Abstract
The WNT signaling system plays an important but paradoxical role in the regulation of pluripotency. In the cow, IWR-1, which inhibits canonical WNT activation and has WNT-independent actions, promotes the derivation of primed pluripotent embryonic stem cells from the blastocyst. Here, we describe a series of experiments to determine whether derivation of embryonic stem cells could be generated by replacing IWR-1 with other inhibitors of WNT signaling. Results confirm the importance of inhibition of canonical WNT signaling for the establishment of pluripotent embryonic stem cells in cattle and indicate that the actions of IWR-1 can be mimicked by the WNT secretion inhibitor IWP2 but not by the tankyrase inhibitor XAV939 or WNT inhibitory protein dickkopf 1. The role of Janus kinase-mediated signaling pathways for the maintenance of pluripotency of embryonic stem cells was also evaluated. Maintenance of pluripotency of embryonic stem cells lines was blocked by a broad inhibitor of Janus kinase, even though the cells did not express phosphorylated signal transducer and activator of transcription 3 (pSTAT3). Further studies with blastocysts indicated that IWR-1 blocks the activation of pSTAT3. A likely explanation is that IWR-1 blocks differentiation of embryonic stem cells into a pSTAT3+ lineage. In conclusion, results presented here indicate the importance of inhibition of WNT signaling for the derivation of pluripotent bovine embryonic stem cells, the role of Janus kinase signaling for maintenance of pluripotency, and the participation of IWR-1 in the inhibition of activation of STAT3.
Keywords: embryonic stem cells, pluripotency, WNT, JAK/STAT, bovine
Derivation of pluripotent embryonic stem cells from bovine blastocysts depends upon blocking WNT signaling; maintenance of pluripotency depends upon JAK signaling in a STAT3-independent manner.
Introduction
Success in the derivation of pluripotent embryonic stem cells (ESCs) from embryos reflects the species-specific mechanisms for the self-renewal of pluripotent cells. Discovery of the 2i derivation method, which involves the inhibition of glycogen synthase kinase-3 (GSK3) and mitogen-activated protein kinase (MAPK) kinase, allowed for the derivation of ESCs in the naïve state of pluripotency from rodent embryos [1–3]. These conditions were not sufficient for the derivation of naïve human ESC (hESC), and additional inhibition of c-Jun N-terminal kinase (JNK) and p38 MAPK was required [4]. Use of the same inhibitors has not been successful for the derivation of putative ESCs from other species, such as cattle [5, 6], pigs [7], and zebrafish [8]. The resultant cells either displayed limited features of pluripotency or failed to survive after a few passages. Accordingly, preservation of the naïve state of the inner cell mass (ICM) has not been achieved in species other than rodents and humans.
Recently, stable pluripotent ESC lines were efficiently derived from bovine and porcine embryos under serum-free conditions [9, 10]. These cells could self-renew for over 40 passages, displayed a gene expression profile characteristic of the primed pluripotency state, and could be differentiated into cell types of each of the three germ layers. Pluripotent ESCs such as these provide models to understand the control of pluripotency but also have promise for realizing schemes for in vitro breeding and cellular agriculture [11, 12].
The WNT signaling system plays an important but paradoxical role in the regulation of pluripotency in ESCs. Activation of WNT signaling has been a requirement for the generation of naïve mouse ESC (mESC) and hESC that maintain ICM ground state under serum-free conditions [3, 4, 13]. Blocking WNT signals by the addition of the WNT secretion inhibitor IWP2 caused both mESC and hESC to shift from the naïve to the primed state [13, 14]. Furthermore, treatment with the WNT inhibitor XAV939 made naïve hESCs more responsive for differentiation signals [15]. WNT signaling also participates in the modulation of primed pluripotency. WNT agonists promoted hESCs’ self-renewal in the presence of feeder cell-conditioned medium and fibroblast growth factor 2 (FGF2) [16, 17] but reduced the expression of pluripotency markers when hESCs were cultured on feeder cells [18]. Addition of IWR-1, a canonical WNT antagonist, to the culture medium boosted the efficiency of derivation of mouse epiblast stem cells (mEpiSC, usually considered as primed pluripotent cells) [19–21]. Treatment with IWR-1 also allowed the development of ESCs with primed pluripotency in human, chimpanzee, cattle, and pig [9, 10, 21].
Simultaneous treatment with the WNT agonist CHIR99021 [an inhibitor of GSK3 that promotes destruction of β-catenin (CTNNB1)] and the WNT antagonist IWR-1 maintained the self-renewal of mEpiSC, hESC, and pig ESC [10, 22, 23]. WNT signaling is a complex process, with 19 ligands, 13 receptors, several signaling pathways, and a variety of proteins, such as dickkopf 1 (DKK1) and R-spondins, that modulate signaling [24]. One explanation for the synergistic actions of WNT agonists and antagonists is that specific components of WNT signaling promote pluripotency, whereas other components inhibit it. Alternatively, IWR-1 may affect multiple cellular pathways besides WNT signaling. IWR-1 was first identified as an inhibitor of the canonical, CTNNB1-mediated pathway for WNT signaling from an in vitro chemical screen of 200 000 compounds [25]. Reduction in CTNNB1 is achieved by the stabilization of AXIN2, one of the members of the CTNNB1 destruction complex [25]. IWR-1 can interact directly with AXIN2 [25] and presumably stabilizes AXIN2 protein conformation. However, IWR-1 is a tankyrase inhibitor and protects AXIN2 from ubiquitination and degradation caused by tankyrase-mediated poly-ADP-ribosylation (PARylation) [26]. Both IWR-1 and another tankyrase inhibitor, XAV939, have been broadly used to maintain ESCs at primed, naïve, and extended pluripotency states in various species [9, 10, 22, 27–30]. These actions may involve WNT-independent effects because tankyrases can cause post-translational modifications to a diverse array of molecules, including components of the HIPPO and NOTCH signaling pathways, and can modulate autophagy, RNA biogenesis, telomere length, cell metabolism, cell proliferation, and survival through PARylation-dependent and -independent mechanisms [31].
As mentioned previously, derivation of stable primed ESCs from bovine blastocysts can be routinely achieved using a culture system based on the presence of IWR-1 [9]. Here, it was tested whether primed bovine embryonic stem cells (bESCs) could be generated by replacing IWR-1 with other inhibitors of WNT signaling, including XAV939 [22, 26], dickkopf WNT signaling pathway inhibitor 1 (DKK1) [32–34], and IWP2 [25]. Maintenance of expression of the pluripotency factor, SRY-box transcription factor 2 (SOX2), was used as a marker of pluripotency. SOX2 was chosen rather than other core pluripotency factors, POU class 5 homeobox 1 (POU5F1) (also known as OCT4) and NANOG homeobox (NANOG), because POU5F1 is not exclusively limited to the ICM of bovine blastocysts [35–37] and NANOG is not highly transcribed in the pluripotent bovine ESCs [9]. Another objective was to determine whether, as in the mouse [38], Janus kinase (JAK)-mediated signaling pathways are important for the maintenance of the pluripotency of ESCs, and if so, whether the regulation of JAK signal transducer and activator of transcription (STAT) signaling is involved in the actions of IWR-1 to promote the generation of primed bESCs. Results confirm the importance of inhibition of WNT signaling for the establishment of pluripotent ESCs in cattle and indicate that the actions of IWR-1 can be mimicked by the WNT secretion inhibitor IWP2 but not by the tankyrase inhibitor XAV939 or WNT inhibitory protein DKK1. Data also indicate a role for JAK in the maintenance of pluripotency and a previously unknown role for IWR-1 in the inhibition of signal transducer and activator of transcription 3 (STAT3) phosphorylation.
Materials and methods
Materials
Details and references for the chemicals and growth factors used in the experiments are listed in Supplementary Table S1. Rabbit immunoglobulin G (IgG) monoclonal anti-human SOX2 (clone EP103) was from Biogenex (Fremont, CA, USA), rabbit IgG polyclonal anti-human CTNNB1 (clone E247) was from Abcam (Cambridge, MA, USA), and rabbit monoclonal anti-human phospho-STAT3 (Tyr705) (clone D3A7) was from Cell Signaling Technology (Danvers, MA, USA). Secondary antibodies (all cross-absorbed) were purchased from Thermo Fisher and were goat polyclonal IgG anti-rabbit IgG (H + L) coupled to Alexa Fluor 488 (catalog number A-11008), goat polyclonal IgG anti-mouse IgG (H + L) coupled to Alexa Fluor 488 (catalog number A-11001), goat polyclonal IgG anti-rabbit IgG (H + L) coupled to Alexa Fluor555 (catalog number A-21428), and goat polyclonal IgG anti-mouse IgG (H + L) coupled to Alexa Fluor 647 (catalog number A-21236).
Other chemicals were purchased from Thermo Fisher Scientific or Sigma-Aldrich unless otherwise stated.
Embryo production
Bovine embryos were produced in vitro as described in detail elsewhere [39]. In brief, cumulus–oocyte complexes were recovered from bovine ovaries (Bos taurus, Bos indicus or admixtures of the two genotypes) collected from an abattoir, matured for 22 h in maturation medium either supplied by IVF Bioscience (Falmouth, UK) or produced in the laboratory as Tissue Culture Medium 199 containing 10% (v/v) fetal bovine serum, 0.2 mM sodium pyruvate, 1% (v/v) alanyl-glutamine (GlutaMAX; Thermo Fisher), 50 ng/ml epidermal growth factor, 5 μg/ml follicle stimulating hormone, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. Fertilization was carried out with spermatozoa pooled from three bulls in a medium termed in vitro fertilization-Tyrode’s albumin lactate pyruvate (TALP) for 16–18 h. The initiation of fertilization was considered as day 0. Putative zygotes (i.e., oocytes exposed to sperm) were subjected to cumulus cell removal by hyaluronidase digestion and then cultured in groups of up to 30 in 50 μl microdrops of synthetic oviduct fluid bovine embryo 2 medium covered with mineral oil at 38.5°C in an atmosphere of 5% (v/v) oxygen and 5% CO2 in a humidified atmosphere until day 7.5.
Derivation and maintenance of ESCs
Procedures followed the protocol for the establishment of stable primed pluripotent bovine ESC lines [9]. The basal medium was a custom mTeSR1 medium without transforming growth factor-β that has been used for hESC and mEpiSC culture [21, 40]. Blastocysts of all stages except for early blastocysts (including non-expanded, expanded, hatching, and hatched) were harvested from culture drops at day 7.5 of development, rinsed in HEPES-TALP medium [39] four times, and subjected to zona pellucida removal by dissection with two 30-Ga needles in HEPES-TALP medium under a stereomicroscope. For each experiment, blastocysts were randomly assigned to treatment after blocking by the blastocyst stage. Each zona pellucida-free blastocyst was individually transferred into a well that was plated 16~36 h earlier with irradiated CF1 mouse embryonic fibroblasts (MEFs) (Thermo Fisher) in Nunc four-well dishes (Thermo Fisher). The feeder cells were cultured in Dulbecco Modified Eagle Medium supplemented with 10% (v/v) fetal bovine serum, 1% (v/v) alanyl-glutamine, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. On the day of seeding the blastocysts, the feeder cells were rinsed with Dulbecco phosphate-buffered saline (DPBS), and the medium was replaced with the ESC culture medium prior to blastocyst seeding. The standard ESC culture medium was the custom mTeSR1 medium supplemented with 20 ng/ml recombinant human FGF2, 2.5 μM IWR-1, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. This medium was modified for some treatments as described for each experiment. For all treatments, a Rho-associated coiled-coil containing protein kinase (ROCK) inhibitor Y-27632 (10 μM) was added to the ESC culture medium on the day of blastocyst seeding and was replaced by medium without Y-27632 the day after. The ROCK inhibitor was also added for 24 h each time the cells were passaged. At 24 h after seeding, blastocysts that had not attached to the bottom of the well were manually assisted to attach by positioning them using a 30-gauge needle. Culture medium was refreshed each day. On day 7 after blastocyst seeding, cells were passaged onto 24-well plates with new feeder cells that were prepared a day in advance. All cultures were passaged regardless of the observed emergence of outgrowth from the blastocyst. A second passage was performed 7 days later. Subsequently, passages were performed every 3–6 days depending on the degree of confluency. For cell passage, wells were rinsed with calcium- and magnesium-free DPBS, treated with 1X TrypLE Select Enzyme (Thermo Fisher) for 2–5 min in a cell culture incubator followed by centrifugation and resuspension into single cells for plating.
Immunolabeling
Procedures for immunolabeling of blastocysts were performed as described elsewhere [41]. Blastocysts were harvested at day 7.5, rinsed in DPBS containing 0.2% (w/v) polyvinyl alcohol (PVA), and then fixed in DPBS containing 4% (w/v) paraformaldehyde at room temperature for 15 min. For pSTAT3 labeling only, blastocysts were permeabilized in ice-cold methanol for 10 min at −20°C and then rinsed in DPBS between the fixation and blocking steps. Blastocysts were then permeabilized for 30 min in 0.25% (v/v) Triton X-100 in DPBS-PVA, incubated in a blocking buffer consisting of DPBS containing 5% (w/v) bovine serum albumin (BSA) for 1 h at room temperature. Primary antibody incubation was performed at 4°C overnight. Primary antibodies used were rabbit IgG monoclonal anti-human SOX2 diluted at 1:300 (v/v), 1 μg/ml rabbit IgG polyclonal anti-human CTNNB1, and rabbit monoclonal anti-human phospho-STAT3 (Tyr705) diluted at 1:100 (v/v). After washing, blastocysts were incubated with a solution of 2 μg/ml Alexa-labeled secondary antibody and Hoechst 33343 (10 μg/ml). Both primary and secondary antibodies were diluted in blocking buffer except for the labeling for pSTAT3 in which case the buffer was DPBS containing 1% (w/v) BSA and 0.3% (v/v) Triton-X 100. Following washing, blastocysts were mounted on glass slides with a coverslip using anti-fade medium (Thermo Fisher).
Immunolabeling of cells was carried out on cells that were seeded onto a Nunc Lab-Tek II Chamber Slide (Thermo Fisher) that was plated with feeder cells a day before. Steps for immunocytochemistry were followed as previously reported [9]. For pSTAT3 labeling only, cells were permeabilized in ice-cold methanol for 10 min at −20°C and then rinsed in DPBS after fixation. Cells were fixed in 4% (w/v) paraformaldehyde in DPBS for 15 min and incubated in DPBS containing 3% (v/v) normal goat serum and 0.3% (v/v) Triton X-100 for 1 h. Primary antibody incubation was for 2 h at room temperature and secondary antibody was incubated for 1 h. Primary and secondary antibodies were used at the concentrations described for blastocysts. Hoechst 33342 was added at 10 μg/ml during the 1 h secondary antibody incubation. The buffer for dilution of all antibodies was DPBS containing 1% (v/v) normal goat serum and 0.3% (v/v) Triton X-100. Cells were covered with a glass coverslip using anti-fade medium after rinsing in DPBS.
Images of immunolabeled blastocysts and cells were obtained using either an epifluorescent microscope (Axioplan2 imaging, Zeiss, White Plains, NY, USA) with AxioVision software (Ver. 4.7.1) or with a spinning disk (Andor DSD2, Oxford Instruments, Tubney Woods, Abingdon, UK) confocal microscope (Axioobserver.Z1, Zeiss) controlled by Andor IQ3 software (Oxford Instruments). The epifluorescent microscope was equipped with Plan Neo-fluor 20X/0.5 and 40X/0.75 lens and a CCD camera (AxioCam MR, Zeiss). The confocal microscope was equipped with a Plan-APOCHROMAT 20X/0.8 objective (Zeiss) and an Andor Zyla sCMOS camera (Oxford Instruments). Z-stack images were taken at 1-μm intervals. Uniform exposure times, light intensities, and gains were used to observe the samples for an individual experiment.
Image analysis
ImageJ software (Ver. 1.52a, Wayne Rasband, NIH, Bethesda, MD, USA) was used for image analysis. Cell counting was achieved by the use of the multi-point tool and manual counting. Quantification of intensity of labeling for SOX2 in confocal images of blastocysts was performed with maximum projections generated from z-stack images of the SOX2 and Hoechst channels. The area corresponding to the blastocyst was selected using the freehand tool, and the threshold feature for the Hoechst channel was used to select nuclei only. The selected areas were then applied to the images for the SOX2 channels to measure the fluorescent intensity of the nuclei. Intensity was corrected by subtracting the local background signals.
Experimental design and statistical analysis
A summary of the design of each experiment is included in the Results section. Statistical analysis of data was performed using the Statistical Analysis System (Ver. 9.4, SAS Institute, Cary, NC). Data on blastocyst characteristics were analyzed by analysis of variance using PROC GLM with treatment and replicate as fixed effects in the model. Effects of blastocyst stage on the efficiency of ESC derivation were determined by logistic regression using the GLIMMIX procedure of SAS and with the distribution set to binary.
Results
The canonical WNT inhibitor DKK1 was not able to replace IWR-1 for establishment of bovine ESCs
One function of DKK1 is to block canonical WNT signaling by internalizing the Frizzled co-receptors LDL receptor-related protein 5 (LRP5) and LRP6 [32–34]. Accordingly, it was hypothesized that IWR-1 could be replaced by DKK1 for the derivation of ESCs. Cell lines were derived from bovine blastocysts by the culture on feeder cells in the presence of 100 ng/ml DKK1, 2.5 μM IWR-1 (positive control), or vehicle (negative control) (Figure 1A). The concentration of DKK1 used has been shown to inhibit the actions of a WNT agonist on embryonic development and to reduce immunoreactive CTNNB1 in the blastocyst [42, 43]. Cell lines that displayed robust growth after 5~6 passages could be derived from the embryos in DKK1 and vehicle conditions with similar derivation efficiencies as for IWR-1-treated cells (Figure 1B). Island-like cell colonies with defined borders were observed in all treatments at passages 1 and 2. Subsequently, however, cells treated with DKK1 or vehicle spread over the plate and the boundaries defining specific colonies were lost after several more passages (Figure 1C). Expression of the pluripotency marker SOX2 was assessed as an indication of pluripotency. Cells treated with DKK1 or vehicle failed to maintain SOX2 expression (Figure 1D), although cells treated with IWR-1 were positive for SOX2. Interestingly, CTNNB1 was localized at the plasma membrane of IWR-1-treated cells (Figure 1E), which closely resembles the pattern for mESC and hESC [17, 44]. Cells treated with DKK1 or vehicle had much lower abundance of CTNNB1 (Figure 1E). Collectively, results indicate that replacement of IWR-1 with DKK1 was not able to maintain bovine pluripotency in vitro.
Figure 1 .

The WNT inhibitor DKK1 was not able to replace IWR-1 for the establishment of bovine ESC. (A) Experimental design. Zona-free blastocysts were seeded onto MEF with the culture medium consisting of base medium mTeSR, FGF2 and with additional treatments of 2.5 μM IWR-1, 100 ng/ml recombinant human DKK1, or vehicle. Outgrowths were passaged on day 7 and the subculture was performed at least five times before the immunolabeling of cells grown in glass slide chambers. The total number of blastocysts analyzed is given in (B). Blastocysts were produced in four embryo production replicates. (B) Efficiency of derivation of cell lines. There was no significant difference among treatments. Subsequent observations were carried out on the number of cell lines indicated. (C) Morphological characteristics of cells at passages 5~6 on week 5. (D, E) Immunolabeling for SOX2 and CTNNB1 after 5–6 weeks of treatment. Scale bar = 100 μm.
Derivation of bESCs could not be achieved by treatment with the tankyrase inhibitor XAV939 but could be with IWP2, an inhibitor of WNT secretion
It was next tested whether IWR-1 could be replaced with XAV939, which like IWR-1, can attenuate the tankyrase activity and stabilize AXIN to interrupt WNT signaling mediated by CTNNB1 [22, 26]. An additional question was whether IWP2, which blocks WNT secretion and thereby abrogates canonical and non-canonical WNT signaling from the endogenous sources of WNT [25], would allow derivation of cells with pluripotent features.
The design of the experiment involved derivation of ESC lines from blastocysts on feeder cells with either 2.5 μM IWR-1 (positive control), 2 μM XAV939, or 2 μM IWP2 (Figure 2A). The efficiency of cell line derivation was highest for IWR-1 and similar for XAV939 and IWP2 (Figure 2B). After four passages, cells derived and maintained in the presence of XAV939 grew out of colonies and spread into the space between colonies (Figure 2C). These cells were also negative for immunoreactive SOX2 and CTNNB1 for 9 of 11 cell lines (Figure 2D and E). For the other two cell lines, SOX2 expression was lost in a subset of cells at the endpoint of the experiment. The differentiation of XAV939-treated cells suggested that IWR-1 could not be replaced by another tankyrase inhibitor XAV939.
Figure 2 .

Derivation of pluripotent bESC could not be achieved by treatment with the WNT and tankyrase inhibitor XAV939 but could by with an inhibitor of WNT secretion. (A) Experimental design. Zona-free blastocysts were seeded onto MEF cells with the culture medium consisting of base medium mTeSR, FGF2 and with additional treatments of 2.5 μM IWR-1, 2.0 μM XAV939, or 2.0 μM IWP2. Outgrowths were passaged on day 7 and the subculture was performed at least five times before the immunolabeling of cells grown in glass slide chambers. The total number of blastocysts analyzed is given in (B). Blastocysts were produced in two embryo production replicates. (B) Efficiency of derivation of cell lines. There was no significant difference among treatments. Subsequent observations were carried out on the number of cell lines indicated. (C) Morphological characteristics of cells at passages 5~6 on week 5. (D, E) Immunolabeling for SOX2 and CTNNB1 after 5–6 weeks of treatment. Scale bar = 100 μm.
In contrast to the results with XAV939, treatment with IWP2 resulted in the derivation of cells that were similar in key aspects to those derived from the culture with IWR-1. Cells cultured with IWP2 showed clear boundaries between colonies and feeder cells (Figure 2C). Cells treated with IWP2 appeared to be more flattened than cells from IWR-1-treated cultures (Figure 2C). Labeling for immunoreactive SOX2 and CTNNB1 resembled that for IWR-1 for 6 of 8 of the cell lines derived with IWP2 (Figure 2D and E). A minority of cells in one IWP2 line eventually lost SOX2 expression. These results indicate that suppressing endogenous WNT production could preserve some pluripotency features during ESC derivation.
Maintenance of pluripotency in established ESCs depends on a JAK-dependent mechanism, but IWR-1 suppresses STAT3 phosphorylation
Besides regulating the transcription of WNT target genes, CTNNB1 is a component of the cadherin–catenin complex that can promote the pluripotency of ESCs through modulation of cell–cell adhesion complexes in a JAK-/STAT3-dependent mechanism [45]. Given that IWR-1 causes accumulation of membrane-associated CTNNB1 (Figures 1E and 2E), it is possible that the pluripotency-promoting actions of IWR-1 involve the activation of JAK/STAT3 signaling. Accordingly, it was tested whether the inhibition of JAK/STAT signaling blocked the ability of IWR-1 to maintain ESCs in a pluripotent state.
The design of the experiment involved treatment of three established bovine ESC lines with 2.5 μM IWR-1, 2.5 μM IWR-1, and JAK inhibitor I (JAKi), or vehicle for 7 weeks (Figure 3A). The JAKi was used at 2 μM because cells degenerated at higher concentrations (results not shown). Culture of ESCs with 2 μM JAKi slowed cell growth (data not shown) and changed the morphology of ESCs to an appearance similar to that of cells cultured with vehicle (Figure 3B). Labeling for SOX2, which was abundant for IWR-1 treated cells, was absent or low for cells treated with IWR-1 + JAKi and for cells treated with vehicle (Figure 3C). Thus, JAK signaling was required for the maintenance of pluripotency in the presence of IWR-1. Surprisingly, IWR-1-treated cells were negative for pSTAT3, while some cells treated with vehicle exhibited a moderate pSTAT3 signal within the nuclear region (Figure 3D). The pSTAT3+ cells were unlikely MEF because their homogenic nuclear counterstaining (Figure 3D) was different from that of MEF [46, 47]. Thus, phosphorylation of STAT3 was blocked in ESCs cultured with IWR-1, and the withdrawal of IWR-1 led to differentiation into lineages with activated STAT3. Results are consistent with the primed pluripotent state of bovine ESCs [9] because primed ESCs are not responsive to stimulation with JAK/STAT [48].
Figure 3 .

IWR-1 suppresses STAT3 phosphorylation in established ESC. (A) Experimental design. A total of three bESC cell lines were each treated with 2.5 μM IWR, 2.5 μM IWR + 2 μM JAK inhibitor I or vehicle. Treatment was initiated at either passage 5 (cell line V2-1), 12 (S8W1), or 14 (S9W4). Cells were grown in glass slide chambers with MEF for immunolabeling. (B) Morphological characteristics of cells after 6 weeks of treatment. (C, D) Immunolabeling for SOX2 and pSTAT3Tyr705 at week 7 of culture. The arrow and arrowhead denote a pSTAT3+ and negative cell, respectively. Scale bars = 100 μm in B and C and 50 μm in D.
One interpretation of the above results was that IWR-1 had direct effects on the phosphorylation of STAT3. This idea was tested by treating bovine embryos with IWR-1 from day 4 to day 7.5 post-fertilization. Treatment with 2.5 μM IWR-1 decreased the number of pSTAT3+ cells in the resultant blastocysts by 37% (18.4 ± 2.2 vs. 11.5 ± 1.6, P = 0.0159), the percent of cells in the blastocyst that were pSTAT3+ by 42% (19.4 ± 2.3 vs. 11.3 ± 1.7, P = 0.0062), and the pSTAT3 fluorescent intensity by 30% (518.4 ± 51.3 vs. 362.9 ± 37.6, P = 0.0176) without affecting the total cell number of the embryos (Figure 4). Thus, IWR-1 can inhibit the phosphorylation of STAT3.
Figure 4 .
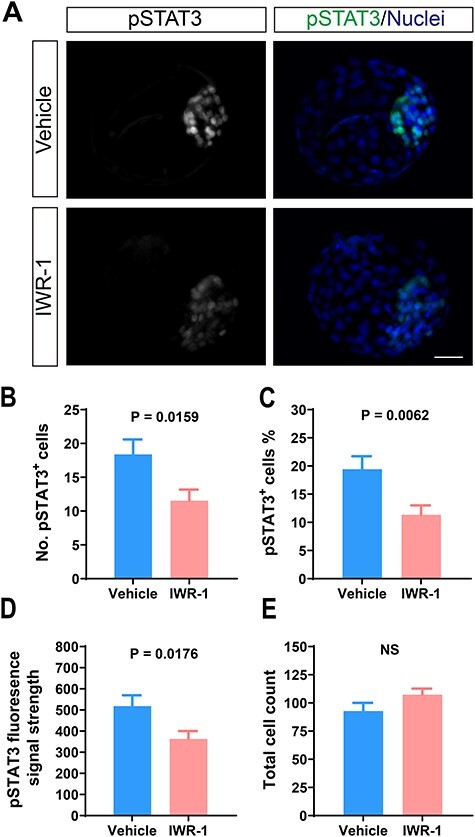
Treatment of bovine embryos with 2.5 μM IWR-1 from days 4–7.5 after fertilization reduced pSTAT3+ cell population in bovine blastocysts. (A) Immunolabeling of pSTAT3Tyr705. Scale bar = 50 μm. (B–E) Quantification of number of pSTAT3+ cells (B), percent of cells that were pSTAT3+ (C), pSTAT3Tyr705 immunofluorescent intensity (D), and total cell number (E). The number of observations was n = 39 for IWR-1 and n = 21 for vehicle from four embryo production replicates. Levels of significance are indicated in each graph. NS = non-significant.
Effect of stage of blastocyst on derivation of ESC
Using data from all the above-mentioned experiments, it was evaluated whether the morphological characteristics of the blastocyst affected the efficiency of ESC derivation under standard conditions (with IWR-1, but without experimental treatments). A greater proportion of expanded blastocysts (P < 0.05) yielded ESC (63%, n = 54) than non-expanded blastocysts (47%, n = 15) or blastocysts that were hatching or hatched (33%, n = 18).
Discussion
A primary focus of this research was to evaluate the role of WNT signaling in the derivation of primed pluripotent ESC in cattle. A second objective was to assess the role of JAK/STAT signaling in the maintenance of pluripotency. Results support the idea that canonical WNT signaling is antagonistic to pluripotency under the conditions used and that derivation of pluripotent ESC in the cow requires inhibition of WNT signaling. Nonetheless, not all WNT inhibitors allow the derivation of pluripotent ESC, with IWR-1 and IWP2 being effective and XAV939 and DKK1 being ineffective. Results also indicate that the activation of JAK-mediated pathways is also important for the derivation of ESC. IWR-1 showed inhibitory effects on STAT3 activation so that actions of JAK to maintain pluripotency are STAT3-independent.
The culture system in which ESCs were generated contains a variety of WNT signaling ligands. Canonical and non-canonical WNT ligands are transcribed by ICM and TE of bovine blastocysts [49], and the MEFs used as feeder cells also express multiple WNT genes [17]. The earlier finding that the addition of the WNT antagonist IWR-1 was required for the generation of primed pluripotent ESC from the cow [9] is suggestive that the inhibition of canonical WNT signaling is a requirement for the derivation of ESC in the cow. The importance of IWR-1 for ESC derivation was confirmed in the present experiments. It was also shown that IWR-1 is required for the maintenance of pluripotency in established ESC lines. These findings by themselves are not conclusive about the role of WNT signaling in pluripotency of embryonic cells, however. IWR-1 inhibits canonical WNT signaling by stabilizing AXIN2 of the CTNNB1 destruction complex through its actions as a tankyrase inhibitor [26]. Tankyrases can exert many effects on cells by catalyzing PARylation of proteins and have been implicated in the regulation of a large number of cellular functions [31].
The approach taken to address the question of whether it was the WNT-inhibitory or WNT-independent actions of IWR-1 that allow the establishment of ESC was to test whether IWR-1 could be replaced with other WNT inhibitors. Neither DKK1, which inhibits WNT signaling and the concomitant accumulation of CTNNB1 by blocking the recruitment of the co-receptor LRP5/6 to the WNT receptor Frizzled [33, 49], nor the tankyrase inhibitor XAV939, which also leads to the degradation of CTNNB1 [26] and can promote the derivation of pluripotent cells in other species [20, 21, 50], could be used in place of IWR-1. By contrast, ESCs that were largely similar in characteristics to those that developed in IWR-1-treated cultures could be derived in the presence of the WNT secretion inhibitor IWP2. IWP2 attenuates the secretion of both canonical and non-canonical WNT by blocking palmitoylation [25], which is important for preventing the differentiation of mEpiSC and hESC [51, 52].
The most likely interpretation of findings is that the inhibition of WNT signaling is required for the derivation of ESC. This can be achieved by IWR-1 and IWP2 but not by XAV939 and DKK1 either because they do not inhibit specific WNT signaling important for differentiation or they have additional actions that are inhibitory to the ESC derivation. In fact, DKK1 and XAV939 can affect cell function in a canonical WNT-independent manner. DKK1 can either activate or inhibit the non-canonical planar cell polarity pathway of WNT signaling [53, 54] and can inhibit Ca++-dependent WNT signaling [54]. In addition, DKK1 regulates the proliferation of cancer cells by binding to the cell surface cytoskeleton-associated protein 4 [55] and blocks apoptosis in the developing neural plate by binding and inactivating its receptor Kremen1 [56]. XAV939 is more potent than IWR-1, acts on different parts of the tankyrase molecule, and has inhibitory activity toward a broader range of poly(ADP-ribose) polymerases than IWR-1 [26, 57–59].
It was surprising that XAV939 did not replace IWR-1 for the derivation of bovine ESC. Both IWR-1 and XAV939 have been used for the derivation of mEpiSC [20, 21, 50] and showed nearly identical effects in other processes such as somatic cell reprogramming [60] and cardiomyocyte differentiation [61, 62]. Perhaps pathways that are regulated by XAV939 and IWR-1 have different actions on pluripotency in cattle than in other species. It could also be possible that 2 μM XAV939 was not sufficient to maintain bovine pluripotency, although the same or lower concentration of XAV939 was used previously in mouse, human, and bovine cells [22, 26, 63]. There are also other studies that used higher concentrations during the derivation of mEpiSC [20, 21, 50].
Although IWP2 allowed the derivation of bESC with nuclear localization of SOX2, it is possible that the WNT secretion inhibitor does not completely recapitulate the phenotype of bESC caused by being cultured with IWR-1. In particular, two of eight cell lines produced with IWP2 exhibited labeling patterns for immunoreactive SOX2 and CTNNB1 which were distinct from that of IWR-1, and one cell line derived with IWP2 experienced partial loss of SOX2 expression. It may be, therefore, that IWR-1 exerts actions important for maintaining pluripotency independent of WNT signaling.
The current experiment demonstrated that, like naïve mESC and hESC [4, 38, 64] but different from mEpiSC and primed hESC [4, 65–67], JAK-mediated signaling pathways are important for the maintenance of pluripotency of bovine ESC. In particular, treatment of established ESC lines with a JAK inhibitor caused the cells to differentiate as evaluated by the morphological appearance and loss of SOX2 labeling. In experiments where JAK was not required for the maintenance of pluripotency [4, 65–67], JAKi treatments were applied for 1–2 weeks, whereas JAKi treatment in the present study was prolonged for at least 1 month.
The JAK inhibitor used in the current experiment and that of Ozawa et al. [38] can block JAK1, JAK2, JAK3, and tyrosine kinase 2 with high affinity [68], and therefore, the specific JAK involved in pluripotency is not known. The most likely activator of JAK in the culture system was FGF2 because this growth factor signals via the JAK/STAT signaling pathway [69, 70]. Similarly, addition of JAK inhibitor reduced the expression of pluripotency markers by mESC cultured with FGF2 in the absence of IWR-1 [38]. The involvement of JAK in the maintenance of pluripotency is presumably independent of STAT3 because nuclear pSTAT3 was not detected in the ESC lines. Inhibition of STAT3 phosphorylation is likely a result of actions of IWR-1 because labeling for pSTAT3 was more extensive for established ESC cultured without IWR-1 and because treatment of the preimplantation embryo with IWR-1 reduced pSTAT3 labeling in the blastocyst. Signaling by FGFs can involve the activation of STAT1 as well as STAT3 [71, 72], and it is possible that JAK acts to promote pluripotency in bESCs through STAT1. Alternatively, JAK functions through a pathway independent of STATs as shown for other cell types [73].
The IWR-1/FGF2 system used to produce bESCs in this study results in cells with characteristics of the primed pluripotent state [9]. It is possible that the achievement of a naïve state requires some activation of WNT pathways, at least based on studies in the mouse and human [3, 4, 13]. In the presence of FGF2, addition of a WNT agonist allowed the establishment of pluripotent stem cells in the mouse, human, and horse which were at an intermediate state close to the naïve state and were germ-line competent [67, 74]. Activation of WNT signaling in the presence of FGF2 was successful in inducing ESCs in the buffalo [75] but not in the bovine [76], so species differences in requirements for pluripotency exist. Further work is warranted in studying the actions of WNT signaling in the bESCs in the absence of FGF2 signaling.
In conclusion, results presented here indicate the importance of WNT signaling for the differentiation of the bovine embryo and the requirement for inhibition of that pathway for derivation of bESC. In addition, JAK signaling is important for the maintenance of pluripotency, and IWR-1 can interact with that signaling pathway by suppressing the differentiation of ESC into a pSTAT3+ lineage.
Supplementary Material
Footnotes
† Grant Support: Research was supported by National Institutes of Health grant R01 HD088352, grant No. 2017-67015-26452 from the United States Department of Agriculture National Institute of Food and Agriculture, and by the L.E. “Red” Larson Endowment to PJH and United States Department of Agriculture multistate project W-4171 to PJR. The Salk Stem Cell Core Facility received philanthropic support from the Helmsley Charitable Trust.
Contributor Information
Yao Xiao, Department of Animal Sciences, Donald Henry Barron Reproductive and Perinatal Biology Research Program, and Genetics Institute, University of Florida, Gainesville, FL, USA.
Thiago F Amaral, Department of Animal Sciences, Donald Henry Barron Reproductive and Perinatal Biology Research Program, and Genetics Institute, University of Florida, Gainesville, FL, USA.
Pablo J Ross, Department of Animal Science, University of California, Davis, CA, USA.
Delia A Soto, Department of Animal Science, University of California, Davis, CA, USA.
Kenneth E Diffenderfer, Stem Cell Core, Salk Institute for Biological Studies, La Jolla, CA, USA.
Aimee R Pankonin, Stem Cell Core, Salk Institute for Biological Studies, La Jolla, CA, USA.
Surawich Jeensuk, Department of Animal Sciences, Donald Henry Barron Reproductive and Perinatal Biology Research Program, and Genetics Institute, University of Florida, Gainesville, FL, USA; Department of Livestock Development, Bureau of Biotechnology in Livestock Production, Pathum Thani, Thailand.
Paula Tríbulo, Department of Animal Sciences, Donald Henry Barron Reproductive and Perinatal Biology Research Program, and Genetics Institute, University of Florida, Gainesville, FL, USA.
Peter J Hansen, Department of Animal Sciences, Donald Henry Barron Reproductive and Perinatal Biology Research Program, and Genetics Institute, University of Florida, Gainesville, FL, USA.
Data availability
Data are available upon request.
Authors’ contributions
Y. X. and P.J.H. designed the research; Y.X., T.F.A., and P.T. performed the experiments; Y.X. and P.J.H. analyzed the results; Y.X., P.J.R., D.A.S., and P.J.H. interpreted the results; Y.X. and P.J.H. wrote the paper; and K.E.D. and A.R.P. prepared the medium for derivation of ESCs.
Acknowledgments
Authors thank Eddie Cummings and Elizabeth Jannaman for technical assistance.
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1. Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying QL, Smith A.. Capture of authentic embryonic stem cells from rat blastocysts. Cell 2008; 135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 2. Mulas C, Kalkan T, Meyenn F, Leitch HG, Nichols J, Smith A. Defined conditions for propagation and manipulation of mouse embryonic stem cells. Development 2019; 146:dev173146. doi: 10.1242/dev.173146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A.. The ground state of embryonic stem cell self-renewal. Nature 2008; 453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, Rais Y, Shipony Z et al. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013; 504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 5. Furusawa T, Ohkoshi K, Kimura K, Matsuyama S, Akagi S, Kaneda M, Ikeda M, Hosoe M, Kizaki K, Tokunaga T.. Characteristics of bovine inner cell mass-derived cell lines and their fate in chimeric conceptuses. Biol Reprod 2013; 89:28. doi: 10.1095/biolreprod.112.106641. [DOI] [PubMed] [Google Scholar]
- 6. Ozawa M, Sakatani M, Hankowski KE, Terada N, Dobbs KB, Hansen PJ. Importance of culture conditions during the morula-to-blastocyst period on capacity of inner cell-mass cells of bovine blastocysts for establishment of self-renewing pluripotent cells. Theriogenology 2012; 78:1243–1251. doi: 10.1016/j.theriogenology.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 7. Haraguchi S, Kikuchi K, Nakai M, Tokunaga T. Establishment of self-renewing porcine embryonic stem cell-like cells by signal inhibition. J Reprod Dev 2012; 58:707–716. doi: 10.1262/jrd.2012-008. [DOI] [PubMed] [Google Scholar]
- 8. Robles V, Marti M, Izpisua Belmonte JC. Study of pluripotency markers in zebrafish embryos and transient embryonic stem cell cultures. Zebrafish 2011; 8:57–63. doi: 10.1089/zeb.2010.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bogliotti YS, Wu J, Vilarino Met al. Efficient derivation of stable primed pluripotent embryonic stem cells from bovine blastocysts. Proc Natl Acad Sci U S A 2018; 115:2090–2095. doi: 10.1089/zeb.2010.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi KH, Lee DK, Kim SW, Woo SH, Kim DY, Lee CK. Chemically defined media can maintain pig pluripotency network in vitro. Stem Cell Rep 2019; 13:221–234. doi: 10.1016/j.stemcr.2019.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goszczynski DE, Cheng H, Demyda-Peyras S, Medrano JF, Wu J, Ross PJ. In vitro breeding: application of embryonic stem cells to animal production. Biol Reprod 2019; 100:885–895. doi: 10.1093/biolre/ioy256. [DOI] [PubMed] [Google Scholar]
- 12. Navarro M, Soto DA, Pinzon CA, Wu J, Ross PJ. Livestock pluripotency is finally captured in vitro. Reprod Fertil Dev 2019; 32:11–39. doi: 10.1071/RD19272. [DOI] [PubMed] [Google Scholar]
- 13. ten Berge D, Kurek D, Blauwkamp T, Koole W, Maas A, Eroglu E, Siu RK, Nusse R.. Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat Cell Biol 2011; 13:1070–1075. doi: 10.1038/ncb2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu Z, Robitaille AM, Berndt JD, Davidson K, Fischer KA, Mathieu J, Potter JC, Ruohola-Baker H, Moon RT.. Wnt/β-catenin signaling promotes self-renewal and inhibits the primed state transition in naïve human embryonic stem cells. Proc Natl Acad Sci U S A 2016; 113:E6382–E6390. doi: 10.1073/pnas.1613849113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rostovskaya M, Stirparo GG, Smith A. Capacitation of human naive pluripotent stem cells for multi-lineage differentiation. Development 2019; 146:dev172916. doi: 10.1242/dev.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cai L, Ye Z, Zhou BY, Mali P, Zhou C, Cheng L. Promoting human embryonic stem cell renewal or differentiation by modulating Wnt signal and culture conditions. Cell Res 2007; 17:62–72. doi: 10.1038/sj.cr.7310138. [DOI] [PubMed] [Google Scholar]
- 17. Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 2004; 10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 18. Davidson KC, Adams AM, Goodson JM, McDonald CE, Potter JC, Berndt JD, Biechele TL, Taylor RJ, Moon RT.. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc Natl Acad Sci U S A 2012; 109:4485–4490. doi: 10.1073/pnas.1118777109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu K, Sun Y, Liu D, Ye S. Inhibition of Wnt/β-catenin signaling by IWR1 induces expression of Foxd3 to promote mouse epiblast stem cell self-renewal. Biochem Biophys Res Commun 2017; 490:616–622. doi: 10.1016/j.bbrc.2017.06.086. [DOI] [PubMed] [Google Scholar]
- 20. Sugimoto M, Kondo M, Koga Y, Shiura H, Ikeda R, Hirose M, Ogura A, Murakami A, Yoshiki A, Chuva de Sousa Lopes SM, Abe K.. A simple and robust method for establishing homogeneous mouse epiblast stem cell lines by Wnt inhibition. Stem Cell Rep 2015; 4:744–757. doi: 10.1016/j.stemcr.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu J, Okamura D, Li M, Suzuki K, Luo C, Ma L, He Y, Li Z, Chris B, Tamura I, Krause MN, Nery JR et al. An alternative pluripotent state confers interspecies chimaeric competency. Nature 2015; 521:316–321. doi: 10.1038/nature14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim H, Wu J, Ye S, Tai CI, Zhou X, Yan H, Li P, Pera M, Ying QL.. Modulation of β-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal. Nat Commun 2013; 4:2403. doi: 10.1038/ncomms3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou X, Chadarevian JP, Ruiz B, Ying QL. Cytoplasmic and nuclear TAZ exert distinct functions in regulating primed pluripotency. Stem Cell Rep 2017; 9:732–741. doi: 10.1016/j.stemcr.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Ortiz MA, Kotula L. The physiological role of Wnt pathway in normal development and cancer. Exp Biol Med (Maywood) 2020; 245:411–426. doi: 10.1177/1535370220901683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 2009; 5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009; 461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 27. Gao X, Nowak-Imialek M, Chen X, Chen D, Herrmann D, Ruan D, Chun A, Chen H, Eckersley-Maslin MA, Ahmad S, Lee YL, Kobayashi T et al. Establishment of porcine and human expanded potential stem cells. Nat Cell Biol 2019; 21:687–699. doi: 10.1038/s41556-019-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang J, Ryan DJ, Wang W, Tsang CH, Lan G, Masaki H, Gao X, Antunes L, Yu Y, Zhu Z, Wang J, Aleksandra A et al. Establishment of mouse expanded potential stem cells. Nature 2017; 550:393–397. doi: 10.1038/nature24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang Y, Liu B, Xu J, Wang J, Wu J, Shi C, Xu Y, Dong J, Wang C, Lai W, Zhu J, Xiong L et al. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell 2017; 169:243–257.e225. doi: 10.1016/j.cell.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zimmerlin L, Park TS, Huo JS, Verma K, Pather SR, Talbot CC Jr, Agarwal J, Steppan D, Zhang YW, Considine M, Guo H, Zhong X et al. Tankyrase inhibition promotes a stable human naive pluripotent state with improved functionality. Development 2016; 143:4368–4380. doi: 10.1242/dev.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zimmerlin L, Zambidis ET. Pleiotropic roles of tankyrase/PARP proteins in the establishment and maintenance of human naive pluripotency. Exp Cell Res 2020; 390:111935. doi: 10.1016/j.yexcr.2020.111935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahn VE, Chu ML, Choi HJ, Tran D, Abo A, Weis WI. Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6. Dev Cell 2011; 21:862–873. doi: 10.1016/j.devcel.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 2001; 11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 34. Wang K, Zhang Y, Li X, Chen L, Wang H, Wu J, Zheng J, Wu D.. Characterization of the Kremen-binding site on Dkk1 and elucidation of the role of Kremen in Dkk-mediated Wnt antagonism. J Biol Chem 2008; 283:23371–23375. doi: 10.1074/jbc.M802376200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirchhof N, Carnwath JW, Lemme E, Anastassiadis K, Scholer H, Niemann H. Expression pattern of Oct-4 in preimplantation embryos of different species. Biol Reprod 2000; 63:1698–1705. doi: 10.1095/biolreprod63.6.1698. [DOI] [PubMed] [Google Scholar]
- 36. Ozawa M, Sakatani M, Yao J, Shanker S, Yu F, Yamashita R, Wakabayashi S, Nakai K, Dobbs KB, Sudano MJ, Farmerie WG, Hansen PJ.. Global gene expression of the inner cell mass and trophectoderm of the bovine blastocyst. BMC Dev Biol 2012; 12:33. doi: 10.1186/1471-213X-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eijk MJ, Rooijen MA, Modina S, Scesi L, Folkers G, van Tol HTA, Bevers MM, Fisher SR, Lewin HA, Rakcolli D, Galli C, de Vaureix C et al. Molecular cloning, genetic mapping, and developmental expression of bovine POU5F1. Biol Reprod 1999; 60:1093–1103. doi: 10.1095/biolreprod60.5.1093. [DOI] [PubMed] [Google Scholar]
- 38. Ozawa M, Kawakami E, Sakamoto R, Shibasaki T, Goto A, Yoshida N. Development of FGF2-dependent pluripotent stem cells showing naive state characteristics from murine preimplantation inner cell mass. Stem Cell Res 2014; 13:75–87. doi: 10.1016/j.scr.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 39. Tríbulo P, Rivera RM, Ortega Obando MS, Jannaman EA, Hansen PJ. Production and culture of the bovine embryo. In: Herrick JR (ed.), Comparative Embryo Culture: Methods and Protocols. New York: Springer; 2019:115-129. DOI 10.1007/978-1-4939-9566-0_8. [DOI] [PubMed] [Google Scholar]
- 40. Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods 2006; 3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 41. Carvalheira LR, Tribulo P, Borges AM, Hansen PJ. Sex affects immunolabeling for histone 3 K27me3 in the trophectoderm of the bovine blastocyst but not labeling for histone 3 K18ac. PLoS One 2019; 14:e0223570. doi: 10.1371/journal.pone.0223570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Denicol AC, Dobbs KB, McLean KM, Carambula SF, Loureiro B, Hansen PJ. Canonical WNT signaling regulates development of bovine embryos to the blastocyst stage. Sci Rep 2013; 3:1266. doi: 10.1038/srep01266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tribulo P, Leão BCDS, Lehloenya KC, Mingoti GZ, Hansen PJ. Consequences of endogenous and exogenous WNT signaling for development of the preimplantation bovine embryo. Biol Reprod 2017; 96:1129–1141. doi: 10.1093/biolre/iox048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anton R, Kestler HA, Kuhl M. β-catenin signaling contributes to stemness and regulates early differentiation in murine embryonic stem cells. FEBS Lett 2007; 581:5247–5254. doi: 10.1016/j.febslet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 45. Pieters T, Roy F. Role of cell-cell adhesion complexes in embryonic stem cell biology. J Cell Sci 2014; 127:2603–2613. doi: 10.1242/jcs.146720. [DOI] [PubMed] [Google Scholar]
- 46. Hagos EG, Ghaleb AM, Dalton WB, Bialkowska AB, Yang VW. Mouse embryonic fibroblasts null for the Kruppel-like factor 4 gene are genetically unstable. Oncogene 2009; 28:1197–1205. doi: 10.1038/onc.2008.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kennedy AL, McBryan T, Enders GH, Johnson FB, Zhang R, Adams PD. Senescent mouse cells fail to overtly regulate the HIRA histone chaperone and do not form robust senescence associated heterochromatin foci. Cell Div 2010; 5:16. doi: 10.1186/1747-1028-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell 2009; 4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 49. Tribulo P, Moss JI, Ozawa M, Jiang Z, Tian XC, Hansen PJ. WNT regulation of embryonic development likely involves pathways independent of nuclear CTNNB1. Reproduction 2017; 153:405–419. doi: 10.1530/REP-16-0610. [DOI] [PubMed] [Google Scholar]
- 50. Sumi T, Oki S, Kitajima K, Meno C. Epiblast ground state is controlled by canonical Wnt/β-catenin signaling in the postimplantation mouse embryo and epiblast stem cells. PLoS One 2013; 8:e63378. doi: 10.1371/journal.pone.0063378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kurek D, Neagu A, Tastemel M, Tüysüz N, Lehmann J, van de Werken HJG, Philipsen S, van der Linden R, Maas A, van IJcken WFJ, Drukker M, ten Berge D.. Endogenous WNT signals mediate BMP-induced and spontaneous differentiation of epiblast stem cells and human embryonic stem cells. Stem Cell Rep 2015; 4:114–128. doi: 10.1016/j.stemcr.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Osteil P, Studdert JB, Goh HN, Wilkie EE, Fan X, Khoo PL, Peng G, Salehin N, Knowles H, Han JDJ, Jing N, Fossat N et al. Dynamics of Wnt activity on the acquisition of ectoderm potency in epiblast stem cells. Development 2019; 146:dev172858. doi: 10.1242/dev.172858. [DOI] [PubMed] [Google Scholar]
- 53. Killick R, Ribe EM, Al-Shawi R, Malik B, Hooper C, Fernandes C, Dobson R, Nolan PM, Lourdusamy A, Furney S, Lin K, Breen G et al. Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol Psychiatry 2014; 19:88–98. doi: 10.1038/mp.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhuang X, Zhang H, Li X, Li X, Cong M, Peng F, Yu J, Zhang X, Yang Q, Hu G.. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol 2017; 19:1274–1285. doi: 10.1038/ncb3613. [DOI] [PubMed] [Google Scholar]
- 55. Bhavanasi D, Speer KF, Klein PS. CKAP4 is identified as a receptor for Dickkopf in cancer cells. J Clin Invest 2016; 126:2419–2421. doi: 10.1172/JCI88620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Causeret F, Sumia I, Pierani A. Kremen1 and Dickkopf1 control cell survival in a Wnt-independent manner. Cell Death Differ 2016; 23:323–332. doi: 10.1038/cdd.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kulak O, Chen H, Holohan B, Wu X, He H, Borek D, Otwinowski Z, Yamaguchi K, Garofalo LA, Ma Z, Wright W, Chen C et al. Disruption of Wnt/β-catenin signaling and telomeric shortening are inextricable consequences of tankyrase inhibition in human cells. Mol Cell Biol 2015; 35:2425–2435. doi: 10.1128/MCB.00392-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Narwal M, Venkannagari H, Lehtio L. Structural basis of selective inhibition of human tankyrases. J Med Chem 2012; 55:1360–1367. doi: 10.1021/jm201510p. [DOI] [PubMed] [Google Scholar]
- 59. Thorsell AG, Ekblad T, Karlberg T, Löw M, Pinto AF, Trésaugues L, Moche M, Cohen MS, Schüler H.. Structural basis for potency and promiscuity in poly(ADP-ribose) polymerase (PARP) and tankyrase inhibitors. J Med Chem 2017; 60:1262–1271. doi: 10.1021/acs.jmedchem.6b00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kimura M, Nakajima-Koyama M, Lee J, Nishida E. Transient expression of WNT2 promotes somatic cell reprogramming by inducing β-catenin nuclear accumulation. Stem Cell Rep 2016; 6:834–843. doi: 10.1016/j.stemcr.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Minami I, Yamada K, Otsuji TG, Yamamoto T, Shen Y, Otsuka S, Kadota S, Morone N, Barve M, Asai Y, Tenkova-Heuser T, Heuser JE et al. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep 2012; 2:1448–1460. doi: 10.1016/j.celrep.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 62. Willems E, Spiering S, Davidovics H, Lanier M, Xia Z, Dawson M, Cashman J, Mercola M.. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res 2011; 109:360–364. doi: 10.1161/CIRCRESAHA.111.249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li S, Liu D, Fu Y, Zhang C, Tong H, Li S, Yan Y.. Podocan promotes differentiation of bovine skeletal muscle satellite cells by regulating the Wnt4-β-catenin signaling pathway. Front Physiol 2019; 10:1010. doi: 10.3389/fphys.2019.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bao S, Tang F, Li X, Hayashi K, Gillich A, Lao K, Surani MA.. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature 2009; 461:1292–1295. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, Mckay RDG. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007; 448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 66. Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci U S A 2010; 107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tsukiyama T, Ohinata Y. A modified EpiSC culture condition containing a GSK3 inhibitor can support germline-competent pluripotency in mice. PLoS One 2014; 9:e95329. doi: 10.1371/journal.pone.0095329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR, Cameron PM, Meinke PT, Liverton N, Weng Y, DeMartino JA.. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg Med Chem Lett 2002; 12:1219–1223. doi: 10.1016/s0960-894x(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 69. Brewer JR, Mazot P, Soriano P. Genetic insights into the mechanisms of Fgf signaling. Genes Dev 2016; 30:751–771. doi: 10.1101/gad.277137.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Deo DD, Axelrad TW, Robert EG, Marcheselli V, Bazan NG, Hunt JD. Phosphorylation of STAT-3 in response to basic fibroblast growth factor occurs through a mechanism involving platelet-activating factor, JAK-2, and Src in human umbilical vein endothelial cells. Evidence for a dual kinase mechanism. J Biol Chem 2002; 277:21237–21245. doi: 10.1074/jbc.M110955200. [DOI] [PubMed] [Google Scholar]
- 71. Frutos CA, Vega S, Manzanares M, Flores JM, Huertas H, Martínez-Frías ML, Nieto MA. Snail1 is a transcriptional effector of FGFR3 signaling during chondrogenesis and achondroplasias. Dev Cell 2007; 13:872–883. doi: 10.1016/j.devcel.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 72. Karuppaiah K, Yu K, Lim J, Chen J, Smith C, Long F, Ornitz DM. FGF signaling in the osteoprogenitor lineage non-autonomously regulates postnatal chondrocyte proliferation and skeletal growth. Development 2016; 143:1811–1422. doi: 10.1242/dev.131722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bousoik E, Aliabadi HM. “Do we know Jack” about JAK? A closer look at JAK/STAT signaling pathway. Front Oncol 2018; 8:287. doi: 10.3389/fonc.2018.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yu L, Wei Y, Sun H, Mahdi AK, Pinzon Arteaga CA, Sakurai M, Schmitz DA, Zheng C, Ballard ED, Li J, Tanaka N, Kohara Aet al. Derivation of intermediate pluripotent stem cells amenable to primordial germ cell specification. Cell Stem Cell 2021; 28:1–18. doi: 10.1016/j.stem.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 75. Deng Y, Lao Y, Ruan Q, Zhang J, Luo C, Shi D, Lu F. Activation of Wnt/β-catenin signaling pathway enhances the derivation of buffalo (Bubalus bubalis) embryonic stem cell-like cells. Cell Reprogram 2020; 22:217–225. doi: 10.1089/cell.2020.0027. [DOI] [PubMed] [Google Scholar]
- 76. Warzych E, Pawlak P, Lechniak D, Madeja ZE. WNT signalling supported by MEK/ERK inhibition is essential to maintain pluripotency in bovine preimplantation embryo. Dev Biol 2020; 463:63–76. doi: 10.1016/j.ydbio.2020.04.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.


