Abstract
A decarboxylated form of L-arginine, agmatine, preferentially antagonizes NMDArs containing Glun2B subunits within the spinal cord and lacks motor side effects commonly associated with non-subunit-selective NMDAr antagonism, namely sedation and motor impairment. Spinally delivered agmatine has been previously shown to reduce the development of tactile hypersensitivity arising from spinal nerve ligation. The present study interrogated the dependence of agmatine’s alleviation of neuropathic pain (spared nerve injury (SNI) model) on GluN2B-containing NMDArs. SNI-induced hypersensitivity was induced in mice with significant reduction of levels of spinal GluN2B subunit of the NMDAr and their floxed controls. Agmatine reduced development of SNI-induced tactile hypersensitivity in controls but had no effect in subjects with reduced levels of GluN2B subunits. Ifenprodil, a known GluN2B-subunit-selective antagonist, similarly reduced tactile hypersensitivity in controls but not in the GluN2B-deficient mice. In contrast, MK-801, an NMDA receptor channel blocker, reduced hypersensitivity in both control and GluN2B-deficient mice, consistent with a pharmacological pattern expected from a NMDAr antagonist that does not have preference for GluN2B subtypes. Additionally, we observed that spinally delivered agmatine, ifenprodil and MK-801 inhibited nociceptive behaviors following intrathecal delivery of NMDA in control mice. By contrast, in GluN2B-deficient mice, MK-801 reduced NMDA-evoked nociceptive behaviors, but agmatine had a blunted effect and ifenprodil had no effect. These results demonstrate that agmatine requires the GluN2B subunit of the NMDA receptor for inhibitory pharmacological actions in pre-clinical models of NMDA receptor-dependent hypersensitivity.
Keywords: Agmatine, NMDA, chronic pain, neuropathic pain, ifenprodil, GluN2B
Introduction
Rising mortality following use of natural and synthetic prescription opioids, as well as illicit opioids, has led to the pursuit of novel, safe, and effective strategies for management of chronic pain, 1 including non-opioid analgesics. Agmatine, the decarboxylated form of L-arginine, is endogenously synthesized in mammals by arginine decarboxylase 2 and meets many of the criteria of acting as a neurotransmitter/neuromodulator, including synthesis in neurons, 3 release from nerve terminals, 4 and binding to post-synaptic receptors.5,6 Agmatine has been demonstrated to reverse pain behaviors in models of inflammation, neuropathy, and spinal cord injury,7–11 but is not analgesic in acute measures of nociception. Agmatine also reduces nocifensive behaviors and thermal hypersensitivity that arises from spinal delivery of NMDA. 7 Agmatine is an antagonist of the N-methyl-D-aspartate receptor (NMDAr),6,12 making it likely that agmatine’s inhibitory analgesic effects on chronic pain are due to NMDAr antagonism. However, it has not been evaluated previously for in vivo NMDAr subunit selectivity.
The NMDA receptor is composed of four subunits, 13 typically composed of two GluN1 and two GluN2 subunits. 14 Of the GluN2 subunits, four subtypes exist (A-D), with each having differential expression and functional properties across the central nervous system (CNS) and throughout development, 15 and each encoded by a separate gene. 16 The subunit composition of the NMDAr determines its pharmacological and physiological characteristics; 17 NR1/NR2A receptors display a faster inactivation rate than NR1/NR2B receptors. 18 NMDAr activity is altered in several ways following peripheral nerve injury. The NR1 subunit experiences a significantly increased phosphorylation level in dorsal spinal cord and gracile nucleus ipsilateral to the site of injury as compared to the contralateral side.19,20 Long-term potentiation (LTP) is a defining electrophysiological signature of central nervous system neuroplasticity. In the spinal cord, LTP requires the activation of NMDA receptors 21 and can inhibited by pre-treating subjects with NMDA receptor antagonists, such as AP5. 22 NMDA receptor-dependent spinal LTP can be induced either through repetitive electrical stimulation of the sural nerve, nociceptive stimulation of the area of sural nerve innervation or acute nerve injury. 23 The NR2B subunit of the NMDA receptor is known to contribute to LTP; the GluN2B subunit selective antagonist, Ro-25‐6981 has been shown to concentration-dependently reduce the induction of spinal LTP in rats by high frequency stimulation of the sciatic nerve. 24 Similarly, GluN2B subunit selective antagonist, ifenprodil has been shown to block the induction of spinal LTP in rats by BDNF application. 25 The demonstration that GluN2B subunit contributes to LTP in the spinal cord is consistent with evidence for its role in the development of neuropathic pain.
Analysis of the GluN2B subunit-selective NMDAr antagonists indicates that GluN2B-containing NMDArs are critical to the development of neuropathic pain at early stages following injury, and for the development of long-lasting enhanced spinal excitability.26,27 Further evidence supporting the involvement of GluN2B-containing NMDArs in the spinal plasticity and central sensitization of pain lies in the tyrosine phosphorylation of this 2B subunit and its increase in multiple pain states.28–31 The responsiveness of ionotropic glutamate receptors to glutamate agonists is potentiated following phosphorylation. 32 Following injection of complete Freund’s adjuvant (CFA) to mouse hindpaws, a prolonged increase in tyrosine phosphorylation is seen in GluN2B- but not GLuN2A-containing NMDArs that is correlated to the temporal expression of hyperalgesia and inflammation. 31 Hindpaw injection of saline results in only a transient increase in tyrosine phosphorylation of GluN2B subunits, indicating that the phosphorylation of GluN2B is maintained by primary afferent input from the site of injury. Taken in total, these data indicate that prolonged antagonism of GluN2B-containing NMDArs following neuropathic pain is a viable strategy to reduce neuropathic pain.
We have previously demonstrated in a spinal cord slice preparation that agmatine inhibition of NMDAr-mediated excitatory postsynaptic currents (EPSCs) in small lamina II dorsal horn neurons requires the GluN2B subunit. 33 Targeting the GluN2B subunit is of interest for therapeutic drug development for the treatment of chronic pain states due to the more restricted nature of the GluN2B subunit containing NMDA receptors; such an expression pattern favors an improved therapeutic index. Specifically, it is anticipated that motor dysfunction commonly associated with non-selective NMDA receptor antagonists is not observed with antagonists selective for the GluN2B subunit-containing NMDA receptors. To determine whether agmatine inhibition of chronic pain responses in the intact and nerve-injured animal demonstrates a requirement for GluN2B receptors and reduced motor dysfunction, we tested agmatine for agmatine reduction of tactile hypersensitivity and impact on motor performance in nerve injury-induced neuropathic pain. We also evaluated the anti-allodynic effect of agmatine in a model of NMDA-evoked nociceptive behavior for dependency on NMDArs containing GluN2B subunits.
Materials and methods
Animals
Male and female ICR mice (21–24 g, Harlan) or C57Bl/6 (as described below) were housed with continuous access to water and food in a humidity- and temperature-controlled environment. Four male and five female mice were housed per cage with 12-hour light/dark cycles. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Generation of GluN2B-deficient mice
Generation of the GluN2B knock-down (KD) mouse was initiated by Dr. E. Delpire (Vanderbilt University), as previously described. 34 The GluN2B-mutant mouse was generated by the Gene-Targeted Mouse Core of the INIA-stress consortium. They are on a C57Bl/6 genetic background. This Integrative Neuroscience Initiative on Alcoholism examines the link between stress and alcohol. The consortium is supported by the National Institute on Alcohol Abuse and Alcoholism. The Gene-Targeted Mouse Core is supported by NIH grant U01 AA013514 (to E.D.). A breeding colony of homogenous GluN2B-floxed mice was established. At time of weaning (p21), all subjects received either an intrathecal injection of 5 microliters of 0.9% saline (floxed control subjects) or AAV9.hSYN.HI.eGFP-Cre.WPRE.SV40 (GluN2B-KD subjects) (Penn Vector Core, University of Pennsylvania).
Rt-qPCR confirmation of GluN2B-deficiency
A decrease of GluN2B subunits in lumbar dorsal horn of AAV9-hSYN-Cre-injected subjects as compared to saline-injected controls was performed as previously described. 33 Briefly, lumbar spinal cord tissue was collected in TRIzol® Reagent (phenol and guanidine isothiocyanate solution) and the expression levels of GluN2B subunits were determined by estimating the messenger RNA copy number through quantitative real-time reverse transcription (RT-qPCR). The oligonucleotide primers used were mouse GluN2B: F 5′-ATGAAGAGGGGCAAGGAGTT-3′ and R 5′-CGATGATGGAGGAGACTTGG-3′, and mouse 18S F 5′-AAGACGATCAGATACCGTCGTAG-3′ and R 5′-TCCGTCAATTCCTTTAAGTTTCA-3′.35,36 18S was used as an endogenous control as it has been validated as a stable normalization gene for RT-qPCR. 37 To obtain the ΔCt value for each of the samples, the Ct value of 18S was subtracted from the Ct value of target (GluN2B). The ΔΔCt was obtained by using the ΔCt experimental value (AAV9-hSYN-Cre-injected) minus the ΔCt control value (saline-injected). Then the fold change (2−ΔΔCt) was calculated.
Chemicals and reagents
Agmatine sulfate, dizocilpine maleate ((+)-MK-801), ifenprodil, and putrescine dihydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in 0.9% saline.
Intrathecal injections
All drugs were delivered in 5 μL volumes via intrathecal injection in conscious mice. 38 Briefly, the mice were held by the iliac crest and a 30-gauge, 0.5-inch needle attached to a 50 µL Luer-hub Hamilton syringe delivered 5 µL of injectate into the intrathecal space of the awake mice.
NMDA-evoked responses
Intrathecally-delivered NMDA induces both a transient thermal hypersensitivity, measured by warm water tail immersion, and caudally directed scratching and biting behaviors that persist for 1 minutes. NMDA responses are typically induced by a single intrathecal injection (0.3 nmol-1 nmol) of NMDA.39,40 The animal’s scratching and biting responses in the first minute after injection were counted. Increasing doses of intrathecally-delivered NMDA were injected in control (0.03, 0.1, 0.3 nmol, i.t.) and in GluN2B-KD (0.3, 0.4, 0.5, 1 nmol, i.t.) subjects. This information was used to select single doses against which doses of agmatine, MK-801, and ifenprodil were tested for inhibition of the NMDA-induced scratching and biting response. This was accomplished by intrathecal co-administration of a dose of NMDA together with a dose of either of the three antagonists.
Spared nerve injury
Tactile hypersensitivity was induced using the spared nerve injury (SNI) model described by Decosterd and Woolf. 41 Subjects are placed under isoflurane anesthesia and the left sciatic nerve is exposed, along with its three terminal branches. The common peroneal and tibial nerves were ligated with 5.0 silk suture. These two nerves were then sectioned 2 mm distal to the ligation site. The sural nerve remained uninjured.
Tactile hypersensitivity
Mice were placed on a wire mesh grid under a glass enclosure and allowed to acclimate for 30 minutes prior to testing. Hypersensitivity was tested by using an electronic von Frey (vF) device (Life Sciences, IITC). The left and right hindpaws were stimulated by the tip of the stimulator with enough force to cause the mouse to withdraw its paw. The amount of force required for withdrawal was recorded and reported in grams. Baseline responses before SNI were collected, as well as responses on alternating days following injury. A maximum of 30 days of vF testing was performed after injury. The experimenter was blinded to genotype and pharmacological treatment of the mice.
Motor coordination
Motor coordination was assessed via an accelerating rotarod (Ugo Basile, Carese, Italy). After a training session, mice were given the opportunity to walk on the accelerating (4–40 rpm) rotarod for a maximum of 300 seconds. Latency to fall off the rotarod was recorded and compared between treatment groups.
Data analysis
All statistical analyses were considered significant at α = 0.05. Mechanical paw withdrawal thresholds collected by von Frey filament stimulation were analyzed by repeated measures ANOVA with a Bonferroni post-hoc correction. Analysis of maladaptive neuroplasticity was performed by calculating the AUC for matched saline controls and the study drug and analyzed by unpaired Student’s t-test. NMDA-evoked behavior for each compound was compared to a subject receiving a saline control injection and was analyzed by ANOVA with Dunnett’s post-hoc test for multiple comparisons to a control. Analysis of RT-qPCR was performed by normalizing the data to a housekeeping gene, 18S, and compared by unpaired Student’s t-test.
Results
Agmatine inhibits injury-induced neuroplasticity
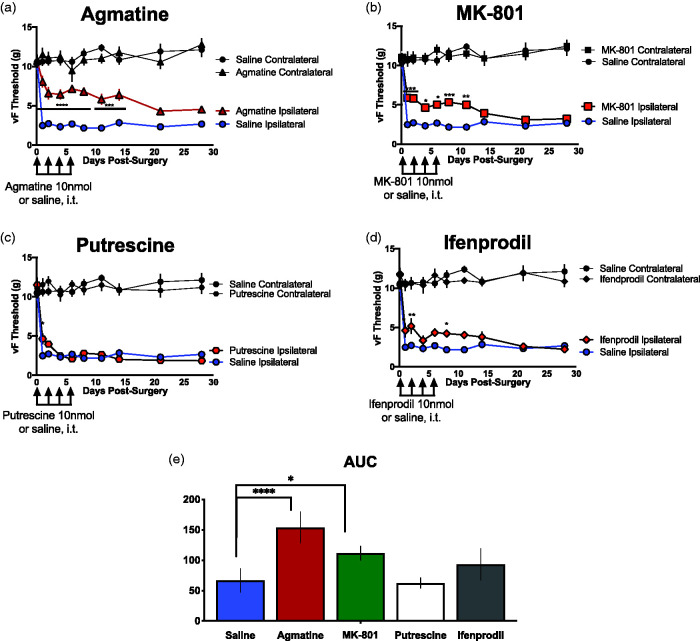
The first goal of this study was to determine agmatine’s efficacy at reversing neuropathic pain behaviors as measured by von Frey threshold. To address this question, we utilized the spared nerve injury (SNI) neuropathic pain model, a well-characterized model of neuropathic pain in rodents. 41 Immediately prior to surgery, each mouse was injected intrathecally with a 5 µL solution of 10 nmol agmatine, as well as on alternating days following surgery (days 2, 4 and 6). Subjects were assessed for their von Frey thresholds prior to surgery and on alternating days after surgery (days 1, 3, 5 and 7) on both the injured (ipsilateral) and non-injured (contralateral) hindpaws. Additional von Frey testing continued weekly for a maximum of 30 days following injury. These data are presented in Figure 1(a).
Figure 1.
Centrally-delivered agmatine attenuates the development of neuropathic pain behaviors following spared nerve injury. Tactile hypersensitivity of both injured (ipsilateral) and non-injured (contralateral) hindpaws of mice was measured prior to and following spared nerve injury (SNI) in saline controls (blue circles, n = 8) and subjects treated with experimental compounds (red symbols). (a) 10 nmol agmatine (red triangles, n = 8) or saline (blue circles n = 8), (b) 10 nmol MK-801(red squares, n = 8), (c) 10 nmol putrescine (red hexagons, n = 9), or (d) 10 nmol ifenprodil (red diamonds, n = 8). All experimental cohorts were injured and tested simultaneously. All drugs were delivered intrathecally immediately prior to and on days 2, 4 and 6 following spared nerve injury. Data are expressed in grams of force required to elicit a behavioral response. * represents significant difference from saline control with p < 0.05, ** with p < 0.01, *** with p < 0.001, **** with p < 0.0001. Statistical significance was tested using ANOVA with Bonferroni’s post-hoc analysis. All cohorts were injured, injected, and assessed for vF thresholds simultaneously and therefore the saline control is represented in (a) to (d). AUC was calculated for each of the pharmacological conditions (saline control, agmatine, MK-801, putrescine, or ifenprodil) and compared between the saline and each study drug using a Student’s t-test. *p < 0.05, ****p < 0.0001.
Additional cohorts were generated and tested in the same manner as the agmatine cohort, but with different pharmacological compounds. Dizocilpine (MK-801), ifenprodil and putrescine were all intrathecally delivered immediately prior to surgery as well as on days 2, 4 and 6 after injury. Behavioral testing was conducted on days 1, 3, 5 and 7, as well as weekly until a maximum of 30 days following injury. These data are presented in Figure 1(b) to (d). AUC was calculated for the saline control and four study drugs (agmatine, MK-801, putrescine, or ifenprodil) and compared between the saline and each study drug using a Student’s t-test, as presented in Figure 1(e).
Agmatine does not impair motor performance in neuropathic subjects
In addition to reflexive (von Frey) behavioral testing, we assessed whether central delivery of agmatine attenuated motor performance. To this end, we performed SNI on male ICR mice. Following injury, mice were assessed for their motor performance on the rotarod assay and separated into groups of equal rotarod performance. They were then injected i.t. with 10 nmol agmatine, 10 nmol MK-801 or saline control. Fifteen minutes following injection, mice were again placed on the accelerating rotarod and their latency to falling off of the rotarod recorded (Figure 2). SNI reduced time spent on the rotarod. Subjects treated with agmatine spent significantly more time than saline-treated subjects on the rotarod, whereas MK-801 manifested its established motor impairment.
Figure 2.
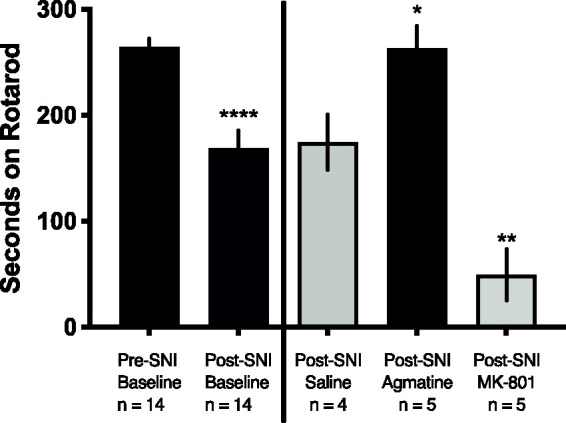
Agmatine does not induce motor impairment characteristic of widespread NMDAR antagonism. All mice (n = 14, male) were assessed for their baseline motor coordination by rotarod performance with a cutoff time of 300 seconds, then given spared nerve injury (SNI) to induce local hypersensitivity and assessed for their decrement in performance. Students t test, ****p < 0.0001. Following injury, subjects were injected i.t. with saline (n = 4), 10 nmol agmatine (n = 5) or 10 nmol MK-80 (n = 5) and placed on an accelerating rotating rod 30 minutes following injection. Latency to fall was recorded, and data were analyzed by one-way ANOVA with reference to the saline control: *p < 0.05 and **p < 0.01.
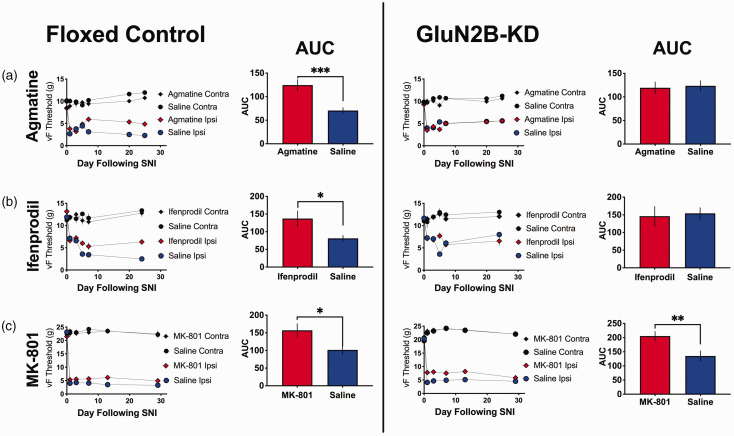
Agmatine requires GluN2B-Containing NMDA receptors to attenuate neuropathic pain
Two GluN2B-floxed cohorts were run in parallel, one that had received an injection of saline at time of weaning (floxed control) and another that had received an injection of AAV9-Cre at time of weaning (GluN2B-KD). A minimum of 4 weeks following this injection, every subject in both cohorts received SNI to establish a state of chronic neuropathic pain. All subjects were assessed for their von Frey thresholds prior to surgery and alternating days after surgery (days 1, 3, 5 and 7). Agmatine (10 nmol) or saline was delivered intrathecally immediately prior to surgery and on days 2, 4 and 6 post-surgery. Additional von Frey testing continued weekly for a maximum of 30 days following injury (Figure 3(a)). Following completion of behavioral testing, spinal cords were extracted and analyzed for GluN2B mRNA levels (Figure 5). We observed that nerve injury reduced vF thresholds in both GluN2B-deficient and floxed control mice. During the induction phase (first post-operative week), intrathecal treatment with agmatine had little effect in either group. During the maintenance phase (period of established chronic pain, week 2 and beyond) agmatine demonstrated no effect in the GluN2B-deficient mice whereas it reduced tactile hypersensitivity in the floxed control mice. These results suggest that the anti-allodynic effects of agmatine require the GluN2B subunit of the NMDA receptor.
Figure 3.
Agmatine attenuates neuropathic pain responses in floxed control, but not GluN2B-KD mice. All subjects were given spared nerve injury to induce local hypersensitivity. Immediately prior to surgery, subjects received saline control or (a) 10 nmol agmatine (floxed control: n = 9, male and female, GluN2B-KD: n = 9, male and female), (b) 10 nmol ifenprodil (floxed control: n = 6, male and female, GluN2B-KD: n = 9, male and female), or (c) 10 nmol MK-801 (floxed control: n = 10, male and female, GluN2B-KD: n = 10, male and female), i.t. Subjects also received saline or study drug (agmatine, ifenprodil or MK-801) on days 2, 4 and 6 following injury. von Frey thresholds were measured prior to and up to 30 days following injury for both ipsilateral (injured) and contralateral (non-injured) hindpaws. Each study drug was assessed once in a cohort of floxed control animals and once in GluN2B-kD animals. Each experimental cohort had a matched saline control with male and female subjects. The n of the saline cohort (identified by their matched pharmacological cohort) are as follows; floxed control agmatine: n = 8, floxed control ifenprodil: n = 12, floxed control MK-801: n = 7, GluN2B-KD agmatine: n = 9, GluN2B-KD ifenprodil: n = 9, GluN2B-KD MK-801: n = 8. AUC was calculated and compared between the saline and study drug groups using a Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 5.
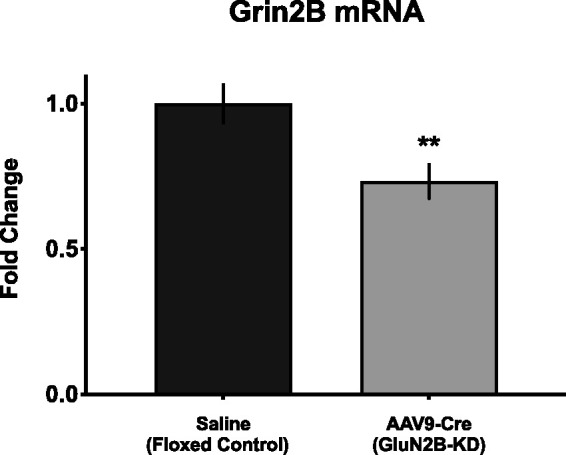
GluN2B analysis of mouse lumbar spinal cord. Quantitative real-time reverse transcription (RT-qPCR) analysis of mouse lumbar spinal cord mRNA to confirm GluN2B-knockdown. At the time of weaning, GluN2B-floxed mice were injected with saline (floxed control) or adeno-associated virus serotype 9 vectors expressing Cre recombinase (AAV9-Cre; GluN2B-knockdown). RT-qPCR indicates a significant decrease in grin2B mRNA in GluN2B-KD mice compared with controls. **p < 0.01; significant difference from saline control (unpaired Student’s t-test).
Ifenprodil requires GluN2B-containing NMDA receptors to attenuate neuropathic pain
Ifenprodil has been demonstrated to be selective for the 2B subunit of the NMDA receptor.42–44 As such, we examined ifenprodil’s effect in this experimental paradigm (Figure 3(b)) and compared this effect to what was observed with agmatine treatment (Figure 3(a)). We observed that, like in the agmatine-treated cohort, ifenprodil had little impact on hypersensitivity thresholds during the induction phase of tactile hypersensitivity following SNI. Similar to agmatine, during the maintenance phase of the injury, ifenprodil showed no effect in the GluN2B-deficient mice but did reduce tactile hypersensitivity in the wild type mice, consistent with ifenprodil’s established requirement for GluN2B-subunits of NMDArs.
Mk-801 does not require the GluN2B subunit of the NMDA receptor to attenuate neuropathic pain
Based on agmatine and ifenprodil’s lack of efficacy in GluN2B-deficient mice in the SNI model of neuropathic pain, we expanded this work to include an additional, gold standard NMDA antagonist. It is well established that MK-801 blocks the open channel of NMDA receptors,45,46 and as such should not require the presence of GluN2B subunits in order to have efficacy in reversing pain behaviors. Again, two GluN2B-floxed cohorts were run in parallel, a floxed control cohort and a GluN2B-deficient cohort. All subjects were behaviorally assessed prior to SNI and days 1, 3, 5 and 7 following SNI (Figure 3(c)). We observed moderate efficacy of MK-801 in both the wild type and GluN2B-deficient mice at reversing long-term hypersensitivity following SNI. These data suggest that, unlike agmatine, the GluN2B receptor subunit is not required for MK-801’s anti-hyperalgesic effects in nerve-injured mice.
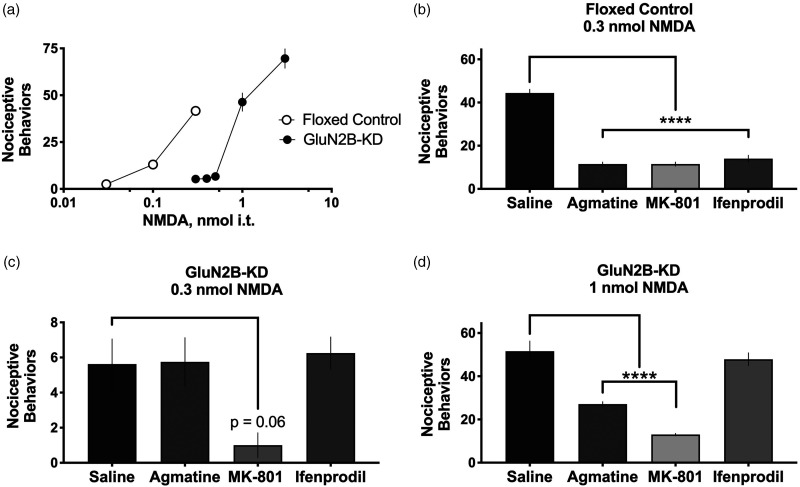
Impact of GluN2B reduction on spinal agmatine, MK-801, and ifendprodil inhibition of NMDA responses in vivo
From previous biochemical,47,48 electrophysiological,6,7 and pharmacological6,7,49,50 studies of agmatine’s mechanism of action, agmatine likely exerts its effect on chronic pain through inhibition of the NMDAr/nitric oxide synthase cascade. We have previously shown that agmatine inhibits thermal hyperalgesia and nociceptive behavior induced when NMDA is delivered intrathecally to mice. 7 However, the impact of agmatine inhibition on responses induced by intrathecal NMDA has not heretofore been assessed in GluN2B-deficient subjects. Therefore, we performed dose-response analysis in floxed control and GluN2B-KD mice (n = 4–11 mice/dose, male and female) following i.t. NMDA and quantified the number of caudally directed scratching and biting nociceptive behaviors characteristic of this assay (Figure 4(a)). We observed that GluN2B-KD mice require an increased dose of i.t. NMDA in order to elicit the same number of nociceptive behaviors as their floxed control controls.
Figure 4.
Pharmacological assessment of subunit-specific NMDAr antagonism in floxed control and GluN2B-KD subjects using changes in NMDA-evoked behavior. Intrathecal NMDA produces transient nociceptive scratching and biting behaviors. (a) The effect of increasing doses of NMDA (i.t.) in floxed control (white circles) and GluN2B-KD (black circles) mice, n = 4–11 mice (male and female)/dose. (b) to (d) Pharmacological assessment of NMDA antagonists (agmatine, MK-801 and ifenprodil, 10 nmol, i.t.) at reducing the nociceptive behaviors following injection of NMDA at doses identified from the dose response curves generated in Panel (a). (b) A 0.3 nmol i.t. dose of NMDA was administered to control mice because it corresponds to the dose identified in Panel (a) that produces approximately 44 ± 1.8 behaviors in control mice (n = 11), providing a standard stimulation range against which the antagonists agmatine, MK-801, and ifenprodil could be tested. (c) A 0.3 nmol i.t. dose of NMDA was delivered to GluN2B-KD mice (n = 8) in order to directly compare to the dose delivered to the control mice. However, the scale is different because, as shown in Panel (a), the 0.3 nmol dose produces only an average of 5.6 ± 1.4 behaviors in GluN2B-KD mice, narrowing the stimulation range against which the antagonists could be assessed. (d) A 1.0 nmol i.t. dose of NMDA i.t. was delivered to GluN2B-KD mice because it produces 52 ± 4.7 behaviors in GluN2B-KD mice (n = 10) providing a comparable range of stimulation as the control mice, as shown in the dose-response curves in Panel (a). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 as evaluated by ANOVA followed by Dunnett’s post hoc test for multiple comparisons to a control (saline, leftmost column).
Using the results from Figure 4(a), we then quantified the nociceptive behaviors following co-injection of 0.3 nmol NMDA with saline, agmatine, MK-801, or ifenprodil in floxed control and GluN2B-KD subjects. In the floxed control subjects (n = 4–11 mice/treatment, male and female), agmatine, MK-801 and ifenprodil were all effective at reducing the numbers of nociceptive behaviors (Figure 4(b)) compared to NMDA co-injected with saline. However, in GluN2B-KD subjects injected with 0.3 nmol NMDA (n = 4–8 per treatment, male and female), none of the compounds were effective at reducing nociceptive behaviors (Figure 4(c)). While MK-801 reached a significance value of p = 0.06, the lack of significant reduction of nociceptive behaviors is likely due to a floor effect due to the limited number of behaviors expressed by GluN2B-KD subjects at 0.3 nmol NMDA; therefore, we performed this assay with 1.0 nmol NMDA (Figure 4(d)). In this assay (n = 8–10 mice/treatment, male and female), ifenprodil was ineffective at reducing the number of nociceptive behaviors in these GluN2B-KD mice as compared to mice injected with 1.0 nmol NMDA co-injected with saline. Agmatine was partially effective at reducing nociceptive behaviors; finally, MK-801 was effective at reducing nociceptive behaviors.
Post-hoc tissue analysis
The spinal cord was collected, and lumbar spinal cord was dissected from GluN2B-floxed, saline-injected control mice and AAV9-hSYN-Cre injected GluN2B-KD mice. Levels of grin2B mRNA, the gene encoding GluN2B subunits, were evaluated using RT-qPCR in both GluN2B-KD and control mice. Grin2b mRNA expression was reduced in AAV9-hSYN-Cre injected subjects as compared to saline controls (Figure 5). These results confirm reduction of spinal GluN2B in the AAV9-hSYN-Cre injected mice.
Discussion
Relief of chronic neuropathic pain remains a substantial public health concern and burden for patients, 51 and increasing concern over the long-term use of opioids has led to the call for development of non-opioid therapeutics.
Mechanism of action of agmatine in neuropathic pain relief
A key observation in these experiments is that central administration of agmatine results in the reduction of chronic neuropathic pain behavior. Most notably, Figure 1 demonstrates the reduction in chronic pain behaviors for days to weeks following the cessation of intrathecal injections of agmatine. Agmatine was delivered a minimum of 12 hours prior to sensory testing, suggesting that the impact of agmatine in this paradigm is likely due to inhibition of neuroplasticity rather than to an acute analgesic effect. The rationale for this timing of central delivery of agmatine and behavioral assessment of sensitivity thresholds is partially due to agmatine’s 12 hour half-life following central delivery. 49 In addition, we determined that agmatine delivery was sufficient to attenuate the neuroplasticity involved in development of neuropathic pain consistent with the effect of the gold standard NMDA receptor antagonist, MK-801, and greater than the magnitude of the GluN2B subunit-selective antagonist ifenprodil. It is important to note that, while agmatine attenuated the development of chronic neuropathic pain, its primary metabolite, putrescine, had no effect. This result indicates that the pharmacological effect observed results from agmatine’s action rather than from any action of its metabolic product.
Side effects assessment of NMDAr antagonism
Due to the NMDAr’s extensive history as a pharmacological target, the side effect profile of a classic NMDAr antagonist is well characterized.52–54 Motor impairment, psychotic symptoms, and memory impairment are seen in both animal modeling and clinically available NMDA antagonist therapeutics.52,54 In consideration of these established concerns, we conducted the most widely used assay for motor coordination, rotarod performance, and saw a significant increase as compared to saline controls in the time that neuropathic pain animals intrathecally injected with agmatine were able to walk and balance on the accelerating rotarod (Figure 2). In contrast to agmatine’s increase, intrathecally delivered MK-801 significantly decreased each subject’s motor coordination, as indicated by a decrease in the amount of time they were able to remain on the accelerating rotarod. In addition to time spent on the rotarod, a notable behavioral phenotype was observed where animals dropped their injured paw off of the rotarod and used only their three uninjured paws, presumably due to pain-related sparing of the injured paw. This observation aligns with recent publications seeking to characterize non-reflexive measures of pain in animal models, including voluntary wheel running, 55 grid-climbing, 56 voluntary movement such as rearing or distance traveled, 57 exploratory behavior, 58 and dynamic weight bearing. 59 Intrathecal delivery of agmatine, shown to be antihyperalgesic in evaluations of mechanical hypersensitivity as measured by von Frey stimulation, significantly increased rather than decreased the time that neuropathic animals were able to remain on the rotarod. It is probable that agmatine reduced the hypersensitivity of the paw at a resting state, leading to less pain-related sparing of the injured paw and an increase in the injured paw’s use on the rotarod, thus increasing the time spent on the rotarod as compared to the saline-injected neuropathic controls. In contrast, intrathecal delivery of MK-801 resulted in motor discoordination, a known characteristic of wide-spread NMDAr antagonism.
Development of targeted GluN2B reduction
An important component of this study was the use of a viable knockdown of the GluN2B subunit of the NMDA receptor in lumbar spinal cord while avoiding the characterized side effects of a global knockout from birth. Pharmacologically, compounds such as ifenprodil, 42 Ro25–6981, 60 and polyamines and protons 61 demonstrate selectivity for the 2B over the 2A subunits of the NMDA receptor. Probing the physiological function and relevance of the 2B subunit of the NMDA receptor has historically been difficult to resolve, as a global knockout of this gene demonstrates severe and even lethal side effects. One early study utilizing mutant mice deficient in this subunit concluded that GluN2B was essential for synaptic plasticity and neuronal pattern formation; these mice lacked a suckling response and died shortly after birth unless hand fed, 62 likely due to the absence of NMDA-mediated developmental regulation. 63 The hippocampus of these mutant mice did not respond to a standard long-term depression (LTD) protocol and lacked synaptic NMDA responses in the trigeminal nucleus, indicating the GluN2B subunit is required for development, synaptic plasticity and neuronal pattern formation such as in the formation of memory.
Advances in gene editing technology led to the development of GluN2B-floxed mice. 34 These mice were initially crossed with transgenic mice expressing CAMKII-driven Cre recombinase, which enabled the creation of mice with reduced GluN2B expression in neurons of the cortex and CA1 region of the hippocampus. Further studies utilizing these mice have demonstrated their viability for use in a site-specific knockdown of GluN2B.64,65 In order to knock down GluN2B in areas of interest to the development and maintenance of neuropathic pain and analgesic therapeutic development, we delivered by direct intrathecal injection the AAV9 virus carrying the gene for Cre-recombinase driven by a human synapsin gene 1 (hSYN) promotor. This approach enabled us to selectively knock down GluN2B in a temporally- and anatomically-restricted manner (Figure 5). The hSYN promoter drives expression of the cre-recombinase in neurons. Viral vectors delivered by direct intrathecal injection are likely to distribute to both spinal cord and dorsal root ganglion neurons. In our post-hoc tissue analysis we evaluated mRNA from extracted spinal cord, but not mRNA from dorsal root ganglia. Consequently, the decrement observed in our spinal tissue may not fully reflect the total source of the GluN2B reduction associated with the loss of agmatine effect. Additionally, we measured total GluN2B mRNA from spinal cord; AAV9-hSYN-cre construct would not impact any non-neuronal GluN2B expression, such as has been reported in astrocytes, 21 microglia or satellite glial cells. 66 We feature the data from the spinal cord, however, as proof of principle that the intrathecal delivery of the AAV9-hSYN-cre vector results in reduction of GluN2B mRNA.
Loss of GluN2B impacts nociceptive processing of intrathecal NMDA
Intrathecally delivered excitatory amino acids (EAAs) including N-methyl-D-aspartate (NMDA) are well characterized as eliciting distinct response profiles: an early-onset behavioral expression of grooming and scratching behaviors and a transient thermal hyperalgesia. 67 These behaviors can be inhibited by pre- or co-treatment with NMDA antagonists, norepinephrine or opioid agonists. 39 These documented behaviors can be used to interrogate the spinal circuitry underlying the initiation and modification of distinct nociceptive signaling. We therefore characterized the effect of intrathecally-delivered NMDA and the ability of co-administered NMDA receptor antagonists including MK-801, ifenprodil, and agmatine to inhibit these NMDA-elicited responses in both floxed control and GluN2B-knockdown mice. We first observed that an equivalent dose of NMDA elicited fewer nociceptive behaviors in GluN2B-KD mice as compared to floxed control, and that this decrease in efficacy could be overcome by increasing the intrathecal dose of NMDA (Figure 4(a)). MK-801 was able to inhibit nociceptive behaviors in both floxed control and GluN2B-KD animals, but ifenprodil was not effective at inhibiting nociceptive behaviors in GluN2B-KD animals given 1.0 nmol of NMDA. It is of interest that agmatine partially reduced the number of nociceptive behaviors in the GluN2B-KD mice given 1.0 nmol NMDA. It is noteworthy that in the cohort of floxed control animals, agmatine prevented 74% of nociceptive behaviors expressed by the group co-injected with saline + NMDA (Figure 4(b)). However, in GluN2B-KD animals that received 1.0 nmol NMDA, co-injection with agmatine only prevented 47% of nociceptive behaviors expressed by the group co-injected with saline + NMDA (Figure 4(d)). This difference, indicating that agmatine is less effective at preventing nociceptive behaviors in GluN2B-KD as compared to floxed control subjects, was found to be statistically significant by Student’s two-tailed t-test (p = 0.0017). We interpret this to be consistent with the proposal that agmatine requires GluN2B-subunits of NMDArs for its full anti-hyperalgesic effect. The partial rather than full reduction of nociceptive behaviors in the GluN2B-KD subjects may be due to agmatine’s actions at receptors other than GluN2B-containing NMDArs 68 or activity at GluN2B subunits still present in lumbar spinal cord due to the viral knockdown strategy.
Summary
These data provide integrated in vivo pharmacological evidence that agmatine preferentially antagonizes GluN2B-containing NMDA receptors. Previously reported electrophysiological studies, 33 as well as the presently reported behavioral pharmacology and molecular data all support this mechanism of action. This report also demonstrates that agmatine is effective at inhibiting maladaptive neuroplasticity induced by chronic neuropathic pain. This characteristic of agmatine, as well as its lack of side effects characteristic to NMDAr antagonism, makes it an attractive and viable structure upon which to develop therapeutics for translation to clinical use.
Footnotes
Author contributions: C.D.P. and C.A.F. conceived and designed research; E.D. contributed essential specialized mouse strains; C.D.P, K.K., H.V., and K.P. performed experiments; C.D.P. and K.P. analyzed the data; C.D.P, C.A.F., and G.L.W. interpreted results of experiments; C.D.P. and K.P. prepared the figures; C.D.P. drafted the manuscript; C.D.P., C.A.F. and G.L.W. edited and revised the manuscript; C.D.P., G.L.W. and C.A.F. approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by R01DA035931 (CAF), R01DA015438 (GLW), NIDA training grant T32-DA007097 supported CDP, and NIDA training grant T32-DA07234 supported KP. The NR2B-flox mouse was generated by the Gene-Targeted Mouse Core of the INIA-stress consortium (supported by NIH grant U01 AA013514 to ED).
ORCID iD: Cristina D Peterson https://orcid.org/0000-0001-7132-5310
References
- 1.Gomes T, Tadrous M, Mamdani MM, Paterson JM, Juurlink DN. The burden of opioid-related mortality in the United States. JAMA Netw Open 2018; 1: e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science 1994; 263: 966–969. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Ying W, Dunlap KA, Lin G, Satterfield MC, Burghardt RC, Wu G, Bazer FW. Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod 2014; 90: 84. [DOI] [PubMed] [Google Scholar]
- 4.Goracke-Postle CJ, Overland AC, Riedl MS, Stone LS, Fairbanks CA. Potassium- and capsaicin-induced release of agmatine from spinal nerve terminals. J Neurochem 2007; 102: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 5.Gibson DA, Harris BR, Rogers DT, Littleton JM. Radioligand binding studies reveal agmatine is a more selective antagonist for a polyamine-site on the NMDA receptor than arcaine or ifenprodil. Brain Res 2002; 952: 71–77. [DOI] [PubMed] [Google Scholar]
- 6.Yang XC, Reis DJ. Agmatine selectively blocks the N-methyl-D-aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J Pharmacol Exp Ther 1999; 288: 544–549. [PubMed] [Google Scholar]
- 7.Fairbanks CA, Schreiber KL, Brewer KL, Yu CG, Stone LS, Kitto KF, Nguyen HO, Grocholski BM, Shoeman DW, Kehl LJ, Regunathan S, Reis DJ, Yezierski RP, Wilcox GL. Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proc Natl Acad Sci U S A 2000; 97: 10584–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu CG, Marcillo AE, Fairbanks CA, Wilcox GL, Yezierski RP. Agmatine improves locomotor function and reduces tissue damage following spinal cord injury. Neuroreport 2000; 11: 3203–3207. [DOI] [PubMed] [Google Scholar]
- 9.Karadag HC, Ulugol A, Tamer M, Ipci Y, Dokmeci I. Systemic agmatine attenuates tactile allodynia in two experimental neuropathic pain models in rats. Neurosci Lett 2003; 339: 88–90. [DOI] [PubMed] [Google Scholar]
- 10.Regunathan S. Agmatine: biological role and therapeutic potentials in morphine analgesia and dependence. AAPS J 2006; 8: E479–E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotagale NR, Shirbhate SH, Shukla P, Ugale RR. Agmatine attenuates neuropathic pain in sciatic nerve ligated rats: modulation by hippocampal sigma receptors. Eur J Pharmacol 2013; 714: 424–431. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds IJ. Arcaine uncovers dual interactions of polyamines with the N-methyl-D-aspartate receptor. J Pharmacol Exp Ther 1990; 255: 1001–1007. [PubMed] [Google Scholar]
- 13.Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature 2005; 438: 185–192. [DOI] [PubMed] [Google Scholar]
- 14.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 2013; 14: 383–400. [DOI] [PubMed] [Google Scholar]
- 15.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 2010; 62: 405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M. Molecular diversity of the NMDA receptor channel. Nature 1992; 358: 36–41. [DOI] [PubMed] [Google Scholar]
- 17.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 1992; 256: 1217–1221. [DOI] [PubMed] [Google Scholar]
- 18.Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol 1998; 79: 555–566. [DOI] [PubMed] [Google Scholar]
- 19.Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett 2006; 399: 85–90. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain 2005; 116: 62–72. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Yan H, Li X, Liu J, Cao S, Huang B, Huang D, Wu L. Inhibition of connexin 43 and phosphorylated NR2B in spinal astrocytes attenuates bone cancer pain in mice. Front Cell Neurosci 2018; 12: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svendsen F, Tjølsen A, Hole K. AMPA and NMDA receptor-dependent spinal LTP after nociceptive tetanic stimulation. Neuroreport 1998; 9: 1185–1190. [DOI] [PubMed] [Google Scholar]
- 23.Sandkühler J, Liu X. Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci 1998; 10: 2476–2480. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen LM, Gjerstad J. Spinal cord long-term potentiation is attenuated by the NMDA-2B receptor antagonist Ro 25-6981. Acta Physiol 2008; 192: 421–427. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Cai J, Feng Z-B, Jin Z-R, Liu B-H, Zhao H-Y, Jing H-B, Wei T-J, Yang G-N, Liu L-Y, Cui Y-J, Xing G-G. BDNF contributes to spinal Long-Term potentiation and mechanical hypersensitivity via Fyn-Mediated phosphorylation of NMDA receptor GluN2B subunit at tyrosine 1472 in rats following spinal nerve ligation. Neurochem Res 2017; 42: 2712–2729. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki R, Matthews EA, Dickenson AH. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain 2001; 91: 101–109. [DOI] [PubMed] [Google Scholar]
- 27.Qu X-X, Cai J, Li M-J, Chi Y-N, Liao F-F, Liu F-Y, Wan Y, Han J-S, Xing G-G. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol 2009; 215: 298–307. [DOI] [PubMed] [Google Scholar]
- 28.Luo X-Q, Cai Q-Y, Chen Y, Guo L-X, Chen A-Q, Wu Z-Q, Lin C. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord contributes to chronic visceral pain in rats. Brain Res 2014; 1542: 167–175. [DOI] [PubMed] [Google Scholar]
- 29.Liang X, Wang S, Qin G, Zie J, Tan G, Zhou J, McBride DW, Chen Let al. Tyrosine phosphorylation of nr2b contributes to chronic migraines via increased expression of CGRP in rats. Biomed Res Int 2017; 2017: 7203458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bu F, Tian H, Gong S, Zhu Q, Xu G-Y, Tao J, Jiang X. Phosphorylation of NR2B NMDA subunits by protein kinase C in arcuate nucleus contributes to inflammatory pain in rats. Sci Rep 2015; 5: 15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci 2002; 22: 6208–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou HY, Chen SR, Pan HL. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol 2011; 4: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waataja JJ, Peterson CD, Verma H, Goracke-Postle CJ, Séguéla P, Delpire E, Wilcox GL, Fairbanks CA. Agmatine preferentially antagonizes GluN2B-containing N-methyl-D-aspartate receptors in spinal cord. J Neurophysiol 2019; 121: 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci 2010; 30: 4590–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tajerian M, Sahbaie P, Sun Y, Leu D, Yang HY, Li W, Huang TT, Kingery W, David Clark J. Sex differences in a murine model of complex regional pain syndrome. Neurobiol Learn Mem 2015; 123: 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhar SS, Wong-Riley MT. Coupling of energy metabolism and synaptic transmission at the transcriptional level: role of nuclear respiratory factor 1 in regulating both cytochrome c oxidase and NMDA glutamate receptor subunit genes. J Neurosci 2009; 29: 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunkapiller T, Kaiser RJ, Koop BF, Hood L. Large-scale and automated DNA sequence determination. Science 1991; 254: 59–67. [DOI] [PubMed] [Google Scholar]
- 38.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 1980; 67: 313–316. [DOI] [PubMed] [Google Scholar]
- 39.Aanonsen LM, Wilcox GL. Nociceptive action of excitatory amino acids in the mouse: effects of spinally administered opioids, phencyclidine and sigma agonists. J Pharmacol Exp Ther 1987; 243: 9–19. [PubMed] [Google Scholar]
- 40.Kitto KF, Haley JE, Wilcox GL. Involvement of nitric oxide in spinally mediated hyperalgesia in the mouse. Neurosci Lett 1992; 148: 1–5. [DOI] [PubMed] [Google Scholar]
- 41.Decosterd I, Allchorne A, Woolf CJ. Progressive tactile hypersensitivity after a peripheral nerve crush: non-noxious mechanical stimulus-induced neuropathic pain. Pain 2002; 100: 155–162. [DOI] [PubMed] [Google Scholar]
- 42.Gallagher MJ, Huang H, Pritchett DB, Lynch DR. Interactions between ifenprodil and the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem 1996; 271: 9603–9611. [DOI] [PubMed] [Google Scholar]
- 43.Chenard BL, Menniti FS. Antagonists selective for NMDA receptors containing the NR2B subunit. Curr Pharm Des 1999; 5: 381–404. [PubMed] [Google Scholar]
- 44.Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Curr Drug Targets 2001; 2: 285–298. [DOI] [PubMed] [Google Scholar]
- 45.Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A 1988; 85: 1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A 1986; 83: 7104–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demady DR, Jianmongkol S, Vuletich JL, Bender AT, Osawa Y. Agmatine enhances the NADPH oxidase activity of neuronal NO synthase and leads to oxidative inactivation of the enzyme. Mol Pharmacol 2001; 59: 24–29. [DOI] [PubMed] [Google Scholar]
- 48.Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J 1996; 316: 247–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts JC, Grocholski BM, Kitto KF, Fairbanks CA. Pharmacodynamic and pharmacokinetic studies of agmatine after spinal administration in the mouse. J Pharmacol Exp Ther 2005; 314: 1226–1233. [DOI] [PubMed] [Google Scholar]
- 50.Wade CL, Eskridge LL, Nguyen HOX, Kitto KF, Stone LS, Wilcox G, Fairbanks CA. Immunoneutralization of agmatine sensitizes mice to micro-opioid receptor tolerance. J Pharmacol Exp Ther 2009; 331: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Mil Med 2016; 181: 397–399. [DOI] [PubMed] [Google Scholar]
- 52.Olney JW. Neurotoxicity of NMDA receptor antagonists: an overview. Psychopharmacol Bull 1994; 30: 533–540. [PubMed] [Google Scholar]
- 53.Neznanova ON, Blokhina EA, Sukhotina IA, Bespalov AY. Motor impairment produced by ethanol and site-selective NMDA receptor antagonists in mice: tolerance and cross-tolerance. Alcohol 2000; 20: 31–36. [DOI] [PubMed] [Google Scholar]
- 54.Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx 2004; 1: 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grace PM, Strand KA, Maier SF, Watkins LR. Suppression of voluntary wheel running in rats is dependent on the site of inflammation: evidence for voluntary running as a measure of hind paw-evoked pain. J Pain 2014; 15: 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falk S, Gallego-Pedersen S, Petersen NC. Grid-climbing behaviour as a pain measure for cancer-induced bone pain and neuropathic pain. In Vivo 2017; 31: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho H, Jang Y, Lee B, Chun H, Jung J, Kim SM, Hwang SW, Oh U. Voluntary movements as a possible non-reflexive pain assay. Mol Pain 2013; 9: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu CZ, Mills CD, Hsieh GC, Zhong C, Mikusa J, Lewis LG, Gauvin D, Lee C-H, Decker MW, Bannon AW, Rueter LE, Joshi SK. Assessing carrageenan-induced locomotor activity impairment in rats: comparison with evoked endpoint of acute inflammatory pain. Eur J Pain 2012; 16: 816–826. [DOI] [PubMed] [Google Scholar]
- 59.Laux-Biehlmann A, Boyken J, Dahllöf H, Schmidt N, Zollner TM, Nagel J. Dynamic weight bearing as a non-reflexive method for the measurement of abdominal pain in mice. Eur J Pain 2016; 20: 742–752. [DOI] [PubMed] [Google Scholar]
- 60.Lynch DR, Shim SS, Seifert KM, Kurapathi S, Mutel V, Gallagher MJ, Guttmann RP. Pharmacological characterization of interactions of RO 25-6981 with the NR2B (epsilon2) subunit. Eur J Pharmacol 2001; 416: 185–195. [DOI] [PubMed] [Google Scholar]
- 61.Gallagher MJ, Huang H, Grant ER, Lynch DR. The NR2B-specific interactions of polyamines and protons with the N-methyl-D-aspartate receptor. J Biol Chem 1997; 272: 24971–24979. [DOI] [PubMed] [Google Scholar]
- 62.Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron 1996; 16: 333–344. [DOI] [PubMed] [Google Scholar]
- 63.Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature 1992; 357: 686–689. [DOI] [PubMed] [Google Scholar]
- 64.Radke AK, Jury NJ, Delpire E, Nakazawa K, Holmes A. Reduced ethanol drinking following selective cortical interneuron deletion of the GluN2B NMDA receptors subunit. Alcohol 2017; 58: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wills TA, Klug JR, Silberman Y, Baucum AJ, Weitlauf C, Colbran RJ, Delpire E, Winder DG. GluN2B subunit deletion reveals key role in acute and chronic ethanol sensitivity of glutamate synapses in bed nucleus of the stria terminalis. Proc Natl Acad Sci U S A 2012; 109: E278–E287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norcini M, Sideris A, Adler SM, Hernandez LAM, Zhang J, Blanck TJJ, Recio-Pinto E. NR2B expression in rat DRG is differentially regulated following peripheral nerve injuries that lead to transient or sustained stimuli-evoked hypersensitivity. Front Mol Neurosci 2016; 9: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aanonsen LM, Wilcox GL. Phencyclidine selectively blocks a spinal action of N-methyl-D-aspartate in mice. Neurosci Lett 1986; 67: 191–197. [DOI] [PubMed] [Google Scholar]
- 68.Piletz JE, Aricioglu F, Cheng J-T, Fairbanks CA, Gilad VH, Haenisch B, Halaris A, Hong S, Lee JE, Li J, Liu P, Molderings GJ, Rodrigues ALS, Satriano J, Seong GJ, Wilcox G, Wu N, Gilad GM. Agmatine: clinical applications after 100 years in translation. Drug Discov Today 2013; 18: 880–893. [DOI] [PubMed] [Google Scholar]