Abstract
Oxytocin (OT), a neuropeptide produced in the supraoptic (SON) and paraventricular (PVN) nuclei, is not only essential for lactation and maternal behavior but also for normal immunological activity. However, mechanisms underlying OT regulation of maternal behavior and its association with immunity around parturition, particularly under mental and physical stress, remain unclear. Here, we observed effects of OT on maternal behavior in association with immunological activity in rats after cesarean delivery (CD), a model of reproductive stress. CD significantly reduced maternal interests to the pups throughout postpartum day 1-8. On postpartum day 5, CD decreased plasma OT levels and thymic index but increased vasopressin, interleukin (IL)-1β, IL-6 and IL-10 levels. CD had no significant effect on plasma adrenocorticotropic hormone and corticosterone levels. In the hypothalamus, CD decreased corticotropin-releasing hormone contents in the PVN but increased OT contents in the PVN and SON and OT release from hypothalamic implants. CD also increased c-Fos expression, particularly in the cytoplasm of OT neurons. Lastly, CD depolarized resting membrane potential and increased spike width while increasing the variability of the firing rate of OT neurons in brain slices. Thus, CD can increase hypothalamic OT contents and release but reduce pituitary release of OT into the blood, which is associated with depressive-like maternal behavior, increased inflammatory cytokine release and decreased relative weight of the thymus.
Keywords: cytokine, hypothalamus, immunology, postpartum depression, rat
Introduction
Oxytocin (OT), a neuropeptide mainly synthesized in OT neurons in the supraoptic (SON) and paraventricular (PVN) nuclei in the hypothalamus. During parturition and suckling, OT is released into the hypothalamus from cell bodies and dendrites, and into the blood from the posterior pituitary and maternal behavior-regulating brain regions from axonal collaterals, thereby facilitating maternal behavior and lactation (Russell et al., 2001; Hatton and Wang, 2008; Hou et al., 2016). The key role of OT in the milk-ejection reflex and maternal behavior is supported by using OT receptor (OTR) antagonist in the hypothalamus that blocks activation of OT neurons (Freund-Mercier and Richard, 1984) and the resultant milk ejections during suckling (Leng and Russell, 2016) and maternal interest in the infant (Boccia et al., 2007).
Healthy maternal behavior depends on immune homeostasis. Peripartum stress can disrupt maternal behavior (Bystrova et al., 2009; Dumas et al., 2013; Liu et al., 2019) in association with maternal inflammation (Gustafsson et al., 2018). OT regulates development of the thymus and bone marrow, and participates in immune surveillance, defense and homeostasis (P. Wang et al., 2015; Y. F. Wang, 2016). For example, OT significantly reduced lipopolysaccharide-induced release of interleukin (IL)-1β, and IL-6 and acute lung injury in mice, which can be blocked by pretreatment with OTR antagonist (An et al., 2019). While these facts support a pivotal role of activating OT neurons in maternal behavior and immunological activities, how OT modulates maternal behavior and immunity at same time remains unclear.
By contrast, immunological diseases can change the activity of OT neurons. For example, human immunodeficiency virus reduces OT immunoreactivity in hypothalamic neurons (Langford et al., 2011). Both the maternal behavior- and immune-regulating functions of OT are associated with activities of the hypothalamic-pituitary adrenal (HPA) axis (T. Li et al., 2017) that can suppress maternal behaviors and cause depression (Klampfl et al., 2018). However, interactions between OT and immune system as well as their influence on maternal behavior during lactation remain poorly understood. For example, persistent social stress causes OT increase in the hypothalamus but disrupts normal maternal behavior and lactation (D. Li et al., 2020, 2021). The responses of HPA axis to various stresses are reduced markedly during lactation (Smotherman et al., 1976) and OT levels are negatively correlated with the symptom of postpartum depression among women (Thul et al., 2020). However, it was also reported that in breastfeeding women with depressive-like symptoms, there is a positive correlation between OT and corticosterone levels (Cox et al., 2015). Thus, it is important to investigate effects of social stress around parturition on maternal behaviors and OT neuronal activity in association with immunological activities.
To answer these questions, we prepared a rat model of cesarean delivery (CD) that has a high prevalence among pregnant women globally (Bateman et al., 2016; Liang et al., 2018) and causes higher incidence of postpartum depression and central inflammation (Miller et al., 2019). Using this stress model, we investigated the involvement of OT neurons in CD-associated aberrant maternal behavior and immunological activities during lactation.
Materials and Methods
All procedures in this study were carried out in accordance with the guidelines on the Use and Care of Laboratory Animals set by National Institutes of Health and were approved by the Institutional Animal Care and Use Committees of Harbin Medical University (No. 31471113).
Preparation of CD rats and sampling
Adult Sprague-Dawley rats were purchased from the animal center of the second affiliated hospital of Harbin Medical University and housed in 21-23°C with free access to water and food. Pregnant rats were randomly divided into natural labor (control and foster dams) and CD groups. CD was performed in the morning of gestational day 19-21. Conventional CD operation (J. Wang et al., 2010) was performed under chloral hydrate anesthesia (10%, 0.3 mL/100 g, i.p.). In brief, the laparotomy was performed under sterile condition. A 3 cm long incision at the midline was made in the low abdomen to expose the uteri, and then longitudinal incision of 1 cm was made along the anti-mesenteric side in the middle portion of each uterine horn. Pups and placentas were extruded out gently. Uterine, muscles and skin were sutured layer by layer and 2% tincture of iodine was pasted on the wound. Postsurgical care was applied to keep body temperature at ~37 °C. Dams were allowed to access water and food freely. Given the fact that premature newborns were less efficient suckers, to ensure that this factor did not affect outcomes, a litter of 10 pups fostered by control dams at the same age (70.9 ± 2.3 g in control; 72.1 ± 4.2 g in CD) was given to the CD dams when the dams recovered from anesthesia.
The blood, SON, PVN, thymus and spleen of the dams were sampled on the morning on the 5th day after labor or CD operation. Among them, trunk blood (0.5-1.0 mL) was collected during decapitation with heparinized tubes, the plasma was separated by centrifugation (3000 rpm, 4°C, 15 min), and plasma aliquots were stored at −20°C. The brain was dissected in ice-cold artificial cerebrospinal fluid (aCSF) and then fixed in 4% paraformaldehyde for 72 h. The hypothalami were then sectioned into 50 µm-thick slices with a microtome and prepared for immunohistochemistry. Hypothalamic tissues were stored at −80°C for Western blot and assay of hormone release. The relative weights (or index) of the thymus and the spleen were calculated by dividing the weight (mg) of thymus and spleen by the body weight (g).
Observation of maternal behavior
Maternal behaviors are different from common social behaviors. Maternal behaviors are a set of behaviors of the mother towards the offspring including nest building, retrieval, contact, nursing, anogenital licking and maternal attack, etc. A reduction in maternal interest toward the pups is the core sign of maternal depression, particularly reduced suckling and anogenital licking (Pedersen and Boccia, 2002; D. Wang et al., 2007). In this study, maternal behaviors of dams were evaluated on the morning at postpartum day 1, 4 and 8 as previously described (Liu et al., 2019). Observations included the latency of pups’ retrieval and anogenital licking, and the number of anogenital licking throughout 1 h after 1 h separation from the pups to let pups eager to sucking.
Assaying hormone and cytokine levels
Assaying plasma levels of different hormones and cytokines mainly performed by Beijing Sino-UK Institute of Biological technology (Beijing, China) using enzyme-linked immunosorbent assay (ELISA). The average recovery rates were > 96% in all assays. The sensitivities for OT, vasopressin, adrenocorticotropic hormone (ACTH), corticosterone, IL-1β, IL-6, and IL-10 were < 0.9 pg/mL, < 0.5 pg/mL, < 0.4 pg/mL, < 0.5 ng/mL, < 0.5 pg/mL, < 3 pg/mL, and < 0.5 pg/mL, respectively. Intra-group and inter-group variations were less than 5% and 9%, respectively.
In assaying OT and CRH levels in the SON and PVN of dams, the SON and PVN were dissected separately from control/natural delivery and CD dams and then transferred into the aCSF (100 μL) in the presence of protease inhibitors (P8340, 1:100, Sigma, Shanghai, China, MDL number: MFCD00677817). The tissues were homogenized, insoluble components were removed through centrifugation, and the supernatants were collected. The supernatant was then frozen until assay. The assay was performed using kits from ImmunoClone (product of I&C, USA and distributed by YBio, Shanghai, China) including a general OT assay kit (IC-OT-Ge) and a rat CRH assay kit (IC-CRH-Ra) as instructed by the manual, which is similar to our previous publication (D. Li et al., 2020). The minimum detectable limit was 5.8 pg/mL for OT and 4.58 pg/mL for CRH, respectively.
The methods of assaying extracellular OT levels in the incubation of the hypothalamus were the same as previously described (D. Li et al., 2020). That is, hypothalamic tissue blocks (4 × 2.5 × 2.5 mm3, ∼20 mg, containing two SONs and two PVNs and their associated accessory nuclei) were dissected from control and CD dams and then separately incubated in aCSF (305 mOsm/kg, 200 μl, 35 °C, 1 h) in the presence of protease inhibitors (0.5x from a 100x stocking solution, P8340, Sigma) and 12 mM KCl. The aCSF contained (in mmol/L) 126 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.3 NaH2PO4, 20 NaHCO3, 10 glucose, 305 mOsm/kg, pH 7.4, gassed with a mixture of 5% CO2 and 95% O2. The solution was then frozen until assay as described above.
Western blots
The methods of Western blots were the same as previously reported (Y. F. Wang et al., 2013). In brief, extracted protein from SON lysate was separated on 10% SDS-PAGE gels and transferred onto PVDF membranes. Protein membranes were pretreated with a blocking buffer containing 5% bovine serum albumin (w/v in TBS) for 1.5 h at room temperature (21-23°C), and then incubated with antibodies against target proteins at 4°C overnight. The primary antibodies were c-Fos antibody (Omnimabs, Alhambra, CA, OM25693, rabbit, 1:1000) and antibody against glyceraldehyde 3-phosphate dehydrogenase (OM254102, rabbit, 1:1500, loading control). The primary antibodies were further detected with HRP-conjugated secondary antibodies for 2 h; protein bands were visualized with an automated ECL imaging analysis system (Tanon 5200, Shanghai, China).
Immunohistochemistry
The methods of fluorescent immunostaining were the same as previously described (Y. F. Wang and Hatton, 2009). Briefly, the hypothalamic sections were permeabilized with 0.3% Triton X-100 for 60 min and then 0.3% gelatin for 60 min to block non-specific binding sites. Sections were incubated at 4°C overnight with the presence of primary antibodies against OT-neurophysin (OT-NP, MAbN844, mouse, 1:3000, Merck Millipore, RRID: AB_2315026, Shanghai, China), and vasopressin-neurophysin (VP-NP, sc-27093, goat, 1:200, RRID: AB_2061964), and c-Fos (OM25693, rabbit, 1:1000). The primary antibodies were further detected with donkey anti-mouse IgG (Alexa Fluor® 555, ab150106, RRID: AB_2857373 or Alexa Fluor® 647, ab150107), donkey anti-rabbit IgG (Alexa Fluor® 488, ab150073, RRID: AB_2636877) and Alexa Fluor® 488-conjugated Streptavidin (35103ES60, Yeasen, Shanghai, China), respectively. Finally, Hoechst (bisbenzimide, B2261, 0.25 µg/mL, Sigma, CAS# 28718-90-3, Shanghai, China) was applied to label the nuclei. Sections were examined with a fluorescence microscope (Eclipse FN1, Nikon, Tokyo, Japan) through a charge-coupled device camera (DS Ri2, Nikon, Tokyo, Japan) or a confocal microscope (Eclipse Ti, Nikon, Tokyo, Japan).
Patch-clamp recordings
The methods of whole-cell patch-clamp recordings were the same as previously described (Y. F. Wang and Hatton, 2004). In brief, 300 µm thick coronal brain slices containing the SON were cut after decapitating the dam rats on the morning of the 5th day after labor and CD operation. Whole-cell patch-clamp recordings were obtained from the soma of magnocellular neurons in the SON under visual guidance of an upright microscope (Eclipse FN1, Nikon, Tokyo, Japan) through an infrared charge-coupled device camera (G-2358 ASG, Manta, German) during perfusion of aCSF. The aCSF contained (in mM): 126 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.3 NaH2PO4, 26 NaHCO3, 10 Glucose, 305 mEq/Kg water, pH 7.4, and gassed with a mixture of 95% O2 and 5% CO2. Patch-pipette filling solution contained the following components (in mM): 145 K-gluconate, 10 KCl, 1MgCl2, 10 HEPES, 1 EGTA, 0.01 CaCl2, 2 Mg-ATP, 0.5 Na2-GTP, 295 mEq/Kg water, pH 7.3 adjusted with KOH. Biotin (B4261, Sigma, Shanghai, China, PubChem Substance ID: 24891762) was added to the pipette solution for identification of the chemical nature of recorded SON neurons. A 700B amplifier (Molecular Devices, CA, USA) was used for collecting electrical signals that were filtered and sampled at 5 kHz by Clampex 10 software through an analogue-to-digital converter (1550, Molecular Devices, CA, USA). The parameters that were evaluated included the resting membrane potential (RMP), firing rate, spike amplitude (Ampl), spike width at 50% amplitude, spike rise time constant (Tau) and decay time constant, respectively.
Data collection and analysis
Analyses of behaviors, Western blots, imaging and patch-clamp recordings have been described in previous reports (D. Li et al., 2020). In immunohistochemistry, the number of c-Fos-positive cells and OT neurons was counted only in the SON somatic area excluding the ventral glial lamina wherein astrocytic somata are located. The c-Fos-positive neurons were counted from those cells that had c-Fos puncta overlapped with or surrounding a “neuronal nucleus” while OT neurons had a diameter ≥20 µm. The membrane potential was recorded without any clamping current initially.
Statistical analyses were performed using SigmaStat program (SPSS19, Chicago, IL, USA). Chi-square test was used for comparison of the rate; Student’s t-test or Welch's t-test (for data of unequal variances), Mann-Whitney test (for non-normally distributed data) and paired t-test were used for comparisons between two groups. P < 0.05 was considered significant. All measures were expressed as mean ± SEM in actual value or in percentage.
Results
Effects of CD on Maternal Behavior
Maternal behavior reflects maternal activities oriented to the care of offspring. Looking for the newborns and anogenital licking can largely represent maternal interest in rat dams toward the pups and thus, we observed the retrieval and anogenital licking behaviors at postpartum day 1, 4 and 8, respectively. The latency of pup retrieval among CD dams showing retrieval behavior was longer than controls at day 1 (335.9 ± 81.4 s, n = 8 in CD vs. 55.8 ± 21.8 s, n = 19 in control, P < 0.001 by Mann-Whitney Test) and at day 4 (240.6 ± 103.4 s, n = 14 in CD vs. 13.0 ± 3.4 s, n = 18 in control, P < 0.001 by Mann-Whitney Test).
Among CD dams showing licking behavior, the latency of anogenital licking was significantly longer than the controls at day 1 (912.8 ± 264.3 s, n = 10 in CD vs. 203.4 ± 44.4 s, n = 19 in control, P = 0.006 by Mann-Whitney Test). The latency decreased gradually in CD dams from day 1 to day 8 (Figure 1B). However, the frequency or numbers of licking in the CD dams (Figure 1C) became significantly lower than those in the controls throughout the observations (4.4 ± 1.4, n = 17 in CD vs. 8.6 ± 1.6, n = 19 in control at day 1, P = 0.026 by Mann-Whitney Test; 6.0 ± 1.1, n = 17 in CD vs. 9.9 ± 1.2, n = 19 in control at day 4, P = 0.032 by Mann-Whitney Test; 5.1 ± 1.1, n = 16 in CD vs. 17.5 ± 2.2, n = 17 in control at day 8, P < 0.001 by Welch's t-test). Consistently, the average latency of suckling in CD dams was significantly longer than that in control dams at day 4 (565.9 ± 103.6 s, n = 13 in CD vs. 235 ± 26.5 s, n = 15 in control, P = 0.0082 by t-test, Figure 1D). In addition, maternal body weight gain in CD dams (1.2 ± 3.3 g, n = 8) was significantly lower than the control (25.2 ± 8.5 g, n = 6, P= 0.037 by t-test) measured at day 8.
Figure 1.
Effects of cesarean delivery (CD) on Maternal Behavior. Graphs with box-whiskers and scattered plots summarizing the latency of pup retrieval (A), latency (B) and number (C) of anogenital licking, and latency of suckling (D), respectively. Note that: *, P < 0.05, **, P < 0.01 vs. control (CTR) by t-test or Mann-Whitney Test.
Effects of CD on Plasma Hormones and Inflammatory Cytokines
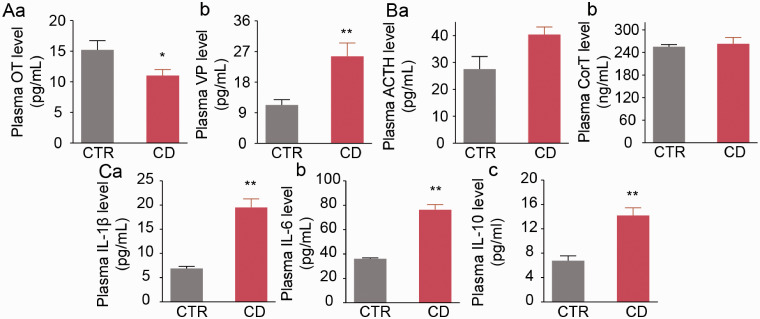
To establish the association of CD-modulated maternal behavior with immune-neuroendocrine activity, the activity of HPA axis and immunologic activities, we further assayed plasma levels of OT, vasopressin, ACTH, corticosterone, IL-1β, IL-6 and IL-10 at day 5. Selection of this time point was because the latency of retrieval and number of licking in CD dams have significant difference from control dams around this time and pro-inflammatory and anti-inflammatory cytokines are released in high levels in general surgery (Zubaidi et al., 2010; Alzoghaibi et al., 2014). Thus, these indices can reflect the immune-neuroendocrine status of the CD dams when depressive-like behavior occurs. As shown in Figure 2, plasma OT concentration was lower in CD group than control group (11.0 ± 1.0 pg/mL, n = 6 in CD vs. 15.2 ± 1.5 pg/mL, n = 6 in control, P = 0.038 by Student's t-test; Figure 2Aa). By contrast, CD significantly increased plasma vasopressin concentrations (25.6 ± 3.9 pg/mL, n = 5 in CD vs. 11.3 ± 1.5 pg/mL, n = 8 in control, P = 0.002 by Student's t-test; Figure 2Ab). Moreover, CD did not affect plasma ACTH levels (40.4 ± 2.8 pg/mL, n = 5 in CD vs. 27.5 ± 4.7 pg/mL in control, n = 7, P = 0.060 by Student's t-test; Figure 2Ba) or corticosterone (263.3 ± 16.7 ng/mL, n = 5 in CD vs. 255.3 ± 5.9 ng/mL, n = 7 in control, P = 0.617 by Student's t-test; Figure 2Bb). Lastly, CD significantly increased plasma levels of IL-1β (19.5 ± 1.8 pg/mL, n = 5 in CD vs. 6.9 ± 0.5 pg/mL in control, n = 8, P = 0.002 by Welch's t-test; Figure 2Ca), IL-6 (76.4 ± 4.2 pg/mL, n = 5 in CD vs. 36.1 ± 0.7 pg/mL, n = 8 in control, P = 0.0005 by Welch's t-test; Figure 2Cb) and IL-10 (14.2 ± 1.3 pg/mL, n = 5 in CD vs. 6.8 ± 0.8 pg/mL, n = 8 in control, P < 0.001 by Student’s t-test; Figure 2Cc).
Figure 2.
Effects of CD on Plasma Neurohypophysial Hormones, adrenocorticotropic hormone (ACTH), corticosterone (CorT) and Inflammatory Cytokine Levels. A: Enzyme-linked immunosorbent assay (ELISA) of oxytocin (OT, a) and vasopressin (VP, b) levels. B: ACTH (a) and CorT (b) levels. C: Interleukin (IL)-1β (a), IL-6 (b) and IL-10 (c) levels. Other annotations are the same as Figure 1.
Effects of CD on Thymic Index
We compared the relative weight of immune organs between control and CD dams. The result showed that thymic index decreased significantly in CD dams (0.50 ± 0.17, n = 4 in CD vs. 0.72 ± 0.13, n = 4 in control, P = 0.03 by Paired t-test); however, there was no significant change in the splenic index (1.68 ± 0.28, n = 4 in CD vs. 1.44 ± 0.23, n = 4 in control, P = 0.52 by Paired t-test). Notably, there is moderate correlation between litter’s body weight gain in 8 h suckling and thymic index without considering the groups (r = 0.592, n = 15, P = 0.03) although there was no correlation between dams body weight and thymic index (r = 0.023, n = 32, P = 0.901).
Effects of CD on Hypothalamic CRH and OT Contents
Inflammatory reaction is closely associated with the activity of HPA axis and OT functions (T. Li et al., 2017). Thus, we further analyzed the expression of CRH in the PVN at day 5. CD significantly reduced CRH levels in the PVN (2.8 ± 0.1 pg/mL, n = 3 in CD vs. 7.6 ± 0.4 pg/mL, n = 4 in control, P = 0.047 by Student's t-test; Figure 3A).
Figure 3.
Effects of CD on corticotrophin-releasing hormone (CRH) and OT Levels in the Hypothalamic Implants. ELISA of CRH in the paraventricular nucleus (PVN, A), OT in the supraoptic nucleus (SON, B), in the PVN (C) and in the medium of hypothalamic incubation (D) at day 5. HIM, hypothalamic incubation medium; other annotations are the same as Figures 1 and 2.
To determine potential involvement of OT in the effect of CD, we further assayed OT levels in the hypothalamus. In the SON, OT level was higher in CD dams than control dams (213.8 ± 46.1 pg/mL, n = 6 in CD vs. 93.7 ± 15.6 pg/mL, n = 10 in control, P = 0.01 by Student’s t-test; Figure 3B). In the PVN, CD dams also had higher level of OT (52.0 ± 7.6 pg/mL, n = 6 in CD vs. 28.7 ± 3.7 pg/mL, n = 9 in control, P = 0.009 by Student's t-test; Figure 3C).
The presence of OT in the SON and PVN may not represent their release into the hypothalamus. Thus, we measured OT levels in the incubation medium in the presence of 12 mM K+ and protease inhibitor for 1 h with ELISA. The concentration of OT in hypothalamus increased in CD group compared to the control (37.9 ± 2.4 pg/mg in CD, n = 6 vs. 14.2 ± 0.9 pg/mg in control, n = 6; P < 0.001 by Student’s t-test; Figure 3D).
Effects of CD on c-Fos Expression in the SON
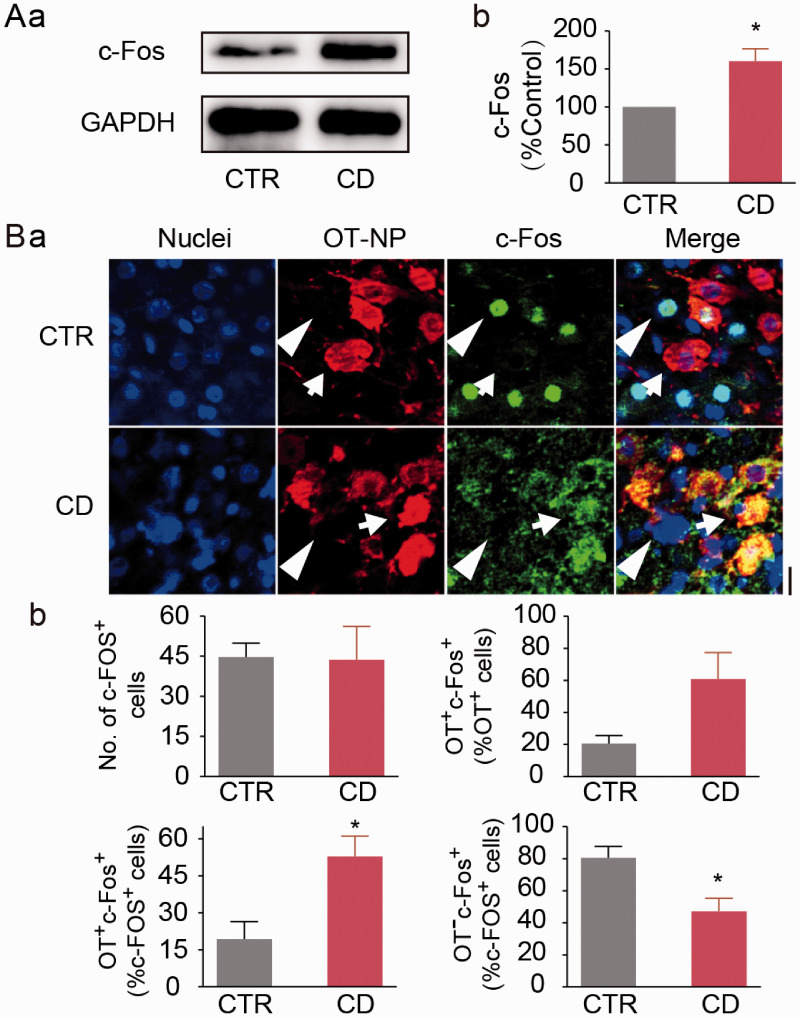
Increased c-Fos expression represents increase in OT production (Jimenez and Gonzalez-Mariscal, 2019). Hence, we analyzed the effects of CD on c-Fos expressions in the SON. In Western blots, CD significantly increased c-Fos protein levels (160.2 ± 16.4% of the control, n = 6 in CD, P = 0.014 to control by Paired t-test, Figure 4A). In immunohistochemistry (Figure 4B), there was no significant difference between CD and control dams in the total number of c-Fos-positive neurons in the SON (43.7 ± 12.4%, n = 3 in CD vs. 44.7 ± 5.2%, n = 3 in control, P = 0.945 by Student's t-test). In the SON of control dams, c-Fos protein was mainly present in the nuclei of OT neurons; however, c-Fos protein in CD dams mainly accumulated in cytosolic compartments of OT neurons in the SON (Figure 4Ba). Further analyzing cell type-associated c-Fos distribution (Figure 4Bb) revealed that CD significantly increased the percentage of OT neurons among c-Fos-positive cells relative to their controls (52.9 ± 8.2%, n = 3 in CD vs. 19.4 ± 7.0%, n = 3 in control, P = 0.036 by Student's t-test), and decreased the percentage of OT-negative neurons (i.e. vasopressin neurons) among c-Fos-positive cells (47.1 ± 8.2%, n = 3 in CD vs. 80.6 ± 7.0%, n = 3 in control, P = 0.036 by Student's t-test).
Figure 4.
Effects of CD on c-Fos Expression in the SON. A: Representative Western blotting bands (a) and the summary graph (b) showing c-Fos protein expression at day 5. B: Representative fluorescent images of immunohistochemistry showing nuclei (in blue), OT neurophysin (OT-NP, in red), c-Fos (in green), and their merges (a) and the summaries (b). Arrows and arrowheads point to OT and putative VP neurons, respectively. The scale bar equals to 20 µm. Other annotations are the same as Figures 1 and 2.
Effects of CD on the Electrical Activity of SON Neurons
The increased hypothalamic and decreased plasma OT levels suggest differential secretory activities of somatodendritic site from neural terminals of OT neurons (D. Li et al., 2020). Thus, we made whole-cell patch-clamp recordings of OT neurons in the SON in brain slices taken from dams at day 5 to determine the reason for this differential secretory activity (Figure 5). Compared to the control, CD significantly depolarized the membrane potential of OT neurons (-42.6 ± 1.9 mV, n = 13 in CD vs. -52.0 ± 2.5 mV, n = 10 in control, P = 0.007 by Student's t-test) and increased spike width measured at 50% amplitude (2.9 ± 0.3 ms, n = 14 in CD vs. 2.0 ± 0.2 ms, n = 8 in control, P = 0.040 by Student's t-test). The variation in the firing rate in CD dams was relatively high although there was no significant difference between the two groups (2.9 ± 0.7 Hz, ranging 0.003-8.287 Hz, n = 13 in CD vs. 2.0 ± 0.5 Hz, ranging 0.027-5.303 Hz, n = 10 in control, P = 0.347 by Student's t-test).
Figure 5.
Effects of CD on the Electrical Activity of OT Neurons. A: Exemplary firing episodes (a) and the features of spikes (b, control in black, CD type I in blue and CD type II in red) of OT neurons. B: Bar-graphs summarizing the resting membrane potential (RMP), firing rate, spike amplitude (Ampl), width at 50% amplitude, rise time constant (Tau) and decay time constant, respectively. The inset is a set of representative images of post hoc identification of OT neuron labeled with biotin. Other annotations refer to Figure 1 and 4.
Discussion
In the present study, we found that CD can cause depressive-like maternal behavior. This effect is likely due to increase in hypothalamic OT contents and decrease in blood OT levels. The differential central and peripheral effects of CD on OT secretion are likely because of over-excitation and the subsequent inhibition. The reduction of plasma OT levels is associated with increased blood IL-1β and reduced thymic index. Hypothalamic OT likely suppresses CRH production; however, effect of CRH on its downstream ACTH is likely neutralized by increased vasopressin secretion. The disorders in OT neuronal activity and inflammatory reactions in CD dams possibly contribute to postpartum depression.
Effects of CD on Maternal Behavior and the Activity of HPA Axis
Maternal behavior in mammals manifests as maternal care of the offspring and suckling (Pérez-Torrero and Rubio-Navarro, 2015). The delayed suckling, reduced anogenital licking in CD dams and poor dams’ body weight gain indicate the occurrence of depressive-like maternal behavior as shown in rat dams separated from their newborns (Liu et al., 2019; D. Li et al., 2020, 2021). CD dams partially recover from the depression as shown in the shortening of latencies of the retrieval, anogenital licking and suckling; however, the number of licking, a core sign of maternal care (D. Wang et al., 2007), remains significantly lower than the control dams. This finding is consistent with previous reports in women (Miller et al., 2019; Hompoth et al., 2020). Since intranasal application of OT reversely increased anogenital licking in CD dams and acinar area of the mammary glands by changing OT neuronal activity and OT secretion (Y. F. Wang et al., 2018), these depressive signs in CD dams are not a simple effect of surgical stress. They together indicate that CD can cause postpartum depression.
To establish maternal depression in CD dams, it is necessary to identify the difference between CD and general surgery. Major depressive disorder is associated with postoperative pain, infections, progression of malignant tumors, poor health-related quality of life as well as other complications (Ghoneim and O'Hara, 2016). In obstetrics, hysterectomy with bilateral salpingo-oophorectomy in women may also cause depression (Mantani et al., 2010), largely because of estrogen deficiency (Parker et al., 2009). However, CD women usually do not have pre-existing depression-associated complications and need no removal of the uteri and ovaries. The key factor is that preterm delivery through surgical approach interrupts normal maturation of OT secretion machinery (Russell et al., 2001; Hou et al., 2016), and thus delays the generation of OT pulsatility (Hatton and Wang, 2008). Thus, although acute postoperative pain may contribute to the postpartum depression, the extensive presence of maternal depressive-like signs in CD dams after CD dams recovered from surgery is inherent to the parturition physiology.
The partial recovery of maternal behavior is likely due to the intimate contacts of dams with pups (Q. Jiang and Wakerley, 1997) as well as the maturation of OT secretory machinery (Albin-Brooks et al., 2017) following dams’ recovery from surgical stress. The remained lower maternal interest to the offspring is consistent with the lower plasma OT levels in CD dams, suggesting insufficient recovery of OT neuronal activity.
In addition to the reduced OT actions, CRH can be another disrupting factor of normal maternal behavior (Klampfl and Bosch, 2019). However, it is not likely that an increased activity of HPA axis is responsible for CD-evoked postpartum depression since CD reduced CRH release in the hypothalamus but did not increase CRH, ACTH and corticosterone. Although we cannot exclude the possibility that HPA axis activity increases transiently at the very early postpartum stage in CD dams, the intra-hypothalamic action of the HPA axis decreases in CD dams at day 5. Together with the general view that the activity of HPA axis is blunted during lactation (Smotherman et al., 1976), we tentatively believe that CD-evoked postpartum depression is not result from the activation of HPA axis that occurs in major depression (Holsen et al., 2013).
Effects of CD on Maternal Behavior and Activity of OT Neurons at Different Compartments
Maternal behavior is regulated by many brain areas including the parvocellular division of the PVN, olfactory bulb, the pyriform cortex, the medial amygdala (MeA), bed nucleus of the stria terminalis, the nucleus accumbens, the medial prefrontal cortex and others (Renier et al., 2016; Jurek and Neumann, 2018). Notably, the OT-secreting system plays a coordinated role in the prevention of postpartum depression. For instance, intranasal OT administration can alleviate postpartum depression by activation of OT neurons (Liu et al., 2019) and inhibition of the amygdala (Liu et al., 2017) that exerts an inhibitory role in maternal behavior (Gao et al., 2018). Thus, increased OT neuronal activity provides tonic inhibition of maternal depression.
As stated in the Introduction, OTR blockade in the hypothalamus disrupts suckling evoked burst firing in OT neurons, milk ejections and maternal behavior; however, OTR blockade on intra-hypothalamic OT secretion remains unknown. The prolonged increase in hypothalamic OT in CD dams could inhibit the ability of OT neurons to release OT in bolus or discharge action potentials in burst and maternal depression after shortly increasing OTR activation and the milk-ejection reflex in a short period of time as observed in rat dams separated from their offspring (D. Li et al., 2020). Thus, when maternal depression occurs in CD dams, the OTR should be inhibited although it remains to be verified in future.
In CD dams, OT contents in the SON and PVN increased significantly, which was accompanied with the reduction of circulating OT levels. This paradoxical phenomenon has been identified in rat dams that are separated from their pups (D. Li et al., 2020, 2021) and likely results from differential secretion of OT from somatodendritic sites and from axonal terminals. This proposal is consistent with the finding that using thapsigargin to deplete intracellular Ca2+ store does not block OT-evoked burst firing in OT neurons (Y. F. Wang and Hatton, 2007) although it blocks somatodendritic K+-evoked OT release (Ludwig et al., 2002). The increase in hypothalamic OT contents is also in agreement with the increased c-Fos expression among OT neurons. This is supported by the depolarization and spike broadening that can result from activation of OTR (Y. F. Wang et al., 2006). Moreover, that CD increased plasma vasopressin levels but reduced c-Fos-positive vasopressin neurons supports that biochemical activity of intrahypothalamic neuroendocrine neurons can be separated from spike-driven neurosecretion from neural terminals (Dayanithi et al., 2012).
It is likely that pain stimulation in CD dams increases brain OT levels (W. Q. Jiang et al., 2019; Nishimura et al., 2019) while OT exerts analgesic role in CD injury likely by acting on the periaqueductal gray area and the spinal cord (Xin et al., 2017). The later effect likely accounts for why the incidence of persistent pain after CD is much lower than after comparable surgeries (Sun and Pan, 2019). In view of OT neuronal activity, persistent increase in OT levels could cause persistent inhibition of the firing activity, particularly burst firing (Y. F. Wang and Hatton, 2007). As shown in patch-clamp recording, while a few cells showed higher firing rate, more OT neurons showed lower firing rate in CD dams, which makes activities of different OT neurons de-coordinated and thus reduces OT release from neural terminals as previously discussed (Hatton and Wang, 2008; Hou et al., 2016). As a result, firing-driven OT release from neural terminals is reduced in the posterior pituitary and in maternal behavior-regulating brain regions, thereby disrupting maternal behavior.
OT Regulation of Immunity and Maternal Behavior
In the present study, CD dams showed increases in both the pro-inflammatory cytokine IL-1β and IL-6 and the anti-inflammatory cytokine IL-10 at day 5. This is similar to a previous report that IL-1β expression upregulated with peak at days 3 to 7 in small bowel and colonic wounds where IL-10 showed high expression at day 5 in rats (Zubaidi et al., 2010; Alzoghaibi et al., 2014). Thus, CD-evoked changes in these immune cytokines could reflect the general response of the immune system to surgical stress. However, the changes in thymic index are beyond the effect of general surgery.
Mechanistically, CD-evoked abnormal OT secretion can disrupt the immunological defense functions of OT (P. Wang et al., 2015; Y. F. Wang, 2016). This is supported by the findings that OTR deficiency mice are more susceptible to inflammatory reactions compared with the wild mice (Tang et al., 2019) and that blocking OTR reduces endotoxin-evoked IL-1β release (An et al., 2019). It is consistent with that disruption of maternal behavior is associated with reproductive injury (i.e. perinea lacerations), inflammation, and stress (Dunn et al., 2015). In addition, increased plasma vasopressin could also contribute to the increase in IL-6 since stimulation that increases vasopressin secretion also increases IL-6 levels (Rohleder et al., 2006). Thus, the increased IL-1β and IL-6 levels in CD dams result from reduced plasma OT and increased vasopressin.
Along with increases in inflammatory cytokines, CD also influences adaptive immunity as indicated by the reduction of thymic index. These findings are in agreement with the reports that OT can act on OTR on the thymus to promote the differentiation of T-lymphocytes (Hansenne et al., 2004) and activate T-lymphocytes (Ndiaye et al., 2008). This action occurs at the peripheral sites since CD-reduced plasma OT was accompanied with increase in hypothalamic OT that should inhibits inflammatory reaction in the hypothalamus. Taken together, we tentatively believe that OT is an essential humoral factor regulating immunity and maternal behavior (Figure 6).
Figure 6.
Summary Diagram of the Effects of CD on OT Neuronal Activity and Its Association With Maternal Behavior and Immunity. A-B: Showing effects of natural delivery (A) and CD (B) at the levels of the hypothalamo-neurohypophysial system (a) and OT neurons (b), respectively. Abbreviations: ACTH, adrenocorticotropic hormone; CTR, control; CD, cesarean delivery; CRH, corticotrophin-releasing hormone; IL, interleukin; OT, oxytocin; Pit, pituitary; PVN, paraventricular nucleus; SON, supraoptic nucleus; VP, vasopressin.
Implications
This study for the first time highlights the immune-neuroendocrine mechanisms underlying CD-evoked postpartum depression. It reveals that abnormally increased hypothalamic OT secretion is a key for CD-associated maternal depression, release of inflammatory cytokines and decrease in lymphocytes. Many of the present findings remain to be further verified in a more specific condition, which is important not only for treatment of abnormal maternal behaviors but also for a variety of diseases with immune disorders like COVID-19 (S. C. Wang and Wang, 2021).
Acknowledgments
We thank Dr. Runsheng Jiao for advice, Jiawei Yu, Hongyang Wang, Shuo Ling, Yunhao Jiang and Guichuan Chen for critical reading.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant No. 31471113, YFW), the fund of “Double-First-Class” Construction of Harbin Medical University (key laboratory of preservation of human genetic resources and disease control in China).
ORCID iD: Yu-Feng Wang https://orcid.org/0000-0001-8543-8906
References
- Albin-Brooks C., Nealer C., Sabihi S., Haim A., Leuner B. (2017). The influence of offspring, parity, and oxytocin on cognitive flexibility during the postpartum period. Horm Behav, 89, 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzoghaibi M. A., Al-Oraini A. I., Al-Sagheir A. I., Zubaidi A. M. (2014). Temporal expression of IL-1beta and IL-10 in rat skin, muscle, small bowel, and colon wounds: A correlative study. Journal of Basic and Clinical Physiology and Pharmacology, 25, 205–210. [DOI] [PubMed] [Google Scholar]
- An X., Sun X., Hou Y., Yang X., Chen H., Zhang P., Wu J. (2019). Protective effect of oxytocin on LPS-induced acute lung injury in mice. Sci Rep, 9, 2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman B. T. Franklin J. M. Bykov K. Avorn J. Shrank W. H. Brennan T. A. Landon J. E. Rathmell J. P. Huybrechts K. F. Fischer M. A., Choudhry N. K. (2016). Persistent opioid use following cesarean delivery: Patterns and predictors among opioid-naive women. Am J Obstet Gynecol, 215, 353 e351–353 e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia M. L. Goursaud A. P. Bachevalier J. Anderson K. D., Pedersen C. A. (2007). Peripherally administered non-peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: A new pharmacological tool for the study of social motivation in non-human primates. Horm Behav, 52, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrova K., Ivanova V., Edhborg M., Matthiesen A. S., Ransjo-Arvidson A. B., Mukhamedrakhimov R., Uvnas-Moberg K., Widstrom A. M. (2009). Early contact versus separation: Effects on mother-infant interaction one year later. Birth, 36, 97–109. [DOI] [PubMed] [Google Scholar]
- Cox E. Q., Stuebe A., Pearson B., Grewen K., Rubinow D., Meltzer-Brody S. (2015). Oxytocin and HPA stress axis reactivity in postpartum women. Psychoneuroendocrinology, 55, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanithi G., Forostyak O., Ueta Y., Verkhratsky A., Toescu E. C. (2012). Segregation of calcium signalling mechanisms in magnocellular neurones and terminals. Cell Calcium, 51, 293–299. [DOI] [PubMed] [Google Scholar]
- Dumas L., Lepage M., Bystrova K., Matthiesen A. S., Welles-Nystrom B., Widstrom A. M. (2013). Influence of skin-to-skin contact and rooming-in on early mother-infant interaction: A randomized controlled trial. Clin Nurs Res, 22, 310–336. [DOI] [PubMed] [Google Scholar]
- Dunn A. B., Paul S., Ware L. Z., Corwin E. J. (2015). Perineal injury during childbirth increases risk of postpartum depressive symptoms and inflammatory markers. Journal of Midwifery & Women's Health, 60, 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. (1984). Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol, 352, 447–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Wu R., Davis C., Li M. (2018). Activation of 5-HT2A receptor disrupts rat maternal behavior. Neuropharmacology, 128, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoneim M. M., O'Hara M. W. (2016). Depression and postoperative complications: An overview. BMC Surgery, 16, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson H. C., Sullivan E. L., Nousen E. K., Sullivan C. A., Huang E., Rincon M., Nigg J. T., Loftis J. M. (2018). Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain, Behavior, and Immunity, 73, 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansenne I., Rasier G., Charlet-Renard C., DeFresne M. P., Greimers R., Breton C., Legros J. J., Geenen V., Martens H. (2004). Neurohypophysial receptor gene expression by thymic T cell subsets and thymic T cell lymphoma cell lines. Clin Dev Immunol, 11, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton G. I., Wang Y. F. (2008). Neural mechanisms underlying the milk ejection burst and reflex. Prog Brain Res, 170, 155–166. [DOI] [PubMed] [Google Scholar]
- Holsen L. M., Lancaster K., Klibanski A., Whitfield-Gabrieli S., Cherkerzian S., Buka S., Goldstein J. M. (2013). HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience, 250, 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hompoth E. A. Peto Z. Fureszne, B.alogh V., Toreki A. (2020). Associations between depression symptoms, psychological intervention and perinatal complications. Journal of Clinical Psychology in Medical Settings, 27, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D., Jin F., Li J., Lian J., Liu M., Liu X., Xu Y., Zhang C., Zhao C., Jia S., Jiao R., Liu X. Y., Wang X., Zhang Y., Wang Y.-F. (2016). Model roles of the hypothalamo-neurohypophysial system in neuroscience study. Biochem Pharmacol (Los Angel), 5, 211. [Google Scholar]
- Jiang Q., Wakerley J. B. (1997). The milk-ejection reflex in the peri-partum rat: Effects of oestradiol and progesterone on basal milk-ejection frequency and the facilitatory response to central oxytocin. J Neuroendocrinol, 9, 9–16. [DOI] [PubMed] [Google Scholar]
- Jiang W. Q. Bao L. L. Sun F. J. Liu X. L., Yang J. (2019). Oxytocin in the periaqueductal gray mainly comes from the hypothalamic supraoptic nucleus to participate in pain modulation. Peptides, 121, 170153. [DOI] [PubMed] [Google Scholar]
- Jimenez A., Gonzalez-Mariscal G. (2019). Maternal responsiveness to suckling is modulated by time post-nursing: A behavioural and c-Fos/oxytocin immunocytochemistry study in rabbits. J Neuroendocrinol, 31, e12788. [DOI] [PubMed] [Google Scholar]
- Jurek B., Neumann I. D. (2018). The oxytocin receptor: From intracellular signaling to behavior. Physiol Rev, 98, 1805–1908. [DOI] [PubMed] [Google Scholar]
- Klampfl S. M., Bosch O. J. (2019). Mom doesn't care: When increased brain CRF system activity leads to maternal neglect in rodents. Front Neuroendocrinol, 53, 100735. [DOI] [PubMed] [Google Scholar]
- Klampfl S. M., Schramm M. M., Gassner B. M., Hubner K., Seasholtz A. F., Brunton P. J., Bayerl D. S., Bosch O. J. (2018). Maternal stress and the MPOA: Activation of CRF receptor 1 impairs maternal behavior and triggers local oxytocin release in lactating rats. Neuropharmacology, 133, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D., Baron D., Joy J., Del Valle L., Shack J. (2011). Contributions of HIV infection in the hypothalamus and substance abuse/use to HPT dysregulation. Psychoneuroendocrinology, 36, 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G., Russell J. A. (2016). The peptide oxytocin antagonist F-792, when given systemically, does not act centrally in lactating rats. J Neuroendocrinol, 28(4). https://doi.org/ 10.1111/jne.12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. Liu H. Liu X. Wang H. Li T. Wang X. Jia S. Wang P., Wang Y. F. (2020). Involvement of hyperpolarization-activated cyclic nucleotide-gated channel 3 in oxytocin neuronal activity in lactating rats with pup deprivation. ASN Neuro, 12, 1759091420944658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Liu X., Liu H., Li T., Jia S., Wang X., Wang P., Qin D., Wang Y.-F. (2021). Key roles of cyclooxygenase 2-protein kinase A-HCN3 pathway in the regulation of oxytocin neuronal activity in lactating rats with intermittent pup-deprivation. Neuroscience, 452, 13–25. [DOI] [PubMed] [Google Scholar]
- Li T. Wang P. Wang S. C., Wang Y. F. (2017). Approaches mediating oxytocin regulation of the immune system. Front Immunol, 7, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J. Mu Y. Li X. Tang W. Wang Y. Liu Z. Huang X. Scherpbier R. W. Guo S. Li M. Dai L. Deng K. Deng C. Li Q. Kang L. Zhu J., Ronsmans C. (2018). Relaxation of the one child policy and trends in caesarean section rates and birth outcomes in China between 2012 and 2016: Observational study of nearly seven million health facility births. Br Med J, 360, k817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Y. Cui D. Li D. Jiao R. Wang X. Jia S. Hou D. Li T. Liu H. Wang P., Wang, Y. F. (2017). Oxytocin removes estrous female vs. male preference of virgin male rats: Mediation of the supraoptic nucleus via olfactory bulbs. Front Cell Neurosci, 11, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Y. Li D. Li T. Liu H. Cui D. Liu Y. Jia S. Wang X. Jiao R. Zhu H. Zhang F. Qin D., Wang Y. F. (2019). Effects of intranasal oxytocin on pup deprivation-evoked aberrant maternal behavior and hypogalactia in rat dams and the underlying mechanisms. Front Neurosci, 13, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M., Sabatier N., Bull P. M., Landgraf R., Dayanithi G., Leng G. (2002). Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature, 418, 85–89. [DOI] [PubMed] [Google Scholar]
- Mantani A., Yamashita H., Fujikawa T., Yamawaki S. (2010). Higher incidence of hysterectomy and oophorectomy in women suffering from clinical depression: Retrospective chart review. Psychiatry and Clinical Neurosciences, 64, 95–98. [DOI] [PubMed] [Google Scholar]
- Miller E. S., Sakowicz A., Roy A., Yang A., Sullivan J. T., Grobman W. A., Wisner K. L. (2019). Plasma and cerebrospinal fluid inflammatory cytokines in perinatal depression. American Journal of Obstetrics and Gynecology, 220, 271 e271–271 e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye K., Poole D. H., Pate J. L. (2008). Expression and regulation of functional oxytocin receptors in bovine T lymphocytes. Biol Reprod, 78, 786–793. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Kawasaki M., Suzuki H., Matsuura T., Motojima Y., Ohnishi H., Yamanaka Y., Yoshimura M., Maruyama T., Saito R., Ueno H., Sonoda S., Nishimura K., Onaka T., Ueta Y., Sakai A. (2019). Neuropathic pain up-regulates hypothalamo-neurohypophysial and hypothalamo-spinal oxytocinergic pathways in oxytocin-monomeric red fluorescent protein 1 transgenic rat. Neuroscience, 406, 50–61. [DOI] [PubMed] [Google Scholar]
- Parker W. H., Jacoby V., Shoupe D., Rocca W. (2009). Effect of bilateral oophorectomy on women's long-term health. Women's Health, 5, 565–576. [DOI] [PubMed] [Google Scholar]
- Pedersen C. A., Boccia M. L. (2002). Oxytocin links mothering received, mothering bestowed and adult stress responses. Stress, 5, 259–267. [DOI] [PubMed] [Google Scholar]
- Pérez-Torrero E., Rubio-Navarro L. (2015). Maternal behaviour their adjustments and implicated factors. Journal of Behavioural and Brain Science, 5, 54244, 54215. [Google Scholar]
- Renier N., Adams E. L., Kirst C., Wu Z., Azevedo R., Kohl J., Autry A. E., Kadiri L., Umadevi Venkataraju K., Zhou Y., Wang V. X., Tang C. Y., Olsen O., Dulac C., Osten P., Tessier-Lavigne M. (2016). Mapping of brain activity by automated volume analysis of immediate early genes. Cell, 165, 1789–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N., Otto B., Wolf J. M., Klose J., Kirschbaum C., Enck P., Klosterhalfen S. (2006). Sex-specific adaptation of endocrine and inflammatory responses to repeated nauseogenic body rotation. Psychoneuroendocrinology, 31, 226–236. [DOI] [PubMed] [Google Scholar]
- Russell J. A., Douglas A. J., Ingram C. D. (2001). Brain preparations for maternity–adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog Brain Res, 133, 1–38. [DOI] [PubMed] [Google Scholar]
- Smotherman W. P., Wiener S. G., Mendoza S. P., Levine S. (1976). Pituitary–adrenal responsiveness of rat mothers to noxious stimuli and stimuli produced by pups. Ciba Found Symp, 5–25. [DOI] [PubMed] [Google Scholar]
- Sun K. W., Pan P. H. (2019). Persistent pain after cesarean delivery. Int J Obstet Anesth, 40, 78–90. [DOI] [PubMed] [Google Scholar]
- Tang Y., Shi Y., Gao Y., Xu X., Han T., Li J., Liu C. (2019). Oxytocin system alleviates intestinal inflammation by regulating macrophages polarization in experimental colitis. Clin Sci (Lond), 133, 1977–1992. [DOI] [PubMed] [Google Scholar]
- Thul T. A., Corwin E. J., Carlson N. S., Brennan P. A., Young L. J. (2020). Oxytocin and postpartum depression: A systematic review. Psychoneuroendocrinology, 120, 104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Noda Y., Tsunekawa H., Zhou Y., Miyazaki M., Senzaki K., Nabeshima T. (2007). Behavioural and neurochemical features of olfactory bulbectomized rats resembling depression with comorbid anxiety. Behav Brain Res, 178, 262–273. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhao M., Liang R., Zhang Z., Zhao , H., Zhang J., Li T., Li Y. (2010). Whey peptides improve wound healing following caesarean section in rats. Br J Nutr, 104, 1621–1627. [DOI] [PubMed] [Google Scholar]
- Wang P., Yang H. P., Tian S., Wang L., Wang S. C., Zhang F., Wang Y. F. (2015). Oxytocin-secreting system: A major part of the neuroendocrine center regulating immunologic activity. J Neuroimmunol, 289, 152–161. [DOI] [PubMed] [Google Scholar]
- Wang S. C., Wang Y. F. (2021). Cardiovascular protective properties of oxytocin against COVID-19. Life Sci, 270, 119130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. F. (2016). Center role of the oxytocin-secreting system in neuroendocrine-immune network revisited. Journal of Clinical & Experimental Neuroimmunology, 1:1000102. [Google Scholar]
- Wang Y. F., Hatton G. I. (2004). Milk ejection burst-like electrical activity evoked in supraoptic oxytocin neurons in slices from lactating rats. J Neurophysiol, 91, 2312–2321. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Hatton G. I. (2007). Dominant role of betagamma subunits of G-proteins in oxytocin-evoked burst firing. J Neurosci, 27, 1902–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. F., Hatton G. I. (2009). Astrocytic plasticity and patterned oxytocin neuronal activity: Dynamic interactions. J Neurosci, 29, 1743–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. F., Li T., Jia S., Wang P. (2018). Effects of intranasal oxytocin on stress-evoked by cesarean section in rats. In The 9th international congress of neuroendocrinology. The International Neuroendocrine Federation, #483, July 15-18, 2018, published by the International Neuroendocrine Federation (INF) and the Society for Behavioural Neuroendocrinology (SBN).
- Wang Y. F., Ponzio T. A., Hatton G. I. (2006). Autofeedback effects of progressively rising oxytocin concentrations on supraoptic oxytocin neuronal activity in slices from lactating rats. Am J Physiol Regul Integr Comp Physiol, 290, R1191–R1198. [DOI] [PubMed] [Google Scholar]
- Wang Y. F., Sun M. Y., Hou Q., Parpura V. (2013). Hyposmolality differentially and spatiotemporally modulates levels of glutamine synthetase and serine racemase in rat supraoptic nucleus. Glia, 61, 529–538. [DOI] [PubMed] [Google Scholar]
- Xin Q., Bai B., Liu W. (2017). The analgesic effects of oxytocin in the peripheral and central nervous system. Neurochem Int, 103, 57–64. [DOI] [PubMed] [Google Scholar]
- Zubaidi A., Buie W. D., Hart D. A., Sigalet D. (2010). Temporal expression of cytokines in rat cutaneous, fascial, and intestinal wounds: A comparative study. Digestive Diseases and Sciences, 55, 1581–1588. [DOI] [PubMed] [Google Scholar]