetes mellitus | × | × | × | ||||
NR: not reported, BMI: body mass index, MetS: metabolic syndrome.
Fig. 5. Forest plot of estimated odds ratio (OR) (95% confidence intervals) for body composition and metabolic changes (A), cardiovascular (CV) mortality and morbidity (B), stroke (C), and thromboembolic events (D) in men under androgen deprivation therapy (ADT) when compared to baseline (body composition; panel A upper panels) or controls (panel A lower panels and as panels B–D). Data are derived from available meta-analyses. LL: lower levels, UL: upper levels, BMI: body mass index, OR: odds ratio, AMI: acute myocardial infarction, GnRH: gonadotropin releasing hormone, AA: anti-androgen, CHD: coronary artery disease, DVT: deep venous thrombosis, NR: not reported.
The underlying mechanisms of ADT-related body composition and metabolic impairment are far from having been elucidated. In contrast to what was observed in the aforementioned preclinical model, data from clinical studies showed that ADT is associated with an increased risk of both MetS and T2DM, as well as with an altered body composition. Conversely, no significant changes in glucose levels and glucose tolerance (as derived from OGTT) was observed in the rabbit model, although visceral fat increased two-fold. Several studies have clearly documented that body composition changes occur very early on during ADT, whereas fasting hyperglycemia and risk of diabetes need a year or more to develop [17]. In addition, worsened glycemic control and increased glycosylated hemoglobin (HbA1c) levels have been also reported in patients with pre-existing diabetes [32,33]. The short duration of ADT characterizing our animal model can explain, at least partially, the differences observed. In addition, rabbits under GnRH analogue were exposed to the same life-style factors (including diet) as the control and were all without glucose alterations at baseline. In line with what has been derived from animal evidence, clinical data have shown that visceral fat content is deeply affected by ADT [17]. AR is present on visceral adipocytes and AR signalingis directly involved in fatty acid mobilization, as documented by data derived from our preclinical models [22,23,25]. The latter, along with the liver dysfunction, represent the first step in inducing insulin-resistance and T2DM development [17]. Similar data have been reported in aging men with hypogonadism not ADT-related [10,34,35]. In the latter population, T replacement therapy (TRT) can improve, at least partially, body composition, whereas more conflicting results are observed when glycometabolic parameters are considered [35,36,37,38,39].
Besides fat accumulation, ADT is also associated with a reduction of lean mass. The final picture identifies a condition also known as sarcopenic obesity which, in turn, is associated with an increased risk of all cause mortality [40].
2) Cardiovascular risk
Up to now, seven systematic meta-analyses have investigated the relationship between ADT and CV mortality and morbidity (Table 2) [41,42,43,44,45,46,47]. The number of the studies included ranges from four to 13, with the patients under ADT considered from 2,000 to 189,202 (Table 2). Among the available studies, three presented data according to the different types of therapy used, whereas only data derived from a mixed population was reported in the latter four papers (Table 2). In addition, the vast majority of the studies analyzed data from observational or mixed studies (observational and RCTs trials) whereas just one meta-analysis included only RCTs (Table 2). The relationship between ADT and overall CV events or CHD, as well as CV mortality, was analyzed in three studies, whereas only one study reported data on sudden cardiac death (Table 2). In addition, four studies investigated the risk of stroke related to ADT (Table 2). Finally, one meta-analysis reported data of comparisons between continuous or intermittent ADT on CV risk (Table 2) [48]. Independently of the meta-analysis considered, ADT was associated with an increased risk of CV morbidity with a trend toward a higher risk of CV mortality or sudden cardiac death (Fig. 5B). Similarly, a higher risk of stroke was also observed (Fig. 5C). When the effects of the different ADT options were analyzed, the combined therapy (GnRH analogues+AA) resulted in the worst impact on CV risk, whereas the use of AA was associated with the less negative effects (Fig. 5B, 5C). When the subtype of strokes was considered, a higher impact of ADT on the risk of ischemic stroke (odds ratio [OR]=1.19 [1.05–1.34]) when compared to hemorrhagic (OR=1.00 [0.29–3.45]), was observed (Fig. 5C). Finally, no differences were found in specific CV mortality and morbidity when intermittent was compared to continuous ADT, although CV death seems to be, at least partially, reducedby intermittent regimens (Supplement Fig. A).
Table 2. Comparisons of the available meta-analyses evaluating the relationship between androgen deprivation therapy (ADT) and cardiovascular (CV) or thromboembolic risk cognitive impairment.
| Inclusion criteria | Nguyen et al (2011) [41] | Carneiro et al (2015) [42] | Zhao et al (2014) [43] | Bosco et al (2015) [44] | Meng et al (2016) [45] | Poorthuis et al (2017) [46] | Liang et al (2020) [47] | Jin et al (2016) [48] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of trials included | 8 | 13 | 7 | 8 | 6 | 4 | 5 | 6 | |||||||||
| No. of patients analyzed | 2,200 | 60,696 | 129,802 | 189,202 | 74,538 | NR | 170,851 | 4,180 | |||||||||
| No. of controls analyzed | 1,941 | 83,001 | 165,605 | 274,810 | 85,947 | NR | 256,794 | - | |||||||||
| Observational studies | × | × | × | ||||||||||||||
| Randomized controlled studies | × | × | × | ||||||||||||||
| Mixed population | × | × | × | × | × | ||||||||||||
| Outcomes evaluated | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| CV death | × | × | × | × | × | × | × | × | |||||||||
| Acute myocardial infarction | × | × | × | × | × | × | × | × | |||||||||
| CV events | × | × | × | × | × | × | × | × | |||||||||
| Coronary heart diseases | × | × | × | × | × | × | × | × | |||||||||
| Sudden death | × | × | × | × | × | × | × | × | |||||||||
| Stroke | × | × | × | × | × | × | × | × | |||||||||
| ADT subtype analysis | × | × | × | × | × | × | × | ||||||||||
| Continuous vs. intermittent ADT | × | × | × | × | × | × | × | × | |||||||||
| Inclusion criteria | Guo et al (2018) [56] | Nead et al (2018) [57] | |||||||||||||||
| No. of trials included | 5 | 10 | |||||||||||||||
| No. of patients analyzed | 170,851 | 32,409 | |||||||||||||||
| No. of controls analyzed | 256,794 | 48,159 | |||||||||||||||
| Outcomes evaluated | Yes | No | Yes | No | |||||||||||||
| Deep venous thrombosis | × | × | |||||||||||||||
| Pulmonary embolism | × | × | |||||||||||||||
| Overall thromboembolic events | × | × | |||||||||||||||
| ADT subtype analysis | × | × | |||||||||||||||
NR: not reported.
Despite the aforementioned evidence, the relationship between ADT and CV mortality and morbidity remains controversial. First of all it should be recognized that properly powered placebo-controlled RCTs with a primary CV endpoint, in men with ADT, are not yet available. Similar considerations can be drawn for the association between low T and CV risk in the general population [11,49,50,51,52]. The increased risk of obesity, T2DM and MetS associated with ADT can explain, at least partially, the documented increased CV mortality and morbidity. However, the real mechanisms through which low T can impact atherosclerosis risk are still mostly unknown. There is evidence, from preclinical data (see above), that low T could trigger immunomodulatory actions by decreasing production of anti-atherosclerotic (such as interleukin [IL]-10) and increasing pro-atherosclerotic (such as tumor necrosis factor-alpha, IL-1beta, and IL-6) cytokines [53,54] in tissue involved in metabolic derangements, such as the liver. In addition, direct positive effects of T on endothelial function have been also documented [55]. However, data deriving from clinical studies are still conflicting. The largest updated meta-analysis published so far confirmed an association between low T and increased CV risk [13]. However, the same study showed that the risk was lower in older patients and in those with a higher prevalence of associated morbidities, supporting the possibility that low T represents only an early marker of poor health [11]. Accordingly, available data did not support reduced CV risk after TRT [49,50,51,52]. In line with this hypothesis, it should be recognized that the data here presented are mainly derived from observational studies, which should be considered with caution, due to the lack of completeness of follow-up and of the management of missing data. Accordingly, when only RCTs were considered, the risk related to ADT was less evident or not significant (Supplement Fig. B). In addition, the contribution of preexisting morbidities to ADT-related CV risk was not investigated either when RCTs or observational studies were analyzed. The latter represents a crucial limitation in the data analysis.
The impact of ADT duration and the role of the new AA on CV risk were investigated only in one study [47]. Interestingly, the negative effects of ADT were more evident in those studies lasting less than five years, although the differences were not statistically significant (Supplement Fig. C). However, it is important to recognize that this represents only an arbitrary criteria selected by the authors and available evidence cannot clarify the specific temporal cutoff value favoring an increased ADT-related CV risk. This point needs to be better clarified in further studies.
Abiterone and enzalutamide are relatively new AA approved by US Food and Drug Administration for the treatment of advanced PC, especially for patients with castration resistance [2]. However, concerns related to CV toxicity for both drugs have been raised [2]. By performing a specific sub-analysis comparing the effects of traditional AA to abiterone and enzalutamide, Liang et al [47] showed that the latter were associated with an increased CV risk, although not statistically different when compared to traditional AA (Supplement Fig. C). Further studies are needed to better clarify these points.
3) Venous thromboembolism risk
Up to now, two systematic meta-analyses have evaluated the relationship between ADT and the risk of thromboembolic events (Table 2). The first study included five retrospective population-based cohort studies, enrolling 170,851 patients and 256,794 controls [56]. The second study included ten studies with more than 80,000 patients [57]. Independently of the meta-analysis considered, ADT resulted in a significant increase of thromboembolic risk either when overall events were considered [57] or when deep venous thrombosis and pulmonary embolism were separately evaluated (Fig. 5D) [56]. In addition, similarly to what was observed for ADT-related CV risk, the combination of GnRH analogues and AA resulted in a higher venous thromboembolism (VTE) risk, when compared to GnRH alone (Fig. 5D). Nead et al [57] also showed that the use of estrogen therapy was associated with an increased risk of thromboembolic events, when compared to ADT without estrogen use (relative risk=1.53; 95% confidence interval=1.07–2.18; p=0.021).
Although the contribution of sex steroids in the pathogenesis of VTE risk has been suggested, the specific contribution of T on VTE in men is still controversial [58]. T levels have been positively related to antithrombin-3, which counteracts the enzymatic activity ofthrombin [44]. In addition, T suppression has been associated with a hypercoagulable state, at least partially mediated through AR [59]. Despite this evidence, however, no association between circulating levels of T and VTE has been reported in the available population-based studies [60,61]. In addition, in the last few years concerns have been raised regarding the possible association between TRT and higher risk of VTE. Possible advocated mechanisms include hematocrit increase (with associated increased blood viscosity), platelet aggregation and increased thromboxane A2 concentrations in platelets [57]. However, most updated meta-analyses did not confirm a possible association between TRT and VTE risk [58,62].
FURTHER DIRECTIONS
The preclinical studies summarized herein indicate that GnRH analogue-induced hypogonadism is not enough to elicit per se the full spectrum of alterations characterizing MetS. It is conceivable that unhealthy lifestyle factors or underlying morbidities are necessary to deteriorate further the ADT-induced condition. In other words, ADT-induced hypogonadism could be a facilitating but not a determining factor, for an increased CV and metabolic risk.
According to the European Association of Urology Guidelines [2], all patients who are candidates for ADT should be screened at baseline for T2DM and checked for glucose and lipid profile before and during ADT therapy. In addition, subjects with impaired fasting glucose or T2DM should be referred to an endocrinological consultation. Similarly, cardiological evaluation and periodic controls are strongly advised [2]. Preventive lifestyle modifications including physical exercise, weight loss, quality of diet improvement and smoking cessation are strongly suggested [2]. Accordingly, a recent meta-analysis including 15 studies and 1,135 patients documented that, in men on ADT, physical exercise can significantly improve muscle strength, exercise tolerance as well as body fat mass, BMI, and ADT-related fatigue and quality of life [63]. In a recent study from our laboratory, we demonstrated that performing regular aerobic physical exercise is enough to counteract some negative effects of MetS [64]. However, the main question is: are subjects with ADT able to perform regular physical exercise? Another study in our preclinical model of MetS-induced hypogonadism indicates that low T is associated with reduced muscle strength and with a worsened ability to run on a treadmill [65]. A reduced lean mass is also a hallmark of ADT in the clinical studies summarized herein. Although data from intermittent ADT regimens on muscle mass are not enough to be meta-analyzed, it is conceivable that intermittent therapies will affect lean mass less, favoring the possibility to engage in healthier lifestyles, including physical activity.
CONCLUSIONS
Current evidence clearly indicates that ADT is associated with a worsened body composition and metabolic profile. Similar results are derived from a preclinical model. In addition, a tight association between ADT and both CV morbidity and VTE risk has been alsodocumented. However, a direct pathogenic role of ADT-induced low bioactive T has yet to be elucidated. Accordingly, the vast majority of the available evidence was derived from observational studies and the effect was less evident when RCTs were considered. In other words, the possibility cannot be excluded that the association between ADT, CV and VTE risk derives from a selection bias, rather than from a causal association. Accordingly, a large number of studies included either local or metastatic diseases, with the subjects with a more advanced PC more likely to have a poor health status and a higher probability of being treated with ADT [56].
Footnotes
Conflict of Interest: The authors have nothing to disclose.
- Conceptualization: GC, MM.
- Data curation: GR, NB, MD, SF.
- Formal analysis: GC, SF, GR, MM.
- Investigation: GC, MM.
- Methodology: GC, MM.
- Project administration: GC, MM.
- Supervision: GC, MM.
- Validation: GC, MM.
- Visualization: GC, MM.
- Writing — original draft: GC, MM.
- Writing — review & editing: GC, SF, GR, MM, SC, AS.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.200109.
Forest plot of estimated odds ratio (OR) (95% confidence intervals) for cardiovascular (CV) mortality and morbidity in men under continuous vs. intermittent androgen deprivation therapy (ADT) (A); when only randomized controlled trials were considered (B) or according ADT duration or type (C). AMI: acute myocardial infarction, CVD: CV diseases.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Mottet N, Cornford P, van den Bergh RCN, Briers E, De Santis M, Fanti S, et al. Prostate cancer [Internet] Arnhem: European Association of Urology; c2019. [cited 2020 Apr 20]. Available from: https://uroweb.org/guideline/prostate-cancer/ [Google Scholar]
- 3.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corona G, Gacci M, Baldi E, Mancina R, Forti G, Maggi M. Androgen deprivation therapy in prostate cancer: focusing on sexual side effects. J Sex Med. 2012;9:887–902. doi: 10.1111/j.1743-6109.2011.02590.x. [DOI] [PubMed] [Google Scholar]
- 5.Corona G, Baldi E, Maggi M. Androgen regulation of prostate cancer: Where are we now? J Endocrinol Invest. 2011;34:232–243. doi: 10.1007/BF03347072. [DOI] [PubMed] [Google Scholar]
- 6.Kuykendal AR, Hendrix LH, Salloum RG, Godley PA, Chen RC. Guideline-discordant androgen deprivation therapy in localized prostate cancer: patterns of use in the medicare population and cost implications. Ann Oncol. 2013;24:1338–1343. doi: 10.1093/annonc/mds618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandaglia G, Sun M, Popa I, Schiffmann J, Abdollah F, Trinh QD, et al. The impact of androgen-deprivation therapy (ADT) on the risk of cardiovascular (CV) events in patients with non-metastatic prostate cancer: a population-based study. BJU Int. 2014;114:E82–E89. doi: 10.1111/bju.12732. [DOI] [PubMed] [Google Scholar]
- 8.Rastrelli G, Lotti F, Reisman Y, Sforza A, Maggi M, Corona G. Metabolically healthy and unhealthy obesity in erectile dysfunction and male infertility. Expert Rev Endocrinol Metab. 2019;14:321–334. doi: 10.1080/17446651.2019.1657827. [DOI] [PubMed] [Google Scholar]
- 9.Lotti F, Rastrelli G, Maseroli E, Cipriani S, Guaraldi F, Krausz C, et al. Impact of metabolically healthy obesity in patients with andrological problems. J Sex Med. 2019;16:821–832. doi: 10.1016/j.jsxm.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Grossmann M, Ng Tang Fui M, Cheung AS. Late-onset hypogonadism: metabolic impact. Andrology. 2019 doi: 10.1111/andr.12705. [Epub] [DOI] [PubMed] [Google Scholar]
- 11.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Endogenous testosterone levels and cardiovascular risk: meta-analysis of observational studies. J Sex Med. 2018;15:1260–1271. doi: 10.1016/j.jsxm.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Rastrelli G, Carter EL, Ahern T, Finn JD, Antonio L, O'Neill TW, et al. EMAS Study Group. Development of and recovery from secondary hypogonadism in aging men: prospective results from the EMAS. J Clin Endocrinol Metab. 2015;100:3172–3182. doi: 10.1210/jc.2015-1571. [DOI] [PubMed] [Google Scholar]
- 13.Corona G, Rastrelli G, Maseroli E, Fralassi N, Sforza A, Forti G, et al. Low testosterone syndrome protects subjects with high cardiovascular risk burden from major adverse cardiovascular events. Andrology. 2014;2:741–747. doi: 10.1111/j.2047-2927.2014.00241.x. [DOI] [PubMed] [Google Scholar]
- 14.Tajar A, Forti G, O'Neill TW, Lee DM, Silman AJ, Finn JD, et al. EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95:1810–1818. doi: 10.1210/jc.2009-1796. [DOI] [PubMed] [Google Scholar]
- 15.Corona G, Rastrelli G, Monami M, Melani C, Balzi D, Sforza A, et al. Body mass index regulates hypogonadism-associated CV risk: results from a cohort of subjects with erectile dysfunction. J Sex Med. 2011;8:2098–2105. doi: 10.1111/j.1743-6109.2011.02292.x. [DOI] [PubMed] [Google Scholar]
- 16.Corona G, Maseroli E, Rastrelli G, Francomano D, Aversa A, Hackett GI, et al. Is late-onset hypogonadotropic hypogonadism a specific age-dependent disease, or merely an epiphenomenon caused by accumulating disease-burden? Minerva Endocrinol. 2016;41:196–210. [PubMed] [Google Scholar]
- 17.Tzortzis V, Samarinas M, Zachos I, Oeconomou A, Pisters LL, Bargiota A. Adverse effects of androgen deprivation therapy in patients with prostate cancer: focus on metabolic complications. Hormones (Athens) 2017;16:115–123. doi: 10.14310/horm.2002.1727. [DOI] [PubMed] [Google Scholar]
- 18.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 19.Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 20.D'Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, Lamb DS, et al. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 21.Levine GN, D'Amico AV, Berger P, Clark PE, Eckel RH, Keating NL, et al. American Heart Association Council on Clinical Cardiology and Council on Epidemiology and Prevention; the American Cancer Society; the American Urological Association. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filippi S, Morelli A, Vignozzi L, Vannelli GB, Marini M, Ferruzzi P, et al. Oxytocin mediates the estrogen-dependent contractile activity of endothelin-1 in human and rabbit epididymis. Endocrinology. 2005;146:3506–3517. doi: 10.1210/en.2004-1628. [DOI] [PubMed] [Google Scholar]
- 23.Filippi S, Vignozzi L, Morelli A, Chavalmane AK, Sarchielli E, Fibbi B, et al. Testosterone partially ameliorates metabolic profile and erectile responsiveness to PDE5 inhibitors in an animal model of male metabolic syndrome. J Sex Med. 2009;6:3274–3288. doi: 10.1111/j.1743-6109.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 24.Morelli A, Comeglio P, Filippi S, Sarchielli E, Cellai I, Vignozzi L, et al. Testosterone and farnesoid X receptor agonist INT-747 counteract high fat diet-induced bladder alterations in a rabbit model of metabolic syndrome. J Steroid Biochem Mol Biol. 2012;132:80–92. doi: 10.1016/j.jsbmb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Maggi M, Filippi S, Vignozzi L, Rastrelli G. Controversial aspects of testosterone in the regulation of sexual function in late-onset hypogonadism. Andrology. 2020 doi: 10.1111/andr.12794. [Epub] [DOI] [PubMed] [Google Scholar]
- 26.Xia N, Li H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br J Pharmacol. 2017;174:3425–3442. doi: 10.1111/bph.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154:2992–3000. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- 28.Vignozzi L, Filippi S, Comeglio P, Cellai I, Sarchielli E, Morelli A, et al. Nonalcoholic steatohepatitis as a novel player in metabolic syndrome-induced erectile dysfunction: an experimental study in the rabbit. Mol Cell Endocrinol. 2014;384:143–154. doi: 10.1016/j.mce.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Haseen F, Murray LJ, Cardwell CR, O'Sullivan JM, Cantwell MM. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. J Cancer Surviv. 2010;4:128–139. doi: 10.1007/s11764-009-0114-1. [DOI] [PubMed] [Google Scholar]
- 30.Bosco C, Crawley D, Adolfsson J, Rudman S, Van Hemelrijck M. Quantifying the evidence for the risk of metabolic syndrome and its components following androgen deprivation therapy for prostate cancer: a meta-analysis. PLoS One. 2015;10:e0117344. doi: 10.1371/journal.pone.0117344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Sun X, Zhao L, Chen X, Zhao J. Androgen deprivation therapy is associated with diabetes: evidence from meta-analysis. J Diabetes Investig. 2016;7:629–636. doi: 10.1111/jdi.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keating NL, Liu PH, O'Malley AJ, Freedland SJ, Smith MR. Androgen-deprivation therapy and diabetes control among diabetic men with prostate cancer. Eur Urol. 2014;65:816–824. doi: 10.1016/j.eururo.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morote J, Gómez-Caamaño A, Alvarez-Ossorio JL, Pesqueira D, Tabernero A, Gómez Veiga F, et al. ANAMET Investigators Group. The metabolic syndrome and its components in patients with prostate cancer on androgen deprivation therapy. J Urol. 2015;193:1963–1969. doi: 10.1016/j.juro.2014.12.086. [DOI] [PubMed] [Google Scholar]
- 34.Rastrelli G, Filippi S, Sforza A, Maggi M, Corona G. Metabolic syndrome in male hypogonadism. Front Horm Res. 2018;49:131–155. doi: 10.1159/000485999. [DOI] [PubMed] [Google Scholar]
- 35.Pizzocaro A, Vena W, Condorelli R, Radicioni A, Rastrelli G, Pasquali D, et al. King, Klinefelter ItaliaN Group. Testosterone treatment in male patients with Klinefelter syndrome: a systematic review and meta-analysis. J Endocrinol Invest. 2020 doi: 10.1007/s40618-020-01299-1. [Epub] [DOI] [PubMed] [Google Scholar]
- 36.Corona G, Maseroli E, Maggi M. Injectable testosterone undecanoate for the treatment of hypogonadism. Expert Opin Pharmacother. 2014;15:1903–1926. doi: 10.1517/14656566.2014.944896. [DOI] [PubMed] [Google Scholar]
- 37.Corona G, Vignozzi L, Sforza A, Maggi M. Risks and benefits of late onset hypogonadism treatment: an expert opinion. World J Mens Health. 2013;31:103–125. doi: 10.5534/wjmh.2013.31.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rastrelli G, Maggi M, Corona G. Pharmacological management of late-onset hypogonadism. Expert Rev Clin Pharmacol. 2018;11:439–458. doi: 10.1080/17512433.2018.1445969. [DOI] [PubMed] [Google Scholar]
- 39.Corona G, Torres LO, Maggi M. Testosterone therapy: What we have learned from trials. J Sex Med. 2020;17:447–460. doi: 10.1016/j.jsxm.2019.11.270. [DOI] [PubMed] [Google Scholar]
- 40.Tian S, Xu Y. Association of sarcopenic obesity with the risk of all-cause mortality: a meta-analysis of prospective cohort studies. Geriatr Gerontol Int. 2016;16:155–166. doi: 10.1111/ggi.12579. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306:2359–2366. doi: 10.1001/jama.2011.1745. [DOI] [PubMed] [Google Scholar]
- 42.Carneiro A, Sasse AD, Wagner AA, Peixoto G, Kataguiri A, Neto AS, et al. Cardiovascular events associated with androgen deprivation therapy in patients with prostate cancer: a systematic review and meta-analysis. World J Urol. 2015;33:1281–1289. doi: 10.1007/s00345-014-1439-6. [DOI] [PubMed] [Google Scholar]
- 43.Zhao J, Zhu S, Sun L, Meng F, Zhao L, Zhao Y, et al. Androgen deprivation therapy for prostate cancer is associated with cardiovascular morbidity and mortality: a meta-analysis of population-based observational studies. PLoS One. 2014;9:e107516. doi: 10.1371/journal.pone.0107516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, Van Hemelrijck M. Quantifying observational evidence for risk of fatal and nonfatal cardiovascular disease following androgen deprivation therapy for prostate cancer: a meta-analysis. Eur Urol. 2015;68:386–396. doi: 10.1016/j.eururo.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 45.Meng F, Zhu S, Zhao J, Vados L, Wang L, Zhao Y, et al. Stroke related to androgen deprivation therapy for prostate cancer: a meta-analysis and systematic review. BMC Cancer. 2016;16:180. doi: 10.1186/s12885-016-2221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poorthuis MH, Algra AM, Algra A, Kappelle LJ, Klijn CJ. Female- and male-specific risk factors for stroke: a systematic review and meta-analysis. JAMA Neurol. 2017;74:75–81. doi: 10.1001/jamaneurol.2016.3482. [DOI] [PubMed] [Google Scholar]
- 47.Liang Z, Zhu J, Chen L, Xu Y, Yang Y, Hu R, et al. Is androgen deprivation therapy for prostate cancer associated with cardiovascular disease? A meta-analysis and systematic review. Andrology. 2020;8:559–574. doi: 10.1111/andr.12731. [DOI] [PubMed] [Google Scholar]
- 48.Jin C, Fan Y, Meng Y, Shen C, Wang Y, Hu S, et al. A meta-analysis of cardiovascular events in intermittent androgen-deprivation therapy versus continuous androgen-deprivation therapy for prostate cancer patients. Prostate Cancer Prostatic Dis. 2016;19:333–339. doi: 10.1038/pcan.2016.35. [DOI] [PubMed] [Google Scholar]
- 49.Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, et al. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology. 2020 doi: 10.1111/andr.12770. [Epub] [DOI] [PubMed] [Google Scholar]
- 50.Corona G, Rastrelli G, Reisman Y, Sforza A, Maggi M. The safety of available treatments of male hypogonadism in organic and functional hypogonadism. Expert Opin Drug Saf. 2018;17:277–292. doi: 10.1080/14740338.2018.1424831. [DOI] [PubMed] [Google Scholar]
- 51.Corona G, Sforza A, Maggi M. Testosterone replacement therapy: long-term safety and efficacy. World J Mens Health. 2017;35:65–76. doi: 10.5534/wjmh.2017.35.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corona G, Rastrelli G, Di Pasquale G, Sforza A, Mannucci E, Maggi M. Testosterone and cardiovascular risk: meta-analysis of interventional studies. J Sex Med. 2018;15:820–838. doi: 10.1016/j.jsxm.2018.04.641. [DOI] [PubMed] [Google Scholar]
- 53.Corrales JJ, Almeida M, Burgo R, Mories MT, Miralles JM, Orfao A. Androgen-replacement therapy depresses the ex vivo production of inflammatory cytokines by circulating antigen-presenting cells in aging type-2 diabetic men with partial androgen deficiency. J Endocrinol. 2006;189:595–604. doi: 10.1677/joe.1.06779. [DOI] [PubMed] [Google Scholar]
- 54.Liva SM, Voskuhl RR. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol. 2001;167:2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- 55.Alexandersen P, Haarbo J, Christiansen C. The relationship of natural androgens to coronary heart disease in males: a review. Atherosclerosis. 1996;125:1–13. doi: 10.1016/0021-9150(96)05864-9. [DOI] [PubMed] [Google Scholar]
- 56.Guo Z, Huang Y, Gong L, Gan S, Chan FL, Gu C, et al. Association of androgen deprivation therapy with thromboembolic events in patients with prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2018;21:451–460. doi: 10.1038/s41391-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 57.Nead KT, Boldbaatar N, Yang DD, Sinha S, Nguyen PL. Association of androgen deprivation therapy and thromboembolic events: a systematic review and meta-analysis. Urology. 2018;114:155–162. doi: 10.1016/j.urology.2017.11.055. [DOI] [PubMed] [Google Scholar]
- 58.Corona G, Dicuio M, Rastrelli G, Maseroli E, Lotti F, Sforza A, et al. Testosterone treatment and cardiovascular and venous thromboembolism risk: what is ‘new’? J Investig Med. 2017;65:964–973. doi: 10.1136/jim-2017-000411. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Li X, Li J, Deng X, Li Y, Cong Y. Experimental arterial thrombosis regulated by androgen and its receptor via modulation of platelet activation. Thromb Res. 2007;121:127–134. doi: 10.1016/j.thromres.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Svartberg J, Braekkan SK, Laughlin GA, Hansen JB. Endogenous sex hormone levels in men are not associated with risk of venous thromboembolism: the Tromso study. Eur J Endocrinol. 2009;160:833–838. doi: 10.1530/EJE-08-0888. [DOI] [PubMed] [Google Scholar]
- 61.Holmegard HN, Nordestgaard BG, Schnohr P, Tybjaerg-Hansen A, Benn M. Endogenous sex hormones and risk of venous thromboembolism in women and men. J Thromb Haemost. 2014;12:297–305. doi: 10.1111/jth.12484. [DOI] [PubMed] [Google Scholar]
- 62.Houghton DE, Alsawas M, Barrioneuvo P, Tello M, Farah W, Beuschel B, et al. Testosterone therapy and venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2018;172:94–103. doi: 10.1016/j.thromres.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yunfeng G, Weiyang H, Xueyang H, Yilong H, Xin G. Exercise overcome adverse effects among prostate cancer patients receiving androgen deprivation therapy: an update meta-analysis. Medicine (Baltimore) 2017;96:e7368. doi: 10.1097/MD.0000000000007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morelli A, Filippi S, Comeglio P, Sarchielli E, Cellai I, Pallecchi M, et al. Physical activity counteracts metabolic syndrome-induced hypogonadotropic hypogonadism and erectile dysfunction in the rabbit. Am J Physiol Endocrinol Metab. 2019;316:E519–E535. doi: 10.1152/ajpendo.00377.2018. [DOI] [PubMed] [Google Scholar]
- 65.Sarchielli E, Comeglio P, Filippi S, Cellai I, Guarnieri G, Guasti D, et al. Testosterone improves muscle fiber asset and exercise performance in a metabolic syndrome model. J Endocrinol. 2020;245:259–279. doi: 10.1530/JOE-19-0532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot of estimated odds ratio (OR) (95% confidence intervals) for cardiovascular (CV) mortality and morbidity in men under continuous vs. intermittent androgen deprivation therapy (ADT) (A); when only randomized controlled trials were considered (B) or according ADT duration or type (C). AMI: acute myocardial infarction, CVD: CV diseases.