Abstract
Purpose
This study was designed to analyze the risk factors for poor survival after recurrence of hepatocellular carcinoma after liver transplantation.
Methods
Patients who underwent liver transplantation for hepatocellular carcinoma during the period of 2007 to 2018 were reviewed and patients who experienced recurrence were included. Multivariable Cox proportional hazard ratios were performed for potential risk factors for survival after recurrence.
Results
A total of 151 recipients experienced hepatocellular carcinoma recurrence after liver transplantation. The median of the recurrence-free period was 9.3 months (0.89–97.25 months). The median follow-up after recurrence was 13.4 months (0.59–118.28 months). One-, 3-, and 5-year survival after recurrence were 65.2%, 34.0% and 20.5%, respectively. Multivariable Cox analysis showed that, graft from living donor (hazard ratio [HR], 0.430; 95% confidence interval [CI], 0.210–0.882; P = 0.021), recurrence-free interval of ≥9 months (HR, 0.257; 95% CI, 0.164–0.403; P < 0.001), alphafetoprotein of ≥100 ng/mL at the time of recurrence (HR, 1.689; 95% CI, 1.059–2.695; P = 0.028), and recurrence in bone (HR, 2.304; 95% CI, 1.399–3.794; P = 0.001) and everolimus within 3 months after recurrence (HR, 0.354; 95% CI, 0.141–0.889; P = 0.027) were related to survival after recurrence.
Conclusion
Although survival was generally poor after recurrence of hepatocellular carcinoma in liver transplantation recipients, prolonged survival can be achieved in certain patients with better prognostic factors.
Keywords: Hepatocellular carcinoma, Liver transplantation, Recurrence
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common malignancy with a high recurrence rate [1,2,3]. Although surgical resection, as well as locoregional therapies including radiofrequency ablation, transarterial chemoembolization, and radiotherapy, which increased the prognosis of HCC, liver transplantation (LT) still remains as the most efficient treatment not only to remove cancer from the body but also to replace the organ with a healthy liver. The Milan criteria was introduced as criteria for LT to minimize recurrence and maximize the success of LT [4]. However, since the Milan criteria was considered too restrictive, many centers adjusted the criteria to expand it to perform LT for many HCC patients [5,6,7]. However, there are still patients who experience HCC recurrence after LT, and management for these patients also requires focus of clinicians. These specific patient groups are under immunosuppression and therefore have a poor prognosis due to the rapid progression of the recurrent tumor [8,9]. Unfortunately, evidence regarding these patients is lacking. The study of Shin et al. [10] reported that bone metastasis was a risk factor for poor prognosis in these specific patients. However, it only included 28 recurred patients out of 138 living donor LT cases. Therefore, we designed this study to summarize our experience with patients with HCC recurrence after LT and analyze the factors related to prognosis in this specific study group.
METHODS
Patients and data
Patients who underwent LT for hepatocellular carcinoma during the period of 2007 to 2018 were reviewed and those who experienced recurrence were included in the study. Patients' demographical data as well as medical history, LT-related data, recurrence-related data were collected. Medical history included treatments received prior to LT. LT-related data included histology of the extracted liver at the time of LT. Recurrence-related data included site, number, and date of recurrence as well as the treatment received for the recurrence after LT. This study was approved by the Institutional Review Board (IRB) of Samsung Medical Center in Seoul, Korea (No.2020-10-158). The acquisition of informed consent from the study subjects was waived by the IRB since the study was designed as retrospective study.
Statistical analysis
Patients' characteristics were compared based on their recurrence-free duration. To find a cutoff point of recurrence-free duration, receiver operating characteristics with Youden's index was used, along with a cutoff with the highest sensitivity and specificity for survival after 12 months post-LT. For comparison, numerical variables were expressed as mean ± standard deviation or median and interquartile range (IQR) and were compared with Student t-test or Mann-Whitney test, respectively. Categorical variables were compared using the chi-square test, Fisher exact test, or linear-to-linear association.
To characterize the prognosis according to the site and number of recurrences, median recurrence-free duration, and median follow-up after recurrence were analyzed. Kaplan-Meier survival was used to analyze the overall survival after the recurrence. For the analysis, follow-up duration was measured between the time of recurrence and the last follow-up. The multivariable Cox proportional hazard model was used to analyze risk factors related to overall survival.
Two-sided P-values of <0.05 were used as an indicator of statistical significance. All statistical analyses were done using IBM SPSS Statistics ver. 20.0 (IBM Corp., Armonk, NY, USA).
RESULTS
During the study period, 1,469 patients underwent LT at our medical center and 712 patients were diagnosed with hepatocellular carcinoma. Among those patients, 151 patients (21.2%) experienced recurrence and were included in the study. Area-under-curve (AUC) was 0.796 (95% confidence interval [CI], 0.717–0.875; P < 0.001) for predicting survival 12 months after LT, and 8.9 months showed the highest sensitivity of 0.848 and specificity of 0.689 based on Youden's index. Therefore, we used 9 months of recurrence-free duration as a cutoff.
Comparison based on 9 months of recurrence-free duration after liver transplantation
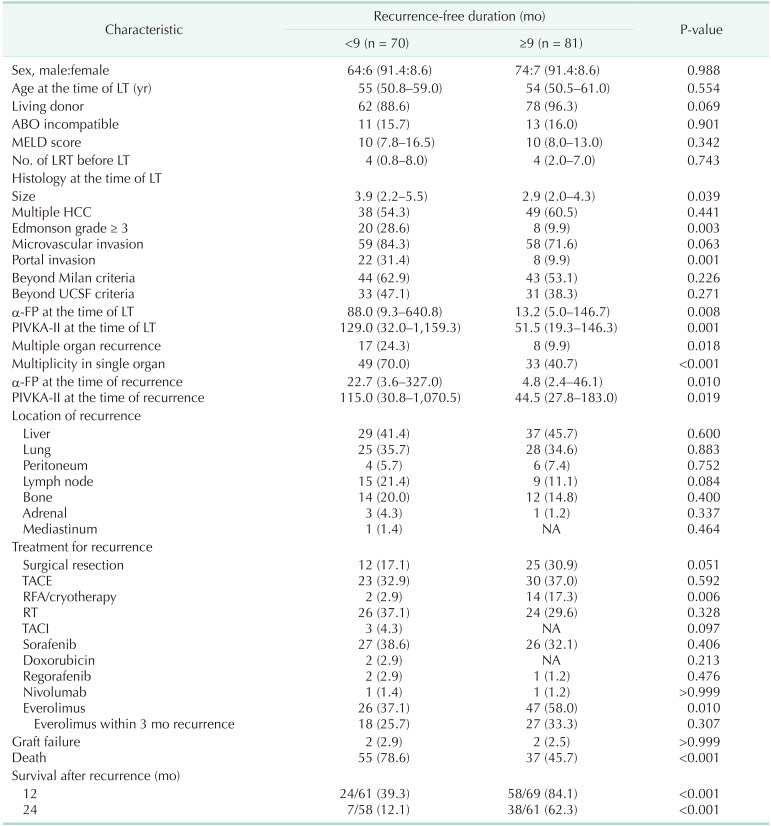
Table 1 summarizes the comparison of baseline characteristics according to the recurrence-free duration divided by 9 months posttransplantation. Recurrence-free duration was shorter than 9 months in 70 patients (46.4%) while 81 patients (53.6%) had recurrence-free duration equal to or longer than 9 months. Histology at the time of LT showed aggressive features including larger median size (3.9 cm [IQR, 2.2–5.5 cm] in <9-month group vs. 2.9 cm [IQR, 2.0–4.3 cm] in ≥9-month group, P = 0.039), higher rate of Edmonson grade ≥ 3 (n = 30 [28.6%] in <9-month group vs. n = 8 [9.9%] in ≥9-month group, P = 0.003), and higher rate of portal invasion (n = 22 [31.4%] in <9-month group vs. n = 8 [9.9%] in ≥9-month group, P = 0.001). Median α-FP level (22.7 U/L [IQR, 3.6–327.0 U/L] in <9-month group vs. 4.8 U/L [IQR, 2.4–46.1 U/L] in ≥9-month group, P = 0.010) and median protein induced by vitamin K absence or antagonists II level (115.0 mAU/mL [IQR, 30.8–1,070.5 mAU/mL] in <9-month group vs. 44.5 mAU/mL [IQR, 27.8–183.0 mAU/mL] in ≥9-month group, P = 0.019) at the time of recurrence were higher in the patients with less than 9 months recurrence-free duration.
Table 1. Comparison of baseline characteristics according to the recurrence-free duration divided by 9-month posttransplantation.
Values are presented as number (%) or median (interquartile range).
MELD, model for end-stage liver disease; LRT, locoregional therapy; LT, liver transplantation; HCC, hepatocellular carcinoma; UCSF, University of California San Francisco; PIVKA-II, protein induced by vitamin K absence or antagonists II; NA, not available; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; RT, radiotherapy; TACI, transarterial chemoinfusion.
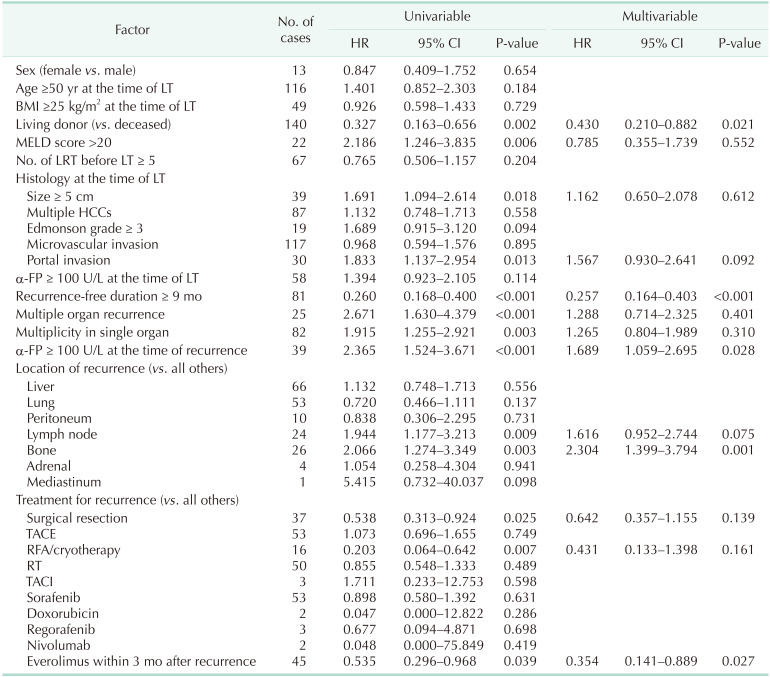
Risk factors for survival after recurrence
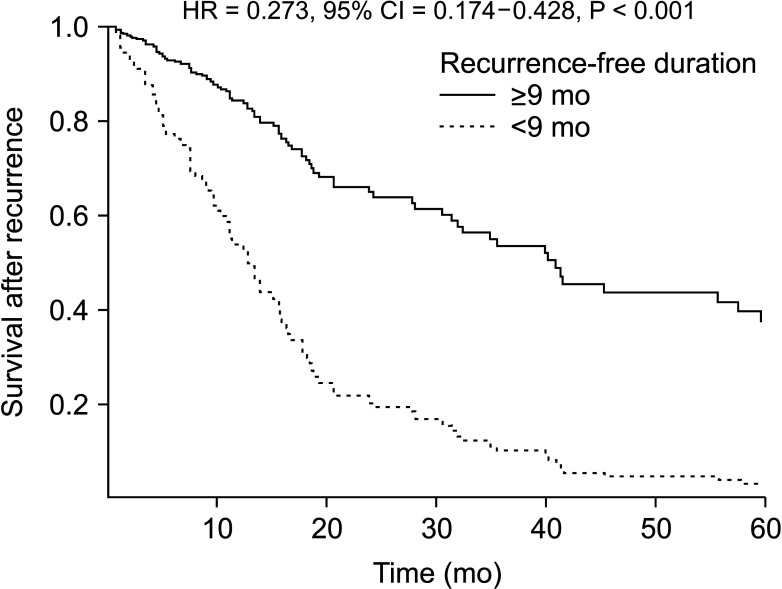
One-year, 3-year, and 5-year survival rates of patients who experienced recurrence of HCC after LT were 65.2%, 34.0%, and 20.5%, respectively. Table 2 shows the result of multivariable Cox proportional hazard model for survival after recurrence. Recurrence-free duration of ≥9 months showed significant relationship to overall survival (hazard ratio [HR], 0.257; 95% CI, 0.164–0.403; P < 0.001), living donor (HR, 0.430; 95% CI, 0.210–0.882; P = 0.021) along with α-FP ≥ 100 U/L at the time of recurrence (HR, 1.689; 95% CI, 1.059–2.695; P = 0.028), recurrence in bone (HR, 2.304; 95% CI, 1.399–3.794; P = 0.001), and everolimus within 3 months after recurrence (HR, 0.354; 95% CI, 0.141–0.889; P = 0.027). Fig. 1 shows the survival curve of patients according to the recurrence-free duration after LT divided by 9 months.
Table 2. Multivariable Cox proportional HR of potential risk factors for survival after recurrence.
HR, hazard ratio; CI, confidence interval; BMI, body mass index; MELD, model for end-stage liver disease; LRT, locoregional therapy; LT, liver transplantation; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; RT, radiotherapy; TACI, transarterial chemoinfusion.
Fig. 1. Survival curves after recurrence according to the recurrence-free duration divided by 9 months analyzed by multivariable Cox proportional hazard ratio (HR). CI, confidence interval.
Everolimus treatment within 3 months after recurrence in survival
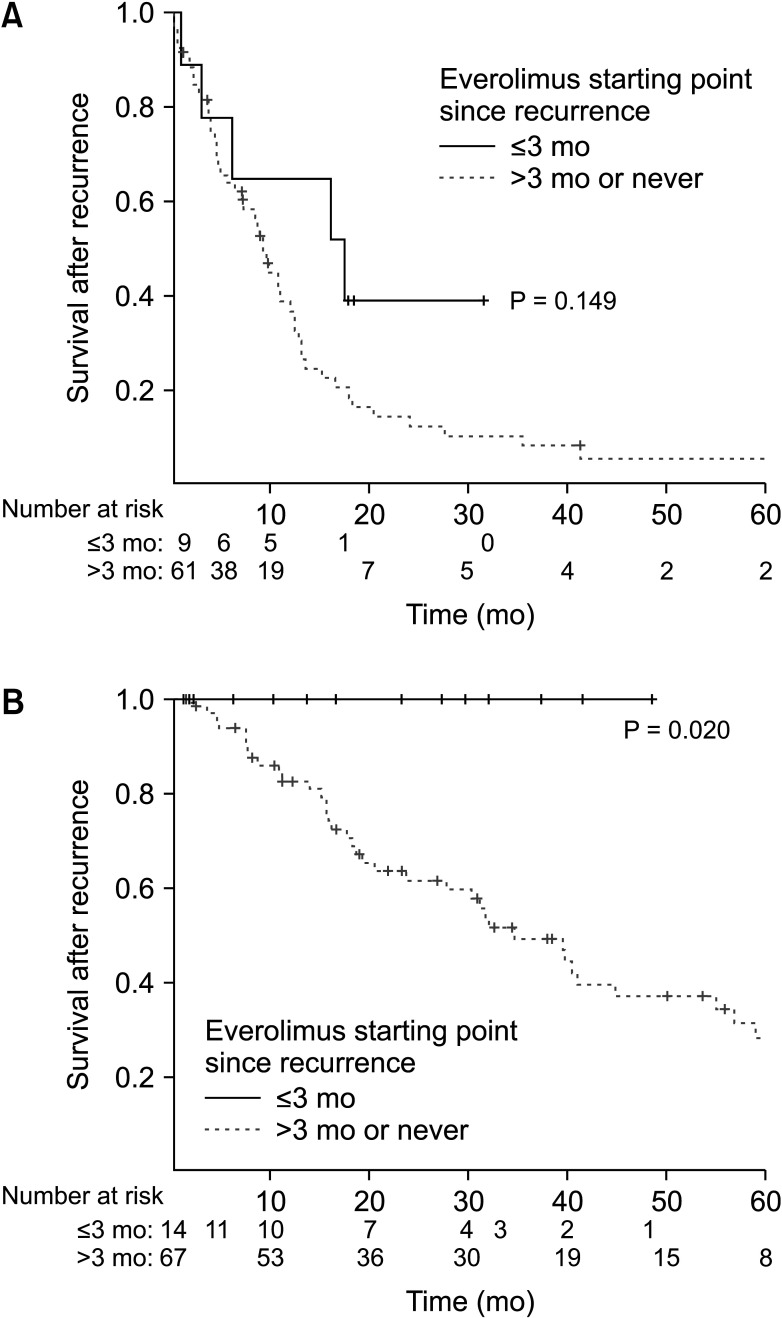
When subgroup analyses by dividing the study group based on the recurrence-free duration of 9 months, everolimus treatment within 3 months after recurrence showed a different impact on survival. In patients with recurrence within 9 months posttransplantation, everolimus treatment within 3 months did not show improved outcome based on Kaplan-Meier log-rank test (P = 0.149). However, in patients with recurrence-free duration of more than 9 months, everolimus treatment within 3-months after recurrence showed improved outcome (P = 0.020, Fig. 2).
Fig. 2. Survival curves based on Kaplan-Meier log-rank test according to the early initiation of everolimus within 3 months after recurrence, stratified by recurrence-free duration of 9 months. Recurrence within 9 months (A) and after 9 months (B) after liver transplantation.
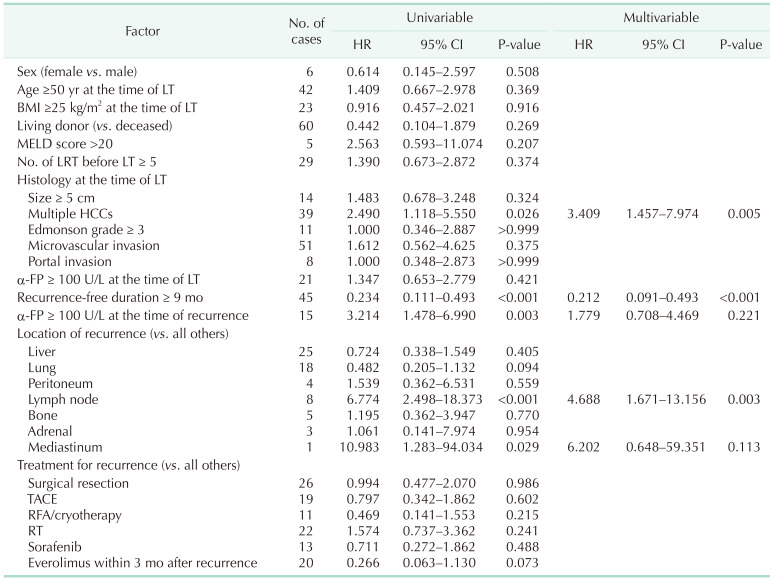
Risk factors for survival after recurrence in patients with solitary recurrence in single organ
Table 3 shows the result of multivariable Cox proportional hazard model for survival after recurrence in patients with solitary recurrence in single organ. Recurrence-free duration of ≥9 months showed significant relationship to overall survival (HR, 0.212; 95% CI, 0.091–0.493; P < 0.001), multiple HCC at the time of LT (HR, 3.409; 95% CI, 1.457–7.974; P = 0.005) and solitary recurrence in lymph node (HR, 4.688; 95% CI, 1.671–13.156; P = 0.003).
Table 3. Multivariable Cox proportional HR of potential risk factors for survival after recurrence in patients with solitary recurrence in a single organ.
HR, hazard ratio; CI, confidence interval; BMI, body mass index; MELD, model for end-stage liver disease; LRT, locoregional therapy; LT, liver transplantation; HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; RT, radiotherapy.
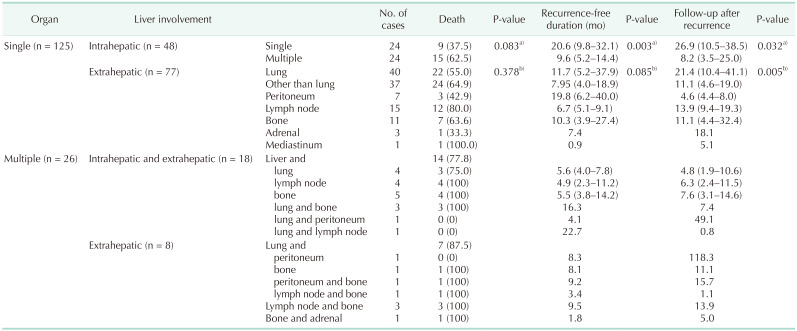
Median follow-up durations after recurrence according to the site and number of recurrence
Table 4 shows the median recurrence-free durations and median follow-up durations after recurrence according to the site and number of recurrences. In a patient subgroup with single organ recurrence, patients with single intrahepatic recurrence had longer median recurrence-free duration (20.6 months; IQR, 9.8–32.1 months) compared to patients with multiple intrahepatic recurrence (median, 9.6 months; IQR, 5.2–14.4 months; P = 0.003). Patients with single intrahepatic recurrence also had longer median follow-up duration after recurrence (26.9 months; IQR, 10.5–38.5 months) compared to patients with multiple intrahepatic recurrence (8.2 months; IQR, 3.5–25.0 months; P = 0.032). In a patient subgroup with single recurrence, patients with lung recurrence had longer median follow-up duration (21.4 months; IQR, 10.4–41.1 months) compared to patients with single extrahepatic recurrence other than lung (11.1 months; IQR, 4.6–19.0 months; P = 0.005).
Table 4. Deaths, median recurrence-free duration, and median follow-up after recurrence according to the involved organ.
Values are presented as number only, number (%), median only, or median (interquartile range).
a)Comparison between single intrahepatic metastasis and multiple hepatic metastasis; b)comparison between lung metastasis and extrahepatic metastasis other than lung.
DISCUSSION
This study analyzed the risk factors related to survival in patients who experienced recurrence of HCC after LT. While LT for HCC is performed in many centers worldwide, reports focusing on the prognosis of patients who experience recurrence after LT are scarce. The study published by Shin et al. [10] reported that major vascular invasion, poorly differentiated tumor, unresectable disease, and bony metastasis were significant factors related to prognosis. Time of recurrence divided by 6 months was only significant in the univariable analysis.
While most of the studies on HCC recurrence after LT focused on the risk factors of HCC recurrence after LT, this study focused on the prognosis after the HCC recurrence after LT. Among the 712 patients who underwent LT for HCC during the period, recurrence occurred in 151 patients (21.2%). The reason we focused on the prognosis of patients with HCC recurrence after LT was that management of those patients is still the point of interest for transplant clinicians. There is no settled guideline for these patients and they undergo treatment and management on a case-by-case basis.
The key finding of this study is that patients who have a recurrence-free duration of ≥ 9 months show significantly superior survival compared to patients who do not. Nine months as a cutoff was analyzed using received operating characteristics (AUC, 0.796; 95% CI, 0.717–0.875; P < 0.001; sensitivity of 0.848 and specificity of 0.689). Multivariable Cox proportional hazard model also revealed that recurrence-free duration of ≥9 months has a positive impact on survival after recurrence (HR, 0.257; 95% CI, 0.164–0.403; P < 0.001); it was also significant in patients with solitary recurrence in a single organ (HR, 0.212; 95% CI, 0.091–0.493; P < 0.001). Living donor LT (HR, 0.430; 95% CI, 0.210–0.882; P = 0.021) and everolimus treatment within 3 months after recurrence (HR, 0.354; 95% CI, 0.141–0.889; P = 0.027) also showed to be a positive impact on postrecurrence survival while α-FP ≥ 100 U/L at the time of recurrence (HR, 1.689; 95% CI, 1.059–2.695; P = 0.028), and recurrence in bone (HR, 2.304; 95% CI, 1.399–3.794; P = 0.001) were negative prognostic factors. In patients with solitary recurrence in a single organ, multiple HCC at the time of LT (HR, 3.409; 95% CI, 1.457–7.974; P = 0.005), and recurrence in lymph node (HR, 4.688; 95% CI, 1.671–13.156; P = 0.003) were significant risk factors for survival. Treatment modalities received by the patients were not factors related to survival.
The limitation of this study is that this study is a retrospectively-designed study. There are various confounding factors that interfere in analyzing their true impact on outcome. A certain amount of recurrence-free duration being a risk factor for outcome shows that statistically analyzing the impact of prognostic factors was a difficult task. The small number of patients included with wide spectrum of background characteristics is also a reason for this result. Living donor LT showed favorable outcome compared to deceased donor LT, while deceased donor LT group had progressive tumor-related factors. The reason living donor LT showed favorable outcome is derived from the difference in the baseline characteristics and the low number of patients with deceased donor LT (n = 11). However, while recurrence-free duration is the outcome of prognostic factors, the duration itself can be considered as a prognostic factor starting from the time point of recurrence.
Generally, the location and number of recurrences are considered significant factors for prognosis. However, multiple organ recurrence (HR, 1.288; 95% CI, 0.714–2.325; P = 0.401) and multiplicity in single organ (HR, 1.265; 95% CI, 0.804–1.989; P = 0.310) were not statistically significant. To analyze the true risk of these factors requires more detailed analysis of a subgroup of patients. Although, we did not have a great enough number of patients for a multivariable analysis in certain subgroups, patients with a single intrahepatic recurrence had significantly longer median follow-up after recurrence (median, 26.9 months; IQR, 10.5–38.5 months) compared to multiple intrahepatic recurrence (median, 8.2 months; IQR, 3.5–25.0 months; P = 0.032). Patients who only had lung recurrence had longer median follow-up after recurrence (median, 21.4 months; IQR, 10.4–41.1 months) compared to extrahepatic metastasis other than lung (median, 11.1 months; IQR, 4.6–19.0 months; P = 0.005). These findings can be useful information for clinicians to manage patients.
Unfortunately, this study failed to reveal which treatment is effective for elongating the survival of recurred patients; besides early initiation of everolimus within 3 months after recurrence. While treatment strategies were planned on a case-by-case basis, the strategy was mainly based on using the most effective treatment for the patients. Surgical removal was planned when it was considered possible, while local ablative therapies or chemoembolization was used for deep intrahepatic recurrences. Patients with extrahepatic recurrence had similar strategies. When surgical resection was possible, surgery was performed, and systemic therapies were used when definite treatment was impossible. Everolimus was started as an immunosuppressant with an expectation of the anti-tumorigenic impact of the drug itself and lowering the dosage of tacrolimus. However, it is difficult to examine the effect of everolimus regarding the dosage and duration of the drug as well as selection bias for prescribing the drug for selected patients with a less invasive nature of recurrence. Therefore, the finding that everolimus in early-treated patients had a favorable outcome should be interpreted with caution. However, stratifying the patients based on recurrence-free duration of 9 months, the early everolimus-treated group showed a significantly different outcome. In patients expected with relatively favorable outcome after recurrence, early initiation of everolimus can be beneficial and needs further study.
The recurrence of HCC can be devastating both for the patient and clinician. However, every recurred case is different and there are still possibilities to improve the outcome for those patients. The aggressiveness of the original HCC at the time of LT, recurrence-free duration, α-FP level at the time of recurrence, and site of recurrence can become a clue for the clinician to estimate the prognosis of the patient. Although there are many aspects to be studied for this specific patient group, clinicians should do their best to plan treatment strategies for individual patients who experience recurrence of HCC after LT.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization, Project Administration: MK, JR, JWJ.
- Formal Analysis: MK, JR, JMK.
- Investigation: MK, JR, JMK, GSC.
- Methodology: MK, JR, GSC.
- Writing — Original Draft: All authors.
- Writing — Review & Editing: All authors.
References
- 1.Rustgi VK. Epidemiology of hepatocellular carcinoma. Gastroenterol Clin North Am. 1987;16:545–551. [PubMed] [Google Scholar]
- 2.Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, et al. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536–543. doi: 10.1097/01.SLA.0000059988.22416.F2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790–800. doi: 10.1097/00000658-199906000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 5.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Kwon CH, Kim JM, Joh JW, Paik SW, Kim BW, et al. Time of hepatocellular carcinoma recurrence after liver resection and alpha-fetoprotein are important prognostic factors for salvage liver transplantation. Liver Transpl. 2014;20:1057–1063. doi: 10.1002/lt.23919. [DOI] [PubMed] [Google Scholar]
- 7.Rhu J, Kim JM, Choi GS, Kwon CHD, Joh JW. Validation of the α-fetoprotein model for hepatocellular carcinoma recurrence after transplantation in an Asian Population. Transplantation. 2018;102:1316–1322. doi: 10.1097/TP.0000000000002136. [DOI] [PubMed] [Google Scholar]
- 8.Escartin A, Sapisochin G, Bilbao I, Vilallonga R, Bueno J, Castells L, et al. Recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2007;39:2308–2310. doi: 10.1016/j.transproceed.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 10.Shin WY, Suh KS, Lee HW, Kim J, Kim T, Yi NJ, et al. Prognostic factors affecting survival after recurrence in adult living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2010;16:678–684. doi: 10.1002/lt.22047. [DOI] [PubMed] [Google Scholar]