Abstract
Uterine leiomyosarcoma (LMS) is a rare malignant neoplasm of the female genital tract poorly responsive to conventional chemotherapy and radiotherapy, with an overall poor prognosis. Pazopanib is at the moment the only FDA-approved targeted molecular therapy for uterine LMS, given the exceedingly rare occurrence of actionable genetic mutations in this type of cancer. Here, we describe the first reported case of metastatic uterine LMS with an FN1-anaplastic lymphoma kinase (ALK) fusion mutation occurring in a 63-year-old woman with a history of uterine leiomyomas. The patient progressed on several lines of therapy, including conventional chemotherapy, pazopanib, and the first-generation ALK inhibitor crizotinib. Interestingly, the patient showed a remarkable 16-month response to second generation ALK inhibitors alectinib and lorlatinib. This case demonstrates that ALK inhibitors can be an effective therapeutic strategy for patients with ALK fusion-positive uterine LMS that has progressed on conventional chemotherapy.
Keywords: Leiomyosarcoma, FN1-ALK fusion, Alectinib, Lorlatinib
Introduction
Uterine sarcomas are a rare and heterogeneous group of neoplasms, comprising 1% of all female genital tract cancers, most commonly diagnosed between the ages of 35 and 75. They can be classified based on histologic features into 3 groups: leiomyosarcoma (LMS), endometrial stromal sarcoma, and adenosarcoma, with LMS being the most common.
Despite the rarity of the diagnosis, uterine sarcomas are extremely aggressive cancers. Five-year overall survival for stage III and stage IV disease at diagnosis was 45% and 29%, respectively [1]. Commonly used systemic regimens for metastatic uterine LMS are gemcitabine/docetaxel, doxorubicin, trabectedin, dacarbazine, and pazopanib.
There are very few reports of clinically actionable targetable mutations in LMSs. The most common genetic alterations in LMSs (both uterine and extrauterine) are 13q and 10q deletions, with loss of function of PTEN and RB1, respectively, both present in 75% of all cases of LMS. The most frequent single gene mutation is inactivation of PTEN, found in 58% of the u-LMS [2]. Anaplastic lymphoma kinase (ALK) rearrangements are extremely rare in LMSs, found in around 2.4% of patients [3]. To our knowledge, this is the first described case of uterine LMS harboring an ALK fusion mutation that was responsive to ALK inhibitors.
Case Report
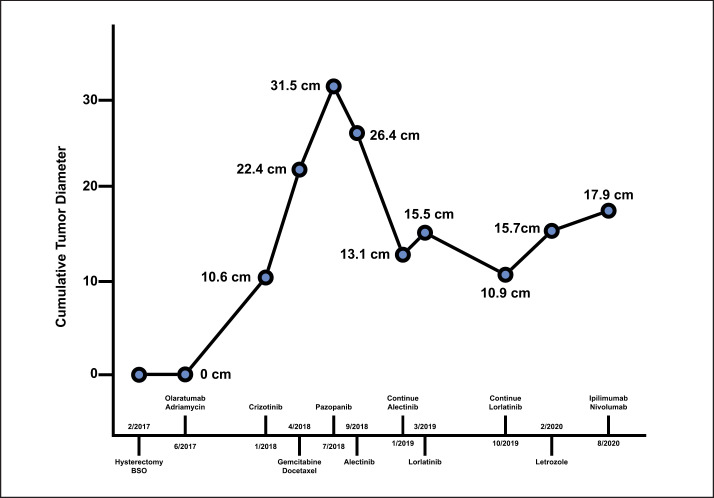
A 63-year-old woman with a history of uterine leiomyomas presented with 4 weeks of abnormal uterine bleeding associated with constipation and severe lower abdominal pain. CT scan of the abdomen and pelvis showed a 15 × 9 × 9 cm uterine mass, concerning for malignancy. She underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy. The surgical pathology demonstrated a highly cellular spindled cell sarcoma with histologic features most compatible with uterine LMS (Fig. 1a, b). No loose myxoid or edematous stroma, thin-walled vessels, or ganglion-like cells were present. In addition, there was no lymphocytic, plasma cellular, eosinophilic, or histiocytic inflammatory infiltrate. The tumor cells were strongly and diffusely positive for desmin (Fig. 1c) and h-caldesmon (Fig. 1d), a marker that is specific for smooth muscle differentiation. In addition, ALK expression was evident on IHC and an ALK gene rearrangement was confirmed on FISH. Pathological staging showed a tumor arising from the uterus, extending to the cervix, left adnexa, and colon with no lymph node or omental involvement. There was no evidence of extra-abdominal metastatic spread at diagnosis. Given her strong family history of cancer, germline testing was performed with a multigene panel, but no germline mutations were detected (Multi-Cancer Panel; Invitae Laboratories, San Francisco, CA, USA). Molecular profiling with next-generation sequencing of the tumor specimen showed FN1-ALK fusion and FBXW7 473 X > R mutation (FoundationOne Heme assay; Foundation Medicine, Cambridge, MA). There was CDKN2C loss, CCNE1 amplification, and TLL2 I164fs*21 mutation, all of unknown significance. Neither microsatellite instability nor PD-L1 expression was detected, and low-tumor mutational burden combined to predict poor response to immune checkpoint inhibitors. After surgery, the patient was started on doxorubicin-olaratumab with 7-month follow-up CT showing no evidence of disease. Four months later, she was found to have locally recurrent disease with multiple new peritoneal masses on imaging (total diameter: 10.6 cm). Given initial ALK positivity of cancer, she received crizotinib 250 mg twice daily with progression of disease on 2-month follow-up imaging (total diameter: 22.4 cm). She was switched to Gemcitabine-docetaxel, but this regimen was stopped after 2 months for local disease progression on follow-up CT (total diameter: 31.5 cm). The patient was switched to pazopanib 200 mg 4 times daily with progression of disease at 2-month radiologic follow-up. Her treatment was switched again to alectinib 600 mg 2 times daily with a 55% decrease in pelvic disease burden at 5-month radiologic follow-up (total diameter: 13.1 cm, Fig. 2). However, follow-up CT imaging 3 months later showed a mixed response with growth of some pelvic masses and significant reduction in size of others (total diameter: 15.5 cm). A decision was made to start lorlatinib 100 mg once a day, with concurrent palliative radiotherapy to 2 dominant pelvic masses. Follow-up imaging response at 4 months showed significant reduction of disease burden to lorlatinib (total diameter: 12.6 cm). Subsequent 7-month and 9-month follow-up CT imaging showed continuing response and stable disease, respectively (total diameter: 10.9 cm, Fig. 3). At 11-month follow-up imaging, she was found to have local progression of disease (total diameter: 15.7 cm). Letrozole 2.5 mg daily was added to lorlatinib given >90% estrogen receptor expression in the tumor at diagnosis. 16-month follow-up imaging demonstrated stable disease. Subsequently, she presented to the emergency department 17 months later with new hematochezia and was found to have progression of disease in a dominant pelvic mass which was invading into the rectal mucosa on CT imaging (total diameter: 17.9 cm). Lorlatinib was stopped, and she was started on salvage immunotherapy with ipilimumab/nivolumab with progression of disease at 3-month follow-up. At this point, alectinib was restarted together with nivolumab and letrozole. At the last follow-up, 4 months later, CT imaging was showing a mixed response. The patient died 1 month later for a traumatic head injury secondary to an accidental fall (Fig. 4, 5).
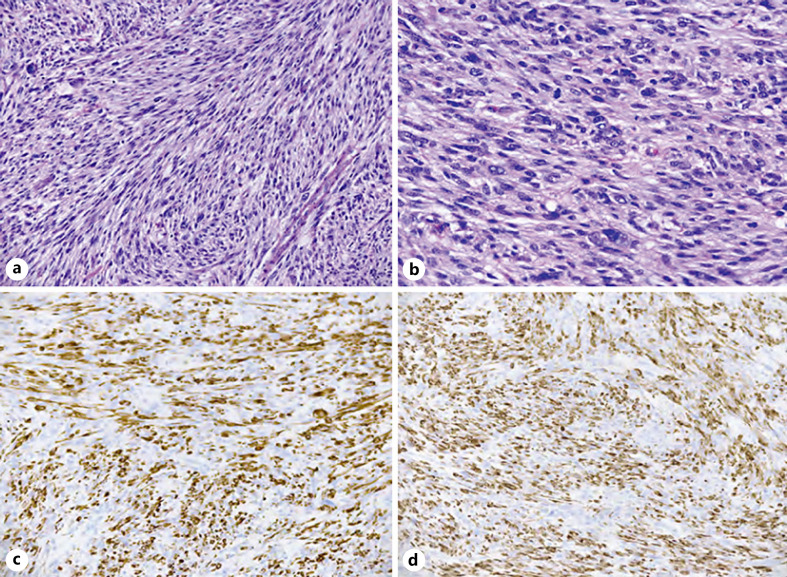
Fig. 1.
Uterine LMS with ALK rearrangement. a The tumor is highly cellular and composed of interlacing long fascicles of spindle cells with diffuse moderate to focally severe cytologic atypia. b Mitotic figures are increased. The tumor cells are positive for desmin (strong and diffuse) (c) and h-caldesmon (strong and diffuse) (d). LMS, leiomyosarcoma; ALK, anaplastic lymphoma kinase.
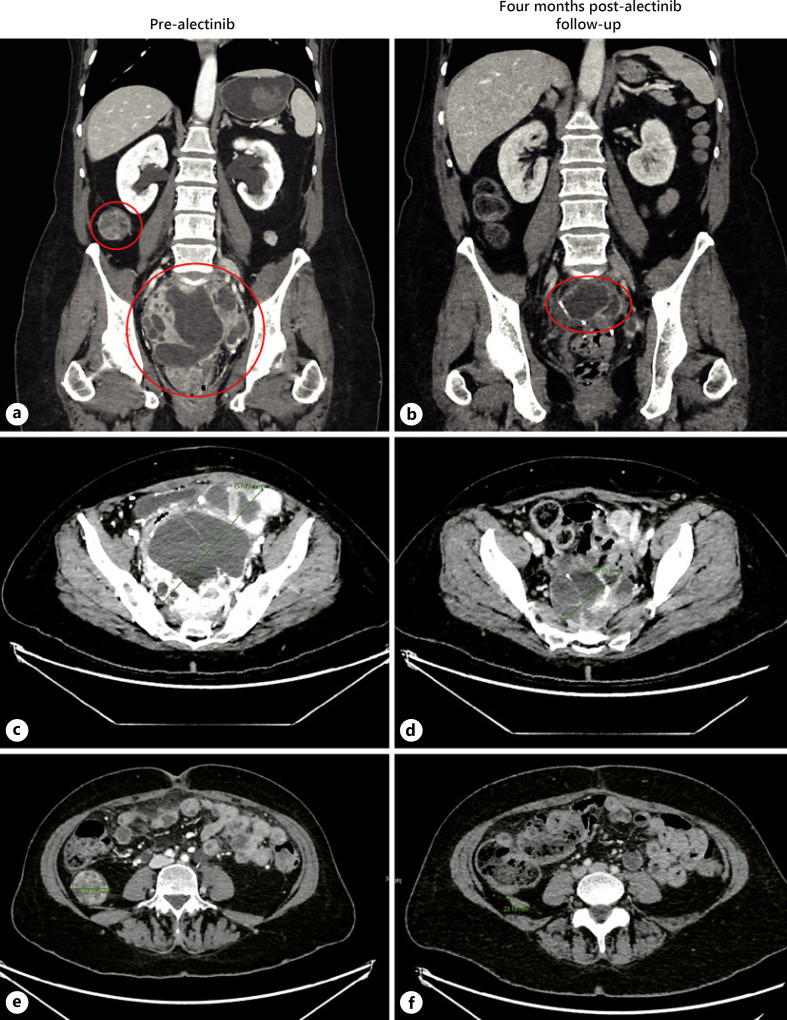
Fig. 2.
Computed tomography imaging illustrating response to alectinib. In the first row, we see circled in red the dominant midline pelvic mass measuring 15.8 × 12.3 cm pre-alectinib (a) and 9.0 × 6.6 cm post-alectinib 3 months later (b). The smaller red circle in A shows the second-largest intrabdominal mass located in the right paracolic gutter pre-alectinib. In the second row, we can see the same dominant midline pelvic mass pre-alectinib (c) and post-alecitnib (d) on the transverse plane. In the third row, we can see the right paracolic gutter mass. Reducing in size from 3.8 cm pre-alectinib (e) to 2.3 cm post-alectinib (f).
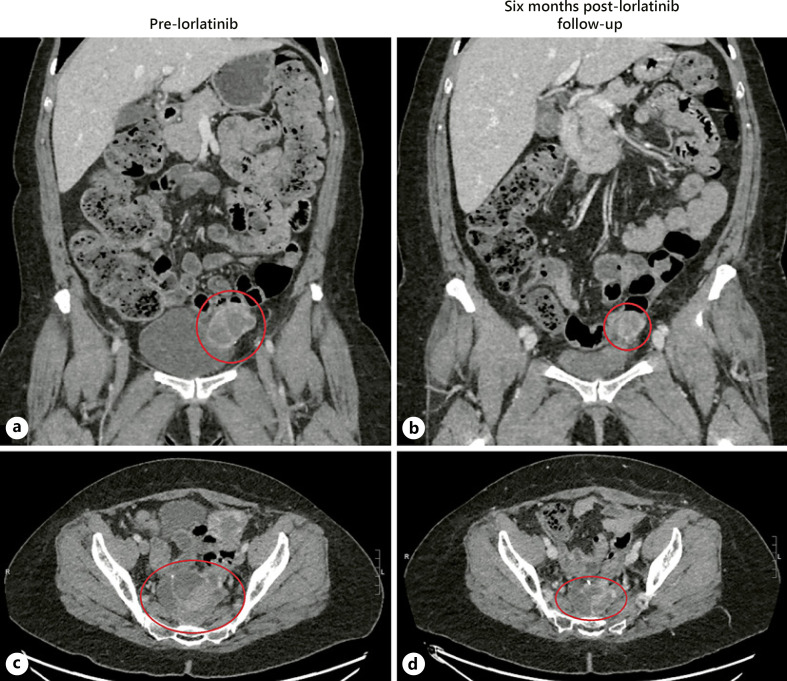
Fig. 3.
Computed tomography imaging illustrating response to lorlatinib. In the first row, we can see a mass abutting the left bladder wall that was measuring 3.7 × 4.4 cm pre-lorlatinib (a) and 3.1 × 3.4 cm post-lorlatnib 6 months later (b). In the second row, we see circled in red the dominant midline pelvic mass measuring 6.0 × 7.8 cm pre-lorlatinib (c) and 5.6 × 4.1 cm post-lorlatinib (d).
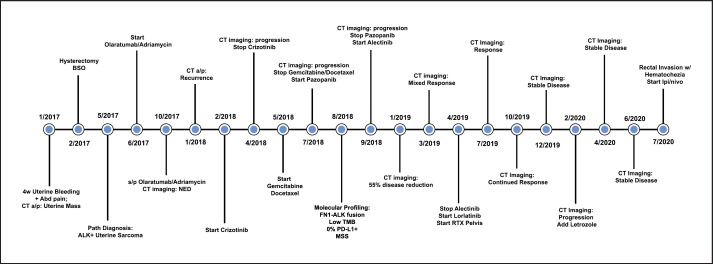
Fig. 4.
Graphical representation of timeline of events including treatments and responses.
Fig. 5.
Change in cumulative tumor diameter over time relative to different treatments adopted.
Discussion
In this case report, we describe the first reported case of uterine LMS harboring the FN1-ALK fusion mutation that was refractory to chemotherapy and VEGF therapy but responded to ALK inhibitor therapy.
ALK is a receptor tyrosine kinase first discovered as a fusion partner in the oncogenic translocation t(2;5) found in a subset of anaplastic large cell lymphoma (ALCL) from which it takes its name. ALK oncogenic mutations are mostly translocations with several partner genes that cause constitutive signaling of the kinase domain of the receptor with activation of several downstream pathways like the MAPK, PI3K-AKT, and JAK-STAT pathway. As of today, more than 30 different ALK fusion partners have been described, with the most common being NPM-ALK in ALCL and EML4-ALK in nonsmall-cell lung cancer (NSCLC). More rarely, point mutations in the kinase domain of ALK can cause constitutive activation of the full-length transmembrane receptor. These mutations are observed mostly in the pediatric population and specifically in neuroblastoma [4]. In the adult population, ALK translocations are seen most commonly in NSCLC, where they account for 4–5% of cases, with approximately 40,000 new cases per year worldwide [5]. ALK fusion genes have been also identified in diffuse large B-cell lymphoma, inflammatory myofibroblastic tumor (IMT), breast cancer, colon cancer, thyroid cancer, and rarely, in pancreatic cancer [4, 5, 6]. ALK oncogenic rearrangements are uncommon in LMSs, with an incidence of less than 3%. Among these, uterine LMSs comprise only a fraction of all the LMSs that harbor ALK fusion genes [3].
The first ALK inhibitor to enter the clinic was crizotinib, which received accelerated approval by the FDA in 2011 based on phase I/II studies showing excellent activity in advanced NSCLC [7]. Later data from phase III trials showed that crizotinib was superior to standard chemotherapy in ALK + NSCLC, with objective response rates of 60–74% and progression-free survival of 8–11 months, depending on the trial considered [8, 9, 10]. Unfortunately, patients treated with crizotinib developed resistance through escape mutations and ultimately progressed. Subsequently, 2nd generation inhibitors, alectinib and ceritinib were developed. Alectinib is currently used as first-line therapy for ALK-positive NSCLC based on the results of the ALEX phase III trial that showed a 12-month event-free survival rate of 68% for the alectinib group compared to 45% in the crizotinib group [11]. However, resistance to ALK inhibitors, which is usually acquired due to point mutations in the tyrosine kinase domain, continues to pose a problem in the treatment of these patients.
Lorlatinib is a third-generation ALK inhibitor active against several alectinib-resistant ALK mutations. A phase II trial showed that lorlatinib has activity in ALK-positive lung cancer. In particular, lorlatinib is active both in treatment naïve and previously ALK inhibitor-treated patients, with an objective response rate of 90% and 47%, respectively [12]. A phase III randomized trial shown lorlatinib to be superior to crizotinib as first-line treatment for patients with ALK-positive NSCLC, with a 1-year progression-free survival of 78% in the lorlatinib group versus 39% in the crizotinib group. Also, lorlatinib has higher activity on CNS metastases given higher penetration diffusion to the blood-brain barrier with an intracranial response rate of 82% and 23% in the lorlatinib and crizotinib groups, respectively [13].
Of note, our case is unique in that our patient responded to second- and third-generation ALK inhibitors, alectinib and lorlatinib, but did not respond to the first-generation ALK inhibitor, crizotinib. One possibility for this could be the presence of pretreatment point mutations in the FN1-ALK gene that confer resistance to crizotinib, but not to second- and third-generation ALK inhibitors. Another possibility is that the fusion partner of ALK could potentially alter the structure of the binding site for crizotinib on ALK.
Our patient demonstrated a 16-month response before progressing and switched to immunotherapy. Notably, the duration of response is shorter compared to those seen in NSCLC but longer than reported for some disease sites other than NSCLC. An APC phase II trial showed that both adults and children with ALK-positive ALCL respond to crizotinib with an objective response rate of 67% and a median progression-free survival of 11.6 months [14]. Another cancer with frequent ALK oncogenic translocations is IMT, a mesenchymal neoplasm frequently arising in the female genital tract. In a multicentric phase II trial, crizotinib has been shown to be highly effective in patients with metastatic ALK-positive IMT compared to patients with advanced ALK-negative IMT, with an objective response rate of 50% versus 14%, respectively [15].
In summary, to our knowledge, this is the first described case of uterine LMS harboring an ALK fusion mutation with 16-month response to second and third-generation ALK inhibitors. It is possible that the shorter duration of response observed in this case of LMS compared to ALK fusion NSCLC is secondary to the development of ALK resistance mutations more rapidly than what it is usually observed in patients with NSCLC.
Statement of Ethics
The patient's next of kin provided written informed consent for the publication of this case report and any accompanying images.
Conflict of Interest Statement
We declare no conflicts of interest.
Funding Sources
No funding was received.
Author Contributions
S.T. drafted the manuscript but was not directly involved in the care of the patient. N.B., L.M., and T.L. were involved in the care of the patient and critically reviewed the manuscript.
References
- 1.George S, Serrano C, Hensley ML, Ray-Coquard I. Soft tissue and uterine leiomyosarcoma. J Clin Oncol. 2018 Jan;36((2)):144–50. doi: 10.1200/JCO.2017.75.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuyoshi H, Yoshida Y. Molecular biomarkers for uterine leiomyosarcoma and endometrial stromal sarcoma. Cancer Sci. 2018 Jun;109((6)):1743–52. doi: 10.1111/cas.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis LE, Nusser KD, Przybyl J, Pittsenbarger J, Hofmann NE, Varma S, et al. Discovery and characterization of recurrent, targetable ALK fusions in leiomyosarcoma. Mol Cancer Res. 2019 Mar;17((3)):676–85. doi: 10.1158/1541-7786.MCR-18-1075. [DOI] [PubMed] [Google Scholar]
- 4.Hallberg B, Palmer RH. The role of the ALK receptor in cancer biology. Ann Oncol. 2016 Sep;27((Suppl 3)):iii4–iii15. doi: 10.1093/annonc/mdw301. [DOI] [PubMed] [Google Scholar]
- 5.Chia PL, Mitchell P, Dobrovic A, John T. Prevalence and natural history of ALK positive non-small-cell lung cancer and the clinical impact of targeted therapy with ALK inhibitors. Clin Epidemiol. 2014 Nov;6:423–32. doi: 10.2147/CLEP.S69718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin E, Li L, Guan Y, Soriano R, Rivers CS, Mohan S, et al. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol Cancer Res. 2009 Sep;7((9)):1466–76. doi: 10.1158/1541-7786.MCR-08-0522. [DOI] [PubMed] [Google Scholar]
- 7.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010 Oct;363((18)):1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013 Jun;368((25)):2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 9.Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N Engl J Med. 2014 Dec;371((23)):2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 10.Lin JJ, Riely GJ, Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017 Feb;7((2)):137–55. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017 Aug;377((9)):829–38. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 12.Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018 Dec;19((12)):1654–67. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AT, Bauer TM, de Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung Ccancer. N Engl J Med. 2020 Nov;383((21)):2018–29. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- 14.Brugières L, Houot R, Cozic N, De La Fouchardière C, Morschhauser F, Brice P, et al. Crizotinib in advanced ALK+ anaplastic large cell lymphoma in children and adults: results of the Acs© phase II trial. Blood. 2017 Dec;130((Suppl 1)):2831. [Google Scholar]
- 15.Schöffski P, Sufliarsky J, Gelderblom H, Blay JY, Strauss SJ, Stacchiotti S, et al. Crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumours with and without anaplastic lymphoma kinase gene alterations (European Organisation for Research and Treatment of Cancer 90101 CREATE): a multicentre, single-drug, prospective, non-randomised phase 2 trial. Lancet Respir Med. 2018 Jun;6((6)):431–41. doi: 10.1016/S2213-2600(18)30116-4. [DOI] [PubMed] [Google Scholar]