Abstract
Here we describe the neurodevelopmental outcomes of very low birth weight (VLBW) infants (birth weight ≤1,500 g) at 3 years of age in the Neonatal Research Network of Japan (NRNJ) database in the past decade and review the methodological issues identified in follow-up studies. The follow-up protocol for children at 3 years of chronological age in the NRNJ consists of physical and comprehensive neurodevelopmental assessments in each participating center. Neurodevelopmental impairment (NDI)—moderate to severe neurological disability—is defined as cerebral palsy (CP) with a Gross Motor Function Classification System score ≥2, visual impairment such as uni- or bilateral blindness, hearing impairment requiring hearing amplification, or cognitive impairment with a developmental quotient (DQ) of Kyoto Scale of Psychological Development score <70 or judgment as delayed by pediatricians. We used death or NDI as an unfavorable outcome in all study subjects and NDI in survivors using number of assessed infants as the denominator. Follow-up data were collected from 49% of survivors in the database. Infants with follow-up data had lower birth weights and were of younger gestational age than those without follow-up data. Mortality rates of 40,728 VLBW infants born between 2003 and 2012 were 8.2% before discharge and 0.7% after discharge. The impairment rates in the assessed infants were 7.1% for CP, 1.8% for blindness, 0.9% for hearing impairment, 15.9% for a DQ <70, and 19.1% for NDI. The mortality or NDI rate in all study subjects, including infants without follow-up data, was 17.4%, while that in the subjects with outcome data was 32.5%. The NRNJ follow-up study results suggested that children born with a VLBW remained at high risk of NDI in early childhood. It is important to establish a network follow-up protocol and complete assessments with fewer dropouts to enable clarification of the outcomes of registered infants.
Keywords: Cerebral palsy, Cognitive impairment, Follow-up, Neurodevelopmental impairments, Very low birth weight infants
Introduction
Recent perinatal and neonatal intensive care advances have resulted in marked improvements in the survival of very preterm and low birth weight (BW) infants [1-4]. Such improvements have been the most significant among infants at the limit of viability born at 22–23 weeks of gestation or with a BW <500 g [5,6]. The goal of neonatal intensive care unit (NICU) admission is not only to ensure survival but to improve the long-term outcomes of these high-risk infants.
The Neonatal Research Network of Japan (NRNJ) database was established in 2003 for all live-born very low birth weight (VLBW) infants with a BW ≤1,500 g to achieve improvements in nationwide NICU care and treatment [7]. Participating centers were originally level III NICU and a few equivalent centers. Level II centers officially joined in 2010 [8]. More than 150 NICU centers are now participating, and the number of registered infants exceeds 5,000 each year. Data from the NRNJ database showed that the survival rate of infants born at 22 weeks increased from 35% in 2003–2007 to 46% in 2008–2012 [9]. However, improvements in survival have raised concerns among physicians and families regarding increases in the rate of neurodevelopmental impairments (NDI) among survivors. Precise and timely outcome information is needed to counsel parents and choose intervention strategies [10-12]. Follow-up data were collected into the NRNJ database from registered infants assessed at 18–24 months of corrected age and 3 years of chronological age [5,9,13]. Neurodevelopmental outcomes evaluated in follow-up included neurological, neurosensory, and cognitive impairments. These impairments have a major long-term impact on children and their families and require long-term support and interventions [14]. Parents need to be provided with information about long-term impairments that their child may develop.
This review describes the follow-up protocol and characteristics of data collected by the NRNJ and presents the up-to-date neurodevelopmental outcomes of VLBW infants at 3 years of age in the NRNJ database over the past decade. This review discusses the methodological issues in follow-up studies, such as data collection, the option of a denominator for the impairment rates, and the use of BW versus gestational age (GA) to group subjects.
NRNJ follow-up assessment protocol
There is no established standard for outcome assessments of VLBW infants. The NRNJ follow-up protocol consists of physical and neurological evaluations and developmental assessments at 18–24 months’ corrected age and 3 years’ chronological age in each participating center according to the Japanese Society for Follow-up Study of High-risk Infants protocol [13,15].
The neurological evaluation included signs and symptoms of cerebral palsy (CP) and sensory abnormalities. CP was defined as a nonprogressive central nervous system disorder characterized by abnormal muscle tone in at least one extremity and the abnormal control of movement and posture [16]. The motor ability of infants with CP was graded using the Gross Motor Function Classification System (GMFCS) [17]. Children with any type of CP and a GMFCS level ≥II were judged to have CP because they were functionally impaired. Visual impairments were defined as blindness with no functional vision in at least one eye. Bilateral amblyopia was diagnosed when children responded to light or hand movement but not other movements or objects regardless of the use of glasses. A hearing impairment was defined as the need for sound amplification.
Cognitive function was evaluated using the Kyoto Scale of Psychological Development (KSPD) test by psychologists working in each participating center [18]. The KSPD, published in 2002, is an individualized face-to-face test that assesses a child’s development in the following 3 areas: Postural–Motor (fine and gross motor functions); Cognitive–Adaptive (nonverbal reasoning or visuospatial perceptions assessed using materials); and Language–Social (interpersonal relationships, socialization, and verbal abilities). Each score for these 3 areas and the sum of the scores were converted to represent each developmental age and the overall developmental age [18]. It takes 30–40 minutes to complete the assessment.
The developmental quotient (DQ) is calculated by dividing the overall developmental age by the corrected age for prematurity in 18- to 24-month evaluation or by chronological age in 3-year evaluation and then multiplying the quotient by 100. The DQ score of a KSPD <70, which represents a 70% achievement of standardized performance for chronological age, was interpreted as significantly delayed. If the KSPD assessment was not available, the pediatrician estimated the child’s developmental level as delayed or not delayed. The KSPD test is a standardized and validated developmental test available at all participating centers, but it has not been published or standardized in English [13,18]. On the other hand, the Bayley Scales of Infant and Toddler Development, third edition (Bayley III) is used globally to assess the developmental/cognitive function of preterm children in early childhood, but it is not standardized for Japanese children or commercially available [19]/A comparison of the KSPD and the Bayley III for VLBW infants revealed that the DQ of KSPD was strongly correlated with the corresponding composite score of the Bayley III [20]. Developmental delays defined by an overall DQ of a KSPD <70 were identified as equivalent to a Bayley III Cognitive composite score of <85.
NDI in the NRNJ follow-up studies was defined as any of the following: CP with a GMFCS level ≥II, visual impairment defined as uni- or bilateral blindness, hearing impairment requiring amplification, or cognitive impairment with a DQ of a KSPD <70 or judged as delayed by a pediatrician (Table 1). Although the definition of NDI differs slightly between follow-up studies of preterm and/or VLBW infants, it represents a moderate to severe neurological disability [2,3,5,21]. Thus, the NDI rate may be a benchmark of the outcomes of a study cohort.
Table 1.
Summary of definitions of neurodevelopmental impairments used in assessments at 3 years of age included in the NRNJ database
| Domain | Criteria for NDI |
|---|---|
| Motor | Cerebral palsy with GMFCS level 2 |
| Visual | Blindness with no functional vision in at least one eye |
| Hearing | Hearing amplification required |
| Cognitive | DQ score of KSPD test <70, or judged as delayed by a pediatrician if the KSPD test was not available |
NRNJ, Neonatal Research Network of Japan; NDI, neurodevelopmental impairment; GMFCS, Gross Motor Function Classification System; DQ, developmental quotient; KSPD, Kyoto Scale of Psychological Development.
Follow-up data collection and importance of a denominator for presenting outcomes
As of Jan 22, 2020, 56,143 VLBW infants born between January 1, 2003 and December 31, 2015 were registered in the NRNJ database (2003–2015). A total of 55,444 infants remained after the exclusion of cases for which data were missing for BW, GA, or outcomes at hospital discharge and infants born at a GA <22 weeks or with a BW <200 g. Fig. 1 shows the mortality and survival rates for cases with versus those without follow-up data. A significant decrease in mortality until 3 years of age was observed over time from 11.9% in 2003 to 5.6% in 2015 (P<0.0001, Cochrane–Armitage χ2 test). Mortality rates for all subjects before and after discharge were 7.5% and 0.6%, respectively. The rate of infants with follow-up data at 3 years of age was approximately 50% of survivors between 2003 and 2012; thereafter, this rate decreased to 25% of survivors born in 2015. There may have been a time lag between clinical follow-up and database registration. Therefore, the study subjects in this review were VLBW infants born between January 1, 2003 and December 31, 2012 and registered in the NRNJ database (2003–2012) as of January 22, 2020.
Fig. 1.
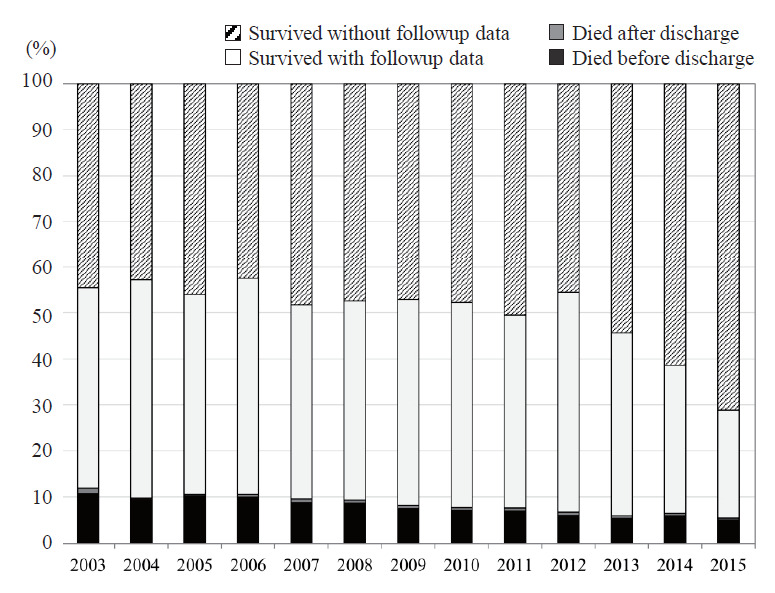
Mortality rates and proportions of survivors with versus without follow-up data in the Neonatal Research Network of Japan database.
When discussing neurodevelopmental outcomes, the mortality rate of a study population is crucially important because death is the most unfavorable outcome [22]. A higher mortality rate may be related to fewer NDI cases among survivors as well as baseline study subjects. Therefore, we used a combined outcome, death or NDI, as an unfavorable outcome for all study subjects. On the other hand, clinicians and parents may want to determine not only the mortality rate but also the possibility of survival with or without NDI [11,12]. The NDI rate of survivors will be used in these cases. In addition, in the majority of follow-up studies, NDI was not assessed in all survivors. Some survivors may drop out from the study protocol, while others may not be able to complete the developmental tests. Therefore, we used the number of baseline study subjects, survivors, or infants with assessments as the denominator for presenting the outcomes. Fig. 2 shows the differences in mortality and NDI rates among VLBW infants in the NRNJ database according to different denominators. NDI rates in all study subjects, subjects with outcome data, all survivors, and survivors with assessments were 8.5%, 15.9%, 9.3%, and 19.1%, respectively.
Fig. 2.
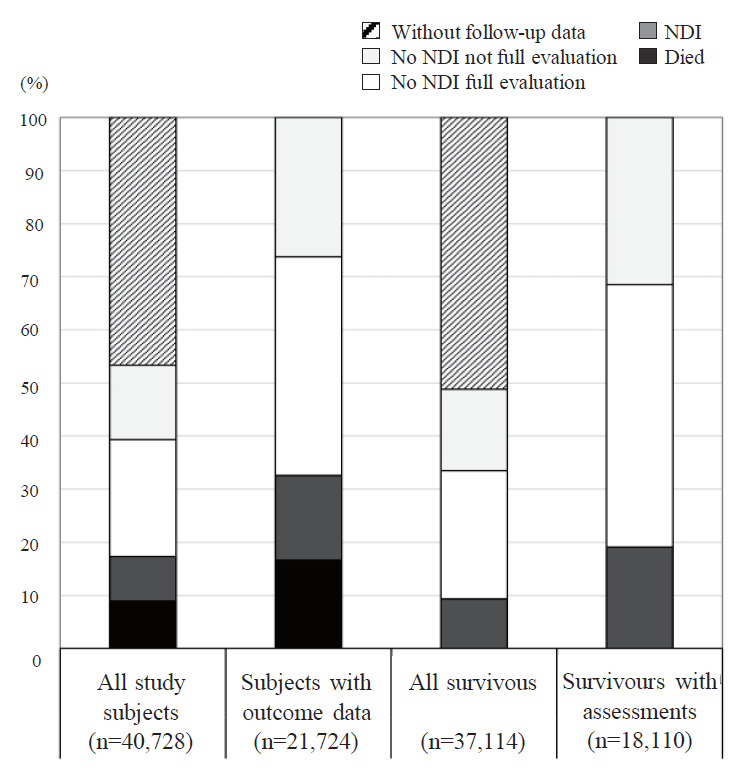
Mortality and neurodevelopmental impairments (NDI) rates among very low birth weight infants born in 2003–2012 in the Neonatal Research Network of Japan database using different denominators. The x-axis shows the different denominators.
Demographic, perinatal, and neonatal characteristics of study subjects in the NRNJ database: comparison of surviving infants with and without follow-up data
Due to selection bias, differences in characteristics between infants with and without follow-up data are important when evaluating neurodevelopmental outcomes [23]. Among 40,801 VLBW infants registered in the NRNJ database (2003–2012), 40,728 were eligible study subjects after the exclusion of cases with missing data for BW, GA, or outcomes at hospital discharge as well as infants born at a GA <22 weeks or with a BW <200 g. Of 37,114 survivors at 3 years of age, follow-up data were collected for 18,110 (48.8% of survivors). Among 202 centers that provided data for the NRNJ database, 63 did not participate in the follow-up study at 3 years of age. After the exclusion of 3,725 cases from the 63 centers, follow-up data were collected from 53.7% of the survivors.
Table 2 compares the demographic, perinatal, and neonatal characteristics between surviving infants with and without follow-up data from all centers. Infants with follow-up data had a lower mean BW and a younger mean GA. Pregnancy-related hypertension and clinical chorioamnionitis was more prevalent in the mothers of infants with follow-up data. Accordingly, infants with follow-up data had more neonatal morbidities related to prematurity, such as respiratory distress syndrome, patent ductus arteriosus, chronic lung disease, and sepsis. They underwent further treatments for retinopathy of prematurity or parental nutrition and home oxygen therapy at discharge. However, the rates of congenital anomalies and grade 3 and 4 intraventricular hemorrhage (IVH) were lower in infants with than in those without follow-up data. More infants without follow-up data were transferred after birth. These differences suggest that more infants who were premature or had a lower BW were followed up by neonatologists registered to the database. On the other hand, infants with congenital anomalies or severe IVH were speculated to be followed up by specialists rather than neonatologists. Previous studies suggested that impaired infants are more likely to be returned for follow-up evaluations, while others speculated that healthier infants are more likely to participate [24-27]. Transfer after birth was another main reason for the lack of follow-up data. Infants may be transferred back and followed up by other hospitals.
Table 2.
Demographic, prenatal, and neonatal characteristics of surviving infants with and without follow-up data at 3 years of age included in the NRNJ database
| Variable | With follow-up data (n=18,110) | Without follow-up data (n=19,004) | P value |
|---|---|---|---|
| Maternal age >35 yr | 4,152/17,472 (23.8) | 4,191/18,228 (23.0) | 0.087 |
| Birth weight (g) | 1,034±294 | 1,090±288 | <0.001 |
| Birth weight distribution | |||
| <500 g | 517/18,110 (2.9) | 357/19,004 (1.9) | <0.001 |
| <1,000 g | 7615/18,110 (42.0) | 6,674/19,004 (35.1) | |
| 1,500 g | 9,978/18,110 (55.1) | 11,973/19,004 (63.0) | |
| GA (wk) | 28.9±3.1 | 29.5±3.2 | <0.001 |
| GA distribution | <0.001 | ||
| 22–27 wk | 7,002/18,110 (38.7) | 6,021/19,004 (31.7) | |
| 28–33 wk | 9,972/18,110 (55.1) | 11,218/19,004 (59) | |
| 34 wk | 1,136/18,110 (6.3) | 1,765/19,004 (9.3) | |
| Male sex | 9,273/18,110 (51.2) | 9,595/19,004 (50.5) | 0.376 |
| Multiple births | 4,468/18,110 (24.7) | 4,885/19,004 (25.7) | 0.022 |
| Life-threatening congenital anomalies | 29/17,903 (0.2) | 87/18,673 (0.5) | <0.001 |
| Non-life-threatening congenital anomalies | 702/17,903 (3.9) | 929/18,673 (5.0) | <0.001 |
| Pregnancy-related hypertension | 3,739/18,031 (20.7) | 3,706/18,649 (19.9) | 0.04 |
| Clinical CAM | 3,002/17,734 (16.9) | 2,728/17,946 (15.2) | <0.001 |
| Antenatal steroids | 8,571/17,972 (47.7) | 8,049/18,466 (43.6) | <0.001 |
| Cesarean section | 14,312/18,044 (79.3) | 14,581/18,761 (77.7) | <0.001 |
| Transferred after birth | 1,126/18,110 (6.2) | 1,698/19,004 (8.9) | <0.001 |
| RDS | 10,059/17,947 (56.0) | 9,631/18,732 (51.4) | <0.001 |
| PDA | 6,284/17,940 (35.0) | 5,901/18,733 (31.5) | <0.001 |
| CLD on day 28 | 6,502/17,484 (37.2) | 5,669/17,601 (32.2) | <0.001 |
| CLD at 36 weeks | 3,359/17,332 (19.4) | 2,838/17,358 (16.3) | <0.001 |
| NEC and/or intestinal perforation | 423/18,110 (2.3) | 479/19,004 (2.5) | 0.252 |
| Sepsis | 1,218/17,930 (6.8) | 976/18,620 (5.2) | <0.001 |
| IVH (grades 3–4) | 486/17,979 (2.7) | 594/18,650 (3.2) | 0.007 |
| Cystic periventricular leukomalacia | 556/18,022 (3.1) | 643/18,675 (3.4) | 0.056 |
| Retinopathy of prematurity, treated | 2,840/17,521 (16.2) | 2428/18,015 (13.5) | <0.001 |
| Parenteral nutrition | 11,687/18,006 (64.9) | 10,588/18,649 (56.8) | <0.001 |
| Home oxygen therapy at discharge | 962/17,937 (5.4) | 871/18,506 (4.7) | 0.004 |
Values are shown as number/number of measurements (%) or mean±standard deviation.
NRNJ, Neonatal Research Network of Japan; GA, gestational age; CAM, chorioamnionitis; RDS, respiratory distress syndrome; PDA, patent ductus arteriosus; CLD on day 28, chronic lung disease received supplemental oxygen on the 28th day after birth; CLD at 36 weeks, chronic lung disease received supplemental oxygen at 36 weeks postmenstrual age; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage.
Boldface indicates a statistically significant difference with P<0.05.
Bias in reported outcomes increases with losses to follow-up. Therefore, efforts to increase follow-up rates are needed to enable critical evaluations of the long-term outcomes of VLBW infants [28]. Strategies to maintain good compliance include: (1) the identification of a specific contact person before NICU discharge, (2) multiple back-up addresses and phone numbers of family members, including grandparents, (3) repeat explanations presented to parents about the importance of long-term follow-up, (4) the provision of a handbook for medical information during the NICU stay that includes information for hospitals, primary physicians, and reginal health care providers after discharge, (5) notes on the schedule of comprehensive follow-ups until school age or adolescence, (6) annual contact with subjects by a coordinator (i.e., birthday cards), (7) medical referral to network study participating centers when patients are moving, and (8) the provision of incentives to parents, physicians, and medical staff to participate and complete follow-ups.
Mortality
Mortality rates in 40,728 VLBW infants in the NRNJ database (2003–2012) before and after discharge until 3 years of age were 8.2% (n=3348) and 0.7% (n=266), respectively. Among the 266 infants who died after discharge, 26 had lethal congenital anomalies (i.e., trisomy 18, trisomy 13, and anencephaly). The mortality rate before discharge of the VLBW infants was inversely associated with BW, ranging from 85.5% in infants with a BW of 200–300 g to 3.5% in those with a BW 1,400–1,500 g. When stratified by gestational weeks, the mortality rate proportionally decreased from 59.6% at 22 weeks’ gestation to 3.2% at 31 weeks’ gestation. It increased after 32 weeks’ gestation to 50% at 39 weeks’ gestation.
We must consider whether to present mortality and morbidity rates based on BW or GA. The outcomes of infants with the same BW but different GA may differ markedly due to differences in background variables [29]. The management of maternal conditions, including infection, premature rupture of membranes, and preterm labor, may influence GA at birth, resulting in prematurity. Maternal hypertension, nutritional status, and tobacco or alcohol exposure may cause fetal growth restrictions, resulting in a low BW and the birth of a small for GA (SGA) infant [30]. Therefore, both BW and GA must be considered in outcome studies, as well as whether SGA infants are included in studies grouped by BW or GA. The higher mortality rate of infants in later gestational weeks in the NRNJ database may be related to the higher rate of SGA infants [1]. Rysavy et al. [31], who reported the outcomes of extremely preterm infants, strongly recommended the stratification of outcomes by GA at birth because the degree of fetal maturation is the most important factor affecting outcomes.
Improvements in survival among extremely premature infants, such as those born at <25 weeks’ gestation, have been attracting increasing attention. The survival rates of infants born at 22 and 23 weeks’ gestation in the NRNJ database increased from 26.5% and 66.3% in 2003 to 51.8% and 73.6% in 2012, respectively. Recent studies from the United States, Sweden, and Germany indicated that mortality rates decreased in extremely premature infants who were provided active treatment [3,32,33]. The National Institute of Child Health and Human Development (NICHD) Neonatal Research Network reported increased survival rates among infants born at <25 weeks’ gestation in 2000–2011. The percentage of surviving infants increased from 30% (424 of 1,391 infants) in 2000–2003 to 36% (487 of 1,348 infants) in 2008–2011 [3]. Among live births at 22 to 24 weeks’ GA in Sweden, 1-year survival rates increased between 2004–2007 and 2014–2016 from 52% to 62%, respectively [32]. A recent study from a level III NICU center in Germany in which infants born in 2010–2014 received active prenatal and postnatal care reported survival rates of 61% (17 of 28) at 22 weeks’ gestation and 71% (41 of 58) at 23 weeks’ gestation [33].
Neurodevelopmental outcomes
The numbers and rates of infants with each NDI among the survived infants with assessments in the NRNJ database are summarized in Table 3. Among 18,110 VLBW infants followed up at 3 years of age, 17,078 were assessed for CP and 1,204 (7.1%) were found to have CP with a GMFCS level ≥II. The proportions of uni- or bilateral blindness and bilateral amblyopia were 1.8% and 1.2% of measurements for visual function, respectively. A severe hearing impairment requiring hearing aids was detected in 0.9% of the infants. A DQ score of a KSPD <70 was observed in 15.9% of the infants. Developmental delay was judged in 21.5% of pediatricians’ assessments. The rates of all impairments were higher in the BW <1,000 g group than in the BW ≥1,000 g group.
Table 3.
NDI of VLBW infants followed up at 3 years of age included in the NRNJ
| Type of impairment | Total | <1,000 g | 1,000–1,500 g |
|---|---|---|---|
| Death by 3 years of age | 3,614/40,728 (8.9) | 2,727/17,890 (15.2) | 887/22,838 (3.9) |
| Survivors with follow-up data | 18,110/37,114 (48.8) | 8,132/15,163 (53.6) | 9,978/21,951 (45.5) |
| Cerebral palsy (GMFCS 2) | 1,204/17,078 (7.1) | 725/7,631 (9.5) | 479/9,447 (5.1) |
| Uni- or bilateral blindness | 301/16,418 (1.8) | 234/7,346 (3.2) | 67/9,072 (0.7) |
| Any blindness or amblyopia | 497/16,418 (3.0) | 400/7,346 (5.4) | 97/9,072 (1.1) |
| Hearing amplification | 118/13,114 (0.9) | 90/5,886 (1.5) | 28/7,228 (0.4) |
| DQ of KSPD <70 | 2,085/13,100 (15.9) | 1,369/5,938 (23.1) | 716/7,162 (10.0) |
| Judged as delayed by pediatricians | 409/1,898 (21.5) | 268/881 (30.4) | 141/1,017 (13.9) |
| NDI full evaluation | 2,066/11,015 (18.8) | 1,329/4,927 (27.0) | 737/6,088 (12.1) |
| NDI not full evaluation | 1,392/7,095 (19.6) | 908/3,205 (28.3) | 484/3,890 (12.4) |
| NDI in survivors with follow-up data | 3,458/18,110 (19.1) | 2,237/8,132 (27.5) | 1,221/9,978 (12.2) |
| Death or NDI in all study subjects | 7,072/40,728 (17.4) | 4,964/17,890 (27.7) | 2,108/22,838 (9.2) |
Values are presented as no. of infants with impairment/no. of infants with assessments.
NDI, neurodevelopmental impairment; VLBW, very low birth weight; NRNJ, Neonatal Research Network of Japan; GMFCS, Gross Motor Function Classification System; DQ, developmental quotient; KSPD, the Kyoto Scale of Psychological Development.
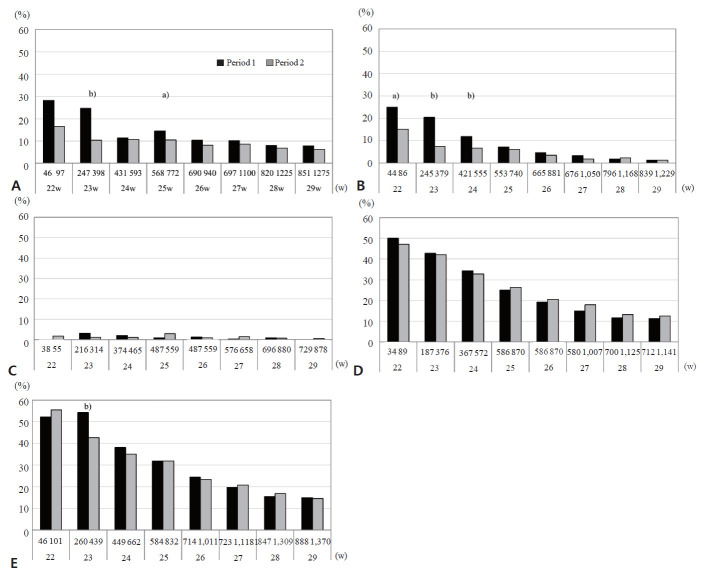
Figs. 3A–D compare the rates of each neurological impairment in VLBW infants in period 1 (2003–2007) and period 2 (2008–2012) stratified by weeks of GA <30 weeks. The numbers of infants with assessments in each period as denominators for rates are presented under the x-axis. The rates of all impairments except hearing impairment were inversely associated with gestational weeks. The mortality rate of infants at 22- to 24-week GA markedly decreased between period 1 and period 2. The CP rates of survivors also decreased at 22–24 weeks of GA, although a significant difference was only observed at 23 weeks. Similarly, the rate of visual impairment, including both blindness and amblyopia, among survivors decreased in period 2 at 22 to 24 weeks’ GA. No significant changes were observed in the rates of hearing impairment or developmental delay. These results are consistent with recent findings showing increases in survival without disabilities. The decrease in the CP rate was significant, whereas that in cognitive function was not in these studies [3,9,21,34].
Fig. 3.
Neurodevelopmental impairments (NDI) in infants assessed at 3 years of age by gestational weeks (22–29 weeks) in period 1 (2003–2007, black bar) and period 2 (2008–2012, gray bar) in the Neonatal Research Network of Japan database. (A) Cerebral palsy, (B) blindness or amblyopia, (C) hearing impairment, (D) developmental delay including a developmental quotient <70 or delay judged by a pediatrician, (E) neurodevelopmental impairment. Numbers under the x-axis show the number of infants assessed for each impairment in periods 1 and 2. a)P<0.05, b)P<0.01 between the periods.
Neurodevelopmental impairment
Among the 18,110 infants followed up at 3 years of age, 3,458 (19.1%) had NDI (Table 3). No significant differences were observed in NDI rates between infants with and those without a full evaluation (P=0.33). The NDI rates of the assessed infants stratified by gestational weeks (22–29 weeks) in periods 1 and 2 are shown in Fig. 3E. NDI among survivors in period 2 was inversely associated with gestational weeks, ranging from 54.4% at 22 weeks to 14.6% at 29 weeks. The morbidity rates of infants assessed for NDI did not change between the 2 periods except for a significant decrease in infants born at 23 weeks’ GA. In infants born at 23 weeks’ GA, rates of both CP and visual impairment in survivors decreased, resulting in a decreased NDI rate at 3 years of age. Although the reason for the decrease in these impairments remains unclear, we speculated that more strategies were used to prevent hypoxic, hyperoxic, or ischemic damage related to IVH, periventricular leukomalacia, or retinopathy of prematurity in the second period, contributing to the improved outcome of survivors at 23 weeks’ GA [9]. An increase in parenteral nutrition in the NICU between the 2 periods may have also contributed to the better outcomes of survivors [35]. For infants born at 22 weeks’ gestation, however, extreme prematurity itself may be critical. In addition, because the number of survivors born at 22 weeks’ GA was relatively small, a significant improvement would be attained when larger numbers of survivors were evaluated.
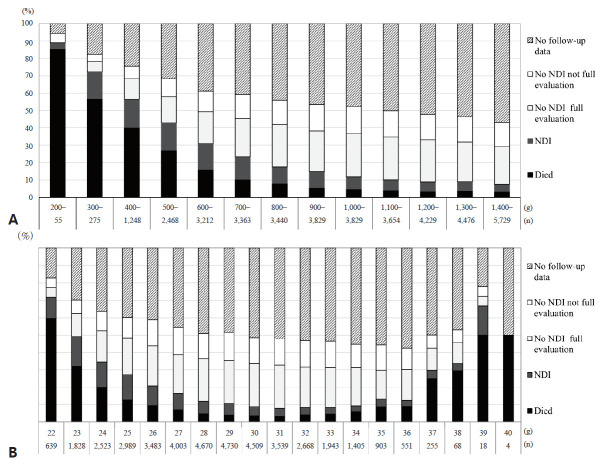
Among the outcome cohort consisting of 21,724 infants with data for death or survival and follow-up assessments at 3 years of age, the survival rate of those without NDI was 67.5% (Fig. 2). In a reevaluation of 40,728 baseline study subjects, 3,458 (8.5%) had NDI and 14,652 (36.0%) did not, while 19,004 (46.7%) did not have follow-up data. The mortality or NDI rate, which represents an unfavorable outcome, in all study subjects including infants without follow-up data was 17.4%, while that in the subjects with outcome data was 32.5% (Table 3, Fig 2). When stratified by BW, death or NDI rates of the total population decreased as BW increased from 200–300 g (89.1%) to 1,400–1,500 g (7.6%) (Fig. 4A). When stratified by gestational weeks, the mortality or NDI rate decreased with an increase in gestational weeks from 22 weeks (71.8%) to 31 weeks (7.8%) and increased to 66.7% at 39 weeks (Fig. 4B). The NDI rate of the total population was the highest in infants born at 23 weeks’ gestation and decreased toward 34 weeks’ gestation from 17.2% to 3.5%.
Fig. 4.
Death versus survival outcomes of infants with versus without neurodevelopmental impairments (NDI) by birth weight (A) and gestational weeks (B) for all study subjects born in 2003–2012 included in the Neonatal Research Network of Japan database.
A recent study from the NICHD Neonatal Research Network that completed neurodevelopmental follow-up assessments of infants born at ≤27 weeks’ GA between 2011 and 2015 reported that the moderate to severe NDI rate of survivors was 19% using the Bayley III cognitive score of <70 and 32% using the Bayley III cognitive cutoff of <85 [21]. The corresponding rate of the NRNJ database cohort in period 2 to that using the Bayley III cognitive cutoff of <85 was 32%. A national cohort study of children born in Canada at <29 weeks’ GA showed that 46% had NDI using the Bayley III cognitive cutoff of <85 and 17% had severe NDI with Bayley III composite scores of <70 at 18–21 months [36]. Thus, NDI in survivors and death or NDI in all study subjects may be used as universal indicators of NICU graduate outcomes.
Long-term outcomes
In a review of neurodevelopmental outcomes, Jarjour [37] reported high long-term disability rates in school-age children and young adults born extremely preterm, with moderate to severe NDI in 17%–48%, CP in 9%–20%, and intellectual disability in 4%–36%. Furthermore, milder degrees of disabilities, including mild cognitive impairment with IQ scores of 70–84, behavioral issues such as autism spectrum disorder or attention-deficit hyperactivity disorder, and learning difficulties were detected in school-age children and young adults in long-term follow-up [38-42]. Based on the high prevalence of mild to severe NDI as well as behavioral issues in school-age children born preterm or at a VLBW, it is necessary to evaluate and manage neurodevelopmental outcomes before these children enter school. Earlier behavioral difficulties are related to cognitive impairments and later low functioning [43]. Early intervention programs for preterm infants positively influence cognitive and motor outcomes in infants and preschoolers [44]. Various social environment factors, including familial support for parental mental health and well-being, will also be interventional targets.45) Further consideration is needed to establish a follow-up protocol for school-age children and adolescents registered in the NRNJ database.
Conclusion
Follow-up studies from the NRNJ database suggested that children born preterm at a VLBW remain at high risk of developing NDI in early childhood. It is important to establish a common protocol within the network and complete assessments with fewer dropouts to clarify the neurodevelopmental outcomes of the registered infants. All possible strategies should be employed to maintain good compliance after NICU discharge for the precise outcome information and because early assessments and interventions positively influence long-term outcomes.
Acknowledgments
I thank Professor Satoshi Kusuda, members of the Neonatal Research Network of Japan, and the investigators of the participating institutions.
Key message
· Very low birth weight infants remain at high risk of developing neurodevelopmental impairments in early childhood.
· It is important to establish a network follow-up protocol and complete assessments with fewer dropouts to enable clarification of the outcomes of registered infants.
· All possible strategies should be employed to maintain good compliance after neonatal intensive care unit discharge.
Footnotes
Ethics statement
The NRNJ protocol was approved by the Ethics Review Committees of Tokyo Women’s Medical University and Jichi Medical University.
No potential conflict of interest relevant to this article was reported.
References
- 1.Itabashi K, Horiuchi T, Kusuda S, Kabe K, Itani Y, Nakamura T, et al. Mortality rates for extremely low birth weight infants born in Japan in 2005. Pediatrics. 2009;123:445–50. doi: 10.1542/peds.2008-0763. [DOI] [PubMed] [Google Scholar]
- 2.Stensvold HJ, Klingenberg C, Stoen R, Moster D, Braekke K, Guthe HJ, et al. Neonatal morbidity and 1-year survival of extremely preterm infants. Pediatrics. 2017;139:e20161821. doi: 10.1542/peds.2016-1821. [DOI] [PubMed] [Google Scholar]
- 3.Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. 2017;376:617–28. doi: 10.1056/NEJMoa1605566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson-Costello D, Friedman H, Minich N, Siner B, Taylor G, Schluchter M, et al. Improved neurodevelopmental outcomes for extremely low birth weight infants in 2000-2002. Pediatrics. 2007;119:37–45. doi: 10.1542/peds.2006-1416. [DOI] [PubMed] [Google Scholar]
- 5.Ishii N, Kono Y, Yonemoto N, Kusuda S, Fujimura M, Neonatal Research Network, Japan Outcomes of infants born at 22 and 23 weeks' gestation. Pediatrics. 2013;132:62–71. doi: 10.1542/peds.2012-2857. [DOI] [PubMed] [Google Scholar]
- 6.Inoue H, Ochiai M, Sakai Y, Yasuoka K, Tanaka K, Ichiyama M, et al. Neurodevelopmental outcomes in infants with birth weight ≤500 g at 3 years of age. Pediatrics. 2018;142:e20174286. doi: 10.1542/peds.2017-4286. [DOI] [PubMed] [Google Scholar]
- 7.Kusuda S, Fujimura M, Sakuma I, Aotani H, Kabe K, Itani Y, et al. Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics. 2006;118:e1130–8. doi: 10.1542/peds.2005-2724. [DOI] [PubMed] [Google Scholar]
- 8.Kusuda S, Fujimura M, Uchiyama A, Totsu S, Matsunami K, Neonatal Research Network, Japan Trends in morbidity and mortality among very-low-birth-weight infants from 2003 to 2008 in Japan. Pediatr Res. 2012;72:531–8. doi: 10.1038/pr.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kono Y, Yonemoto N, Nakanishi H, Kusuda S, Fujimura M. Changes in survival and neurodevelopmental outcomes of infants born at <25 weeks' gestation: a retrospective observational study in tertiary centres in Japan. BMJ Paediatr Open. 2018;2:e000211. doi: 10.1136/bmjpo-2017-000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372:1801–11. doi: 10.1056/NEJMoa1410689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle LW, Anderson PJ, Battin M, Bowen JR, Brown N, Callanan C, et al. Long term follow up of high risk children: who, why and how? BMC Pediatr. 2014;14:279. doi: 10.1186/1471-2431-14-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemyre B, Moore G. Counselling and management for anticipated extremely preterm birth. Paediatr Child Health. 2017;22:334–41. doi: 10.1093/pch/pxx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kono Y, Mishina J, Yonemoto N, Kusuda S, Fujimura M. Outcomes of very-low-birthweight infants at 3 years of age born in 2003-2004 in Japan. Pediatr Int. 2011;53:1051–8. doi: 10.1111/j.1442-200X.2011.03480.x. [DOI] [PubMed] [Google Scholar]
- 14.Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. 2015;(11):CD005495. doi: 10.1002/14651858.CD005495.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Japan Neonatal Follow-up Study Group . Japan Neonatal Follow-up Study Group; Protocol for the multicenter follow-up study of VLBW infants (in Japanese) [Internet] [cited 2020 Jun 10]. Available from: http://highrisk-follow-up.jp/schedule/ [Google Scholar]
- 16.Bax MC. Terminology and classification of cerebral palsy. Dev Med Child Neurol. 1964;6:295–7. doi: 10.1111/j.1469-8749.1964.tb10791.x. [DOI] [PubMed] [Google Scholar]
- 17.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 18.Society for the Kyoto Scale of Psychological Development Test . Shinpan K Shiki Hattatsu Kensahou 2001 Nenban [The Kyoto Scale of Psychological Development Test 2001] Kyoto (Japan): Nakanishiya Shuppan; 2008. (Japanese) [Google Scholar]
- 19.Bayley N. Bayley scales of infant and toddler development. 3rd ed. San Antonio (TX): Harcourt Assessment; 2006. [Google Scholar]
- 20.Kono Y, Yonemoto N, Kusuda S, Hirano S, Iwata O, Tanaka K, et al. Developmental assessment of VLBW infants at 18 months of age: a comparison study between KSPD and Bayley III. Brain Dev. 2016;38:377–85. doi: 10.1016/j.braindev.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Adams-Chapman I, Heyne RJ, DeMauro SB, Duncan AF, Hintz SR, Pappas A, et al. Neurodevelopmental Impairment Among Extremely Preterm Infants in the Neonatal Research Network. Pediatrics. 2018;141:e20173091. doi: 10.1542/peds.2017-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen MC, Cristofalo EA, Kim C. Outcomes of preterm infants: morbidity replaces mortality. Clin Perinatol. 2011;38:441–54. doi: 10.1016/j.clp.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Wolke D, Ratschinski G, Ohrt B, Riegel K. The cognitive outcome of very preterm infants may be poorer than often reported: an empirical investigation of how methodological issues make a big difference. Eur J Pediatr. 1994;153:906–15. doi: 10.1007/BF01954744. [DOI] [PubMed] [Google Scholar]
- 24.Fewtrell MS, Kennedy K, Singhal A, Martin RM, Ness A, Hadders-Algra M, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child. 2008;93:458–61. doi: 10.1136/adc.2007.127316. [DOI] [PubMed] [Google Scholar]
- 25.Castro L, Yolton K, Haberman B, Roberto N, Hansen NI, Ambalavanan N, et al. Bias in reported neurodevelopmental outcomes among extremely low birth weight survivors. Pediatrics. 2004;114:404–10. doi: 10.1542/peds.114.2.404. [DOI] [PubMed] [Google Scholar]
- 26.Callanan C, Doyle L, Rickards A, Kelly E, Ford G, Davis N. Children followed with difficulty: how do they differ? J Paediatr Child Health. 2001;37:152–6. doi: 10.1046/j.1440-1754.2001.00621.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim NH, Youn YA, Cho SJ, Hwang JH, Kim EK, Kim EA, et al. The predictors for the non-compliance to follow-up among very low birth weight infants in the Korean neonatal network. PLoS One. 2018;13:e0204421. doi: 10.1371/journal.pone.0204421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vohr BR, Wright LL, Hack M, Aylward G, Hirtz D. Follow-up care of high-risk infants. Pediatrics. 2004;114(Suppl):1377–97. [Google Scholar]
- 29.Vohr BR. How should we report early childhood outcomes of very low birth weight infants? Semin Fetal Neonatal Med. 2007;12:355–62. doi: 10.1016/j.siny.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 30.McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23:779–93. doi: 10.1016/j.bpobgyn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Rysavy MA, Marlow N, Doyle LW, Tyson JE, Serenius F, Iams JD, et al. Reporting outcomes of extremely preterm births. Pediatrics. 2016;138:e20160689. doi: 10.1542/peds.2016-0689. [DOI] [PubMed] [Google Scholar]
- 32.Norman M, Hallberg B, Abrahamsson T, Björklund LJ, Domellöf M, Farooqi A, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004-2007 and 2014-2016. JAMA. 2019;321:1188–99. doi: 10.1001/jama.2019.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehler K, Oberthuer A, Keller T, Becker I, Valter M, Roth B, et al. Survival among infants born at 22 or 23 weeks' gestation following active prenatal and postnatal care. JAMA Pediatr. 2016;170:671–7. doi: 10.1001/jamapediatrics.2016.0207. [DOI] [PubMed] [Google Scholar]
- 34.Pierrat V, Marchand-Martin L, Arnaud C, Kaminski M, Resche-Rigon M, Lebeaux C, et al. Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks' gestation in France in 2011: EPIPAGE-2 cohort study. BMJ. 2017;358:j3448. doi: 10.1136/bmj.j3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruyama H, Yonemoto N, Kono Y, Kusuda S, Fujimura M, Neonatal Research Network of Japan Weight growth velocity and neurodevelopmental outcomes in extremely low birth weight infants. PLoS One. 2015;10:e0139014. doi: 10.1371/journal.pone.0139014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Synnes A, Luu TM, Moddemann D, Church P, Lee D, Vincer M, et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child Fetal Neonatal Ed. 2017;102:F235–F234. doi: 10.1136/archdischild-2016-311228. [DOI] [PubMed] [Google Scholar]
- 37.Jarjour IT. Neurodevelopmental outcome after extreme prematurity: a review of the literature. Pediatr Neurol. 2015;52:143–52. doi: 10.1016/j.pediatrneurol.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. 2011;69(5 Pt 2):11R–18R. doi: 10.1203/PDR.0b013e318212faa0. [DOI] [PubMed] [Google Scholar]
- 39.Johnson S, Wolke D. Behavioural outcomes and psychopathology during adolescence. Early Hum Dev. 2013;89:199–207. doi: 10.1016/j.earlhumdev.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 40.Pinto-Martin JA, Levy SE, Feldman JF, Lorenz JM, Paneth N, Whitaker AH. Prevalence of autism spectrum disorder in adolescents born weighing <2000 grams. Pediatrics. 2011;128:883–91. doi: 10.1542/peds.2010-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanabe K, Tamakoshi K, Kikuchi S, Murotsuki J. Learning disability in 10- to 16-year-old adolescents with very low birth weight in Japan. Tohoku J Exp Med. 2014;232:27–33. doi: 10.1620/tjem.232.27. [DOI] [PubMed] [Google Scholar]
- 42.Farooqi A, Adamsson M, Serenius F, Hägglöf B. Executive functioning and learning skills of adolescent children born at fewer than 26 weeks of gestation. PLoS One. 2016;11:e0151819. doi: 10.1371/journal.pone.0151819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123:1485–92. doi: 10.1542/peds.2008-1216. [DOI] [PubMed] [Google Scholar]
- 44.Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. 2015;(11):CD005495. doi: 10.1002/14651858.CD005495.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Treyvaud K, Lee KJ, Doyle LW, Anderson PJ. Very preterm birth influences parental mental health and family outcomes seven years after birth. J Pediatr. 2014;164:515–21. doi: 10.1016/j.jpeds.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]