Abstract
Purpose
Long-term oncologic differences in outcome between groups of patients with Lynch syndrome (LS) colorectal cancer (CRC) and sporadic CRC with microsatellite instability-high (MSI-H) are the focus of investigation in the current study.
Methods
Patients registered in the Korean Hereditary Tumor Registry and 2 tertiary referral hospitals treated for stage I–III CRC between 2005 and 2015 were retrospectively analyzed. Detection for both groups was performed using pedigree, microsatellite instability, and mismatch repair (MMR) gene testing. Multivariate analyses for overall survival (OS) and disease-free survival (DFS) were conducted.
Results
Cases of LS (n = 77) and sporadic CRC with MSI-H (n = 96) were identified. LS CRC patients were younger in age and displayed tumor sidedness, typically involving left-sided colon and rectum, compared to patients with sporadic CRC with MSI-H. OS and DFS were lower for LS CRC relative to CRC with MSI-H (OS, 72.7% vs. 93.8%, P = 0.001; DFS, 71.4% vs. 88.5%, P = 0.001). In multivariate analyses, tumor sidedness, stage, and chemotherapy were independent factors for OS and DFS. LS CRC was a prognostic factor for poorer OS (hazard ratio, 2.740; 95% confidence interval, 1.003–7.487; P = 0.049), but not DFS.
Conclusion
Our findings indicate that LS CRC is associated with poorer outcomes compared to sporadic CRC with MSI-H, presenting distinct clinical features. In view of the current lack of knowledge on genetic and molecular mechanisms, appropriate management taking into consideration the difficulty of identification of CRC with hypermutable tumors harboring heterogeneity is essential.
Keywords: Colorectal neoplasms, Lynch syndrome, Microsatellite instability
INTRODUCTION
Genetic assessment of hereditary colorectal cancer (CRC) has evolved significantly since the molecular discovery of microsatellite instability (MSI), the first DNA marker associated with defective mismatch repair (dMMR) via germline mutations in MMR genes or epigenetic inactivation of MLH1 to leading a subset of ‘mutator’ phenotype tumors [1,2]. These different pathways reflect the existence of the hypermutable MSI phenotype in a broad spectrum of tumors, supporting the need for a tailored approach to differentiate patients with Lynch syndrome (LS) from those with related MSI-high (MSI-H)/dMMR tumors [3].
Hypermutable phenotype tumors comprise ~15% of all CRCs [1]. Several studies have reported clinical and oncogenic differences of these tumors, with LS-associated d/MMR CRC patients showing better prognosis relative to sporadic CRC patients [4,5]. Furthermore, chemotherapy with addition of oxaliplatin to fluoropyrimidine is reported to provide survival benefits for high-risk stage II or stage III CRC patients with dMMR compared to matched stage patients with non-hypermutable tumors [6]. However, oncologic differentiation of hereditary and sporadic CRC in the setting of dMMR tumors only is yet to be achieved, considering the outcomes of patients with metastatic CRC [7]. Furthermore, comparative data of both tumors have not yet been reported in Korea. With concern as to the difficulty of diagnostic approach based on genomic assessment [2], a comparative study between these tumor types may aid in differential diagnosis of LS-related and sporadic tumors in clinical practice [8].
The main objective of the current study was to compare the clinicopathologic features and long-term oncologic outcomes between groups of patients with LS CRC and sporadic CRC with MSI-H.
METHODS
Study population
Retrospective analysis was performed using clinical data from LS CRC patients registered at the Korean Hereditary Tumor Registry (KHTR) from 2005 to 2015 [9] and CRC patients presenting MSI-H with stage I–III who underwent curative resection at 2 tertiary referral hospitals. For surgical management of LS, prophylactic colectomy was recommended as a standard procedure using either open or minimally invasive techniques. The surgical, adjuvant/neoadjuvant therapy and postoperative surveillance procedures were determined by the attending physician based on pathologic stage and general condition of the patient in accordance with the National Comprehensive Cancer Network (NCCN) guidelines [10]. All cases were restaged retrospectively according to the 7th edition of the American Joint Committee on Cancer (AJCC) TNM staging system [11]. Ethical approval was provided by the Institutional Review Board of Seoul National Bundang Hospital (No. B-1407-258-010). Written informed consent was waived due to its retrospective nature.
Diagnosis of Lynch syndrome colorectal cancer and sporadic colorectal cancer with microsatellite instability-high
The diagnostic approaches for identification of LS CRC and sporadic CRC patients with MSI-H included pedigree, MSI, and MMR gene analyses. The hypermutable phenotype of CRC was determined via immunohistochemistry of MMR proteins or MSI testing [2]. Pedigree analysis used the Amsterdam II Criteria proposed by the International Collaborative Group on Hereditary nonpolyposis CRC and revised criteria of suspected hereditary nonpolyposis colorectal cancer [12]. The method for MSI testing was consistent at each institution and involved the use of 5 microsatellite markers (BAT-25, BAT-26, D2S123, D5S346, and D17S250) based on previous studies [13]. Polymerase chain reaction (PCR) analyses were performed using DNA extracted from paraffin-embedded tumor and surrounding normal tissues with comparison of shifted alleles of PCR products between tumor and normal colonic mucosa. Tumors with at least 2 of the 5 microsatellite markers displaying shifted alleles were classified as MSI-H. Diagnosis of LS CRC was confirmed in cases fulfilling Amsterdam criteria II or with germline mutations in MMR genes. In addition, we detected cases of LS among suspected patients who did not fulfill Amsterdam criteria II with MSI-H or loss of immunostaining, when confirmed a germline mutation in an MMR gene or displayed a loss of expression of MSH2 or MSH6 (or both), or PMS2 alone. Cases that did not fulfill Amsterdam criteria II with positive MLH1 hypermethylation in MSI tests were classified as sporadic CRC with MSI-H.
Statistical methods
Continuous or categorical variables were analyzed with the chisquare and Fisher exact tests. Null hypothesis of no difference was rejected in cases where P-values were less than 0.05. Overall survival (OS) and disease-free survival (DFS) were defined as described previously [14]. Oncologic outcomes, including OS and DFS, were compared with Kaplan-Meier analysis. The Cox proportional hazards model was applied to estimate the crude and adjusted hazard ratios (HR) with 95% confidence intervals (CI) of OS and DFS. In addition, subgroup analyses for patients diagnosed from 2010 to 2015 were conducted by matching the MSI test period for reducing bias due to different MSI testing time-frames. Oncologic evaluation of LS patients diagnosed during 1990–2004 and 2005–2014 was additionally included to investigate the oncologic effect of the KHTR [9]. Data were analyzed with IBM SPSS Statistics ver. 26.0 (IBM Corp., Seoul, Korea) and R statistical software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org).
RESULTS
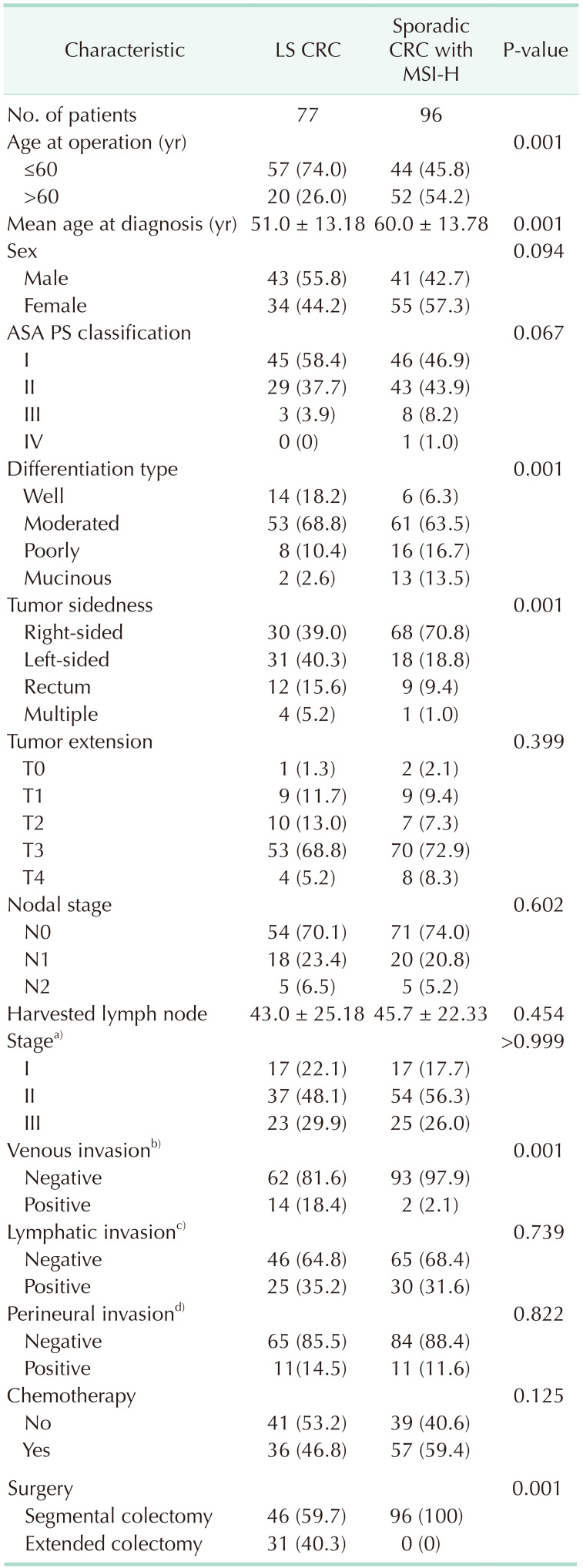
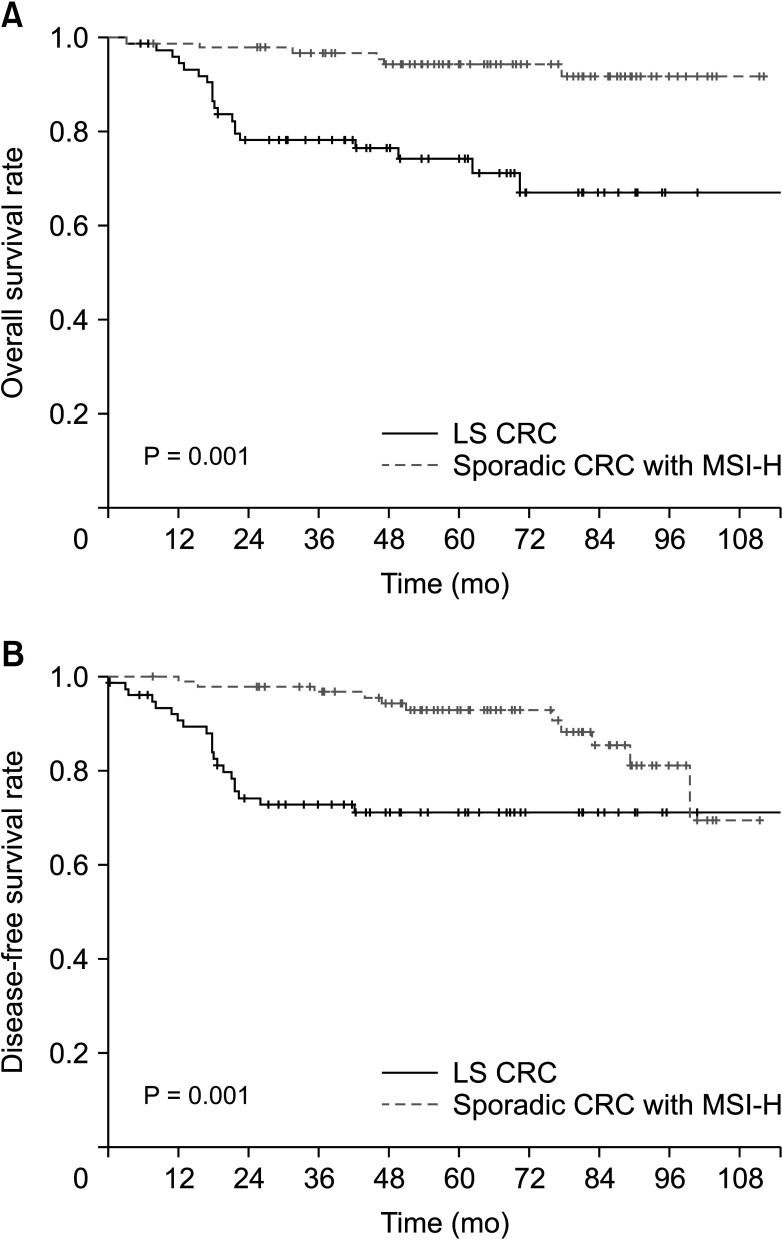
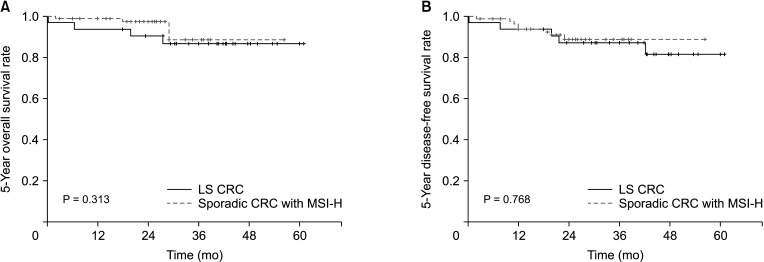
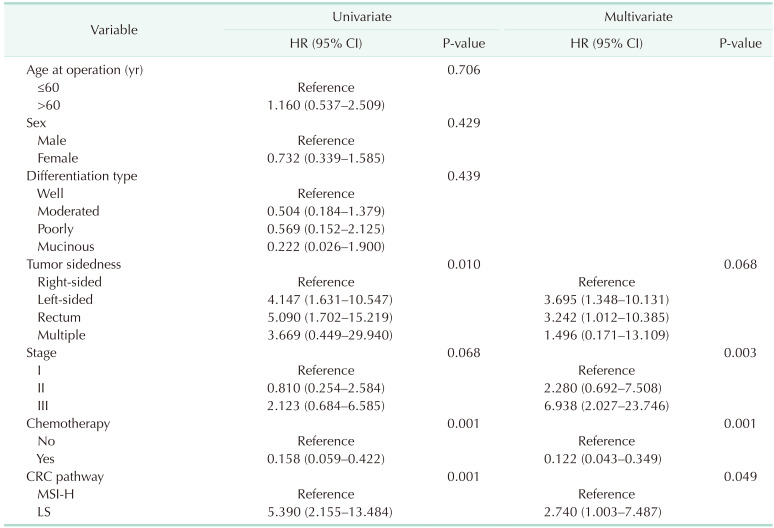
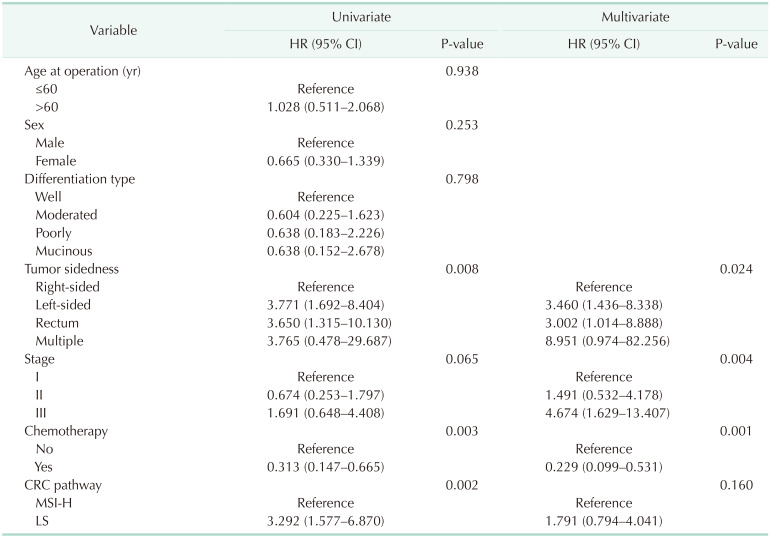
In terms of baseline characteristics, age at operation was significantly lower in the LS CRC than sporadic CRC with the MSI-H group (mean age, 51.0 years vs. 60.0 years; P = 0.001) (Table 1). With regard to tumor sidedness, sporadic CRC with MSI-H was associated with right-sided colon cancer while LS CRC frequently occurred in the left-sided colon or rectum. Segmental colectomy was performed for more cases of sporadic CRC with MSI-H than LS CRC. During the median follow-up period of 60.0 months (range, 1–118 months), OS and DFS were lower in patients with LS CRC than sporadic CRC with MSI-H (OS: 72.7% vs. 93.8%, P = 0.001, Fig. 1A; DFS: 71.4% vs. 88.5%, P = 0.001, Fig. 1B). The oncologic outcomes with adjustment for the MSI testing time-frame showed comparable 5-year overall and DFS rates between 32 LS CRC and 84 sporadic CRC with MSI-H cases (Fig. 2A: 5-year OS, 87.5% vs. 95.4%, respectively, P = 0.313; Fig. 2B: 5-year DFS, 84.4% vs. 90.5%, respectively, P = 0.768). In multivariate analysis, tumor sidedness, stage, and chemotherapy were independent factors for OS and DFS, respectively. LS CRC was independently associated with poorer OS (HR, 2.740; 95% CI, 1.003–7.487; P = 0.049), but not DFS (HR, 1.791; 95% CI, 0.099–0.531; P = 0.160) (Table 2, 3).
Table 1. Clinical and pathologic variable between LS and sporadic CRC with MSI-H.
Values are presented as number only, mean ± standard deviation, and number (%).
LS, Lynch syndrome; CRC, colorectal cancer; MSI-H, microsatellite instability-high; ASA, American Society of Anesthesiologists; PS, physical status.
a)The 7th edition of the American Joint Committee on Cancer TNM staging system; b)not available patients (n = 2); c)not available patients (n = 7); d)not available patients (n = 2).
Fig. 1. The long-term outcomes between Lynch syndrome (LS) and sporadic colorectal cancer (CRC) with microsatellite instability-high (MSI-H). The Kaplan-Meier curves for overall survival (A) and disease-free survival (B).
Fig. 2. Five-year survival outcomes with adjustment for the microsatellite instability (MSI) testing time-frame. The Kaplan-Meier curve 5-year overall survival (A) and 5-year disease-free survival (B) between Lynch syndrome (LS) colorectal cancer (CRC) patients (n = 32) and sporadic CRC patients with MSI-high (MSI-H, n = 84) performed the MSI testing from 2010 to 2015.
Table 2. Univariate and multivariate analyses for overall survival.
HR, hazard ratio; CI, confidence interval; CRC, colorectal cancer; MSI-H, microsatellite instability-high; LS, Lynch syndrome.
Table 3. Univariate and multivariate analyses for disease-free survival.
HR, hazard ratio; CI, confidence interval; LS, Lynch syndrome; CRC, colorectal cancer; MSI-H, microsatellite instability-high.
Using data from LS CRC patients only diagnosed from 1990 to 2014, OS and DFS were lower in the earlier (1990–2004) than later (2005–2014) diagnosis period (OS; 65.5% vs. 75.3%, P < 0.001; DFS: 62.1% vs. 74.0%, P < 0.001). Furthermore, OS and DFS between segmental and extended colectomy groups were comparable (OS, 74.2% vs. 68.5%, P = 0.338; DFS, 72.6% vs. 65.8%, P = 0.396). Consistently, segmental resection was not an independent factor for OS and DFS in multivariate analysis. However, diagnosis period (2005–2014) was an independent factor for OS and DFS in multivariate analysis (OS: HR, 0.089; 95% CI, 0.02–0.38; P = 0.001; DFS: HR, 0.155; 95% CI, 0.47–0.51; P = 0.002).
DISCUSSION
Here, we attempted to distinguish LS CRC from sporadic CRC patient groups with tumors harboring dMMR. Although the clinical outcomes of our study are concordant with previous findings [15,16], survival patterns of the 2 groups are a matter of debate, considering our results are converse to the widely accepted opinion that survival of LS patients is better than that of patients with sporadic CRC [4,5,7]. The morphological distinctions, of which poorly and mucinous differentiated types were observed infrequently in both groups, may also contribute to different prognosis compared to previous studies [17,18]. Here, we propose that our outcomes analysis using dMMR tumors only, except stage IV, have potential utility in the management of patients with hypermutable tumors.
In this study, oncologic evaluation may have been influenced by switching to regimens with fluoropyrimidine plus oxaliplatin in the early 2000s [19] and the registry for LS patients during long-term follow-up. Given that LS CRC has been diagnosed since 1989 while MSI testing has been conducted since 2003, the impact of the registry may be inferred from the oncologic outcome by adjusting the MSI testing time-frame, which is related to reduction of LS CRC mortality as a result of establishing the hereditary registration and surveillance system [20]. Consistently, survival improvement since 2005 in subgroup analyses of LS patients only has been reported. In terms of tumor sidedness, masked high prevalence and limited response to fluoropyrimidine-based therapy of LS-related dMMR/MSI left-sided colon or rectal cancer [21,22] were the outcomes observed by our group compared to previous studies [4,5,7]. Additionally, half of the LS CRC patients subjected to segmental colectomy were at risk of synchronous or metachronous malignancy [23], though segmental colectomy did not show a statistical significance in subgroup survival analyses. However, studies on the potential of intensive colonoscopic surveillance as an alternative to extended colectomy are lacking. These oncologic hazards warrant further investigation, with consideration of comparable local and distant recurrence pattern in sporadic CRC with MSI-H as in this study.
Clinical detection of actual LS from suspected cases can be very difficult due to Lynch-like syndrome representing a heterogeneous group between sporadic CRC with MSI-H and true LS patients. Lynch-like syndrome is defined as a two-hit combination mutation coupled with loss of heterozygosity followed by 2 somatic mutations, which is nearly impossible to differentiate from actual LS, unlike sporadic CRC with MSI-H [23,24]. The only distinctive feature between Lynch-like and LS is the lower standardized incidence ratio of CRC in the former cancer type [8]. The traditional prediction models may not be ideal due to overlapping syndromes, atypical phenotypes associated with MSH6 and PMS2, and familial CRC type X [25,26], with initial recommendation for germline mutation testing regardless of tumor type and family history [3,27]. However, the NCCN guidelines have also suggested caution and uncertainty in implementing routine multi-gene panel testing with weak evidence [10]. The different ways in which ordering physicians and genetic counselors deal with examination of a single suspected gene or the entire genome in real clinical practice owing to the lack of a formal testing policy is currently a matter of concern [2]. For instance, a previous study using data from 22 Korean institutions reported delayed confirmation of LS after surgery [28]. These issues highlight the urgent need for a nationwide hereditary cancer registry [20].
This study has several limitations. The major drawback is its retrospective design and small sample size. There are some missing data for recurrence pattern of the LS group. Our study did not control bias of all parameters, including neo- or adjuvant treatment or chemotherapy regime shifts according to current guidelines, despite the significance of chemotherapy in statistical analysis. These problems should be investigated in future analyses with gene testing for BRAF, KRAS, and NRAS, and EPCAM mutations. Furthermore, MMR gene testing of all patients with suspected LS could not be performed, though it is already known for diagnostic algorithms of LS, which involve a complex multistep molecular testing process [28]. However, the MMR gene was confirmed in all LS CRC patients. We believe that separate identification of true LS from heterogeneous hypermutable tumors poses a significant burden for individual physicians without the aid of the hereditary cancer registry [29].
In conclusion, LS CRC and sporadic CRC patients with MSI-H with distinctive clinical features were successfully diagnosed. In our efforts to establish a hereditary registry, poor longterm oncologic outcomes of LS CRC were observed compared to sporadic CRC patients with MSI-H, though genetic and molecular research to sufficiently support our conclusions is currently lacking. In practice, identification of LS CRC at a local registry or single institution in Korea remains difficult, often resulting in inappropriate diagnosis or management. Therefore, our findings warrant further investigation to facilitate appropriate management for CRC patients with hypermutable tumors harboring heterogeneity.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: ITS, DWK.
- Formal Analysis: ITS, DWK, MHK, YKS, JLK.
- Investigation: ITS, DWK, HKO, SBK, SYJ, KJP.
- Methodology: ITS, DWK, YKS, JLK.
- Project Administration: HKO, SBK, SYJ, KJP.
- Writing — Original Draft: ITS, DWK.
- Writing — Review & Editing: All authors.
References
- 1.Boland CR, Goel A. Microsatel lite inst abi l it y in colorec t al cancer. Gastroenterology. 2010;138:2073–2087. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carethers JM. DNA testing and molecular screening for colon cancer. Cl in Gastroenterol Hepatol. 2014;12:377–381. doi: 10.1016/j.cgh.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latham A, Srinivasan P, Kemel Y, Shia J, Bandlamudi C, Mandelker D, et al. Microsatellite instability is associated with the presence of lynch syndrome pan-cancer. J Clin Oncol. 2019;37:286–295. doi: 10.1200/JCO.18.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sankila R, Aaltonen LA, Järvinen HJ, Mecklin JP. Better survival rates in patients with MLH1-associated hereditary colorectal cancer. Gastroenterology. 1996;110:682–687. doi: 10.1053/gast.1996.v110.pm8608876. [DOI] [PubMed] [Google Scholar]
- 5.You JF, Hsieh LL, Changchien CR, Chen JS, Chen JR, Chiang JM, et al. Inverse effects of mucin on survival of matched hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer patients. Clin Cancer Res. 2006;12(14 Pt 1):4244–4250. doi: 10.1158/1078-0432.CCR-06-0202. [DOI] [PubMed] [Google Scholar]
- 6.Tougeron D, Mouillet G, Trouilloud I, Lecomte T, Coriat R, Aparicio T, et al. Efficacy of adjuvant chemotherapy in colon cancer with microsatellite instability: a large multicenter AGEO Study. J Natl Cancer Inst. 2016:108. doi: 10.1093/jnci/djv438. [DOI] [PubMed] [Google Scholar]
- 7.Cohen R, Buhard O, Cervera P, Hain E, Dumont S, Bardier A, et al. Clinical and molecular characterisation of hereditary and sporadic metastatic colorectal cancers harbouring microsatellite instability/DNA mismatch repair deficiency. Eur J Cancer. 2017;86:266–274. doi: 10.1016/j.ejca.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:404–412. doi: 10.1158/1055-9965.EPI-16-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son IT, Kim DW, Jeong SY, Shin YK, Ihn MH, Oh HK, et al. Clinicopathological features and type of surgery for lynch syndrome: changes during the past two decades. Cancer Res Treat. 2016;48:605–611. doi: 10.4143/crt.2015.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Provenzale D, Regenbogen SE, Hampel H, Slavin TP, Hall MJ, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 3.2017. J Natl Compr Canc Netw. 2017;15:1465–1475. doi: 10.6004/jnccn.2017.0176. [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 12.Park JG, Vasen HF, Park YJ, Park KJ, Peltomaki P, de Leon MP, et al. Suspected HNPCC and Amsterdam criteria II: evaluation of mutation detection rate, an international collaborative study. Int J Colorectal Dis. 2002;17:109–114. doi: 10.1007/s003840100348. [DOI] [PubMed] [Google Scholar]
- 13.Lee SY, Kim DW, Lee HS, Ihn MH, Oh HK, Min BS, et al. Low-level microsatellite instability as a potential prognostic factor in sporadic colorectal cancer. Medicine (Baltimore) 2015;94:e2260. doi: 10.1097/MD.0000000000002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Punt CJ, Buyse M, Köhne CH, Hohenberger P, Labianca R, Schmoll HJ, et al. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst. 2007;99:998–1003. doi: 10.1093/jnci/djm024. [DOI] [PubMed] [Google Scholar]
- 15.Lynch HT, Boland CR, Gong G, Shaw TG, Lynch PM, Fodde R, et al. Phenotypic and genotypic heterogeneity in the Lynch syndrome: diagnostic, surveillance and management implications. Eur J Hum Genet. 2006;14:390–402. doi: 10.1038/sj.ejhg.5201584. [DOI] [PubMed] [Google Scholar]
- 16.Konishi M, Kikuchi-Yanoshita R, Tanaka K, Muraoka M, Onda A, Okumura Y, et al. Molecular nature of colon tumors in hereditary nonpolyposis colon cancer, familial polyposis, and sporadic colon cancer. Gastroenterology. 1996;111:307–317. doi: 10.1053/gast.1996.v111.pm8690195. [DOI] [PubMed] [Google Scholar]
- 17.Jass JR, Walsh MD, Barker M, Simms LA, Young J, Leggett BA. Distinction between familial and sporadic forms of colorectal cancer showing DNA microsatellite instability. Eur J Cancer. 2002;38:858–866. doi: 10.1016/s0959-8049(02)00041-2. [DOI] [PubMed] [Google Scholar]
- 18.Dionigi G, Bianchi V, Villa F, Rovera F, Boni L, Annoni M, et al. Differences between familial and sporadic forms of colorectal cancer with DNA microsatellite instability. Surg Oncol. 2007;16(Suppl 1):S37–S42. doi: 10.1016/j.suronc.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 19.André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplat in, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 20.Barrow P, Khan M, Lalloo F, Evans DG, Hill J. Systematic review of the impact of registration and screening on colorectal cancer incidence and mortality in familial adenomatous polyposis and Lynch syndrome. Br J Surg. 2013;100:1719–1731. doi: 10.1002/bjs.9316. [DOI] [PubMed] [Google Scholar]
- 21.Cercek A, Dos Santos Fernandes G, Roxburgh CS, Ganesh K, Ng S, Sanchez-Vega F, et al. Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res. 2020;26:3271–3279. doi: 10.1158/1078-0432.CCR-19-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Rosa N, Rodriguez-Bigas MA, Chang GJ, Veerapong J, Borras E, Krishnan S, et al. DNA mismatch repair deficiency in rectal cancer: benchmarking its impact on prognosis, neoadjuvant response prediction, and clinical cancer genetics. J Clin Oncol. 2016;34:3039–3046. doi: 10.1200/JCO.2016.66.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim TJ, Kim ER, Hong SN, Kim YH, Huh JW, Park YA, et al. Survival outcome and risk of metachronous colorectal cancer after surgery in Lynch syndrome. Ann Surg Oncol. 2017;24:1085–1092. doi: 10.1245/s10434-016-5633-1. [DOI] [PubMed] [Google Scholar]
- 24.Carethers JM. Differentiating Lynch-like from Lynch syndrome. Gastroenterology. 2014;146:602–604. doi: 10.1053/j.gastro.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 26.Yurgelun MB, Allen B, Kaldate RR, Bowles KR, Judkins T, Kaushik P, et al. Identification of a variety of mutations in cancer predisposition genes in patients with suspected Lynch syndrome. Gastroenterology. 2015;149:604–613. doi: 10.1053/j.gastro.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinicrope FA. Lynch syndrome-associated colorectal cancer. N Engl J Med. 2018;379:764–773. doi: 10.1056/NEJMcp1714533. [DOI] [PubMed] [Google Scholar]
- 28.Jun SY, Lee EJ, Kim MJ, Chun SM, Bae YK, Hong SU, et al. Lynch syndrome-related small intestinal adenocarcinomas. Oncotarget. 2017;8:21483–21500. doi: 10.18632/oncotarget.15277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasen HF, Velthuizen ME, Kleibeuker JH, Menko FH, Nagengast FM, Cats A, et al. Hereditary cancer registries improve the care of patients with a genetic predisposition to cancer: contributions from the Dutch Lynch syndrome registry. Fam Cancer. 2016;15:429–435. doi: 10.1007/s10689-016-9897-1. [DOI] [PMC free article] [PubMed] [Google Scholar]