Abstract
High-grade gliomas are aggressive tumors that require multimodal management and gross total resection is considered to be the first crucial step of treatment. Because of their infiltrative nature, intraoperative differentiation of neoplastic tissue from normal parenchyma can be challenging. For these reasons, in the recent years, neurosurgeons have increasingly performed this surgery under the guidance of tissue fluorescence. Sodium fluoresceine and 5-aminolevulinic acid represent the 2 main compounds that allow real-time identification of residual malignant tissue and have been associated with improved gross total resection and radiological outcomes. Though presenting different profiles of sensitivity and specificity and further investigations concerning cost-effectiveness are need, Sodium fluoresceine, 5-aminolevulinic acid and new phluorophores, such as Indocyanine green, represent some of the most important tools in the neurosurgeon’s hands to achieve gross total resection.
Keywords: fluorescence, intraoperative imaging, glioblastoma, surgery, resection
Introduction
High-grade gliomas (HGGs), which account for about 80% of all primary malignant cerebral neoplasms, are aggressive tumors that require multimodal management. 1 -5 When achievable, gross total resection (GTR) is considered to be the first crucial step of treatment; as known, resections of ≥98% of the enhancing HGG-lesions have been associated with very significant survival advantage when compared to excisions performed below that limit. 6 -12 However, the prognosis of HGG remains bleak because of the infiltrative nature of the HGGs on one hand and the high local relapse rate on the other, given that more than 80% of HGG-recurrences occurred within 2 cm of the resection margins. 2,13,14 HGGs disrupts the integrity of the blood-brain barrier (BBB), resulting in extravascular leakage of gadolinium contrast agents. Conventionally, the tumor territory is regarded as the gadolinium enhancing region seen on Magnetic Resonance Imaging (MRI) sequences. Accurate intraoperative differentiation of neoplastic tissue from normal parenchyma can be challenging, since there is no definitive way to distinguish tumor from normal brain during the course of the procedure other than by gross visualization and tactile consistency. This is particularly true at the invasive tumor margins: an appreciable amount of tumor cells usually infiltrates around the radiologically enhancing region, where the blood brain barrier is not yet destroyed, compromising the specificity of gadolinium. 9 For these reasons in the recent years, neurosurgeons have increasingly performed this surgery under the guidance of tissue fluorescence that have been developed to face the after mentioned challenges. 15 -19 Two main compounds have been used so far in malignant glioma surgery: 5-amilovelulinic acid (5-ALA) and Sodium Fluoresceine (SF). 5-ALA is a cellular metabolic phluorophore. SF is able to penetrate the tumor because of the impaired BBB and tends not to diffuse into normal brain, although It can extend beyond gadolinium contrast-enhancing regions probably because of its smaller molecular weight. The aim of this review is to underline the usefulness of these 2 compounds, highlighting their impact on glioma surgery, and to provide an overview on other intraoperative techniques, such as the Second Window Indocyanine Green (SWIG) that could represent a new available weapon in the field of Fluorescence Guided Surgery (FGS).
Methods
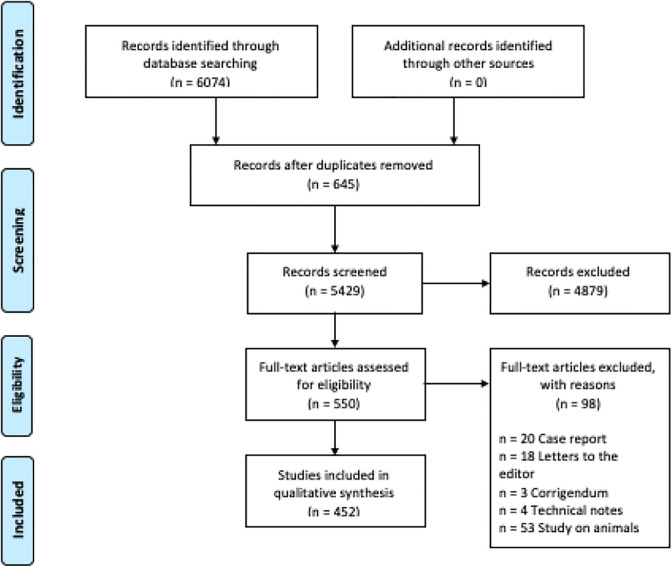
An extensive search of the English Medical Literature has been performed on MEDLINE/PubMed service, using the MeSH (medical subject heading) terms “high grade glioma,” “glioma,” “ALA,” “5-aminlevulinic acid,” “sodium fluorescein,” “indocyanine green,” “second window indocianine green,” “intraoperative,” “image guidance,” and “brain tumor” in various combinations. Inclusion of the references was based on the scope of this review, according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Figure 1). 20 The initial wide-net search produced a total of 6074 publications of which only 452 studies were considered to be relevant.
Figure 1.
PRISMA flow chart.
Background and Mechanism of Action
The application of 5-ALA to patients undergoing surgery for HGGs was first described by Stummer et al in 1998 in a series of 10 patients. 21 5-ALA is the first natural precursor metabolite in the heme biosynthesis pathway and is usually synthesized from succinyl-CoA and alanine. Protoporphyrin IX (PPIX) is a strongly fluorescent molecule in the red range that represents the final metabolite before heme biosynthesis and its selective accumulation within glioma cells is related to the reduced level of the ferrochelatase enzyme and impaired cellular clearance by an ATP-binding cassette transporter (ABCB6). 21 -24,25 5-ALA induced fluorescence is also influenced by the vascularity of the tumor, BBB permeability, tumor cell proliferative activity and cellular density. 22,26 Its take-up in WHO grade III and IV glioma is almost 6 times more than that of normal brain tissue, while approximately 20% of grade II are fluorescent after 5-ALA application. 27,28 SF is a phluorophore discovered in 1871 by Adolf von Baeyer and has been evaluated in different pathologies of the central nervous system. 29,30 The presumed mechanism of action is a passive staining of the extracellular space in areas with disrupted BBB, then it corresponds, according to Neira et al, to gadolinium uptake on magnetic resonance imaging. Moreover, SF enhancement could extend beyond gadolinium contrast-enhancing regions, probably because of the smaller molecular weight of SF. 31 Vascular leakage and breakdown of BBB are caused by increased blood vessels formation that occurred in the context of GBMs. 26,32 It is readily available, inexpensive and FDA approved, having been used by ophthalmologists since the 1960s for retinal angiography.
Practical Use and Safety
5-ALA is a metabolic, high-cost tracer approved in June 2017 by the FDA for resection of HGGs; a proper visualization of PPIX fluorescence requires xenon filters to give blue-violet light with a wave-length of 375 to 440 nm and an emission filter, allowing visualization of pink-red fluorescence, which has a peak at 635 and 704 nm. 5-ALA is administered as oral solution at a dose of 20 mg/kg body weight and the peak of fluorescence can be expected after about 6-8 h, with fluorescence beginning to be visible after about 3 hours. 24 Due to the small size of this molecule, it is rapidly absorbed from the intestine and is cleared from plasma within 2 hours after administration, being fluorescence impression unperturbed even beyond 12 h. 28,33 5-ALA is a well-tolerated agent and associated with low rates of adverse events, the most common involving liver function, a temporary hypotension and, because of the transient accumulation of porphyrins in the skin, a light sensitivity for the first 24 hours after application. This requires to maintain patients away from direct light before and after surgery. 34,35 Differently from 5-ALA, the administration and use of SF in HGG-surgery has been less extensively described because its less-specific mechanism of action, the heterogeneity of dose, timing and non-related tumor factors such as tissue manipulation and edema bulk flow. 36,37 This fluorescent tracer is excited by a light wavelength ranging from 460 to 500 nm and emits fluorescent radiation in wavelengths ranging from 540 to 690 nm. The current literature supports the use of low dose SF (1-4 mg/kg) with a Yellow 560 filter, that should be administered right after the induction of general anesthesia using a central venous line. 38 SF is completely excreted within 24 hours and has very few side effects; in the English Literature adverse events after the administration of SF have been extensively evaluated after SF angiography procedures. Kwiterovich et al showed, in a prospective study involving 2789 angiographic procedures and 2025 patients, an overall adverse reaction in 4.8% of all cases after receiving a dose ranging from 3 to 5 ml of SF (10%) in 6 seconds. 39 These reactions included nausea (2.9%), vomiting (1.2%), flushing/itching/hives (0.5%), and others (dyspnea, syncope, excessive sneezing) (0.2%). No cases of anaphylaxis, myocardial infarction, pulmonary edema, or seizures were described. The percentage of reactions was lower (1.8%) in patients who have had a previous angiography without adverse effects. 32,40 In a more recent retrospective study on 2247 patients with 3381 consecutive SF angiography, the overall adverse reaction rate after administration of 5 ml SF (10%) was 3.3%. In this case the most common adverse reactions reported were nausea (2,7%) and vomiting (0,6%) while anaphylactic reactions involved only 1 patients (0.04%). 41 Some authors have started then to perform allergy skin tests in order to easily screen out susceptible patients avoiding then the occurrence of anaphylactic reactions during surgery. 42 -44
Correlation Between Fluorescence and Malignant Cells
Many studies in the available literature focused on the positive correlation between fluorescence intensity and tumor cellularity (Ki67-MIB-1) as showed by several intraoperative biopsies at tumor areas with different fluorescent intensity later compared with histology examinations. 45 -48 It is important to highlight that 5-ALA presents 3 different patterns of fluorescence when observed under the blue-light filter: necrotic areas usually showed no fluorescence or just parts of non-omogeneous pink-red fluorescence 49,50 ; solid tumor areas, on the other hand, display a clear and shining fluorescence of solid intensity, while transitional areas of infiltrating brain tissue usually appears as regions of faint fluorescence (Figure 2). 51,52 These aspects should be kept in mind by the surgeon who knows that areas of faint fluorescence usually surround areas of bright uptake (which are approximately related to Gadolinium uptake at MRI). 53 The reduction of fluorescence observed at the lesion borders usually corresponds with gradual entrance into the areas of healthy brain tissue. A prospective Phase II clinical trial of 5-ALA demonstrated that the Positive Predictive Value (PPV) utilizing the higher grade of fluorescence (bright) as a predictor for the presence of tumor was 97.4%. 5-ALA faint fluorescence intensity showed not to be related with specific tumor cellular densities as bright fluorescence. On the other hand, in the case of absence of 5-ALA uptake, the probability of having absence of tumoral tissue i.e. the Negative Predictive Value (NPV) was 37.7%. 21,50,54 Ferraro et al conducted a recent systematic review about the use of 5-ALA in brain tumor surgery, extracting data on 1163 patients from 22 studies. His conclusion revealed that 5-ALA-mediated detection of HGGs was associated with a sensitivity of 85% and a specificity of 82%. 55 In a prior study, on the contrary, Roberts et al showed that 73.7% of non-fluorescent samples were able to show the presence of tumor. 53 These findings could hint the presence of particular neoplastic cells that do not consistently take up 5-ALA in a way that ends up showing a visible fluorescence. However, the absence of macroscopic 5-ALA uptake does not definitely exclude the presence of 5-ALA or fluorescent porphyrins: it is also suggested, for example, that macrophages and other inflammatory cells could accumulate and convert ALA and display PPIX associated fluorescence. 56 Tissue malignancy could be underestimated for other reasons: firstly, the raw fluorescence emissions could contain contributions in the background coming from other sources (leakage of excitation light, photoproducts and autofluorescence) which could alter the inferior limit of sensitivity to PPIX 57 ; secondly, raw fluorescence intensity could be related by optical absorption of the tissue and scattering effect, which substantially distort the remitted signal, and not only by the concentration of PPIX. 58 The most critical moment involving these distortions could be located near the end of surgery, when remaining disease must be recognized within a surgical cavity. Widhalm et al showed how fluorescence could be directly calculated with a handheld spectroscopic probe, and then spectra used in order to calculate the level of PPIX accumulated in the tissue. In this way, it could be possible to detect spectrometric fluorescence in marginal tissue even without the presence of a macroscopic fluorescence. 59 These algorithms of spectroscopy, and subsequently the hyper-spectral data processing, 60 have been studied in order to decouple PPIX emissions from other phluorophores and then avoiding the presence of the effects of distortion of background optical properties. In this way, it is possible to quantify the fluorescence that is really related to the presence of tissutal PPIX. More recently, a novel filter system with higher background illumination has been evaluated with the aim to determine the diagnostic accuracy and perception of PPIX fluorescence without obtaining a spectral analysis. 61 In this study a total of 128 biopsies from 32 patients with HGGs were collected using a new filter which enhanced the brightness of the surgical field, allowing superior discrimination of brain anatomy. The authors found that the sensitivity of fluorescence to detect tumor tissue was 70.89% (95% CI 59.43%-79.21%) and specificity 97.37% (95% CI 86.19%-99.93%); the PPV was 98.44% (95% CI 90.06%-99.77%), whereas the NPV was 57.81% (95% CI 49.88%-65.36%); the last one higher than previously reported. This study suggests a potential benefit despite its limitation concerning the absence of validation for this method, a low sample size and the obvious disparity between images and what surgeon directly observes during surgery. The use of SF had a progressive affirmation in the field of FGS after the overcoming of its limitation. A reliable identification of the fluorescence pattern, mechanism of action and the dose and administration timing have represented the biggest challenge before the definition of SF as comparable alternative to 5-ALA. Using SF, a different fluorescence pattern within the tumor is not clear and this can make it more difficult to recognize the tumor border, while central necrosis is usually evident. 52 Furthermore, non-neoplastic structures can be fluorescent: tissue manipulation, edema bulk flow, dose and timing administration can play a role in fluorescence distribution because of SF extravagation. 37 These last 2 factors, in particular, are the most important bias that cause heterogeneity in the evaluation and comparison of the SF between the various studies: in some of these sodium fluorescein (diluted at 10%) was intravenously administered at high dose (15-20 mg/kg) and subsequently observed with white light illumination, 38,62 in others it was administered at medium dose (5-10 mg/kg) and observed with dedicated Yellow 560 filter. 39,63 Giving an high dose of SF has the potential benefit of reducing the shifting from filtered to white light during surgery. On the other hand, the passive staining of the dye through the disrupted BBB, the surgery-related damage of the BBB associated with the small molecule weight and the administration timing, could result in the extravagation beyond the tumor margin in the healthy tissue especially in long lasting surgery then reducing the accuracy of visual evaluation. 62,63 Acerbi et al suggested that giving SF intravenously too late (e.g., during craniotomy or dura opening) can provide different results in terms of tissue fluorescence if compared with earlier administrations. 64 The current literature supports the use of low dose of SF (1-4 mg/kg) with dedicated Yellow 560 filter, through a central venous line immediately after the induction of general anesthesia. 65 SF may accumulate in any region where there is blood, edema, surgical injury or trauma as above mentioned, giving a potentially poor positive predictive value of tumor tissue and low specificity though the good results registered in achieving GTR in preliminary studies. 37,38,62 Despite these limitations, evident especially in the early use of fluorescein in the FGS, the progressive and extensive use of SF in has led to better exploit all its potentials. Neira et al demonstrated indeed that intraoperative SF staining correlated with histopathological alteration in both contrast-enhancing and non-contrast enhancing regions, with a PPV greater than 96% in non-contrast-enhancing regions, suggesting that SF can be used as a visual marker for glioma resection in both regions of GBM. 31 FLUOGLIO is a Multicentric Prospective Phase II Study which reported that the sensitivity and specificity of SF in identifying tumor tissue was respectively 80.8% and 79.1% (Table 1). 65
Figure 2.
Fluorescent guided surgery. A and B, A 33-year-old man harboring a left frontal HGG with relevant mass effect underwent surgery. C, A combined fluorescent guided surgery allowed to achieve GTR on the immediate post-operative MRI image. D, E and F, A left frontal craniotomy was performed and, once, reached the subcortical enhancing node (D), a bright pink nodule was observed under the Blue 400 nm filter light (E) and a brilliant green nodule was observed under the Yellow 560 nm filter light (F). Picture taken from the University of Turin, Department of Neurosurgery’s Archive.
Table 1.
Phluorophore Characteristics.
| GTR (on average) | PFS (after surgery) | Sensitivity (on average) | Specificity (on average) | PPV | NPV | Safety | Cost | Unfavorable features | |
|---|---|---|---|---|---|---|---|---|---|
| 5 ALA | 69.1% | 8 months | 85% | 82% | 95% | 40% | + | +++ | Light sensitizer, cost |
| SF | 84.4% | 7 months | 80.8% | 79.1% | 98.4% | 57.8% | + | + | Can cause atopic reactions (rare), less specific |
| SWIG | N/A | N/A | 97% | 56% | 82% | 90% | + | ++ | Adequate filter, center expertise |
Abbreviations: N/A, not applicable; GTR, gross total resection; PFS, period free survival; PPV, positive predictive value; NPV, negative predictive value.
Simultaneous Administration of 5-ALA and SF
There are only 3 studies (and small case series) in the available literature that studied the administration of both 5-ALA and SF. 66 Della Puppa et al, despite the main limitation of a study represented by a very low sample size (only 3 cases), reported that sensitivity was high for both 5-ALA and SF (especially for 5-ALA, 100%, while SF was 93%). More specifically, PPV and specificity of 5-ALA resulted higher than SF (67% vs. 33% and 94% vs. 87%, respectively). The NPV as well was higher for 5-ALA when compared with SF (100% vs. 50%, respectively). Specificity was 100% and 67% when bright and faint 5-ALA fluorescence was considered respectively. Regarding areas where faint fluorescence was present (peritumoral infiltration), both 5-ALA and SF presented a false-positive rate; however, 5-ALA showed to have a higher specificity than SF (67% 5-ALA while 33% for SF). 51 Yano et al reported 8 patients with GBMs, suggesting more sensitivity of 5-ALA compared to SF in detecting tumor cells at the boundaries. The study however showed to present 2 limitations: SF was used considering a high dosage (15-20 mg/kg administered at the dura opening), then in contrast with the studies that now suggest to use a lower dose during induction; Furthermore, a non-specific BLUE 400 filter was used to detect yellow fluorescence instead of a specific YELLOW 560 filter. 67 Suero Molina et al focused as well on administration of both SF and 5-ALA. However, in order to obtain a simultaneous visualization, an external blue light (375-440 nm) illumination was obtained to detect 5-ALA fluorescence, while the YELLOW 560 module alone was utilized to capture both SF and 5-ALA fluorescence patterns. Finally, no histological data were reported to compare the 2 techniques. 68
5-ALA False Positive Fluorescence
Despite the importance of distinguish neoplastic from non-neoplastic tissue, thus the possibility of decrypting false negatives using 5-ALA, in the last years few authors focused on the 5-ALA false positive-induced fluorescence. In a recent review of the literature La Rocca et al classified the 5-ALA false positive induced fluorescence in oncological setting and non-oncological setting. 69,70 Autofluorescence of normal brain tissue due to peritumoral inflammation, 71 HGGs cases with high inflammatory infiltrations, 71 radiation necrosis and reactive gliosis as the results of chemo-radiotherapies treatments, are the main causes of 5-ALA false positive fluorescence reported in literature in the oncological setting. This phenomenon seems to be related, mostly in HGGs recurrences, to the presence of peritumoral inflammation, probably as the results of increased reactive mitotic activity and responsive astrocytosis associated to surgical and radiation intervention. 72,73 In the literature a single report described 5-ALA false positive fluorescence during first surgery for HGG. This last case was characterized by a remarkable neutrophils infiltration on the histological examination. 71,74 Although 5-ALA false positive fluorescence seems to be related also to the peritumoral inflammation due to 5-ALA uptake and metabolization by inflammatory cells, it is important to highlight that the heme synthase pathways may not always be affected in secondary GBM, and thus 5-ALA may be particularly useful in those cases. 24,50 On the contrary SF usually stains both primary and secondary GBM where the BBB is broken. 75 These reports stress the importance of considering alternative fluorescence source in tumor recurrence surgeries, especially after medical and radio-treatment in the possible context of pseudoprogression. In a phase II multicentric prospective trial, Nabavi et al showed how, in recurrent glioblastoma, where tissue scarring and changes induced by previous radiotherapy and chemotherapy were present, 5-ALA-guided resection was still shown to be effective with a PPV of 99.5%. 76 A debated topic concern 5-ALA-induced fluorescence in the ventricular wall, not primary involved in GBM. Hayashi et al found that most of samples taken from the ventricular wall, presented 5-ALA-induced fluorescence and histological disruption of ependymal cell layers, suggesting ventricular involvement or environmental-induced changes. 77 On the contrary, Tejada-Solis et al showed that no one of the cases with 5-ALA-induced fluorescence, presented disruption of the ependymal layer or tumor cells invasion. 78 More recently Moon et al collected 25 samples from the ventricular wall of 19 patient with primary GBM without ventricular enhancement. Eleven out of the 25 samples (44%) displayed intraoperative fluorescence and 5 of these (45.5%) resulted positive for the presence of tumoral cells (2 cases corresponded to a low-grade glioma (LLG) and 3 cases corresponded to HHG), while the remaining 6 (54.5%) were negative for the presence of tumor cells. The 14 samples (56%) that did not displayed intraoperative fluorescence, resulted negative for the presence of tumor cells. Moreover, no reactive astrocytes, inflammatory cells infiltration or necrosis were detected in the setting of false positive 5-ALA-induced fluorescence as well as it is known that the majority of LGG did not usually exhibit intraoperative fluorescence. 79 This results, despite the low sample size and subjective fluorescence evaluation-related bias, need further investigation especially in light of recent evidences under which primary isocitrate dehydrogenase (IDH) wild-type GBM, originated from mutated neural stem cells in the astrocytic ribbon of subventricular zone. 79 -81 Considering non-oncological settings, 5-ALA fluorescence has been observed in multiple sclerosis, 82 neurodegenerative diseases 71,83 and infectious conditions (several bacterial species could elaborate porphyrin precursor like cofactor of growth, explaining the reason behind bacterial abscesses macroscopic fluorescence). 69,70,84,85 It is important to underline that all the oncological and non-oncological settings share a common characteristic consisting in a high number of immune cells that could show different degrees of fluorescence due to 5-ALA metabolism and uptake.
Impact of Fluorescence-Guided Surgery for High-Grade Glioma
Patients with GBM share an inevitable destine that is the recurrence of the disease, despite a first favorable surgery. 6,13,86 The key-role of surgery relies on the established benefit of the extent of resection (EOR) on progression-free survival (PFS) and overall survival (OS). 87,88 GTR is, according to increasing evidence, superior to nonsurgical treatment in recurrent GBM, while incomplete resection showed to be non-inferior to the best medical treatment. 89 The administration of 5-ALA with the aid of a dedicated BLUE 400 filter in a phase 3 randomized trial showed concrete results in increasing extent of resection and progression-free survival. Complete resection was achieved in 65% of patients with the aid of 5-ALA guided surgery compared with 33% of the control group of patients operated under white light. Progression-free survival improved from 20% to 40%. 36 In a recent review of literature, the analysis of the postoperative MRI of 1163 cases involving 22 studies underwent 5-ALA FGS, showed that 65% of patients who received 5-ALA group reported GTR of the contrast-enhancing tumor, which was an higher percentage if compared to patients in the control group (36% without FGS). In 5-ALA cohort, 6-month PFS rate of 41% was significantly higher if compared to that of the white light surgery control group (21%). 55 More recent studies regarding 5-ALA impact on the extent of resection, have reported higher rates of GTR, even exceeding 80% in some reports. This could be also the result of surgeon improving learning curve through the years and this consideration should be taken into account. 48 Regarding SF, a recent multicentric controlled prospective phase II trial has been published reporting an 82.6% of patients that experienced GTR. PFS at 6 and 12 months was, respectively, 56.6% and 15.2%. 65 Katsevman et al showed that patients who underwent surgery with SF had a statistically significantly smaller median tumor volume of 17 cm3 left compared to 32.5 cm3 in the non-SF group. Overall, GTR (≥98%) was achieved in 73% of SF procedures compared to 53% (21/40) of patients when surgery was performed without SF (OR 2.5, 95% CI 1.09-5.75; P < 0.05). 90 In a recent mata-analyses conducted by Eljamel concerning the effectiveness and cost-effectiveness of intraoperative imaging for glioma surgery, the author found comparable primary outcome endpoints (GTR rate of 69.1% and 84.4%, using respectively 5-ALA and SF (P > 0.05). The impact of intraoperative fluorescence-guided surgery on PFS was found to be a valuable endpoint after GTR. Resection after 5-ALA administration showed to be associated with a PFS of 8 months if compared to 7 months following the use of SF during surgery. 91 Finally, the use of fluorescence was able to change surgeons decision in order to continue or stop resection in over 59% and improved functional outcomes in at least 19% of patients, according to a meta-analyses conducted by Mahboob et al. The decreasing of the neurological functional status was reported in only 13% or less and the neurological deficit described was usually mild and often short-lived. 92
New Perspectives—Second Window Indocyanine Green
Indocyanine Green (ICG) is a tricarbocyanine near-infrared (NIR) fluorophore used as an angiographic agent since the 1960s and for assessment of cardiac and hepatic function. More recently, ICG has been used for tracing tumor tissue with a new technique known as SWIG. Unlike the traditional use in videoangiography procedures, where the tracer is used to visualize the vasculature within few minutes after the injection, this method has allowed the intraoperative visualization of tumors about 24 hours after high-dose intravenous administration. This relatively new application has been used in numerous different cancer types such lung, prostate, breast, ovarian, colorectal, pancreatic, esophageal and metastatic cancer 93 before its wide application in different brain tumor such as meningiomas, 94 metastases, pituitary adenomas, 95 chordomas, craniopharyngiomas, lymphoma. 96,97 and glioma. 94,98,99 The real mechanism of action of the SWIG is not entirely clear but the hypothesis is that the accumulation of ICG happens according to the described “enhanced permeability and retention” (EPR) effect, which states that solid tumors usually are characterized by high vascular permeability because of the presence of defective vascular structures feeded by mediators causing an increased permeability. 100 This is why ICG is able then to accumulate in tumoral areas being visualized even 24 hours later. 101 Indocyanine green peak of emission and excitation is 780 and 810 nm respectively and requires a dedicated NIR imaging device for visualization in the operative field. It is removed by biliary excretion with half-life of <180 sec and without significative side effects. For SWIG application, ICG is administered at a dose of 5.0 mg/kg up to 24 hours before surgery. 94,102 In neurosurgery, in contrast to PPIX or fluorescein, SWIG imaging utilizes NIR fluorescence, increasing then the level of tissue penetration, especially in less dense tissues. NIR light can pass through the brain tissue more than 10 mm, then allowing a proper visualization of brain tumors through the dura. This is functional in the intraoperative planning before the dural opening and corticectomy, because it can minimize normal brain tissue damage. Furthermore, NIR imaging is achieved in real-time, then SWIG is not affected by the intraoperative dynamic changes of the brain during the tumor excision. For these reasons, this phluorophore could be a useful tool in order to improve the extent of surgical resection through FGS for brain tumors and in particular for HGGs, considering also that SWIG appears to correlate with tumor contrast enhancement in MRI sequences. 98 One of the limits of SWIG consists in the weakness of dye’s signal and the consequent need to use a camera system that increase the exposure. This probably contributes to increase the number of false-positive biopsies in particular considering that conventional surgical microscopes with add-on NIR modules showed a significant lower sensitivity for NIR fluorescence, if compared to microscopes with dedicated NIR imaging modules. In order to minimize this problem, it could be useful to register the fluorescence intensity when the tumor is first exposed and then to keep it throughout the rest of the surgery. Any NIR fluorescence intensity at the same level of the starting one increases the neoplastic characteristics of the examined area. 103 The second limit of SWIG, in the same way as SF, is represented by the low specificity due to its mechanism of action of passive-staining through the areas of blood-brain-barrier breakdown. 93,95 Moreover ICG is generally bound to plasma albumin or others carriers and this could limit the bioavailability of the tracer. There is lack of strong evidence in the literature concerning the SWIG for HGG. The first study published by Li et al on SWIG for gliomas, included 15 patients: 10 WHO grade IV, 1 WHO grade III anaplastic astrocytoma, 2 WHO grade II astrocytomas and 1 WHO grade I juvenile pilocytic astrocytoma. The authors found that 12 out of 15 analyzed gliomas demonstrated positive NIR fluorescence, while the 3 gliomas that did not demonstrate NIR fluorescence did not enhance also after gadolinium administration on the preoperative MRI. During surgery, diagnostic test on 71 specimens were taken from the 12 patients with positive NIR fluorescence and the authors reported that SWIG showed about 98% of sensitivity, 45% of specificity, 82% of PPV and 90% of negative predictive value NPV. 98 Updated data published from Cho et al on 78 specimens show that SWIG has 97% sensitivity, 56% specificity, 94% PPV, 71% NPV, and 92% accuracy. 103 Moreover, the authors hypothesize that SWIG could predict the location of gadolinium uptake on postoperative MRI. SWIG could be considered a promising tool for brain surgery and especially for gliomas, because it could potentially combine the advantages of FGS with the possibility of detecting the neoplastic tissue through the dura and the brain parenchyma without significant side effects. Future investigation studies would compare SWIG to the current approved phluorophore for the FGS, and a cost-benefits analysis would be mandatory (Table 1).
Conclusion
Fluorescence guided surgery represents an important advancement in the management of HGGs. The real-time identification of residual malignant tissue has shown to facilitate GTR and has been associated with improved radiological outcomes. 5-ALA and SF present different profiles of sensitivity and specificity which need to be considered and further investigated in cost-effectiveness analysis. New techniques and new phluorophores such as ICG and SWIG might play an important role in FGS.
Abbreviations
HGGs, High‐grade gliomas; LGG, Low-grade glioma; GTR, Gross total resection; MRI, Magnetic Resonance Imaging; 5-ALA, 5-amilovelulinic acid; SF, Sodium Fluoresceine; BBB, Blood-brain barrier; SWIG, Second Window Indocianine Green; FGS, Fluorescence Guided Surgery; GBMs, Glioblastomas; PPV, Positive Predictive Value; PPIX, Protoporphyrin IX; NPV, Negative Predictive Value; EOR, Extent of resection; PFS, Progression-free survival; OS, Overall survival; ICG, Indocyanine Green; NIR, Near-infrared; EPR, Enhanced permeability and retention.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Giuseppe Palmieri  https://orcid.org/0000-0001-5554-4900
https://orcid.org/0000-0001-5554-4900
Giuseppe Di Perna  https://orcid.org/0000-0002-7381-9194
https://orcid.org/0000-0002-7381-9194
References
- 1. Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842-1850. [DOI] [PubMed] [Google Scholar]
- 2. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190-198. [DOI] [PubMed] [Google Scholar]
- 3. Stummer W, Reulen H-J, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564-576. [DOI] [PubMed] [Google Scholar]
- 4. Pichlmeier U, Bink A, Schackert G, Stummer W. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. 2008;10(6):1025-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Della Pepa GM, Sabatino G, la Rocca G. ‘Enhancing vision’ in high grade glioma surgery: a feasible integrated 5-ALA + CEUS protocol to improve radicality. World Neurosurg. 2019;129:401-403. [DOI] [PubMed] [Google Scholar]
- 6. Altieri R, Raimondo S, Tiddia C, et al. Glioma surgery: from preservation of motor skills to conservation of cognitive functions. J Clin Neurosci. 2019;70:55-60. [DOI] [PubMed] [Google Scholar]
- 7. Altieri R, Melcarne A, Junemann C, et al. Inferior fronto-occipital fascicle anatomy in brain tumor surgeries: from anatomy lab to surgical theater. J Clin Neurosci. 2019;68:290-294. [DOI] [PubMed] [Google Scholar]
- 8. Pesce A, Palmieri M, Cofano F, et al. Standard awake surgery versus hypnosis aided awake surgery for the management of high grade gliomas: a non-randomized cohort comparison controlled trial. J Clin Neurosci. 2020;77:41-48. [DOI] [PubMed] [Google Scholar]
- 9. Chang EL, Akyurek S, Avalos T, et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys. 2007;68(1):144-150. [DOI] [PubMed] [Google Scholar]
- 10. Altieri R, Melcarne A, Soffietti R, et al. Supratotal resection of glioblastoma: is less more? Surg Technol Int. 2019;35:432-440. [PubMed] [Google Scholar]
- 11. Schucht P, Seidel K, Beck J, et al. Intraoperative monopolar mapping during 5-ALA-guided resections of glioblastomas adjacent to motor eloquent areas: evaluation of resection rates and neurological outcome. Neurosurg Focus. 2014;37(6):E16. [DOI] [PubMed] [Google Scholar]
- 12. Monticelli M, Zeppa P, Altieri R, et al. Exploring the anatomy of negative motor areas (NMAs): findings in awake surgery. J Clin Neurosci. 2020;73:219-223. [DOI] [PubMed] [Google Scholar]
- 13. Altieri R, Hirono S, Duffau H, et al. Natural history of de novo high grade glioma: first description of growth parabola. J Neurosurg Sci. 2020;64(4):399-403. [DOI] [PubMed] [Google Scholar]
- 14. Monticelli M, Zeppa P, Zenga F, et al. The post-surgical era of GBM: how molecular biology has impacted on our clinical management. A review. Clin Neurol Neurosurg. 2018;170:120-126. [DOI] [PubMed] [Google Scholar]
- 15. Hadjipanayis CG, Widhalm G, Stummer W. What is the surgical benefit of utilizing 5-aminolevulinic acid for fluorescence-guided surgery of malignant gliomas? Neurosurgery. 2015;77(5):663-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ewelt C, Nemes A, Senner V, et al. Fluorescence in neurosurgery: its diagnostic and therapeutic use. Review of the literature. J Photochem Photobiol B Biol. 2015;148:302-309. [DOI] [PubMed] [Google Scholar]
- 17. Altieri R, Zenga F, Fontanella MM, et al. Glioma surgery: technological advances to achieve a maximal safe resection. Surg Technol Int. 2015;27:297-302. [PubMed] [Google Scholar]
- 18. Altieri R, Meneghini S, Agnoletti A, et al. Intraoperative ultrasound and 5-ALA: the two faces of the same medal? J Neurosurg Sci. 2019;63(3):258-264. [PubMed] [Google Scholar]
- 19. La Rocca G, Della Pepa GM, Menna G, et al. State of the art of fluorescence guided techniques in neurosurgery. J Neurosurg Sci. 2019;63(6):619-624. [DOI] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700-b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stummer W, Stocker S, Wagner S, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998;42(3):525-526. [DOI] [PubMed] [Google Scholar]
- 22. Stummer W, Stepp H, Möller G, Ehrhardt A, Leonhard M, Reulen HJ. Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir (Wien). 1998;140(10):995-1000. [DOI] [PubMed] [Google Scholar]
- 23. Idoate MA, Díez Valle R, Echeveste J, Tejada S. Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology. 2011;31(6):575-582. [DOI] [PubMed] [Google Scholar]
- 24. Stummer W, Stocker S, Novotny A, et al. In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B Biol. 1998;45(2-3):160-169. [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto K, Hagiya Y, Endo Y, et al. Effects of plasma membrane ABCB6 on 5-aminolevulinic acid (ALA)-induced porphyrin accumulation in vitro: tumor cell response to hypoxia. Photodiagnosis Photodyn Ther. 2015;12(1):45-51. [DOI] [PubMed] [Google Scholar]
- 26. Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer. 1996;77(2):362-372. [DOI] [PubMed] [Google Scholar]
- 27. Jaber M, Wölfer J, Ewelt C, et al. The value of 5-aminolevulinic acid in low-grade gliomas and high-grade gliomas lacking glioblastoma imaging features: an analysis based on fluorescence, magnetic resonance imaging, 18F-fluoroethyl tyrosine positron emission tomography, and tumor molecular factors. Neurosurgery. 2016;78(3):401-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ennis SR, Novotny A, Xiang J, et al. Transport of 5-aminolevulinic acid between blood and brain. Brain Res. 2003;959(2):226-234. [DOI] [PubMed] [Google Scholar]
- 29. Stummer W. Fluorescein for vascular and oncological neurosurgery. Acta Neurochir (Wien). 2013;155(8):1477-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stummer W. Fluorescein in brain metastasis and glioma surgery. Acta Neurochir (Wien). 2015;157(12):2199-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neira JA, Ung TH, Sims JS, et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J Neurosurg. 2017;127(1):111-122. [DOI] [PubMed] [Google Scholar]
- 32. Nduom EK, Yang C, Merrill MJ, Zhuang Z, Lonser RR. Characterization of the blood-brain barrier of metastatic and primary malignant neoplasms. J Neurosurg. 2013;119(2):427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stummer W, Stepp H, Wiestler OD, Pichlmeier U. Randomized, prospective double-blinded study comparing 3 different doses of 5-aminolevulinic acid for fluorescence-guided resections of malignant gliomas. Neurosurgery. 2017;81(2):230-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chung IWH, Eljamel S. Risk factors for developing oral 5-aminolevulinic acid-induced side effects in patients undergoing fluorescence guided resection. Photodiagnosis Photodyn Ther. 2013;10(4):362-367. [DOI] [PubMed] [Google Scholar]
- 35. Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392-401. [DOI] [PubMed] [Google Scholar]
- 36. Stummer W. Factors confounding fluorescein-guided malignant glioma resections: edema bulk flow, dose, timing, and now: imaging hardware? Acta Neurochir (Wien). 2016;158:327-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koc K, Anik I, Cabuk B, Ceylan S. Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. Br J Neurosurg. 2008;22(1):99-103. [DOI] [PubMed] [Google Scholar]
- 38. Acerbi F, Broggi M, Eoli M, et al. Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: preliminary results in 12 cases. Acta Neurochir (Wien). 2013;155(7):1277-1286. [DOI] [PubMed] [Google Scholar]
- 39. Kwiterovich KA, Maguire MG, Murphy RP, et al. Frequency of adverse systemic reactions after fluorescein angiography. Results of a prospective study. Ophthalmology. 1991;98(7):1139-1142. [DOI] [PubMed] [Google Scholar]
- 40. Russell SM, Elliott R, Forshaw D, Golfinos JG, Nelson PK, Kelly PJ. Glioma vascularity correlates with reduced patient survival and increased malignancy. Surg Neurol. 2009;72(3):246-247. [DOI] [PubMed] [Google Scholar]
- 41. Xu K, Tzankova V, Li C, Sharma S. Intravenous fluorescein angiography-associated adverse reactions. Can J Ophthalmol. 2016;51(5):321-325. [DOI] [PubMed] [Google Scholar]
- 42. Fan C, Jiang Y, Liu R, et al. Safety and feasibility of low-dose fluorescein-guided resection of glioblastoma. Clin Neurol Neurosurg. 2018;175:57-60. [DOI] [PubMed] [Google Scholar]
- 43. Dilek O, Ihsan A, Tulay H. Anaphylactic reaction after fluorescein sodium administration during intracranial surgery. J Clin Neurosci. 2011;18(3):430-431. [DOI] [PubMed] [Google Scholar]
- 44. Tanahashi S, Lida H, Dohi S. An anaphylactoid reaction after administration of fluorescein sodium during neurosurgery. Anesth Analg. 2006;103(2):503. [DOI] [PubMed] [Google Scholar]
- 45. Lau D, Hervey-Jumper SL, Chang S, et al. A prospective phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. JNS. 2016;124(5):1300-1309. [DOI] [PubMed] [Google Scholar]
- 46. Coburger J, Engelke J, Scheuerle A, et al. Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. FOC. 2014;36(2):E3. [DOI] [PubMed] [Google Scholar]
- 47. Díez Valle R, Tejada Solis S, Idoate Gastearena MA, García de Eulate R, Domínguez Echávarri P, Aristu Mendiroz J. Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neurooncol. 2011;102(1):105-113. [DOI] [PubMed] [Google Scholar]
- 48. Stummer W, Tonn J-C, Goetz C, et al. 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery. 2014;74(3):310-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Valdés PA, Leblond F, Kim A, et al. Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg. 2011;115(1):11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg. 2000;93(6):1003-1013. [DOI] [PubMed] [Google Scholar]
- 51. Hauser SB, Kockro RA, Actor B, Sarnthein J, Bernays R-L. Combining 5-aminolevulinic acid fluorescence and intraoperative magnetic resonance imaging in glioblastoma surgery. Neurosurgery. 2016;78(4):475-483. [DOI] [PubMed] [Google Scholar]
- 52. Della Puppa A, Ciccarino P, Lombardi G, Rolma G, Cecchin D, Rossetto M. 5-Aminolevulinic acid fluorescence in high grade glioma surgery: surgical outcome, intraoperative findings, and fluorescence patterns. Biomed Res Int. 2014;2014:232561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roberts DW, Valdés PA, Harris BT, et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters: clinical article. JNS. 2011;114(3):595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Novotny A, Xiang J, Stummer W, Teuscher NS, Smith DE, Keep RF. Mechanisms of 5-aminolevulinic acid uptake at the choroid plexus. J Neurochem. 2000;75(1):321-328. [DOI] [PubMed] [Google Scholar]
- 55. Ferraro N, Barbarite E, Albert TR, et al. The role of 5-aminolevulinic acid in brain tumor surgery: a systematic review. Neurosurg Rev. 2016;39(4):545-555. [DOI] [PubMed] [Google Scholar]
- 56. Dietze A, Berg K. ALA-induced porphyrin formation and fluorescence in synovitis tissue: in-vitro and in vivo studies. Photodiagnosis Photodyn Ther. 2005;2(4):299-307. [DOI] [PubMed] [Google Scholar]
- 57. Mansfield JR, Gossage KW, Hoyt CC, Levenson RM. Autofluorescence removal, multiplexing, and automated analysis methods for in-vivo fluorescence imaging. J Biomed Opt. 2005;10(4):41207. [DOI] [PubMed] [Google Scholar]
- 58. Müller MG, Georgakoudi I, Zhang Q, Wu J, Feld MS. Intrinsic fluorescence spectroscopy in turbid media: disentangling effects of scattering and absorption. Appl Opt. 2001;40(25):4633-4646. [DOI] [PubMed] [Google Scholar]
- 59. Widhalm G, Olson J, Weller J, et al. The value of visible 5-ALA fluorescence and quantitative protoporphyrin IX analysis for improved surgery of suspected low-grade gliomas. J Neurosurg. 2019;133(1):79-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bravo JJ, Olson JD, Davis SC, Roberts DW, Paulsen KD, Kanick SC. Hyperspectral data processing improves PpIX contrast during fluorescence guided surgery of human brain tumors. Sci Rep. 2017;7(1):9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Suero Molina E, Stögbauer L, Jeibmann A, Warneke N, Stummer W. Validating a new generation filter system for visualizing 5-ALA-induced PpIX fluorescence in malignant glioma surgery: a proof of principle study. Acta Neurochir (Wien). 2020;162(4):785-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen B, Wang H, Ge P, et al. Gross total resection of glioma with the intraoperative fluorescence-guidance of fluorescein sodium. Int J Med Sci. 2012;9(8):708-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Catapano G, Sgulò FG, Seneca V, Lepore G, Columbano L, di Nuzzo G. Fluorescein-guided surgery for high-grade glioma resection: an intraoperative ‘contrast-enhancer’. World Neurosurg. 2017;104:239-247. [DOI] [PubMed] [Google Scholar]
- 64. Acerbi F, Broggi M, Broggi G, Ferroli P. What is the best timing for fluorescein injection during surgical removal of high-grade gliomas? Acta Neurochir (Wien). 2015;157(8):1377-1378. [DOI] [PubMed] [Google Scholar]
- 65. Acerbi F, Broggi M, Schebesch K-M, et al. Fluorescein-guided surgery for resection of high-grade gliomas: a multicentric prospective phase II study (FLUOGLIO). Clin Cancer Res. 2018;24(1):52-61. [DOI] [PubMed] [Google Scholar]
- 66. Schwake M, Stummer W, Suero Molina EJ, Wölfer J. Simultaneous fluorescein sodium and 5-ALA in fluorescence-guided glioma surgery. Acta Neurochir (Wien). 2015;157(5):877-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yano H, Nakayama N, Ohe N, Miwa K, Shinoda J, Iwama T. Pathological analysis of the surgical margins of resected glioblastomas excised using photodynamic visualization with both 5-aminolevulinic acid and fluorescein sodium. J Neurooncol. 2017;133(2):389-397. [DOI] [PubMed] [Google Scholar]
- 68. Suero Molina E, Wölfer J, Ewelt C, Ehrhardt A, Brokinkel B, Stummer W. Dual-labeling with 5-aminolevulinic acid and fluorescein for fluorescence-guided resection of high-grade gliomas: technical note. J Neurosurg. 2018;128(2):399-405. [DOI] [PubMed] [Google Scholar]
- 69. La Rocca G, Sabatino G, Menna G, et al. 5-Aminolevulinic acid false positives in cerebral neuro-oncology: not all that is fluorescent is tumor. A case-based update and literature review. World Neurosurg. 2020;137:187-193. [DOI] [PubMed] [Google Scholar]
- 70. Ando T, Kobayashi E, Liao H, et al. Precise comparison of protoporphyrin IX fluorescence spectra with pathological results for brain tumor tissue identification. Brain Tumor Pathol. 2011;28(1):43-51. [DOI] [PubMed] [Google Scholar]
- 71. Utsuki S, Oka H, Sato S, et al. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir (Tokyo). 2007;47(5):210-214. [DOI] [PubMed] [Google Scholar]
- 72. Panciani PP, Fontanella M, Schatlo B, et al. Fluorescence and image guided resection in high grade glioma. Clin Neurol Neurosurg. 2012;114(1):37-41. [DOI] [PubMed] [Google Scholar]
- 73. Hickmann A-K, Nadji-Ohl M, Hopf NJ. Feasibility of fluorescence-guided resection of recurrent gliomas using five-aminolevulinic acid: retrospective analysis of surgical and neurological outcome in 58 patients. J Neurooncol. 2015;122(1):151-160. [DOI] [PubMed] [Google Scholar]
- 74. Miyatake S, Kuroiwa T, Kajimoto Y, Miyashita M, Tanaka H, Tsuji M. Fluorescence of non-neoplastic, magnetic resonance imaging-enhancing tissue by 5-aminolevulinic acid: case report. Neurosurgery. 2007;61(5):E1101-E1104. [DOI] [PubMed] [Google Scholar]
- 75. La Rocca G, Barresi V, Sabatino G, et al. 5-ALA false-positive in anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted. Surg Technol Int. 2020;36:453-456. [PubMed] [Google Scholar]
- 76. Nabavi A, Thurm H, Zountsas B, et al. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase II study. Neurosurgery. 2009;65(6):1070-1077. [DOI] [PubMed] [Google Scholar]
- 77. Hayashi Y, Nakada M, Tanaka S, et al. Implication of 5-aminolevulinic acid fluorescence of the ventricular wall for postoperative communicating hydrocephalus associated with cerebrospinal fluid dissemination in patients with glioblastoma multiforme: a report of 7 cases: clinical article. JNS. 2010;112(5):1015-1019. [DOI] [PubMed] [Google Scholar]
- 78. Tejada-Solis S, Díez-Valle R. Letter to the editor: diffuse glioma detection. JNS. 2013;119(2):530-531. [DOI] [PubMed] [Google Scholar]
- 79. Moon JH, Kim SH, Shim J-K, et al. Histopathological implications of ventricle wall 5-aminolevulinic acid-induced fluorescence in the absence of tumor involvement on magnetic resonance images. Oncol Rep. 2016;36(2):837-844. [DOI] [PubMed] [Google Scholar]
- 80. Yoon S-J, Park J, Jang D-S, et al. Glioblastoma cellular origin and the firework pattern of cancer genesis from the subventricular zone. J Korean Neurosurg Soc. 2020;63(1):26-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Piccirillo SGM, Spiteri I, Sottoriva A, et al. Contributions to drug resistance in glioblastoma derived from malignant cells in the sub-ependymal zone. Cancer Res. 2015;75(1):194-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ji SY, Kim JW, Park C-K. Experience profiling of fluorescence-guided surgery II: non-glioma pathologies. Brain Tumor Res Treat. 2019;7(2):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nestler U, Warter A, Cabre P, Manzo N. A case of late-onset multiple sclerosis mimicking glioblastoma and displaying intraoperative 5-aminolevulinic acid fluorescence. Acta Neurochir. 2012;154(5):899-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Voellger B, Klein J, Mawrin C, Firsching R. 5-aminolevulinic acid (5-ALA) fluorescence in infectious disease of the brain. Acta Neurochir (Wien). 2014;156(10):1977-1978. [DOI] [PubMed] [Google Scholar]
- 85. Solis WG, Hansen M. Fluorescence in a cryptococcoma following administration of 5-aminolevulinic acid hydrochloride (Gliolan). BMJ Case Rep. 2017;2017:bcr2017219469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Panciani PP, Fontanella M, Garbossa D, Agnoletti A, Ducati A, Lanotte M. 5-aminolevulinic acid and neuronavigation in high-grade glioma surgery: results of a combined approach. Neurocirugia (Astur). 2012;23(1):23-28. [DOI] [PubMed] [Google Scholar]
- 87. Altieri R, Agnoletti A, Quattrucci F, et al. Molecular biology of gliomas: present and future challenges. Transl Med UniSa. 2014;10:29-37. [PMC free article] [PubMed] [Google Scholar]
- 88. Altieri R, Zenga F, Ducati A, et al. Tumor location and patient age predict biological signatures of high-grade gliomas. Neurosurg Rev. 2018;41(2):599-604. [DOI] [PubMed] [Google Scholar]
- 89. Seystahl K, Wick W, Weller M. Therapeutic options in recurrent glioblastoma—an update. Crit Rev Oncol Hematol. 2016;99:389-408. [DOI] [PubMed] [Google Scholar]
- 90. Katsevman GA, Turner RC, Urhie O, Voelker JL, Bhatia S. Utility of sodium fluorescein for achieving resection targets in glioblastoma: increased gross- or near-total resections and prolonged survival. J Neurosurg. 2019;132(3):914-920. [DOI] [PubMed] [Google Scholar]
- 91. Eljamel S. 5-ALA Fluorescence image guided resection of glioblastoma multiforme: a meta-analysis of the literature. Int J Mol Sci. 2015;16(5):10443-10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mahboob S, McPhillips R, Qiu Z, et al. Intraoperative ultrasound-guided resection of gliomas: a meta-analysis and review of the literature. World Neurosurg. 2016;92:255-263. [DOI] [PubMed] [Google Scholar]
- 93. Holt D, Okusanya O, Judy R, et al. Intraoperative near-infrared imaging can distinguish cancer from normal tissue but not inflammation PLoS One. 2014;9(7):e103342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lee JYK, Thawani JP, Pierce J, et al. Intraoperative near-infrared optical imaging can localize gadolinium-enhancing gliomas during surgery. Neurosurgery. 2016;79(6):856-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lee JYK, Cho SS, Zeh R, et al. Folate receptor overexpression can be visualized in real time during pituitary adenoma endoscopic transsphenoidal surgery with near-infrared imaging. J Neurosurg. 2018;129(2):390-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xia L, Zeh R, Mizelle J, et al. Near-infrared intraoperative molecular imaging can identify metastatic lymph nodes in prostate cancer. Urology. 2017;106:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Henderson F, Brem S, Hussain J, et al. Second window indocyanine green localizes CNS lymphoma in real time in the operating room: report of two cases. Br J Neurosurg. 2020;1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li C, Sullivan PZ, Cho S, et al. Intraoperative molecular imaging with second window indocyanine green facilitates confirmation of contrast-enhancing tissue during intracranial stereotactic needle biopsy: a case series. World Neurosurg. 2019;126:e1211-e1218. [DOI] [PubMed] [Google Scholar]
- 99. Cho SS, Salinas R, De Ravin E, et al. Near-infrared imaging with second-window indocyanine green in newly diagnosed high-grade gliomas predicts gadolinium enhancement on postoperative magnetic resonance imaging. Mol Imaging Biol. 2020;22(5):1427-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1-2):271-284. [DOI] [PubMed] [Google Scholar]
- 101. Ergin A, Wang M, Zhang JY, et al. The feasibility of real-time in vivo optical detection of blood-brain barrier disruption with indocyanine green. J Neurooncol. 2012;106(3):551-560. [DOI] [PubMed] [Google Scholar]
- 102. Jiang JX, Keating JJ, Jesus EMD, et al. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am J Nucl Med Mol Imaging. 2015;5(4):390-400. [PMC free article] [PubMed] [Google Scholar]
- 103. Cho SS, Salinas R, Lee JYK. Indocyanine-green for fluorescence-guided surgery of brain tumors: evidence, techniques, and practical experience. Front Surg. 2019;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]