Abstract
COVID-19 vaccination related adverse events is an evolving field. Here we present a case of acute myocardial injury that developed as a result of an acute immune response following the second dose of COVID-19 vaccination (Moderna) in a 67-year-old man who presented in acute congestive heart failure. His clinical course improved over 3 days. Review of the Vaccine Adverse Events Reporting System (VAERS) in the Centers for Disease Control and Prevention websites identified 37 vaccine recipients who developed myocarditis as an adverse event following COVID-19 vaccination. With the mass expansion of COVID-19 vaccine administration, physicians need to be vigilant about the possibility of new adverse events.
Keywords: COVID-19 vaccine, adverse events, myocarditis
Introduction
The ongoing COVID-19 pandemic has affected more than 159 million lives across the world. 1 The absence of definitive treatment for the disease and an associated mortality of 2.19% have led to significant challenges to the world economy. The introduction of vaccines in less than a year has offered hope for overcoming the pandemic. The United States Food and Drug Administration (FDA) has granted emergency authorization to the Pfizer/BioNTech (BNT162b2) and Moderna (mRNA-1273) COVID-19 vaccines on December 11, 2020 and December 18, 2020, respectively. Both the vaccines were safe and immunogenic leading to more than 94% protection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in clinical trials.2,3 The proposed regimen for the Pfizer/BioNTech vaccine is 2 doses, administered 21 days apart; the schedule for the Moderna vaccine is 2 doses, administered 28 days apart. A third vaccine, Ad26.COV2.S, also known as the Janssen COVID-19 vaccine developed by Johnson and Johnson, was approved by FDA for emergency use on February 27, 2021. Its dosing schedule includes a single dose, with a reported efficacy of 66.9%. 4 Commonly reported side effects of the vaccines include pain, redness and swelling at the administration site, malaise, headache, myalgias, chills, nausea, and fever. The Centers for Disease Control and Prevention (CDC) maintains a Vaccine Adverse Events Reporting System (VAERS) for self-reporting of adverse events. 5 In this article, we describe the clinical course of a patient developing acute myocardial injury following administration of second dose of COVID-19 vaccine. We also reviewed the existing evidence for similar cases reported in the VAERS database and summarize the characteristics of patients developing COVID-19 vaccine induced myocarditis.
Case
A 67-year-old man was transferred from another medical facility for worsening shortness of breath, fever, and chills that started approximately 6 h after administration of his second dose of the Moderna COVID-19 vaccine. He also had nausea, orthopnea, and increasing fatigue that developed 12 h following vaccine administration. He denied chest pain, palpitations, and syncope. His medical history was significant for hypertension, type 2 diabetes mellitus, hyperlipidemia, coronary artery disease with history of multiple stent placements and coronary artery bypass grafting surgery, congestive heart failure (CHF) with preserved ejection fraction, chronic obstructive pulmonary disease without any home oxygen requirement, hypothyroidism, and gastro-esophageal reflux disease. For these ailments, he was taking low-dose aspirin (81 mg daily), atorvastatin (80 mg daily), clopidogrel (75 mg daily), furosemide (80 mg twice daily), isosorbide mononitrate (120 mg daily), levothyroxine (25 mcg daily), lisinopril (40 mg daily), metformin (500 mg twice daily), metoprolol tartrate (50 mg twice daily), potassium chloride (10 mEq daily) along with albuterol and tiotropium inhalers. He had been compliant to all his medications. He had no similar symptoms after the first dose of vaccine. He denied history of previous COVID infection or contact with a patient with known COVID-19 infection.
At his presentation to the emergency room, he had oxygen saturations down to 86% on 6 L/min of oxygen via nasal canula. He was also hypertensive (169/96 mmHg), tachycardic (141 beats/min), tachypneic (40 breaths/min), and febrile (101.2°F) and was speaking in short sentences due to dyspnea (Table 1). He had coarse crackles in both lung bases, elevated jugular venous pressure, and 1+ pitting edema in his lower extremities. Initial labs were significant for leukocytosis (WBC count of 17.86 × 103/μL), an elevated ESR of 41 mm/h, an elevated proBNP of 43134 pg/mL, and an elevated high sensitivity troponin of 180.8 ng/L. His blood gases showed a pH of 7.294, HCO3 of 21 mmole/L, and PaCO2 of 39.9 mmHg. His serum lactate level (5.7 mmol/L) and total bilirubin (1.9 mg/100 mL) were elevated. His Hb was 18 g/dL at presentation. A chest x-ray showed cardiomegaly and pulmonary edema without any focal consolidation (Figure 1). An ECG revealed sinus tachycardia with non-specific ST/T wave changes. A transthoracic echocardiogram showed a mildly dilated left atrium, a left ventricle ejection fraction of 50%-54%, mild hypokinesia in the mid-septal and mid-anterior walls, and grade 2 diastolic dysfunction. He tested negative for COVID-19 and influenza by PCR. His urinalysis was normal, and sputum and blood cultures were negative; however, his CRP level was elevated (15.5 mg/dL).
Table 1.
Vital Signs and Laboratory Marker Trend during the Hospital Course.
| Parameters | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| Vital signs | |||
| BP, mmHg | 169/96 | 134/61 | 129/73 |
| HR, beats/min | 141 | 92 | 87 |
| Temperature (°F) | 101.2 | 98.6 | 98.3 |
| RR, breaths/min | 26 | 21 | 22 |
| Oxygen saturation, %; oxygen requirement | 92%; 15 L oxygen by NRB mask | 99%; BiPAP (12/8); 40% FiO2 | 96%; 2 L/min by NC |
| Laboratory parameters (with reference values) | |||
| ESR, mm/h (0-20 mm/h) | 41 | N/A | 34 |
| CRP, mg/dL (0-0.5 mg/dL) | 15.5 | N/A | 11.5 |
| Troponin, ng/L (<19 ng/L) | 180.8 | 117 | 85.2 |
| Ferritin, ng/dL (30-400 ng/dL) | N/A | 550 | 454 |
| LD, units/L (135-225 units/L) | N/A | N/A | 239 |
| D-dimer HS, ng/mL (<500 ng/mL) | N/A | N/A | 2010 |
| BNP, pg/mL (<124 pg/mL) | 43134 | 39813 | 4796 |
Abbreviations: BNP, brain natriuretic peptide; BP, Blood pressure; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HR, heart rate; HS, high sensitivity; LD, lactate dehydrogenase; NRB, non-rebreather; RR, respiratory rate.
Figure 1.
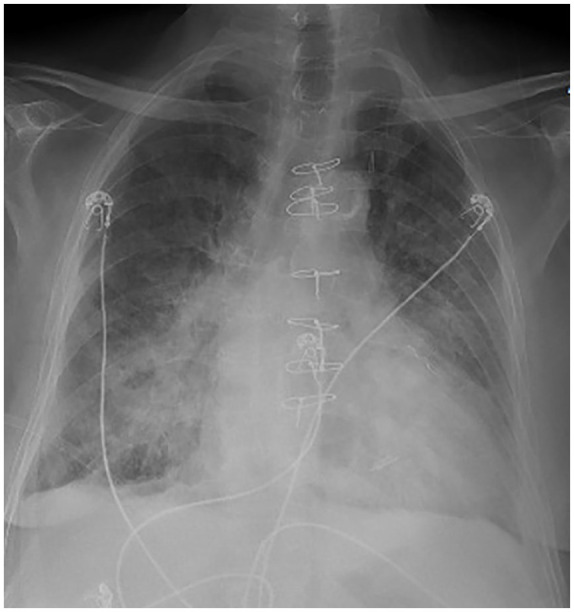
This A-P film reveals cardiomegaly, bilateral alveolar infiltrates, and sternal wires from prior surgery.
The patient was treated for his acute exacerbation of CHF with diuretics and supplemental oxygen therapy. His hypoxia and dyspnea significantly improved after placing him on BiPAP (12/8 cm H2O setting). His tachycardia and tachypnea improved with intravenous furosemide and bronchodilators, and his hypertension improved with hydralazine. Table 1 summarizes his vitals and some inflammatory markers during the course of his hospital stay which showed gradual improvement. He was also empirically treated for sepsis with ceftriaxone and azithromycin to cover for community-acquired pneumonia as a possible source of infection. Despite antibiotic treatment, he continued to have heart failure symptoms and the pro-BNP remained elevated, suggesting an alternative etiology for his CHF exacerbation. Elevated troponin levels and mild hypokinesia of the ventricular wall in 2 regions on TTE with an acute presentation, including fever, led to the clinical diagnosis of myocarditis rather than type 2 non-ST elevation myocardial infarction associated with heart failure. The temporal association of his symptoms with COVID-19 vaccination and the absence of preceding symptoms of viral infection suggested that the acute myocardial injury was associated with his vaccination.
His subsequent clinical course showed remarkable improvement with resolution of symptoms over the next 2 days. His oxygen requirement decreased to 2 L/min of oxygen by nasal cannula. At a 2-month follow-up, he was at his baseline with normalization of troponin and inflammatory markers.
Myocarditis after COVID-19 Vaccination: Review of Current Evidence from VAERS Database
PubMed, Scopus, Embase, Science Direct, and Web of Science literature databases were searched for reports of cases of myocarditis or cardiac complications following COVID-19 vaccination using the search strategy as described in Supplementary Table 2. Only articles in English language were considered. The search yielded 7 unique results, out of which 4 were review article,6-9 1 was an editorial, 10 1 was an original research paper, 11 and 1 was a case report of multi-system inflammatory syndrome (MIS) following COVID-19 vaccination. 12 The original article and the case report did not report any myocardial injury or myocarditis related clinical presentation.
We then looked for evidence of similar adverse events following COVID-19 vaccination that have been reported in the VAERS database in the CDC website. We used the following query criteria for this search: Adverse Event Description: “myocarditis” AND Age: “18-29 years,” “30-39 years,” “40-49 years,” “50-59 years,” “60-64 years,” “65+ years,” “Unknown”; Date Report Completed: “2021”; Vaccine Products: “COVID19 VACCINE (COVID19)”;VAERS ID: “All”; Group By: “Symptoms,” “VAERS ID”; Show Totals: “False”; Show Zero Values: “Disabled.” This search was completed on 3-30-2021 and resulted in 37 unique cases (See Supplementary Table 1 for full characteristics).
A diagnosis of myocarditis in these cases was made based on clinical presentation, laboratory tests, and radiologic findings. The most common clinical presentation included fever, malaise, chills, chest pain, and shortness of breath. Commonly associated abnormalities included elevated troponin levels, ECG changes with ST/T wave abnormalities, and echocardiography showing wall motion abnormalities. Cardiac MRIs in 8 of the 37 patients were diagnostic of myocarditis. Endomyocardial biopsy was done in 1 patient with confirmation of the diagnosis. Workup also included ruling out other causes of myocarditis, such as viral infections in 4 of these 37 patients.
The characteristics of the study subjects in the VAERS database who developed myocarditis following vaccination are summarized in Table 2. Out of the 37 subjects, 25 were male and the median age was 35 years. Two doses of vaccine were received by 22 of these patients. The median time to onset of myocarditis symptoms was 3 days following vaccination. The majority (22 out of 37) of the patients required hospitalization with median length of hospital stay of 3 days (interquartile range 4 days). One of the 37 patients died.
Table 2.
Summary of Characteristics of Vaccinees Developing Myocarditis Reported in the VAERS Database.
| Characteristics of subjects | Number of subjects |
|---|---|
| Sex | |
| Male | 25 |
| Female | 12 |
| Past medical condition | |
| Prior cardiac comorbidity | 5 |
| Previous history of COVID-19 | 3 |
| Previous history of COVID-19 myocarditis | 1 |
| Type of vaccine | |
| Moderna | 22 |
| Pfizer-BioNTech | 15 |
| Number of vaccine dose | |
| Single dose of vaccine | 15 |
| Two doses of vaccine | 22 |
| Outcome | |
| Hospitalization | 31 |
| Death | 1 |
| Characteristics of subjects | Median (interquartile range) |
| Age in years | 35 (16.5) |
| Days from vaccination to symptom onset | 3 (6.25) |
| Length of hospital stay in days | 3 (4) |
Discussion
Cardiovascular complications commonly associated with COVID-19 infection include myocarditis, pericardial effusion, and pericarditis. Although isolated cases of acute myocarditis in COVID-19 infection have been reported by some authors,13-16 myocarditis has been increasingly recognized as a late sequala identified in up to 46% to 78% of recovering COVID-19 patients.17-19 The ACE-2 protein serves as the receptor for binding to the spike proteins of SARS-CoV-2 virus and is found in cardiomyocytes, 20 which may result in direct myocyte injury from the virus. However, immune activation and cytokine storm induced multi-system inflammation occur in severe COVID-19 infections and can contribute to both acute heart failure as well as to the development of late-onset myocarditis. 19 Multi-system inflammatory syndrome resulting from a severe inflammatory response to COVID-19 infection has been frequently reported in the literature. Multi-system inflammatory syndrome in children (MIS-C) leads to shock, acute heart failure, abdominal pain, and elevated inflammatory markers. Although initially reported more commonly in the pediatric population,21-23 MIS has also been increasingly recognized in adults (MIS-A) infected with COVID-19. 24 A FDA document raised the caution that although vaccination is highly effective against symptomatic COVID-19 infection, it may not prevent asymptomatic infections or late sequalae of infections such as myocarditis. 25 Recently, a case of MIS-A following COVID-19 vaccination was reported in which increased 18 F-fluorodeoxyglucose uptake was noticed in deltoid muscle, axillary lymph nodes and spleen, without any observed cardiac changes or symptoms. 12
Myocarditis following COVID-19 vaccine administration is a possible vaccine related adverse effect that has rarely been reported in the VAERS database. Our patient developed symptoms after his second dose of vaccine and had elevation of non-specific inflammatory markers. A second dose of the vaccine is associated with stronger immune response compared to the first dose, 26 which might explain why our patient did not develop myocarditis symptoms after his initial vaccine dose. In the VAERS data vaccine induced myocarditis developed in a majority (22 of 37) of patients after their second doses. Out of these 37 patients developing myocarditis following COVID-19 vaccination, 32 were hospitalized (Supplementary Table 1). Between January to March 2021, 16 325 adverse events following COVID-19 vaccination were reported to CDC; 1133 cases required hospitalization, indicating that severe events can occur post vaccination.
Analysis of our case requires several considerations. This patient had several important chronic diseases and possibly had an acute exacerbation. However, routine cardiac or respiratory exacerbations would not explain his elevated inflammatory markers. Our patient could have had a remote COVID-19 infection and later developed the myocarditis, but there is no clinical history supporting a prior COVID-19 infection. However, a previous asymptomatic infection cannot be completely ruled out. It is possible the patient had a prior COVID-19 infection and that the vaccine triggered an acute immune response. Again, historical details from the patient would not support this possible explanation. The temporal relationship with vaccination makes a vaccine-associated adverse event a plausible explanation in our patient, and this patient might have MIS-A following vaccination. This possibility will require more careful measurement of biochemical and immunologic markers in patients who appear to have acute vaccine related clinical syndromes.
Supplemental Material
Supplemental material, sj-docx-1-jpc-10.1177_21501327211029230 for Acute Myocardial Injury Following COVID-19 Vaccination: A Case Report and Review of Current Evidence from Vaccine Adverse Events Reporting System Database by Anasua Deb, John Abdelmalek, Kenneth Iwuji and Kenneth Nugent in Journal of Primary Care & Community Health
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Anasua Deb  https://orcid.org/0000-0002-1049-5882
https://orcid.org/0000-0002-1049-5882
Kenneth Iwuji  https://orcid.org/0000-0001-5489-233X
https://orcid.org/0000-0001-5489-233X
Kenneth Nugent  https://orcid.org/0000-0003-2781-4816
https://orcid.org/0000-0003-2781-4816
Patient Permission: Obtained.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. WHO coronavirus (COVID-19) dashboard. Accessed December 5, 2021. https://CoVID19.who.int.
- 2. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Accessed March 30, 2021. https://wonder.cdc.gov/vaers.html.
- 6. Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39(22):3037-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adeyinka A, Bailey K, Pierre L, et al. COVID 19 infection: pediatric perspectives. J Am Coll Emerg Physicians Open. 2021;2(1):e12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krumm ZA, Lloyd GM, Francis CP, et al. Precision therapeutic targets for COVID-19. Virol J. 202;18(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alfawaz DA, Alfawaz M, Almutawa AM. Impact of COVID-19 on cardiovascular patients and review of current COVID-19 treatment strategies. J Pharm Res Int. 2021;32(42):69-79. [Google Scholar]
- 10. Blumenthal JA, Burns JP. Complexities of the COVID-19 vaccine and multisystem inflammatory syndrome in children. Pediatr Investig. 2020;4(4):299-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kroshus E, Hawrilenko M, Tandon PS, Christakis DA. Plans of US parents regarding school attendance for their children in the fall of 2020: a national survey. JAMA Pediatr. 2020;174(11):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinberg J, Thomas A, Iravani A. (18)F-fluorodeoxyglucose PET/CT findings in a systemic inflammatory response syndrome after COVID-19 vaccine. Lancet. 2021;397(10279):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imazio M, Klingel K, Kindermann I, et al. COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106(15):1127-1131. [DOI] [PubMed] [Google Scholar]
- 14. Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41(19):1861-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peretto G, Sala S, Caforio ALP. Acute myocardial injury, MINOCA, or myocarditis? Improving characterization of coronavirus-associated myocardial involvement. Eur Heart J. 2020;41(22):2124-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajpal S, Tong MS, Borchers J, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bajaj R, Sinclair HC, Patel K, et al. Delayed-onset myocarditis following COVID-19. Lancet Respir Med. 2021;9(4):e32-e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goulter AB, Goddard MJ, Allen JC, Clark KL. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004;2:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. J Am Med Assoc. 2020;324(3):259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belot A, Denise A, Renolleau S, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25(22):2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dean R, Narayanan M, Nix E, Stepka K, Mattamal R. First reported case of Multisystem Inflammatory Syndrome in children in West Texas. Southwest Respir Crit Care Chronicles. 2021;9(37):66-69. [Google Scholar]
- 24. Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection—United Kingdom and United States, March–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1450-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting FDA Briefing Document. Pfizer-BioNTech COVID-19 Vaccine. FDA; 2020: 1-53. [Google Scholar]
- 26. Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594-599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jpc-10.1177_21501327211029230 for Acute Myocardial Injury Following COVID-19 Vaccination: A Case Report and Review of Current Evidence from Vaccine Adverse Events Reporting System Database by Anasua Deb, John Abdelmalek, Kenneth Iwuji and Kenneth Nugent in Journal of Primary Care & Community Health


