Abstract
Objective(s):
Prostate-specific membrane antigen (PSMA) ligand positron emission tomography/computed tomography (PET/CT) is an emerging modality to detect metastatic disease in patients with prostate cancer (PCa). This prospective study aimed to evaluate the role of [68Ga]-PSMA PET/CT in the initial workup of intermediate and high-risk PCa.
Methods:
Twenty-five patients with newly transrectal ultrasound biopsy-proven, untreated intermediate- and high-risk PCa (mean age, 68.5±6.2 years; range 55–83 years) were enrolled in this prospective study between September 2018 and June 2020 and underwent a [68Ga]-PSMA PET/CT examination. All images were analyzed both visually and semiquantitatively by measuring the maximum standardized uptake value (SUVmax) of the primary prostatic tumor and metastatic lesions. The diagnostic sensitivity of [68Ga]-PSMA PET/CT for the diagnosis of PCa was established by histopathology as the reference standard. The associations between SUVmax of the primary tumors and prostate-specific antigen (PSA) levels, Gleason scores (GSs), and metastatic extent of the disease were studied.
Results:
All patients had a positive [68Ga]-PSMA PET/CT exam. Seventeen patients (58%) showed [68Ga]-PSMA avidity in both prostate lobes and 8 (32%) had unilateral uptake. SUVmax in the primary tumor significantly correlated with serum PSA values (r=0.57, P=0.003). PSMA PET/CT depicted regional lymph node metastases in 32% of patients, distant lymph node metastases in 20%, osseous metastases in 16% and pulmonary metastases in 8% of patients. Sixty percent of PSMA-positive bone metastases and 21.4% of intraprostatic tumoral lesions were missed on the contemporaneous bone scintigraphy and magnetic resonance imaging, respectively.
Conclusion:
[68Ga]-PSMA PET/CT shows promise as a valuable imaging modality with high diagnostic sensitivity in the setting of intermediate and high-risk PCa. Moreover, the SUVmax of the primary tumor has a positive correlation with PSA levels at the time of the scan.
Key Words: Prostate cancer, PSMA PET/CT, Primary staging
Introduction
Prostate cancer (PCa) is the most commonly known malignancy in men and a leading cause of cancer-related death (1). Diagnosis of PCa is mainly based on histopathology results following prostate biopsy, which also aids in patients’ stratification into distinct risk groups (low-, intermediate-, and high-risk) according to serum prostate-specific antigen (PSA) levels and Gleason scores. Precise local and systemic prostate cancer staging is an important diagnostic procedure for patient stratification and a key to designing the optimum personalized management strategy for the individual patient. Despite careful selection of patients before curative intent treatments, including surgery and radiotherapy, relapse following treatment is not uncommon (2, 3), and this could be partly explained due to the insufficient accuracy of existing standard-of-care conventional imaging (4) with CT and bone scan to detect non-localized disease (5). Novel whole-body tumor-specific imaging techniques developed based on targeting of prostate-specific membrane antigen (PSMA), a cell surface glycoprotein overexpressed on prostate cancer cells, might enable a more tailored multimodal treatment plan by more accurate definition of disease extent at the outset.
Although the role of PSMA-based PET/CT is well documented in patients with biochemical evidence of recurrent PCa (6-10), there are few studies, mostly retrospective in design, on the initial evaluation and staging of newly diagnosed PCa (11-14). Accordingly, this prospective study was aimed at addressing the diagnostic sensitivity of [68Ga]-PSMA PET/CT for the diagnosis and initial staging of intermediate and high-risk prostate cancer.
Methods
Participants
Twenty-five patients (mean age, 68.5±6.2 years; range 55–83 years) with newly diagnosed, treatment naïve, intermediate- and high-risk prostate cancer, defined according to the D’Amico risk stratification (15), were prospectively recruited (September 2018 to June 2020) for primary staging in this single-center study. The Ethics Committee of Tehran University of Medical Sciences approved the study protocol (IR.TUMS.VCR.REC.1397.203) and written informed consent was obtained from all participants. Inclusion criteria included age of more than 18 years, no prior PCa treatment, histologically proven PCa with transrectal ultrasound (TRUS)-guided biopsy, Gleason score ≥6 accompanied by the prostate-specific antigen (PSA) ≥10, or Gleason score ≥7 regardless of the PSA level. Exclusion criteria included the inability to lie still for imaging, exceeding the safe weight of the PET/CT bed (200 kg), claustrophobia and medical instability. PSA values, Gleason scores, relevant clinical history and any other available imaging reports were recorded prior to being submitted to the PET/CT scan. Once enrolled, all patients underwent [68Ga]-PSMA PET/CT.
Image Acquisition
The [68Ga]-PSMA was provided in a ready-to-use from the Pars Isotope Company, Tehran, Iran. Quality controls were carried out according to the standard protocols provided by the manufacturer. Patients were not subjected to any special preparation prior to imaging. PET/CT imaging was acquired in all patients, using Biograph 6 True Point HD PET/CT scanner (Siemens Medical Solutions, Erlangen, Germany), 60 minutes following the injection of a bodyweight adjusted dose of 2-4 MBq/kg (170±22 MBq). Patients were instructed to void immediately prior to the study. Image acquisition was performed with a whole-body field of view (vertex to mid-thigh). The CT exposure factors for all examinations were 80 mAs, 120-130 keV, pitch of 1.3 and slice thickness of 5 mm. Maintaining patient position, a whole-body PET scan was performed immediately after CT acquisition and covered an area identical to that covered by CT from mid-thigh to vertex. PET scan was carried out in 3D with an acquisition time of 4 min per bed position. PET images were reconstructed using CT for attenuation correction. Transaxial PET data were reconstructed using ordered subsets expectation maximization algorithm (2 iterations and 21 subsets) and post-reconstruction smoothing with a Gaussian filter (4.0 mm full-width at half maximum (FWHM).
Image Interpretation and Reference Standard
Expert nuclear medicine specialists analyzed images prospectively. Analysis was performed using Syngo software TrueD (Siemens Medical Systems), which allowed the review of PET, CT and fused imaging data in axial, coronal and sagittal slices. Physicians with access to clinical data completed a qualitative assessment and recorded the number of positive lesions (0, 1, 2, 3, 4, 5, 6-10, >10) and their anatomic sites (local tumor, regional nodes, distant nodes (i.e., nodes located cranial to the bifurcation of the common iliac vessels), bone, lung,). The European Association of Nuclear Medicine (EANM) criteria have been used to improve objectivity and accuracy in image interpretation and to classify scans as positive (pathologic) or negative (normal) (16); according to which, all areas of increased radiotracer uptake in sites not expected to show physiological uptake (e.g., lacrimal glands, salivary glands, liver, spleen, small intestine, colon, kidney, autonomic ganglia, etc.) were considered “anomalous”. Increased uptake was characterized as focal tracer uptake higher than the adjacent background. Subsequently, all anomalous findings suggestive of primary prostate cancer and its metastases based on the combination of imaging and clinical features were noted as “pathologic” (positive scan) unless another explanation for the increased uptake could be postulated (e.g. Paget’s bone disease, synchronous malignancy, etc.), in which case the findings would not be described as pathologic (negative scan). According to these definitions, baseline classification as positive (pathologic) or negative (normal) was thus largely based on readers’ experience.
Quantitative data, comprising the maximum standardized uptake value (SUVmax) of the primary prostatic tumor and the five most active extraprostatic metastases were measured by placing a circular region of interest (ROIs) on the area of the highest activity consistent with the extraprostatic metastases or primary tumoral lesion.
We did not mandate the management of patients following the PET/CT imaging and left the decision at the discretion of individual surgeons. The definitive diagnosis was validated by biopsy-proven histopathological findings, examined by experienced pathologists, which were obtained by transrectal ultrasound (TRUS) directed procedure within one month before [68Ga]-PSMA PET/CT scan. The histopathology results from radical prostatectomy specimens following PET/CT were used as the standard-of-reference in 12 (48%) participants.
Statistical Analysis
The normalcy of data was assessed using the Kolmogorov-Smirnov test. Descriptive values were expressed as the mean (±SD) or median (range) if data were not normally distributed. Associations between the GS, PSA and number of PSMA positive metastases with the SUVmax of the primary intraprostatic tumor were described using the non-parametric Spearman’s rank correlation. A patient-based sensitivity evaluation was also carried out. The patient-based calculation of specificity, positive predictive value (PPV), and negative predictive value (NPV) could not be determined in the entire cohort as all our participants had histologically proven PCa and therefore the cohort did not include true-negative patients. However, sensitivity, specificity, PPV, NPV, and accuracy were calculated in the subgroup of patients who underwent radical prostatectomy following PET/CT. A p value of less than 0.05 was considered to represent statistical significance. Statistical analyses were performed using SPSS version 24.0 software (IBM Corp., Armonk, NY) and R (version 3.6.0; The R Foundation for Statistical Computing, General Public License).
Results
Baseline patients’ characteristics are summarized in Table 1. Twenty-five patients with intermediate- and high-risk disease were enrolled. Of these, two subjects (8%) had Gleason score of 6, eleven (44%) had Gleason score of 7, eight (32%) had Gleason score of 8 and four (16%) were categorized as the Gleason score 9-10 group. The median PSA at the time of PSMA PET scanning was 15.5 ng/mL [6.2-172 ng/mL]. None of the patients was receiving treatments prior to or at the time of PSMA PET scanning.
Table 1.
Patient characteristics (n=25)
| Characteristic | Value |
|---|---|
| Age (years) | 68.5±6.2 |
| Weight (kg) | 75.8±10.5 |
| Height (cm) | 171.6±7.4 |
| Injected activity (mCi) | 4.6±0.6 |
| PSA at PET/CT (ng/ml), median (range) | 15.5 (6.2-172.0) |
| Initial Gleason score | |
| 6 | 2 (8.0%) |
| 7 (3+4) | 7 (28.0%) |
| 7 (4+3) | 4 (16.0%) |
| 8 | 8 (32.0%) |
| 9-10 | 4 (16.0%) |
| Prior Imaging† | |
| Bone Scintigraphy | 19 (76.0%) |
| Abdominopelvic MRI | 14 (56.0%) |
† Categories are not mutually exclusive.
PSA, Prostate specific antigen; MRI, magnetic resonance imaging; CT, computed tomography
Interpretation of PSMA PET/CT images was feasible in all participants. The detection rate of [68Ga]-PSMA PET/CT was not different between different groups of patients in terms of Gleason score and PSA values. Primary intraprostatic tumoral lesions were detected in all 25 enrolled patients. The patient-based analysis showed that all patients had a positive PSMA PET/CT scan (sensitivity of 100%). Among the 25 positive scans, PSMA PET/CT depicted local intraprostatic tumors in 100% of patients, regional lymph node metastases in 32% of patients, distant lymph node metastases in 20% of patients, osseous metastases in 16% of patients, and pulmonary metastases in 8% of patients (Table 2). Of these 25 patients with local tumors, seventeen (68%) had pathological [68Ga]-PSMA uptake in bilateral prostate lobes; eight (32%) had unilateral disease, and seven patients (28%) had increased uptake in the seminal vesicles. A number of participants had disease in more than one site. Per-patient subgroup analysis of the cases who underwent radical prostatectomy following PET/CT is provided in Table 3, suggesting the high accuracy of the PSMA PET/CT to detect the involvement of seminal vesicles (91.7%), regional lymph nodes’ involvement (91.7%), followed by extraprostatic extension of the primary tumoral lesion (83.3%), as well as a lower accuracy in detection of tumors’ laterality within the prostate gland (66.7%). Figures 1 and 2 demonstrate representative [68Ga]-PSMA uptake in primary and secondary tumor sites in two patients.
Table 2.
Qualitative assessment of scans (n=25)
| Parameter | Number of Patients (%) |
|---|---|
| Final PET/CT result | |
| Negative | 0 (0%) |
| Positive | 25 (100%) |
| Number of lesions | |
| 1 | 14 (56.0%) |
| 2 | 1 (4.0%) |
| 3 | 3 (12.0%) |
| 4 | 1 (4.0%) |
| 5 | 1 (4.0%) |
| 6-10 | 0 (0%) |
| > 10 | 5 (20.0%) |
| Site of lesions† | |
| Local tumor | 25 (100%) |
| Regional nodes | 8 (32.0%) |
| Distant nodes | 5 (20.0%) |
| Bone | 4 (16.0%) |
| Lung | 2 (8.0%) |
| Number of organs involved with distant metastasis | N=8 |
| 1 | 5 (62.5%) |
| 2 | 3 (37.5%) |
| 3 | 0 (0%) |
† Categories are not mutually exclusive
Table 3.
Per-patient analysis of [68Ga]-PSMA PET/CT scans based on tumor histopathology in the subgroup of patients who underwent radical prostatectomy following the PET/CT (N=12)
| Primary Tumor’s Characteristics | TP | TN | FP | FN | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| Extraprostatic extension | 0 | 10 | 1 | 1 | 0% | 90.9% | 0% | 90.9% | 83.3% |
| Seminal vesicle(s)’ involvement | 0 | 11 | 0 | 1 | 0% | 100% | N/A | 91.7% | 91.7% |
| Regional lymph node(s)’ involvement | 1 | 10 | 1 | 0 | 100% | 90.9% | 50.0% | 100% | 91.7% |
| Bilateral tumoral involvement | 8 | 0 | 0 | 4 | 66.7% | N/A | 100% | 0% | 66.7% |
[68Ga]-PSMA PET/CT, prostate-specific membrane antigen positron emission tomography/computed tomography; TP, true positive; FP, false positive; TN, true negative; FN, false negative; PPV, positive predictive value; NPV, negative predictive value
Figure 1.
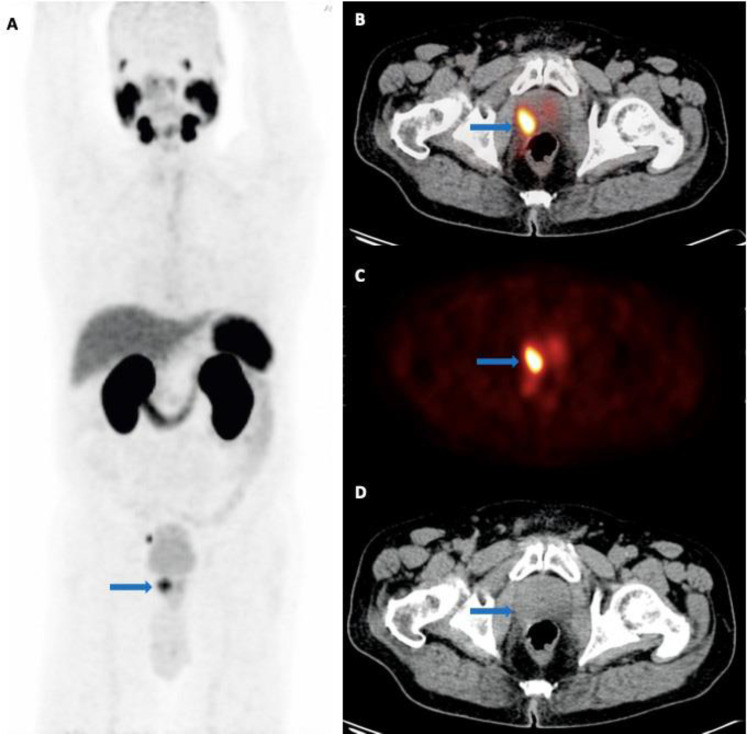
A 76-year-old man with newly diagnosed prostate cancer referred for initial staging (PSA level: 23.2 ng/mL; GS: 5 + 5). (A) The maximum intensity projection (MIP), along with trans-axial (B) fused [68Ga]-PSMA PET/C, (C) PET and (D) CT images showing PSMA uptake in the primary tumor site in the prostate (arrow), located to the lateral right mid-gland, SUVmax 8.4, with suspicious invasion to the right rectal wall. Metastatic involvement of the right pararectal and right external iliac lymph nodes are not shown here
Figure 2.
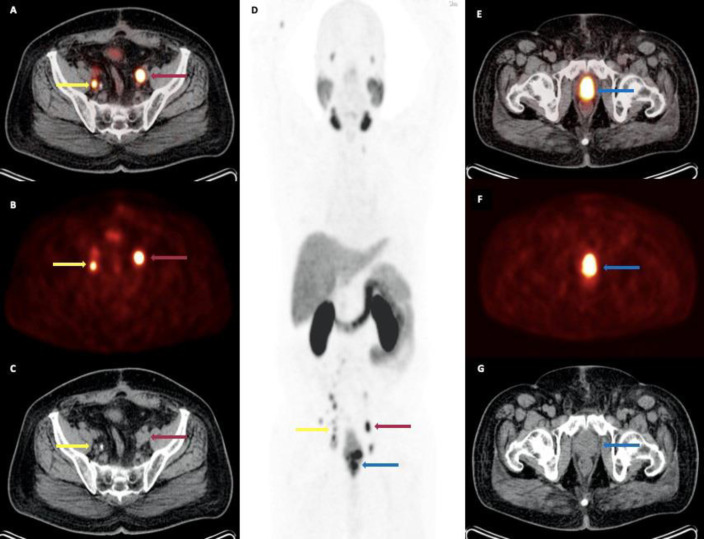
A 74-year-old man with newly diagnosed prostate cancer referred for initial staging (PSA level: 10.2 ng/mL; GS: 5 + 4). Trans-axial (A) fused [68Ga]-PSMA PET/C, (B) PET and (C) CT images showing PSMA uptake in bilateral metastatic external iliac lymph nodes (red and yellow arrows); (D) the maximum intensity projection (MIP) demonstrating the extent of disease, including primary prostate malignancy and lymph node metastases involving the para-aortic, pre-caval, para-caval, bilateral common iliac and bilateral external iliac regions; trans-axial (E) fused PET/C, (F) PET and (G) CT images showing PSMA uptake in the primary tumor site in the prostate (blue arrow), located at the base and mid-portion, SUVmax 19.1
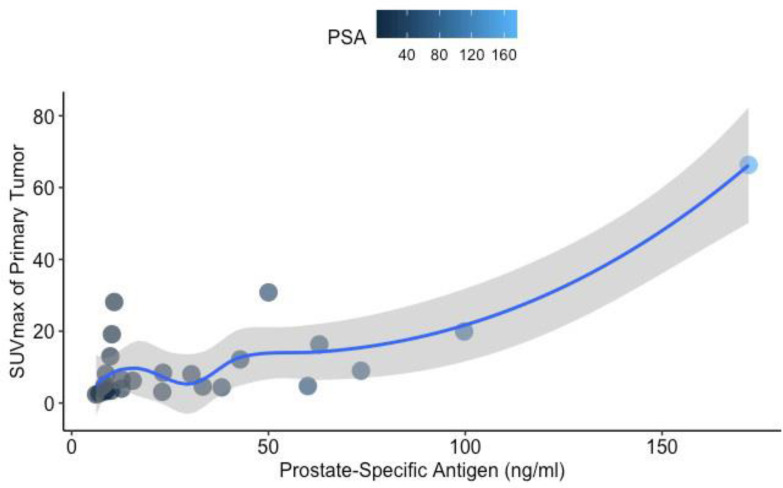
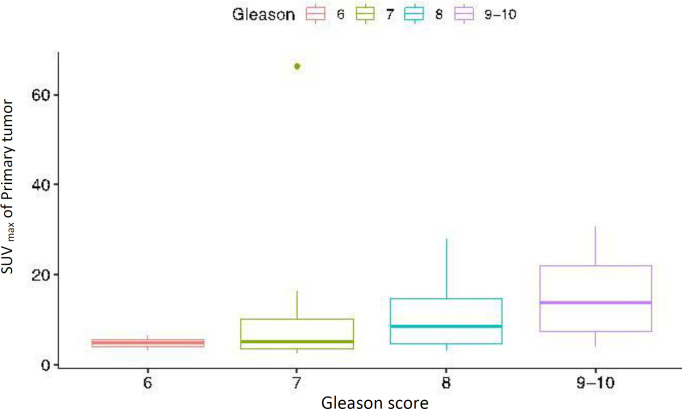
The median SUVmax value of the primary tumoral lesion in the prostate was 6.5 [2.4–66.3]. SUVmax of the primary intraprostatic tumor correlated with the PSA values (Spearman r=0.57, P=0.003) (Figure 3). However, there were no significant associations between the SUVmax of the primary intraprostatic tumor and Gleason score (P=0.08) (Figure 4) or the number of extraprostatic metastases (P=0.25). No significant correlation was observed between the Gleason scores and serum PSA levels (P=0.35). Prior imaging included bone scintigraphy (76%) and abdominopelvic magnetic resonance imaging (MRI) (56%), with some participants having undergone more than one type of imaging. A total of 20 positive bone lesions were identified on PSMA PET/CT in 4 of 25 patients (16%), and only 8 of 20 (40%) of those PSMA-avid lesions were also evident on 99mTc-methylene diphosphonate (MDP) bone scintigraphy. Three lesions identified as metastases on bone scintigraphy revealed no corresponding PSMA avidity and were deemed false positive lesions based on correlation with other conventional imaging findings. Compared to 14 contemporaneous abdominopelvic MRI, intraprostatic primary tumoral lesions were solely visible on PSMA PET/CT in 3 patients (21.4%).
Figure 3.
Scatterplot showing the correlation between the primary tumor related [68Ga]-PSMA uptake, expressed as SUVmax, and baseline prostate-specific antigen (PSA) values
Figure 4.
Comparison of the primary tumor SUVmax for different Gleason scores
Discussion
The study presented here was carried out on 25 patients with newly diagnosed, treatment naïve, intermediate- and high-risk prostate cancer to assess the diagnostic sensitivity of [68Ga]-PSMA PET/CT in this setting. Despite the fact that the enrollment of patients with primary prostate cancer precluded the calculation of patient-based specificity, PPV, and NPV, our overall results were promising and depicted a high diagnostic sensitivity of [68Ga]-PSMA PET/CT in newly diagnosed intermediate- and high risk PCa, highlighting the promising ability of this modality as a diagnostic tool for these patients. Our results with an overall sensitivity of 100% are in line with the overall sensitivity in studies published earlier (17-24), ranging from 77 to 100%. The meta-analysis conducted by Perera et al. also showed a 77% per-patient sensitivity of [68Ga]-PSMA PET/CT for primary staging purposes with respect to histology-proven disease (25).
Our findings revealed a correlation between the intensity of [68Ga]-PSMA uptake in the primary tumor, measured as SUV max, and PSA levels. Basha et al. (23), Kuten et al. (26), Sachpekidis et al. (18), Fendler et al. (27) and Uprimny et al. (28) suggested the same results, previously. In line with the findings of the study performed by Kuten et al. (26), but in contrast to the previously reported findings by Uprimny et al. (28), we observed no significant correlation between the SUVmax of the primary tumor and the number of metastatic lesions or with Gleason score.
Extraprostatic metastases were shown on PSMA PET/CT in 11 cases (44%), reinforcing the value of [68Ga]-PSMA PET/CT in the initial workup of intermediate- and high-risk patients, providing more accurate staging of newly diagnosed PCa and identifying the ideal treatment strategy.
We found that [68Ga]-PSMA PET/CT outperformed both bone scintigraphy and MRI by detecting 60% more osseous metastases and 21.4% more intraprostatic primary tumors, respectively. In keeping with our results, a meta-analysis performed by Han et al. (29) reported superior detection rates of [68Ga]-PSMA PET/CT over conventional imaging modalities and a subsequent change in management in 54% of patients. Recently, the multicenter, two-arm, randomized proPSMA study of 302 patients with high-risk PCa reported a 27% greater accuracy of [68Ga]-PSMA PSMA PET-CT than that of conventional imaging with CT or bone scanning (92% vs. 65%; p<0·0001), as well as a higher sensitivity (85% vs. 38%) and specificity (98% vs. 91%) for PSMA PET-CT, along with the superiority of PSMA PET-CT for patients with pelvic nodal and distant metastases (30). Furthermore, some retrospective single-center studies have proposed that PSMA PET-CT might be more accurate than CT or MRI in the staging of pelvic lymph nodes before prostatectomy, using histopathology as the standard-of-reference (11, 31). Maurer et al. retrospectively showed the superior performance of PSMA PET/CT over CT or MRI in 130 patients with intermediate- to high-risk prostate cancer, with a diagnostic accuracy of 0·83 versus 0·69 (11). Our data support previous observations by Pyka et al. (32) and Kuten et al. (24) that the PSMA PET/CT is superior to bone scanning in detecting skeletal metastases.
Main limitations of our study include the small sample size and lack of validating biopsy in case of suspected distant metastatic lesions, which was not possible owing to practical and ethical reasons. Moreover, it was impossible to establish the specificity, PPV, and NPV of PSMA PET/CT in this study, as we did not have negative cases for analysis.
Conclusion
[68Ga]-PSMA PET/CT is a valuable tool with high diagnostic sensitivity in patients with newly diagnosed intermediate- and high-risk prostate cancer before curative-intent therapy and should be included (if not considered as a replacement) for conventional imaging modalities, during initial workup in this context. The cost of PSMA PET-CT varies considerably by geographical locations; however, the high costs of this modality might limit its utility to cases with suspicious findings on conventional imaging methods. Future prospective studies with larger sample size are required to confirm these findings.
Conflicts of Interest
Authors declare no conflicts of interest.
Acknowledgements
This research has been carried out as part of a nuclear medicine specialty thesis and supported by Tehran University of Medical Sciences, grant no. 36976, Tehran, Iran.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Eggener SE, Scardino PT, Walsh PC, Han M, Partin AW, Trock BJ, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185(3):869–75. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumsteg ZS, Spratt DE, Romesser PB, Pei X, Zhang Z, Polkinghorn W, et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol. 2015;67(6):1009–16. doi: 10.1016/j.eururo.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohler JL, Antonarakis ES, Armstrong AJ, D’Amico AV, Davis BJ, Dorff T, et al. Prostate cancer, version 2019, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network. 2019;17(5):479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 5.Briganti A, Abdollah F, Nini A, Suardi N, Gallina A, Capitanio U, et al. Performance characteristics of computed tomography in detecting lymph node metastases in contemporary patients with prostate cancer treated with extended pelvic lymph node dissection. European urology. 2012;61(6):1132–8. doi: 10.1016/j.eururo.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Calais J, Ceci F, Eiber M, Hope TA, Hofman MS, Rischpler C, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. The Lancet Oncology. 2019;20(9):1286–94. doi: 10.1016/S1470-2045(19)30415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. Journal of Nuclear Medicine. 2018;59(1):82–8. doi: 10.2967/jnumed.117.197160. [DOI] [PubMed] [Google Scholar]
- 8.Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. European journal of nuclear medicine and molecular imaging. 2017;44(8):1258–68. doi: 10.1007/s00259-017-3711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA oncology. 2019;5(6):856–63. doi: 10.1001/jamaoncol.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur Urol. 2020;77(4):403–17. doi: 10.1016/j.eururo.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 11.Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of 68gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. The Journal of urology. 2016;195(5):1436–43. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Ferraro DA, Schüler HIG, Muehlematter UJ, Eberli D, Müller J, Müller A, et al. Impact of 68 Ga-PSMA-11 PET staging on clinical decision-making in patients with intermediate or high-risk prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging. 2020;47(3):652–64. doi: 10.1007/s00259-019-04568-1. [DOI] [PubMed] [Google Scholar]
- 13.Yaxley JW, Raveenthiran S, Nouhaud FX, Samaratunga H, Yaxley WJ, Coughlin G, et al. Risk of metastatic disease on 68gallium‐prostate‐specific membrane antigen positron emission tomography/computed tomography scan for primary staging of 1253 men at the diagnosis of prostate cancer. BJU international. 2019;124(3):401–7. doi: 10.1111/bju.14828. [DOI] [PubMed] [Google Scholar]
- 14.Van Leeuwen PJ, Emmett L, Ho B, Delprado W, Ting F, Nguyen Q, et al. Prospective evaluation of 68Gallium‐prostate‐specific membrane antigen positron emission tomography/ computed tomography for preoperative lymph node staging in prostate cancer. BJU international. 2017;119(2):209–15. doi: 10.1111/bju.13540. [DOI] [PubMed] [Google Scholar]
- 15.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. Jama. 1998;280(11):969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 16.Fanti S, Minozzi S, Morigi JJ, Giesel F, Ceci F, Uprimny C, et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur J Nucl Med Mol Imaging. 2017;44(10):1622–35. doi: 10.1007/s00259-017-3725-1. [DOI] [PubMed] [Google Scholar]
- 17.Berger I, Annabattula C, Lewis J, Shetty D, Kam J, Maclean F, et al. 68Ga-PSMA PET/CT v mpMRI for locoregional prostate cancer staging: correlation with final histopathology. Prostate cancer and prostatic diseases. 2018;21(2):204–11. doi: 10.1038/s41391-018-0048-7. [DOI] [PubMed] [Google Scholar]
- 18.Sachpekidis C, Kopka K, Eder M, Hadaschik BA, Freitag MT, Pan L, et al. 68Ga-PSMA-11 dynamic PET/CT imaging in primary prostate cancer. Clinical nuclear medicine. 2016;41(11):e473–e9. doi: 10.1097/RLU.0000000000001349. [DOI] [PubMed] [Google Scholar]
- 19.Budäus L, Leyh-Bannurah S-R, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of 68Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. European urology. 2016;69(3):393–6. doi: 10.1016/j.eururo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Kabasakal L, Demirci E, Ocak M, Akyel R, Nematyazar J, Aygun A, et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nuclear medicine communications. 2015;36(6):582–7. doi: 10.1097/MNM.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 21.Rahbar K, Weckesser M, Huss S, Semjonow A, Breyholz H-J, Schrader AJ, et al. Correlation of intraprostatic tumor extent with 68Ga-PSMA distribution in patients with prostate cancer. Journal of Nuclear Medicine. 2016;57(4):563–7. doi: 10.2967/jnumed.115.169243. [DOI] [PubMed] [Google Scholar]
- 22.Kallur KG, Ramachandra PG, Rajkumar K, Swamy SS, Desai I, Rao RM, et al. Clinical utility of gallium-68 PSMA PET/CT scan for prostate cancer. Indian journal of nuclear medicine: IJNM: the official journal of the Society of Nuclear Medicine, India. 2017;32(2):110. doi: 10.4103/0972-3919.202255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basha MAA, Hamed MAG, Hussein O, El-Diasty T, Abdelkhalek YI, Hussein YO, et al. 68Ga-PSMA-11 PET/CT in newly diagnosed prostate cancer: diagnostic sensitivity and interobserver agreement. Abdominal Radiology. 2019;44(7):2545–56. doi: 10.1007/s00261-019-02006-2. [DOI] [PubMed] [Google Scholar]
- 24.Hedva Lerman M, Charles Levine M, Barnes S. 68Ga-PSMA PET/CT Staging of Newly Diagnosed Intermediate-and High-Risk Prostate Cancer [Google Scholar]
- 25.Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer—updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. European urology. 2020;77(4):403–17. doi: 10.1016/j.eururo.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 26.Kuten J, Mabjeesh NJ, Lerman H, Levine C, Barnes S, Even-Sapir E. Ga-PSMA PET/CT Staging of Newly Diagnosed Intermediate-and High-Risk Prostate Cancer. The Israel Medical Association journal: IMAJ. 2019;21(2):100–4. [PubMed] [Google Scholar]
- 27.Fendler WP, Schmidt DF, Wenter V, Thierfelder KM, Zach C, Stief C, et al. 68Ga-PSMA PET/CT detects the location and extent of primary prostate cancer. Journal of Nuclear Medicine. 2016;57(11):1720–5. doi: 10.2967/jnumed.116.172627. [DOI] [PubMed] [Google Scholar]
- 28.Uprimny C, Kroiss AS, Decristoforo C, Fritz J, von Guggenberg E, Kendler D, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. European journal of nuclear medicine and molecular imaging. 2017;44(6):941–9. doi: 10.1007/s00259-017-3631-6. [DOI] [PubMed] [Google Scholar]
- 29.Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. European urology. 2018;74(2):179–90. doi: 10.1016/j.eururo.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 30.Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multi-centre study. The Lancet. 2020 doi: 10.1016/S0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]
- 31.Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, et al. 68Ga-PSMA positron emission tomography/ computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. European urology. 2016;70(4):553–7. doi: 10.1016/j.eururo.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 32.Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. European journal of nuclear medicine and molecular imaging. 2016;43(12):2114–21. doi: 10.1007/s00259-016-3435-0. [DOI] [PubMed] [Google Scholar]