Abstract
Objective
Previous literatures have demonstrated widely variable clinical results after transsacral epiduroscopic laser decompression (SELD) and the factors predicting outcomes are not yet established. Therefore, we analyzed the clinical outcome and associated predictive factors of SELD in patients with lumbar disc herniation.
Methods
Between 2015 and 2018, 82 patients who underwent single-level SELD and followed up at least 6 months were enrolled. The overall success rate (excellent or good results at final follow-up) was 58.5% according to Odom’s criteria. Based on this result, patients were divided to 2 groups: a favorable group (n = 48) and an unfavorable group (n = 34). A retrospective review of the baseline characteristics and clinical outcome were conducted to reveal the predictive factors.
Results
As expected, improvement of pain and patient satisfaction, was more favorable in the favorable group (p < 0.05). Moreover, the rate of additional procedure was lower in the favorable group (4.2%, 2 of 48 patients) than in the unfavorable group (35.3%, 12 of 34 patients) (p = 0.011). Among the various baseline characteristics, the only significant predictive factor for favorable outcome was the presence of a high-intensity zone (HIZ) on preoperative magnetic resonance imaging (50.0% [24 of 48 patients] in the favorable group vs. 11.8% [4 of 34 patients] in the unfavorable group; odds ratio, 15.67; p = 0.024).
Conclusion
Although SELD for lumbar disc herniation resulted in a less favorable clinical outcome than that reported in previous studies, in patients with a HIZ, SELD can be an effective minimally invasive surgery to relieve low back pain and/or leg pain.
Keywords: Disc, High-intensity zone, Low back pain, Lumbar spine, Predictive factor, Transsacral epiduroscopic laser decompression
INTRODUCTION
The trans-sacral epiduroscopic laser decompression (SELD) was introduced to resolve a symptomatic epidural lesion of the lumbosacral spine with the development of small-caliber endoscope, flexible video-guided catheters, and less invasive laser technology since 2000s [1-5]. This minimally invasive spinal surgery has been performed as an option among various surgical techniques for treatment of diverse lumbar spinal diseases [4,6]. Many previous literatures have reported the clinical application of SELD in various epidural lesions of the lumbo-sacral spine, such as disc herniation, spinal stenosis, and failed back surgery [6-15].
In particular, in terms of the principle of lasers to condense hydrated materials, soft disc herniation with mild to moderate degree has been suggested as the appropriate indication of SELD [16-18]. According to previous studies, the clinical results of SELD for lumbar disc herniation was so varied that some reports suggested favorable outcome with a greater than 80% success rate [16,18-23], while others insisted unfavorable outcome with a lower than 60% success rate [17, 24].
However, to date, no reports have examined the reason of variations and predictive factors affecting clinical results after SELD in lumbar disc herniation. Therefore, we reviewed the patients with lumbar disc herniation after SELD with follow-up data of at least 6 months and analyzed the predictive factors affecting the outcomes.
MATERIALS AND METHODS
1. Indication and Patient Population
This study was approved by the Institutional Review Board of Gil Medical Center (GAIRB2018-214). The ethics committee waived the requirement for informed consent due to its retrospective character and all data were fully anonymized before we accessed them.
As demonstrated in author’s previous study about the clinical results of SELD [17], the indications of SELD were soft disc herniation with mild to moderate features on magnetic resonance imaging (MRI) concordant with low back pain and/or radiating leg pain despite sufficient conservative treatment at least 2 weeks or with severe pain making daily life impossible. The contraindication for SELD included cauda equina syndrome or motor weakness, hard calcified disc herniation, significant spinal stenosis, infection, hemorrhagic diathesis, and anatomical variations including closed sacral hiatus and peridural cyst [17].
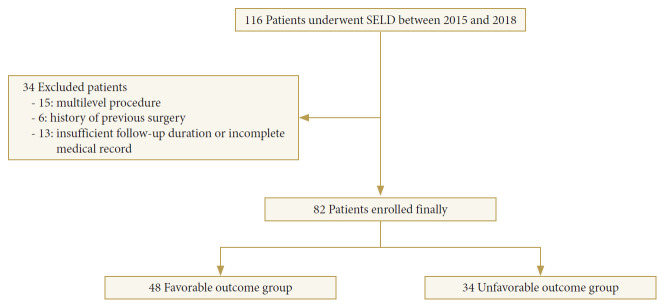
A total of 116 patients who underwent SELD by 1 surgeon in a single institution between November 2015 and November 2018 were analyzed retrospectively. To minimize the selection bias, patients with multilevel procedure, previous history of lumbar spine surgery, and incomplete data of 6-month follow-up were excluded, and eventually, 82 patients were enrolled in final study cohort. Based on patient’s satisfaction at 6 months after surgery, final cohort was allocated to 2 groups; favorable group (n = 48) determined as “excellent” or “good” according to Odom’s criteria, and unfavorable group (n = 34) determined as “fair” or “poor” according Odom’s criteria (Fig. 1).
Fig. 1.
Selection of the final study cohort. SELD, sacral epiduroscopic laser decompression.
2. Operative Technique
Under local anesthesia of the sacral hiatus after prone position of the patients, a 5-mm skin incision and insertion of trocar via sacral hiatus were made under fluoroscopic guidance. After the entering of the trocar to the S2–3 level, a 3.2-mm diameter video-guided catheter containing 2 lumens was inserted through the trocar to the ventral epidural space of the target level using bidirectional steering characteristics. Through the video-guided catheter, a 1.0-mm diameter flexible epiduroscope and a 550-μm diameter flexible fiber of the Ho:YAG laser were advanced to the end of the catheter. The Ho:YAG laser with a 0.4-mm penetration depth and a 2,100-nm wavelength leads to effective ablation of the hydrated soft disc herniation without thermal injury to the adjacent neural structures including nerve root or thecal sac [5,25]. Protruded or ruptured discs was shrunk by a high-intensity laser of 8–10 W (0.8–1.0 J, 10 Hz) until the sufficient decompression of the nerve root. Direct visualization of the widening of the epidural space through the epiduroscope and epidurographic images showing flattened disc outlines and free flow beyond the lesion was considered to be the point of sufficient decompression. A 5–10 mL of solution mixture of lidocaine, dexamethasone, and methylprednisolone was injected into the epidural space at the end of the procedure.
3. Outcome Evaluation
The baseline characteristics such as demographic data including age and sex, body mass index, trauma history, previous history of nerve block, preoperative symptom duration, and surgical level were investigated.
Preoperative lumbar MRI and simple radiographs were performed in all patients. Based on these radiographic findings, disc degeneration based on the Pfirrmann grade [26], presence of high-intensity zone (HIZ) implying annular tearing, morphology of disc herniation (bulging, protruded, or extruded), location of the pathology (central, right, or left), degree of canal compromise (mild, moderate, or severe), grade of root compression (abutting, displace, near obliteration, or obliteration), degree of combined stenosis (none, mild, moderate, or severe), and volume index of the herniated disc were evaluated. The volume index of disc herniation was calculated as height of disc herniation× depth× transverse diameter× 1/2 of the protruded or ruptured disc fragment on MRI. In addition, degree of adhesion during surgery was subjectively classified according to the operator’s experience as mild, moderate, or severe.
The clinical outcomes based on visual analogue scale (VAS) of low back pain, VAS of radiating leg pain, and Odom’s criteria for patient’s satisfaction were collected preoperatively and at every follow-up visit (at 1 week, 1 month, and 6 months after surgery).
The surgical outcomes were assessed based on operation time, surgical failure, complications, hospital stay, and duration of return-to-work. In addition, the requirement of additional procedures including nerve block or revision surgery during follow-up were surveyed.
Plain and dynamic radiographies were performed at preoperation and at 6 months after surgery to assess the radiographic effect. Disc height was measured as an average of anterior and posterior disc height, and corrected using the ratio of disc height to the anteroposterior diameter of the L5 vertebral body to overcome any variations of x-ray magnification. Segmental angle and range of motion at the index level, and total lumbar lordotic angle were determined using Cobb method to assess the change in lumbar alignment.
4. Statistical Analysis
Data management and statistical analysis were performed using IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA). Pearson chi-square test, independent t-test, and nonparametric Mann-Whitney U-test for comparison according to characteristics of the factors. Also, we performed a dichotomous logistic regression analysis of variables that were assumed to have a p-value less than 0.2 in univariate analysis. Results were expressed as means ± standard deviations, means with 95% confidence interval (CI), median with range, or odds ratio (OR), and statistical significance was accepted for p-values of < 0.05.
RESULTS
1. Clinical Outcomes, Surgical Outcomes, and Radiographic Outcomes Between the 2 Groups
A total of 82 patients were comprised of 52 men and 30 women, with a mean age of 40.78 ± 15.24 years.
In terms of the clinical outcome, as expected, low back pain in 1 week and 1 month after surgery; leg pain in 1 week, 1 month, and 6 moths; and Odom’s criteria in 1 week, 1 month, and 6 months were significantly better in the favorable group than in the unfavorable group (p < 0.05; independent t-test and Pearson chi-square test) (Table 1).
Table 1.
Difference in the clinical outcomes between the 2 groups
| Variable | Favorable group (n = 48) | Unfavorable group (n = 34) | Difference | 95% CI | p-value |
|---|---|---|---|---|---|
| VAS for back | |||||
| Preoperation | 5.55 ± 1.80 | 5.34 ± 1.78 | 0.21 ± 0.45 | -0.790 to 1.419 | 0.579† |
| 1 Week | 2.73 ± 0.79 | 3.77 ± 1.36 | -1.04 ± 0.47 | -2.009 to 0.075 | 0.036† |
| 1 Month | 1.73 ± 0.90 | 4.00 ± 1.22 | -2.27 ± 0.45 | -3.200 to 1.346 | < 0.001† |
| 6 Months | 2.36 ± 1.43 | 3.23 ± 1.36 | 0.87 ± 0.57 | -2.053 to 0.319 | 0.144† |
| VAS for leg | |||||
| Preoperation | 5.72 ± 2.15 | 6.62 ± 1.04 | 0.89 ± 0.67 | -2.283 to 0.506 | 0.200† |
| 1 Week | 2.82 ± 1.66 | 4.77 ± 1.53 | -1.95 ± 0.65 | -3.306 to 0.596 | 0.007† |
| 1 Month | 1.64 ± 1.43 | 5.69 ± 1.18 | -4.056 ± 0.53 | -5.162 to 2.649 | < 0.001† |
| 6 Months | 2.27 ± 1.62 | 4.69 ± 1.80 | -2.42 ± 0.70 | -3.879 to 0.960 | 0.002† |
| Odom’s criteria, excellent:good:fair:poor | |||||
| 1 Week | 10:32:6:0 | 0:8:24:2 | - | - | < 0.001‡ |
| 1 Month | 20:28:0:0 | 0:2:28:4 | - | - | < 0.001‡ |
| 6 Months | 16:32:0:0 | 0:0:30:4 | - | - | < 0.001‡ |
Values are presented as mean±standard deviation or number.
CI, confidence interval; VAS, visual analogue scale.
Independent t-test.
Pearson chi-square test.
In terms of the surgical outcome, although the complication rate was not significantly different between the groups. Complications included 1 case of dura puncture, 2 cases of transient lower extremity weakness, and 4 cases of transient headaches or nuchal pain. The rate of additional procedure (revision surgery or additional nerve block), implying surgical failure or recurrence, were significantly lower in the favorable group than in the unfavorable group (4.2% [2 of 48 patients] vs. 35.3% [12 of 34 patients], p = 0.011; Pearson chi-square test) (Table 2).
Table 2.
Difference in the surgical outcomes between the 2 groups
| Variable | Favorable group (n = 48) | Unfavorable group (n = 34) | OR or difference | 95% CI | p-value |
|---|---|---|---|---|---|
| Median operation time (min) | 50.00 (95% CI, 43.87–60.00) | 52.33 (95% CI, 45.02–59.65) | 0.39 ± 4.01 | -0.749 to 1.354 | 0.848† |
| Hospital stay (day) | 3.5 ± 0.9 | 3.7 ± 1.2 | 0.2 ± 0.8 | -0.847 to 1.247 | 0.854‡ |
| Return-to-work | 15.0 ± 7.1 | 15.6 ± 4.2 | 0.6 ± 2.0 | -4.178 to 5.378 | 0.645‡ |
| Complication (n) | 3 | 4 | 0.727 | 0.042–12.518 | 0.826§ |
| Additional procedure | 2 (4.2) | 12 (35.3) | 0.083 | 0.009–0.781 | 0.011§ |
| Additional nerve block | 2 | 6 | 0.212 | 0.020–2.247 | 0.166§ |
| Revision surgery | 0 | 6 | 0.824 | 0.661–1.026 | 0.036§ |
Values are presented as mean±standard deviation or number (%) unless otherwise indicated.
OR, odds ratio; CI, confidence interval.
Nonparametric Mann-Whitney U-test,
Independent t-test,
Pearson chi-square test.
There was no difference in radiographic outcome between the 2 groups (Table 3).
Table 3.
Difference in the radiological outcomes between the 2 groups
| Variable | Favorable group (n = 48) | Unfavorable group (n = 34) | Difference | 95% CI | p-value |
|---|---|---|---|---|---|
| Disc height (mm) | |||||
| Preoperation | 17.41 ± 3.86 | 18.46 ± 1.33 | 1.06 ± 0.98 | -3.031 to 0.921 | 0.377† |
| 6 Months | 17.21 ± 1.54 | 18.35 ± 1.32 | 1.14 ± 1.45 | -3.351 to 1.601 | 0.260 |
| Segmental angle (°) | |||||
| Preoperation | 6.56 ± 3.99 | 9.08 ± 5.30 | 2.51 ± 1.97 | -6.648 to 1.612 | 0.218† |
| 6 Months | 6.78 ± 3.59 | 9.42 ± 4.61 | 2.64 ± 1.74 | -6.281 to 1.009 | 0.147† |
| Range of motion (°) | |||||
| Preoperation | 5.82 ± 4.06 | 6.09 ± 5.16 | 0.27 ± 1.96 | -4.372 to 3.825 | 0.891† |
| 6 Months | 10.57 ± 3.05 | 10.39 ± 3.29 | 2.65 ± 2.64 | -8.152 to 2.843 | 0.326† |
| Total lumbar lordosis (°) | |||||
| Preoperation | 33.88 ± 15.08 | 34.10 ± 13.90 | 0.22 ± 6.23 | -0.647 to 1.087 | 0.670† |
| 6 Months | 32.21 ± 10.57 | 40.18 ± 10.39 | 7.96 ± 4.49 | -17.330 to 1.408 | 0.092† |
Values are presented as mean±standard deviation.
CI, confidence interval.
Independent t-test.
2. Univariate Simple Analysis to Find Predictive Factor
With the exception of low back pain as a dominant symptom, almost none of the baseline characteristics were significantly different between the 2 groups. Low back pain dominance was a significant predictive factor for the favorable group, (45.8% [22 of 48 patients] in the favorable group vs. 11.8% [4 of 34 patients] in the unfavorable group; OR, 6.35; p = 0.021; Pearson chi-square test) (Table 4).
Table 4.
Demographic data and symptom-related characteristics of the 2 groups
| Variable | Favorable group (n = 48) | Unfavorable group (n = 34) | OR or difference | 95% CI | p-value |
|---|---|---|---|---|---|
| Age (yr) | 40.38 ± 14.26 | 41.35 ± 16.97 | -0.97 ± 4.89 | -10.877 to 8.924 | 0.256† |
| Male ratio | 30 (62.5) | 22 (64.7) | 1.18 | 0.321 to 4.326 | 0.804‡ |
| Smoking | 16 (33.3) | 10 (29.4) | 1.28 | 0.332 to 4942 | 0.720‡ |
| Height (cm) | 169.47 ± 10.40 | 169.44 ± 10.33 | 0.04 ± 3.29 | -6.61 to 6.69 | 0.991† |
| Weight (kg) | 69.67 ± 13.87 | 69.24 ± 14.04 | 0.42 ± 4.42 | -8.512 to 9.361 | 0.924† |
| Body mass index (kg/m2) | 24.23 ± 4.36 | 24.02 ± 3.53 | 0.21 ± 1.28 | -2.383 to 2.814 | 0.869† |
| Diabetes | 2 (4.2) | 4 (11.8) | 0.34 | 0.109 to 2.354 | 0.379‡ |
| Hypertension | 8 (16.7) | 10 (29.4) | 0.50 | 0.113 to 2.265 | 0.368‡ |
| Previous block | 30 (62.5) | 18 (52.9) | 1.67 | 0.463 to 6.006 | 0.433‡ |
| Trauma history | 8 (16.7) | 4 (11.8) | 1.58 | 0.254 to 9.817 | 0.622‡ |
| Median symptom duration (wk) | 1.00 (95% CI, 1.04–5.10) | 2.00 (95% CI, 1.67–2.60) | 0.64 ± 1.06 | -0.349 to 2.378 | 0.132§ |
| Dominant symptom, back pain:leg pain | 22:26 | 4:30 | 6.346 | 1.183 to 30.042 | 0.021‡ |
Values are presented as mean±standard deviation or number (%) unless otherwise indicated.
OR, odds ratio; CI, confidence interval.
Independent t-test.
Pearson chi-square test.
Nonparametric Mann-Whitney U-test.
Among the characteristics determined by preoperative MRI and intraoperative findings, the presence of HIZ (50.0% [24 of 48 patients] in the favorable group vs. 11.8% [4 of 34 patients] in the unfavorable group; OR, 7.52; p = 0.011; Pearson chi-square test) and the degree of nerve root compression (p = 0.048; Pearson chi-square test) were significantly different between the 2 groups (Table 5).
Table 5.
Dichotomous logistic regression analysis of various variables between the 2 groups
| Variable | Favorable group (n = 48) | Unfavorable group (n = 34) | OR or difference | 95% CI | p-value |
|---|---|---|---|---|---|
| Surgical level, L3–4:L4–5:L5–S1 | 2:16:30 | 4:6:24 | - | - | 0.386† |
| Pfirrmann grade, I:II:III:IV | 0:14:26:8 | 0:8:24:2 | - | - | 0.504† |
| Disc height ratio to vertebral body | 0.38 ± 0.10 | 0.39 ± 0.08 | 0.01 ± 0.09 | -0.102 to 0.111 | 0.917‡ |
| High intensity zone, n (%) | 24 (50.0) | 4 (11.8) | 7.52 | 1.401–40.0 | 0.011† |
| Morphology of lesion, bulging:protruded:extruded | 6:22:20 | 4:24:6 | - | - | 0.190† |
| Location of herniation, central:right:left | 14:10:24 | 12:10:12 | - | - | 0.517† |
| Degree of canal compromise, mild:moderate:severe | 30:18:0 | 30:4:0 | 4.82 | 0.884–26.300 | 0.055† |
| Degree of nerve compression, abutting:displace:near obliteration:obliteration | 16:24:6:2 | 26:4:4:0 | - | - | 0.048† |
| Herniated disc volume (mm3) | 33.14 ± 11.52 | 38.25 ± 8.96 | 5.11 ± 10.52 | -7.56 to 18.59 | 0.854‡ |
| Degree of stenosis, none:mild:moderate:severe | 32:14:2:0 | 22:12:0:0 | - | - | 0.667† |
| Adhesion, mild:moderate:severe | 3:13:32 | 2:9:23 | - | - | 0.749† |
Values are presented as number or mean±standard deviation unless otherwise indicated.
OR, odds ratio; CI, confidence interval.
Pearson chi-square test.
Independent t-test.
3. Dichotomous Logistic Regression Analysis for Finding Predictive Factor
We performed a regression analysis to screen out clear predictive factors among the various baseline characteristic for the favorable group. In the previous univariate simple analysis, symptom duration, low back pain as a dominant symptom, presence of HIZ, morphology of lesion, degree of canal compromise, and degree of root compression showed meaningful values with p-value less than 0.2.
According to regression analysis of these meaningful factors, the HIZ on MRI (OR, 15.67; 95% CI, 1.425–172.385; p = 0.024) was the only significant predictive factor for the favorable group (Table 6). The correlation test showed no correlation between various factors.
Table 6.
Dichotomous logistic regression analysis of various variables between the 2 groups
| Variable | Favorable group (n = 48) | Unfavorable group (n = 34) | OR or difference | 95% CI | p-value |
|---|---|---|---|---|---|
| High intensity zone | 24 (50.0) | 4 (11.8) | 15.67 | 1.425–172.385 | 0.024 |
| Dominant symptom, back pain:leg pain | 22:26 | 4:30 | 16.95 | 0.570–200.0 | 0.122 |
| Morphology of lesion, bulging:protruded:extruded | 6:22:20 | 4:24:6 | - | - | 0.431 |
| Degree of canal compromise, mild:moderate:severe | 30:18:0 | 30:4:0 | 2.64 | 0.016–9.009 | 0.548 |
| Median symptom duration (wk) | 1.00 (95% CI, 1.04–5.10) | 2.00 (95% CI, 1.67–2.60) | 1.10 | 0.810–1.484 | 0.553 |
| Degree of nerve compression, abutting:displace:near obliteration:obliteration | 16:24:6:2 | 26:4:4:0 | - | - | 0.735 |
Values are presented as number (%) or number unless otherwise indicated.
OR, odds ratio; CI, confidence interval.
DISCUSSION
Some previous literatures reporting the clinical results of SELD for lumbar disc herniation showed that the outcome was favorable, even compared to that of open discectomy or full endoscopic discectomy, in terms of the significant improvement in pain and the high patient’s satisfaction rate (more than 80%) [16,20,22,25,27]. However, according to the author’s previous study, the clinical result was different with that of previous literature as the lower patient’s satisfaction rate (58.5%) and the higher symptom recurrence rate (17.1%) during a minimum 6-month follow-up [17]. This result was not favorable compared to other minimally invasive surgical techniques for lumbar disc herniation.
There are a number of possible explanations for this in inconsistency with previous reports. First, the surgical proficiency of surgeons for SELD could affect the clinical result. We speculated that the outcome is not favorable in the early case series compared to the late case series, and this variation of surgical skill could affect the overall outcomes. However, in our previous study, both the surgical outcome and clinical outcome were not different between the early and late surgery groups [28]. Therefore, we speculated that the patient characteristics could affect clinical outcomes after SELD. For example, differences in detailed baseline characteristics such as demographic data, disc level, morphology of pathology could cause varied clinical outcomes. To find out which factors influence the prognosis after SELD, we compared the various factors between the favorable and unfavorable group.
In our study, as expected reasonably, the clinical outcome, including improvement of pain and patient satisfaction, and surgical outcome, including surgical failure or recurrence, were different between the 2 groups; the favorable group showed more favorable outcome than the unfavorable group. Consequently, we analyzed various factors that could influence the clinical result. Among these factors, according to regression analysis, the existence of a HIZ on preoperative MRI was the only significant predictive factor of the clinical outcome. If MRI showed a HIZ at the pathologic disc level, the effect of SELD was maximized, resulting in favorable outcomes after SELD.
A HIZ is defined as focal high signal intensity in the dorsal side of the disc beneath the posterior longitudinal ligament on T2-weighted MRI [29-31]. This bright area surrounded by a low signal intensity of the annulus fibrosus is clearly dissociated from the signal of nucleus pulposus and appreciably brighter than the water signal at the same level on sagittal T2-weighted MRI [29-31]. A HIZ on T2-weighted MRI may represent damage or tearing of the annulus fibrosus and hydrated inflammation of the tearing site [32-34]. Damage or inflammation of the annulus fibrosus can cause low back pain due to irritation of the sinuvertebral nerve or cause radiating leg pain due to irritation or compression of the concordant nerve root, although occasionally there are no related symptoms [35]. Consequently, according to previous studies, a HIZ is known to be correlated with discogenic low back pain [32,33,36].
With regard to mechanism of laser ablation, SELD could be effective when there are more focal lesions than diffuse lesions and more hydrated lesions than dehydrated lesions. Based on this concept, focal HIZ, i.e., a focal hydrated lesion, could be an optimal target of laser ablation, and the effect of laser ablation can be maximized compared to other soft disc herniation without HIZ. In other words, mild to moderate soft lumbar disc herniation with HIZ can be an optimal indication in performing SELD.
There are several limitations in this study. Because of its retrospective study design, it was difficult to control for all factors related to outcomes. In addition, the number of patients was relatively small and the study was limited in a single institute. However, this single-institute research could keep the quality of data and preclude the diversity of surgeon’s skill.
To the best of our knowledge, this is the first study to report on the predictive factor for successful SELD. Further studies with a larger number of patients or prospective studies are required to confirm the correlation between a specific predictive factor and the clinical result of SELD.
CONCLUSION
The only significant baseline predictive factor for the favorable outcome of SELD was the presence of a HIZ in the pathological disc on preoperative T2-weighted MRI. A favorable outcome can be expected when the patient is selected based on this optimal predisposing factor.
Acknowledgments
This research was supported by the Gachon University and Gil Medical Center (Grant number: FRD2020-20).
Footnotes
The authors have nothing to disclose.
REFERENCES
- 1.Blomberg R. The dorsomedian connective tissue band in the lumbar epidural space of humans: an anatomical study using epiduroscopy in autopsy cases. Anesth Analg. 1986;65:747–52. [PubMed] [Google Scholar]
- 2.Leu H, Schreiber A. Endoscopy of the spine: minimally invasive therapy. Orthopade. 1992;21:267–72. [PubMed] [Google Scholar]
- 3.Shutse G, Kurtse G, Grol O, et al. Endoscopic method for the diagnosis and treatment of spinal pain syndromes. Anesteziol Reanimatol. 1996;(4):62–4. [PubMed] [Google Scholar]
- 4.Sircus W. Milestones in the evolution of endoscopy: a short history. J R Coll Physicians Edinb. 2003;33:124–34. [PubMed] [Google Scholar]
- 5.Ruetten S, Meyer O, Godolias G. Application of holmium: YAG laser in epiduroscopy: extended practicabilities in the treatment of chronic back pain syndrome. J Clin Laser Med Surg. 2002;20:203–6. doi: 10.1089/104454702760230528. [DOI] [PubMed] [Google Scholar]
- 6.Ruetten S, Meyer O, Godolias G. Endoscopic surgery of the lumbar epidural space (epiduroscopy): results of therapeutic intervention in 93 patients. Minim Invasive Neurosurg. 2003;46:1–4. doi: 10.1055/s-2003-37962. [DOI] [PubMed] [Google Scholar]
- 7.Ruetten S, Meyer O, Godolias G. Epiduroscopic diagnosis and treatment of epidural adhesions in chronic back pain syndrome of patients with previous surgical treatment: first results of 31 interventions. Z Orthop Ihre Grenzgeb. 2002;140:171–5. doi: 10.1055/s-2002-31536. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi T, Hirabayashi Y, Seo N, et al. Lysis of adhesions and epidural injection of steroid/local anaesthetic during epiduroscopy potentially alleviate low back and leg pain in elderly patients with lumbar spinal stenosis. Br J Anaesth. 2004;93:181–7. doi: 10.1093/bja/aeh201. [DOI] [PubMed] [Google Scholar]
- 9.Dashfield AK, Taylor MB, Cleaver JS, et al. Comparison of caudal steroid epidural with targeted steroid placement during spinal endoscopy for chronic sciatica: a prospective, randomized, double-blind trial. Br J Anaesth. 2005;94:514–9. doi: 10.1093/bja/aei084. [DOI] [PubMed] [Google Scholar]
- 10.Donato AD, Fontana C, Pinto R, et al. The effectiveness of endoscopic epidurolysis in treatment of degenerative chronic low back pain: a prospective analysis and follow-up at 48 months. Acta Neurochir Suppl. 2011;108:67–73. doi: 10.1007/978-3-211-99370-5_11. [DOI] [PubMed] [Google Scholar]
- 11.Jo DH, Yang HJ. The survey of the patient received the epiduroscopic laser neural decompression. Korean J Pain. 2013;26:27–31. doi: 10.3344/kjp.2013.26.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jo DH, Yang HJ, Kim JJ. Approach for epiduroscopic laser neural decompression in case of the sacral canal stenosis. Korean J Pain. 2013;26:392–5. doi: 10.3344/kjp.2013.26.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jo DH, Kim ED, Oh HJ. The comparison of the result of epiduroscopic laser neural decompression between FBSS or not. Korean J Pain. 2014;27:63–7. doi: 10.3344/kjp.2014.27.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GW, Jang SJ, Kim JD. The efficacy of epiduroscopic neural decompression with Ho:YAG laser ablation in lumbar spinal stenosis. Eur J Orthop Surg Traumatol. 2014;24 Suppl 1:S231–7. doi: 10.1007/s00590-013-1407-7. [DOI] [PubMed] [Google Scholar]
- 15.Jeon S, Lee GW, Jeon YD, et al. A preliminary study on surgical navigation for epiduroscopic laser neural decompression. Proc Inst Mech Eng H. 2015;229:693–702. doi: 10.1177/0954411915599801. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Lee SH, Lim KT. Trans-sacral epiduroscopic laser decompression for symptomatic lumbar disc herniation: a preliminary case series. Photomed Laser Surg. 2016;34:121–9. doi: 10.1089/pho.2015.4000. [DOI] [PubMed] [Google Scholar]
- 17.Son S, Lee SG, Ahn Y, et al. Clinical outcomes of trans-sacral epiduroscopic laser decompression (SELD) in patients with lumbar disc herniation. Pain Res Manag. 2020;2020:1537875. doi: 10.1155/2020/1537875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon BJ, Yi S, Ha Y, et al. Clinical efficacy and safety of transsacral epiduroscopic laser decompression compared to percutaneous epidural neuroplasty. Pain Res Manag. 2019;2019:2893460. doi: 10.1155/2019/2893460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi KC, Lee DC, Park CK. A novel combination of percutaneous endoscopic lumbar discectomy and epiduroscopic laser neural decompression for down-migrated disc herniation. Pain Physician. 2017;20:E605–9. [PubMed] [Google Scholar]
- 20.Kim SK, Lee SC, Park SW, et al. Complications of lumbar disc herniations following trans-sacral epiduroscopic lumbar decompression: a single-center, retrospective study. J Orthop Surg Res. 2017;12:187. doi: 10.1186/s13018-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park CH, Lee SH. Endoscopic epidural laser decompression versus transforaminal epiduroscopic laser annuloplasty for lumbar disc herniation: a prospective, randomized trial. Pain Physician. 2017;20:663–70. [PubMed] [Google Scholar]
- 22.Kim SK, Lee SC, Park SW. Trans-sacral epiduroscopic laser decompression versus the microscopic open interlaminar approach for L5-S1 disc herniation. J Spinal Cord Med. 2018:1–11. doi: 10.1080/10790268.2018.1442285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh J, Jo D. Epiduroscopic laser neural decompression as a treatment for migrated lumbar disc herniation: case series. Medicine. 2018;97:e0291. doi: 10.1097/MD.0000000000010291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Son S, Lee SG, Ahn Y, et al. Outcomes of epiduroscopic laser ablation in patients with lumbar disc herniation. Medicine. 2020;99:e23337. doi: 10.1097/MD.0000000000023337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jo D, Lee DJ. The extent of tissue damage in the epidural space by Ho/YAG laser during epiduroscopic laser neural decompression. Pain Physician. 2016;19:E209–14. [PubMed] [Google Scholar]
- 26.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001;26:1873–8. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 27.Hazer DB, Acarbas A, Rosberg HE. The outcome of epiduroscopy treatment in patients with chronic low back pain and radicular pain, operated or non-operated for lumbar disc herniation: a retrospective study in 88 patients. Korean J Pain. 2018;31:109–15. doi: 10.3344/kjp.2018.31.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Son S, Yoo CJ, Yoo BR, et al. Learning curve of trans-sacral epiduroscopic laser decompression in herniated lumbar disc disease. BMC Surg. 2021;21:39. doi: 10.1186/s12893-020-00949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teraguchi M, Samartzis D, Hashizume H, et al. Classification of high intensity zones of the lumbar spine and their association with other spinal MRI phenotypes: the Wakayama Spine Study. PLoS One. 2016;11:e0160111. doi: 10.1371/journal.pone.0160111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aprill C, Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361–9. doi: 10.1259/0007-1285-65-773-361. [DOI] [PubMed] [Google Scholar]
- 31.Carragee EJ, Paragioudakis SJ, Khurana S. 2000 Volvo Award winner in clinical studies: Lumbar high-intensity zone and discography in subjects without low back problems. Spine (Phila Pa 1976) 2000;25:2987–92. doi: 10.1097/00007632-200012010-00005. [DOI] [PubMed] [Google Scholar]
- 32.Schellhas KP, Pollei SR, Gundry CR, et al. Lumbar disc high-intensity zone. Correlation of magnetic resonance imaging and discography. Spine (Phila Pa 1976) 1996;21:79–86. doi: 10.1097/00007632-199601010-00018. [DOI] [PubMed] [Google Scholar]
- 33.Dongfeng R, Hou S, Wu W, et al. The expression of tumor necrosis factor-α and CD68 in high-intensity zone of lumbar intervertebral disc on magnetic resonance image in the patients with low back pain. Spine (Phila Pa 1976) 2011;36:E429–33. doi: 10.1097/BRS.0b013e3181dfce9e. [DOI] [PubMed] [Google Scholar]
- 34.Teraguchi M, Yim R, Cheung JP, et al. The association of high-intensity zones on MRI and low back pain: a systematic review. Scoliosis Spinal Disord. 2018;13:22. doi: 10.1186/s13013-018-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang C, Zhang W, Chen L, et al. The correlation between the high-intensity zone on a T2-weighted MRI and positive outcomes of discography: a meta-analysis. J Orthop Surg Res. 2017;12:26. doi: 10.1186/s13018-017-0523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Li Z, Zhang C, et al. Correlation between high-intensity zone on MRI and discography in patients with low back pain. Medicine. 2017;96:e7222. doi: 10.1097/MD.0000000000007222. [DOI] [PMC free article] [PubMed] [Google Scholar]