Abstract
Introduction
Roux-en-Y gastric bypass (RYGB) is the most common surgical procedure for morbid obesity. However, it can present serious late complications, like postprandial hyperinsulinemic hypoglycemia (PHH). Recent data suggested an increase in intestinal SGLT-1 after RYGB. However, there is no data on the inhibition of SGLT-1 to prevent PHH in patients with prior RYBG. On this basis, we aimed to evaluate (a) the effect of canagliflozin 300 mg on the response to 100 g glucose overload (oral glucose tolerance test [OGTT]); (b) the pancreatic response after intra-arterial calcium stimulation in the context of PHH after RYGB.
Materials and Methods
This is a prospective pilot study including patients (n = 21) with PHH after RYGB, matched by age and gender with healthy controls (n = 5). Basal OGTT and after 2 weeks of daily 300 mg of canagliflozin was performed in all cases. In addition, venous sampling after intra-arterial calcium stimulation of the pancreas was performed in 10 cases.
Results
OGTT after canagliflozin showed a significant reduction of plasma glucose levels (minute 30: 161.5 ± 36.22 vs. 215.9 ± 58.11 mg/dL; minute 60: 187.46 ± 65.88 vs. 225.9 ± 85.60 mg/dL, p < 0.01) and insulinemia (minute 30: 95.6 ± 27.31 vs. 216.35 ± 94.86 mg/dL, p = 0.03; minute 60: 120.85 ± 94.86 vs. 342.64 ± 113.32 mIU/L, p < 0.001). At minute 180, a significant reduction (85.7%) of the rate of hypoglycemia was observed after treatment with canagliflozin (p < 0.00001). All cases presented normal pancreatic response after intra-arterial calcium administration.
Conclusion
Canagliflozin (300 mg) significantly decreased glucose absorption and prevented PHH after 100 g OGTT in patients with RYGB. Our results suggest that canagliflozin could be a new therapeutic option for patients that present PHH after RYGB.
Keywords: Bariatric surgery, Obesity, Pharmacological therapy, Postprandial hypoglycemia
Introduction
Roux-en-Y gastric bypass (RYGB) is one of the best known and commonly performed bariatric surgery (BS) procedures [1]. However, despite the excellent results in sustainable weight loss and resolution of comorbidities [2], late complications have been reported. One of these complications is reactive hyperglycemia, also known as postprandial hyperinsulinemic hypoglycemia (PHH).
Recent data in a randomly selected population 4 years after primary RYGB surgery showed that about 50% of the patients developed a hypoglycemic event during a mixed meal test [3]. PHH is characterized by an exaggerated and rapid postprandial peak of insulin followed by a hypoglycemia within 2 or 3 h after the meal, normal fasting plasma glucose and fasting plasma insulin [4, 5]. It is usually described in patients who underwent BS at least 12 months before [6]. However, most of the patients are diagnosed with severe neuroglycopenia symptoms, such as loss of consciousness or seizures [7], suggesting that this alteration may begin earlier after BS [8, 9]. At present, there are no standardized diagnostic methods for PHH. In 2020, the Delphi expert consensus was published in order to shed light on this issue [3]. The authors agreed that a modified oral glucose tolerance test (OGTT) is a useful diagnostic test for PHH. The test is recommended to be considered positive in case of hypoglycemia (<50 mg/dL) 60–180 min after ingestion of glucose (75 or 100 g) after a 12-h fasting.
The physiopathology of PHH is very complex and remains to be elucidated. Several hypotheses have been proposed to explain the appearance of PHH in the context of RYGB. The first hypothesis was an increase in the mass of β cells and development of nesidioblastosis as a result of the exaggerated secretion of GLP-1 [10]. However, this possibility has been questioned because a higher sensitivity of the β cells to GLP-1 was not demonstrated in the pancreas of affected subjects, and due to the failure of pancreatic partial pancreatectomy to resolve hypoglycemia in most patients [5, 11]. At present, the most accepted hypothesis is the ingestion-induced insulin secretion of the β cells due to a greater sensitivity of the β cells to increased glucose levels [12] and less insulin suppression in hypoglycemic conditions.
Apart from the dysregulation of the mechanisms involved in lowering blood glucose levels, the mechanisms related to glucose absorption may also contribute to PHH. It should be noted that in RYGB, the passage of nutrients from the gastric reservoir to the jejunum is accelerated up to 100 times, while the hypertrophy of the intestinal mucosa favors a faster absorption of glucose [5]. Recent trials have suggested the role of intestinal SGLT-1 in the pathophysiology of reactive hypoglycemia [13, 14], and several studies have shown a dysregulation of these transporters in obesity, diabetes, or in patients having a high sugar diet [15, 16]. Additionally, Nguyen et al. [17] found an upregulation of intestinal glucose transporters, SGLT-1, after RYGB. These observations open up the hypothesis of using SGLT-1 blockade as a therapeutic target for PHH.
Canagliflozin is an SGLT-2 inhibitor approved for the treatment of patients with type 2 diabetes (T2D) both in a 100- or 300-mg daily dose [18, 19]. The main accepted mechanism for lowering blood glucose is by increasing urinary glucose excretion via inhibition of renal SGLT-2. However, in healthy subjects treated with escalating doses of canagliflozin given before a mixed meal, doses of canagliflozin higher than 200 mg produced an additional reduction in postprandial plasma glucose and insulin concentrations than lower doses, but with similar urinary glucose excretion during the postprandial period [20]. These results point to extra-renal effects of high doses of canagliflozin. Since canagliflozin at a dose of 300 mg has an SGLT-1 inhibitory action, and these transporters are abundantly expressed in the intestinal mucosa of the small intestine, it seems reasonable to postulate that this drug could be useful in treating PHH. In fact, SGLT-1 represents the primary pathway involved in intestinal glucose and galactose absorption [21].
On this basis, we performed a pilot study that aimed to evaluate the response to OGTT in basal conditions and after treatment with canagliflozin 300 mg in patients with PHH after RYGB. Additionally, we examined the presence of nesidioblastosis by evaluating the pancreatic response to intra-arterial calcium stimulation.
Materials and Methods
A prospective open, placebo-uncontrolled, pilot interventional study was performed at the EASO-accredited COM Morbid Obesity Unit of the Vall Hebron University Hospital, including patients that had previously undergone RYGB at our site and were diagnosed with PHH between July 2018 and December 2019. The control group comprised healthy normal-weight persons matched by age. Most of them were family members of patients included in the study. The study was registered in ClinicalTrails.gov (NCT 04720859), approved by the local Ethics Committee (PR[AG]320/2018), and carried out in accordance with the Declaration of Helsinki.
The patients that agreed to participate in the study signed the informed consent before any procedure related to the study. All patients underwent a complete medical history, anthropometric measurements, and biochemical analysis. We calculated body mass index (BMI) as body weight (kg)/height (m)2. The percentage of excess weight loss (%EWL) was calculated as: (Pre-BS weight − actual weight)/(Pre-BS weight − ideal weight for BMI 25 kg/m2) × 100. Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) was calculated with the following formula: HOMA-IR = fasting insulin (mIU/L) × fasting plasma glucose (mg/dL)/405.
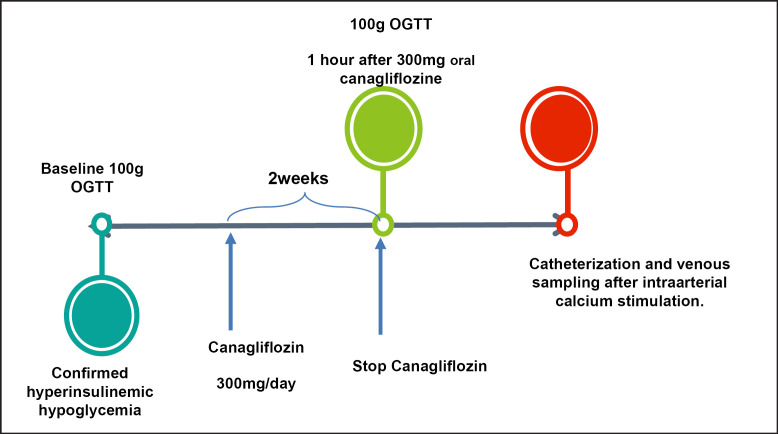
The OGTT was performed as follows: a solution of 100 g glucose was administered at 8 a.m. after 10 h of fasting. Plasma glucose and serum insulin were measured at minute −5, +30, +60, +120, and +180 after the ingestion of the glucose solution. A first OGTT was performed in basal conditions. The control group only performed the basal OGTT. A value of plasma glucose below 50 mg/dL at 60–180 min after the administration of the glucose solution was considered positive for PHH. The patients that additionally presented a value of plasma glucose >200 mg/dL during the OGTT were selected for continuing in the study and were prescribed canagliflozin 300 mg orally daily, as described in Figure 1.
Fig. 1.
Study design.
Patients unwilling to take canagliflozin 300 mg orally during 2 weeks or having any contraindication to this medication were excluded from the study. The patients were asked to sign an additional informed consent for the pancreatic intra-arterial calcium-stimulating test with hepatic venous sampling. The procedure was performed as follows: a 4.1-Fr catheter with a side hole at the tip was positioned through the left femoral vein into the middle hepatic vein. The right femoral artery was catheterized with another 4.1-Fr catheter, followed by standard pancreatic arteriography, with selective injections of nonionic contrast agent into the superior mesenteric artery, gastroduodenal artery, splenic artery, and common hepatic artery. Following each selective arteriogram, 10% calcium gluconate solution was diluted with normal saline into a 5-mL bolus and injected into the selective artery at a dose of 0.010–0.025 mEq Ca2+/kg. Venous blood sample was collected from the hepatic vein during the probe. A >2-fold gradient in insulin concentration at 30, 60, 90, or 120 s after the arterial calcium injection and baseline in any of the superior mesenteric artery, gastroduodenal artery, splenic artery, and common hepatic artery was defined as a positive response for endogenous hyperinsulinism.
Statistical Analysis
To assess differences between groups, the χ2 test for qualitative variables and ANOVA followed by DMS post hoc tests for quantitative variables were used. To evaluate the correlations Spearman's correlation test and regression analysis were performed. Significance was accepted at the level of p < 0.05. Logistic regression was used to predict the ROC curves and the χ2 test was used for ROC area comparison. Statistical analyses were performed with SPSS 24.0 statistical package.
Results
After the basal OGTT, a total of 21 patients that fulfilled the criteria were included in the study. Five healthy normal-weight persons matched by age served as a control group. Ten out of 21 patients consented to the additional pancreatic sampling. The patients who underwent BS had a pre-BS BMI of 42.38 ± 4.87 kg/m2. According to the surgical history records, the RYGB technique, performed by the same team formed by 3 trained bariatric surgeons, had the following characteristics: food loop length: 150–180 cm, bilio-pancreatic loop length: 120 cm, gastric pouch: 30 mL3.
The clinical evolution after the BS was favorable: BMI nadir after 12 months was 28.05 ± 3.47 kg/m2, %EWL 77.95 ± 23.64. Before BS, 5 (23.80%) patients had T2D (1 patient was under insulin treatment and had 7 years of diabetes duration, while the rest of the patients were on diet or metformin, with a T2D duration <2 years), 5 (23.80%) patients had arterial hypertension, and 4 (19.4%) patients had dyslipidemia. After the BS, all patients discontinued the T2D treatment and maintained remission during the follow-up. Pre-BS, the patients presented HbA1c 5.88 ± 0.89% and HOMA-IR 5.88 ± 3.8. These parameters significantly improved during follow-up (HbA1c 5.1 ± 0.95%, p < 0.001, and HOMA-IR 1.64 ± 0.2, p = 0.02; respectively), as reflected by Table 1.
Table 1.
Baseline characteristics of patients and control group
| Cases | Controls | p value | |
|---|---|---|---|
| Subjects, n | 21 | 5 | na |
| Age, years | 47.0±8.41 | 47.1±6.53 | ns |
| Female gender, % | 85 | 80 | ns |
| HbA1c (DCCT), % | 5.1±0.95 | 5.24±0.86 | ns |
| HOMA-IR | 1.64±0.2 | 1.38±0.5 | ns |
| BMI, kg/m2 | 28.9±3.99 | 22.31±2.83 | 0.001 |
The mean time between BS performance and PHH diagnosis was 39.29 ± 31.86 months (ranging between 6 months and 5.7 years). Most of the patients (83%) referred to dizziness and blurry vision as the main symptoms at 3 h after a meal rich in carbohydrates, almost weekly, for at least 3–6 months prior to the diagnosis. It should be noted that 6 patients out of 21 had at least 1 severe episode of 3 h of postprandial loss of consciousness prior to the diagnosis by means of OGTT.
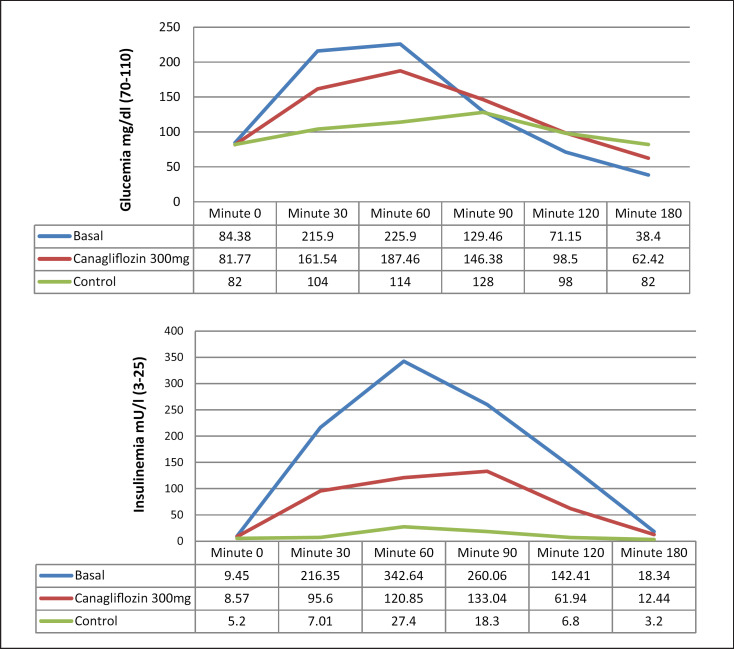
Results from the OGTTs are presented in Figure 2. Basal OGTT in cases showed a pattern of glycemia and insulin secretion that was different from the control group. The patients that underwent BS presented a significantly higher peak of glycemia (>200 mg/dL) and insulinemia (8–14 times above the normal level) at 30–60 min, followed by a hypoglycemia at 180 min (38.4 ± 11.17 mg/dL), while the controls remained in normal range for OGTT during the whole test.
Fig. 2.
Plasma glucose and serum insulin responses during OGTT: basal and after administration of canagliflozin 300 mg.
Canagliflozin administration resulted in a significant reduction in plasma glucose at 30 min (161.5 ± 36.22 vs. 215.9 ± 58.11 mg/dL, p < 0.01) and 60 min (187.46 ± 65.88 vs. 225.9 ± 85.60 mg/dL baseline, p < 0.01) when compared to baseline levels. The same pattern was seen in insulinemia (minute 30: 95.6 ± 27.31 vs. 216.35 ± 94.86 mg/dL, p = 0.03; minute 60: 120.85 ± 94.86 vs. 342.64 ± 113.32 mIU/L, p <0.001). In addition, glucose levels at 180 min were significantly higher than in naïve patients (62.42 ± 13.79 [35–84] vs. 38.4 ± 11.17 [17–55] mg/dL, p < 0.01).
It should be noted that during basal OGTT 20 out of 21 patients (95.2%) presented with blood glucose levels lower than 50 mg/dL. By contrast, during OGTT after canagliflozin only 2 out of 21 patients (9.5%) presented with blood glucose levels lower than 50 mg/dL. Therefore, a significant reduction (85.7%) in the rate of hypoglycemia was observed after treatment with canagliflozin (p < 0.01).
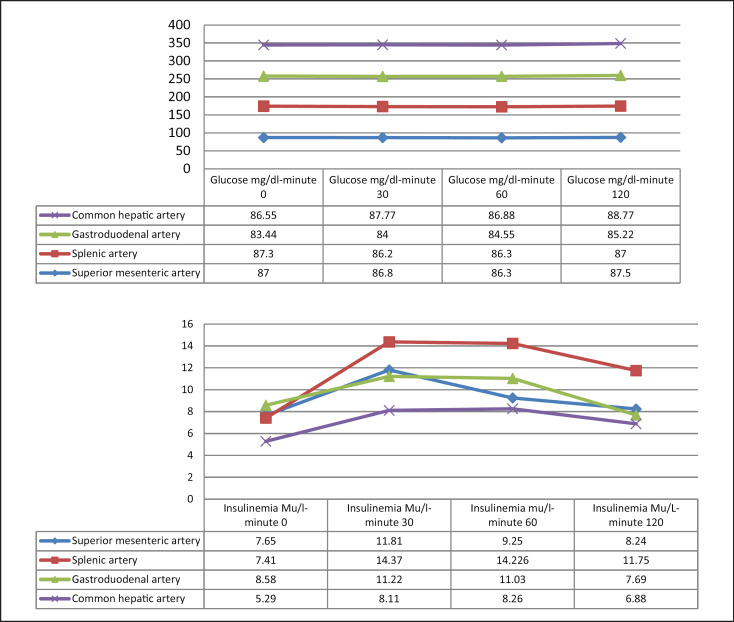
Regarding the pancreatic venous sampling, all patients presented a normal insulin response after intra-arterial calcium administration as reflected by Figure 3. No gradient >2 was registered in any of the territories of the common hepatic, superior mesenteric, gastroduodenal, and splenic arteries.
Fig. 3.
Glucose and insulin levels in venous sampling after intra-arterial calcium stimulation.
Discussion/Conclusion
In this pilot study, we found a reduction of the PHH (according to current recommendations [3]) in 85.7% of the cases after treatment with canagliflozin 300 mg. As far as we know, this is the first study that shows that treatment with canagliflozin is able to prevent reactive hypoglycemia after an OGTT in patients that underwent RYGB.
The mechanisms involved in PHH remain to be elucidated. One of the first hypotheses was an increase in the mass of β cells and the development of nesidioblastosis as a result of the exaggerated secretion of GLP-1 in the time after the BS [10]. However, in the present study, the pancreatic intra-arterial calcium-stimulating test performed in 10 patients with a mean follow-up of around 3 years after BS showed a normal insulin response. These results support the current concept that the exaggerated insulin secretion during OGTT can be attributed to extra-pancreatic factors rather than a nesidioblastosis. In addition, the inhibitory effect of canagliflozin on SGLT-1 transporters expressed in the small intestine seems the most reliable explanation of our findings and suggests an important role of SGLT-1 in the kinetics of glucose absorption and the consequent insulin response. Moreover, since the SGLT-1 channels were shown to be overexpressed after RYGB [17], the early effect of SGLT-1 inhibition after canagliflozin 300 mg administration can represent an important therapeutic target for patients that present PHH after RYGB.
There is scarce literature regarding the effect of SGLT-1 inhibition on insulin response after oral glucose overload. Polidori et al. [22] studied the effects of canagliflozin 300 mg on glycemic and insulinemic excursions after a 600-calorie mixed meal in 20 healthy individuals. These authors found that canagliflozin treatment reduced postprandial plasma glucose and insulin excursions during OGTT. More recently, Martinussen et al. [13] suggested that SGLT-1-mediated glucose absorption contributed to incretin hormone secretion after RYGB. In this study, the authors showed a significant reduction in postprandial levels of glucose and insulin during OGTT using 600 mg of canagliflozin. Taken together, these reported results reinforce the rationale of the present study.
Our pilot study is the first study that aimed to explore the role of canagliflozin 300 mg in PHH after RYGB. We included patients that underwent RYGB and had a previous diagnosis of PHH by showing plasma glycemia <50 mg/dL after a 100-g OGTT. Canagliflozin 300 mg was administered 1 h before the glucose intake solution in order to obtain the maximal effect according to drug pharmacokinetics [19]. During OGTT after the intake of 300 mg of canagliflozin, we found a significant reduction both in glucose levels compared to baseline (25% at minute 30 and 17% at minute 60) and in insulinemia (55.81% at minute 30 and 64.72% at minute 60), followed by prevention of hypoglycemia at minute 180, defined as per Delphi consensus [3] (<50 mg/dL) in 90.47% of the cases. Therefore, canagliflozin can be regarded as an effective and safe therapeutic option for patients that present with PHH after RYGB.
Currently, the main treatment of PHH after RYBG is to decrease the pancreatic response by decreasing glucose absorption or inhibiting the pancreatic response. Most cases have a satisfactory response to a low-carbohydrate diet [23]. However, some cases require medical therapies, such as acarbose, diazoxide, or somatostatin analogs [24], and sometimes even surgical therapies, such as reversal of surgery [25] or pancreatectomy. Our results open up a new therapeutic strategy based on the inhibition of SGLT-1 intestinal transporters.
It could be argued that SGLT-2 inhibition could also participate in the observed results and, therefore, the lack of data regarding urinary excretion of glucose could be a limitation of our study. However, it has been shown in healthy individuals that the main effect of SGLT-1 inhibition using 300 mg of canagliflozin occurs in the first 2 h during the OGTT, while the urinary glucose excretion effect occurs mainly after 2 h[22]. Therefore, a significant contribution of SGLT-2 inhibition to our results is very unlikely.
Finally, we observed that, even under treatment with canagliflozin, patients who underwent RYBG presented significant differences in the TTOG values from healthy controls. This finding underlines the complex pathophysiology of PHH and suggests that SGLT-1-mediated overabsorption of glucose is only one of the involved mechanisms.
In conclusion, we showed a significant reduction in plasma glucose and serum insulin levels after 300 mg canagliflozin during OGTT, thus resulting in prevention of PHH in patients that underwent RYGB. Our results point to inhibition of SGLT-1 channels as the primary underlying mechanism of this effect. Further studies to confirm this pilot study, as well as to examine the pharmacokinetics and the complete mechanism of action of canagliflozin, are needed. Meanwhile, canagliflozin seems a valid option for treating PHH after RYGB in those patients without response to dietetic recommendations.
Statement of Ethics
The study was approved by the local Ethics Committee (PR[AG]320/2018), registered in ClinicalTrails.gov (NCT 04720859), and carried out in accordance with the Declaration of Helsinki. The patients that agreed to participate in the study signed the informed consent before any procedure related to the study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There were no funding sources.
Author Contributions
A.C., R.S., and M.S.: conceptualization; A.C., M.S., and C.H.: methodology; A.C., M.S., and E.F.: software; R.S. and A.C.: validation; A.C., M.S., and E.F.: formal analysis; A.C., M.S., I.H., E.C., M.C., C.G., N.L., R.V., M.G., and R.F.: investigation; A.C. and R.S.: resources; A.C. and E.F.: data curation; A.C. and M.S.: writing; A.C., C.H., and R.S.: writing − review and editing; A.C. and R.S.: supervision: A.C. and R.S.: project administration. All authors have read and agreed to the published version of the manuscript.
References
- 1.Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, et al. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes Surg. 2019 Mar;29((3)):782–95. doi: 10.1007/s11695-018-3593-1. [DOI] [PubMed] [Google Scholar]
- 2.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Swedish Obese Subjects Study Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007 Aug;357((8)):741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 3.Scarpellini E, Arts J, Karamanolis G, Laurenius A, Siquini W, Suzuki H, et al. International consensus on the diagnosis and management of dumping syndrome. Nature Reviews Endocrinology Nature Research. 2020;Volume 16:pp. 448–66. doi: 10.1038/s41574-020-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raverdy V, Baud G, Pigeyre M, Verkindt H, Torres F, Preda C, et al. Incidence and predictive factors of postprandial hyperinsulinemic hypoglycemia after Roux-en-Y Gastric bypass a five year longitudinal study. Ann Surg. 2016 Nov;264((5)):878–85. doi: 10.1097/SLA.0000000000001915. [DOI] [PubMed] [Google Scholar]
- 5.Honka H, Salehi M. Postprandial hypoglycemia after gastric bypass surgery: From pathogenesis to diagnosis and treatment [Internet]. Vol. 22, Current Opinion in Clinical Nutrition and Metabolic Care. Lippincott Williams and Wilkins; 2019:p. 295–302. doi: 10.1097/MCO.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Service FJ, Natt N, Thompson GB, Grant CS, van Heerden JA, Andrews JC, et al. Noninsulinoma pancreatogenous hypoglycemia: a novel syndrome of hyperinsulinemic hypoglycemia in adults independent of mutations in Kir6.2 and SUR1 genes. J Clin Endocrinol Metab. 1999 May;84((5)):1582–9. doi: 10.1210/jcem.84.5.5645. [DOI] [PubMed] [Google Scholar]
- 7.Marsk R, Jonas E, Rasmussen F, Näslund E. Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia. 2010 Nov;53((11)):2307–11. doi: 10.1007/s00125-010-1798-5. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen JB, Pedersen AM, Gribsholt SB, Svensson E, Richelsen B. Prevalence, severity, and predictors of symptoms of dumping and hypoglycemia after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016 Sep-Oct;12((8)):1562–8. doi: 10.1016/j.soard.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Marques AR, Lobato CB, Pereira SS, Guimarães M, Faria S, Nora M, et al. Insights from the Impact of Meal Composition on Glucose Profile Towards Post-bariatric Hypoglycemia Management. Obes Surg. 2020 Jan;30((1)):249–55. doi: 10.1007/s11695-019-04147-1. [DOI] [PubMed] [Google Scholar]
- 10.Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005 Jul;353((3)):249–54. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- 11.Reubi JC, Perren A, Rehmann R, Waser B, Christ E, Callery M, et al. Glucagon-like peptide-1 (GLP-1) receptors are not overexpressed in pancreatic islets from patients with severe hyperinsulinaemic hypoglycaemia following gastric bypass. Diabetologia. 2010 Dec;53((12)):2641–5. doi: 10.1007/s00125-010-1901-y. [DOI] [PubMed] [Google Scholar]
- 12.Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: Current concepts and controversies [Internet]. Vol. 103, Journal of Clinical Endocrinology and Metabolism. Oxford University Press; 2018:p. 2815–26. doi: 10.1210/jc.2018-00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinussen C, Veedfald S, Dirksen C, Bojsen-Møller KN, Svane MS, Wewer Albrechtsen NJ, et al. The effect of acute dual SGLT1/SGLT2 inhibition on incretin release and glucose metabolism after gastric bypass surgery. Am J Physiol Endocrinol Metab. 2020 Jun;318((6)):E956–64. doi: 10.1152/ajpendo.00023.2020. [DOI] [PubMed] [Google Scholar]
- 14.Ohgaki R, Wei L, Yamada K, Hara T, Kuriyama C, Okuda S, et al. Interaction of the sodium/glucose cotransporter (SGLT) 2 inhibitor canagliflozin with SGLT1 and SGLT2: inhibition kinetics, sidedness of action, and transporter-associated incorporation accounting for its pharmacodynamic and pharmacokinetic featuress. J Pharmacol Exp Ther. 2016 Jul;358((1)):94–102. doi: 10.1124/jpet.116.232025. [DOI] [PubMed] [Google Scholar]
- 15.Bhutta HY, Deelman TE, le Roux CW, Ashley SW, Rhoads DB, Tavakkoli A. Intestinal sweet-sensing pathways and metabolic changes after Roux-en-Y gastric bypass surgery. Am J Physiol Gastrointest Liver Physiol. 2014 Sep;307((5)):G588–93. doi: 10.1152/ajpgi.00405.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Gall M, Thenet S, Aguanno D, Jarry AC, Genser L, Ribeiro-Parenti L, et al. Intestinal plasticity in response to nutrition and gastrointestinal surgery. Nutr Rev. 2019 Mar;77((3)):129–43. doi: 10.1093/nutrit/nuy064. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen NQ, Debreceni TL, Bambrick JE, Chia B, Deane AM, Wittert G, et al. Upregulation of intestinal glucose transporters after Roux-en-Y gastric bypass to prevent carbohydrate malabsorption. Obesity (Silver Spring) 2014 Oct;22((10)):2164–71. doi: 10.1002/oby.20829. [DOI] [PubMed] [Google Scholar]
- 18.Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, et al. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem. 2010 Sep;53((17)):6355–60. doi: 10.1021/jm100332n. [DOI] [PubMed] [Google Scholar]
- 19.Devineni D, Curtin CR, Polidori D, Gutierrez MJ, Murphy J, Rusch S, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013 Jun;53((6)):601–10. doi: 10.1002/jcph.88. [DOI] [PubMed] [Google Scholar]
- 20.Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011 Jul;13((7)):669–72. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 21.Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012 Jan;61((1)):187–96. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polidori D, Sha S, Mudaliar S, Ciaraldi TP, Ghosh A, Vaccaro N, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013 Aug;36((8)):2154–61. doi: 10.2337/dc12-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasser M, Meier C, Herren S, Aubry E, Steffen R, Stanga Z. Is testing for postprandial hyperinsulinemic hypoglycemia after gastric bypass necessary? Clin Nutr. 2019 Feb;38((1)):444–9. doi: 10.1016/j.clnu.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Øhrstrøm CC, Worm D, Højager A, Andersen D, Holst JJ, Kielgast UL, et al. Postprandial hypoglycaemia after Roux-en-Y gastric bypass and the effects of acarbose, sitagliptin, verapamil, liraglutide and pasireotide. Diabetes Obes Metab. 2019 Sep;21((9)):2142–51. doi: 10.1111/dom.13796. [DOI] [PubMed] [Google Scholar]
- 25.Dar R, Hershko D, Nevo HA, Mokary SE, Sakran N. Laparoscopic Conversion of Roux-en-Y Gastric Bypass to Sleeve Gastrectomy for Postprandial Hyperinsulinemic Hypoglycemia. Obes Surg. 2020 Mar;30((3)):1171–2. doi: 10.1007/s11695-019-04339-9. [DOI] [PubMed] [Google Scholar]