Abstract
Introduction
Weight loss in old age increases the risk of sarcopenia caused by the age-related reduction of fat-free mass (FFM). Due to the strong correlation between FFM and resting energy expenditure (REE), the maintenance of this must also be considered. Besides, the physical function (PF) must be maintained.
Objective
The impact of protein intake on changes in FFM, REE, and PF during weight loss in overweight postmenopausal women was investigated.
Methods
Fifty-four postmenopausal women (BMI 30.9 ± 3.4; age 59 ± 7 years) were randomized into 2 groups receiving energy-restricted diets with either 0.8 g (normal protein; NP) or 1.5 g protein/kg body weight (high protein; HP) for 12 weeks, followed by a 6-month follow-up phase with an ad libitum food intake. FFM, REE, and PF (strength, endurance, and balance) were measured at baseline, after weight loss, and after follow-up.
Results
Forty-six women completed the weight loss intervention and 29 were followed up. The weight loss was −4.6 ± 3.6 kg (HP) and −5.2 ± 3.4 kg (NP; both p < 0.001) and the weight regain during follow-up was 1.3 ± 2.8 kg (HP; p = 0.03) and 0.4 ± 2.5 kg (NP; p = 0.39), with no differences between groups. Similar decreases in FFM (−0.9 ± 1.1 [HP] vs. −1.0 ± 1.3 kg [NP]) and REE (−862 ± 569 [HP] vs. −1,000 ± 561 kJ [NP]; both p < 0.001) were observed in both groups. During follow-up, no changes in FFM were detected in either group, whereas in the NP group the REE increased again (+138 ± 296; p = 0.02). The main determinants of FFM loss were the energy deficit and the speed of weight loss. In the NP group, the Short Physical Performance Battery score improved with weight loss (+0.6 ± 0.8; p < 0.001) and handgrip strength decreased (−1.7 ± 3.4 kg; p < 0.001), whereas no changes were observed in the HP group.
Conclusions
An HP weight-loss diet without exercise had no impact on preservation of FFM and REE but may help to maintain muscle strength in postmenopausal women.
Keywords: Weight loss, Protein, Fat-free mass, Resting energy expenditure, Physical function
Introduction
In Germany 61% of women aged 50–59 years are overweight and 27% are obese, and the prevalence increases with age [1]. Obesity can be associated with reduced muscle mass and strength, especially in older people [2]; this is known as “sarcopenic obesity” [3] and it increases the risk of low physical function (PF) [4]. Weight reduction in particular is also associated with a loss of lean muscle mass [4] and thus increases the risk of sarcopenia in older adults [3]. The combination of a higher prevalence of obesity and sarcopenia in women increases the gender-specific risk [5, 6]. Nonetheless, intentional weight loss can improve the PF [7, 8] and metabolic profile [7, 9, 10]. Therefore, suitable scientifically based dietary recommendations for weight reduction in older people should be generated to minimize the risk of FFM loss. Previous studies have shown that weight loss with a high-protein (HP) diet (1.4 vs. 0.8 g/kg protein/day) may help to preserve FFM in adults aged 20–80 years [9, 10, 11]. Two studies in older adults (age 50–70 years) showed a better preservation of FFM with a higher protein intake (30 vs. 15% protein of the total energy intake or 1.11 vs. 0.85 g/kg) during a weight loss of about 9 kg in 20 weeks [12] and 3 kg in 13 weeks, respectively, but the latter with an exercise program [13]. In contrast, some other studies did not show an improved preservation of lean mass with a higher protein content in a weight loss diet (10 weeks to 6 months) in older people (age > 55 years; 0.8–1.0 vs. 1.2–1.7 g/kg protein) [14, 15], one of them with exercise [16]. Also after bariatric surgery, which is accompanied by a significant loss of muscle mass, an HP diet does not prevent muscle mass loss [17]. The designs of the available studies (intervention period, gender, age, and with and without exercise), however, are very heterogeneous and do not allow clear conclusions. In contrast to middle-aged adults, the presence of significant effects in older persons is missing. Furthermore, not all of them were randomized controlled studies (RCT) and only 1 of them had a blinded design. For this issue, no double blinded RCT in postmenopausal women is available.
Due to the association between FFM and resting energy expenditure (REE) [18], the weight loss-induced decrease in FFM also impairs REE and thus increases the risk of weight regain [19]. In addition, Porter Starr et al. [7] showed that a high protein intake (1.2 vs. 0.8 g/kg) can help to improve or maintain PF during weight reduction even without physical exercise in adults aged > 60 years.
The aim of this study was to investigate the effect of an HP diet without exercise during weight loss on changes in FFM, REE, and PF in overweight postmenopausal women. We hypothesized that postmenopausal women on an HP diet experience less of a decrease in muscle mass, REE, and PF during weight reduction compared to the control group. The primary outcome was FFM. Secondary outcomes included REE and PF.
Subjects and Methods
This study was conducted at the Department Nutritional, Food and Consumer Sciences of the Fulda University of Applied Sciences. Fifty-four women were recruited via local flyers or advertisement and randomized in a 1: 1 ratio to 2 parallel arms, i.e., a normal protein group (NP; 0.8 g/kg body weight/day, based on current recommendations at the time of the start of the study [20]) and a HP group (HP; 1.5 g/kg). In order to be included in this study, participants aged ≥50 years and with a BMI ≥30 or ≥27 and waist circumference > 88 cm had to be able to walk without auxiliaries, climb 10 stairs, and to do their typical daily activities. The estimated physical activity level (PAL) had to be < 1.8 and constant during the last 3 months. All women provided written informed consent before randomization. Participants were excluded for the following reasons: BMI ≥35; creatinine > 2.0 mg/dL; weight change > ±5% in the last 6 months; type 2 diabetes; thyroid disease; medication (steroids, diuretics, thyroid drugs, statins, weight loss medication, and β-blockers); participation in other weight reduction programs; kidney, heart, or liver failure; protein supplementation during the last 3 months; neurological disorders; electronic implants; active prostheses; life-sustaining electronic devices; aversion or allergy to whey products (self-information).
A 1: 1 randomization with random block sizes between 12 and 16 was done by an independent computer scientist. A computer-generated list with random numbers was used and, relevant to block size, allocated to a ranking. The study participants and study staff were blinded to the allocation into groups. Annulment of blinding was done after completion of the study.
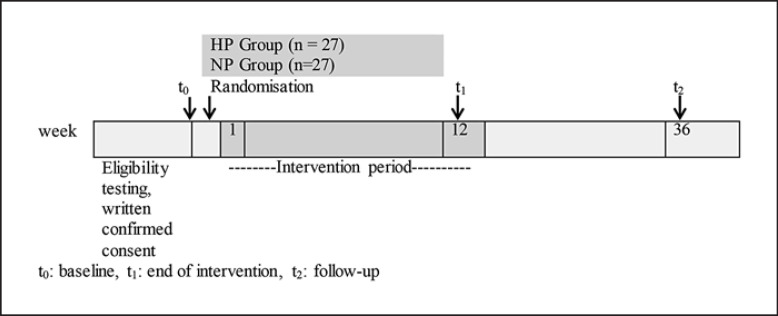
The intervention period of 12 weeks included 4 nutrition training sessions for both groups separately and telephone interviews to enhance compliance. The weight loss period was followed by an ad libitum food intake for 6 months without intervention (follow-up phase). The primary outcome was the change in FFM after the 12-week weight loss period, and secondary outcome parameters were changes in REE and PF after 12 weeks. An outline of the study protocol is given in Figure 1.
Fig. 1.
Schematic representation of this study.
The individual total energy expenditure was calculated as the measured REE multiplied by the PAL (estimated based on a 7-day activity diary) and reduced by about 3,139 kJ (750 kcal) per day for both groups to derive the individual prescription of energy intake. At baseline, dietary history gave information about the preferences and aversions toward food. The individually prescribed energy intake was translated into a meal plan as follows: the participants received a meal replacement (Precon shake; Darmstadt, Germany; 1,565 kJ [374 kcal], 12.2 g fat, 8.7 g carbohydrates, and 46.6 g protein per 100 g) 2 times a day prepared with 300 mL milk (1.5% fat). The third meal and/or snack should be chosen from the individual diet plan. The shakes of the HP group were fortified with whey powder (Primal State, Berlin, Germany; 1,686 kJ [403 kcal], 8.5 g fat, 5.7 g carbohydrates, 76 g protein per 100 g) to achieve the target amount of protein. The differences in protein intake were compensated for by the caloric load on carbohydrate intake in the control group. There were no restrictions of timing for meal intake. For the first 3 weeks, women obtained individual daily meal plans and at each following session they received 10 new plans. Two dietitians were trained in advance to ensure the same content and methods were used during training in all of the groups. Participants had to keep a food diary (after the first and third training sessions for 7 consecutive days) and food checklists (on the remaining days).
Anthropometry, REE, and PF measurements were done by a trained nutritionist at baseline (t0), after the intervention time (t1), and after the ad libitum phase (t2).
REE was measured under standardized conditions after an overnight fast in the morning using indirect calorimetry (Quark RMR; Cosmed, Germany). Before REE was recorded for at least 20 min, the subjects lay supine on a patient couch for 30 min. The first 10 min of data were discarded. The calorimeter was calibrated each morning according to the manufacturer's guidelines.
Body height was determined barefoot in a standing position using a stadiometer (seca 274; seca GmbH, Hamburg, Germany). Body weight was recorded after an overnight fast barefoot in light, everyday clothing using a bioelectrical impedance analysis (seca medical body composition analyzer; seca mBCA 515/514). FFM and fat mass were measured with the patient in a standing position and barefoot, with 8 electrodes for both hands and feet (seca mBCA 515/514), under standardized conditions, with an empty bladder, minimal fasting time, and abstinence from strenuous physical exercise of 8 h, respectively. Waist circumference was measured in an upright position between the lower rib and the iliac crest using a seca tapeline.
The Short Physical Performance Battery (SPPB) [21] was used to measure 3 categories, i.e., balance, gait speed, and strength. Women had to walk a distance of 400 m as fast as they could without running. A Jamar hand dynamometer was used to measure isometric hand grip strength using the standardized Southampton protocol [22].
Fasting blood samples were analyzed at the Fulda clinic for concentrations of serum urea nitrogen in order to assess differences in protein intake among study groups. To assess kidney and liver function, the glomerular filtration rate and creatinine and albumin levels were measured.
Target weight loss was calculated using a body weight planner [23] based on weight, sex, age, height, PAL, % fat mass, % calories from carbohydrates and REE and compared with the actual weight loss for evaluation of compliance.
To detect differences in changes in FFM with weight loss between groups, 27 women per group were needed at a significance level of α = 0.05 and a power of 80%. This calculation was based on an effect size of 0.69 and an SD of 1.5 [10]. Data are presented as means ± SD. An intention-to-treat analysis with the last observation carried forward for all of the participants and a per protocol analysis for the participants who completed the study were conducted. Between-subject analyses served to compare outcomes between groups over time (independent t test), and within-subject analyses were used to measure effects over time (dependent t test, t0 − t1, t1 − t2). An analysis of variance (ANOVA), if applicable with a Greenhouse-Geisser correction, was also done with Bonferroni-corrected pairwise comparisons to detect specific time differences. The significance level was set at α = 0.05.
Results
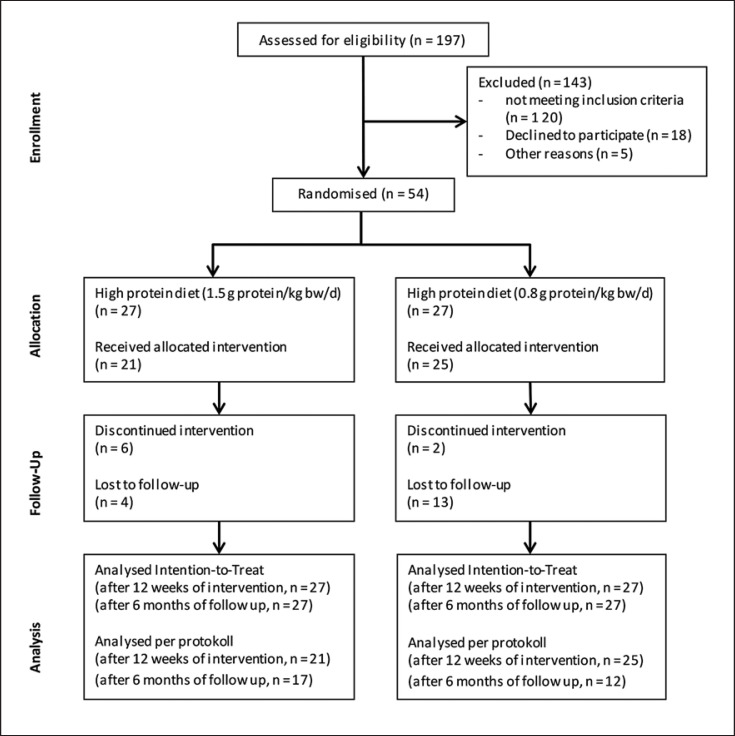
After screening 197 subjects for eligibility (October 2016 to April 2017), 54 women were randomized into 2 groups (Fig. 2). Eight women dropped out during the intervention because of adverse events not related to this study (n = 5) and nonacceptance of the shakes (n = 3). Twenty-nine women completed this study, including the follow-up. Baseline characteristics are shown in Table 1. There were no significant differences in baseline characteristics between the groups, except for a higher serum urea in the HP group (p = 0.003). During the intervention, the HP group consumed on average 113 ± 17 g protein (1.4 ± 0.1 g/kg) and the NP group 63 ± 9 g protein per day (0.8 ± 0.1 g/kg; Table 2). The results of the per protocol analysis were not different from the results of the intention-to-treat analysis. So, only the results of the intention-to-treat analysis are presented.
Fig. 2.
CONSORT flow chart of the study design.
Table 1.
Baseline characteristics of the study participants by treatment group
| HP group (n = 27) | NP group (n = 27) | p valuea | |
|---|---|---|---|
| Age, years | 59.0±6 | 58.7±6 | 0.566 |
| PAL | 1.4±0.1 | 1.4±0.1 | 0.377 |
| Body weight, kg | 85.1±8.6 | 84.9±9.8 | 0.929 |
| BMI | 30.5±2.8 | 31.3±4.0 | 0.439 |
| FFM, kg | 46.8±6.9 | 46.7±5.0 | 0.940 |
| Fat mass, kg | 38.3±5.6 | 38.2±6.9 | 0.947 |
| Waist circumference, cm | 98.2±6.9 | 98.4±10.8 | 0.878 |
| REE, kJ (kcal) | 7,174±707 | 7,082±837 | |
| (1,714±169) | (1,692±200) | 0.665 | |
| SPPB score | 9.4±1.1 | 9.9±1.0 | 0.119 |
| Handgrip strength, kg | 28.7±7.2 | 29.0±4.9 | 0.652 |
| 400-m walk, min:s | 4:10±0:33 | 04:11±0:31 | 0.692 |
| Urea, mg/dL | 33.2±7.6 | 27.5±5.6 | 0.003 |
Data are presented as means ± SD.
Significance level for an independent t test.
Table 2.
Dietary energy and macronutrient intake data in the first and third quarters of the intervention
| Energy, kJ/day (kcal/day) | Protein, g/day | Carbohydrates, g/day | Fat, g/day | ||
|---|---|---|---|---|---|
| Intervention weeks 1–3 | HP (n = 21) NP (n = 25) |
6,048±695 (1,445±166) 5,249±917 (1,254±219)* |
114±16 61±9* |
127±22 134±37 |
45±8 46±14 |
| Intervention weeks 7–9 | HP (n = 20) NP (n = 25) |
6,082±992 (1,453±237) 5,584±1101 (1,334±263) |
112±22 65±11* |
126±20 138±33 |
48±12 51±12 |
| Mean | HP (n = 20) NP (n = 25) |
6,065±737 (1,449±176) 5,417±904 (1,294±216)* |
113±17 63±9* |
127±18 136±29 |
46±9 48±11 |
Data are presented as means ± SD.
Significant difference between groups (p < 0.05, independent t test).
No differences were found between the predicted and real energy deficits calculated by the body weight planner [23]; the values were: −179 ±1,117 kJ/day (−43 ± 267 kcal/day; p = 0.468) in the HP group and 105 ± 1,326 kJ/day (25 ± 317 kcal/day; p = 0.686) in the NP group. However, there was a difference between the real and predicted changes in FFM in both groups (1.7 ± 1.1 kg; p < 0.001 [HP] and 1.4 ± 1.4 kg; p < 0.001 [NP]). The recorded total energy intake was higher in the HP group than in the NP group. The deviation of the recorded energy intake from the planned one was smaller in the HP group than in the NP group, but this was not significant (p = 0.44).
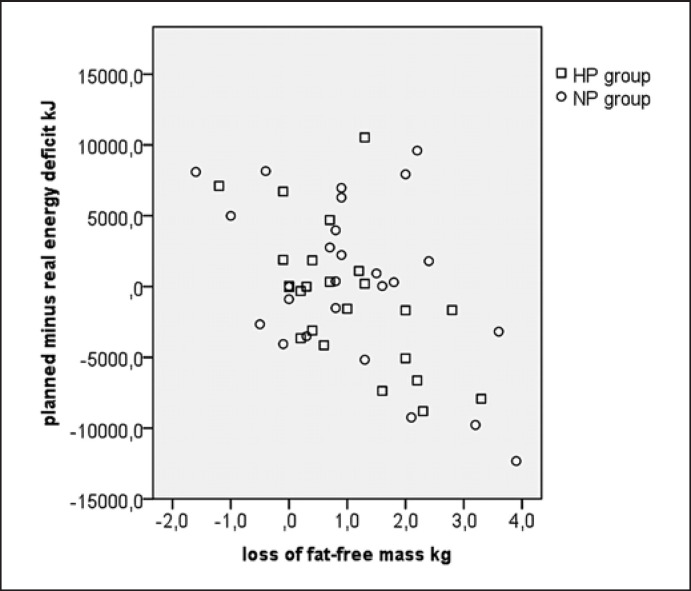
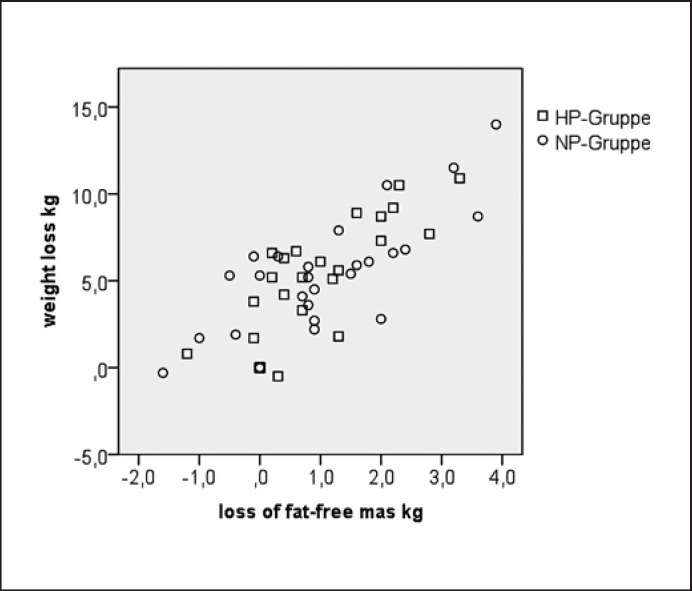
The intervention led to a similar weight loss in both groups (Table 3), which was still significant at the end of the follow-up phase. ANOVA showed no within-group differences during follow-up (t1 vs. t2; p = 0.074) and no between-group differences (t1: p = 0.538, t2: p = 0.264, and over time: p = 0.343). A higher energy deficit and a higher weight loss, respectively, were associated with a higher loss of FFM (r = −0.49; p < 0.001, r = 0.78; p < 0.001; Fig. 3, 4). No significant between-group differences were observed for changes in FFM, fat mass, and waist circumference at t1 and t2 (all p > 0.05). The decrease in FFM did not correlate with protein consumption (in g/kg FFM). Thirty-nine percent of the variance in FFM loss was explained by the speed of weight loss during the first 3 weeks and the energy deficit.
Table 3.
Absolute changes in body weight, FFM, fat mass, waist circumference, REE, SPPB, 400-m walk, and handgrip with weight loss and follow-up by treatment group
| HP group | NP group |
p value (HP × NP)b |
|||
|---|---|---|---|---|---|
| mean ± SD | p valuea | mean ± SD | p valuea | ||
| Body weight, kg | |||||
| Baseline | 85.1±8.6 | 84.9±9.8 | |||
| Change at t1 | −4.6±3.6 | <0.001 | −5.2±3.4 | <0.001 | 0.538 |
| Change at t2 | + 1.3±2.8 | 0.028 | +0.4±2.5 | 0.392 | 0.253 |
| FFM, kg | |||||
| Baseline | 46.8±6.9 | 46.7±5.0 | |||
| Change at t1 | −0.9±1.1 | <0.001 | −1.0±1.3 | <0.001 | 0.575 |
| Change at t2 | +0.4±1.4 | 0.146 | −0.0±1.6 | 0.890 | 0.181 |
| FM, kg | |||||
| Baseline | 38.3± 5.6 | 38.2±6.9 | |||
| Change at t1 | −3.8±2.8 | <0.001 | −4.0±2.7 | <0.001 | 0.718 |
| Change at t2 | +0.9±2.1 | 0.040 | +0.3±2.1 | 0.478 | 0.315 |
| Waist circumference, cm | |||||
| Baseline | 98.2±6.9 | 98.4±10.8 | |||
| Change at t1 | −6.5±4.2 | <0.001 | −7.2±4.0 | <0.001 | 0.669 |
| Change at t2 | −0.6±4.6 | 0.525 | −0.3±2.4 | 0.522 | 0.756 |
| REE, kJ (kcal) | |||||
| Baseline | 7,175±707 (1,714±169) | 7,083±837 (1,692±200) | |||
| Change at t1 | −862±569 (−206±136) | <0.001 | −1,000±561 (−239±134) | <0.001 | 0.369 |
| Change at t2 | +25±276 (+ 6±66) | 0.650 | +138±297 (+33±71) | 0.022 | 0.148 |
| SPPB, score | |||||
| Baseline | 9.4±1.1 | 9.9 ±1.0 | |||
| Change at t1 | +0.4±0.9 | 0.051 | +0.6±0.8 | <0.001 | 0.463 |
| Change at t2 | +0.4±0.7 | 0.015 | +0.1±0.4 | 0.327 | 0.073 |
| 400-m walk, min:s | |||||
| Baseline | 4:10±0:33 | 04:11±0:31 | |||
| Change at t1 | −0:00±0:07 | 0.876 | −0:05±0:12 | 0.045 | 0.281 |
| Change at t2 | +0:01±0:19 | 0.630 | +0:02±0:26 | 0.636 | 0.919 |
| Handgrip, kg | |||||
| Baseline | 28.7±7.2 | 29.0±4.9 | |||
| Change at t1 | +0.1±2.6 | 0.798 | −1.6±3.3 | <0.001 | 0.041 |
| Change at t2 | +0.1±1.9 | 0.864 | −0.2±1.5 | 0.493 | 0.577 |
Significance level for dependent t test within-group changes (t0−t1; t1−t2).
Significance level for independent t test between-group changes.
Fig. 3.
Loss of lean mass vs. the planned minus real energy deficit. Correlation coefficient of the HP group: r = −0.58; p = 0.002. Correlation coefficient of the NP group: r = −0.45; p = 0.019. Correlation coefficient overall: r = −0.49; p < 0.001.
Fig. 4.
Loss of lean mass vs. weight loss. Correlation coefficient of the HP group: r = −0.80; p < 0.001. Correlation coefficient of the NP group: r = −0.78; p < 0.001. Correlation coefficient overall: r = −0.78; p < 0.001.
REE showed a similar decrease in weight loss in both groups (p = 0.369). At t2 significant reductions remained without a group effect (p = 0.871). After adjusting REE for FFM using regression analysis, REE remained reduced with weight loss (p < 0.001).
The SPPB revealed improvements with weight loss (HP: p = 0.051; NP: p < 0.001) and follow-up (both groups: p < 0.001), with no differences between groups. Results from the 400-m test remained unchanged in both groups with weight loss and follow-up. Handgrip strength remained unchanged with weight loss (p = 0.798) in the HP group but decreased in the NP group (p = 0.019). This group effect was significant (p = 0.041).
Discussion
The hypothesis that an increased protein intake without exercise during weight loss in postmenopausal women promotes the preservation of FFM was not confirmed. Reasons for the lack of group effects in the weight loss phase may be the large difference between the predicted and real FFM changes. However, compliance was the same in both groups, so there is no systematic bias. Limitations in measurement precision and the validity of the bioelectrical impedance analysis during the instable phase of weight loss, i.e., because of a decrease in FFM hydration [24], would be another reason, though there needs to be at least a minimal difference, which could not be shown. Furthermore, our results are consistent with studies that used dual X-ray absorptiometry or air displacement plethysmography (Table 4); Backx et al. [15] used 1.7 vs. 0.9 g/kg protein in their 12-week RCT and achieved a weight loss of about 9 kg in 61 subjects aged > 55 years, without differences in FFM loss between the groups. Similarly, in the POWR-UP study, 60 women aged > 45 years were randomized to either an HP group (1.3 g/kg) or an NP group (0.8 g/kg) and achieved a weight loss of 6% within 6 months, without group effects on FFM loss [8]. In addition, in the MEASURE-UP study, equal losses of FFM were observed in 67 older subjects (> 60 years) over a 6-month period of weight loss regardless of the protein amount (0.8 vs. 1.2 g/kg) [7]. Layman et al. [25] also achieved similar FFM losses after 4 months of weight loss in 130 subjects (age 40–56 years) with either 0.8 or 1.6 g/kg protein. In a study with 110 adults (age 25–70 years) a significant greater weight loss but without differences in FFM loss was observed after 3 months of weight loss and after 9 months of weight maintenance in the HP group (0.8 vs. 1.34 g/kg) [26]. Also a study on bariatric patients (age 18–65 years) showed no better preservation of lean mass after a 6-month weight loss follow-up with a high protein intake (25.4 ± 3.7 vs. 15.8 ± 4.4% of the energy intake; p < 0.001) [17]. Other studies, however, have reported contradictory findings (Table 4). Verreijen et al. [13] reported preservation of appendicular muscle mass in 88 obese adults aged > 55 years on a 13-week high-whey protein hypocaloric diet (1.1 vs. 0.85 g/kg), which was confirmed in a 6-week study on adults (age 20–75 years) with insulin resistance (15 vs. 23% of energy intake) [27]. A recent meta-analysis concluded improved maintenance of FFM during weight loss without exercise in older adults (mean > 50 years) consuming an HP diet (≥25% of the total energy or ≥1.0 g/kg) for a time of 8–104 weeks (mean 25 weeks) [28]. Nevertheless, the included studies had different primary outcomes (e.g., bone density, IGF level, and cardiovascular risk factors) and were very heterogeneous. Only 4 or 2 of 20 articles provided details on randomization or blinding procedures. In addition, certain studies were considered as 2 separate studies, which may have artificially increased the protein effect. Only 3 of 20 studies, however, showed a significant difference in FFM loss between groups and only 1 of them included adults aged > 50 years. Furthermore, the studies also included adults aged < 50 years. Of major importance for the evaluation of FFM loss is weight loss over time [29, 30, 31]. Because energy deficit showed a positive association with FFM loss, the results also support that energy deficit is an important predictor and therefore a confounder in studies analyzing the effect of protein on weight loss. Improved satiety and an increased thermic effect of food on an HP diet [32, 33] facilitate weight loss on an HP diet and might therefore contribute to a higher energy deficit which prevents/or acts against the FFM-preserving effects of an HP diet. Weight loss was, however, not significantly higher in the HP diet group (p = 0.54).
Table 4.
Intervention studies on the effects of protein intake during weight loss on FFM
| Study | Proband characteristics | Protein intake | Energy restriction | Weight reduction | FFM change |
|---|---|---|---|---|---|
| Johnston et al. [27] | 21 subjects Male and female Age 20–75 years BMI 20–42 |
125 g/day 80 g/day |
−500 kcal/day from the TEE (calculated using a formula) 6 weeks | −2.3±2.9 kg (P) −2.0±1.8 kg (C) Group difference n.s. |
+ 1.5±3.8 kg (P) −0.5±1.5 kg (C) Group difference p = 0.008 |
| Verreijen et al. [13] | 88 subjects Male and female Age >55 years BMI >30 (men) or >28 (women) and waist circumference >88 cm (women) or >102 cm (men) |
1.11 g/kg BW/day 0.85 g/kg BW/day |
−600 kcal/day from the TEE (REE measured by indirect calorimetry + PAL measured using a 3-day movement diary) 13 weeks | −3.4±3.6 kg −2.8±2.8 kg Group difference n.s. |
Appendicular muscle mass + 0.4±1.2 kg (P) −0.5±2.1 kg (C) Group difference p = 0.03 Leg muscle mass +0.3±1.2 kg (P) −0.6±1.8 kg (C) Group difference p = 0.01 |
| Backx et al. [15] | 61 subjects Male and female Age 55–70 years BMI 27–40 and waist circumference >88 cm (women) or >102 cm (men) |
1.7 g/kg BW/day 0.9 g/kg BW/day |
−25% of the baseline energy intake calculated from a validated 177-item food frequency questionnaire 12 weeks | From 92.8±11.0 to 83.9±10.1 kg (P) From 90.5±10.0 to 81.5±9.7 kg (C) Group difference n.s. |
From 54.8±12.2 to 53.1±11.4 kg (P) From 54.5±9.3 to 52.4±9.1 kg (C) Group difference n.s. |
| Bales et al. [8] | 80 women Age ≥45 years BMI ≥3θ |
1.2 g/kg BW/day 0.8 g/kg BW/day |
−500 kcal/day from the TEE (calculated using a formula) 6 months | −6.2±5.9 kg (P) −6.4±4.9 kg (C) Group difference n.s. |
−0.6±1.1 kg (P) −1.0±1.1 kg (C) Group difference n.s. |
| Porter Starr et al. [7] | 67 subjects Male and female Age >60 years BMI ≥30 |
1.2 g/kg BW/day 0.8 g/kg BW/day |
−500 kcal/day 6 months |
−8.7±7.4 kg (P) −7.5±6.2 kg (C) Group difference n.s. |
−1.1±1.5 kg (P) −1.8±2.9 kg (C) Group difference n.s. |
| Layman et al. [25] | 130 subjects Male and female Age 40–56 years BMI >27 |
1.6 g/kg BW/day 0.8 g/kg BW/day |
−500 kcal/day 4-month weight reduction 8 months with a stable weight |
After 4 months: −8.2±0.5 kg (P) −7.0±0.5 kg (C) Group difference n.s. |
−2.6±0.2 kg (P) −2.4±0.2 kg (C) Group difference n.s. |
| Flechtner-Mors et al. [26] | 110 subjects Male and female Age 25–70 years BMI 27–45 |
1.34 g/kg BW/day 0.8 g/kg BW/day |
−500 kcal from the REE (formula) 3-month weight reduction 9 months with a stable weight | After 3 months: −7.4±4.6 kg (P) −4.8±4.0 kg (C) Group difference p < 0.01 |
After 3 months −2.8±3.6 kg (P) −3.2±2.7 kg (C) Group difference n.s. |
TEE, total energy expenditure; n.s., nonsignifcant; EN%, percentage of energy; P, protein group; C, control group; BW, body weight.
Our assumption that the REE is better maintained following an increased protein intake during weight loss could not be confirmed. This is due to the lack of an effect of the HP diet on preservation of FFM. In addition, weight reduction may lead to an adaptive thermogenesis, indicating that the REE decline is independent of the loss of FFM [29, 34, 35, 36]. Here, there was a difference after adjustment of REE for FFM, and therefore an adaptive thermogenesis was present and the risk of weight regain was higher. Tang et al. [10] showed a significant better preservation of FFM after 12 weeks of weight loss in the HP group (1.4 vs. 0.8 g/kg), and macronutrients did not affect the reduction in REE in 43 overweight middle aged men. Luscombe et al. [37] also did not find an effect of diet composition (27 vs. 16% protein, 12-week intervention) on REE in adults (age 35–65 years). By contrast, Baba et al. [38] found a significant lower decrease in REE in the HP group (45 vs. 12%) in 13 men (−132 ± 51 vs. −384 ± 85 kcal) after a 4-week energy-restricted diet. Data of FFM changes, however, were not available (Table 5).
Table 5.
Intervention studies on the effects of protein intake during weight loss on REE
| Study | Proband characteristics | Protein intake | Energy restriction | Weight reduction, kg | REE change |
|---|---|---|---|---|---|
| Tang et al. [10] | 43 men Age >21 years BMI 25–39.9 |
1.4 g/kg BW/day 0.8 g/kg BW/day |
−750 kcal/day from REE × 1.5 estimated by the Harris-Benedict formula 12 weeks | −9.1±0.7 (P) −10.6±0.6 (C) Group difference n.s. |
−104±77 kcal/day (P) −171±71 kcal/day (C) Group difference n.s. |
| Luscombe et al. [37] | 36 subjects Male and female Age 34–65 years Hyperinsulinemia BMI 27–43 |
27 EN% 16 EN% |
−30% from TEE (measured via the bicarbonate-urea method) 16 weeks, thereof 4 weeks of balanced energy | −7.9±1.1 (P) −8.0±0.7 (C) Group difference n.s. |
−650±171 kJ/day (P) −780±132 kJ/day (C) Group difference n.s. |
| Baba et al. [38] | 13 men Hyperinsulinemia BMI >31 |
45% 12% |
80 E% from REE (measured by indirect calorimetry) 4 weeks | −8.3±0.7 (P) −6.0±0.6 (C) Group difference p < 0.05 |
−132±51 kcal/day (P) −384±85 kcal/day (C) Group difference p < 0.05 |
TEE, total energy expenditure; n.s., nonsignificant; EN%, percentage of energy; P, protein group, C, control group; BW, body weight.
Similar to the present study, improvements in PF (SPPB score: +2.4 ± 1.7 [HP] vs. +0.9 ± 1.7 [NP]; p < 0.01), but with group effects in favor of the HP group (1.2 vs. 0.8 g/kg) in adults aged > 60 years, were shown after 6 months of weight reduction by 8 kg [7]. Verreijen et al. [13] also observed a significant improvement in walking speed and hand grip strength in 88 older subjects (age > 55 years) after a weight loss of 3.5 kg within 13 weeks, without an impact of protein intake (1.11 and 0.85 g/kg). An HP diet (1.3 g/kg) in combination with physical training led to greater improvements of these parameters within 10 weeks of weight reduction compared to an HP diet or training alone in older adults (age > 55 years) [16]. In contrast, other studies found no improvement in walking speed in a weight loss group with 1.2 g/kg protein versus a stable-weight group with 0.8 g/kg in older adults (age > 65 years, 6-month intervention) [39]. Also muscle strength or PF changes remained unaffected after 12 weeks of energy restriction (−25%) by protein intake (1.7 vs. 1.0 g/kg) in 61 adults aged ≥55 years [15] (Table 6).
Table 6.
Intervention studies on the effects of protein intake during weight loss on PF
| Study | Proband characteristics | Protein intake | Energy restriction | Weight reduction, kg | PF change |
|---|---|---|---|---|---|
| Porter Starr et al. [7] | 67 subjects Male and female Age >60 years BMI ≥30 |
1.2 g/kg BW/day 0.8 g/kg BW/day |
−500 kcal/day 6 months |
−8.7±7.4 (P) −7.5±6.2 (C) Group difference n.s. |
SPPB: +2.4±1.7 (P) +0.9±1.7 (C) p = 0.02 group difference Hand grip strength was unchanged in both groups |
| Wycherley et al. [43] | 43 men Age 20–45 years BMI 27–39.9 |
35% (1.3 g/kg BW/day) 17% (0.8 g/kg BW/day) |
−7,000 kJ/day 12 weeks |
−10.7±5.3 (P) −8.7±3.5 (C) Group difference n.s. |
Hand grip strength: −1.9±4.2 kg (P) −1.4±4.1 kg (C) Group differences n.s. Isometric knee strength: +16.8 ± 35.2 (P) + 11.5 ± 36.8 (C) Group difference n.s. |
| Verreijen et al. [13] | 88 probands Male and female Age >55 years BMI >30 (men) or >28 (women) and waist circumference >88 cm (women) or >102 cm (men) |
1.11 g/kg BW/day 0.85 g/kg BW/day |
−600 kcal/day from TEE (REE measured by indirect calorimetry + PAL measured by a 3-day movement diary) 13 weeks |
−3.4±3.6 −2.8±2.8 Group difference n.s. |
Hand grip strength: +2.0 ± 4.6 kg (P) +2.2 ± 4.1 kg (C) Group difference n.s. 4-m walking speed: +0.11±0.25 m/s (P) +1.04±0.22 m/s (C) Group difference n.s. 400-m walking speed: +0.04±0.1 m/s (P) +0.05±0.11 m/s (C) Group difference n.s. Chair rising test: −2.4±4.0 s (P) −1.4±3.1 s (C) Group difference n.s. |
| Backx et al. [15] | 61 subjects Male and female Age 55–70 years BMI 27–40 and waist circumference >88 cm (women) or >102 cm (men) |
1.7 g/kg BW/day 0.9 g/kg BW/day |
−25% from the baseline energy intake calculated from a validated 177-item food frequency questionnaire 12 weeks | From 92.8±11.0 to 83.9±10.1 (P) From 90.5±10.0 to 81.5±9.7 (C) Group difference n.s. |
Hand grip strength: −3±6 kg (P) −1±5 kg (C) Group difference n.s. SPPB score: +0.1±0.7 (P) +0.1±0.7 (C) Group difference n.s. 400-m walking speed: +0.05±0.09 m/s (P) +0.02±0.08 m/s (C) Group difference n.s. Leg press: −9±14 kg (P) −9±13 kg (C) Group difference n.s. |
| Beavers et al. [39] | 69 subjects Male and female Age 65–79 years BMI 30–40 |
1.2 g/kg BW/day (weight loss) 0.8 g/kg BW/day (weight stable) |
−500 kcal/day Weight loss group Stable-weight group |
−6.6±0.4 (weight loss) −0.2±0.5 (stable weight) |
Gait speed: +0.01 (−0.02 to 0.04) m/s (weight loss) −0.02 (−0.05 to 0.01) m/s (weight stable) Group difference n.s. |
| Verreijen et al. [16] | 100 subjects Male and female Age 55–80 years BMI ≥28 (men) or >25 (women) and waist circumference >88 cm (women) or >102 cm (men) |
1.3 g/kg BW/day 0.8 g/kg BW/day |
−600 kcal/day from TEE (REE measured by indirect calorimetry + PAL measured by a 3-day movement diary) 10 weeks |
−2.1±3.6 (P) −1.7±1.8 (C) −2.6±2.9 (exercise) −2.0±2.2 (exercise + P) |
Hand grip strength: −1.7±6.5 kg (P) +1.8±6.6 kg (C) −1.8±11.6 kg (exercise) −2.0±6.0 kg (exercise + P) Protein × exercise interaction p = 0.03 4-m walking speed: +0.08±0.26 m/s (P) +0.13±0.24 m/s (C) +0.08±0.13 m/s (exercise) +0.20±0.24 m/s (exercise + P) Protein × exercise interaction p = 0.045 400-m walking speed: +0.07±0.1 m/s (P) +0.04±0.15 m/s (C) +0.07±0.07 m/s (exercise) +0.08±0.15 m/s (exercise + P) Group differences n.s. Chair rising test: −1.6±1.7 s (P) −1.6±2.1 s (C) −1.0±2.7 s (exercise) −1.4±2.7 s (exercise + P) Group differences n.s. |
EE, total energy expenditure; n.s., nonsignificant; EN%, percentage of energy; P, protein group, C, control group; BW, body weight.
Strengths and Limitations
The present study protocol was based on the recommendations of Bellg et al. [40] for enhancement of treatment fidelity. We recorded a dropout rate of 15% during the intervention phase, which is quite similar to the rate of 15–20% in other studies [13, 39, 41] and below 20%, a value above which Schulz and Grimes [42] postulate a limited validity of study results. However, during follow-up the loss of subjects was 46%. Therefore, these results should be interpreted with caution and further studies with a long-term follow-up period are needed. Nevertheless, this study was characterized by good compliance, demonstrated by the protein-induced increase in serum urea levels in the HP compared to the NP group as well as the low difference between the measured and the predicted weight loss. Dietary records indicated that the target protein intakes of 1.5 and 0.8 g/kg were almost achieved, with 1.4 ± 0.1 and 0.8 ± 0.1 g/kg of protein per day in both groups. The differences in the recorded energy intake illustrate the problem of under- or overreporting, as there was no difference in weight loss. This double-blind RCT is characterized by an intensive assessment of dietary history and individual nutritional plans. The a priori measurement of REE by indirect calorimetry and the assessment of PAL allowed estimation of individual energy requirements and a personalized prescription for energy intake.
In conclusion, this study indicates that an energy-reduced HP diet in combination with 2 meal replacements and high-quality whey protein without physical exercise was more effective for maintaining PF measured by hand grip strength compared to an isocaloric weight loss diet with a normal protein intake in overweight, postmenopausal women. The energy deficit and the speed of weight loss have an important influence on preservation of FFM and should be more considered as a confounder in further studies. Physical exercise should be integrated into weight loss interventions whenever feasible for the subjects.
Statement of Ethics
This study complies with the guidelines for human studies and was conducted in accordance with the World Medical Association Declaration of Helsinki. The Ethics Committee of the Fulda University of Applied Sciences endorsed the study protocol, and written informed consent was obtained from all of the subjects. This study is registered in the German Clinical Trial Register (DRKS00011238).
Conflict of Interest Statement
The authors have no conflict of interests to declare.
Funding Sources
We acknowledge support from the Open Access Publishing Fund of the Fulda University of Applied Sciences. The shake powder was sponsored by the company Precon, and the whey protein powder was sponsored by the company Primal State. The preparation of data and this paper was carried out independently of the sponsors.
Author Contributions
I.E. created the study design, conducted this study, performed the statistical analysis, and wrote this paper. K.K.-M. supervised this study. A.B.-W., S.C.B., and K.K.-M. critically revised this paper. All of the authors read and approved the final version of this work.
Acknowledgement
We thank all of the women for their participation. We would also like to thank the students and dieticians for their active support in conduction of this study.
References
- 1.Mensink GB, Schienkiewitz A, Haftenberger M, Lampert T, Ziese T, Scheidt-Nave C. [Overweight and obesity in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013 May;56((5-6)):786–94. doi: 10.1007/s00103-012-1656-3. [DOI] [PubMed] [Google Scholar]
- 2.Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010 Jan;13((1)):46–51. doi: 10.1097/MCO.0b013e32833309cf. [DOI] [PubMed] [Google Scholar]
- 3.Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin Nutr. 2012 Oct;31((5)):583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011 Mar;364((13)):1218–29. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yadigar S, Yavuzer H, Yavuzer S, Cengiz M, Yürüyen M, Döventaş A, et al. Primary Sarcopenia in Older People with Normal Nutrition. J Nutr Health Aging. 2016 Mar;20((3)):234–8. doi: 10.1007/s12603-015-0562-4. [DOI] [PubMed] [Google Scholar]
- 6.Drey M, Maetzler W, Ferrari U, Sarkopenie . In: Neurogeriatrie: ICF-basierte Diagnose und Behandlung. Maetzler W, Dodel R, Jacobs AH, editors. Berlin, Heidelberg: Springer Berlin Heidelberg; 2019. pp. pp. 69–84. [Google Scholar]
- 7.Porter Starr KN, Pieper CF, Orenduff MC, McDonald SR, McClure LB, Zhou R, et al. Improved Function With Enhanced Protein Intake per Meal: A Pilot Study of Weight Reduction in Frail, Obese Older Adults. J Gerontol A Biol Sci Med Sci. 2016 Oct;71((10)):1369–75. doi: 10.1093/gerona/glv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bales CW, Porter Starr KN, Orenduff MC, McDonald SR, Molnar K, Jarman AK, et al. Influence of Protein Intake, Race, and Age on Responses to a Weight-Reduction Intervention in Obese Women. Curr Dev Nutr. 2017 May;1((5)):e000703. doi: 10.3945/cdn.117.000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007 Feb;15((2)):421–9. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 10.Tang M, Armstrong CL, Leidy HJ, Campbell WW. Normal vs. high-protein weight loss diets in men: effects on body composition and indices of metabolic syndrome. Obesity (Silver Spring) 2013 Mar;21((3)):E204–10. doi: 10.1002/oby.20078. [DOI] [PubMed] [Google Scholar]
- 11.Farnsworth E, Luscombe ND, Noakes M, Wittert G, Argyiou E, Clifton PM. Effect of a high-protein, energy-restricted diet on body composition, glycemic control, and lipid concentrations in overweight and obese hyperinsulinemic men and women. Am J Clin Nutr. 2003 Jul;78((1)):31–9. doi: 10.1093/ajcn/78.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Gordon MM, Bopp MJ, Easter L, Miller GD, Lyles MF, Houston DK, et al. Effects of dietary protein on the composition of weight loss in post-menopausal women. J Nutr Health Aging. 2008 Oct;12((8)):505–9. doi: 10.1007/BF02983202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJ. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. 2015 Feb;101((2)):279–86. doi: 10.3945/ajcn.114.090290. [DOI] [PubMed] [Google Scholar]
- 14.Beavers KM, Gordon MM, Easter L, Beavers DP, Hairston KG, Nicklas BJ, et al. Effect of protein source during weight loss on body composition, cardiometabolic risk and physical performance in abdominally obese, older adults: a pilot feeding study. J Nutr Health Aging. 2015 Jan;19((1)):87–95. doi: 10.1007/s12603-015-0438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Backx EM, Tieland M, Borgonjen-van den Berg KJ, Claessen PR, van Loon LJ, de Groot LC. Protein intake and lean body mass preservation during energy intake restriction in overweight older adults. Int J Obes. 2016 Feb;40((2)):299–304. doi: 10.1038/ijo.2015.182. [DOI] [PubMed] [Google Scholar]
- 16.Verreijen AM, Engberink MF, Memelink RG, van der Plas SE, Visser M, Weijs PJ. Effect of a high protein diet and/or resistance exercise on the preservation of fat free mass during weight loss in overweight and obese older adults: a randomized controlled trial. Nutr J. 2017 Feb;16((1)):10. doi: 10.1186/s12937-017-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schollenberger AE, Karschin J, Meile T, Küper MA, Königsrainer A, Bischoff SC. Impact of protein supplementation after bariatric surgery: A randomized controlled double-blind pilot study. Nutrition. 2016 Feb;32((2)):186–92. doi: 10.1016/j.nut.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Bosy-Westphal A, Wolf A, Bührens F, Hitze B, Czech N, Mönig H, et al. Familial influences and obesity-associated metabolic risk factors contribute to the variation in resting energy expenditure: the Kiel Obesity Prevention Study. Am J Clin Nutr. 2008 Jun;87((6)):1695–701. doi: 10.1093/ajcn/87.6.1695. [DOI] [PubMed] [Google Scholar]
- 19.Bosy-Westphal A, Schautz B, Lagerpusch M, Pourhassan M, Braun W, Goele K, et al. Effect of weight loss and regain on adipose tissue distribution, composition of lean mass and resting energy expenditure in young overweight and obese adults. Int J Obes. 2013 Oct;37((10)):1371–7. doi: 10.1038/ijo.2013.1. [DOI] [PubMed] [Google Scholar]
- 20.Deutsche Gesellschaft für Ernährung Referenzwerte für die Nährstoffzufuhr. 2. Auflage, 2., aktualisierte Ausgabe. Frankfurt am Main: Umschau-Verl; Deutsche Gesellschaft für Ernährung; 2015 [Google Scholar]
- 21.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49((2)):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 22.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011 Jul;40((4)):423–9. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 23.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011 Aug;378((9793)):826–37. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goele K, Bosy-Westphal A, Kossel E, Glüer C, Heller M, Rümcker B, et al. Relative Validität und Präzision der Bioelektrischen Impedanzanalyse zur Erfassung von Veränderungen in der Körperzusammensetzung bei adipösen Patientinnen vor und nach einer Gewichtsreduktion. Akt Ernähr Med. 2008;33((06)):284–90. [Google Scholar]
- 25.Layman DK, Evans EM, Erickson D, Seyler J, Weber J, Bagshaw D, et al. A moderate-protein diet produces sustained weight loss and long-term changes in body composition and blood lipids in obese adults. J Nutr. 2009 Mar;139((3)):514–21. doi: 10.3945/jn.108.099440. [DOI] [PubMed] [Google Scholar]
- 26.Flechtner-Mors M, Boehm BO, Wittmann R, Thoma U, Ditschuneit HH. Enhanced weight loss with protein-enriched meal replacements in subjects with the metabolic syndrome. Diabetes Metab Res Rev. 2010 Jul;26((5)):393–405. doi: 10.1002/dmrr.1097. [DOI] [PubMed] [Google Scholar]
- 27.Johnston CS, Sears B, Perry M, Knurick JR. Use of Novel High-Protein Functional Food Products as Part of a Calorie-Restricted Diet to Reduce Insulin Resistance and Increase Lean Body Mass in Adults: A Randomized Controlled Trial. Nutrients. 2017 Oct;9((11)):E1182. doi: 10.3390/nu9111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JE, O'Connor LE, Sands LP, Slebodnik MB, Campbell WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr Rev. 2016 Mar;74((3)):210–24. doi: 10.1093/nutrit/nuv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Müller MJ, Enderle J, Braun W, Pourhassan M, Bosy-Westphal A. Gibt es die Adaptive Thermogenese? Adipositas - Ursachen, Folgeerkrankungen. Therapie. 2015;09((04)):210–6. [Google Scholar]
- 30.Garthe I, Raastad T, Refsnes PE, Koivisto A, Sundgot-Borgen J. Effect of two different weight-loss rates on body composition and strength and power-related performance in elite athletes. Int J Sport Nutr Exerc Metab. 2011 Apr;21((2)):97–104. doi: 10.1123/ijsnem.21.2.97. [DOI] [PubMed] [Google Scholar]
- 31.Ashtary-Larky D, Ghanavati M, Lamuchi-Deli N, Payami SA, Alavi-Rad S, Boustaninejad M, et al. Rapid Weight Loss vs. Slow Weight Loss: Which is More Effective on Body Composition and Metabolic Risk Factors? Int J Endocrinol Metab. 2017 May;15((3)):e13249. doi: 10.5812/ijem.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bendtsen LQ, Lorenzen JK, Bendsen NT, Rasmussen C, Astrup A. Effect of dairy proteins on appetite, energy expenditure, body weight, and composition: a review of the evidence from controlled clinical trials. Adv Nutr. 2013 Jul;4((4)):418–38. doi: 10.3945/an.113.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leidy HJ, Tang M, Armstrong CL, Martin CB, Campbell WW. The effects of consuming frequent, higher protein meals on appetite and satiety during weight loss in overweight/obese men. Obesity (Silver Spring) 2011 Apr;19((4)):818–24. doi: 10.1038/oby.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goele K, Bosy-Westphal A, Rumcker B, Lagerpusch M, Muller MJ. Influence of changes in body composition and adaptive thermogenesis on the difference between measured and predicted weight loss in obese women. Obes Facts. 2009;2((2)):105–9. doi: 10.1159/000210369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995 Mar;332((10)):621–8. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 36.Fothergill E, Guo J, Howard L, Kerns JC, Knuth ND, Brychta R, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 2016 Aug;24((8)):1612–9. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luscombe ND, Clifton PM, Noakes M, Farnsworth E, Wittert G. Effect of a high-protein, energy-restricted diet on weight loss and energy expenditure after weight stabilization in hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 2003 May;27((5)):582–90. doi: 10.1038/sj.ijo.0802270. [DOI] [PubMed] [Google Scholar]
- 38.Baba NH, Sawaya S, Torbay N, Habbal Z, Azar S, Hashim SA. High protein vs high carbohydrate hypoenergetic diet for the treatment of obese hyperinsulinemic subjects. Int J Obes Relat Metab Disord. 1999 Nov;23((11)):1202–6. doi: 10.1038/sj.ijo.0801064. [DOI] [PubMed] [Google Scholar]
- 39.Beavers KM, Nesbit BA, Kiel JR, Sheedy JL, Arterburn LM, Collins AE, et al. Effect of an Energy-Restricted, Nutritionally Complete, Higher Protein Meal Plan on Body Composition and Mobility in Older Adults With Obesity: A Randomized Controlled Trial. J Gerontol A Biol Sci Med Sci. 2019 May;74((6)):929–35. doi: 10.1093/gerona/gly146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Treatment Fidelity Workgroup of the NIH Behavior Change Consortium Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004 Sep;23((5)):443–51. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- 41.Wright CS, Zhou J, Sayer RD, Kim JE, Campbell WW. Effects of a High-Protein Diet Including Whole Eggs on Muscle Composition and Indices of Cardiometabolic Health and Systemic Inflammation in Older Adults with Overweight or Obesity: A Randomized Controlled Trial. Nutrients. 2018 Jul;10((7)):E946. doi: 10.3390/nu10070946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz KF, Grimes DA. Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet. 2002 Mar;359((9308)):781–5. doi: 10.1016/S0140-6736(02)07882-0. [DOI] [PubMed] [Google Scholar]
- 43.Wycherley TP, Buckley JD, Noakes M, Clifton PM, Brinkworth GD. Comparison of the effects of weight loss from a high-protein versus standard-protein energy-restricted diet on strength and aerobic capacity in overweight and obese men. Eur J Nutr. 2013 Feb;52((1)):317–25. doi: 10.1007/s00394-012-0338-0. [DOI] [PubMed] [Google Scholar]