Abstract
Background:
Twenty-four hour fasting periods are being used to scrutinize basal insulin infusion rates for pump-treated patients with type 1 diabetes.
Methods:
Data from 339 consecutive in-patients with adult type 1 diabetes on insulin pump therapy undergoing a 24-hour fast as a basal rate test were retrospectively analyzed. Hourly programmed basal insulin infusion rates and plasma glucose concentrations within, below, or above arbitrarily defined target ranges were assessed for periods of the day of special interest (eg, 01:00-07:00 am, “dawn” period, 04:00-07:00 pm, and “dusk” period). Statistics: χ2-tests, paired t-tests were used.
Results:
Basal rates (mean: 0.90 ± 0.02 IU/h) showed circadian variations with peaks corresponding to “dawn” (1.07 ± 0.02 IU/h from 01:00 to 07:00 am) and, less prominently, “dusk” (0.95 ± 0.02 IU/h from 03:00 to 07:00 pm). Individual mean plasma glucose concentrations averaged 6.6 ± 0.1 mmol/L, with 53.1% in the predefined “strict” (4.4-7.2 mmol/L) target range. Interestingly, during the “dawn” period, plasma glucose was significantly higher (by 0.5 ± 0.1 mmol/L [95% confidence interval: 0.3-0.8 mmol/L; P < .0001]) and the odds ratio for hypoglycemia was significantly lower compared to the reference period.
Interpretation:
Twenty-four hour fasting periods as basal rate tests frequently unravel periods with inappropriate basal insulin infusion rates potentially responsible for fasting hyper- or hypoglycemia. Notably, the higher basal insulin infusion rate found during the “dawn” period seems to be justified and may need to be accentuated.
Keywords: type 1 diabetes, insulin pump (CSII: continuous subcutaneous insulin infusion), basal rate profiles, dawn phenomenon, dusk phenomenon
Introduction
The severe insulin deficiency in patients with type 1 diabetes necessitates full insulin replacement therapy. Intensified insulin therapy has been established as the standard treatment. This regimen typically involves division of the overall insulin requirement into a basal insulin component, covering the insulin demand under fasting conditions, and a prandial/bolus insulin component, aiming to cover postprandial glucose excursions or to lower plasma glucose in the case of hyperglycemia (correction algorithms).1
One major advantage of insulin pump therapy is the opportunity to precisely program basal insulin infusion requirements at hourly (or even shorter) intervals to address diurnal variations in the individual insulin needs.2,3
Basal insulin infusion rates usually are programmed according to published recommendations initially.4,5 Later, they should be optimized empirically, eg, in response to overnight plasma glucose concentrations (the longest fasting period during a normal day),6-8 according to glucose concentrations determined after omission of individual meals (short fasting periods),8 or depending on the glucose profiles obtained during a full 24-hour fasting period (basal rate tests).9,10 Such basal rate tests appear necessary, because on a day with regular meal rhythm, rapid-acting insulin may compensate for deficits in basal insulin and vice versa.
Despite the fact that many patients nowadays are using sensor-augmented pumps,11-13 which can address low plasma glucose concentrations by temporarily stopping basal insulin infusion, the question is whether this obviates the need for an individually optimized basal insulin infusion rate, which may be too low at times as well. Most likely, a custom-tailored basal insulin infusion rate plus technical solutions to account for variations in physical activity will provide the best results.
In specialized German diabetes centers with inpatient service, it has become customary to perform 24-hour fasting periods in pump-treated patients with type 1 diabetes, if there appears to be a chance to optimize the diurnal profile of insulin administration, and if there is a suspicion that basal insulin infusion rates are principally or temporarily too high or too low and thus contribute to a risk for fasting hypo- or hyperglycemia. To gain more insight into the relationship between hourly basal insulin infusion profiles used in insulin pumps and the resulting 24-hour fasting plasma glucose profiles, we analyzed data from a large cohort of patients attending the Diabetes Center Bad Lauterberg between 2008 and 2014, with a special emphasis on diurnal changes in insulin administration and plasma glucose concentrations. Our hypothesis was that valuable insights can be gained from analyzing the relationship of hourly basal insulin infusion rates and the resulting plasma glucose profiles during a 24-hour fast in patients with type 1 diabetes on insulin pump treatment.
Patients and Methods
Study Design
This retrospective analysis was conducted based on patients who had been inpatients at the diabetes center Bad Lauterberg, aiming at an optimization of their glycemic control using insulin pumps. Pump-treated patients with type 1 diabetes were retrieved from a dedicated database (collected between 2008 and 2014). These patients’ data were extracted from their hospital charts, using a case report form specifying the parameters of interest. All patients had given permission for the analysis of their clinical data for scientific purposes, and the present study was performed fully complying with pertinent ethical and data protection regulations. Each patient was studied applying his/her basal insulin infusion rate as it had developed empirically. When starting insulin pump therapy, recommendations for basal insulin infusion rates issued by Renner and coworkers4,14 were, as a rule, used as guidance for programming hourly insulin infusion rates. They correspond to the Roche Basal Rate Calculator widely distributed across Europe. Later, individual basal insulin infusion rates were optimized in clinical practice (guided by plasma glucose measurements) and, in the cohort of patients described in the present analysis, by performing 24-hour fasting basal rate tests. Meal-related insulin needs were optimized empirically, based on carbohydrate counting and individual dosing algorithms developed for each major meal, taking into account diurnal variations in insulin sensitivity.15
Selection of Study Patients
Patients were included in the present retrospective analysis, if they had type 1 diabetes and were treated with continuous subcutaneous insulin infusion (using any approved model of insulin pump: Disetronic H-tron, n = 39 [11.5%]; Disetronic D-tron, n = 19 [5.3%]; Roche Accu Chek, n = 153 [5.1%]; Medtronic Paradigm, n = 97 [28.6%]; Cozmo, n = 19 [5.6%]; and OmniPod, n = 11 [3.2%]) and had an age of 16 to 80 years. Patients were excluded, if their diabetes type was different from type 1 diabetes, if they were pregnant at the time of inpatient treatment, or if no basal rate test (fasting period 24 hours) had been performed. Patients with an age of less than 16 years were excluded, because insulin requirements in children and adolescents are usually different.2,3 If a patient had been admitted to the hospital for the performance of a 24-hour fast (basal rate test) more than once, only the most recent dataset was used to most likely represent the individual optimum. A flow chart describing the patients retrieved from the original database and the reasons for excluding data from the present analysis are shown as supplemental Figure S1.
Data Extraction
Patient characteristics (summarized in Table 1), details of the treatment (supplemental Table S1), and laboratory values were extracted from medical records. Hourly basal insulin infusion rates (insulin pump) used and plasma glucose profiles measured during a 24-hour fasting period were recorded. A representative plasma glucose profile after treatment optimization (ie, determined immediately before hospital discharge) was also obtained.
Table 1.
Subject Characteristics of Patients With Type 1 Diabetes Treated With Continuous Subcutaneous Insulin Infusion (Insulin Pumps) Who Performed a 24-hour Fasting Test During Inpatient Service for Optimization of Plasma Glucose Control.
| Parameter | Unit | Mean ± SD/proportion | Range |
|---|---|---|---|
| Gender | Female/male (% female) | 183/156 (54.0) | n.a. |
| Age | y | 41 ± 14 | 16-80 |
| Body-mass index | kg/cm2 | 26.1 ± 4.9 | 16.2-44.7 |
| Diabetes duration | y | 20 ± 12 | 11-62 |
| Duration of CSII treatment | y | 4 ± 5 | 0-28 |
| Blood pressure | |||
| Systolic | mmHg | 131 ± 17 | 90-190 |
| Diastolic | mmHg | 79 ± 10 | 50-110 |
| Arterial hypertension | Yes/no (% yes) | 184/155 (55.4) | |
| Hypoglycemic episodes | |||
| Any hypoglycemic episode | Yes/no (% yes) | 295/44 (94.6) | n.a. |
| Symptomatic episodes | per week | 3 ± 3 | 0-21 |
| Severe hypoglycaemia | Yes/no (% yes) | 77/262 (27.9) | n.a. |
| Episodes, last 12 months | per year | 0.4 ± 1.5 | 0-20 |
| Diabetic complications | n.a. | ||
| Retinopathy | Yes/no (% yes) | 122/217 (36.0) | n.a. |
| Nephropathy | Yes/no (% yes) | 61/278 (18.0) | n.a. |
| Neuropathy | Yes/no (% yes) | 146/190 (43.4) | n.a. |
| Coronary heart disease | Yes/no (% yes) | 11/327 (3.3) | n.a. |
| Diabetic foot syndrome | Yes/no (% yes) | 29/311 (8.6) | n.a. |
| HbA1c | mmol/mol | 67 ± 17 | 27-160 |
| HbA1c | % | 8.3 ± 1.6 | 4.6-16.8 |
| Serum triacylglycerol concentration | mmol/L | 1.1 ± 0.8 | 0.0-8.8 |
| HDL cholesterol | mmol/L | 1.5 ± 0.4 | 0.0-3.0 |
| Creatinine | µmol/L | 73 ± 42 | 38-641 |
| eGFR | mL/min | 107 ± 17 | 42-159 |
| Albuminuria | g/mol creatinine | 8.8 ± 45.2 | 0.0-458.7 |
| Normal | Yes | 289/50 (85.3) | n.a. |
| Microalbuminuria | Yes | 30/309 (8.8) | n.a. |
| Macroalbuminuria | Yes | 13/326 (3.8) | n.a. |
Abbreviations: BIIR, basal insulin infusion rate; CSII, continuous subcutaneous insulin infusion; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; SD, standard deviation.
Mean ± standard deviation or number of measurements either fulfilling or not fulfilling the criterion in question as well as the proportions (percentages) of all measurements available.;
Optimization of Hourly Basal Insulin Infusion Rates
Basal rate tests were performed in a standardized manner after avoiding strenuous exercise and alcoholic drinks during the prior 24 hours. They were performed over 24 hours, starting at 06:00 pm, omitting dinner, breakfast, and lunch, or any snacks in between, unless carbohydrate intake was necessary to compensate for low plasma glucose values or hypoglycemia (plasma glucose <3.9 mmol/L) or in the case that patients usually ate a late-night snack covered by the basal insulin infusion rate. Fasting was discontinued at 06:00 pm of the next day. Plasma glucose was measured in capillary samples using a glucose oxidase/amperometric method on a Biosen S-line Lab + (EKF Diagnostic GmbH, D-39179 Barleben, Germany) at prespecified time points (06:00, 08:00, and 10:00 pm; midnight; 02:00, 04:00, 06:45, and 09:00 am; noon; 02:00 and 06:00 pm). Information generated by these basal rate tests was used to optimize programming BIIR (basal insulin infusion rate) for each hour of the day, either confirming an “appropriate” BIIR, keeping plasma glucose in the target range for the fasting state,10 tentatively defined as 4.4 to 6.7 mmol/L, or increasing BIIR if plasma glucose was higher and/or decreasing it, if plasma glucose was lower.
Design of the Analysis
The present analysis focuses on the questions: (a) whether empirically derived basal insulin infusion rates lead to (mean) plasma glucose concentrations in the target range for the fasting situation, (b) how plasma glucose concentration during 24-hour fasting tests differs from those obtained during daily profiles when the same patients eat meals (with an emphasis on fasting plasma glucose in the morning and premeal plasma glucose concentrations), and (c) whether typical peaks in the diurnal basal insulin infusion rate profiles representing the early morning (“dawn” phenomenon) or late afternoon (“dusk” phenomenon) rise in insulin resistance are appropriately addressed by transient increments in insulin delivery rates during these periods.
Achievement of Fasting Plasma Glucose Targets During 24-hour Fast (Basal Rate Tests)
Two definitions for the target range of plasma glucose during fasting have been analyzed: First, our more stringent definition of this target range was 4.4 to 7.2 mmol/L (80-130 mg/dL) according to the definition of the fasting target range recently published by the American Diabetes Association.16 This, in our clinical practice, is the range that is aimed for without suggesting or inducing changes in the insulin infusion rates when reached. Second, as a more relaxed definition, we used 3.9-9.0 mmol/L (70-162 mg/dL), because <3.9 mmol/L (<70 mg/dL) is equivalent to hypoglycemia,16 and since for a target HbA1c of 7.5% (58 mmol/mol), this is the upper limit of the fasting plasma glucose range that would be equivalent.16 This latter range appears generally acceptable, however, would lead to attempts of optimizing basal insulin infusion rates, if single or more values lie outside the narrow target range as defined above.
The achievement of plasma glucose in the fasting target range was tested for each measurement taken at any time during the 24-hour fasting period (to fully represent the distribution of individual values) and at the patient level, ie, by calculating mean values for each individual subject in our cohort.
Potential Differences in Plasma Glucose Profiles Obtained During 24-hour Fasting and During Days With a Normal Meal Rhythm
To identify differences in plasma glucose concentrations between profiles obtained during 24-hour fasting periods and days with a normal meal rhythm, mean values for all time points were compared as well as average (mean) glucose concentrations during profiles taken under both conditions. Since fasting plasma glucose in the morning (before breakfast) and premeal plasma glucose concentrations measured before lunch and dinner, respectively, are determined under conditions that resemble those during fasting more than those measured at postmeal time points, a mean of fasting/preprandial (06:45 am, noon, and 06:00 pm) plasma glucose concentrations was also calculated.
The distribution of individual and mean plasma glucose concentrations was analyzed by generating histograms, with categories including glucose concentration ranges 1.0 mmol/L wide, spanning from 0-1.0 to 19.0-20.0 and >20.0 mmol/L, which was also compared between 24-hour fasting periods and fasting/preprandial time points during profiles with regular meal rhythm.
Appropriateness of Diurnal Variations in Hourly Insulin Infusion Rates With Emphasis on the “Dawn” and “Dusk” Phenomenon
Based on the diurnal profiles of basal insulin infusion rates (Figure 1), there were two periods (09:00 pm to 01:00 am and 10:00 am to 02:00 pm) with “flat” and comparable basal insulin infusion rates, whereas there was a progressive rise in hourly basal insulin infusion rates between 01:00 and 07:00 am, tentatively representing the “dawn” phenomenon.17-20 Corresponding standard time points of measuring plasma glucose were at 02:00, 04:00, and 06:45 am. There was another transient rise in hourly basal insulin infusion rates with a peak at 06:00 pm, tentatively representing a “dusk” phenomenon, with a corresponding time point of measuring plasma glucose at 06:00 pm. The flat parts of the basal insulin infusion profile were defined as non-“dawn”-non-“dusk” periods. Corresponding time points for determining plasma glucose were 10:00 pm and midnight as well as noon and 02:00 pm Two time points of measuring plasma glucose, at 08:00 pm and at 08:45 am, could not be convincingly assigned to any of these well-defined periods.
Figure 1.
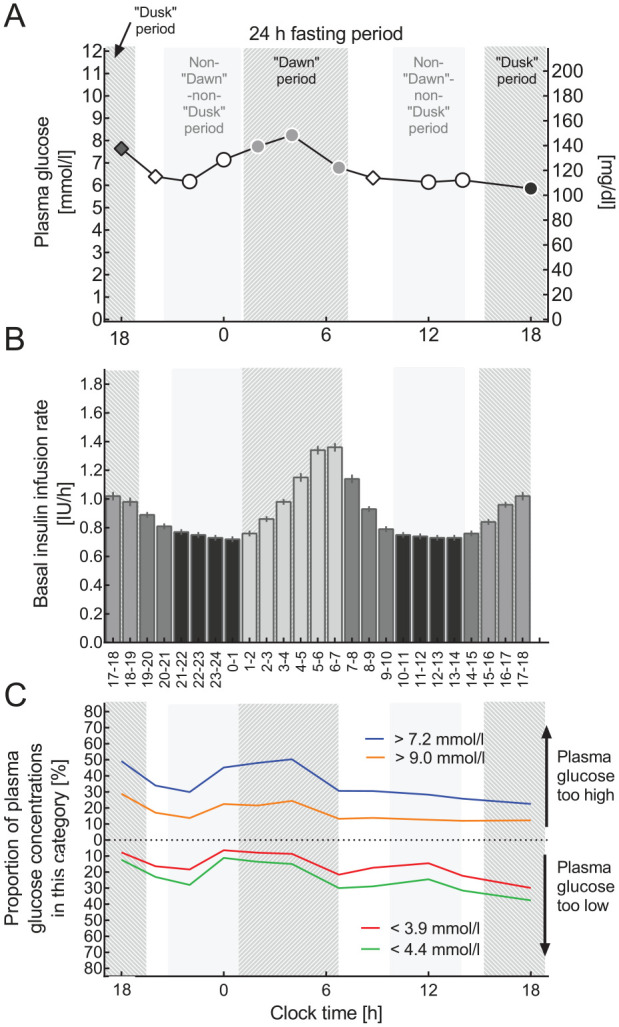
Basal insulin infusion rate profiles and results of 24-hour fasting periods performed as a basal rate test. (A) Plasma glucose profiles during 24-hour fasting periods, (B) the current hour by hour basal insulin infusion profile (IU/h), and (C) proportions of patients with plasma glucose concentrations below thresholds indicating “below the target range” or hypoglycemia (<4.4 or <3.9 mmol/L, respectively) or above thresholds indicating “above the target range” or hyperglycemia (>7.2 or >9.0 mmol/L. (A, B) Means ± standard error of the mean and (C) % of all plasma glucose values available for this time point. Light gray bars highlight the periods of the day as defined for the purpose of the present analysis. The “dawn” period from 01:00 to 07:00 am, the “dusk” period from 03:00 to 07:00 pm, a reference period with a peak-less, flat insulin basal rate profile (10:00 am to 02:00 pm and 08:00 pm to 01:00 am, non-“dawn”-non-“dusk” period). Plasma glucose concentrations are shown with different symbols if belonging to one of these predefined periods of the day. Time points 08:00 pm and 08:45 am were considered not to belong to any of these predefined periods.
For each of these diurnal time periods, mean plasma glucose concentrations were calculated as well as the proportion of plasma glucose concentrations measured below the thresholds for low plasma glucose concentrations (“stringent” definition: <4.4 mmol/L; more “relaxed” definition: <3.9 mmol/L) and above thresholds for plasma glucose concentrations deemed to be too high (“stringent” definition: >7.2 mmol/L; “relaxed” definition: >9.0 mmol/L). The latter was performed as a sensitivity analysis. These proportions were also calculated for all time points when plasma glucose was measured.
The proportion of patients with plasma glucose concentrations in a stringently defined (4.4-7.2 mmol/L) and a relaxed (3.9-9.0 mmol/L) target range was also calculated.
Proportions of plasma glucose concentrations that were too low or too high according to these definitions were calculated and compared between the “dawn” period or “dusk” period and the non-“dawn”-non-“dusk” period. Odds ratios for the probability of glucose concentrations that were too low or too high comparing different periods of interest were determined. The rationale was to determine whether the hourly basal insulin infusion rate, which typically was higher during the “dawn” period than during other periods, leads to a higher probability of corresponding plasma glucose concentrations that were too low. In this case, the temporarily higher hourly basal insulin infusion rate would be judged inappropriate. Similar reasoning would apply to the “dusk” period.
Sample Size Calculation
Since it was not the aim of the study to address a hypothesis regarding differences of any kind, this is an exploratory analysis using a large sample of patients mainly based on their availability. No formal sample size calculation has been performed. Intuitively, the number of patients available for analysis was considered sufficient.
Statistical Analysis
Patients’ characteristics are reported as means ± standard deviation (SD) or counts (n) as well as proportions (%). Results are reported as means ± standard error of the mean (SEM) or means ± 95% confidence intervals (CIs) as described in figure legends. When proportions are reported, the number of patients fulfilling or not fulfilling the criterion in question is reported, thus presenting the total number of observations, allowing the extrapolation of missing data. Significances of differences between two time points or periods were tested by paired t-tests (continuous variables; Statistica Version 12.0, Statsoft Europe, Hamburg, Germany) or contingency table analysis (χ2 test categorical variables, GraphPad Prism version 7.00 for Windows, GraphPad Software, La Jolla California, United States, www.graphpad.com). P-values < .05 were taken to indicate significant differences.
Results
Diurnal Variation in Basal Insulin Infusion Rates
Patients were examined during 24-hour fasting periods using their empirically derived basal insulin infusion rates. Our descriptive analysis of the factual basal insulin infusion rates results in a mean over 24 hours of 0.90 ± 0.02 IU/h (or 21.5 ± 9.2 IU/day or 0.27 ± 0.09 IU/kg body weight per day). Hourly basal insulin infusion rates were lowest during the periods from 09:00 pm to 01:00 am and from 10:00 am to 02:00 pm (Figure 1(B)). Basal insulin infusion rates increased overnight with a peak between 06:00 and 07:00 am (1.36 ± 0.03 IU/h). A substantially smaller peak was present in the late afternoon (1.02 ± 0.03 IU/h) from 05:00 to 06:00 pm.
Plasma Glucose Concentrations During a 24-hour Fasting Period
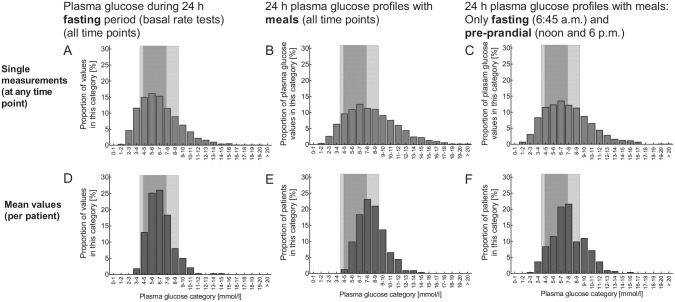
Mean (± SEM) plasma glucose concentrations during the 24-hour fasting period were 6.6 ± 0.1 mmol/L (95% CI: 6.4-6.8 mmol/L) (Figure 1(A)). Analysis of the frequency distribution of individual values across categories of plasma glucose concentrations revealed a wider distribution (Figure 2(A)), with 23.1% of the values below 4.4 mmol/L, 15.9% below 3.9 mmol/L, and 36.0% above 7.2 mmol/L as well as 17.5% above 9.0 mmol/L. The frequency distribution of mean plasma glucose concentrations for the individual patients was narrower (Figure 2(D); P < .0001 vs individual plasma glucose concentrations determined at any time point). Mean plasma glucose concentrations mainly fell into the target ranges (Figure 2(D)), with 4.4% of the values below 4.4 mmol/L, 1.2% below 3.9 mmol/L, and 29.8% above 7.2 mmol/L as well as 8.0% above 9.0 mmol/L.
Figure 2.
Histograms showing the frequency distribution of plasma glucose concentrations across categories ranging from 1.0-2.0 to 19.0-20.0 and >20 mmol/L (each category 1.0 mmol/L wide) during 24-hour fasting periods (A, D), during 24-hour plasma glucose profiles with regular meal rhythm (B, E), and mean fasting (06:45 am) and preprandial (noon and 06:00 pm) plasma glucose concentrations from profiles with meals (C, F). The upper row of panels (a-c) shows the frequency distribution of single measurements (taken at any time point) and the lower row of panels (D-F) shows the frequency distribution of mean values (per patient). Light gray areas indicate the “relaxed” target range (3.9-9.0 mmol/L) and the darker gray area shows the “strict” target range (4.4-7.2 mmol/L).
Proportion of Plasma Glucose Concentrations During a 24-hour Fast Within Predefined Target Ranges
Of all plasma glucose concentrations measured during 24-hour fasting periods, depending on the time point, 34.8% to 47.2% (overall: 40.9%) fell into the strictly defined target range (4.4-7.2 mmol/L), while 57.9% to 73.0% (overall: 67.1%) fell into the target range defined by more relaxed criteria (3.9-9.0 mmol/L; Figure 3). If calculated for mean plasma glucose concentrations for each individual patient, these proportions were significantly higher (P < .0001) (Figure 3(C)).
Figure 3.
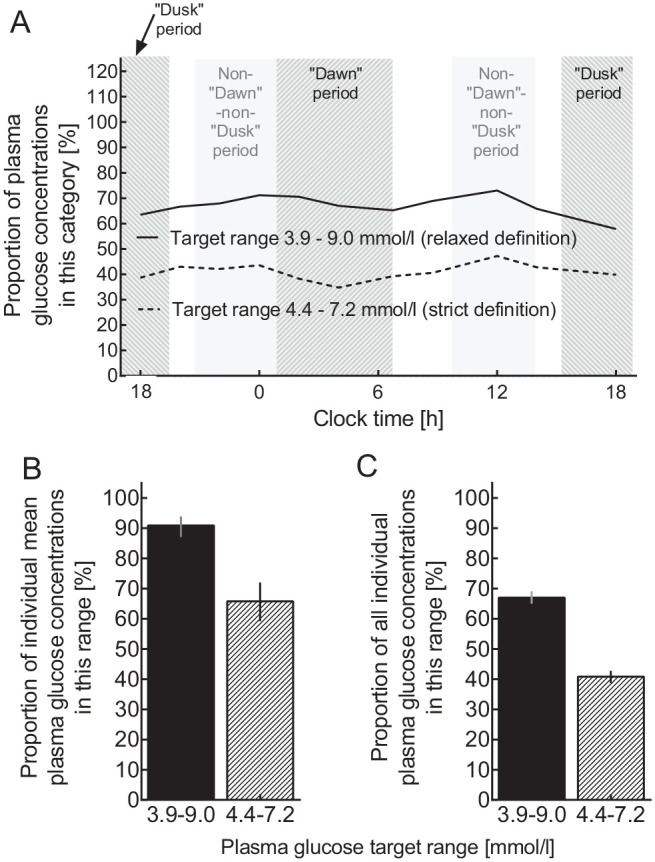
Proportions of plasma glucose measurements within predefined target ranges (“strict”: 4.4-7.2 mmol/L; “relaxed”: 3.9-9.0 mmol/L) for all patients by time point (A), for mean plasma glucose concentrations across all time points in individual patients (B), and for individual plasma glucose concentrations at all time points during 24-hour fasting periods (C). The predefined periods of the day are indicated by light gray bars (for details see Figure 1).
Comparison of Plasma Glucose Concentrations During a 24-hour Fasting Period With Profiles Taken During a Day With Regular Meal Rhythm
Mean (± SEM) plasma glucose concentration during the 24-hour period with a regular meal rhythm was 8.1 ± 0.1 mmol/L (95% CI: 7.9-8.3 mmol/L), which was significantly (P < .0001) higher (by 1.5 ± 0.1 mmol/L [95% CI: 1.2-1.7 mmol/L]) than that during 24-hour fasting (Figures 2 and 4).
Figure 4.
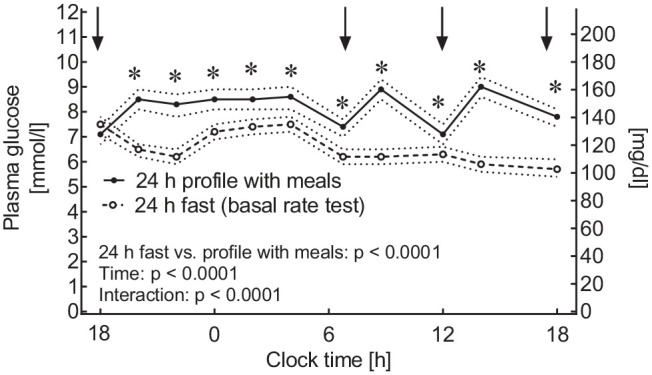
Plasma glucose profiles taken during a 24-hour fasting period (open symbols, broken line) and during a 24-hour plasma glucose profile taken during a day with regular meal rhythm (filled symbols, uninterrupted lines). Means ± 95% confidence intervals. Statistics: Repeated-measures analysis of variance reporting P-values for differences between the two experiments (fasting vs meals), for changes over time, and for their interaction. Asterisks indicate a significant difference (P < .05) between the conditions at individual time points as determined by post hoc testing.
Figure 4 shows that plasma glucose concentrations were higher during 24-hour plasma glucose profiles taken on a day with regular meals than during 24-hour fasting at all time points except 06:00 pm before commencing fasting. These differences are also observed at time points characterized by fasting (in the morning) and before meals (noon and 06:00 pm, before lunch and dinner, respectively). Accordingly, mean ± SEM of these time points still were higher (by 7.4 ± 0.1 [95% CI: 7.2-7.6 mmol/L]) than those measured during fasting (by 0.8 ± 0.1 mmol/L [95% CI: 0.5-1.1 mmol/L]).
Analysis of the frequency distribution of individual values across categories of plasma glucose concentrations during a day with meals (Figure 2(B)) revealed a wider distribution than during fasting (P < .0001; Figure 2(A)), particularly regarding hyperglycemic categories. The same applies to fasting and preprandial plasma glucose concentrations measured during a day with regular meals compared to 24-hour fasting periods (Figure 2; P < .0001). The shift to the right in frequency distributions was obvious also when comparing mean plasma glucose concentrations per individual patients between 24-hour plasma glucose profiles on a regular meal rhythm (Figure 2(B)) or selected fasting and preprandial time points (Figure 2(C)) with 24-hour fasting periods (Figure 2(A); all comparisons P < .0001),
Not only were plasma glucose concentrations during a day with meals generally significantly higher that during 24-hour fasting periods (Figures 2 and 3), but also there was no significant correlation of plasma glucose concentrations at preprandial sampling times (noon and 06:00 pm), when comparing individual plasma glucose measurements determined at these time points. Only at 06:45 am, there was a weak, but significant correlation (P = .045; details not shown).
Magnitude of the Transient Rise in Basal Insulin Infusion Rates During the “Dawn” and “Dusk” Periods
Hourly basal insulin infusion rates increased between 01:00 and 07:00 am (“dawn” period) and between 03:00 and 07:00 pm (“dusk” period) (supplemental Figure S2). A “dawn” and a “dusk” index were calculated by relating the basal insulin infusion rates during the “dawn” or “dusk” periods to the average during a reference period without peaks or troughs. The dimension of the increment (index) was greater for the “dawn” than for the “dusk” period (Figure 1(B); supplemental Figure S2A), however, with substantial interindividual variation. A histogram indicated more subjects without a significant increment in basal insulin infusion rates during the “dusk” period than during the “dawn” period (supplemental Figure S2B). There was a significant correlation between individually determined “dawn” and “dusk” indices (supplemental Figure S2C).
Appropriateness of Increased Basal Insulin Infusion Rates During the “Dawn” Period
Figure 1(c) demonstrates that during the period characterized by progressively rising hourly basal insulin infusion rates (01:00-07:00 am) the proportion of plasma glucose concentrations which were above the target ranges tended to be higher during this period, while those below the target range tended to be reduced when compared to the rest of the day. Table 2 breaks down these proportions below and above the target ranges comparing the period of the day characterized by the “dawn” phenomenon (01:00-07:00 am) with uneventful periods of the 24-hour profile that was used as a reference, because they were unrelated to any noticeable peaks or troughs in hourly basal insulin infusion rates. Remarkably, during the “dawn” period, the proportion of low glucose concentrations (in comparison to both thresholds) was lower, resulting in odds ratios <1.00, which was significant (P < .0001) for a threshold of 4.4 mmol/L, and remained a trend (P = .11) for the threshold of 3.9 mmol/L. Conversely, the proportion of high plasma glucose concentrations was relatively higher during the “dawn” period, resulting in odds ratios >1.00, which were significant for both thresholds of hyperglycemia (Table 2). Mean plasma glucose concentrations, accordingly, were significantly higher (7.0 ± 0.1 mmol/L) during the “dawn” period than during the reference period (6.5 ± 0.1 mmol/L). The difference amounted to 0.5 ± 0.1 mmol/L (95% CI: 0.3-0.8 mmol/L).
Table 2.
Comparison of Plasma Glucose Concentrations and Proportions of Plasma Glucose Concentrations That Were too Low or too High During Periods of a 24-hour Fasting Period (Basal Rate Test) That Were Characterized by the Dawn or Dusk Phenomenon vs Those Measure Outside These Periods.
| Period | “Dawn” period | “Dusk” period | Non-“dawn”-non-“dusk” (reference) period | Dawn vs non-“dawn”-non-“dusk” periods | “Dusk” vs non-“dawn”-non-“dusk” periods | |||
|---|---|---|---|---|---|---|---|---|
| Criterion | Unit | (01:00-07:00 am) | (05:00-06:00 pm) | (09:00 am-01:00 pm and 10:00 pm-02:00 am) | (Odds ratio [95% confidence interval]) | Significance (P-value) | Odds ratio (95% confidence interval) | Significance (P-value) |
| Mean plasma glucose | mmol/L | 7.0 ± 0.1 | 5.8 ± 0.2 | 6.5 ± 0.1 | n.a. | <.0001 | n.a. | <.0001 |
| Difference to reference period | mmol/L | 0.5 ± 0.1 (0.3-0.8) | −0.7 ± 0.1 (−1.0 to −0.4) | n.a. | n.a. | n.a. | n.a. | n.a. |
| Proportion of plasma glucose concentrations that were too low | ||||||||
| <4.4 mmol/L | Yes/no (% yes) | 193/792 (19.6) | 117/194 (37.6) | 297/964 (23.6) | 0.40 (0.31-0.53) | <.0001 | 1.96 (1.50-2.55) | <.0001 |
| <3.9 mmol/L | Yes/no (% yes) | 126/859 (12.8) | 93/218 (29.9) | 191/1070 (15.1) | 0.82 (0.64-1.05) | .11 | 2.39 (1.79-3.19) | <.0001 |
| Proportion of plasma glucose concentrations that were too high | ||||||||
| >7.2 mmol/L | Yes/no (% yes) | 423/562 (42.9) | 70/241 (22.5) | 410/851 (32.5) | 1.56 (1.31-1.86) | <.0001 | 0.60 (0.45-0.81) | .0006 |
| >9.0 mmol/L | Yes/no (% yes) | 194/791 (19.7) | 38/273 (12.2) | 193/1068 (15.3) | 1.36 (1.09-1.69) | .0063 | 0.77 (0.53-1.12) | .17 |
Mean ± standard error of the mean (95% confidence intervals in brackets) or number of measurements either fulfilling or not fulfilling the criterion in question as well as the proportions (percentages) of all measurements available.
Appropriateness of Increased Basal Insulin Infusion Rates During the “Dusk” Period
Plasma glucose concentration during 24-hour fasting was lower at the time of the “dusk” phenomenon (5.8 ± 0.2 mmol/L) compared to the reference period (by −0.7 ± 0.1 mmol/L [95% CI: −1.0 to −0.4 mmol/L]). Along the same line, the proportions of plasma glucose concentrations that were too low were higher during the “dusk” period (P < .0001 for both threshold values), whereas the proportion of plasma glucose concentrations that were too high was lower (significant, P = .0006 in the case of the 7.2 mmol/L threshold; Table 2), with a similar trend for a threshold of 9.0 mmol/L.
Discussion
The present study allows some important insights from performing and analyzing 24-hour fasting periods in insulin pump-treated patients with type 1 diabetes: (A) The majority of plasma glucose concentrations measured was within or near the target ranges applicable to the fasting state (Figures 1 and 2). (B) Individual mean plasma glucose concentrations calculated throughout these 24-hour fasting periods were even more likely to fall within the target range (Figure 2). (C) There were prominent diurnal variations in hourly basal insulin infusion rates, with progressively increasing basal insulin administration between 01:00 and 07:00 am (arbitrarily defined as the “dawn” period;17-20), a smaller rise in the afternoon hours (tentatively defined as the “dusk” period), and two uneventful periods without prominent peaks troughs between 09:00 pm and 01:00 am and between 10.00 am and 02:00 pm (used as the non-“dawn”-non-“dusk” reference period). (d) There also were substantial diurnal variations in the proportions of plasma glucose values that were too high or too low when compared with our thresholds defining the strict and more relaxed target ranges for the fasting condition (Figure 1(C)). (e) Both according to the strict and less stringent criteria, it was more likely to measure plasma glucose concentrations above than below these thresholds (17.5% high vs 15.4% low, P = .016, for 3.9-9.0 mmol/L and 36.0% high vs 23.1% low, P < .0001, for 4.4-7.2 mmol/L; Figure 1(C)), indicating that overall, higher basal insulin infusion rates might be more appropriate in many patients. (f) Between midnight and the early morning hours, relative to other periods of the day, fewer plasma glucose concentrations were too low, and more were too high (see discussion of the “dawn” period below17-20). (g) Only 40.9% of all individually determined plasma glucose concentrations during 24-hour fasting periods fell within the strictly defined target range (4.4-7.2 mmol/L), while 67.1% fell within the less stringently defined target range (3.9-9.0 mmol/L), indicating opportunities for the optimization of basal insulin infusion rates for a significant proportion of patients in our cohort. (h) For mean plasma glucose concentrations by individual patients, these figures significantly increased to 65.8% and 90.9% (Figure 3), indicating, as a rule, inappropriate basal insulin administration rates mainly for circumscript periods of the day in many of our patients. Only for a minority of patients, mean plasma glucose concentrations were outside the target ranges (Figure 3(C)). (i) A frequency distribution (histogram) indicates higher plasma glucose concentrations during plasma glucose profiles taken on day with regular meal rhythm or selected fasting and preprandial plasma glucose concentrations taken from such profiles with regular meal intake (Figure 2) than during 24-hour fasting periods (Figures 2 and 4). Thus, plasma glucose concentrations measured during 24-hour fasting periods seem to be helpful in detecting imbalances in basal insulin infusion rates as used for pump treatment.
We believe that this suggests to optimize hourly basal insulin infusion rates with the help of information gathered during 24-hour fasting tests in order to fully exploit the technical opportunities offered by such insulin infusion devices, which can deliver a separately defined basal insulin infusion rate at least for each 60 minutes segment of the day.2,5,8,21 Therefore, we suggest that using the information provided by plasma glucose profiles measured during 24-hour fasting periods for the optimization of glycemic control in pump-treated patients with type 1 diabetes should be prospectively evaluated against any reasonable other guidance for this process. This may be of particular importance in patients using ultra-fast-acting insulin with meals, because between meals they should rely on their basal insulin more extensively than with slower-acting meal-related insulins.
Prolonged fasting might lead to a rise in nonesterified fatty acids promoting progressive insulin resistance. This may lead to a secondary rise in plasma glucose with extended periods of fasting. Therefore, avoiding 24-hour fasting and substituting it with shorter periods (skipping individual meals) has been advocated to test the appropriateness of basal infusion rates.8 However, plasma glucose concentrations during our 24-hour fasting period did not tend to be reduced over time. We doubt that a period of approximately six hours equivalent to the time elapsing between two meals would suffice to reach a steady state as would be required for judging the appropriateness of basal insulin infusion rates.
One might argue that fasting and preprandial plasma glucose concentrations on a 24-hour profile with a regular meal rhythm might substitute for those determined at the same time of the day during a 24-hour fasting period. This, however, is not supported by our analysis: Premeal plasma glucose was significantly higher during a profile taken during a 24-hour period with regular meal rhythm before breakfast, lunch, and dinner (Figure 4). Moreover, the individual plasma glucose concentrations determined under both conditions (fasting vs eating) did not even correlate convincingly. These differences indicate that plasma glucose concentrations taken at the same time of the day during a 24-hour fasting period and during a 24-hour period with regular meal rhythm are influenced by different factors (eg, meal-related insulin injections), and cannot substitute for each other as determinants of the basal insulin infusion rate. We, therefore, advocate the use of plasma glucose concentrations obtained during prolonged fasting conditions.
This is supported by the fact that during 24-hour fasts, 15.4% of the plasma glucose concentrations taken were <3.9 mmol/L, a proportion significantly higher than during 24-hour profiles with regular meal rhythms (8.3%; P < .0001; during the day: 9.4%; at night: 6.0%); thus, a 24-hour fast helps detect fasting hypoglycemia, which would go undetected during regular plasma glucose profiles taken while the patients eat. Our estimate of the proportion of plasma glucose concentrations <3.9 mmol/L is surprisingly similar to that reported by Ly et al,22 who used continuous plasma glucose measurements in pump-treated patients with type 1 diabetes with and without concomitant glucose sensors (day time: 5.7% and 8.3%; night time 7.3% and 11.1%) . This gives credibility to our estimates of the “proportion in range” (Figure 3) determined without continuous glucose measuring devices. For future studies, the use of continuous glucose measurements would be a helpful addition to provide even more details and precise estimates. While in the past, glycated hemoglobin in the target range was the main criterion for ascertaining acceptable metabolic control, most patients and clinicians want to achieve a maximum of readings in a safe zone, avoiding not only hyper- but also hypoglycemia. The exact value of the range of desired glucose levels is subject of an ongoing debate, but values in the range chosen here are of reasonable clinical importance and have been derived from recommendation by the American Diabetes Association for glycemic targets in the fasting range.16 The doses of insulin needed to stay in such a desired range obviously vary by time of day.
Basal rate insulin infusion profiles in our cohort of insulin pump-treated type 1 diabetic subjects are characterized by substantial diurnal variations corresponding to a higher insulin demand, progressively increasing between 01:00 and 07:00 am, and to a lesser degree, between 03:00 and 07:00 pm. These periods of the day correspond to those previously noted for the so-called “dawn”17-20 and “dusk”23,24 phenomena. The “dawn” phenomenon has mechanistically been explained by overnight surges of growth hormone secretion giving rise to transient insulin resistance, and subsequently, a higher insulin demand.17-20 Based on the frequency of plasma glucose concentrations determined to be either too low (or even frankly hypoglycemic) or too high (or even frankly hyperglycemic), our results indicate relatively high plasma glucose concentrations, and a rather low proportion of values below the fasting glucose target or in the range indicating hypoglycemia, during the period from 01:00 to 07:00 am, when the insulin infusion rates were highest (Table 2). We used the term dawn period for the time between 01.00 and 07:00 am, when there was a continuous rise in basal insulin needs. The “dawn” phenomenon describes a period of relative insulin resistance, which seems to apply to the time between 04:00 and 09:00 am in line with the previous descriptions.18-20 Thus, it appears reasonable to conclude that, for an unselected, mixed cohort of adult subjects with type 1 diabetes, we confirm that basal insulin infusions need to be higher within their diurnal profile during the “dawn” period. If anything, our data suggest that for obtaining an equal mean plasma glucose concentration, and equal proportions of plasma glucose concentrations below the target range, as is characteristic for the remainder of the 24-hour period or our arbitrarily chosen reference period, basal insulin infusion rates programmed for the period lasting from 01:00 to 07:00 am should be even higher than empirically derived in our patients. Additional studies and analyses will be necessary to address interindividual variations in insulin demands during this critical period of 24-hour insulin infusion profile.
The present study has limitations: It is based on a database analyzed in a retrospective manner, with some incomplete datasets. Based on standard operating procedures established at the Diabeteszentrum Bad Lauterberg, plasma glucose was measured only at the time points analyzed, while shorter intervals would be desirable in particular during the night. Unfortunately, this starting and finishing point of 24-hour fasting tests was at 06:00 pm, thus, potentially interfering with our analysis of the “dusk” phenomenon. Our patient selection certainly does not allow to extrapolate toward basal insulin infusion rates for children and adolescents with type 1 diabetes.3,9,25 Other subgroups may also have been under-represented in our cohort.
We used rather arbitrary definitions of plasma glucose target ranges that seemed appropriate for the fasting state in insulin-treated type 1 diabetic subjects. However, we used two definitions of the upper and lower thresholds of target ranges, and qualitatively found similar results with both, indicating a rather robust approach overall (Table 2).
In addition, the periods of the day that were of special interest (eg, because of different patterns of basal insulin infusion rates compared to the rest of the 24-hour period) followed the results obtained and have been arbitrarily defined post hoc, after inspecting the data.
We cannot provide data on the efficacy of modulating the programmed basal insulin infusion rate in response to knowledge gained during 24-hour fasting periods. However, the information gained through such tests would provide a rather rational basis for optimizing basal insulin infusion rates. We also have not compared 24-hour fasting periods with shorter periods of fasting (eg, optimizing basal insulin infusion rates based on overnight plasma glucose profiles or plasma glucose measurements determined when skipping individual meals7).
We cannot rule out that some of our results are explained by certain traditions (eg, the choice of initial basal insulin infusion profile when starting insulin pump treatment with the help of published recommendations, like those widely distributed by manufacturers of insulin pumps, originating from analyses of small numbers of pump-treated patients with type 1 diabetes published by R. Renner and his group.4,14
However, the strength of our study is the relatively large cohort of subjects with type 1 diabetes using insulin pumps, who were subjected to a 24-hour fasting period with the use of an exact, laboratory-based measurement of plasma glucose. We are aware that this may not easily be possible in other health care environments, where in-patient treatment for the optimization of glycemic control is not available or reimbursed.
In conclusion, based on the assessment of 339 adult type 1 diabetic subjects using insulin pumps and undergoing 24-hour fasting periods to scrutinize the appropriateness of their basal insulin infusion rates, we found that, for the whole population, insulin demands were higher in the early morning hours, corresponding to a “dawn” phenomenon, and that such 24-hour fasting tests detect a multitude of plasma glucose concentrations outside the fasting plasma glucose range requiring reprogramming of the basal insulin infusion rate. We suggest that supervised 24-hour fasting periods are a helpful tool to optimize glycemic control in such patients, especially considering interindividual differences in insulin needs and their diurnal profiles.
Supplemental Material
Supplemental material, InsulinPumpSupplement290719 for Twenty-Four Hour Fasting (Basal Rate) Tests to Achieve Custom-Tailored, Hour-by-Hour Basal Insulin Infusion Rates in Patients With Type 1 Diabetes Using Insulin Pumps (CSII) by Michael A. Nauck, Anna M. Lindmeyer, Chantal Mathieu and Juris J. Meier in Journal of Diabetes Science and Technology
Acknowledgments
We acknowledge the contribution of Heike Schulze, Sandra Tepelmann, Annette Varnhorn, and Martin Janert (all Diabeteszentrum Bad Lauterberg, Bad Lauterberg im Harz, Germany) to documenting all insulin pump treatments at this institution, and for helping retrieve patient data from hospital charts, and Melanie Kahle-Stephan (Diabetes center Bochum-Hattingen, Bochum, Germany) for generating the database on insulin-pump-treated subjects with type 1 diabetes.
Footnotes
Author Contributions: MAN and JJM designed the study; MAN and AML analyzed the data, performed the statistical analysis, and wrote the first draft of the manuscript. JJM and CM provided input into the analysis and into designing and writing the manuscript. All authors have seen an approved the final draft of this manuscript and have decided to submit it for publication. MAN is the guarantor who takes full responsibility for the work as a whole, including study design, access to data, and the decision to submit and publish the manuscript.
Declaration of Conflicts Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MAN has been member on advisory boards or has consulted with AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Fractyl, GlaxoSmithKline, Menarini/Berlin Chemie, Merck, Sharp & Dohme, and NovoNordisk. He has received grant support from AstraZeneca, Eli Lilly & Co., Menarini/Berlin-Chemie, Merck, Sharp & Dohme, Novartis Pharma and NovoNordisk. He has also served on the speakers’ bureau of AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Menarini/Berlin Chemie, Merck, Sharp & Dohme, NovoNordisk, and Sun Pharma. AML has no conflicts of interest to declare. CM serves or has served on advisory panels for Novo Nordisk, Sanofi, Merck Sharp and Dohme, Eli Lilly, Novartis, Bristol-Myers Squibb, AstraZeneca, Pfizer, Janssen Pharmaceuticals, Boehringer Ingelheim, Hanmi Pharmaceuticals, Roche Diagnostics, Medtronic, Mannkind, Intrexon, Dianax and UCB, and as a speaker for Novo Nordisk, Sanofi, Merck Sharp and Dohme, Eli Lilly, Boehringer Ingelheim, AstraZeneca and Novartis. Financial compensation for these activities has been received by KU Leuven. KU Leuven has received research support for Chantal Mathieu from Medtronic, Novo Nordisk, Sanofi, Merck Sharp and Dohme, Eli Lilly, Roche Diagnostics, Abbott, Intrexon and Novartis. JJM has received consulting and speaker honoraria from AstraZeneca, BristolMyersSquibb, Eli Lilly, Merck, Sharp & Dohme, Novo Nordisk and Sanofi. He has received research support from Eli Lilly, Boehringer-Ingelheim, Merck, Sharp & Dohme, Novo Nordisk, Novartis and Sanofi. SW has nothing to declare.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Michael A. Nauck  https://orcid.org/0000-0002-5749-6954
https://orcid.org/0000-0002-5749-6954
Access to Original Data: The authors are offering access to original data for research purposes upon reasonable request.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 2. Scheiner G, Boyer BA. Characteristics of basal insulin requirements by age and gender in Type-1 diabetes patients using insulin pump therapy. Diabetes Res Clin Pract. 2005;69(1):14-21. [DOI] [PubMed] [Google Scholar]
- 3. Danne T, Battelino T, Kordonouri O, et al. A cross-sectional international survey of continuous subcutaneous insulin infusion in 377 children and adolescents with type 1 diabetes mellitus from 10 countries. Pediatr Diabetes. 2005;6(4):193-198. [DOI] [PubMed] [Google Scholar]
- 4. Wizemann E, Renner R, Reitberger U, Hepp KD. Prospektive Evaluation einer standardisierten Basalratenverteilung für die CSII bei Typ 1 Diabetes über 6 Monate (abstract). Diabetologie Stoffw. 2001;10:57. [Google Scholar]
- 5. Pickup JC. Insulin-pump therapy for type 1 diabetes mellitus. N Engl J Med. 2012;366(17):1616-1624. [DOI] [PubMed] [Google Scholar]
- 6. Kuroda A, Kaneto H, Yasuda T, et al. Basal insulin requirement is ~30% of the total daily insulin dose in type 1 diabetic patients who use the insulin pump. Diabetes Care. 2011;34(5):1089-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Orszag A, Falappa CM, Lovblom LE, et al. Evaluation of a clinical tool to test and adjust the programmed overnight basal profiles for insulin pump therapy: a pilot study. Can J Diabetes. 2015;39(5):364-372. [DOI] [PubMed] [Google Scholar]
- 8. Wilmot EG, Choudhary P, Grant P, Hammond P. Insulin pump therapy: a practical guide to optimising glycaemic control. Practical Diabetes. 2014;31:121a-125a. [Google Scholar]
- 9. Strich D, Teomim R, Gillis D. The basal insulin dose; a lesson from prolonged fasting in young individuals with type 1 diabetes. Pediatr Diabetes. 2014;16(8):629-633. [DOI] [PubMed] [Google Scholar]
- 10. Mucha GT, Merkel S, Thomas W, Bantle JP. Fasting and insulin glargine in individuals with type 1 diabetes. Diabetes Care. 2004;27(5):1209-1210. [DOI] [PubMed] [Google Scholar]
- 11. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320. [DOI] [PubMed] [Google Scholar]
- 12. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Sensor-augmented pump therapy for A1C reduction (STAR 3) study: results from the 6-month continuation phase. Diabetes Care. 2011;34(11):2403-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hermanides J, Norgaard K, Bruttomesso D, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled Type 1 diabetes; a randomized controlled trial. Diabet Med. 2011;28(10):1158-1167. [DOI] [PubMed] [Google Scholar]
- 14. Lüddeke HJ, Renner R, Hepp KD. Ein Expertensystem zur Ermittlung optimaler Basalratenprofile und Bolusgaben für multiprogrammierbare Pumpen (abstract). Diabetes Stoffw. 1994;3:178-179. [Google Scholar]
- 15. Bell KJ, Barclay AW, Petocz P, Colagiuri S, Brand-Miller JC. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2014;2(2):133-140. [DOI] [PubMed] [Google Scholar]
- 16. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(suppl 1):S61-S70. [DOI] [PubMed] [Google Scholar]
- 17. Campbell PJ, Bolli GB, Cryer PE, Gerich JE. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus: accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N Engl J Med. 1985;312(23):1473-1479. [DOI] [PubMed] [Google Scholar]
- 18. De Feo P, Perriello G, Bolli GB. Somogyi and dawn phenomena: mechanisms. Diabetes Metab Rev. 1988;4(1):31-49. [DOI] [PubMed] [Google Scholar]
- 19. Perriello G, De Feo P, Torlone E, et al. The dawn phenomenon in type 1 (insulin-dependent) diabetes mellitus: magnitude, frequency, variability, and dependency on glucose counterregulation and insulin sensitivity. Diabetologia. 1991;34(1):21-28. [DOI] [PubMed] [Google Scholar]
- 20. Porcellati F, Lucidi P, Bolli GB, Fanelli CG. Thirty years of research on the dawn phenomenon: lessons to optimize blood glucose control in diabetes. Diabetes Care. 2013;36(12):3860-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hunger-Dathe W, Braun A, Müller UA, Schiel R, Femerling M, Risse A. Insulin pump therapy in patients with Type 1 diabetes mellitus: results of the Nationwide Quality Circle in Germany (ASD) 1999-2000. Exp Clin Endocrinol Diabetes. 2003;111(7):428-434. [DOI] [PubMed] [Google Scholar]
- 22. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA. 2013;310(12):1240-1247. [DOI] [PubMed] [Google Scholar]
- 23. Du S, Shi MJ, Sun ZZ, Li W. Clinical diagnosis for dusk phenomenon of diabetes. Medicine (Baltimore). 2018;97(34):e11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li W, Du SN, Shi MJ, Sun ZZ. Spontaneous and transient predinner hyperglycemia in some patients with diabetes: dusk phenomenon. Medicine (Baltimore). 2016;95(47):e5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia. 2013;56(11):2392-2400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, InsulinPumpSupplement290719 for Twenty-Four Hour Fasting (Basal Rate) Tests to Achieve Custom-Tailored, Hour-by-Hour Basal Insulin Infusion Rates in Patients With Type 1 Diabetes Using Insulin Pumps (CSII) by Michael A. Nauck, Anna M. Lindmeyer, Chantal Mathieu and Juris J. Meier in Journal of Diabetes Science and Technology