Abstract
Development of intranasal vaccines for HIV-1 and other mucosal pathogens has been hampered by the lack of adjuvants that can be given safely to humans. We have found that an intranasal Shigella vaccine (Invaplex) which is well tolerated in humans can also function as an adjuvant for intranasal protein and DNA vaccines in mice. To determine whether Invaplex could potentially adjuvant similar vaccines in humans, we simultaneously administered a simian immunodeficiency virus (SIV) envelope (Env) protein and DNA encoding simian-human immunodeficiency virus (SHIV) with or without Invaplex in the nasal cavity of female rhesus macaques. Animals were intranasally boosted with adenoviral vectors expressing SIV env or gag,pol to evaluate memory responses. Anti-SIV antibodies in sera and nasal, genital tract and rectal secretions were quantitated by ELISA. Intracellular cytokine staining was used to measure Th1-type T cells in blood. Macaques given DNA/protein immunizations with 0.5 mg Invaplex developed greater serum IgG, nasal IgA and cervicovaginal IgA responses to SIV Env and SHIV Gag,Pol proteins when compared to non-adjuvanted controls. Rectal IgA responses to Env were only briefly elevated and not observed to Gag,Pol. Invaplex increased frequencies of IFNγ-producing CD4 and CD8 T cells to the Env protein, but not T cell responses induced by the DNA. Ad-SIV boosting increased Env-specific polyfunctional T cells and Env- and Gag,Pol-specific antibodies in serum and all secretions. The data suggest that Invaplex could be highly effective as an adjuvant for intranasal protein vaccines in humans, especially those intended to prevent infections in the genital or respiratory tract.
Abbreviations: Ad, adenovirus; CVS, cervicovaginal secretions; Env, envelope; ICS, intracellular cytokine staining; IM, intramuscular; IN, intranasal; NHP, nonhuman primates; NS, nasal secretions; RS, rectal secretions; S-IgA, secretory IgA; Th, T helper
Keywords: HIV/AIDS, IgA, Mucosal adjuvant, Reproductive, Respiratory tract
1. Introduction
Vaccines for respiratory pathogens are typically administered by the intramuscular (IM) route, which does not induce immune responses specifically in the respiratory tract [1], [2] but does generate serum antibodies that eliminate pathogens inside the host. Intranasal (IN) vaccines may be optimal for preventing infections by respiratory pathogens as they generate local secretory IgA (S-IgA) respiratory tract antibodies that could prevent pathogen entry into the body altogether. Indeed, in animal studies, influenza-specific IgA in nasal secretions has been shown more effective than serum IgG neutralizing antibodies for preventing airborne transmission in the upper respiratory tract [3], [4], [5], [6]. IN immunization has also been found more effective than IM immunization for preventing a variety of respiratory infections in animals [7], [8], [9], [10], [11]. Thus, new nasal vaccines for respiratory syncytial virus and other respiratory pathogens are being tested in humans [12], [13], [14], [15].
The nasal vaccination route is the only mucosal route shown to be capable of inducing humoral and cellular immune responses in multiple mucosal tissues and the systemic compartment of humans and nonhuman primates (NHP) [16], [17], [18], [19]. Tissues populated by effector lymphocytes after nasal immunization include the female genital tract and large intestine [20], [21]. Therefore, delivery of vaccines by the nasal route could be an effective strategy for preventing infections by sexually-transmitted pathogens, such as human immunodeficiency virus type 1 (HIV). Indeed, IN administration of a “T cell-inducing” DNA/recombinant modified vaccinia ankara (MVA) virus vaccine for SIV resulted in greater control of SIVmac251 vaginal and rectal infection in rhesus macaques [22], [23] when compared to IM immunization with the same products. An “antibody-inducing” HIV vaccine given by both IN and IM routes also protected macaques against vaginal simian-human immunodeficiency virus (SHIV) challenge whereas IM vaccination alone did not [24].
The correlates of protection against HIV have not been precisely defined but there is evidence that optimal vaccine-mediated protection will require induction of serum IgG antibodies that neutralize or mediate antibody-dependent cellular cytotoxicity, and Th1-type antiviral CD8 T cells, especially in exposed mucosal tissues [25], [26]. Vaccine-induced mucosal antiviral IgA responses at the site of viral challenge have also been associated with protection or control of infection in several NHP HIV vaccine studies [24], [27], [28], [29]. However, effective induction of mucosal IgA responses will require mucosal delivery of vaccine, and a strong adjuvant to prevent tolerance. Cholera toxin and the closely related E. coli heat-labile toxin have proved most effective as mucosal adjuvants in animals, and many nontoxic derivatives of these enterotoxins have been created for use as mucosal adjuvants in humans [30], [31]. Unfortunately, most cannot be administered in the nasal cavity due to their propensity to bind to nerve endings and cause Bell's Palsy [32]. To our knowledge, the only products that have clearly been demonstrated both safe and effective as nasal adjuvants in humans are chitosan [33] and Protollin, consisting of Shigella flexneri lipopolysaccharide (LPS) and Neisseria meningitides outer membrane proteins [34], [35]. Thus, there is a need to identify more nasal adjuvants for use in humans.
Invaplex 50 is native structure isolated from wild-type Shigella and consists of Shigella flexneri 2a LPS complexed with two invasion plasmid antigen (Ipa) proteins, IpaB and IpaC [36], which are key effector proteins of the type-three secretion system. Invaplex 50, which is retrospectively termed Native Invaplex or InvaplexNAT, induces endocytosis and facilitates cytosolic delivery of co-administered antigens. Invaplex was originally developed as a nasal subunit Shigella vaccine [37], and in a recent Phase I study, dosages as high as 0.69 mg were reported safe in the human nasal cavity [38]. In addition to preventing Shigella infection in mice [36], Invaplex has acted as an IN adjuvant for co-administered protein immunogens or DNA vaccine in this species [39], [40]. However, results in IN-immunized mice do not always extrapolate to humans, possibly because the nasal cavity of rodents contains more immune-inductive lymphoid tissue than primates [41].
To better ascertain whether Invaplex could potentially adjuvant nasal vaccines in humans, we performed a pilot study testing its ability to enhance systemic and mucosal antibody responses in rhesus macaques given simultaneous IN vaccinations with an SIV Env protein and a model DNA vaccine that encodes noninfectious SHIV89.6P particles [22]. The ability to generate B cell memory was evaluated by measuring anamnestic antibody responses after a IN boost with adenoviral type 5 (Ad5) vectors expressing SIV env and SIV gag-pol. Because Invaplex can act, in a non-toxic manner, as a transporter of DNA or complexed immunogens into the cytosol of antigen-presenting cells [40], potentially leading to the activation of antigen-specific CD8 T cells, we also evaluated frequencies of T helper type 1 (Th1)-type SIV-specific CD8 T cells. The results indicate that Invaplex can function as an adjuvant for enhancing serum and mucosal antibodies to IN administered protein immunogens, and it can promote induction of protein immunogen-specific CD8 T cells.
2. Methods
2.1. Animals
Twelve female Indian-origin Macaca mulatta, aged 7–15 years, were cared for in accordance with the Animal Welfare Act and the NIH “Guide for the Care and Use of Laboratory Animals”. All animals were negative for SIV, Simian Retrovirus Type D, and Simian T-Lymphotropic Virus Type 1. From weeks (wks) −2 to 14, animals were given monthly IM injections of 1.5 mg kg−1 Depo-Provera™ (Pfizer, New York, NY). All procedures were approved by Institutional Animal Care and Use Committees at Tulane and LSUHSC.
2.2. Immunogens
Baculovirus-derived SIVmac251 rgp130 Env protein was from ImmunoDiagnostics (Woburn, MA). The pVacc4 plasmid (SHIV89.6P DNA) in saline was produced by Aldevron (Fargo, ND), and is a derivative of pVacc1 [42] that contains the HIV env gene of SHIVKB9, a molecular clone of SHIV89.6P, and mutated SIVmac239 gag and pol genes [43] which result in secretion of noninfectious viral particles. Shigella Invaplex 50 was purified from S. flexneri 2a cultures as described [44]. Purified replication-incompetent human Ad type 5 vectors encoding SIVmac239 Env or SIVmac239 Gag-Pol fusion protein [45] were a generous gift from Dr. Gary Nabel and the NIH Vaccine Research Center, Bethesda, MD.
2.3. Immunizations
Three groups of macaques (each n = 4) were IN immunized with 1.5 mg naked SHIV DNA plus 0.5 mg SIV gp130 Env protein with or without InvaplexNAT in a total volume of 250 µl on wks 0, 4, and 8. Group (Gp) 2 and Gp 3 animals received DNA + protein with 0.25 and 0.5 mg InvaplexNAT, respectively. The individual vaccine components were mixed 1 h before immunization. On wk 28, all animals were IN boosted with 1010 particle units (PU) Ad-SIVenv plus 1010 PU Ad-SIVgag-pol in 200 µl. Immunizations were performed by pipetting half of each solution into each nostril. After application, each nostril was immediately pressed shut and released to ensure coating of internal surfaces.
2.4. Specimen collection and processing
Standard venipuncture was used to collect whole blood for serum or heparinized blood for peripheral blood mononuclear cells (PBMC). The PBMC were isolated by density gradient centrifugation on Lymphocyte Separation Medium (VWR, Radnor, PA) and cryopreserved. Starting on wk 0, cervicovaginal secretions (CVS) and rectal secretions (RS) were sampled at biweekly or monthly intervals by consecutive application of 2 premoistened Weck-Cel sponges (Beaver Visitec, Waltham, MA) in the vagina or rectum as described [46]. Nasal secretions (NS) were collected at biweekly intervals starting on wk-2, except on days when IN immunization was performed. To collect NS, 100 µl DPBS was pipetted into a nostril. The nostril was immediately pressed shut, massaged gently to hydrate surfaces, then released. A sponge premoistened with 50 µl PBS was quickly inserted and allowed to absorb fluid for 5 min. After removal, the procedure was repeated for the other nostril using a second sponge. Tubes containing sponges were maintained on wet ice until storage at −80˚C. To extract secretions, one sponge was placed in the upper chamber of a Corning SpinX filter (VWR) that had holes poked in the filter (to prevent clogging by mucus). The sponge was treated with 100 µl ice-cold PBS containing 0.5% Igepal detergent (Sigma, St. Louis, MO) and protease inhibitors [46], then ultracentrifuged at 20,000g and 4˚C. After 45 min, the upper chamber was replaced with a new upper chamber containing the 2nd sponge, and the procedure was repeated.
2.5. ELISA for total IgA or IgG
These assays were performed as described [46] using microtiter plates coated with goat anti-monkey IgA (Alphadiagnostics, San Antonio, TX) or goat F(ab')2 to monkey IgG (MP Biomedicals, Solon, OH). The standard was a normal rhesus macaque serum containing known amounts of Ig (kindly provided by Dr. M.W. Russell, University at Buffalo, NY). The secondary antibodies were biotinylated goat anti-monkey IgA (Alphadiagnostics) or -human IgG (SouthernBiotech Associates: SBA, Birmingham, AL). Absorbance was recorded at 414 nm after development with streptavidin-peroxidase and azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (both Sigma). Concentrations were interpolated from 4-parameter standard curves constructed with SoftMaxPro software (Molecular Devices, Sunnyvale, CA).
2.6. ELISA for InvaplexNAT or SIV antibodies
Assays for InvaplexNAT antibodies were performed as described [36] using Immulon I plates (Dynatech, Chantilly, VA) coated overnight with 50 ng per well InvaplexNAT in pH 9 carbonate buffer. Assays for SIV antibodies were done as described [22] using Fisherbrand high protein-binding plates coated with 100 ng SIV gp130 (ImmunoDiagnostics) or 250 ng SIVmac251 lysate (Advanced Biotechnologies Inc., Columbia, MD) in PBS per well. Anti-lysate antibodies are referred to as being “Gag,Pol”-specific because no Env protein could be detected in the coated lysate using an anti-gp130 antibody (ImmunoDiagnostics). Pooled serum from InvaplexNAT-immunized and SIV-infected macaques were used as standards. The concentration of antibody in each standard was calibrated relative to the total IgA or IgG in the normal monkey serum by coating different portions of a plate with antigen or Ig capture antibody. Plates were developed as above, except that the substrate was tetramethylbenzidine (SBA), 1 N H2SO4 stop solution was added, and absorbance was read at 450 nm. The specific activity (sp act) in secretions was calculated by dividing the concentration of Invaplex-, SIV Env- or SIV Gag,Pol-specific IgA or IgG by the respective concentration of total IgA or IgG. Secretions were considered antibody-positive if they contained sp act that was both ≥ mean sp act + 3SD for naïve macaque secretions and 3.4-fold above the animal’s preimmune sp act. If a secretion had undetectable antibody, it was assigned the mean sp act of naïve specimens.
2.7. Intracellular cytokine staining
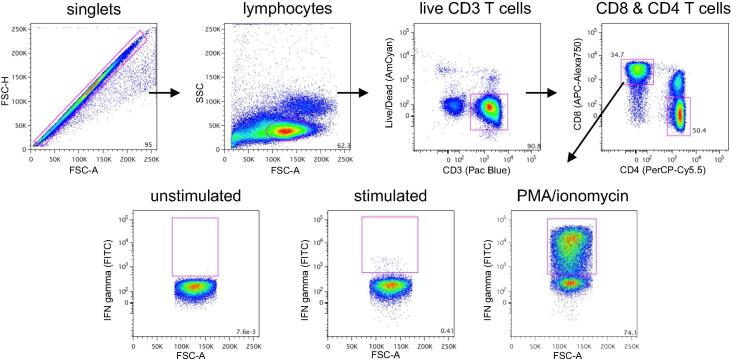
Reagents were from BD Biosciences (San Diego, CA) unless otherwise noted. Cryopreserved PBMC were washed and used for all T cell assays. One million PBMC were suspended in RPMI1640 medium containing 5% FBS, penicillin, streptomycin, L-glutamine and 1 µg ml−1 anti-CD28 (clone 28.2) and anti-CD49d (9F10). The medium was additionally supplemented with DMSO (final 0.2%) or 2 µg ml−1 pooled peptides encompassing the SIVmac239 Env or SIVmac239 Gag protein (NIH AIDS Research and Reference Reagent Program). Positive controls were stimulated with 10 ng ml−1 phorbol myristate acetate (PMA) plus 1 µg ml−1 ionomycin (Sigma). The cultures were incubated at 37˚C in 5% CO2 for 1 h, spiked with monensin, cultured an additional 5 h, then cooled to 10˚C. The following day, PBMC were treated for 5 min with 50 U ml−1 DNase I (Sigma), washed with PBS, and resuspended in 50 µl of Aqua Live/Dead cell stain (Invitrogen) in PBS. After 2 min, the reaction was stopped by adding 10 µl goat serum. The cells were then washed and stained for 30 min with anti-CD3 (SP34-2)-Pacific Blue, anti-CD4 (L200)-PerCP-Cy5.5 and anti-CD8 (RPA-T8)-APC-Alex750 (eBioscience, San Diego, CA). Cells were washed and resuspended in 200 µl Cytofix/Cytoperm. After 20 min, cells were washed with PermWash buffer and stained for 30 min with anti-IFN-γ (B27)-FITC, anti-IL-2 (MQ1-17H12)-PE and anti-TNF-α (MAb11)-PE-Cy7 (eBioscience). Cells were washed, fixed with 1% paraformaldehyde, then analyzed in an LSRII flow cytometer. Maximal events were acquired. Compensation Beads expressing anti-mouse or anti-rat kappa chain antibody were processed in parallel for each fluorophore-conjugated antibody. For Live/Dead cell stain and anti-CD3 antibody, compensation was done using amine-reactive silica beads (Bang's Laboratories, Fishers, IN) incubated with these reagents. The FlowJo computer program (Tree Star, Ashland, OR) was used for data analysis and Boolean gating. Percentages of cytokine-positive cells in unstimulated cultures (typically 0–0.03%) were subtracted from those in stimulated cultures. The latter were considered significant if adjusted percentages were > 0.05% and twice the background.
2.8. Statistics
The GraphPad Prism v9 computer program was used for graphing and statistics. Non-parametric statistics were used because of the small number of animals in each group. Differences between groups were initially sought using the Kruskal Wallis test. If a significant p value was obtained, Group 2 or Group 3 was subsequently compared to Group 1 using the two-tailed Mann-Whitney rank sum test to determine if a significant difference existed. Correlations were done using the two-tailed Spearman rank correlation test. Only p values ≤ 0.05 were considered significant.
3. Results
3.1. Systemic IgG responses to Env protein immunogen and DNA encoded antigens
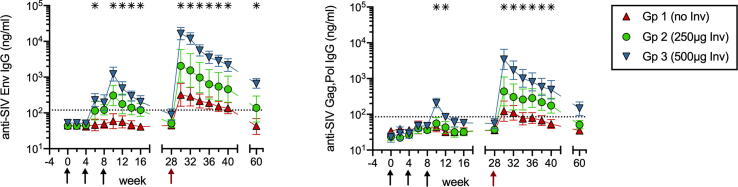
Two doses (0.25 and 0.5 mg) of InvaplexNAT were selected for testing based on the finding that they induced anti-Invaplex antibodies in IN-immunized humans [38]. In Phase 1 of the study, 3 groups of female macaques (each n = 4) were given 3 monthly IN immunizations with SHIV89.6 DNA + SIVmac251 Env protein formulated with either PBS, 0.25 mg or 0.5 mg InvaplexNAT. In Group (Gp) 1 nonadjuvanted control animals, no IgG antibodies to the SIV Env protein or DNA encoded SIV Gag,Pol antigens were detected by ELISA in serum collected up to 20 wks after the 3rd immunization (Fig. 1). In Gp 2 animals that received 0.25 mg InvaplexNAT, the systemic antibody responses were not significantly improved because only 50% of the animals developed SIV-specific IgG antibodies in serum. However, co-administration of vaccine antigens with 0.5 mg InvaplexNAT significantly increased SIV Env-specific IgG in serum of all Gp 3 animals (Fig. 1). Gp 3 animals also had increased Gag,Pol-specific serum IgG, albeit to a lesser extent (Fig. 1). At their Phase 1 peak on wk 10, the geometric mean concentrations of SIV Env- and Gag,Pol-specific IgG in Gp 3 sera were 23– and 9-fold above preimmune levels, respectively.
Fig. 1.
Systemic IgG responses after nasal DNA/protein vaccination with or without Invaplex. ELISA was used to measure concentrations of serum IgG antibodies to SIV Env protein immunogen (left panel) and SIV Gag,Pol antigens (right panel) encoded by the DNA vaccine. Shown are geometric means with SEM. Black arrows indicate when immunizations with DNA/protein ± InvaplexNAT were performed. The red arrow indicates when Ad-SIV boosting was done. Asterisks indicate time points when Gp 3 had significantly greater antibody concentrations than Gp 1 (p ≤ 0.05 by Mann-Whitney). Gp 2 antibody levels did not differ significantly from Gp 1 at any time. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To confirm that immunizations with Invaplex generated B cell memory, all animals were IN boosted during Phase 2 of the study on wk 28 with Ad-SIVgag-pol plus Ad-SIVenv (Ad-SIV). Anamnestic responses in Gp 2 and Gp 3 were clearly evident. In Gp 3, the boost increased Env- and Gag,Pol-specific IgG to levels that were 185- to 62-fold above those measured on wk28, respectively (Fig. 1). In contrast, Gp 1 controls demonstrated only 7- and 3-fold mean increases in these antibodies (Fig. 1). Thus, a 0.5 mg dose of Invaplex was able to enhance systemic IgG responses and to generate antigen-specific memory B cells against both the Env protein immunogen and DNA encoded Gag and Pol proteins.
3.2. Local and distal mucosal IgA responses to Env protein immunogen
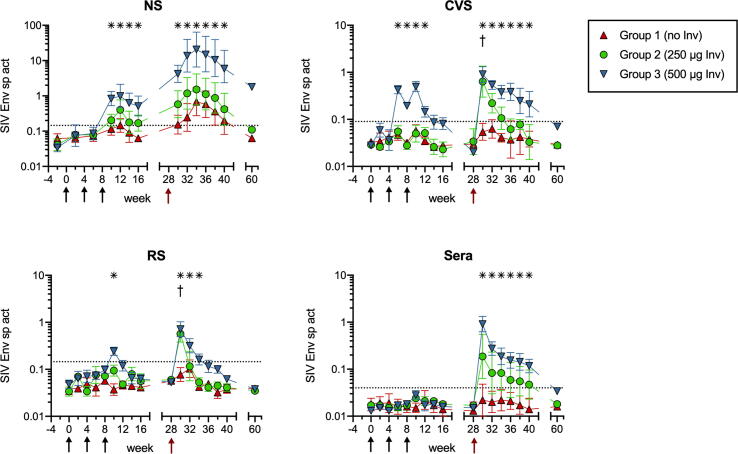
The magnitude of the anti-SIV Env mucosal IgA responses generated in mucosal compartments of immunized animals was compared after calculating the Env IgA sp act (ng anti-Env IgA per µg total IgA) in secretions. As shown in Fig. 2, Gp 1 nonadjuvanted control animals completely failed to develop anti-Env IgA antibodies in distal CVS or RS during both phases of the study. Overall, the Gp 2 animals did not respond better than controls because the same 2/4 serum IgG non-responders also failed to develop mucosal antibodies. However, all Gp 3 animals did have Env IgA in CVS or RS after the 2nd or 3rd immunization, and the magnitude and longevity of these genital tract and intestinal IgA responses was modestly increased by the Ad-SIV boost (Fig. 2).
Fig. 2.
IgA responses to Env protein immunogen. The Env IgA sp act (ng anti-Env IgA antibody per µg total IgA) was calculated after measuring concentrations of Env-specific and total IgA by ELISA. The geometric mean sp act ×/÷ SEM is shown for nasal secretions (NS), cervicovaginal secretions (CVS), rectal secretions (RS), and sera. Note that NS were not collected for antibody analysis on days when IN immunization was performed with DNA/protein ± InvaplexNAT (black arrows) or Ad-SIV (red arrow). Asterisks denote the wks when Gp 3 had significantly greater sp act than Gp 1. Crosses indicate when Gp 2 had significantly greater sp act than Gp 1 (all p ≤ 0.05 by Mann-Whitney). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
NS from Gp 3 animals also contained greater Env IgA sp act than control animals (Fig. 2). For Gp 3, peak increases in Env IgA antibodies during Phase 1 were 30-fold and these were dramatically increased by the Ad-SIV boost to levels 670-fold above preimmune. The nasal IgA response to Env was also surprisingly long-lived. Eight months after the Ad-SIV boost, Env IgA in Gp 3 NS was still elevated by 53-fold (Fig. 2). Interestingly, when compared to CVS, the peak nasal antibody response was delayed by 2–4 weeks, a finding similarly observed in humans [47]. The magnitude of the nasal Env IgA response was also greater than the distal IgA responses (Fig. 2), consistent with reports that greatest mucosal antibody responses are generated at sites of antigen administration in humans [16], [48].
Currently, there are no sufficiently specific and sensitive anti-macaque secretory component antibodies available to confirm that secretions of these animals contained secretory antibodies (polymeric IgA or IgM that has undergone pIgR-mediated basolateral-to-apical transport across epithelial cells). However, no Env IgA was found in serum of any of the Gps during Phase 1 (Fig. 2). Therefore, the Env IgA in secretions of Gp 3 animals during Phase 1 must have come from the mucosae, rather than serum. During Phase 2, a mucosal origin for the Env IgA in RS and CVS could not be confirmed because the Ad-SIV boost generated Env-specific IgA in serum which was similar in levels and correlated with Env-specific IgA in RS or CVS on wks 30–40 (all p ≤ 0.0006). However, a mucosal anti-Env IgA response was elicited in the nasal cavity of Gp 3 animals on wks 32–40 because the Env IgA sp act in NS was significantly greater (p < 0.05 at every time point) and not correlated with the Env IgA sp act in serum (p = 0.1626).
During Phase 1, SIV Env-specific IgG was also measured in Gp 1 and 3 NS (Supplemental Fig. 1). Starting on wk10, Env IgG was detected in NS of both Gps 1 and 3 and peaked on wk12 at levels that were 4.1- and 8.5-fold above preimmune, respectively. These presumably represent local IgG responses because Gp 1 controls did not have significant Env-specific IgG in serum at this time, and the Env IgG in serum versus NS of Gp 3 animals were not correlated (p = 0.1647). Thus, nasal vaccination in the absence of Invaplex induced a weak nasal anti-Env IgG but not IgA response, whereas vaccinations with Invaplex induced a strong nasal IgA response. Invaplex also clearly functioned as an adjuvant for increasing distal mucosal IgA responses to the Env protein.
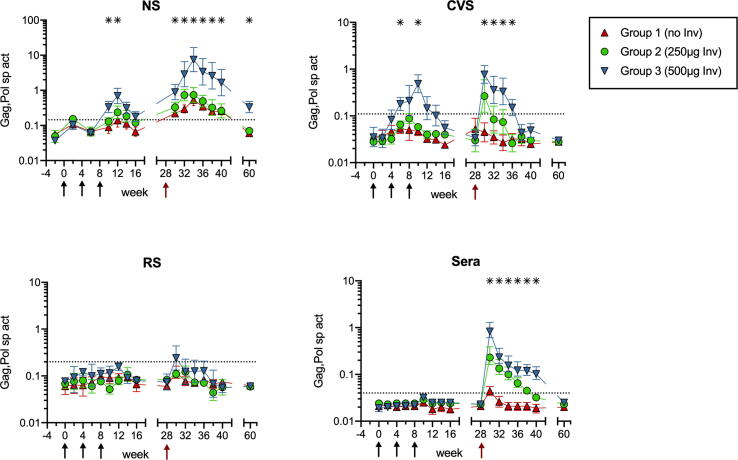
3.3. Local and distal mucosal IgA responses to DNA vaccine antigens
Systemic and mucosal IgA responses to Gag,Pol antigens in the DNA encoded SHIV particles (Fig. 3) developed in parallel with Env IgA responses but were of lower magnitude, as found for IgG in serum. Group 1 nonadjuvanted animals did not develop Gag,Pol IgA in distal CVS and RS, though slight elevations were found in NS of these controls after the Ad-SIV boost (Fig. 3). The CVS and NS of Gp 3 animals contained the greatest Gag,Pol IgA sp act, which again could be attributed to mucosal synthesis during Phase 1 due to the absence of Gag,Pol-specific IgA in serum (Fig. 3).
Fig. 3.
IgA responses to DNA encoded Gag,Pol antigens. The Gag,Pol IgA sp act (ng anti-Gag,Pol IgA antibody per µg total IgA) was calculated after measuring Gag,Pol-specific and total IgA by ELISA. The geometric mean sp act ×/÷ SEM is shown for NS, CVS, RS, and sera. Asterisks denote the wks when Gp 3 had significantly greater sp act than Gp 1 (p ≤ 0.05 by Mann-Whitney). Gp 2 did not have significantly greater sp act than Gp 1 at any time.
In Gp 3, the Ad-SIV IN immunization dramatically boosted the Gag,Pol IgA sp act in NS, with the peak 19-fold Phase 1 increase climbing to 200-fold on wk 34 (Fig. 3). The increases of Gag,Pol IgA sp act in NS of Gp 3 animals were both significantly greater (p < 0.05) and not correlated with those in serum on wks 34–40 (p = 0.5450). The Gag,Pol IgA in Gp 3 NS was still elevated over pre-immune values by 9-fold at 8 months following the Ad-SIV boost (Fig. 3). In contrast to Env IgA results, Gp 3 animals did not develop Gag,Pol IgA in RS (Fig. 3), presumably due to introduction of lower antigen dosages by the DNA vaccine.
For IgG, no Gag,Pol-specific IgG was found in NS of Gp 1 control animals (Supplemental Fig. 1). The NS from Gp 3 animals did have low levels of Gag,Pol IgG on wks 6–16, which peaked at 6-fold on wk 10, but these were likely transudated from serum because the Gag,Pol IgG sp act in NS was both lower and correlated with that in serum (p = 0.0200).
These results indicate that Invaplex can function as an adjuvant to enhance nasal and genital tract IgA antibodies to proteins encoded by a nasal DNA vaccine. Induction of rectal antibodies may require larger vaccine doses or more optimal delivery methods such as a spray device to deposit vaccine and adjuvant more thoroughly on the nasal mucosae.
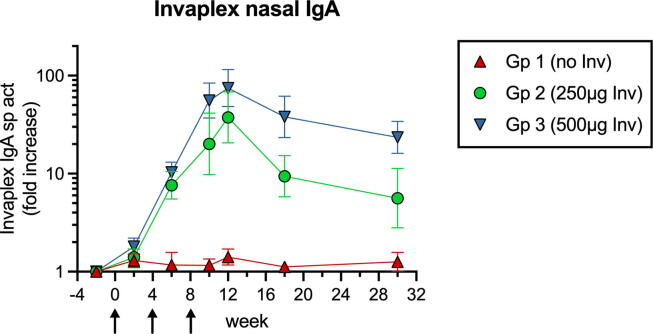
3.4. Induction of Invaplex-specific nasal IgA antibodies
Antibodies generated against Invaplex were also measured in sera and NS collected from wk −2 to wk 30. None of the groups had significant (>3.4-fold) increases of anti-Invaplex IgG or IgA in sera (not shown). NS were also negative for Invaplex-specific IgG (not shown). However, starting on wk 6, Invaplex-specific IgA was detected in NS of Gp 2 and 3 animals (Fig. 4). On wk 12, the Invaplex IgA sp act in Gp 2 and 3 peaked at levels 56- and 94-fold above preimmune, respectively. On wk 30, they were still elevated by 28-fold in Gp 3 NS (Fig. 4). Pre-existing IgA to Invaplex has not prevented Invaplex from acting as an IN adjuvant in mice [39]. Nevertheless, future studies will be required to determine whether the induction of these adjuvant-specific nasal IgA antibodies in humans may preclude use of InvaplexNAT in IN booster vaccinations.
Fig. 4.
Nasal IgA responses to InvaplexNAT. The Invaplex IgA sp act (units anti-Invaplex IgA per µg total IgA) in NS was determined after measuring anti-Invaplex IgA and total IgA by ELISA. The fold increase was then calculated by dividing the postimmunization sp act by the preimmunization sp act. Geometric mean and SEM are presented. Arrows indicate when immunizations with DNA/protein ± InvaplexNAT were performed. Postimmunization fold increases had to be 3.4-fold to be considered significant.
3.5. T Cell responses to protein immunogen
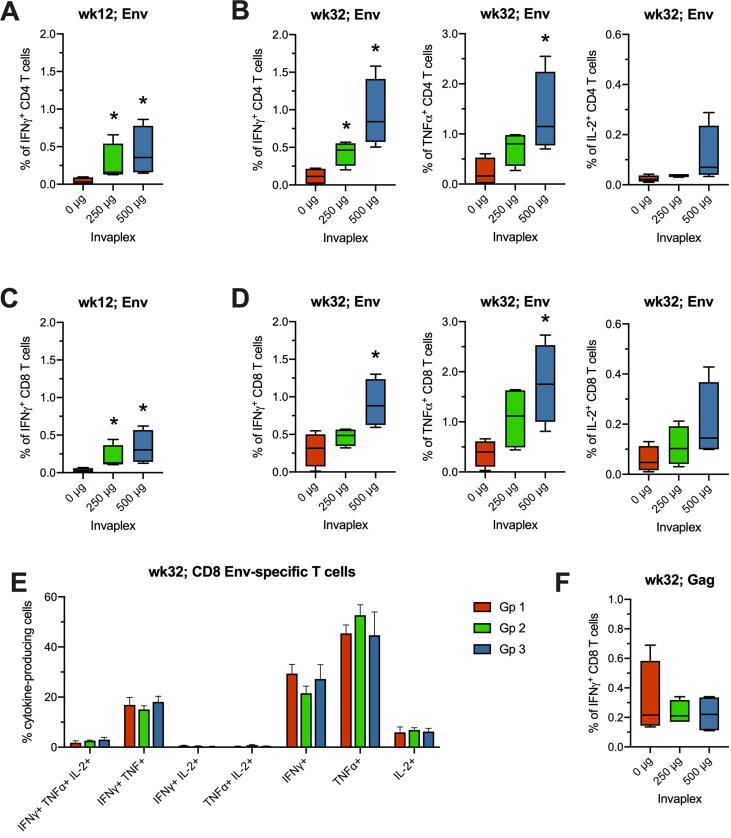
A goal of HIV vaccines is to induce Th1-type antiviral cellular responses. Therefore, we used intracellular cytokine staining (ICS) and flow cytometry to evaluate SIV-specific IFNγ, TNFα and IL-2 producing T cells induced in blood of animals at 2 wk intervals after immunization. The gating strategy and representative results are illustrated in Fig. 5. During Phase 1 of the study, IFNγ-producing Env-specific CD4 and CD8 T cells were observed on wk 12 (Fig. 6A and C), when they peaked, as observed for nasal antibodies. Both Gp 2 and Gp 3 animals had significantly greater frequencies of these cells than Gp 1 controls (all comparisons p = 0.0286; Fig. 6). One month after the Ad-SIV boost (wk 32), IFNγ-secreting Env-specific T cells appeared again, with IL-2- and TNFα-producing Env-specific T cells becoming evident at this time (Fig. 6B and D). On wk 32, Gp 3 had significantly greater frequencies of Env-specific CD4 + and CD8 + T cells secreting either IFN-γ or TNF-α when compared to Gp 1 (all p < 0.05; Fig. 6B and D). Analysis of the wk32 Env-specific T cells by Boolean gating indicated that the majority of the CD4 (not shown) and CD8 T cells (Fig. 6E) were monofunctional, followed by dual producers of IFNγ and TNFα. The frequencies of monofunctional and polyfunctional T cells did not differ significantly between the groups.
Fig. 5.
Flow cytometric gating strategy. For ICS with PBMC, doublets were first excluded. Gating was then done on lymphocytes, CD3 T cells negative for Live/Dead viability dye, and CD8 or CD4 T cells. Each T cell subset was then evaluated for cytokine. Shown are representative results for IFNγ-producing CD8 T cells in wk12 PBMC cultures treated with medium alone (unstimulated), SIV Env peptide (stimulated) or PMA/ionomycin as a positive control.
Fig. 6.
T cell responses after DNA/protein immunization and Ad-SIV boosting. The frequency of SIV Env-specific CD4 (A, B) or CD8 (C, D) T cells producing IFNγ, TNFα, or IL-2 was measured by ICS after peptide stimulation of PBMC. Results are depicted as minimum-to-maximum whisker box plots for wk12 (4 wks after the 3rd DNA/protein immunization with or without Invaplex) and wk32 (4 wks after the Ad-SIV boost). (E) Boolean gating was used to determine the frequencies of monofunctional and polyfunctional Env-specific CD8 T cells on wk32. There were no differences between the groups. (F) Gag-specific IFNγ-secreting CD8 T cells detected on wk32 by ICS. *p ≤ 0.05 by Mann-Whitney when compared to the control group.
During Phase 1, frequencies of SIV Gag-specific T cells exceeding 0.05% were not detected by ICS. After Ad-SIV boosting, IFNγ-producing Gag-specific CD4 (not shown) and CD8 T cells (Fig. 6F) were observed in low frequencies, which did not differ significantly between the groups. These results suggest that Invaplex may not adjuvant T cell responses to naked DNA vaccine co-administered in the nasal cavity, but it can enhance cellular responses, including CD8 T cell responses, to protein immunogens.
4. Discussion
In this study, we show that IN co-delivery of Shigella InvaplexNAT with a SHIV DNA/SIV Env protein formulation in NHP was able to adjuvant mucosal IgA and systemic IgG responses to both the protein immunogen and antigens encoded by the DNA. The IN DNA/protein vaccinations with 0.5 mg InvaplexNAT followed by IN boosting with Ad-SIV induced extremely high levels of SIV Env-specific IgA in NS, which were still elevated over preimmune levels by 56-fold after 8 months. It is well-established that greatest IgA responses are generated in the mucosa at sites of antigen exposure [16], [20], [48], but these nasal IgA responses are notable because HIV- or SIV-specific IgA responses of this magnitude and longevity have not previously been reported in macaques. In fact, generating strong mucosal IgA responses has been somewhat problematic in this species and appears to require larger vaccine dosages when compared to humans [49]. It is for this reason that we used such a high dose of Env immunogen.
It is possible that the induction of HIV- or SIV-specific IgA antibodies in macaques has been difficult because generating IgA responses in this species is dependent on the production of a specific soluble factor that has not been adequately elicited by most vaccines. For example, class-switching to IgA2 in human B cells requires A Proliferating Ligand (APRIL), which is produced by mucosal epithelial cells and myeloid dendritic cells [50]. Interestingly, the synthesis of APRIL by human intestinal epithelial cells can be triggered by Salmonella LPS, a Toll-like receptor 4 (TLR4) ligand [50]. In mice, TLR-4 signaling in intestinal epithelial cells recruits B cells to the intestinal lamina propria [51]. Since Shigella LPS is a major component of Invaplex, an interaction with TLR-4 on nasal epithelium or local dendritic cells may have stimulated secretion of APRIL or another IgA inducing protein [52] that promoted B cell recruitment to nasal tissues and their subsequent differentiation into IgA antibody-secreting cells. An InvaplexNAT-stimulated production of an IgA-inducing factor by epithelial cells in the nasal cavity would explain why the magnitude of nasal IgA responses to SIV Env, SIV Gag,Pol and Invaplex were much greater than the nasal IgG responses to these antigens.
We had hoped that IN immunization with InvaplexNAT would elicit greater IgA responses in the rectum. In a previous study with women, we found that IN vaccination with a highly immunogenic protein, in the absence of adjuvant, could induce specific IgA in secretions of both the distal female genital tract and rectum [16]. Importantly, a 10-fold lower nasal dose of protein was able to generate genital tract IgA responses comparable to those observed in vaginally-immunized women [16]. However, the magnitude of the IgA response in the rectum of IN-immunized women was quite modest. We hypothesized that IN co-delivery of immunogen with a strong adjuvant would improve distal IgA responses by increasing the frequency of activated B cells with homing receptors for distal tissues. Indeed, IN DNA/protein immunizations with InvaplexNAT did generate genital tract IgA responses to both the protein immunogen and DNA encoded Gag,Pol antigens that were comparable to those observed in the nasal cavity. Immunizations with Invaplex also enhanced the distal rectal IgA response to Env protein. However, the magnitude of the rectal IgA response to Env was low, and no Gag,Pol-specific rectal IgA was detected. Therefore, IN administration of vaccines may not induce rectal IgA responses of sufficient magnitude to prevent rectal HIV transmission. Local delivery of vaccine would likely produce superior rectal IgA responses [16], [48], [53]. A potential problem is that rectal immunization with a variety of immunogens [16], [48], [53], [54], including a live attenuated vaccine [55], has proved poorly effective for induction of IgA antibodies in the female genital tract of NHP and women. Vaginal immunization can similarly produce highly restricted local antibody responses [16], [48], [53]. Since IN immunization is more effective for generating antibodies in CVS than in RS, a regimen consisting of IN priming followed by rectal boosting might be most effective for induction of antibodies in both rectal and female genital tract secretions.
In macaques immunized with Invaplex, antibody responses to the Gag,Pol antigens encoded by the DNA vaccine component were of lower magnitude than those to the SIV Env protein immunogen. This was anticipated because doses of SHIV particles budding from pVacc4-transfected cells in the nasal mucosa were probably much lower than the amount of Env protein that was internalized. In addition, nasal immunization of macaques with pVacc4 or similar plasmids has not induced much antibody in sera or secretions, even after nasal boosting with MVA-SHIV [22], [43] and VLP [19]. Thus, we were surprised to find Gag,Pol-specific IgG in serum and IgA in CVS prior to the Ad-SIV boost. Co-delivery of InvaplexNAT with DNA may have stimulated the production of chemokines that recruited or activated immature DC at a time that was optimal for capture of SHIV particles in nasal tissue or draining lymph nodes. We have found that treatment of polarized colonic epithelial cells with InvaplexNAT induces production of GM-CSF, IL-1 and IL-4, which could facilitate maturation of DC (Kaminski and Oaks, unpublished observations).
Although the DNA/protein immunizations with InvaplexNAT did not have apparent effects on development of T cell responses to the DNA encoded Gag protein, InvaplexNAT did enhance CD4 and CD8 Th1-type responses to the Env protein immunogen. We believe InvaplexNAT adjuvanted Env specific-CD8 T cell responses by promoting cellular internalization of the protein by DC and other antigen-presenting cells. The Shigella Ipa proteins are normally utilized by shigellae to invade epithelial cells by inducing actin polymerization and phagocytosis [56]. In previous studies, we have found that the IpaB and IpaC proteins mediate cellular uptake of Invaplex and co-administered proteins or DNA, suggesting that the biological activities of these invasin proteins are retained in InvaplexNAT [40]. The inherent ability of the complex to induce uptake, combined with the TLR-4 ligand activity of the LPS, are likely responsible for the adjuvant activity of Invaplex. More recently, a second generation Invaplex product has been developed [57] which is assembled into a large complex using purified Shigella LPS, IpaB and IpaC. Artificial Invaplex, or InvaplexAR, is more compatible with large scale manufacture, is customizable and well-defined, facilitating regulatory compliance. InvaplexAR also retains the adjuvant properties attributed to InvaplexNAT described here. Phase 1 studies evaluating InvaplexAR delivered IN demonstrated the product was safe, well-tolerated and induced robust systemic and mucosal immune responses directed to the three major product components (Kaminski, manuscript in preparation).
The immunological processes that govern the development of polyfunctional T cells are not understood. However, antigen dose may be a factor. Lower doses of vaccine have been reported to promote polyfunctionality [45], [58], [59]. Larger doses have been found to bias cellular immunity toward monofunctional IFNγ responses [45]. Since we administered a high dose of Env protein immunogen, this may explain why only IFNγ-producing Env-specific T cells were observed during the adjuvant phase of our study. It is possible, though, that polyfunctional T cells were generated in mucosal tissues of our animals. In macaques immunized in the respiratory tract with DNA/MVA- or Ad5-based SIV vaccines, greater frequencies of virus-specific T cells have been detected in mucosal tissues compared to blood [19], [22], [23], [45], and although monofunctional T cells typically dominated in blood, more heterogeneous profiles of IFNγ, TNFα and IL-2 secreting T cells were observed in rectal or vaginal tissues [19].
A caveat of our study is that the animals were given Depo-Provera (medroxyprogesterone) during the adjuvant phase. This was done to prevent ovulation and menses [60] because, at ovulation, total IgG and IgA in CVS of NHP [61], like humans [62], declines dramatically, making antibody detection difficult, and, during menses, the blood in CVS prevents accurate assessment of genital tract antibody responses. CVS collection cannot be scheduled around these menstrual cycle phases at most Primate Centers because exam rooms need to be reserved well in advance. Since we could not afford to exclude CVS with insufficient IgA or blood due to the small numbers of macaques in our study, we opted to administer Depo-Provera (DP). This contraceptive has immune suppressive properties [63], [64], but they appear to be largely confined to the cervicovaginal mucosa in humans and NHP [65], [66], [67]. DP has not altered systemic antibody responses induced by rubella vaccination of women [68] or by intravenous (IV) immunization of macaques with nonpathogenic SHIV89.6 [66]. DP-treated female macaques IV-immunized with SHIV89.6 were also reported to develop strong gag-specific T cell responses with greater polyfunctionality in blood, and, following IV infection with SIV, they better controlled viremia when compared to untreated macaques [67]. In contrast, DP-treated macaques infected with SIV or pathogenic SHIV by the vaginal route have demonstrated less TNFα, more T regulatory cells and delayed appearance of IFNγ-producing gag-specific T cells in the periphery [67], [69], suggesting that DP may dampen immune induction specifically in the female genital tract. On the other hand, administration of DP to mice IN-immunized with a CpG-adjuvanted herpes protein was reported to reduce titers of specific IgA in vaginal secretions [70]. To determine if this might also occur in NHP given DP, we performed a preliminary study in which 12 female rhesus macaques were IN-immunized with the whole-inactivated Dukoral “oral” cholera vaccine (containing 100 µg CTB) [16] at monthly intervals a total of 3 times. Starting 2 weeks before the first immunization, six of these animals were given monthly injections of 1.5 mg kg−1 DP. No significant differences in the kinetics or magnitude of the CTB-specific serum IgG, rectal IgA and vaginal IgA response were observed between the untreated and DP-treated animals when measured by ELISA [16] at biweekly intervals up to week 16, the last time point in the study (Kozlowski, unpublished observations). However, T cell responses were not measured. Therefore, we still cannot exclude the possibility that DP may hinder development of polyfunctional T cells in IN-immunized NHP. Thus, until the current study is repeated in cycling macaques, the immune responses observed here should be considered to best reflect those that would be generated in pre-menopausal women using DP.
In conclusion, InvaplexNAT has good adjuvant activity for augmenting both humoral and cellular immune responses to protein immunogens given in the nasal cavity of NHP. This strongly suggests it would similarly augment mucosal and systemic antibody responses to IN subunit vaccines in humans. InvaplexNAT could also adjuvant mucosal and systemic antibody responses to IN DNA vaccines that encode secreted proteins. In addition to potentially promoting vaccine-mediated protective immunity to sexually-transmitted pathogens, the inclusion of Invaplex in IN vaccines for influenza, SARS CoV-2 or other air-borne pathogens, for which S-IgA antibodies have clearly been associated with protection [71], could be highly beneficial for preventing respiratory tract infections in humans.
Disclaimer
Some authors (RW Kaminski and EV Oaks) are employees of the U.S. Government. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense, nor the U.S. Government.
Copyright Statement
Some authors (RW Kaminski and EV Oaks) are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by National Institutes of Health grants RO1 AI058896 (PAK), AI41365 (AA), RR00164 (Tulane National Primate Research Center) and the Louisiana Vaccine Center and South Louisiana Institute for Infectious Disease Research sponsored by the Louisiana Board of Regents. We thank the veterinary technicians at the Tulane National Research Primate Center, and Kristen Clarkson and K. Ross Turbyfill at Walter Reed Army Institute for excellent technical assistance. The SIV peptides used in ICS assays were obtained from the NIH AIDS Reagent Program.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2021.100105.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Brokstad K.A., Eriksson J.C., Cox R.J., Tynning T., Olofsson J., Jonsson R. Parenteral vaccination against influenza does not induce a local antigen-specific immune response in the nasal mucosa. J Infect Dis. 2002;185:878–884. doi: 10.1086/339710. [DOI] [PubMed] [Google Scholar]
- 2.Clarke E.T., Williams N.A., Findlow J., Borrow R., Heyderman R.S., Finn A. Polysaccharide-specific memory B cells generated by conjugate vaccines in humans conform to the CD27+IgG+ isotype-switched memory B Cell phenotype and require contact-dependent signals from bystander T cells activated by bacterial proteins to differentiate into plasma cells. J Immunol. 2013;191:6071–6083. doi: 10.4049/jimmunol.1203254. [DOI] [PubMed] [Google Scholar]
- 3.Asahi Y., Yoshikawa T., Watanabe I., Iwasaki T., Hasegawa H., Sato Y. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J Immunol. 2002;168:2930–2938. doi: 10.4049/jimmunol.168.6.2930. [DOI] [PubMed] [Google Scholar]
- 4.Renegar K.B., Small P.A., Jr. Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 5.Renegar K.B., Small P.A., Jr., Boykins L.G., Wright P.F. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–1986. doi: 10.4049/jimmunol.173.3.1978. [DOI] [PubMed] [Google Scholar]
- 6.Seibert C.W., Rahmat S., Krause J.C., Eggink D., Albrecht R.A., Goff P.H. Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs. J Virol. 2013;87:7793–7804. doi: 10.1128/JVI.00979-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J., Thorson L., Stokes R.W., Santosuosso M., Huygen K., Zganiacz A. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J Immunol. 2004;173:6357–6365. doi: 10.4049/jimmunol.173.10.6357. [DOI] [PubMed] [Google Scholar]
- 8.Pierantoni A., Esposito M.L., Ammendola V., Napolitano F., Grazioli F., Abbate A. Mucosal delivery of a vectored RSV vaccine is safe and elicits protective immunity in rodents and nonhuman primates. Mol Ther Methods Clin Dev. 2015;2:15018. doi: 10.1038/mtm.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li K, Li Z, Wohlford-Lenane C, Meyerholz DK, Channappanavar R, An D, et al. Single-Dose, Intranasal Immunization with Recombinant Parainfluenza Virus 5 Expressing Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Spike Protein Protects Mice from Fatal MERS-CoV Infection. mBio. 2020;11. [DOI] [PMC free article] [PubMed]
- 10.Kim M.H., Kang J.O., Kim J.Y., Jung H.E., Lee H.K., Chang J. Single mucosal vaccination targeting nucleoprotein provides broad protection against two lineages of influenza B virus. Antiviral Res. 2019;163:19–28. doi: 10.1016/j.antiviral.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Bhide Y., Dong W., Gribonika I., Voshart D., Meijerhof T., de Vries-Idema J. Cross-Protective Potential and Protection-Relevant Immune Mechanisms of Whole Inactivated Influenza Virus Vaccines Are Determined by Adjuvants and Route of Immunization. Front Immunol. 2019;10:646. doi: 10.3389/fimmu.2019.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green C.A., Sande C.J., Scarselli E., Capone S., Vitelli A., Nicosia A. Novel genetically-modified chimpanzee adenovirus and MVA-vectored respiratory syncytial virus vaccine safely boosts humoral and cellular immunity in healthy older adults. J Infect. 2019;78:382–392. doi: 10.1016/j.jinf.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McFarland E.J., Karron R.A., Muresan P., Cunningham C.K., Libous J., Perlowski C. Live Respiratory Syncytial Virus Attenuated by M2–2 Deletion and Stabilized Temperature Sensitivity Mutation 1030s Is a Promising Vaccine Candidate in Children. J Infect Dis. 2020;221:534–543. doi: 10.1093/infdis/jiz603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scaggs Huang F., Bernstein D.I., Slobod K.S., Portner A., Takimoto T., Russell C.J. Safety and immunogenicity of an intranasal sendai virus-based vaccine for human parainfluenza virus type I and respiratory syncytial virus (SeVRSV) in adults. Hum Vaccin Immunother. 2020:1–6. doi: 10.1080/21645515.2020.1779517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin A., Apostolovic D., Jahnmatz M., Liang F., Ols S., Tecleab T. Live attenuated pertussis vaccine BPZE1 induces a broad antibody response in humans. J Clin Invest. 2020;130:2332–2346. doi: 10.1172/JCI135020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozlowski P.A., Williams S.B., Lynch R.M., Flanigan T.P., Patterson R.R., Cu-Uvin S. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J Immunol. 2002;169:566–574. doi: 10.4049/jimmunol.169.1.566. [DOI] [PubMed] [Google Scholar]
- 17.Brekke K., Lind A., Holm-Hansen C., Haugen I.L., Sorensen B., Sommerfelt M. Intranasal administration of a therapeutic HIV vaccine (Vacc-4x) induces dose-dependent systemic and mucosal immune responses in a randomized controlled trial. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0112556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudin A., Riise G.C., Holmgren J. Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect Immun. 1999;67:2884–2890. doi: 10.1128/iai.67.6.2884-2890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manrique M., Kozlowski P.A., Cobo-Molinos A., Wang S.W., Wilson R.L., Montefiori D.C. Immunogenicity of a vaccine regimen composed of simian immunodeficiency virus DNA, rMVA, and viral particles administered to female rhesus macaques via four different mucosal routes. J Virol. 2013;87:4738–4750. doi: 10.1128/JVI.03531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quiding-Jarbrink M., Nordstrom I., Granstrom G., Kilander A., Jertborn M., Butcher E.C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Invest. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson E.L., Rask C., Fredriksson M., Eriksson K., Czerkinsky C., Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manrique M., Kozlowski P.A., Wang S.W., Wilson R.L., Micewicz E., Montefiori D.C. Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal Immunol. 2009;2:536–550. doi: 10.1038/mi.2009.103. [DOI] [PubMed] [Google Scholar]
- 23.Manrique M., Kozlowski P.A., Cobo-Molinos A., Wang S.W., Wilson R.L., Montefiori D.C. Long-term control of simian immunodeficiency virus mac251 viremia to undetectable levels in half of infected female rhesus macaques nasally vaccinated with simian immunodeficiency virus DNA/recombinant modified vaccinia virus Ankara. J Immunol. 2011;186:3581–3593. doi: 10.4049/jimmunol.1002594. [DOI] [PubMed] [Google Scholar]
- 24.Bomsel M., Tudor D., Drillet A.S., Alfsen A., Ganor Y., Roger M.G. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Arunachalam P.S., Charles T.P., Joag V., Bollimpelli V.S., Scott M.K.D., Wimmers F. T cell-inducing vaccine durably prevents mucosal SHIV infection even with lower neutralizing antibody titers. Nat Med. 2020;26:932–940. doi: 10.1038/s41591-020-0858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephenson K.E., Li H., Walker B.D., Michael N.L., Barouch D.H. Gag-specific cellular immunity determines in vitro viral inhibition and in vivo virologic control following simian immunodeficiency virus challenges of vaccinated rhesus monkeys. J Virol. 2012;86:9583–9589. doi: 10.1128/JVI.00996-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marthas M.L., Van Rompay K.K., Abbott Z., Earl P., Buonocore-Buzzelli L., Moss B. Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques. Vaccine. 2011;29:3124–3137. doi: 10.1016/j.vaccine.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai L., Vodros D., Kozlowski P.A., Montefiori D.C., Wilson R.L., Akerstrom V.L. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology. 2007;369:153–167. doi: 10.1016/j.virol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao P., Zhao J., Patterson L.J., Brocca-Cofano E., Venzon D., Kozlowski P.A. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol. 2010;84:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clements JD, Norton EB. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere. 2018;3. [DOI] [PMC free article] [PubMed]
- 31.Bernasconi V., Norling K., Gribonika I., Ong L.C., Burazerovic S., Parveen N. A vaccine combination of lipid nanoparticles and a cholera toxin adjuvant derivative greatly improves lung protection against influenza virus infection. Mucosal Immunol. 2020 doi: 10.1038/s41385-020-0334-2. [DOI] [PubMed] [Google Scholar]
- 32.Lewis D.J., Huo Z., Barnett S., Kromann I., Giemza R., Galiza E. Transient facial nerve paralysis (Bell's palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills K.H., Cosgrove C., McNeela E.A., Sexton A., Giemza R., Jabbal-Gill I. Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin a. Infect Immun. 2003;71:726–732. doi: 10.1128/IAI.71.2.726-732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao W., Kim J.H., Reber A.J., Hoelscher M., Belser J.A., Lu X. Nasal delivery of Protollin-adjuvanted H5N1 vaccine induces enhanced systemic as well as mucosal immunity in mice. Vaccine. 2017;35:3318–3325. doi: 10.1016/j.vaccine.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fries L.F., Montemarano A.D., Mallett C.P., Taylor D.N., Hale T.L., Lowell G.H. Safety and immunogenicity of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine administered intranasally to healthy adults. Infect Immun. 2001;69:4545–4553. doi: 10.1128/IAI.69.7.4545-4553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oaks E.V., Turbyfill K.R. Development and evaluation of a Shigella flexneri 2a and S. sonnei bivalent invasin complex (Invaplex) vaccine. Vaccine. 2006;24:2290–2301. doi: 10.1016/j.vaccine.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 37.Turbyfill K.R., Kaminski R.W., Oaks E.V. Immunogenicity and efficacy of highly purified invasin complex vaccine from Shigella flexneri 2a. Vaccine. 2008;26:1353–1364. doi: 10.1016/j.vaccine.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 38.Riddle M.S., Kaminski R.W., Williams C., Porter C., Baqar S., Kordis A. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine. 2011;29:7009–7019. doi: 10.1016/j.vaccine.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 39.Kaminski R.W., Turbyfill K.R., Oaks E.V. Mucosal adjuvant properties of the Shigella invasin complex. Infect Immun. 2006;74:2856–2866. doi: 10.1128/IAI.74.5.2856-2866.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaminski R.W., Turbyfill K.R., Chao C., Ching W.M., Oaks E.V. Mucosal adjuvanticity of a Shigella invasin complex with dna-based vaccines. Clin Vaccine Immunol. 2009;16:574–586. doi: 10.1128/CVI.00435-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pabst R. Mucosal vaccination by the intranasal route. Nose-associated lymphoid tissue (NALT)-Structure, function and species differences. Vaccine. 2015;33:4406–4413. doi: 10.1016/j.vaccine.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 42.Wang S.W., Kozlowski P.A., Schmelz G., Manson K., Wyand M.S., Glickman R. Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J Virol. 2000;74:10514–10522. doi: 10.1128/jvi.74.22.10514-10522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertley F.M., Kozlowski P.A., Wang S.W., Chappelle J., Patel J., Sonuyi O. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J Immunol. 2004;172:3745–3757. doi: 10.4049/jimmunol.172.6.3745. [DOI] [PubMed] [Google Scholar]
- 44.Turbyfill K.R., Hartman A.B., Oaks E.V. Isolation and characterization of a Shigella flexneri invasin complex subunit vaccine. Infect Immun. 2000;68:6624–6632. doi: 10.1128/iai.68.12.6624-6632.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song K., Bolton D.L., Wei C.J., Wilson R.L., Camp J.V., Bao S. Genetic immunization in the lung induces potent local and systemic immune responses. Proc Natl Acad Sci U S A. 2010;107:22213–22218. doi: 10.1073/pnas.1015536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kozlowski P.A., Lynch R.M., Patterson R.R., Cu-Uvin S., Flanigan T.P., Neutra M.R. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr. 2000;24:297–309. doi: 10.1097/00126334-200008010-00001. [DOI] [PubMed] [Google Scholar]
- 47.Rudin A., Johansson E.L., Bergquist C., Holmgren J. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect Immun. 1998;66:3390–3396. doi: 10.1128/iai.66.7.3390-3396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozlowski P.A., Cu-Uvin S., Neutra M.R., Flanigan T.P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalek S.M., Lackner A.A., Katz J., Russell M.W., Eldridge J.H., Mestecky J. Oral immunization studies with Streptococcus mutans and influenza vaccines in rhesus macaque monkeys. Adv Exp Med Biol. 1995;371B:1423–1429. [PubMed] [Google Scholar]
- 50.He B., Xu W., Santini P.A., Polydorides A.D., Chiu A., Estrella J. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Shang L., Fukata M., Thirunarayanan N., Martin A.P., Arnaboldi P., Maussang D. Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology. 2008;135:529–538. doi: 10.1053/j.gastro.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Endsley M.A., Njongmeta L.M., Shell E., Ryan M.W., Indrikovs A.J., Ulualp S. Human IgA-inducing protein from dendritic cells induces IgA production by naive IgD+ B cells. J Immunol. 2009;182:1854–1859. doi: 10.4049/jimmunol.0801973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eriksson K., Quiding-Jarbrink M., Osek J., Moller A., Bjork S., Holmgren J. Specific-antibody-secreting cells in the rectums and genital tracts of nonhuman primates following vaccination. Infect Immun. 1998;66:5889–5896. doi: 10.1128/iai.66.12.5889-5896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crowley-Nowick P.A., Bell M.C., Brockwell R., Edwards R.P., Chen S., Partridge E.E. Rectal immunization for induction of specific antibody in the genital tract of women. J Clin Immunol. 1997;17:370–379. doi: 10.1023/a:1027312223474. [DOI] [PubMed] [Google Scholar]
- 55.Kantele A., Hakkinen M., Moldoveanu Z., Lu A., Savilahti E., Alvarez R.D. Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: evidence for compartmentalization within the common mucosal immune system in humans. Infect Immun. 1998;66:5630–5635. doi: 10.1128/iai.66.12.5630-5635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zychlinsky A., Perdomo J.J., Sansonetti P.J. Molecular and cellular mechanisms of tissue invasion by Shigella flexneri. Ann N Y Acad Sci. 1994;730:197–208. doi: 10.1111/j.1749-6632.1994.tb44249.x. [DOI] [PubMed] [Google Scholar]
- 57.Turbyfill KR, Clarkson KA, Vortherms AR, Oaks EV, Kaminski RW. Assembly, Biochemical Characterization, Immunogenicity, Adjuvanticity, and Efficacy of Shigella Artificial Invaplex. mSphere. 2018;3. [DOI] [PMC free article] [PubMed]
- 58.Betts M.R., Harari A. Phenotype and function of protective T cell immune responses in HIV. Curr Opin HIV AIDS. 2008;3:349–355. doi: 10.1097/COH.0b013e3282fbaa81. [DOI] [PubMed] [Google Scholar]
- 59.Aagaard C, Hoang TT, Izzo A, Billeskov R, Troudt J, Arnett K, et al. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One. 2009;4:e5930. [DOI] [PMC free article] [PubMed]
- 60.Butler K., Ritter J.M., Ellis S., Morris M.R., Hanson D.L., McNicholl J.M. A Depot Medroxyprogesterone Acetate Dose That Models Human Use and Its Effect on Vaginal SHIV Acquisition Risk. J Acquir Immune Defic Syndr. 2016;72:363–371. doi: 10.1097/QAI.0000000000000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu F.X., Ma Z., Rourke T., Srinivasan S., McChesney M., Miller C.J. Immunoglobulin concentrations and antigen-specific antibody levels in cervicovaginal lavages of rhesus macaques are influenced by the stage of the menstrual cycle. Infect Immun. 1999;67:6321–6328. doi: 10.1128/iai.67.12.6321-6328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kutteh W.H., Prince S.J., Hammond K.R., Kutteh C.C., Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol. 1996;104:538–542. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zalenskaya I.A., Chandra N., Yousefieh N., Fang X., Adedipe O.E., Jackson S.S. Use of contraceptive depot medroxyprogesterone acetate is associated with impaired cervicovaginal mucosal integrity. J Clin Invest. 2018;128:4622–4638. doi: 10.1172/JCI120583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huijbregts R.P., Michel K.G., Hel Z. Effect of progestins on immunity: medroxyprogesterone but not norethisterone or levonorgestrel suppresses the function of T cells and pDCs. Contraception. 2014;90:123–129. doi: 10.1016/j.contraception.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tasker C., Pizutelli V., Lo Y., Ramratnam B., Roche N.E., Chang T.L. Depot medroxyprogesterone acetate administration increases cervical CCR5+CD4+ T cells and induces immunosuppressive milieu at the cervicovaginal mucosa. AIDS. 2020;34:729–735. doi: 10.1097/QAD.0000000000002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abel K., Rourke T., Lu D., Bost K., McChesney M.B., Miller C.J. Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of depo-provera before intravaginal challenge with simian immunodeficiency virus mac239. J Infect Dis. 2004;190:1697–1705. doi: 10.1086/424600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Genesca M., McChesney M.B., Miller C.J. Depo-provera treatment does not abrogate protection from intravenous SIV challenge in female macaques immunized with an attenuated AIDS virus. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0009814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharp D.S., Macdonald H. Use of medroxyprogesterone acetate as a contraceptive in conjunction with early postpartum rubella vaccination. Br Med J. 1973;4:443–446. doi: 10.1136/bmj.4.5890.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trunova N., Tsai L., Tung S., Schneider E., Harouse J., Gettie A. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology. 2006;352:169–177. doi: 10.1016/j.virol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 70.Kaushic C., Ashkar A.A., Reid L.A., Rosenthal K.L. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.