Abstract
Introduction. A growing literature has developed on identifying outcomes that matter to patients. This study demonstrates an approach involving patient and regulatory perspectives to identify outcomes that are meaningful in the context of medical devices for Parkinson’s disease (PD). Methods. A systematic process was used for specifying relevant regulatory endpoints by synthesizing inputs of various sources and stakeholders. First, a literature review was conducted to identify important benefits, risks, and other considerations for medical devices to treat PD; patient discussion groups (n = 6) were conducted to refine the list of considerations, followed by a survey (n = 29) to prioritize them; and patient and Food and Drug Administration (FDA) reviewers informed specification of the final endpoints. Two FDA clinicians gave clinical and regulatory perspectives at each step. Results. Movement symptoms were ranked as most important (ranked 1 or 2 by 72% of participants) and psychological and cognitive symptoms as the next most important (ranked 1 or 2 by 52% of participants). Within movement symptoms, falls, impaired movement, bradykinesia, resting tremor, stiffness, and rigidity were ranked highly. Overall, nine attributes were identified and prioritized as patient-centric for use in clinical trial design and quantitative patient preference studies. These attributes were benefits and risks related to therapeutics for PD as well as other considerations, including time until a medical device is available for patient use. Discussion. This prospective approach identified meaningful and relevant benefits, risks, and other considerations that may be used for clinical trial design and quantitative patient preference studies. Although PD was the focus of this study, the approach can be used to study patient perspectives about other disease or treatment areas.
Keywords: medical devices, Parkinson’s disease, Patient preference, regulatory
There has been a movement in medical care toward better understanding the needs of patients, in order to design clinical trials that measure what matters and inform regulatory and clinical decision making, in the service of better outcomes and increased patient satisfaction. This progress has been encouraged in legislation, including the 21st Century Cures Act, which enhances the U.S. Food and Drug Administration’s (FDA) ability to collect and use patient experience data in decision-making. To evaluate the use and quality of these data properly, the FDA first needs to identify medical products’ attributes, which represent any concept that goes into benefit-risk decision-making, such as benefits, risks, and uncertainty. When identifying these attributes to characterize the safety and effectiveness of a treatment, it is important to include the factors that are relevant to patients, providers, and other decision makers. 1
Examples of benefits may include improvement of a symptom, decreased impact of symptoms on daily activities, or a feeling of better quality of life. Benefit can also incorporate a treatment’s ability to slow or stop disease progression even if the treatment does not affect symptoms. Historical considerations for treatment benefit related to a specific disease provide a starting point for consideration but may not encompass all of the treatment effects valued by a patient population. 2 Risks associated with treatment are included in a benefit-risk assessment to determine the tradeoffs that patients are willing to accept for a given benefit.
A variety of approaches have been used for identification of endpoints relevant to patient care and outcomes, including literature review, 3 surveys of experts in clinical practice and research, 4 and applying principles of health technology assessment to eliciting input from patients. 5 As awareness of the value of patient engagement in medical research has increased, methods for eliciting meaningful patient input have been developed and refined.6–9 Although patient engagement in research has improved patient-centricity of medical product development, there have been limited examples of the impact of patient engagement on medical product benefit-risk assessment.10–14 Such examples are needed to chart a course for expanded patient input in this area.
Parkinson’s disease (PD) currently affects over four million people worldwide, with numbers projected to double in the next few decades. The population prevalence of PD is 0.3%, increasing to 1% in those >60 and 4% >80 years. 15 Symptoms are progressive and inexorable. Core motor features consist of involuntary tremor, bradykinesia, rigidity, and postural instability. 16 Non-motor features include autonomic dysfunction, cognitive impairment, and subsequent dementia, mood, psychiatric, sensory, and sleep disorders. Current treatments include dopamine replacement therapy (including levodopa and dopamine agonists) and deep brain stimulation (DBS). “On time” is the when Parkinson’s treatment is working, and the Parkinson’s symptoms are controlled better. The time each day when Parkinson’s treatment is not working, and symptoms are worse is called “off time.” The non-motor symptoms are often treated empirically with off-label approaches. All current treatments remain symptomatic. No treatments are currently available that are known to slow the progression of the disease.
There are both patient-reported and physician-reported outcome assessments; however, the highly heterogeneous presentation of the disease in the patient population complicates both clinical trial design and benefit-risk assessment for medical products to treat PD. 17 Some of the effects experienced by individuals with PD have been neglected in clinical trial design and outcome assessment, 18 particularly cognitive 19 and other non-motor symptoms 20 of the disease. Therefore, despite existing patient-reported and physician-reported outcome assessments for PD, an activated patient population motivated the Medical Device Innovation Consortium (MDIC) to select this study as a test case of incorporating the patient perspective in clinical trial design and benefit-risk assessments for medical devices.
Early preference elicitation from patients with PD examined preferences associated with the treatment of “off time,” and found that while there was a preference for minimizing “off time,” respondents were averse to a risk of death. 21 Preference-based quality-of-life measurement surveys administered to patients with PD have been found to correlate substantially with clinical measures. 22 However, these measures, which include the EuroQol System EQ-5D, do not provide information about the relative importance to patients of treatment benefits and risks or about the benefit-risk tradeoffs patients are willing to make. Patient preferences about the relative importance of treatment benefits and risks can inform benefit-risk decision-making and clinical trial design.
The design of quantitative patient preference studies for regulatory considerations benefits from input by regulators who are the end users of the study results. This work presents an approach that synthesizes existing literature with the perspectives of patients, health care professionals, and regulatory decision-makers to identify relevant benefits, risks, and other attributes of medical devices used in the treatment of PD. Unlike other comparative effectiveness and patient-centered research mixed methods approaches in PD,23–26 this study was designed within a regulatory framework specifically to inform clinical trial design and benefit-risk assessments. The follow-up quantitative patient preference survey quantified the benefit-risk tradeoffs that are acceptable to patients with PD. 27 The elicited preference weights were then used as input to a model for clinical trial design. While this study focuses on PD, this approach to integrating patient, clinical, and regulatory perspectives can be used to inform patient preference survey design about other disease and/or treatment areas for clinical trial design and benefit-risk assessments for medical devices.
Methods
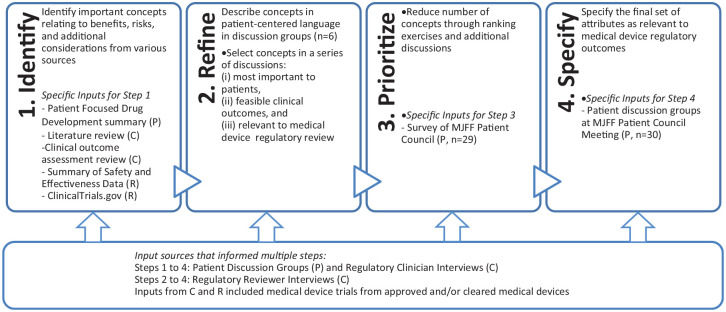
A four-step process was used to specify outcomes of PD treatments that were both meaningful to patients and relevant to regulatory decision making. The process synthesized patient, clinical, and regulatory input through the following steps (see Figure 1):
Figure 1.
A four-step process was used to identify relevant regulatory outcomes, and each step was informed by three types of input sources: patient (P), regulatory (R), and clinical (C) input.
Identify concepts from various sources
Refine the list using various sources of input
Prioritize these concepts through ranking exercise
Specify a set of relevant regulatory outcomes
An initial literature review was used to identify candidate concepts. These were discussed with patients and physicians in an iterative process, then prioritized and specified by patients and FDA regulators collaboratively.
Six members of the Patient Council of The Michael J. Fox Foundation for Parkinson’s Research, all with PD, convened as a working group to participate in this study. Two of the Patient Council members (Margaret Sheehan and AD) were research partners engaged in the entire study design process. The Michael J. Fox Foundation for Parkinson’s Research Patient Council, which was established in 2009, consists of 30 leaders and advocates who act as a channel for the Foundation to solicit input from patients with PD and the broader Parkinson’s community. All participants in the study were either members of the Patient Council or recruited by them from their networks.
Input was obtained from two FDA neurologists (KG and WH, leads) who serve as clinical regulatory reviewers of neurological products with expertise in movement disorders and neural prostheses. The FDA neurologists also obtained feedback from regulatory reviewers with biomedical engineering backgrounds to incorporate regulatory and clinical perspectives into the selection of attributes. Interviews were conducted by FDA investigators (HB, BC, JR, and AS), and input from physicians and engineers was directly transcribed to working documents for patient feedback. These interviews with regulatory clinicians centered on PD symptoms and treatment risks considered during clinical practice, including shared decision making, and regulatory review for medical devices.
Step 1: Identify
A literature review was conducted in PubMed and other available literature from October to December 2016 to generate a list of benefits, risks, and other patient-centered considerations for PD treatments, including drugs and devices. The PubMed search included all articles through December 2016. Search terms included “Parkinson’s disease,”“Parkinson’s treatments,”“Parkinson’s clinical trials,”“Parkinson’s deep brain stimulation,” and “Parkinson’s deep brain stimulation study/trials.” Other literature included the reports of the 2015 FDA Patient Focused Drug Development meeting for Parkinson’s disease, 28 Parkinson’s disease and deep brain stimulation trials on ClinicalTrials.gov, and the publicly posted Summary of Safety and Effectiveness Data document for deep brain stimulation systems approved by the FDA for use in the United States. Search terms for the ClinicalTrials.gov search (October to December 2016) included “Parkinson’s disease” and “deep brain stimulation.”
Attributes were gathered from the reported benefits, risks, and side effects (Supplementary Table A1) and include features of drugs and devices in order to scope all potential attributes relating to PD. We recorded and used the most highly cited clinical investigations that led to repeated themes in the reporting of benefits and adverse events,29–48 and clinical outcome assessments used for PD and side effects of PD treatments.49–63
Step 2: Refine
To further refine the list of important attributes, patient discussion group topics included review, discussion, and prioritization of the list of benefits, risks, and current clinical endpoints obtained from the literature review. Patients participated in both open theme elicitation and direct review of attributes identified in Step 1. Analysis was descriptive rather than interpretive. Discussion groups were recorded, transcribed, and summarized using an approach similar to thematic analysis, in which recurring themes were identified by reviewing transcripts and notes. 64 The Patient Council working group, made up of participants from the Patient Council, participated in a series of discussion groups to assist in identifying clinical outcomes that could be important to patients when making treatment decisions and prioritizing the identified outcomes (maximum of six participants). Patient discussion groups focused on concept elicitation and think-aloud confirmation of disease concepts.
Regulatory clinician and reviewer interviews centered on reviewing concepts identified from the literature review and patient discussions to refine attributes and elucidating how symptoms change during progression of the disease. Attributes were further refined by identifying benefits, risks, and side effects that could be features of medical devices.
Step 3: Prioritize
Once potential attributes were identified through literature review, clinical and regulatory consultation, and initial discussion groups with the Patient Council working group, a survey (29 respondents) with a series of ranking exercises was used to prioritize and classify the draft attribute list. This approach was used to contribute to discussion leading to a final attribute list by patient and regulatory contributors, rather than as a formal development of an item-based scale. The survey used numerical values and required strict ranking; no ties were permitted. In each question, respondents were asked to rank several related potential attributes, such as attributes related to movement symptoms, from most important (1) to least important (5 or lower, depending on number of attributes). In later questions, respondents ranked categories of attributes, such as movement symptoms, cognitive symptoms, and activities of daily living. Important attributes were defined as “things you would consider most carefully when making a treatment decision.” Respondents were asked to rank only the top five attributes in each section (or all attributes if there were fewer than five). Summary statistics of survey responses were compiled and presented to all study collaborators for a final determination about which attributes should be considered for inclusion in clinical trial design.
Regulatory clinician and reviewer interviews centered on the severity of risks appropriate for Parkinson’s treatment with a medical device and affirmation of benefits prioritized by patients.
Step 4: Specify
To confirm the interpretation of the attributes prioritized through the survey results, attribute descriptions were discussed with individuals with PD in small group discussion sessions at the semi-annual meeting of The Michael J. Fox Foundation for Parkinson’s Research Patient Council (30 total participants). Each small group discussion was attended by five to six Patient Council members and facilitated with an interview guide by one of the coauthors (BH or SC), with an additional note-taker in attendance (HB or AS). Discussion group participants viewed one attribute description at a time and commented on the description relevance, completeness, and comprehensibility.
Regulatory clinician interviews and regulatory reviewer interviews were used to confirm clinically relevant values for each attribute.
Results
The four-step process for developing the attributes led to the results we discuss below including specific feedback received from the patients and how that led to the attribute table for the quantitative patient preference survey.
Attributes can represent any concept that goes into benefit-risk decision-making about a treatment option, including both potential side effects and treatment targets. For PD, some attributes may be attributed to the disease and/or a side effect of a treatment.
In Step 1, a common outcome list based on clinicaltrial.gov review was developed (Table 1). From that work, a list of 12 common clinical effectiveness endpoints (benefits of treatment) and 26 safety endpoints (risks of treatment or adverse events) were identified (Supplementary Table A1) to represent potential benefits, risks, and additional considerations. This list of treatment effects was considered in parallel with those mentioned in the Patient Focused Drug Development meeting and clinicaltrials.gov review. The categories and attributes identified for inclusion in the ranking survey are shown in Supplementary Table A2.
Table 1.
Common Outcomes in Parkinson’s Disease Clinical Trials (All Treatment Types)
| Motor Endpoints | Other Endpoints |
|---|---|
| Gait changes | Mental side effects (hallucinations, anxiety, depression) |
| Tremor changes—resting | Cognitive impairment |
| Tremor changes—kinetic | Dementia |
| Motor skills (speech, posture, stability) | Stress changes |
| Bradykinesia changes | Sleep efficiency measures |
| Dyskinesia changes | |
| Dystonia changes | |
| Fine motor coordination | |
| Reaction time changes |
In Step 2, members of The Michael J. Fox Foundation for Parkinson’s Research Patient Council and regulatory physicians provided input to refine the attributes (see Figure 1, multiple discussions with total participants N = 6). The median age of patients on the Patient Council was 58 years (range 40–80), and 36% were female. The estimated median years since PD diagnosis was 9 (range 2–22). The names of the members of the Patient Council are publicly posted. Therefore, additional demographic data on the Patient Council is not publicly available due to privacy concerns. Important comments that contributed to attribute selection were deidentified and summarized below.
On time: When discussing “on time” and movement, Patient Council working group members were asked if some movement functions were more important than others. Responses included, “All are important,” and when further probed, “On/off time is very important—it captures both effects and perception, incorporates all buckets in a summary form,” and “Everybody’s on and off times may be different, but it is a summary of your condition.”
Primary motor symptoms: When discussing the importance of motor skills, Patient Council working group members stated, “Motor skills would create more isolation for me, when I think I can’t keep up,” and “Fine motor skills [would not] trouble me anywhere as much as the major motor skills.” When asked if primary motor symptoms were a critical consideration when weighing treatment options, one patient responded, “Yes, yes, yes, yes, absolutely, they totally interfere with daily living, absolutely.” Further discussion probed whether certain attributes would concern an individual with PD more than others, to which one response was,
Once again if you think about what’s life threatening or severely debilitating, falls would be at the top of the list. I’d say freezing and falls would be the ones that scare me the most. I have very painful muscle spasms; I don’t know if you call that scary or not, but I just, I really don’t like them.
Cognition and depression: In discussing cognition and depression, Patient Council working group members stated, “I’ve not been depressed, but I think if I was depressed that would be the worst, far worse than small handwriting. It affects every aspect of your life,” and “If I had a chance to avoid any possibility of dementia, I’d grab it.” Another Patient Council working group member emphasized the impact of dementia as a risk of treatment and symptom of the disease, saying, “I think the executive function piece is important.”
During discussion of psychological risks and cognition, one patient recognized the severity of these symptoms, saying, “These are big problems. These are big risks. Yeah, cognitive impairment, that could be devastating. Dementia, depression, suicide; this is not a happy list, no.” Another reflected on severity by discussing unwillingness to trade psychological risks for other symptoms: “It’s hard if your state is compromised and you have a significant symptom and one or two other symptoms. You trade all those to be depressed or suicidal; I don’t think so, for myself.”
Independence and activities of daily living: When asking Patient Council working group members about attributes related to independence and their activities of daily living, responses included, “To tell you the truth, if someone else did all my shopping I didn’t mind, especially the groceries, and housework too,” and “These all relate to one’s independence and they’re all in their own way pretty critical. They define whether or not you can make decisions for yourself, take care of yourself.” These discussions indicated that while independence and activities of daily living were considered important, they were complex and dependent upon a variety of factors, including symptoms, support structures, and individual circumstances.
Pain: While apparent in the literature, pain was rarely emphasized relative to other disease symptoms or measured as an outcome in clinical trials. Patient Council working group members stressed the importance of pain with statements such as “I have very painful muscle spasms. I don’t know if you call that scary or not, but I just, I really don’t like them,” and “We lost pain, and I think pain is an important factor.” This attribute was reinforced by physicians as an appropriate attribute in thinking about how to treat and increase quality of life for Parkinson’s patients.
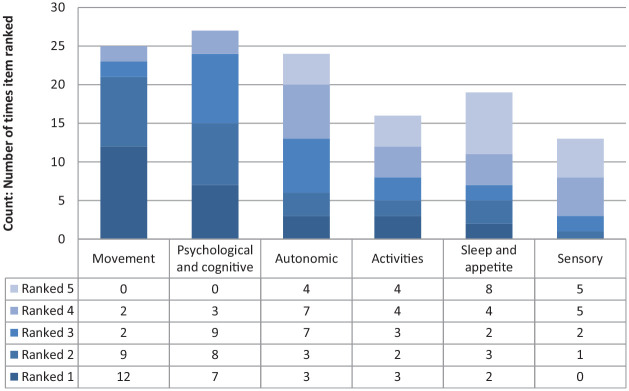
In Step 3 (Prioritize), 29 individuals with PD completed the prioritization survey to inform final attribute selection. Additional information about participants was not recorded to protect participant confidentiality. Results from the prioritization survey are shown in Figure 2 and Supplementary Figure A1. Overall, movement symptoms and psychological and cognitive symptoms were ranked as the most important (1) and next most important (2) categories most frequently. Movement symptoms were ranked as most important (ranked 1 or 2 by 72% of participants) and psychological and cognitive symptoms as the next most important (ranked 1 or 2 by 52% of participants). Within movement symptoms, falls, impaired movement, bradykinesia, resting tremor, stiffness, and rigidity were most frequently ranked highly. Within psychological and cognitive symptoms, depression, dementia, slowed thinking, and memory were most frequently ranked highly.
Figure 2.
Ranked items from the “Overall Symptoms and Activities” section of the prioritization exercise (29 respondents). Attributes are shown from most frequently ranked most important (1) to least (5).
In Step 4, we identified 10 attributes as patient-centric regulatory considerations for medical devices (Table 2). In addition to symptoms and risks, reductions in prescription drug dosage, reflecting patients’ desires to reduce side effects and burden of pharmaceutical treatments, was included because patients in the Patient Focused Drug Development meeting and discussion groups expressed significant interest in reducing prescriptions drugs. The final attribute, time until treatment availability, relates to the impact of the delay in treatment availability due to research and development, clinical trial, and regulatory processes. This attribute was included in order to support the patient preference-based study to optimize clinical trial design by maximizing timely patient access to safe and effective treatments.
Table 2.
Patient-Centric Regulatory Considerations
| Final Attributes with Descriptions | |
|---|---|
| Benefits |
Amount of time your Parkinson’s treatment works each day Relevant to all treatment types Parkinson’s symptoms can include movement symptoms (such as tremor, slowness of movement, unsteadiness, and rigidity) and nonmovement symptoms (such as sleep problems, low blood pressure when standing up, trouble thinking clearly, and mood problems). People with Parkinson’s disease can experience the following situations during the day: • Your Parkinson’s treatment is working and the Parkinson’s symptoms are controlled better. The time each day when the Parkinson’s treatment is working to control your symptoms is called “on time.” • Your Parkinson’s treatment is not working and the Parkinson’s symptoms get worse. The time each day when the Parkinson’s treatment is not working and your symptoms are worse is called “off time.” |
|
Movement symptoms of Parkinson’s disease Relevant to all treatment types People with Parkinson’s disease experience different types of movement symptoms. These symptoms can include • Resting tremor: A trembling in a body part when that body part is not performing an action • Postural instability: Being unsteady or lacking balance when standing upright • Bradykinesia: Slowness of movement and limited range of movement • Rigidity: Unusual stiffness in one more arms, legs, or in another body part Not all people experience the same movement symptoms. | |
|
Pain because of Parkinson’s disease Relevant to all treatment types Some people with Parkinson’s disease experience pain. This pain can include aching or burning muscle pain and severe muscle cramping, sharp nerve pain, numbness, or “pins and needles.” | |
|
Trouble thinking clearly, getting organized, or making plans because of Parkinson’s disease Relevant to all treatment types Some people with Parkinson’s say that the disease affects their ability to think clearly, get or stay organized, or make plans. When this happens, people sometimes need to make lists to help them organize their thoughts. If you have difficulty thinking clearly, it may be hard for you to remember things that you used to be able to remember easily. Sometimes planning your usual daily activities is harder than it used to be. | |
| Risks |
Increased risk of depression or anxiety Relevant to all treatment types Sometimes people with Parkinson’s can experience emotional problems such as depression and anxiety because of their disease. In addition, some devices used to treat Parkinson’s disease can affect people’s mood and cause depression and anxiety. Depression is a serious medical condition that affects the way you feel, think, and how you act. People with depression may lose interest or pleasure in doing things they once enjoyed. They may feel sad and hopeless or have feelings of guilt or low self-worth. Depression can affect how you work and study and how you interact with people. It may lead some people think about suicide. Anxiety is the feeling of being constantly overwhelmed or afraid. Anxiety can impact your ability to do your usual daily activities. The risk of depression or anxiety related to the Parkinson’s device is in addition to the chance of depression or anxiety you may already face because of Parkinson’s disease. |
|
Risk of a bleeding in your brain because of the device Relevant to medical devices There is a risk that you could have bleeding in the brain after getting a device to treat Parkinson’s disease. Bleeding in the brain is a type of stroke and occurs when a blood vessel in the brain bursts. Blood in the brain can kill brain cells and cause permanent damage to the brain. The damage to the brain can cause you to experience sudden weakness, loss of coordination, or difficulty speaking. A severe stroke can lead to permanent paralysis. If you have a stroke caused by bleeding in your brain, you would have to be treated in a hospital, sometimes for many weeks. You cannot do your normal activities while you have brain bleeding. | |
|
Risk of dying within 1 year after getting a device Relevant to medical devices There is a risk that you could die within 1 year after getting a device to treat Parkinson’s disease. Dying could be a result of the operation used to place the device in your brain or a result of the device itself. | |
| Other considerations |
Time until the device is available Relevant to medical devices Some devices that could be used to treat Parkinson’s disease are currently being developed and tested and may not be available to patients yet. The process of developing and testing new device treatments can take years. Sometimes patients are willing to wait to get a new device to treat their disease if the benefits of the device are expected to be better than the treatments that are available to them now. |
|
Number of oral medicines you take each day to treat Parkinson’s disease and the side effects of Parkinson’s medicines Relevant to medicines People with Parkinson’s often need to take a lot of pills or tablets each day to treat the disease. In addition, people with Parkinson’s may need to take additional medicines to treat the side effects of their Parkinson’s medicines. Usually, people need to take oral medicines throughout the day. Often, people need to take these medicines on a very specific schedule in order for the treatment to work well. | |
Attribute Descriptions
Changes in attribute descriptions as a result of The Michael J. Fox Foundation for Parkinson’s Research Patient Council discussion groups, which refined attribute concepts and language, are shown in Supplementary Table A3. For example, the “increase in on time” description was expanded from a concept involving movement symptoms only to one involving both movement and non-movement symptoms of PD. The discussion groups also commented on what would be meaningful changes in symptoms for them and gave insight into the complex interplay between symptoms and medication side effects for individuals with PD.
Discussion
Summary
As patient centricity has received more emphasis in regulatory review65,66 a variety of approaches to patient engagement have been pursued to glean the relevant considerations from patients, clinicians, and regulators led to a prioritized attribute table. Importantly, we learned that much of the clinical trial and current treatment options for patients do not address unmet needs for patients regarding pain and psychological and cognitive issues.
In collaboration with The Michael J. Fox Foundation for Parkinson’s Research Patient Council, we used discussion groups and a ranking survey to identify and prioritize meaningful regulatory endpoints for medical devices for the treatment of PD. We incorporated patient, clinical, and regulatory perspectives throughout the process to increase the likelihood that the results would be both patient-centered and relevant for clinical trial design and regulatory decision-making. As recommended in the literature on good practices, 68 discussion groups were used to further develop an initial literature review and allowed exploration of the breadth and variety of PD symptoms with the Patient Council working group members. The prioritization survey allowed for input from a broader patient network and to focus the initial literature review to the symptoms that most patients ranked highly.
Contribution to Patient Preferences and Patient-Centered Outcome Measures in Parkinson’s Disease
Recent studies on patient-centered health care for patients with PD and patient preferences for PD treatment have focused on the attributes associated with satisfactory clinical care.69–72 As there is now evidence that non-motor symptoms of PD can affect quality of life as much as motor symptoms do, recent Movement Disorders Society evidence-based recommendations include the treatment of non-motor symptoms such as depression and dementia. 73
This study extends the science of patient input in the PD community by identifying psychological and cognitive treatment attributes, which may be used in patient preference studies. Patient preferences about the benefit-risk tradeoffs associated with “on time,” the motor symptoms of PD, and some of the non-motor symptoms of PD, including psychological and cognitive treatment effects and pain, may therefore also be informative for benefit-risk assessments.
The MDS-UPDRS (Movement Disorder Society–Unified Parkinson’s Disease Rating Scale), a PD-specific clinician- and patient-reported outcome measure, is composed of motor and non-motor domains. However, the motor examination domain, part III, is often used alone in clinical trials to assess a patient’s health status. 34 Through the initial literature review and discussion groups with patients and clinicians, we learned that the MDS-UPDRS, part III, may not be sufficiently patient-centric. Stakeholders voiced concerns that UPDRS represents an older model of patient care that may not have evolved alongside new knowledge about PD. Patients proposed ideas to improve existing patient-reported outcome measures that focus on specific symptoms or capture general quality of life.32,34–37,39 Patient-centered endpoints, like the ones identified in this study, can be used to develop patient-centric clinician- and patient-reported outcome measures.
Limitations
Because this study was intended to inform a larger quantitative study with a more generalizable patient population, patient input was obtained from a small convenience sample of highly educated and engaged patients. These patients were well-informed about the progression of PD, but did not themselves have late-stage PD. As a result, the outcomes they emphasized may be different from those that would be emphasized by patients with late-stage PD. Additional limitations with this convenience sample is the lack of other public demographic information on the patients or demographic data collected in the survey that would limit the generalizability of this to the broader PD community.
Rather than engaging in in-depth qualitative analysis, an iterative approach based on the think-aloud method and thematic analysis was used with patients and FDA to update working documents and develop an attribute list. This streamlined approach was selected with an awareness of understanding the importance of creating efficiencies in medical device development programs. The development process for preference surveys requires resources from medical product developers and patient groups interested in conducting patient preference studies, so we aimed to take a pragmatic approach to identifying fit-for-purpose attributes that were both patient-centered and had regulatory relevance. Regulatory relevance especially on the risks of medical devices is important to ensure the deployed survey can have utility in making a regulatory assessment like benefit-risk tradeoffs. Before a patient preference study using these attributes is fielded, it should be pretested with a heterogeneous patient sample to ensure it is clearly understood and appropriately answered. The fielded patient preference survey should include capturing patient demographics to understand pre-specified subgroup preferences and generalizability of the results.
Conclusion
There is inherent value to including the patient voice in medical product development and assessment for both the developer and the regulator, and ultimately for patients. Medical device developers could use structured patient input in designing and evaluating devices based on what matters to patients, which may contribute to increased participation in clinical trials, improved outcomes measurement, and better enrollment and retention. 74
Patient input provides insight for benefit and risk assessments. Clinicians have a great deal of experience with patients but may have assumptions regarding what is important to patients that may benefit from further evaluation and input from patients themselves. Additionally, there can be aspects of the disease that affect the patient in a manner that may not be immediately apparent to a regulator or clinician. This is especially true for diseases with a heterogeneous manifestation within the population. In PD, patient-reported outcome assessments primarily were developed for motor symptoms. Others have shown that, in addition to motor symptoms, mood changes and sleep problems are important to individuals with advanced PD. 20 The work with PD patients presented here has shown that there is a subset of the population for whom the treatment of motor symptoms alone results in residual unmet patient need. In particular, patients identified psychological, cognitive, and pain outcomes as important treatment considerations.
Supplemental Material
Supplemental material, sj-docx-1-mpp-10.1177_23814683211021380 for Patient-Centered Identification of Meaningful Regulatory Endpoints for Medical Devices to Treat Parkinson’s Disease by Heather L. Benz, Brittany Caldwell, John P. Ruiz, Anindita Saha, Martin Ho, Stephanie Christopher, Dawn Bardot, Margaret Sheehan, Anne Donnelly, Lauren McLaughlin, Brennan Mange, A. Brett Hauber, Katrina Gwinn, William J. Heetderks and Murray Sheldon in MDM Policy & Practice
Acknowledgments
The authors thank Drs. Shomesh Chaudhuri and Andrew Lo for discussions about a patient-informed clinical trial design model, the late Dr. Peter Como for early conversations that led to the development of the study, and biomedical engineering reviewers of neurology devices in FDA/CDRH’s Office of Product Evaluation and Quality.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BC, AS, MH, M Sheldon, AD, LM, BM, AH, KG, WH, and M Sheehan have no conflicts of interest to report. HB is employed at Johnson and Johnson; during her contributions to the work, she was employed by the US Food and Drug Administration. JR is employed at Dataprise; during his contributions to the work, he was employed by the US Food and Drug Administration. SC is employed at Currently at National Organization for Rare Disorders (NORD); during her contributions to the work, she was employed by the nonprofit Medical Device Innovation Consortium. DB is employed by Abiomed; during her contributions to the work, she was employed by the nonprofit Medical Device Innovation Consortium BH is currently employed at Pfizer Inc.; during his contributions to the work, he was employed by RTI Health Solutions.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Medical Device Innovation Consortium, a nonprofit, public-private partnership to advance medical device regulatory science. Funding for this project came from MDIC general operating funds. The FDA did not receive funding. Financial support for this study was provided in part by a contract with the Medical Device Innovation Consortium. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. The following authors were employed by the sponsor: Stephanie Christopher and Dawn Bardot.
Authors’ Note: Institutions where the work was done:
US FDA Center for Devices and Radiological Health
The Michael J. Fox Foundation for Parkinson’s Research
RTI Health Solutions
Meetings at which the work was presented
MDIC Patient Centered Clinical Trial Design Workshop (Arlington, Virginia, 2018)
NYC Neuromodulation Conference (New York, New York, 2018)
Patients as Partners (Philadelphia, Pennsylvania, 2018)
American Neurological Association (Atlanta, Georgia, 2018)
American Academy of Neurology (Los Angeles, California, 2018)
World Parkinson Congress (Kyoto, Japan, 2019)
ORCID iDs: Heather L. Benz  https://orcid.org/0000-0002-6707-9695
https://orcid.org/0000-0002-6707-9695
Anindita Saha  https://orcid.org/0000-0003-0306-4264
https://orcid.org/0000-0003-0306-4264
A. Brett Hauber  https://orcid.org/0000-0003-3129-7268
https://orcid.org/0000-0003-3129-7268
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Policy & Practice website at https://journals.sagepub.com/home/mpp.
Contributor Information
Heather L. Benz, US FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
Brittany Caldwell, US FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
John P. Ruiz, US FDA Center for Devices and Radiological Health, Silver Spring, Maryland
Anindita Saha, US FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
Martin Ho, US FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
Stephanie Christopher, Medical Device Innovation Consortium, Arlington, Virginia.
Dawn Bardot, Medical Device Innovation Consortium, Arlington, Virginia.
Margaret Sheehan, The Michael J. Fox Foundation for Parkinson’s Research, New York, New York.
Anne Donnelly, The Michael J. Fox Foundation for Parkinson’s Research, New York, New York.
Lauren McLaughlin, The Michael J. Fox Foundation for Parkinson’s Research, New York, New York.
Brennan Mange, RTI Health Solutions, Research Triangle Park, North Carolina.
A. Brett Hauber, RTI Health Solutions, Research Triangle Park, North Carolina.
Katrina Gwinn, US FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
William J. Heetderks, US FDA Center for Devices and Radiological Health, Silver Spring, Maryland
Murray Sheldon, US FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
References
- 1. Kluetz PG, O’Connor DJ, Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. The Lancet Oncology. 2018;19(5):e267-e74. [DOI] [PubMed] [Google Scholar]
- 2. Pais-Ribeiro J. Quality of life is a primary end-point in clinical settings. Clinical nutrition. 2004;23(1):121-30. [DOI] [PubMed] [Google Scholar]
- 3. Patel KK, Mehdirad AA, Lim MJ, Ferreira SW, Mikolajczak PC, Stolker JM. Beyond warfarin: a patient-centered approach to selecting novel oral anticoagulants for stroke prevention in atrial fibrillation. Journal of hospital medicine. 2014;9(6):400-6. [DOI] [PubMed] [Google Scholar]
- 4. Driver VR, Gould LJ, Dotson P, Gibbons GW, Li WW, Ennis WJ, et al. Identification and content validation of wound therapy clinical endpoints relevant to clinical practice and patient values for FDA approval. Part 1. Survey of the wound care community. Wound Repair and Regeneration. 2017;25(3):454-65. [DOI] [PubMed] [Google Scholar]
- 5. Kinter ET, Schmeding A, Rudolph I, Bridges JF. Identifying patient-relevant endpoints among individuals with schizophrenia: an application of patient-centered health technology assessment. International journal of technology assessment in health care. 2009;25(1):35-41. [DOI] [PubMed] [Google Scholar]
- 6. Mullins CD, Abdulhalim AM, Lavallee DC. Continuous patient engagement in comparative effectiveness research. Jama. 2012;307(15):1587-8. [DOI] [PubMed] [Google Scholar]
- 7. Duffett L. Patient engagement: what partnering with patient in research is all about. Thrombosis Research. 2017;150:113-20. [DOI] [PubMed] [Google Scholar]
- 8. Frank L, Forsythe L, Ellis L, Schrandt S, Sheridan S, Gerson J, et al. Conceptual and practical foundations of patient engagement in research at the patient-centered outcomes research institute. Quality of Life Research. 2015;24(5):1033-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith SK, Selig W, Harker M, Roberts JN, Hesterlee S, Leventhal D, et al. Patient engagement practices in clinical research among patient groups, industry, and academia in the United States: a survey. PloS one. 2015;10(10):e0140232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ho MP, Gonzalez JM, Lerner HP, Neuland CY, Whang JM, McMurry-Heath M, et al. Incorporating patient-preference evidence into regulatory decision making. Surgical endoscopy. 2015;29(10):2984-93. [DOI] [PubMed] [Google Scholar]
- 11. Chaudhuri SE, Ho MP, Irony T, Sheldon M, Lo AW. Patient-centered clinical trials. Drug Discovery Today. 2018;23(2):395-401. [DOI] [PubMed] [Google Scholar]
- 12. Irony T, Ho M, Christopher S, Levitan B. Incorporating patient preferences into medical device benefit-risk assessments. Statistics in Biopharmaceutical Research. 2016;8(3):230-6. [Google Scholar]
- 13. Smith MY, Hammad TA, Metcalf M, Levitan B, Noel R, Wolka AM, et al. Patient engagement at a tipping point-the need for cultural change across patient, sponsor, and regulator stakeholders: insights from the DIA Conference,"Patient engagement in benefit risk assessment throughout the life cycle of medical products”. Therapeutic innovation & regulatory science. 2016;50(5):546-53. [DOI] [PubMed] [Google Scholar]
- 14. Paradise J. 21st Century Citizen Pharma: The FDA & Patient-Focused Product Development. American Journal of Law & Medicine. 2018;44(2-3):309-27. [DOI] [PubMed] [Google Scholar]
- 15. De Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. The Lancet Neurology. 2006;5(6):525-35. [DOI] [PubMed] [Google Scholar]
- 16. Goetz CG. The history of Parkinson’s disease: early clinical descriptions and neurological therapies. Cold Spring Harbor perspectives in medicine. 2011;1(1):a008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewis TB, Standaert DG. Design of clinical trials of gene therapy in Parkinson disease. Experimental neurology. 2008;209(1):41-7. [DOI] [PubMed] [Google Scholar]
- 18. Nisenzon AN, Robinson ME, Bowers D, Banou E, Malaty I, Okun MS. Measurement of patient-centered outcomes in Parkinson’s disease: what do patients really want from their treatment? Parkinsonism & related disorders. 2011;17(2):89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eberling J, Vincent L, Goldman JG, Weintraub D, Kulisevsky J, Marras C, et al. Therapeutic development paths for cognitive impairment in Parkinson’s disease: report of a regulatory roundtable. Journal of Parkinson’s disease. 2014;4(4):585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Politis M, Wu K, Molloy S, G. Bain P, Chaudhuri KR, Piccini P. Parkinson’s disease symptoms: the patient’s perspective. Movement Disorders. 2010;25(11):1646-51. [DOI] [PubMed] [Google Scholar]
- 21. Palmer CS, Schmier JK, Snyder E, Scott B. Patient preferences and utilities for ’off-time’ outcomes in the treatment of Parkinson’s disease. Quality of Life Research. 2000;9(7):819-27. doi: Doi 10.1023/A:1008903126315. PubMed PMID: WOS:000166904200004. [DOI] [PubMed] [Google Scholar]
- 22. Siderowf A, Ravina B, Glick HA. Preference-based quality-of-life in patients with Parkinson’s disease. Neurology. 2002;59(1):103-8. PubMed PMID: WOS:000176622600020. [DOI] [PubMed] [Google Scholar]
- 23. Riggare S, Duncan TS, Hvitfeldt H, Hgglund M. “You have to know why you’re doing this”: a mixed methods study of the benefits and burdens of self-tracking in Parkinson’s disease. BMC medical informatics and decision making. 2019;19(1):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Birtwell K, Dubrow-Marshall L, Dubrow-Marshall R, Duerden T, Dunn A. A mixed methods evaluation of a Mindfulness-Based Stress Reduction course for people with Parkinson’s disease. Complementary therapies in clinical practice. 2017;29:220-8. [DOI] [PubMed] [Google Scholar]
- 25. Cabrera LY, Kelly-Blake K, Sidiropoulos C. Perspectives on Deep Brain Stimulation and Its Earlier Use for Parkinson’s Disease: A Qualitative Study of US Patients. Brain Sciences. 2020;10(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maier F, Lewis CJ, Horstkoetter N, Eggers C, Kalbe E, Maarouf M, et al. Patients’ expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson’s disease: a mixed-method approach. Journal of Neurology, Neurosurgery & Psychiatry. 2013;84(11):1273-81. [DOI] [PubMed] [Google Scholar]
- 27. Hauber B, Mange B, Zhou M, Chaudhuri S, Benz HL, Caldwell B, et al. Parkinson’s Patients’ Tolerance for Risk and Willingness to Wait for Potential Benefits of Novel Neurostimulation Devices: A Patient-Centered Threshold Technique Study. MDM Policy & Practice. 2021;6(1):2381468320978407. doi: 10.1177/2381468320978407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Center for Drug Evaluation and Research USFaDA. The Voice of the Patient: Parkinson’s Disease. In: DHHS, editor. 2016. [Google Scholar]
- 29. Amirnovin R, Williams ZM, Cosgrove GR, Eskandar EN. Experience with microelectrode guided subthalamic nucleus deep brain stimulation. Neurosurgery. 2006;58(1 Suppl):ONS96-102; discussion ONS96-. doi: 10.1227/01.NEU.0000192690.45680.C2.PubMed PMID: 16543878. [DOI] [PubMed] [Google Scholar]
- 30. Binder DK, Rau G, Starr PA. Hemorrhagic complications of microelectrode-guided deep brain stimulation. Stereotact Funct Neurosurg. 2003;80(1-4):28-31. doi: 10.1159/000075156. PubMed PMID: 14745205. [DOI] [PubMed] [Google Scholar]
- 31. Blomstedt P, Hariz MI. Hardware-related complications of deep brain stimulation: a ten year experience. Acta Neurochir (Wien). 2005;147(10):1061-4; discussion 4. doi: 10.1007/s00701-005-0576-5. PubMed PMID: 16041470. [DOI] [PubMed] [Google Scholar]
- 32. Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896-908. doi: 10.1056/NEJMoa060281. PubMed PMID: 16943402. [DOI] [PubMed] [Google Scholar]
- 33. Fraix V, Houeto JL, Lagrange C, Le Pen C, Krystkowiak P, Guehl D, et al. Clinical and economic results of bilateral subthalamic nucleus stimulation in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77(4):443-9. doi: 10.1136/jnnp.2005.077677.PubMed PMID: 16543519; PubMed Central PMCID: PMCPMC2077508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goodman RR, Kim B, McClelland S, 3rd, Senatus PB, Winfield LM, Pullman SL, et al. Operative techniques and morbidity with subthalamic nucleus deep brain stimulation in 100 consecutive patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77(1):12-7. doi: 10.1136/jnnp.2005.069161.PubMed PMID: 16361585; PubMed Central PMCID: PMCPMC2117411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamani C, Lozano AM. Hardware-related complications of deep brain stimulation: a review of the published literature. Stereotact Funct Neurosurg. 2006;84(5-6):248-51. doi: 10.1159/000096499. PubMed PMID: 17063047. [DOI] [PubMed] [Google Scholar]
- 36. Hamani C, Richter E, Schwalb JM, Lozano AM. Bilateral subthalamic nucleus stimulation for Parkinson’s disease: a systematic review of the clinical literature. Neurosurgery. 2005;56(6):1313-21; discussion 21-4. PubMed PMID: 15918948. [DOI] [PubMed] [Google Scholar]
- 37. J. V, Y. W, M. M, R. L, A. K, D. L, et al. Deep-brain stimulation: long-term analysis of complications caused by hardware and surgery—experiences from a single centre. Neurol Neurosurg Psychiatry. 2006;77(7):868-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21 Suppl 14:S290-304. doi: 10.1002/mds.20962. PubMed PMID: 16892449. [DOI] [PubMed] [Google Scholar]
- 39. Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003;349(20):1925-34. doi: 10.1056/NEJMoa035275. PubMed PMID: 14614167. [DOI] [PubMed] [Google Scholar]
- 40. Kumar R, Lozano AM, Kim YJ, Hutchison WD, Sime E, Halket E, et al. Double-blind evaluation of subthalarnic nucleus deep brain stimulation in advanced Parkinson’s disease. American Academy of Neurology. 1998;51:850-5. [DOI] [PubMed] [Google Scholar]
- 41. Louis Benabid A, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol. 2009;8:67-81. [DOI] [PubMed] [Google Scholar]
- 42. Lyons KE, Wilkinson SB, Overman J, Pahwa R. Surgical and hardware complications of subthalamic stimulation: A series of 160 procedures. Neurology. 2004;63. [DOI] [PubMed] [Google Scholar]
- 43. Oh MY, Abosch A, Kim SH, Lang AE, Lozano AM. Long-term Hardware-related Complications of Deep Brain Stimulation. Neurosurgery. 2002;50:1268-76. [DOI] [PubMed] [Google Scholar]
- 44. Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, et al. Management of Referred Deep Brain Stimulation Failures: A Retrospective Analysis From 2 Movement Disorders Centers. Arch Neurol. 2005;62. [DOI] [PubMed] [Google Scholar]
- 45. Pahwa R, Wilkinson SB, Overman J, Lyons KE. Bilateral subthalamic stimulation in patients with Parkinson disease: long-term follow up. J Neurosurg. 2003;99(1):71-7. doi: 10.3171/jns.2003.99.1.0071. PubMed PMID: 12854747. [DOI] [PubMed] [Google Scholar]
- 46. Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005;128(Pt 10):2240-9. doi: 10.1093/brain/awh571. PubMed PMID: 15975946. [DOI] [PubMed] [Google Scholar]
- 47. Seijo FJ, Alvarez-Vega MA, Gutierrez JC, Fdez-Glez F, Lozano B. Complications in subthalamic nucleus stimulation surgery for treatment of Parkinson’s disease. Review of 272 procedures. Acta Neurochir (Wien). 2007;149(9):867-75; discussion 76. doi: 10.1007/s00701-007-1267-1. PubMed PMID: 17690838. [DOI] [PubMed] [Google Scholar]
- 48. Temel Y, Ackermans L, Celik H, Spincemaille GH, van der Linden C, Walenkamp GH, et al. Management of hardware infections following deep brain stimulation. Acta Neurochir (Wien). 2004;146(4):355-61; discussion 61. doi: 10.1007/s00701-004-0219-2. PubMed PMID: 15057529. [DOI] [PubMed] [Google Scholar]
- 49. Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in Parkinson’s disease: Prevalence and characteristics. Pain. 2009;141(1-2):173-7. doi: 10.1016/j.pain.2008.12.004. PubMed PMID: 19100686. [DOI] [PubMed] [Google Scholar]
- 50. de Boer AG, Wijker W, Speelman JD, de Haes JC. Quality of life in patients with Parkinson’s disease: development of a questionnaire. J Neurol Neurosurg Psychiatry. 1996;61(1):70-4. PubMed PMID: 8676165; PubMed Central PMCID: PMCPMC486462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Giladi N, Shabtaia H, Simona ES, Biranb S, Talc J, Korcyna AD. Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism and Related Disorders. 2000;6:165-70. [DOI] [PubMed] [Google Scholar]
- 52. Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-70. doi: 10.1002/mds.22340. PubMed PMID: 19025984. [DOI] [PubMed] [Google Scholar]
- 53. Hobson JP, Edwards NI, Meara RJ. The Parkinson’s Disease Activities of Daily Living Scale: a new simple and brief subjective measure of disability in Parkinson’s disease. Clin Rehabil. 2001;15(3):241-6. Epub 2001/06/02. doi: 10.1191/026921501666767060.PubMed PMID: 11386393. [DOI] [PubMed] [Google Scholar]
- 54. Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age and Ageing. 1997;26:353-7. [DOI] [PubMed] [Google Scholar]
- 55. Martinez-Martin P, Jeukens-Visser M, Lyons KE, Rodriguez-Blazquez C, Selai C, Siderowf A, et al. Health-related quality-of-life scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2011;26(13):2371-80. doi: 10.1002/mds.23834. PubMed PMID: 21735480. [DOI] [PubMed] [Google Scholar]
- 56. Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30(4):459-63. doi: 10.1016/j.gaitpost.2009.07.108. PubMed PMID: 19660949. [DOI] [PubMed] [Google Scholar]
- 57. Schulzer M, Mak E, Calne SM. The psychometric properties of the Parkinson’s Impact Scale (PIMS) as a measure of quality of life in Parkinson’s disease. Parkinsonism Relat Disord. 2003;9(5):291-4. PubMed PMID: 12781596. [DOI] [PubMed] [Google Scholar]
- 58. Hoehn MM, Yahr MD. Parkinsonism : onset, progression, and mortality. Neurology. 1967;17(5):427-42. [DOI] [PubMed] [Google Scholar]
- 59. Fahn S, Tolosa E, Marin C. Clinical Rating Scale for Tremor. In: Jankovic J, Tolosa E, editors. Parkinson’s Disease and Movement Disorders. Baltimore-Munich: Urban & Schwarzenburg; 1988. p. 225-34. [Google Scholar]
- 60. Burke R, Fahn S, CD M, SB B, C M, J F. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35(1):73-7. [DOI] [PubMed] [Google Scholar]
- 61. Elble R, Comella C, Fahn S, Hallett M, Jankovic J, Juncos JL, et al. Reliability of a new scale for essential tremor. Mov Disord. 2012;27(12):1567-9. doi: 10.1002/mds.25162.PubMed PMID: 23032792; PubMed Central PMCID: PMCPMC4157921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Goetz CG, Nutt JG, Stebbins GT. The Unified Dyskinesia Rating Scale: presentation and clinimetric profile. Mov Disord. 2008;23(16):2398-403. doi: 10.1002/mds.22341. PubMed PMID: 19025759. [DOI] [PubMed] [Google Scholar]
- 63. Schwab R, England A, Jr, editors. Projection technique for evaluating surgery in Parkinson’s Disease. Third symposium on Parkinson’s Disease;1969. [Google Scholar]
- 64. Braun V, Clarke V. Thematic analysis. 2012. [Google Scholar]
- 65. Rummel M, Kim TM, Aversa F, Brugger W, Capochiani E, Plenteda C, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20_ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab). Annals of Oncology. 2017;28(4):836-42. doi: 10.1093/annonc/mdw685. PubMed PMID: WOS:000397622100028. [DOI] [PubMed] [Google Scholar]
- 66. Ho MP, Gonzalez JM, Lerner HP, Neuland CY, Whang JM, McMurry-Heath M, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29(10):2984-93. Epub 2015/01/02. doi: 10.1007/s00464-014-4044-2. PubMed PMID: 25552232. [DOI] [PubMed] [Google Scholar]
- 67. Bardes CL. Defining “Patient-Centered Medicine”. New England Journal of Medicine. 2012;366(9):782-3. PubMed PMID: WOS:000300874300003. [DOI] [PubMed] [Google Scholar]
- 68. Coast J, Al-Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health economics. 2012;21(6):730-41. [DOI] [PubMed] [Google Scholar]
- 69. van der Eijk M, Faber MJ, Al Shamma S, Munneke M, Bloem BR. Moving towards patient-centered healthcare for patients with Parkinson’s disease. Parkinsonism & Related Disorders. 2011;17(5):360-4. doi: 10.1016/j.parkreldis.2011.02.012. PubMed PMID: WOS:000292487200012. [DOI] [PubMed] [Google Scholar]
- 70. Marshall T, Pugh A, Fairchild A, Hass S. Patient Preferences for Device-Aided Treatments Indicated for Advanced Parkinson Disease. Value Health. 2017;20(10):1383-93. doi: 10.1016/j.jval.2017.06.001. PubMed PMID: 29241898. [DOI] [PubMed] [Google Scholar]
- 71. Weernink MGM, Groothuis-Oudshoorn CGM, IJzerman MJ, van Til JA. Valuing Treatments for Parkinson Disease Incorporating Process Utility: Performance of Best-Worst Scaling, Time Trade-Off, and Visual Analogue Scales. Value in Health. 2016;19(2):226-32. doi: 10.1016/j.jval.2015.11.011. PubMed PMID: WOS:000373078800014. [DOI] [PubMed] [Google Scholar]
- 72. Weernink MGM, van Til JA, van Vugt JPP, Movig KLL, Groothuis-Oudshoorn CGM, IJzerman MJ. Involving Patients in Weighting Benefits and Harms of Treatment in Parkinson’s Disease. Plos One. 2016;11(8). doi: ARTN e016077110.1371/journal.pone.0160771. PubMed PMID: WOS:000382877200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Okun MS. Management of Parkinson Disease in 2017 Personalized Approaches for Patient-Specific Needs. Jama-J Am Med Assoc. 2017;318(9):791-2. doi: 10.1001/jama.2017.7914. PubMed PMID: WOS:000409183700005. [DOI] [PubMed] [Google Scholar]
- 74. Mullins CD, Vandigo J, Zheng Z, Wicks P. Patient-centeredness in the design of clinical trials. Value in Health. 2014;17(4):471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mpp-10.1177_23814683211021380 for Patient-Centered Identification of Meaningful Regulatory Endpoints for Medical Devices to Treat Parkinson’s Disease by Heather L. Benz, Brittany Caldwell, John P. Ruiz, Anindita Saha, Martin Ho, Stephanie Christopher, Dawn Bardot, Margaret Sheehan, Anne Donnelly, Lauren McLaughlin, Brennan Mange, A. Brett Hauber, Katrina Gwinn, William J. Heetderks and Murray Sheldon in MDM Policy & Practice