Abstract
The dopamine receptors (DRs) family includes 5 members with differences in signal transduction and ligand affinity. Abnormal DRs expression has been correlated multiple tumors with their clinical outcome. Thus, it has been proposed that DRs-targeting drugs—developed for other diseases as schizophrenia or Parkinson’s disease—could be helpful in managing neoplastic diseases. In this review, we discuss the role of DRs and the effects of DRs-targeting in tumor progression and cancer cell biology using multiple high-prevalence neoplasms as examples. The evidence shows that DRs are valid therapeutic targets for certain receptor/disease combinations, but the data are inconclusive or contradictory for others. In either case, further studies are required to define the precise role of DRs in tumor progression and propose better therapeutic strategies for their targeting.
Keywords: dopamine, dopamine receptor, DRD1, DRD2, thioridazine, glioblastoma, breast cancer, NSCLC
Introduction
The family of dopamine receptors (DRs) includes 5 G protein–coupled receptors—DRD1, DRD2, DRD3, DRD4, and DRD5—with different anatomical distribution, expression levels, and functional properties. 1 For example, DRD1 and DRD2 located in the brain play a role in memory and learning, but peripheral DRD1, DRD2, and DRD4 regulate renal function, blood pressure, and intestinal motility. 2,3 Alterations in DR-signaling have been identified in schizophrenia, Parkinson’s disease, Tourette’s syndrome, and attention deficit hyperactivity disorder. 3,4 Thus, multiple drugs that target the dopaminergic system have been developed. 1,2,5
The DRs are classified into 2 subfamilies based on the G proteins that mediate their signal transduction. 1,6 The D1-like subfamily includes DRD1 and DRD5, which are coupled to Gαs or Gαolf proteins, controlling the activation of the enzyme adenylyl cyclase (AC). The D2-like subfamily comprises DRD2, DRD3, and DRD4, coupled to Gαi/o, govern AC inhibition. Because of their opposite signaling, each subfamily seems to play contrary roles in cellular functions. For example, in neurons, DRD1 stimulation triggers PKA activation, leading to the phosphorylation of cAMP-regulated neuronal phosphoprotein (DARPP-32). 7,8 DARPP-32 phosphorylation is coupled to the de-inhibition of the phosphatase PP1, which dephosphorylate histones, regulating gene expression and the activity of multiple effector proteins such as transcription factors, ionotropic receptors, and ionic channels. 8 On the contrary, activation of D2-like receptors negatively regulates PKA and DARPP-32. 6,9
The interplay between DRs is further complicated by the differences in ligand affinity within the members of the subfamilies. DRD1 binds dopamine with 10-times lower affinity than DRD5 (Ki: 1 µM vs. 100 nM). 10 DRD3 and DRD4 display a similar affinity for dopamine (Ki: 10 nM) which is higher than that of DRD2 (Ki: 100 nM). 10 Furthermore, each DR has different pharmacological properties. 1,10
The idea that DRs could be playing key roles in tumor progression was first supported by the finding that cancer patients that consumed antipsychotic drugs simultaneously to the antineoplastic treatment displayed better clinical responses. 5,11 Further studies showed that schizophrenic or Parkinson’s disease patients who receive ziprasidone, asenapine, quetiapine, clozapine, or aripiprazole (agonists or non-selective antagonist of DRs), 11 or levodopa (a prodrug that yields a non-selective agonism of DRs) 12 have a lower risk of developing diverse types of cancer compared to the general population. 13,14 However, female schizophrenic patients treated with DRD2 antagonists of variable selectivity (haloperidol, risperidone, paliperidone, or amisulpride) have a higher risk of developing breast cancer. 15,16
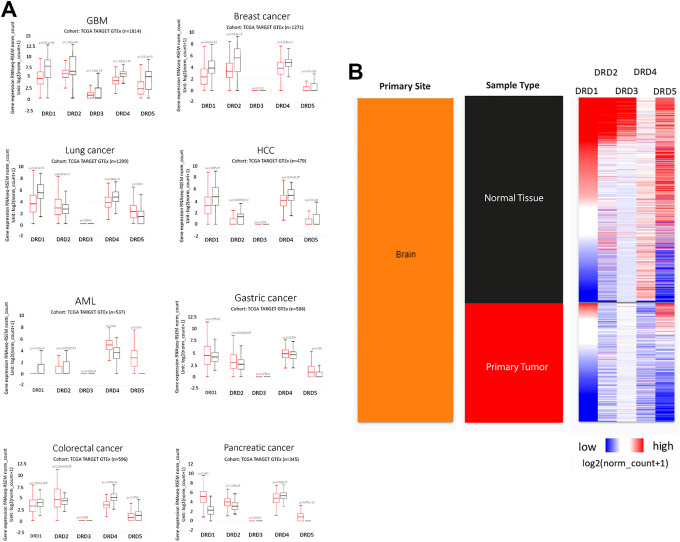
To date, multiple studies have shown that DRs are differentially expressed in several tumors and that each tumor type has a specific pattern of DRs expression. For example, DRD4 is overexpressed only in Acute Myeloid Leukemia (AML), and DRD3 is expressed only in glioblastoma but at lower levels than in normal brain (Figure 1). Alterations in the expression of DRs have been reported not only in cancer cells but also in tumor-associated cells. Furthermore, forced changes in DRs expression alters diverse functions of cancer cells, indicating that modulation of the receptors’ activity impacts tumor biology (Table 1). Thus, DRs have been pointed as potential therapeutic targets to improve clinical response in cancer patients. 2,5,11,14
Figure 1.
Dopamine receptors (DRs) gene expression in tumors. A, mRNA expression of DRs in different tumors (red boxes) and their corresponding normal tissues (black boxes). Statistical analysis was performed using Welch’s t-test. B, Comparison of the expression of DRs in brain tumors vs. normal tissue. Plots were generated using the UCSC Xena platform with data from the TCGA TARGET GTEx database.
Table 1.
Reported Effects of Dopamine Receptor-Targeting in Cancer.
| Target | Stimulus | Tumor type (reference) | Biological response elicited |
|---|---|---|---|
| All DRs | Receptor activation with dopamine | Multiple tumor types (lymphoblastoma, neuroblastoma, non-small cell lung cancer, and breast adenocarcinoma) 17 | Decreased cell viability |
| Colorectal cancer 18 | Increased cell viability | ||
| Gastric cancer 19 | Reduced invasive capacity Decreased migration ability |
||
| Gastric cancer 20 | Inhibition of cell proliferation | ||
| DRD2 | Receptor silencing or knock-out | Breast cancer 21 | Reduction in tumorsphere formation in some triple-negative cells without changes in cellular proliferation |
| Colorectal cancer 18 | No effect on cell viability | ||
| Pancreatic cancer 22 | Cancer cell growth inhibition in vitro and in vivo | ||
| DRD2 | Receptor blockage by thioridazine | Acute myeloid leukemia 23 | Decreased clonogenicity in leukemic stem cells Differentiation of leukemic stem cells No effects on normal HSCs |
| Acute myeloid leukemia 24 | Decreased leukemic burden in patients following 5-day treatment (effect associated with DRD2 expression at baseline) | ||
| Lung 25 | Decreased cell viability Decreased clonogenicity and self-renewal Chemotherapy sensitization Induction of proliferation (reduction of quiescent status) Impaired tumorigenicity in xenografts |
||
| Gastric cancer 26 | Decreased cell viability | ||
| Glioblastoma 27 | Decreased cell viability Decreased clonogenicity Reduced DRD2 expression Autophagy and apoptosis induction in vitro Impaired tumorigenicity in xenografts Autophagy induction in vivo |
||
| Receptor blockage by haloperidol | Multiple tumor types (lymphoblastoma, neuroblastoma, non-small cell lung cancer, and breast adenocarcinoma) 17 | Decreased cell viability | |
| Glioblastoma 28 | Decreased cell viability Decreased clonogenic capacity Sensitization to EGFR inhibition in vitro Inhibition of tumor growth in xenografts and increased survival |
||
| Glioblastoma 27 | No change in cell viability | ||
| Receptor blockage by pimozide | Multiple tumor types (lymphoblastoma, neuroblastoma, non-small cell lung cancer, and breast adenocarcinoma) 17 | Decreased cell viability Decreased clonogenicity |
|
| Pancreatic cancer 22 | Cell-cycle arrest in vitro
Apoptosis induction |
||
| Receptor blockage by ONC201 | Glioblastoma 29 | Apoptosis induction in vitro
Chemo- and radio-sensitization Inhibition of tumor growth in vivo |
|
| Colorectal cancer 18 | Decrease cell viability | ||
| Receptor inhibition by trifluoperazine | Glioblastoma 27 | Decreased cell viability Decreased clonogenicity Reduced DRD2 expression |
|
| DRD2 | Receptor activation by quinpirole | Lung 30 | Inhibition of cell proliferation Decreased clonogenicity Reduced invasive capacity Inhibition of tumor growth in xenografts |
| Breast 21 | Increased self-renewal capacity in CSCs | ||
| Gastric 20 | Inhibition of cell proliferation | ||
| Glioblastoma 28 | Increased cell proliferation | ||
| Receptor activation by bromocriptine | Acute myeloid leukemia 31 | Decreased cell viability Decreased clonogenicity Differentiation (CD11b upregulation) |
|
| DRD4 | Receptor silencing | Glioblastoma 32 | Decreased cell proliferation Cytotoxicity (associated with accumulation of autophagic vacuoles) |
| DRD4 | Receptor blockage by L-741742 | Glioblastoma 32 | Decreased cell viability Decreased clonogenicity Sensitization to temozolomide in clonogenic assays Cell cycle arrest and apoptosis induction Inhibition of tumor growth in xenografts Increased survival of tumor-bearing mice (in combination with temozolomide) |
| Glioblastoma 28 | Decreased clonogenicity | ||
| Receptor blockage by PNU 96145E | Glioblastoma 32 | Decreased cell viability Decreased clonogenicity Sensitization to temozolomide in clonogenic assays Cell cycle arrest and apoptosis induction Inhibition of tumor growth in xenografts Increased survival of tumor-bearing mice (in combination with temozolomide) |
However, the possible application of drugs that target DRs in cancer therapy is still unclear. We found that most of the drugs studied have moderate or low specificity, suggesting that the reported biological effects may be (at least partially) caused by the activity of the drugs on other DRs, other GPCRs, or other cellular targets (see Table 2). For example, pimozide, a DRD2 antagonist employed as second-line therapy for Tourette’s Disorder that has shown antineoplastic effects, can also bind to σ receptors, the serotonin receptor 5-HT7, or calmodulin. 33 Despite their reduced specificity, some of the drugs that target DRs have advantages that make them attractive candidates for developing new anti-cancer therapies, like FDA-approval or specific biodistribution patterns.
Table 2.
Selectivity of Dopamine Receptor-Targeting Drugs Employed in Cancer Studies.
| Drug | Main target | Action | pKi for DRs * | pKi for other targets * | Studied in |
|---|---|---|---|---|---|
| Dopamine | DRs | Non-selective agonist | D4: 7.6 (full) D3: 6.4-7.3 (full) D5: 6.6 (full) D2: 4.7-7.2 (full) D1: 4.3-5.6 (full) |
None | GBM
34
BC 34 -37 NSCLC 38 HCC 39 |
| Chlorprothixene | DRs | Non-selective antagonist | No data | No data | AML 40 |
| Clozapine | DRs | Non-selective antagonist | D4: 7.5 D1: 6.9 D5: 6.6 D2: 5.8-6.9 D3: 5.2-6.3 |
H1: 8.8-9.6 5-HT2B: 8.0-8.8 5-HT2A: 7.6-9.0 (inverse agonist) 5-HT2C: 7.4-8.7 (inverse agonist) 5-HT6: 7.8-8.1 (inverse agonist) 5-HT1D: 8.0 (inverse agonist) 5-HT1A: 6.8-6.9 (full agonist) 5-HT1B: 6.2 (full) |
AML
23
BC 11 |
| A77636 | D1 | Selective agonist | D1: 8.7 (full) | None | BC 41 |
| Fenoldopam | D1 | Selective agonist | D1: 6.5-7.9 (full) D4: 6.5 (full) |
None | BC 37 |
| SCH-23390 | D1 | Selective agonist | D1: 7.5-9.5 D5: 7.4-9.5 |
Ion channels (Kir2.3 y Kir3.2) | BC 36,42 |
| SCH-39166 | D1 | Antagonist | D1: 8.3 D5: 8.3 |
None | BC 37 |
| Bromocriptine | D2 | Agonist | D2: 7.3-8.3 (full) D1: 7.6-8.2 (partial) D3: 7.1-8.2 (partial) D4: 6.4 # D5: 6.3 (full) |
5-HT2B: 8.9 (full) 5-HT2A: 8.2 (full) 5-HT1D: 8.0 (partial) α2A-AR: 8.0 # 5-HT1A: 7.9 (partial) α2C-AR: 7.6 # 5-HT6: 7.5 (full) α2B-AR: 7.5 # 5-HT1B: 6.5 (partial) 5-HT2C: 6.1 (partial) |
HCC
43,44
AML 31,45,46 NSCLC 47 |
| Quinpirole | D2 | Specific agonist | D4: 7.5 (full) D3: 6.4-8.0 (full) D2: 4.9-7.7 (full) |
5-HT1A: 5.8 (full) 5-HT2B: 5.0-6.5 (full) 5-HT2A: 5.0-5.5 (full) 5-HT2C: 5.0-5.5 (full) |
GBM
28
BC 48 NSCLC 30,49,38 |
| Aripiprazole | D2 | Agonist | D2: 9.1 (partial) | 5-HT1A: 8.2 (partial) 5-HT2A: 7.5-8.1 (partial) 5-HT2C: 7.6 (partial) H1: 7.5 # 5-HT1D: 7.2 (full) 5-HT1B: 6.1 (full) |
BC 11,48 |
| Cabergoline | D2 | Agonist | D2: 9.0-9.2 (partial) D3: 9.1 (partial) D5: 7.7 (full) D4: 7.3 (full) D1: 6.7 (full) |
5-HT2B: 8.9 (full) 5-HT2A: 8.2 (full) 5-HT1D: 8.1 (partial) α2A-AR: 7.9 # α2C-AR: 7.7 # 5-HT1A: 7.7 (full) α2B-AR: 7.1 # 5-HT1B: 6.3 (full) 5-HT2C: 6.2 (full) |
BC
37
NSCLC 38 |
| Apomorphine | D2 | Agonist | D4: 8.4 (partial) D5: 6.4-7.8 (partial) D3: 6.1-7.6 (partial) D2: 5.7-7.5 (partial) D1: 5.3-6.2 (full) |
α2C-AR: 7.4 # α2B-AR: 7.2 # 5-HT2C: 7.0 # 5-HT1A: 6.9 (partial) 5-HT2A: 6.9 # 5-HT2B: 6.9 # α2A-AR: 6.9 (partial) |
NSCLC 50 |
| Haloperidol | D2 | Antagonist | D4: 8.7-8.8 D2: 7.4-8.8 D3: 7.5-8.6 D5: 6.3 D1: 6.2 |
5-HT2A: 6.7-7.3 5-HT1D: 6.6 5-HT7: 6.3-6.6 5-HT2B: 5.8-6.4 H1: 5.7-6.1 5-HT1A: 5.7-5.8 |
GBM
28
AML 14 NSCLC 14 BC 14 |
| Ziprasidone | D2 | Antagonist | D2: 8.6 | 5-HT2A: 8.8-9.5 5-HT1D: 9.0 (full agonist) 5-HT2C: 7.9-9.0 (inverse agonist) 5-HT1A: 7.9-8.9 (partial agonist) 5-HT7: 8.4 (inverse agonist) 5-HT1B: 8.3 (full agonist) H1: 7.4-7.8 |
BC 11 |
| Amisulpride | D2 | Antagonist | D2: 7.8-8.0 | None | BC 15 |
| Palperidone | D2 | Antagonist | No data | No data | BC 15 |
| Asenapine | D2 | Antagonist | D2: 8.9 | 5-HT2A: 10.2 H1: 9.8 5-HT1D: 8.4 (full agonist) 5-HT2C: 6.1 (inverse agonist) 5-HT1A: 8.0-8.3 (full agonist) 5-HT1B: 8.1 (full agonist) |
BC 11 |
| Quetiapine | D2 | Antagonist | D2 receptor: 7.2 | H1: 8.0-8.7 5-HT2A: 6.4-7.0 5-HT1A: 6.5-6.6 (full agonist) 5-HT1D: 5.7 (full agonist) |
BC 11 |
| Thioridazine | D2 | Antagonist | D1: 7.0 D5: 5.6 |
5-HT2A: 7.4-8.0 5-HT2C: 7.2-7.3 5-HT6: 7.2 (inverse agonist) 5-HT1A: 7.1 |
GBM
28
HCC 43 BC 21,51 AML 23,24,46 NSCLC 25,52 |
| Risperidone | D2 | Antagonist | D2: 9.4 D3: 7.0 |
5-HT2A: 9.3-10.0 (inverse agonist) α1A-AR: 8.4 5-HT1D: 7.8-8.0 5-HT2C: 6.1 (inverse agonist) 5-HT1B: 6.6-7.3 5-HT1A: 6.4-6.5 5-HT6: 5.6 |
GBM
28
BC 15 |
| Trifluoperazine | D2 | Antagonist | D2: 8.9-9.0 D4: 7.4 |
5-HT2A: 7.9 H1: 7.2 5-HT2C: 6.4 |
GBM
53,54
HCC 55,56 NSCLC 57 |
| l-Stepholidine | D2 | Antagonist | D2: 7.9 | None | BC 42 |
| Domperidone | D2 | Antagonist | D2: 7.9-8.4 D3: 7.1-7.6 |
None | HCC 39 |
| Pimozide | D2 | Antagonist | D2: 7.0-8.8 D3: 7.0-8.6 |
5-HT2A: 7.1-7.7 5-HT1A: 6.8 H1: 6.2 Ion channels (Kir3, Cav3.1, Cav3.3, Cav3.2) |
GBM
14,33
HCC 33,56 BC 14,33,58 AML 33 NSCLC 33,58 |
| L-741742 | D4 | Antagonist | D4: 8.5 | None | GBM 32 |
| PNU 9641E | D4 | Antagonist | No data | No data | GBM 32 |
Abbreviations: 5-HTx, 5-hydroxytryptamine receptors; Hx, histamine receptors; Kir, Inwardly-rectifier potassium channel; CaV, voltage-gated calcium channels; α-AR, alpha-adrenoceptors; GBM, Glioblastoma multiforme; BC, Breast cancer; NSCLC, Non-small cell lung cancer; HCC, hepatocellular carcinoma; AML, acute myeloid leukemia.
* The pKi values were obtained from IUPHAR website 59 and are ordered from highest to lowest.
# The drug functions as antagonist.
Herein we compile and discuss evidence of the role of DRs in cancer. We mainly focused on: i) the expression of the DRs in high-prevalence human tumors and its correlation with the clinical outcome; ii) the functions that DRs can modulate in cancer cells; iii) the differences in DRs expression/function between specific subtypes of a particular tumor; and iv) the role of DRs in the subpopulation of cancer stem cells (CSCs).
DRs in Glioblastoma (GBM)
Gliomas represent 80% of the malignant tumors from the brain. GBM, the most frequent and aggressive form of glioma, has a 1-year survival rate of 37.4% and a median survival time of 18 months. 60 Analysis performed using gene-expression data from The Cancer Genome Atlas (TCGA) showed that increased expression of DRs and enzymes participating in dopamine synthesis correlate with GMB patients survival. 34
The 2 members of the D2 family, DRD2 and DRD4, are upregulated in GBM tumors compared to adjacent normal tissue. 32,61 High expression of DRD2 or DRD4 is an independent predictor of reduced survival in GBM patients. 32,61 Increased DRD2 expression is found more frequently in primary GBM than in secondary GBM—which presents a significantly better prognosis. 61,62
Mechanistic studies have shown that: i) DRD2 expressed by GBM cells is activated through autocrine signaling by dopamine, and ii) DRD2 activation triggers mitogenic signals and induces phenotypic changes that favor tumor progression. 2,18,28,34 Accordingly, DRD2 silencing inhibits proliferation of patient-derived GBM cells in culture and reduces the growth of xenografts in mice. 28
The importance of D2-like receptors in GBM progression has been corroborated in studies employing the antipsychotic drug trifluoperazine, a DRD2/DRD4 antagonist. In vitro, trifluoperazine reduces the proliferation and motility of GBM cells by a calmodulin-dependent mechanism. 53 Trifluoperazine is also effective in GBM xenografts, where it inhibits tumor growth and reduces the number of metastatic lesions. 53 Similarly, the small molecule ONC201, a competitive antagonist of DRD2 and DRD3, 29,63 induces apoptosis of GBM cells, including those resistant to temozolomide, bevacizumab, or radiation. 29 In mouse models, ONC201 crosses the blood-brain barrier, inhibiting GBM growth and increasing the median survival time when combined with radiotherapy. 29,64 To date, clinical trials using ONC201 have shown signs of efficacy in biomarker-defined recurrent GBM patients, 64,65 as well as in pediatric and adult H3 K27M-mutant glioma. 65 -67 Noteworthy, ONC201 is also in Phase II clinical trials for AML, 68,69 breast cancer, 69,70 colorectal cancer, 69,71 lung cancer, 71 endometrial cancer, 69 -71 and multiple myeloma. 72
The key role of D2-like receptors in GBM progression led to the analysis of their participation in CSCs biology. DRD2 is expressed in glioma stem cells incrementing the malignancy of tumors. 2,62 DRD2 silencing, 62 but also DRD4 silencing 32 or trifluoperazine treatment, 53 reduces the clonogenicity of GBM cells, an in vitro subrogate measurement of the CSC content. ONC201 reduces the self-renewal of glioblastoma CSCs in vitro 64 and inhibits the proliferation of CSCs in 3D neurospheres culture established from freshly isolated human glioblastoma tumors. 29 Similarly, the DRD4-selective antagonists L-741742 and PNU-96415E decrease GBM stem cell viability and block their clonogenicity but do not affect normal neural stem cells. 32 Furthermore, L-741742 and PNU-96415E display a synergic effect when combined with the first-line cytotoxic agent temozolomide. 32
Altogether, the above evidence shows that DRD2 and DRD4 can be considered therapeutic targets in GBM and suggest that DRD2/DRD4 antagonist as adjuvant therapy would be beneficial for GBM patients. The efficacy of ONC21 in clinical trials supports this hypothesis, but other drugs are still to be tested in patients.
On the other hand, the role of the D1-like receptors in GBM is much less clear. GBM cell lines overexpress DRD1, DRD2, and DRD5 in culture. 2,18,34 Thus, it is possible that the overexpression of those receptors promotes aggressive phenotypes. It has been reported that reduced levels of DRD1 and DRD5 mRNAs favor the survival of GBM patients. 34 However, DRD5 activation functions as an anti-oncogenic signal in GBM cells, inducing autophagic cell death. 73 This contradictory evidence shows that there is not enough information to consider that modulation of D1-like receptors would be beneficial for GBM patients and calls for further studies.
DRs in Breast Cancer
Breast cancer is the most frequent cancer type in women worldwide, causing more than 600,000 deaths per year. 74 Expression of DRs is higher in malignant breast tumors than in benign ones, which, in turn, have increased levels of the receptors than normal mammary tissue. 13 Exogenous administration of dopamine (the natural agonist for all DRs) reduces tumor growth and angiogenesis in vivo. 35,36,75 However, it is unclear if those effects are mediated by a subfamily or a specific dopamine receptor. Moreover, studies aimed to elucidate the role of specific receptors in breast cancer have shown contradictory results.
Breast cancer patients with tumoral DRD1 overexpression have reduced overall and recurrence-free survival compared to patients that do not express the receptor. 37 DRD1 is overexpressed in breast cancer cell lines negative for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), suggesting that its signaling is important for the biology of triple-negative (TN) cancer cells. 37,41 Accordingly, the compound A77636, a DRD1 selective antagonist, inhibits proliferation and motility, and triggers apoptosis and autophagy in TN breast cancer cells, but lacks effect in normal epithelial cells. A77636 also reduces osteolytic metastasis in vivo, indicating that the D1-mediated dopaminergic signaling is essential for establishing bone metastasis. 41
However, other authors report that DRD1-signaling impairs breast cancer progression. Dopamine or D1-like agonists reduce the viability and promote apoptosis in TN breast cancer cells, but not in the luminal MCF-7 cells. 37 Apoptosis induction is dependent on DRD1 since it is blocked by the DRD1 silencing or by the addition of the antagonist SCH39166. 37 Furthermore, the DRD1 agonist fenoldopam (which has only peripheral effects due to its absence of brain penetration) inhibits the growth of TN xenotransplants by activating the DRD1/cyclic guanosine 3,’ 5’-monophosphate (cGMP)/protein kinase G (PKG) pathway. 37
In luminal breast cancer cells, the use of fenoldopam or l-stepholidine reduces migration and invasion in a DRD1-dependent manner. 42 The same study demonstrated that fenoldopam or l-stepholidine administration to mice isotransplanted with 4T1 breast cancer cells blocks lung metastasis but does not change tumor growth. 42 On the other hand, administration of the DRD1 antagonist SCH-23390 to mice xenotransplanted with doxorubicin-resistant luminal breast cancer cells promotes tumor growth and blocks the synergic inhibitory effect of dopamine and sunitinib (a multi-targeted receptor tyrosine kinase inhibitor) on tumor growth. 36 These results suggest that the role of DRD1 may be different in TN than in luminal breast cancer cells.
Besides, effects elicited by DRD1 activation may vary in different subsets of cancer cells. Dopamine decreases the fraction of cells with the CSC-associated immunophenotype CD44+/CD24- in MCF/Adr cells, 36 whereas the DRD1 antagonist SCH-23390 increases such fraction. In agreement, activation of the DRD1 with fenoldopam reduces the CD44+/CD24−, ALDH+, and mammosphere-forming fractions in 4T1 cells. 42 Thus, it has been suggested that the population of breast cancer stem cells within luminal cell lines is particularly sensitive to the DRD1 activation, an idea supported by the fact that the large majority of CD44+/CD24− cells express DRD1. 36 However, additional studies are still required to fully clarify the role of DRD1 in luminal breast CSCs. Such studies must evaluate the effect of DRD1 activation on transcriptional and functional responses displayed only by breast CSCs, using proper methodologies beyond immunophenotype. 76 Additionally, the role of DRD1 in CSCs from TN or HER2+ tumors is still to be clarified.
DRD2 also plays an important role in breast cancer progression. DRD2 is overexpressed in breast cancer human samples, as well as in cell lines with different ER, PR, and HER2 expression. 13 However, the TN cells displayed the highest DRD2 levels. 13 The inhibition of DRD2 expression with a microRNA (miR-4301) suppresses the proliferation and induces apoptosis in diverse human breast cancer cell lines, suggesting that DRD2 favors breast cancer progression. 13 Yet, the administration of cabergoline, a DRD2 agonist, lacks effect in xenotransplants of TN breast cancer cells. 37
Additional evidence suggests that DRD2 activation is essential for maintaining the CSC pool, especially in TN cell lines. Quinpirole, a DRD2/DRD3 agonist, increases the mammosphere-forming efficiency in SUM-149 cells. 21 Congruously, DRD2 silencing or the exposure to different D2-like antagonists, including thioridazine, inhibits mammosphere formation by impairing activation of STAT3/IL-6 pathway. 21 Interestingly, thioridazine, an antipsychotic drug employed before 2005, is active against leukemia 23 and colorectal cancer stem cells, 77 suggesting that DRD2 may play similar roles in CSC maintenance in other tumor types. Thus, multiple authors have proposed the use of thioridazine or other DRD2-blocking drugs as adjuvant therapy for breast cancer. Nonetheless, it is unclear if those therapies would equally benefit all different subtypes of breast cancer. For example, in the luminal MCF-7/Adr cells, only 5%-15% of the CD44+/CD24− cells express DRD2, 36 suggesting that only a small fraction of cells within the CSC pool would respond to DRD2 inhibition.
DRs in Lung Cancer
Lung cancer is the most common cancer, and it causes more deaths than any other cancer worldwide. 78 Approximately 85% of the human lung tumors are non-small cell lung cancers (NSCLC); for those patients, the 5-year survival rate is below 20%. 79 Lung tumors express DRD2 and DRD4, 49,80 but DRD2 expression is reduced in NSCLC compared to normal lung tissu. 49 Furthermore, low DRD2 expression correlates with increased risk for larger tumors and more advanced TNM stages. 49 In vitro, DRD2 silencing by shRNA promotes cell proliferation and colony formation, whereas DRD2 overexpression has the opposite effect. 49 In agreement, DRD2-activation by apomorphine 50 or nanoparticled bromocriptine 47 decreases NSCLC cell proliferation. In vivo, activation of DRD2-signaling in cancer cells, either by DRD2 forced overexpression or by the administration of agonists, reduces tumor growth 38,49 and inhibits brain metastasis. 50 Similar effects have been reported in the subpopulation of lung CSCs. DRD2 is expressed in the majority of CD133+ CSCs from NSCLC human tumor samples and human cell lines, and the activation of the receptor reduces their viability, clonogenicity, and invasiveness, as well as in vivo tumor growth. 30
DRD2-signaling can also be triggered in non-cancer tumor cells, affecting NSCLC progression. DRD2 is overexpressed in the endothelium of NSCLC tumors, and such expression correlates with tumor stage and smoking history. 38 Administration of dopamine or DRD2 agonists (quinpirole or cabergoline) impairs the in vivo tumor growth of NSCLC iso- and xenotransplants. 38 Such effect is associated with reduced tumor angiogenesis and decreased infiltration of myeloid-derived suppressor cells (MDSCs). 38 So, it seems that endothelial DRD2 plays an anti-tumoral role in NSCLC.
The evidence above demonstrates that DRD2 activation may be beneficial for NSCLC patients since it reduces proliferation and stemness in cancer cells and may impair the activation of endothelial cells required for angiogenesis.
However, other reports have found favorable effects of DRD2 blockage, particularly with the drug thioridazine. Thioridazine reduces viability and clonogenicity, induces cell cycle arrest, and promotes apoptosis in NSCLC cell lines, and impairs tumor initiation and growth in vivo. 25,52 The drug also increases the sensitivity of NSCLC cells to non-targeted chemotherapy. 25 However, those studies did not analyze whether thioridazine effects are caused only by DRD2 blockage or by additional DRD2-independent mechanisms, as previously suggested. 37 Clarification of the thioridazine mechanism of action will promote the identification of drugs with similar activities.
DRs in Hepatocellular Carcinoma (HCC)
HCC is the most frequent hepatic cancer and is the fourth cause of cancer-related deaths worldwide. 81 HCC patients usually have a poor prognosis, with the exemption of those diagnosed early, for whom the 5-year survival rate is above 70%. 82 Only a few studies have analyzed the role of DRs in HCC progression. Normal hepatic cells and HCC cells express all dopamine receptors. 39 DRD5 is overexpressed in HCC tumor samples compared to normal adjacent tissue, whereas DRD1 expression is decreased. 43 However, the functional roles of such changes are still unclear.
On the other hand, there is enough evidence to support the anti-tumoral role of DRD2 in HCC. DRD2 activation inhibits proliferation and invasion, 39,44 promotes apoptosis, and blocks migration of HCC cells. 44 In vivo, dopamine reduces tumor growth and lung metastasis in a DRD2-dependent fashion since the effects are reverted by the simultaneous administration of dopamine and the DRD2 antagonist domperidone. 39 In agreement, the DRD2 agonist bromocriptine reduces tumor mass and increases overall survival of HHC-bearing mice. 39
As for other tumor types, it has been shown that thioridazine impairs tumorsphere formation and reduces the expression of CSC-associated genes, 43 suggesting that the DRD2 signaling may be different in CSCs and tumor-bulk HCC cells.
DRs in Acute Myeloid Leukemia (AML)
AML is the most common (62%) leukemia and generally has a poor prognosis. 83 It has been reported that human AML cell lines overexpress DRD2 and DRD4 and have low DRD1 and DRD3 expression. 45 Similar results have been found in animal models of myelodysplastic syndrome, 45 a condition associated with an increased risk of developing AML. Chlorprothixene, a wide-spectrum antagonist of DRs, reduces growth and induces apoptosis of AML cells from different subtypes and impairs tumor progression in vivo. 40
The available evidence points to DRD2 as a regulator of cell proliferation and cell death in AML, particularly in leukemia CSCs. Sachlos and collaborators identified that the DRD2 antagonist thioridazine induces differentiation in neoplastic pluripotent stem cells and AML blasts, but lacks effect on normal pluripotent or hematopoietic stem cells. 23 Further analysis showed that thioridazine reduces the engraftment of AML cells but not that of hematopoietic stem cells; such differential effect is caused by the absence of DRs expression in normal cells and the expression of multiple DRs in AML blast. 23 This landmark study showed that: i) leukemic stem cells overexpress DRs, ii) DRs expression in leukemic cells could be a prognosis marker, and iii) altering the dopaminergic signaling could be helpful in a subset of AML patients. 23
Subsequent reports analyzed the efficacy and safety of thioridazine, combined with cytarabine, in patients with recurrent or refractory AML. Oral administration of thioridazine gives rise to plasma concentrations similar to those employed in vitro by Sachlos et al, inducing partial responses in the fraction of patients with DRD2-positive disease at the beginning of the trial. 24 Even when thioridazine causes multiple toxic effects that limit its use, the study demonstrates that DR-mediated signaling is important for AML clinical progression. Nevertheless, as discussed above, thioridazine may not elicit its effects only through DRs. Therefore, these data should not be considered definitive proof that DRD2 (or any other DR) is a universal target in leukemia. For example, the treatment of cultures of AML cells with the DRD2 agonist bromocriptine induces apoptosis, reducing cell viability and colony formation. 45 Likewise, the combined treatment with bromocriptine and cytarabine has a synergic cytotoxic effect in AML cells. 45 Thus, the specific role of DRD2 in AML is still to be clarified. Further studies should analyze the participation of DRD2 using different models of AML with special focus on the CSC population.
DRs in Gastric Cancer
Normal gastric cells and gastric cancer cells express all dopamine receptors. 19 DRD2 is overexpressed in tumor samples compared to adjacent healthy tissues, correlating with a shorter survival of patients. 26 In contrast, gastric cancer cells in culture display reduced levels of DRD2. 19,84 In vitro activation of DRD2 with quinpirole, a specific agonist, inhibits the insulin-like growth factor 1 receptor (IGF-1 R)/Protein kinase B (PKB) pathway reducing cell proliferation. 19 Likewise, exogenous administration of dopamine to tumor-bearing mice inhibits tumor growth by activating DRD2 on endothelial cells, leading to activity suppression in the vascular endothelial growth factor-A receptor-2 (VGEFR2). 84 However, inhibition of DRD2 with thioridazine, a DRD2 antagonist, has also been reported to decrease gastric cancer cell growth. 26
In addition, DRD5 has been associated with cell growth of gastric cancer cells. The activation of DRD5 by the agonist SKF83959 suppresses cell growth by inhibiting mTOR functions and inducing autophagy, followed by cell death. 73 Thus, is it possible that DRD5 become a therapeutic target for gastric cancer in the future.
DRs in Colorectal Cancer (CRC)
In CRC, DRD2 is overexpressed in tumor samples. 18 In vitro, DRD2 activation with dopamine or the D2-like agonist sumanirole increase CRC cell number, whereas transient knockdown of DRD2 or DRD2 inhibition with antagonists (L-741626 or PG01037) generate the opposite effect. 18 ONC201, another selective antagonist of DRD2 and DRD3, also reduces cell viability. However, the effect of ONC201 is not altered by DRD2 knockout, indicating a DRD2-independent mechanism of action. 18
In a subgroup of CRC patients, the existence of DRD2 polymorphisms reduces protein expression 85 and is associated with an increased risk of CRC. 85,86 Furthermore, the dopamine content in malignant human colon tissue is reduced 3- to 10-fold compared to normal tissue, depending on the tumor stage. 85 The progressive reduction of dopamine and DRD2 levels caused by the polymorphisms leads to a reduction of intracellular cyclic AMP, an inhibitor of cell growth, promoting CRC progression. 85,86
DRs in Pancreatic Cancer
In pancreatic ductal adenocarcinoma (PDAC), DRD2 protein levels are increased in tumor samples compared with normal tissue. 22 DRD2 silencing by shRNA inhibits pancreatic cancer cell growth in vitro and in vivo. Similarly, treatment of pancreatic cancer cell lines with pimozide, an FDA-approved DRD2 antagonist, induces cell-cycle arrest and apoptosis. 22 L-741626, another DRD2 antagonist, generates similar effects, suggesting that pharmacologic blockade of DRD2 may be a promising therapeutic strategy for pancreatic cancer. A third DRD2 antagonist, olanzapine, sensitizes pancreatic CSCs to chemotherapeutic agents in vitro, but it is unclear if such effect is mediated exclusively by DRD2 inhibition. 87 Thus, independent studies report that DRD2-targeting could be beneficial for pancreatic cancer patients. On the contrary, no reports analyze the role of other DRs in this tumor type, suggesting that future research should focus on receptors beyond DRD2.
Conclusions and Final Considerations
We conclude that certain DRs can be considered therapeutic targets for specific tumor types. For example, DRD2/DRD4 inhibition in GBM or DRD1 activation in luminal breast cancer produces antitumoral effects. In such cases, future efforts should focus on: i) analyzing the efficacy of specific DR-targeting drugs in relevant models of disease; ii) identifying pertinent pharmacodynamic variables for the evaluation of therapeutic and adverse effects in future clinical trials; and iii) developing new drugs with increased specificity for the target.
On the other hand, the evidence is either inconclusive or contradictory for other receptor/disease combinations. For example, the roles of DRD5 in GBM or DRD1 in TN breast cancer need further clarification. Additional studies should define: i) whether DRs expression is associated with tumor stage and/or clinical outcomes; ii) the differences in DRs expression between tumor-bulk cells and CSCs; and iii) the functional consequences of genetic or pharmacological modulation of DRs in cancer cells. We recommend that when performing those studies, researchers take into consideration that: a) the same receptor can generate opposite responses in different subpopulations of cancer cells; b) the receptor expression levels may be crucial for the induction of specific responses; and c) available DRs-modulating drugs generate off-target effects and, thus, additional controls are required to demonstrate their specificity in the model of study. These considerations will increase the relevance of the data generated and facilitate the development of specific therapies for cancer patients.
Footnotes
Authors’ Note: All authors made substantial contributions to conception and design, compilation of published information, or interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by UNAM-PAPIIT IN219719 (M.A.V-V.) and UNAM-PAPIIT IA205421 (N.S-J.). A.R-C. is recipient of a M.Sc. fellowship from CONACYT (1003227).
ORCID iD: Marco A. Velasco-Velázquez, PhD  https://orcid.org/0000-0001-9717-0265
https://orcid.org/0000-0001-9717-0265
References
- 1. Beaulieu J-M, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. Sibley DR, ed. doi:10.1124/pr.110.002642 [DOI] [PubMed] [Google Scholar]
- 2. Wang X, Wang Z-B, Luo C, et al. The prospective value of dopamine receptors on bio-behavior of tumor. J Cancer. 2019;10(7):1622–1632. doi:10.7150/jca.27780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mishra A, Singh S, Shukla S. Physiological and functional basis of dopamine receptors and their role in neurogenesis: possible implication for Parkinson’s disease. J Exp Neurosci. 2018;12. doi:10.1177/1179069518779829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24(1):125–132. doi:10.1016/S0149-7634(99)00063-9 [DOI] [PubMed] [Google Scholar]
- 5. Weissenrieder JS, Neighbors JD, Mailman RB, Hohl RJ. Cancer and the dopamine D2 receptor: a pharmacological perspective. J Pharmacol Exp Ther. 2019;370(1):111–126. doi:10.1124/jpet.119.256818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol. 2019;39(1):31–59. doi:10.1007/s10571-018-0632-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beaulieu JM, Espinoza S, Gainetdinov RR. Dopamine receptors—IUPHAR review 13. Br J Pharmacol. 2015;172(1):1–23. doi:10.1111/bph.12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bateup HS, Svenningsson P, Kuroiwa M, et al. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci. 2008;11(8):932–939. doi:10.1038/nn.2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Romanelli RJ, Williams JT, Neve KA. Dopamine receptor signaling: intracellular pathways to behavior. In: The Dopamine Receptors. Humana Press; 2010:137–173. doi:10.1007/978-1-60327-333-6_6 [Google Scholar]
- 10. Seeman P. Historical overview: introduction to the dopamine receptors. In: The Dopamine Receptors. Humana Press; 2010:1–21. doi:10.1007/978-1-60327-333-6_1 [Google Scholar]
- 11. Roney MSI, Park SK. Antipsychotic dopamine receptor antagonists, cancer, and cancer stem cells. Arch Pharm Res. 2018;41(4):384–408. doi:10.1007/s12272-018-1017-3 [DOI] [PubMed] [Google Scholar]
- 12. Tacik P, Curry S, Fujioka S, et al. Cancer in Parkinson’s disease. Park Relat Disord. 2016;31:28–33. doi:10.1016/j.parkreldis.2016.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gholipour N, Ohradanova-Repic A, Ahangari G. A novel report of MiR-4301 induces cell apoptosis by negatively regulating DRD2 expression in human breast cancer cells. J Cell Biochem. 2018;119(8):6408–6417. doi:10.1002/jcb.26577 [DOI] [PubMed] [Google Scholar]
- 14. Lee JK, Nam DH, Lee J. Repurposing antipsychotics as glioblastoma therapeutics: potentials and challenges. Oncol Lett. 2016;11(2):1281–1286. doi:10.3892/ol.2016.4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu Chou AI, Wang YC, Lin CL, Kao CH. Female schizophrenia patients and risk of breast cancer: a population-based cohort study. Schizophr Res. 2017;188:165–171. doi:10.1016/j.schres.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 16. De Hert M, Peuskens J, Sabbe T, et al. Relationship between prolactin, breast cancer risk, and antipsychotics in patients with schizophrenia: a critical review. Acta Psychiatr Scand. 2016;133(1):5–22. doi:10.1111/acps.12459 [DOI] [PubMed] [Google Scholar]
- 17. Wiklund ED, Catts VS, Catts SV, et al. Cytotoxic effects of antipsychotic drugs implicate cholesterol homeostasis as a novel chemotherapeutic target. Int J Cancer. 2010;126(1):28–40. doi:10.1002/ijc.24813 [DOI] [PubMed] [Google Scholar]
- 18. Kline CLB, Ralff MD, Lulla AR, et al. Role of dopamine receptors in the anticancer activity of ONC201. Neoplasia. 2018;20(1):80–91. doi:10.1016/j.neo.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang H, Wu K, Ma J, Du Y, Cao C, Nie Y. Dopamine D2 receptor suppresses gastric cancer cell invasion and migration via inhibition of EGFR/AKT/MMP-13 pathway. Int Immunopharmacol. 2016;39:113–120. doi:10.1016/j.intimp.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 20. Ganguly S, Basu B, Shome S, et al. Dopamine, by acting through its D2 receptor, inhibits insulin-like growth factor-I (IGF-I)-induced gastric cancer cell proliferation via up-regulation of Krüppel-like factor 4 through down-regulation of IGF-IR and AKT phosphorylation. Am J Pathol. 2010;177(6):2701–2707. doi:10.2353/ajpath.2010.100617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tegowski M, Fan C, Baldwin AS. Thioridazine inhibits self-renewal in breast cancer cells via DRD2-dependent STAT3 inhibition, but induces a G1 arrest independent of DRD2. J Biol Chem. 2018;293(41):15977–15990. doi:10.1074/jbc.RA118.003719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jandaghi P, Najafabadi HS, Bauer AS, et al. Expression of DRD2 is increased in human pancreatic ductal adenocarcinoma and inhibitors slow tumor growth in mice. Gastroenterology. 2016;151(6):1218–1231. doi:10.1053/j.gastro.2016.08.040 [DOI] [PubMed] [Google Scholar]
- 23. Sachlos E, Risueño RM, Laronde S, et al. Identification of drugs including a dopamine receptor antagonist that selectively target cancer stem cells. Cell. 2012;149(6):1284–1297. doi:10.1016/j.cell.2012.03.049 [DOI] [PubMed] [Google Scholar]
- 24. Aslostovar L, Boyd AL, Almakadi M, et al. A phase 1 trial evaluating thioridazine in combination with cytarabine in patients with acute myeloid leukemia. Blood Adv. 2018;2(15):1935–1945. doi:10.1182/bloodadvances.2018015677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yue H, Huang D, Qin L, et al. Targeting lung cancer stem cells with antipsychological drug thioridazine. Biomed Res Int. 2016;2016:6709828. doi:10.1155/2016/6709828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mu J, Huang W, Tan Z, et al. Dopamine receptor d2 is correlated with gastric cancer prognosis. Oncol Lett. 2017;13(3):1223–1227. doi:10.3892/ol.2017.5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng HW, Liang YH, Kuo YL, et al. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis. 2015;6(5):e1753–e1753. doi:10.1038/cddis.2015.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li J, Zhu S, Kozono D, et al. Genome-wide shRNA screen revealed integrated mitogenic signaling between Dopamine Receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget. 2014;5(4):882–893. doi:10.18632/oncotarget.1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ralff MD, Lulla AR, Wagner J, El-Deiry WS. ONC201: a new treatment option being tested clinically for recurrent glioblastoma. Transl Cancer Res. 2017;6(suppl 7):S1239–S1243. doi:10.21037/tcr.2017.10.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roy S, Lu K, Nayak MK, et al. Activation of D2 dopamine receptors in CD133+ve cancer stem cells in non-small cell lung carcinoma inhibits proliferation, clonogenic ability, and invasiveness of these cells. J Biol Chem. 2017;292(2):435–445. doi:10.1074/jbc.M116.748970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lara-Castillo MC, Cornet-Masana JM, Etxabe A, et al. Repositioning of bromocriptine for treatment of acute myeloid leukemia. J Transl Med. 2016;14(1):261. doi:10.1186/s12967-016-1007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dolma S, Selvadurai HJ, Lan X, et al. Inhibition of dopamine receptor D4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell. 2016;29(6):859–873. doi:10.1016/j.ccell.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elmaci I, Altinoz MA. Targeting the cellular schizophrenia. Likely employment of the antipsychotic agent pimozide in treatment of refractory cancers and glioblastoma. Crit Rev Oncol Hematol. 2018;128:96–109. doi:10.1016/j.critrevonc.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 34. Caragher SP, Hall RR, Ahsan R, Ahmed AU. Monoamines in glioblastoma: complex biology with therapeutic potential. Neuro Oncol. 2018;20(8):1014–1025. doi:10.1093/neuonc/nox210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peters MAM, Meijer C, Fehrmann RSN, et al. Serotonin and dopamine receptor expression in solid tumours including rare cancers. Pathol Oncol Res. 2020;26(3):1539–1547. doi:10.1007/s12253-019-00734-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang S, Mou Z, Ma Y, et al. Dopamine enhances the response of sunitinib in the treatment of drug-resistant breast cancer: involvement of eradicating cancer stem-like cells. Biochem Pharmacol. 2015;95(2):98–109. doi:10.1016/j.bcp.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 37. Borcherding DC, Tong W, Hugo ER, et al. Expression and therapeutic targeting of dopamine receptor-1 (D1R) in breast cancer. Oncogene. 2016;35(24):3103–3113. doi:10.1038/onc.2015.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoeppner LH, Wang Y, Sharma A, et al. Dopamine D2 receptor agonists inhibit lung cancer progression by reducing angiogenesis and tumor infiltrating myeloid derived suppressor cells. Mol Oncol. 2015;9(1):270–281. doi:10.1016/j.molonc.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang QB, Zhang BH, Zhang KZ, et al. Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: with reference to nervous system. Oncogene. 2016;35(31):4122–4131. doi:10.1038/onc.2015.484 [DOI] [PubMed] [Google Scholar]
- 40. Du Y, Li K, Wang X, Kaushik AC, Junaid M, Wei D. Identification of chlorprothixene as a potential drug that induces apoptosis and autophagic cell death in acute myeloid leukemia cells. FEBS J. 2020;287(8):1645–1665. doi:10.1111/febs.15102 [DOI] [PubMed] [Google Scholar]
- 41. Minami K, Liu S, Liu Y, et al. Inhibitory effects of dopamine receptor D1 agonist on mammary tumor and bone metastasis. Sci Rep. 2017;7:1–12. doi:10.1038/srep45686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang L, Yao Y, Yong L, et al. Dopamine D1 receptor agonists inhibit lung metastasis of breast cancer reducing cancer stemness. Eur J Pharmacol. 2019;859:172499. doi:10.1016/j.ejphar.2019.172499 [DOI] [PubMed] [Google Scholar]
- 43. Lu M, Li J, Luo Z, et al. Roles of dopamine receptors and their antagonist thioridazine in hepatoma metastasis. Onco Targets Ther. 2015;8:1543–1551. doi:10.2147/OTT.S77373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu XF, Long HJ, Miao XY, Liu GL, Yao HL. Fisetin inhibits liver cancer growth in a mouse model: relation to dopamine receptor. Oncol Rep. 2017;38(1):53–62. doi:10.3892/or.2017.5676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liberante FG, Pouryahya T, McMullin MF, Zhang SD, Mills KI. Identification and validation of the dopamine agonist bromocriptine as a novel therapy for high-risk myelodysplastic syndromes and secondary acute myeloid leukemia. Oncotarget. 2016;7(6):6609–6619. doi:10.18632/oncotarget.6773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wojcicki AV, Kadapakkam M, Frymoyer A, Lacayo N, Chae HD, Sakamoto KM. Repurposing drugs for acute myeloid leukemia: a worthy cause or a futile pursuit? Cancers (Basel). 2020;12(2):441. doi:10.3390/cancers12020441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sheikhpour M, Sadeghizadeh M, Yazdian F, et al. Co-administration of curcumin and bromocriptine nano-liposomes for induction of apoptosis in lung cancer cells. Iran Biomed J. 2020;24(1):24–29. doi:10.29252/ibj.24.1.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee H, Kang S, Sonn JK, Lim Y. Dopamine receptor D 2 activation suppresses the radiosensitizing effect of aripiprazole via activation of AMPK. FEBS Open Bio. 2019;9(9):1580–1588. doi:10.1002/2211-5463.12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu X-Y, Zhang C-X, Deng L-C, et al. Overexpressed D2 dopamine receptor inhibits non-small cell lung cancer progression through inhibiting NF-κB signaling pathway. Cell Physiol Biochem. 2018;48(6):2258–2272. doi:10.1159/000492644 [DOI] [PubMed] [Google Scholar]
- 50. Singh M, Venugopal C, Tokar T, et al. Therapeutic targeting of the premetastatic stage in human lung-to-brain metastasis. Cancer Res. 2018;78(17):5124–5134. doi:10.1158/0008-5472.CAN-18-1022 [DOI] [PubMed] [Google Scholar]
- 51. Yin T, HE S, Shen G, YE T, GUO F, Wang Y. Dopamine receptor antagonist thioridazine inhibits tumor growth in a murine breast cancer model. Mol Med Rep. 2015;12(3):4103–4108. doi:10.3892/mmr.2015.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shen J, Ma B, Zhang X, et al. Thioridazine has potent antitumor effects on lung cancer stem-like cells. Oncol Lett. 2017;13(3):1563–1568. doi:10.3892/ol.2017.5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kang S, Hong J, Lee JM, et al. Trifluoperazine, a well-known antipsychotic, inhibits glioblastoma invasion by binding to calmodulin and disinhibiting calcium release channel IP3 R. Mol Cancer Ther. 2017;16(1):217–227. doi:10.1158/1535-7163.MCT-16-0169-T [DOI] [PubMed] [Google Scholar]
- 54. Pinheiro T, Otrocka M, Seashore-Ludlow B, et al. Reprint of: a chemical screen identifies trifluoperazine as an inhibitor of glioblastoma growth. Biochem Biophys Res Commun. 2018;499(2):136–142. doi:10.1016/j.bbrc.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 55. Jiang J, Huang Z, Chen X, et al. Trifluoperazine activates FOXO1-related signals to inhibit tumor growth in hepatocellular carcinoma. DNA Cell Biol. 2017;36(10):813–821. doi:10.1089/dna.2017.3790 [DOI] [PubMed] [Google Scholar]
- 56. Chen M-H, Yang W-LR, Lin K-T, et al. Gene expression-based chemical genomics identifies potential therapeutic drugs in hepatocellular carcinoma. PLoS One. 2011;6(11):e27186. Agoulnik I, ed. doi:10.1371/journal.pone.0027186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yeh CT, Wu ATH, Chang PMH, et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am J Respir Crit Care Med. 2012;186(11):1180–1188. doi:10.1164/rccm.201207-1180OC [DOI] [PubMed] [Google Scholar]
- 58. Dakir E-H, Pickard A, Srivastava K, et al. The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer. Oncotarget. 2018;9(79):34889–34910. doi:10.18632/oncotarget.26175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. IUPHAR/BPS. Guide to pharmacology. Published 2020. Accessed April 27, 2021. https://www.guidetopharmacology.org/
- 60. Barnholtz-Sloan JS, Ostrom QT, Cote D. Epidemiology of brain tumors. Neurol Clin. 2018;36(3):395–419. doi:10.1016/j.ncl.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 61. Prabhu VV, Madhukar NS, Gilvary C, et al. Dopamine receptor D5 is a modulator of tumor response to dopamine receptor D2 antagonism. Clin Cancer Res. 2019;25(7):2305–2313. doi:10.1158/1078-0432.CCR-18-2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Caragher SP, Shireman JM, Huang M, et al. Activation of dopamine receptor 2 prompts transcriptomic and metabolic plasticity in glioblastoma. J Neurosci. 2019;39(11):1982–1993. doi:10.1523/JNEUROSCI.1589-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Allen JE, Kline CLB, Prabhu VV, et al. Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget. 2016;7(45):74380–74392. doi:10.18632/oncotarget.11814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. He L, Bhat K, Ioannidis A, et al. Synergistic effects of the DRD2/3 antagonist ONC201 and radiation in glioblastoma. BioRxiv. 2020. doi:10.1101/2020.07.23.218446. [Google Scholar]
- 65. Arrillaga-Romany I, Chi AS, Allen JE, Oster W, Wen PY, Batchelor TT. A phase 2 study of the first imipridone ONC 201, a selective DRD 2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget. 2017;8(45):79298–79304. doi:10.18632/oncotarget.17837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chi AS, Tarapore RS, Hall MD, et al. Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neurooncol. 2019;145(1):97–105. doi:10.1007/s11060-019-03271-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hall MD, Odia Y, Allen JE, et al. First clinical experience with DRD2/3 antagonist ONC201 in H3 K27M-mutant pediatric diffuse intrinsic pontine glioma: a case report. J Neurosurg Pediatr. 2019;23(6):719–725. doi:10.3171/2019.2.PEDS18480 [DOI] [PubMed] [Google Scholar]
- 68. Borthakur G, Ishizawa J, DiNardo CD, et al. A phase I/II clinical trial of the first-in-class GPCR antagonist ONC201 in relapsed/refractory acute leukemias. Blood. 2016;128(22):3997–3997. doi:10.1182/blood.v128.22.3997.3997 [Google Scholar]
- 69. Prabhu VV, Morrow S, Rahman Kawakibi A, et al. ONC201 and imipridones: anti-cancer compounds with clinical efficacy. Neoplasia (United States). 2020;22(12):725–744. doi:10.1016/j.neo.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gatti-Mays M, Greer Y, Steinberg S, et al. Abstract OT2-07-04: a phase 2 study of ONC201 in recurrent/refractory metastatic breast cancer and advanced endometrial carcinoma. In: Cancer Research. Vol 78. American Association for Cancer Research (AACR); 2018: OT2-07-04-OT2-07-04. doi:10.1158/1538-7445.sabcs17-ot2-07-04 [Google Scholar]
- 71. Stein MN, Bertino JR, Kaufman HL, et al. First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin Cancer Res. 2017;23(15):4163–4169. doi:10.1158/1078-0432.CCR-16-2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tu Y, sheng He J, Liu H, et al. The imipridone ONC201 induces apoptosis and overcomes chemotherapy resistance by up-regulation of Bim in multiple myeloma. Neoplasia (United States). 2017;19(10):772–780. doi:10.1016/j.neo.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leng ZG, Lin SJ, Wu ZR, et al. Activation of DRD5 (dopamine receptor D5) inhibits tumor growth by autophagic cell death. Autophagy. 2017;13(8):1404–1419. doi:10.1080/15548627.2017.1328347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Harbeck N, Penault-Llorca F, Cortes J, et al. Breast cancer. Nat Rev Dis Prim. 2019;5(1):1–31. doi:10.1038/s41572-019-0111-2 [DOI] [PubMed] [Google Scholar]
- 75. Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. 2008;14(8):2502–2510. doi:10.1158/1078-0432.CCR-07-1778 [DOI] [PubMed] [Google Scholar]
- 76. Velasco-Velázquez MA, Velázquez-Quesada I, Vásquez-Bochm LX, Pérez-Tapia SM. Targeting breast cancer stem cells: a methodological perspective. Curr Stem Cell Res Ther. 2019;14(5):389–397. doi:10.2174/1574888x13666180821155701 [DOI] [PubMed] [Google Scholar]
- 77. Zhang C, Gong P, Liu P, Zhou N, Zhou Y, Wang YI. Thioridazine elicits potent antitumor effects in colorectal cancer stem cells. Oncol Rep. 2017;37(2):1168–1174. doi:10.3892/or.2016.5313 [DOI] [PubMed] [Google Scholar]
- 78. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 79. Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. In: The Lancet, Vol 378. Elsevier BV; 2011:1727–1740. doi:10.1016/S0140-6736(10)62101-0 [DOI] [PubMed] [Google Scholar]
- 80. Campa D, Zienolddiny S, Lind H, et al. Polymorphisms of dopamine receptor/transporter genes and risk of non-small cell lung cancer. Lung Cancer. 2007;56(1):17–23. doi:10.1016/j.lungcan.2006.11.007 [DOI] [PubMed] [Google Scholar]
- 81. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi:10.1038/s41575-019-0186-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21(37):10573–10583. doi:10.3748/wjg.v21.i37.10573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. doi:10.1016/j.blre.2019.04.005 [DOI] [PubMed] [Google Scholar]
- 84. Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10(13):4349–4356. doi:10.1158/1078-0432.CCR-04-0059 [DOI] [PubMed] [Google Scholar]
- 85. Gemignani F, Landi S, Moreno V, et al. Polymorphisms of the dopamine receptor gene DRD2 and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1633–1638. [DOI] [PubMed] [Google Scholar]
- 86. Murphy G, Cross AJ, Sansbury LS, et al. Dopamine D2 receptor polymorphisms and adenoma recurrence in the polyp prevention trial. Int J Cancer. 2009;124(9):2148–2151. doi:10.1002/ijc.24079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sanomachi T, Suzuki S, Kuramoto K, et al. Olanzapine, an atypical antipsychotic, inhibits survivin expression and sensitizes cancer cells to chemotherapeutic agents. Anticancer Res. 2017;37(11):6177–6188. doi:10.21873/anticanres.12067 [DOI] [PubMed] [Google Scholar]